Abstract
Key Clinical Message
Patients with antineutrophil cytoplasmic antibody (ANCA)‐associated vasculitis, specifically with myeloperoxidase (MPO)‐ANCA, would have a risk for developing corneal melt and perforation abruptly in a short period. It is desirable to have a team of collaboration of rheumatologists and other specialties.
Abstract
An 80‐year old man who had been diagnosed 5.5 years previously as ANCA‐associated vasculitis by temporal artery biopsy developed corneal melt and perforation with scleritis in both eyes. He underwent successful cataract surgery and retained ambulatory vision with the aid of intravenous rituximab. Two additional patients with similar manifestations were found in the literature.
Keywords: ANCA‐associated vasculitis, corneal melt and perforation, rituximab, scleritis, temporal artery biopsy
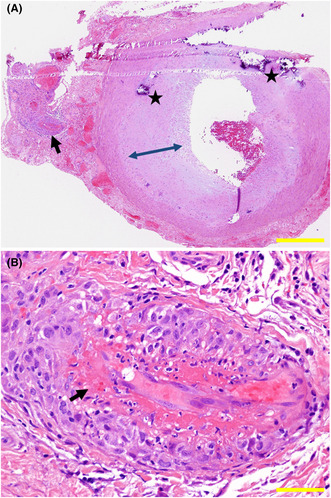
Biopsy of temporal artery on the left side. Overview of biopsy specimen (A), showing normal structure of a large artery with nonspecific calcification (stars) and subendothelial thickening (double arrowheads). Small vessels (arrow in A) as the branch of the large vessel have endothelial fibrinoid necrosis (arrow in B) and perivascular infiltration with mononuclear cells. Hematoxylin–eosin stain. Yellow scale bar = 500 μm in A, 50 μm in B.
1. INTRODUCTION
Vasculitis is currently classified by the size of vessels involved by inflammation: large‐vessel vasculitis such as Takayasu arteritis and giant cell arteritis, medium‐sized‐vessel vasculitis such as polyarteritis nodosa and Kawasaki disease, and small‐vessel vasculitis such as immune complex small‐vessel vasculitis and antineutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. 1 ANCA‐associated vasculitis is characterized by necrotizing small vessels, including capillaries, venules, arterioles, and sometimes small arteries, together with the presence of autoantibodies against neutrophils, myeloperoxidase (MPO)‐ANCA, proteinase 3 (PR3)‐ANCA or both. ANCA‐associated vasculitis has three categories, microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, previously called Wegener granulomatosis), and eosinophilic granulomatosis with polyangiitis (EGPA, previously called Churg‐Strauss syndrome).
Pulmonary lesions and renal glomerular lesions are most common systemic manifestations in ANCA‐associated vasculitis. 1 Scleritis is a rare but famous eye manifestation in ANCA‐associated vasculitis and usually accompanies circumcorneal infiltration with leukocytes. 2 , 3 , 4 , 5 , 6 , 7 The ocular fundus manifestations, such as acute posterior multifocal placoid pigment epitheliopathy (APMPPE) 8 and multifocal choroiditis (subretinal fibrosis and uveitis syndrome), 9 are also noted on rare occasions as the manifestations of choroidal vascular inflammation and occlusion. 2 We experienced a patient with ANCA‐associated vasculitis who had been diagnosed pathologically by temporal artery biopsy and positive serum titers of MPO‐ANCA, and later developed corneal melt and perforation in the active stage of scleritis in both eyes. Cataract surgery was successfully done to maintain ambulatory vision in one eye of this patient under the therapeutic control by intravenous rituximab. We also reviewed two additional similar cases in the literature who showed rapidly progressive corneal melt in ANCA‐associated vasculitis. 10 , 11
2. CASE REPORT
A 75‐year‐old man noticed palpitation and dyspnea, and visited a local hospital. At admission, he had atrial fibrillation and regained regular sinus rhythm after asystole for a few minutes. He showed serum C‐reactive protein (CRP) elevated to 5–7 mg/dL and developed fever at 38°C 2 days later. Oral levofloxacin, then switched to intravenous cefazolin, in a week, did not lead to lowering of the fever. After the antibiotics was discontinued, he still showed fever at 38°C and a high level of serum CRP. Chest and abdominal computed tomographic scan detected nothing abnormal, blood and urine cultures were negative for bacteria. He was referred to University Hospital of Kawasaki Medical School. In the past history, he had undergone appendectomy at 20 years old, hemorrhoid surgery at 52 years old, colectomy for rectal cancer at 55 years old, surgeries for inguinal herniation and hemorrhoids fistula at 59 years old, and intestinal adhesion dissection for ileus at 62 years old. He had been taking warfarin and verapamil for atrial fibrillation since the age 73 years. He was a previous smoker with 20 packs daily for 20 years from the age of 20 to 40 years.
At the referral, he had jaw claudication, cervical and pharyngeal pain, muscular pain in lower extremities and around the hip joints, showed weight loss by 3 kg in 2 weeks. He had no eye symptoms or signs including optic neuropathy. The complete blood cell counts showed white blood cells at 9.33 × 103/μL with neutrophils at 86.5%, red blood cells at 4.01 × 106/μL, and platelets at 335 × 103/μL. The liver and kidney function tests were within normal limits. The urinalysis was also normal. Serum CRP was at a high level of 10.14 mg/dL, soluble interleukin‐2 receptor (sIL‐2R) elevated at 1357 U/mL, ferritin elevated at 390 ng/mL, d‐dimer elevated at 3.5 μg/mL, and brain natriuretic peptide (BNP) elevated at 72.2 pg/mL. Serum rheumatoid factor was elevated at 44 IU/mL, MPO‐ANCA elevated at 19.8 U/mL while PR3‐ANCA or anti‐cyclic citrullinated peptide (CCP) antibody, Sjogren syndrome (SS)‐A antibody or antinuclear antibodies were negative. Screening tests for infectious diseases, such as serological tests for syphilis, hepatitis B virus surface antigen and antibody (HBsAg and HBsAb), hepatitis C virus antibody (HCV‐Ab), and human immunodeficiency virus‐1/2 antigen/antibodies (HIV‐1/2 Ag/Ab) screening test, were all negative. Interferon‐γ‐releasing assay with T‐SPOT was negative as well.
Suspicious of temporal arteritis, he underwent biopsy of the temporal artery on the left side which showed fibrinoid necrosis of small branch vessels with perivascular infiltration with mononuclear cells (Figure 1B) in the background of intact structure of the temporal artery itself (Figure 1A). Together with positive MPO‐ANCA at 19.8 U/mL, he was diagnosed microscopic polyangiitis with no involvement in the other organs including the lungs and kidneys. He began to have oral prednisolone at 50 mg daily which was gradually tapered to 10 mg in half a year (Figure 2). At this dose of oral prednisolone, he began to show elevated levels of MPO‐ANCA at 48.8 U/mL and 129 U/mL, respectively, in a month and in 6 months.
FIGURE 1.
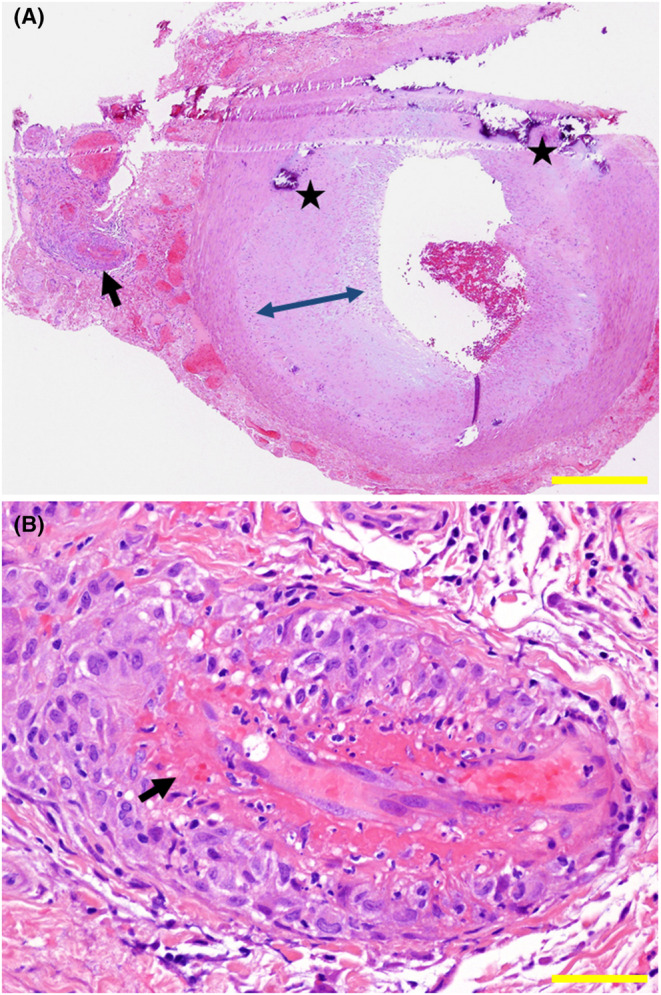
Biopsy of temporal artery on the left side. Overview of biopsy specimen (A), showing normal structure of a large artery with nonspecific calcification (stars) and subendothelial thickening (double arrowheads). Small vessels (arrow in A) as the branch of the large vessel have endothelial fibrinoid necrosis (arrow in B) and perivascular infiltration with mononuclear cells. Hematoxylin–eosin stain. Yellow scale bar = 500 μm in A, 50 μm in B.
FIGURE 2.
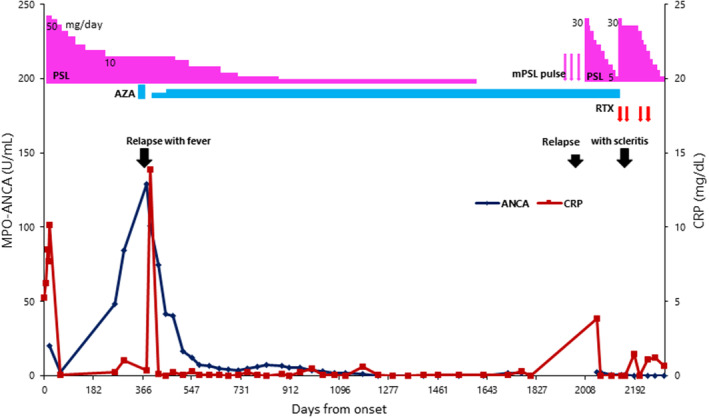
Serum levels of C‐reactive protein (CRP) and myeloperoxidase‐antineutrophil cytoplasmic antibody (MPO‐ANCA) in the entire course under the treatment with oral prednisolone (PSL) and azathioprine (AZA), mini‐pulse therapy with methylprednisolone (mPSL) 500 mg daily for 3 days, and intravenous administration of rituximab (RTX, 375 mg/m2).
One year later from the initial visit at the age of 76 years, oral azathioprine at 100 mg in combination with sulfamethoxazole/trimethoprim was started but discontinued due to skin rashes, fever, and dyspnea which were considered as adverse events of sulfamethoxazole/trimethoprim. In 2 months, he still showed persistent high levels of MPO‐ANCA only with oral prednisolone 10 mg daily, and thus, began to have additional azathioprine at 25 mg daily which was increased to the dose at 50 mg daily in the next 2 months. In the following 2 months, serum MPO‐ANCA decreased to a low level less than 10 U/mL. Oral prednisolone at 10 mg daily was gradually tapered in the period of 3 years and discontinued at the age of 79 years, 4.5 years later from the initial visit while oral azathioprine was maintained at 50 mg daily (Figure 2).
He was stable for next 1 year with oral azathioprine at 50 mg daily only until the age of 80 years, 5.5 years from the initial visit, when he developed bulbar conjunctival injection and corneal haze in both eyes. He had history of peripheral corneal ulcer in both eyes for the previous 2 years and had been using 0.1% betamethasone eye drops 2–4 times daily, prescribed by a local eye doctor. At referral for eye examinations, he showed diffuse scleritis with peripheral corneal infiltration in both eyes. The best‐corrected visual acuity in decimals was 0.02 in the right eye and 0.2 in the left eye. In the background of diffuse scleritis with peripheral corneal infiltration in both eyes, the right eye showed corneal epithelial defect and stromal thinning at the center and crescent‐shaped peripheral corneal ulcer extending from the nasal side to the inferior side (Figure 3A) while the left eye showed the intact central cornea with crescent‐shaped peripheral corneal thinning on the temporal side sequel to the previous ulcer (Figure 3B). He had posterior subcapsular cataract in both eyes. The intraocular pressure was 10 mmHg in both eyes and the ocular fundi in both eyes were normal.
FIGURE 3.
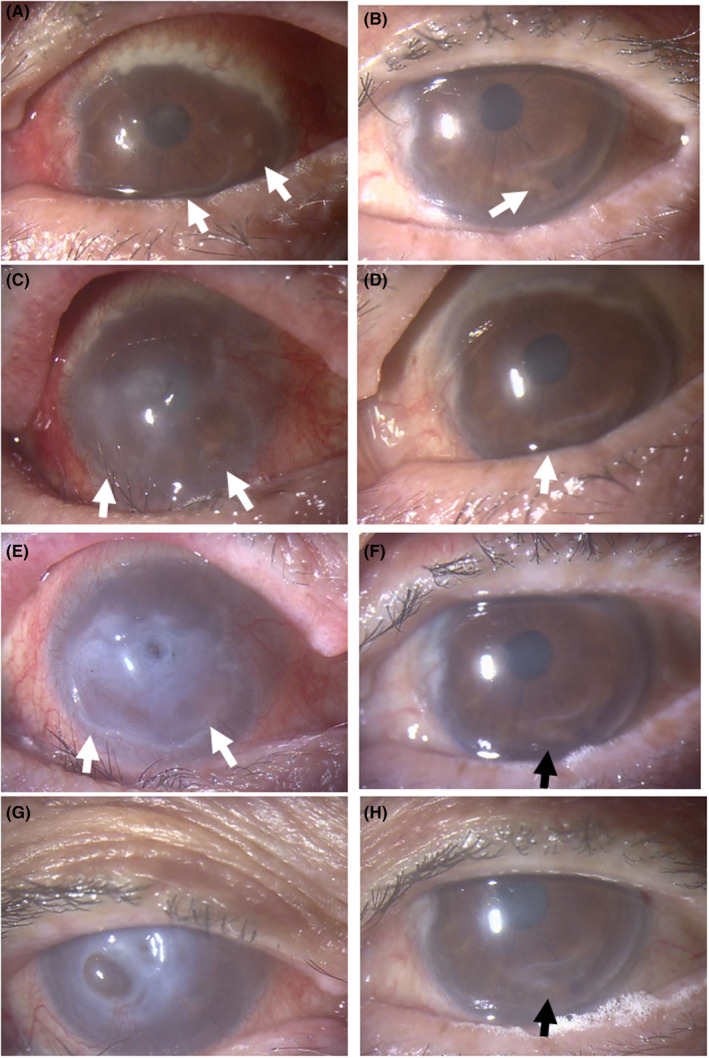
Biomicroscopic images. Diffuse scleritis with massive peripheral corneal infiltration and crescent‐shaped corneal ulcer (arrows) on the nasal to inferior side in the right eye (A) and crescent‐shaped corneal ulcer (arrow) on the temporal to inferior side in the left eye (B) at referral to an ophthalmologist. In a month, note central corneal haze and infiltrates with inferior crescent‐shaped wide ulcer (arrows) in the right eye (C) and peripheral corneal infiltrates with inferior ulcer (arrow) in the left eye (D). In the following 3 weeks, the central cornea has been perforated in the right eye with the inferior wide ulcer (arrows, E) while the corneal ulcer is stable in the left eye (arrow, F). Four months after the referral, the right eye is stable with corneal opacity with iris incarceration (G) and the left eye is stable with healed corneal ulcer (H).
With the diagnosis of ocular manifestations of ANCA‐associated vasculitis, he was consulted with a rheumatologist. In a month or so, the right eye showed central corneal perforation with iris incarceration with active scleritis (Figure 3C,E) while the left eye showed impending corneal melt in the lower part which was the site of the previous ulcer (Figure 3D,F). He stopped using 0.1% betamethasone eye drops and immediately underwent steroid mini‐pulse therapy with drip infusion of methylprednisolone 500 mg daily for 3 days, followed by oral prednisolone 30 mg daily, together with current oral azathioprine 50 mg daily, leading to stable condition (Figure 2): corneal perforation with iris incarceration in the right eye (Figure 3G) and healing peripheral corneal ulcer in the left eye (Figure 3H). When oral prednisolone was tapered to 12.5 mg daily in the next 2 months, the left eye showed corneal melt (Figure 4A) and impending corneal perforation (Figure 4B) in the lower part which was the site of the previous ulcer. The conjunctival sac culture in preparation for cataract surgery was negative at that time in both eyes.
FIGURE 4.
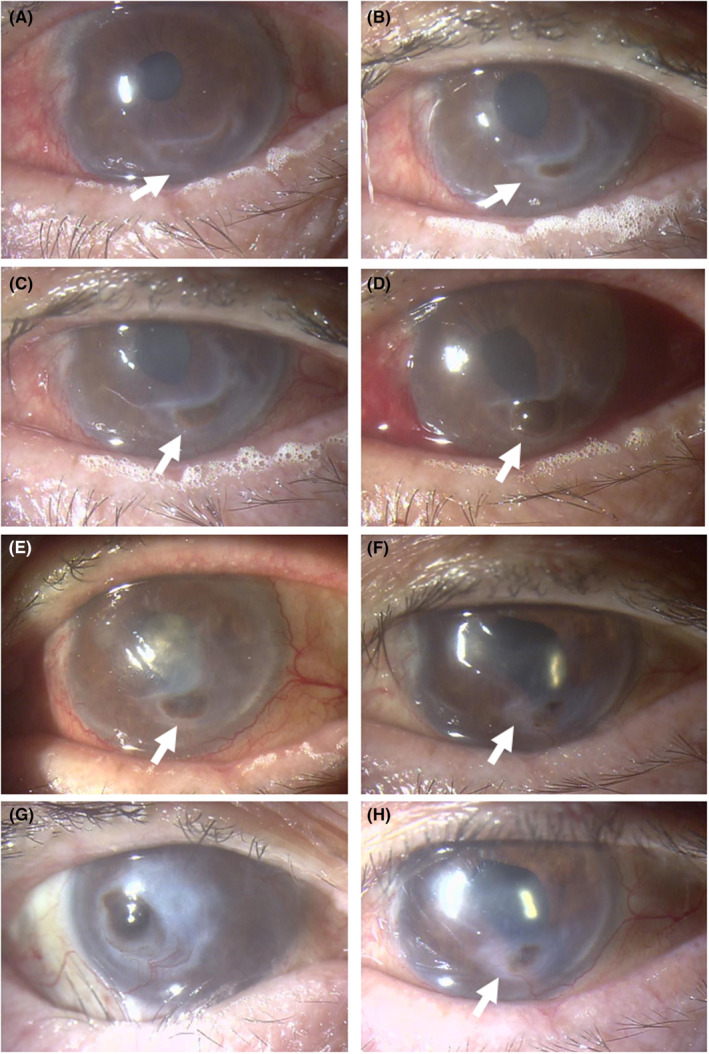
Biomicroscopic images. Crescent‐shaped ulcer in the inferior cornea of the left eye becomes active (arrow, A) in 2 weeks, and has been perforated with iris incarceration in next 1 month (arrow, B). A week later, note epithelialized corneal perforation in the left eye just before cataract surgery (arrow, C) and next day after cataract surgery with intraocular lens implantation (arrow, D). The central corneal haze due to delayed epithelialization 3 weeks after surgery (arrow, E) becomes clear in 2 months (arrow, F). Four months after cataract surgery, scleritis has subsided in the right eye with corneal opacity and iris incarceration (G) and in the left eye with relatively transparent central cornea with iris incarceration and adhesion in the lower part (arrow, H).
Oral prednisolone was thus increased to 30 mg daily and intravenous rituximab (500 mg/body: 375 mg/m2) was given twice in the interval of 2 weeks (Figure 2). Four months later from the initial visit to ophthalmology, the patient underwent successful cataract surgery with intraocular lens implantation (Figure 4C,D) on the next day after the second dose of rituximab. One month after the second dose, the third and fourth doses of intravenous rituximab 500 mg were given in the interval of 1 month, and oral prednisolone was tapered to 20 mg daily. By switching 0.1% betamethasone eye drops to 0.1% fluorometholone eye drops, the central corneal haze in the left eye due to the delayed corneal epithelialization 3 weeks after the surgery (Figure 4E) became clear in 2 months, leading to the recovery of visual acuity at 0.09 in the left eye (Figure 4F). Around this time, the patient had general fatigue and fever. Chest computed tomographic scan showed pulmonary infiltrates on both sides and serum β‐d‐glucan was high at 1344 pg/mL, indicative of pulmonary fungal infection. Oral voriconazole 300 mg daily, tapered to 200 mg daily, was given for a month, oral azathioprine was discontinued, and oral prednisolone was tapered at a fast pace to 5 mg daily in 1 month. At the age of 81 years, 4 months after the cataract surgery, the best‐corrected visual acuity was light perception in the right eye and 0.05 in the left eye. The intraocular pressure was 4 mmHg in both eyes. Scleritis in both eyes subsided with no corticosteroid eye drops. The right eye was stable with corneal opacity with iris incarceration and mature cataract (Figure 4G). The cornea in the left eye was transparent as a whole with stromal thinning in the lower part with iris adhesion (Figure 4H). Throughout the 10 months from the initial visit to the last visit at ophthalmology, serum MPO‐ANCA remained at the low levels less than 1.0 U/mL. He died of pneumonia further 2 months later.
3. DISCUSSION
The present patient is unique at the point that he developed active scleritis and peripheral corneal infiltration in both eyes in the stable systemic condition of ANCA‐associated vasculitis which had been diagnosed 5.5 years previously. He appeared to have two‐year history of peripheral corneal ulcer in both eyes. At the time of ophthalmic presentation, the peripheral corneal ulcer at the healed stage in both eyes became active and finally led to corneal perforation in such a short period as a month or so in the right eye. Under the circumstances, oral prednisolone was resumed at the dose of 30 mg daily and tapered gradually in combination of current oral azathioprine 50 mg daily. Unfortunately, the patient lost the vision in the right eye due to the corneal opacity with iris incarceration and could not undergo cataract surgery in the right eye.
In the course of tapering of oral prednisolone from 30 mg daily to 12.5 mg daily, the corneal ulcer in the left eye became active and tended to be perforated. To save the vision in the left eye, cataract surgery was planned. In consultation with a rheumatologist, two doses of intravenous rituximab in the interval of 2 weeks were scheduled before cataract surgery and oral prednisolone was maintained at the dose of 25 mg daily. At this condition, he underwent cataract surgery in the left eye at a narrow window of safety. After the successful cataract surgery, additional two doses of intravenous rituximab were given in the interval of a month and oral prednisolone was tapered to 5 mg daily in 4 months. We initially planned four doses of weekly intravenous administration of rituximab as the standard of treatment, but changed the interval to 2 weeks before cataract surgery and to 4 weeks after the surgery, based on the patient's systemic condition.
Another point to note in this patient is how to use topical corticosteroid eye drops. Topical corticosteroids can control active inflammation of scleritis but inhibit the corneal epithelialization in case of corneal ulcer, leading to delayed healing of the ulcer. It is, thus, important to take a balance between inflammatory control and corneal healing in case of ulcer formation, sequel to scleritis and peripheral corneal infiltration. To enhance the healing of corneal ulcer at the setting of active scleritis, the number of daily instillation of topical corticosteroids is changed in the range from once to six times daily, and corticosteroid eye drops would be switched from 0.1% betamethasone to 0.1% fluorometholone, based on their adverse events to inhibit corneal epithelialization. In parallel with topical treatment on the ocular surface, systemic treatment can be initiated or strengthened in the repertoire of prednisolone, immunosuppresants such as cyclophosphamide and azathioprine, and biologics as rituximab. 12 , 13 , 14 In the present patient, the introduction of rituximab led to the subsidence of scleritis and prevented corneal melt from deterioration in the left eye even in the condition of the reduced use of topical corticosteroids.
From the viewpoint of the diagnosis as ANCA‐associated vasculitis, the present patient showed symptoms suspicious of temporal arteritis at the initial presentation and underwent the temporal artery biopsy. The pathological examination revealed fibrinoid necrosis of small vessels branched from the temporal artery, but not the picture of giant cell arteritis of large vessels regarding the temporal artery itself. Temporal arteritis‐like symptoms and signs have been known to be caused by ANCA‐associated vasculitis involving small branch vessels from the temporal artery. 15 , 16 In the present patient, MPA in ANCA‐associated vasculitis was diagnosed on the basis of temporal arteritis‐like presentation, pathological findings of the temporal artery biopsy, and blood examinations such as positive titers of MPO‐ANCA and elevated levels of CRP. 1 The patient can be diagnosed MPA with the sum of scores as six in the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for MPA. 17 It is still curious in this patient that scleritis in both eyes occurred in the stable systemic condition, several years after the initial diagnosis of ANCA‐associated vasculitis. Careful follow‐up by rheumatologists and other specialties such as ophthalmologists would be desirable to detect earlier signs of active inflammation in specific organs, even in the stable condition which is monitored by systemic indicators such as CRP. Furthermore, it should be noted in this patient that the titers of MPO‐ANCA remained low throughout the period of eye manifestations.
To analyze similar cases, PubMed, and Google Scholar were searched for the key words “ANCA‐associated vasculitis” and “corneal melt or corneal perforation”. Two patients with sufficient description 10 , 11 were found and summarized, together with the present case, in Table 1. Three patients are all men with the age at the initial visit ranging from 58 to 75 years. It should be noted that all three patients had a common feature as positive titers of MPO‐ANCA. The diagnoses were GPA, EGPA, and MPA in each patient (Table 1). The timing of the diagnosis as ANCA‐associated vasculitis was concurrent with the onset of eye manifestations in one patient while the diagnosis had been established before the onset of eye manifestations in two patients.
TABLE 1.
Review of three patients with ANCA‐associated vasculitis who showed scleritis and corneal melt, including the present patient.
| Case No./Gender/Age at onset | Diagnosis Timing a | Biopsy | MPO‐ANCA at initial visit | PR3‐ANCA at initial visit | Organs involved | Other features | Right eye features | Left eye features | Systemic treatment at the time of eye manifestations | Authors (year) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/Male/72 |
EGPA Previously (unknown period) |
Not done | High titer | Not described | Pneumonia with oral prednisolone 30 mg daily in a year |
Eosinophilia Asthma |
None | Episcleritis Corneal melt & perforation |
Prednisolone tapered four doses of monthly intravenous cyclophosphamide Switch to mycophenolate mofetil with prednisolone 7.5 mg daily |
Fennelly et al. (2019) 10 |
| 2/Male/58 |
GPA Concurrent |
Not done | Positive | Not described |
Bilateral pulmonary nodules Paranasal sinusitis Nasopharyngeal mucosal ulcer |
Macroscopic hematuria Weight loss |
Episcleritis Corneal melt & perforation |
Episcleritis Corneal melt & perforation |
Steroid pulse two doses of intravenous cyclophosphamide Dead of pneumonia in 2 months |
Vargas‐Villanueva et al. (2020) 11 |
| 3/Male/75 |
MPA 5.5 years previously |
Temporal artery on left side |
19.8 U/mL up to 129 U/mL 1 year later |
Undetectable | None | Ileus |
Scleritis Corneal melt & perforation |
Scleritis Corneal melt & perforation |
Azathioprine 50 mg daily Steroid mini‐pulse four doses of rituximab Prednisolone 5 mg daily Dead of pneumonia in a year |
This case |
Note: Steroid pulse therapy with methylprednisolone 1000 mg daily for 3 days. Steroid mini‐pulse therapy with methylprednisolone 500 mg daily for 3 days.
Abbreviations: GPA, granulomatosis with polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Indicates the timing of the diagnosis relative to the onset of eye manifestations.
In conclusion, patients with ANCA‐associated vasculitis, specifically with positive titers of MPO‐ANCA, would have a risk for developing corneal melt and perforation abruptly in a short period. When the corneal melt and perforation would once occur, the visual recovery is limited. It is recommended to detect eye manifestations on the earlier phase and to introduce or reinforce systemic treatment with prednisolone, immunosuppresants, and biologics at the aim of protecting the vision from devastating condition sequel to scleritis and peripheral corneal infiltration. To this end, continued ophthalmic surveillance will be desirable in patients with ANCA‐associated vasculitis. Multidisciplinary management and collaborative care will provide better overall outcomes and reduce complications since ANCA‐associated vasculitis has a wide range of systemic and multiorgan involvement.
AUTHOR CONTRIBUTIONS
Toshihiko Matsuo: Conceptualization; data curation; investigation; methodology; writing – original draft. Sumie Hiramatsu‐Asano: Data curation; investigation; methodology; writing – review and editing. Hiroshi Sawachika: Data curation; investigation. Hirotake Nishimura: Data curation; investigation; writing – review and editing.
FUNDING INFORMATION
The authors receive no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICS STATEMENT
Ethics committee review was not applicable due to the case report design, based on the Ethical Guidelines for Medical and Health Research Involving Human Subjects, issued by the Government of Japan.
CONSENT
Written consent was obtained from the patient for his anonymized information to be published in this article.
ACKNOWLEDGMENTS
None.
Matsuo T, Hiramatsu‐Asano S, Sawachika H, Nishimura H. ANCA‐associated vasculitis with scleritis, corneal melt, and perforation rescued by rituximab: Case report and literature review. Clin Case Rep. 2023;11:e7595. doi: 10.1002/ccr3.7595
DATA AVAILABILITY STATEMENT
Additional data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1‐11. [DOI] [PubMed] [Google Scholar]
- 2. Matsuo T. Eye manifestations in patients with perinuclear antineutrophil cytoplasmic antibody‐associated vasculitis: case series and literature review. Jpn J Ophthalmol. 2007;51:131‐138. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt J, Pulido JS, Matteson EL. Ocular manifestations of systemic disease: antineutrophil cytoplasmic antibody‐associated vasculitis. Curr Opin Ophthalmol. 2011;22:489‐495. [DOI] [PubMed] [Google Scholar]
- 4. Ungprasert P, Crowson CS, Cartin‐Ceba R, et al. Clinical characteristics of inflammatory ocular disease in anti‐neutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. Rheumatology (Oxford). 2017;56:1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshida A, Watanabe M, Okubo A, Kawashima H. Clinical characteristics of scleritis patients with emphasized comparison of associated systemic diseases (anti‐neutrophil cytoplasmic antibody‐associated vasculitis and rheumatoid arthritis). Jpn J Ophthalmol. 2019;63:417‐424. [DOI] [PubMed] [Google Scholar]
- 6. Miyanaga M, Takase H, Ohno‐Matsui K. Anti‐neutrophil cytoplasmic antibody‐associated ocular manifestations in Japan: a review of 18 patients. Ocul Immunol Inflamm. 2021;29:991‐996. [DOI] [PubMed] [Google Scholar]
- 7. Junek ML, Zhao L, Garner S, et al. Ocular manifestations of ANCA‐associated vasculitis. Rheumatology (Oxford). 2022:keac663. doi: 10.1093/rheumatology/keac663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsuo T, Horikoshi T, Nagai C. Acute posterior multifocal placoid pigment epitheliopathy and scleritis in a patient with pANCA‐positive systemic vasculitis. Am J Ophthalmol. 2002;133:566‐568. [DOI] [PubMed] [Google Scholar]
- 9. Matsuo T, Matsuo N. Progressive subretinal fibrosis in patients with rheumatoid arthritis and renal dysfunction. Ophthalmologica. 1998;212:289‐294. [DOI] [PubMed] [Google Scholar]
- 10. Fennelly E, Greenan E, Murphy CC. Corneal melt secondary to eosinophilic granulomatosis with polyangiitis. BMJ Case Rep. 2019;12:e229859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas‐Villanueva A, Carvajal‐Saiz N, Munoz‐Ortiz J, de‐la‐Torre A. Bilateral corneal perforation and iris prolapse as a complication non‐peripheral ulcerative keratitis in a patient with fulminant granulomatosis with polyangiitis. J Ophthalmic Inflamm Infect. 2020;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujita Y, Fukui S, Endo Y, et al. Peripheral ulcerative keratitis associated with granulomatosis with polyangiitis emerging despite cyclophosphamide, successfully treated with rituximab. Intern Med. 2018;57:1783‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weng X, Iwata D, Namba K, et al. Posterior scleritis with anti‐neutrophil cytoplasmic antibody‐associated vasculitis utilizing rituximab therapy to maintain remission: a case report. Am J Ophthalmol Case Rep. 2022;25:101333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nevares A, Raut R, Libman B, Hajj‐Ali R. Noninfectious autoimmune scleritis: recognition, systemic associations, and therapy. Curr Rheumatol Rep. 2022;22:11. [DOI] [PubMed] [Google Scholar]
- 15. Delaval L, Samson M, Schein F, et al. Temporal arteritis revealing antineutrophil cytoplasmic antibody‐associated vasculitides: a case‐control study. Arthritis Rheumatol. 2021;73:286‐294. [DOI] [PubMed] [Google Scholar]
- 16. Suyama Y, Ikeda R, Tanaka S, Hagiwara K. ANCA‐associated small‐vessel vasculitis surrounding the temporal artery. QJM. 2018;111:197‐198. [DOI] [PubMed] [Google Scholar]
- 17. Suppiah R, Robson JC, Grayson PC, et al. 2022 American College of Rheumatology/European Alliance of associations for rheumatology classification criteria for microscopic polyangiitis. Arthritis Rheumatol. 2022;74:400‐406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data are available upon reasonable request to the corresponding author.


