Abstract
Neurodevelopmental disorders (NDDs) are mostly diagnosed around the age of 4–5 years, which is too late considering that the brain is most susceptive to interventions during the first two years of life. Currently, diagnosis of NDDs is based on observed behaviors and symptoms, but identification of objective biomarkers would allow for earlier screening. In this longitudinal study, we investigated the relationship between repetition and change detection responses measured using an EEG oddball task during the first year of life and at two years of age, and cognitive abilities and adaptive functioning during preschool years (4 years old). Identification of early biomarkers is challenging given that there is a lot of variability in developmental courses among young infants. Therefore, the second aim of this study is to assess whether brain growth is a factor of interindividual variability that influences repetition and change detection responses. To obtain variability in brain growth beyond the normative range, infants with macrocephaly were included in our sample. Thus, 43 normocephalic children and 20 macrocephalic children were tested. Cognitive abilities at preschool age were assessed with the WPPSI-IV and adaptive functioning was measured with the ABAS-II. Time–frequency analyses were conducted on the EEG data. Results indicated that repetition and change detection responses in the first year of life predict adaptive functioning at 4 years of age, independently of head circumference. Moreover, our findings suggested that brain growth explains variability in neural responses mostly in the first years of life, so that macrocephalic children did not display repetition suppression responses, while normocephalic children did. This longitudinal study demonstrates that the first year of life is an important period for the early screening of children at risk of developing NDDs.
Subject terms: Neuroscience, Cognitive neuroscience, Learning and memory, Sensory processing
Introduction
Infancy is a crucial period in brain development since the brain is growing fastest and undergoes multiple changes during the first two years of life1. For the brain to develop healthily, dynamic and adaptive interactions between gene expression and environmental input are essential2. In this period, the brain is particularly vulnerable to environmental insults or pathogenic genetic alterations that can disrupt developmental processes. These disruptions can have long-lasting or permanent effects on brain structure and function, thus leading to neurodevelopmental disorders (NDDs)1. NDDs are defined as a heterogeneous group of disorders with onset in the developmental period, i.e. before school age, and include, among others, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), specific learning disorder (SLD), and language disorder (LD)3. Diagnosis of NDDs is based on observed behaviors and symptoms that are specified in either the Statistical Manual of Mental Disorders (DSM) or the World Health Organization (WHO) International Classification of Disease (CIM). For most of NDDs, children do not receive their diagnosis until 4–5 years of age3, which is late considering that the brain is the most susceptible to interventions during the first two years of life4. The WHO stated that the identification of infants at risk for neurodevelopmental disorders is a crucial starting point to provide early intervention5 and thus minimize motor, cognitive and emotional impairments in young children4. There is thus a need to establish objective biological markers for NDDS that would allow for earlier diagnosis. A biomarker, according to the basic definition elaborated by the BEST (Biomarkers, EndpointS, and other Tools) Resource, is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention6. However, in the scope of this article, it would more appropriate to consider the definition of diagnostic biomarkers, which is defined as a biomarker that detects or confirms the presence of a disease or condition of interest, or identifies an individual with a subtype of the disease6. Identification of early biomarkers is challenging given that there is a lot of variability in developmental courses among young infants, this is why biomarkers validity and reliability for early prediction of neurodevelopmental disorders during infancy must be evaluated by criteria such as its relation to typical development and its sensitivity to developmental changes7.
Electroencephalography (EEG) offers several advantages compares to other neuroimaging techniques, such as its high temporal resolution, its usability with a wide range of population and age groups, and its low operating costs8. When it comes to identifying biomarkers, this neuroimaging technique has enabled researchers to establish typical patterns of brain development, then allowing the study of atypical brain development. Many parameters can be derived from EEG signal, whether it is task-locked or spontaneous recordings, such as absolute and relative spectral power, phase coherence, event-related potentials, entropy, cross-frequency coupling, and connectivity9,10. In the past years, researchers have invested efforts in finding EEG biomarkers for different NDDs. For example, in a review conducted by Wang et al.11, it is suggested that children with ASD show a U-shaped profile of electrophysiological power alterations, with excessive power at low-frequency (delta, theta) and high-frequency (beta, gamma) bands. Power in the middle-range frequency band (alpha) is, on the other hand, reduced. The authors attributed this U-shaped profile partially to the abnormal functioning of gamma-aminobutyric acid (GABA)ergic receptors in inhibitory circuitry. In a longitudinal study, the same team investigated the developmental course of resting EEG in infants at high-risk and low-risk for ASD. Using spectral power analyses, they demonstrated that at 6 months of age, spectral power was lower across all frequency bands (delta, theta, low alpha, high alpha, beta, and gamma) in high-risk infants. Moreover, the subsequent rate of change in spectral power in all frequency bands seemed to differ between the two groups, indicating that trajectories of change in EEG power may be a more robust biomarker12. More recently, researchers have found that intertrial coherence in the theta range during visual processing was reduced in infants who had a sibling diagnosed with ASD and who eventually received a diagnosis of ASD themselves. These results suggest that in the first year of life, reduced intertrial coherence in brain activity during face processing is associated with emerging autism13. Regarding children with ADHD or SLD, studies have found an increased theta/beta ratio14,15, which indicates altered top-down control of attention and affect16,17. SLD was also associated with an increased theta/alpha ratio, suggesting a lag in EEG maturation. Altogether, these results point toward a general slowing in EEG oscillations in children with SLD14.
One of the pathological neural processes identified in NDDs18 is the repetition response. This response is considered an experience-dependent neural change, reflecting Hebbian synaptic plasticity, considered to be the synaptic mechanism of learning19,20. With each presentation of the stimulus, the disparity between the ascending sensory input (bottom-up input) and the experience-dependent top-down prediction decreases resulting in more efficient information processing by the primary areas of the brain19,21. The change detection response refers to a larger neural response elicited when a repeated stimulus is followed by an unexpected stimulus (deviant stimulus). Repetition and change detection responses have been observed in young typically developing infants22–24 and they were found to be altered in various clinical populations such as patients with language impairment, ASD and X fragile syndrome25–29. Multiple cerebral factors could affect brain activity in these populations. In the last decade, little attention has been allocated to the frequent abnormal brain growth observed in these populations30,31. Macrocephaly is a relatively common clinical condition (5% of pediatric population32) defined as an abnormally large head with a circumference greater than 2 standard deviations above the mean for a given age and sex33. In most cases, this condition is familial and benign (i.e., idiopathic macrocephaly), and occurs in the absence of other neurological dysfunctions34–36. Results from past research have suggested impairments in the neurodevelopment of children with idiopathic macrocephaly leading to speech and language delay37, motor problems and neurodevelopmental dysfunction38, as well as arithmetic problems and visuomotor difficulty39. Whether brain overgrowth as an isolated trait affect brain activity remains to be studied.
In a recent study published by López‐Arango et al.40, the authors demonstrated modulation of repetition and change detection responses by adaptive abilities during infancy. However, it is still unknown if these neural responses could be associated with cognitive development later in childhood and whether these neural responses could be potential biomarkers for the early identification of children at risk of NDDs. The first aim of this study was to determine the relationship between repetition and change detection responses measured during the first year of life and at two years of age, and cognitive abilities and adaptive functioning during preschool years (4 years old) in normocephalic and macrocephalic infants. We hypothesized that infants who showed greater repetition responses (i.e., greater reduction of brain response between each presentation of the stimulus) and greater change detection responses (i.e., greater neural response to a deviant stimulus) during the two first years of life would have higher scores on the cognitive and adaptive functioning assessment tools 4 years of age.
The second aim of this study was to assess whether brain growth is a factor of interindividual variability that influences repetition and change detection responses, given the possible impacts of macrocephaly on the integrity of brain function, that could lead to sensory processing peculiarities, which the neural responses to repetition and change detection are the foundation. We hypothesized that children with increased brain growth during the first year of life would show less repetition suppression and change detection responses during the two first years of life, compared to normocephalic children.
Materials
Participants and procedure
We recruited 43 normocephalic children (24 males) and 20 macrocephalic children (11 males) aged three to eleven months to participate in this longitudinal study (see Table 1 for demographics). Normocephalic children were recruited at CHU Sainte-Justine’s birth unit, in daycares, and through social networks, while macrocephalic children were recruited at the medical imaging department, following a referral from their pediatrician. Developmental information was gathered from an in-house developmental questionnaire completed by the parents. All children were born at term with no pregnancy or delivery complications and had no significant health problems or suspicions of developmental delay. Parents gave informed written consent for themselves and for their infants prior to the study. The study was approved by the ethics, administrative and scientific committees of the Ste-Justine’s University Hospital Research Center and all experiments were performed in accordance with relevant guidelines and regulations.
Table 1.
Demographics.
| Age at the first visit (mean (SD)) | Age at the second visit (mean (SD)) | Age at the third visit (mean (SD)) | |
|---|---|---|---|
| Control group | |||
| Boys | 5.4 (1.4) | 23.7 (0.5) | 48.3 (1.2) |
| Girls | 4.9 (1.6) | 23.9 (0.5) | 48.1 (0.9) |
| Macrocephalic group | |||
| Boys | 6.7 (3.3) | 24.1 (1.1) | 48.6 (1.8) |
| Girls | 6.4 (2.4) | 24.2 (0.8) | 48.1 (0.3) |
This project is a longitudinal study including three visits to CHU Sainte-Justine at the Neuroscience of Early Development (NED) laboratory. The first visit took place during the infant’s first year of life (between 3–11 months), the second visit at two years of age, and the last visit is at four years of age. The oddball task administered at the first and second visits was analyzed to identify an EEG marker detectable before preschool age that would allow for earlier screening of children. An assessment of intellectual and adaptive abilities was done at the third visit.
Intellectual and adaptive functioning assessment
To assess intellectual abilities of the children at 4 years of age, the Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition (WPPSI-IV)41 was used, allowing us to obtain a Full-Scale IQ (FSIQ) score for each participant. Data for three participants is missing since they turned 4 years old during the COVID-19 confinement in 2020 so it was not possible to do the assessment. Adaptive functioning was assessed using the Adaptive Behavior Assessment System—Second Edition (ABAS-II)42, specifically the Parent/Primary Caregiver Form. The General Adaptive Composite (GAC) score was obtained for each participant. To avoid missing data during the COVID-19 confinement in 2020, this questionnaire was sent by mail to the families.
EEG oddball task
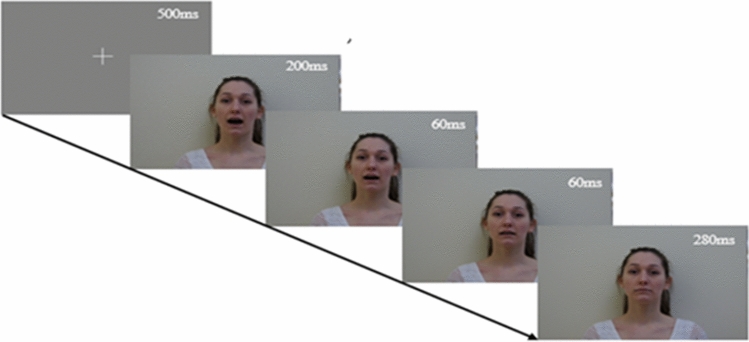
The task consisted of audio-visual stimuli featuring a woman and a man alternating in the articulation of the vowels /a/ or /i/24. The audio-visual design served to maximize children’s attention. Stimuli were generated by a Dell Optiplex 790 PC using E-Prime 2.0 software (Psychology Software Tools Inc., Pittsburgh, PA, USA) on a 17″ screen placed at a viewing distance of 60 cm. A team member was present in the room with the infant to assure the child was looking sufficiently at the screen and that the child seemed attentive. Eye-tracking was monitored, indicating looking behavior to the experimenter outside the shielded room. The sounds were delivered at a sound pressure level of 70 dB through two speakers located laterally at 30 cm distance from the children’s ears. Infants were seated on their parent’s laps, in front of the screen. The onset of the auditory vowel coincided with a visual clip lasting 200 ms of the mouth fully opened. Following the end of the sound, two frames of 60 ms showing the mouth gradually closing were presented. During the next 280 ms, the face with a closed mouth was presented followed by the onset of the next vowel (Fig. 1). The task followed a local/global paradigm and consisted of 96 trials, 80 trials followed an aaaI pattern, which corresponds to a global standard with a local deviant (standard trial) and 16 trials followed an aaaa pattern, corresponding to a global deviant with a local standard. The order of the trials was pseudo-randomized and fixed across participants, making sure no aaaa trials would follow each other. To assess repetition and change detection responses, only standard trials that did not directly follow an aaaa trial were analyzed. See previously published articles from the same author for the original description of this method43,44.
Figure 1.
Experimental design. The task consisted of a man or a woman (pictured) articulating the vowel /a/. On each trial, infants were presented with three consecutive /a/ while the fourth vowel could be either /a/ or /i/. The sound lasted 200 ms, in synchrony with the first frame (mouth opened). Following the end of the sound, two frames of a mouth gradually closing were presented (60 ms). Finally, during the last 280 ms, a face with a closed mouth was presented. *Informed consent to publish identifying images was obtained.
EEG recordings
EEG recordings took place in a dark soundproof experimental chamber. EEG was recorded continuously with a high-density EEG system containing 128 electrodes (Magstim EGI, Eugene, OR, USA). Altogether, the EEG net set-up and the recording took in average 60 min. EEG Signals were acquired and processed by a G4 Macintosh computer using NetStation EEG Software (Version 4.5.4). Data were acquired at a 1000 Hz sampling rate and an analog bandpass filter of 0.1–4000 Hz was applied. Impedances were kept below 40 kΩ45. Off-line signal processing and analyses were performed using MATLAB (Mathworks, Inc., Natick, MA) and the EEGLAB toolbox46. Data were digitally filtered with a lower-bound 0.5 Hz and a higher-bound 150 Hz and a 60 Hz notch filter. Twenty-eight electrodes containing muscular artifacts placed around the neck and face were excluded. Electrodes with a total standard deviation higher than 200 μV and lower than 2 μV were automatically removed. Channels with sporadic behavior were then manually removed during subsequent visual inspection. Data were re-referenced to the average reference. Eye movement and cardiac artifacts were rejected using semi-automatic independent component analysis (ICA) as implemented in the EEGLAB toolbox46. ICAs were calculated automatically, and rejection of the components was done manually. The data was segmented and cleaned in 800 ms time windows (− 200 to 600 ms), representing the time window of each stimulus with a short pre-stimulus segment. Epochs with voltage exceeding ± 200 μV were marked and a visual inspection of the segmented data (− 200 to 600 ms) was performed next to manually reject epochs with significant artefacts. Following epoch exclusion, an average of 79% epochs were kept for analyses. The data of 15 participants (visit 1: 11 participants, visit 2: 4 participants) were excluded from the analysis due to excessive artifacts or unusable recordings. See previously published articles from the same author for the original description of this method43,44.
Regions of interest
Region of interest (ROI) was determined by using a PCA47 which identifies the most relevant and contributing cluster of electrodes while avoiding redundant dimensions48. PCA was used given the rapid maturation of the brain during childhood, which causes many functional changes, and the presence in our study of a clinical group for which almost no EEG literature exists. The analysis was carried out using a Varimax Rotation on IBM SPSS, Version 27 (IBM, Armonk, NY, USA). The observations consisted of the three presentations of the /a/ and the last /i/ in all participants for both testing time points, while the 99 channels were the dependent variables. The spatial PCA yielded 1 main factor, which explained 58% of data variance and included a cluster of 11 electrodes in the central region (E7, E30, E31, E37, E54, E55, E80, E87, E105, E106, E112). Moreover, the five electrodes explaining the most variability (E7, E31, E55, E80, E106) correspond exactly to the five electrodes that constitute the central region reported in the literature. Therefore, time–frequency analyses were conducted on this ROI since central region is known to be related to auditory processing49 and its coherent with past developmental studies showing that increased responses in central slow frequency are associated with learning and attention control in early childhood50,51. See previously published article from the same author for the original description of this method43.
Time–frequency analyses
Time–frequency (TF) analyses were used as they provide temporal information on the activity of specific frequency bands. To avoid overlap between segments, a padding technique was implemented, in which the first spectral power value of each segment was added for the 800 ms period before and the last spectral power value for the 800 ms period after the segment, thus increasing its length to 2400 ms. Complex Gaussian Morlet’s wavelets transformation52 was computed to analyze the signal in the time and frequency domain. This specific wavelet convolution can be expressed as follows:
M(t, f) is a matrix of complex values (vectors) for a given time (t) and frequency (f). S was the signal as a function of time (t) and W corresponds to Morlet’s wavelet which is a complex exponential (Fourier) with a Gaussian envelope that undergoes a series of translations (a) and dilations (b) dependently on the frequency (f). The event-related spectral perturbation (ERSP) computation uses the complex values (amplitude and phase) given by Morlet’s wavelet transform as shown in the following formula calculating the power spectrum for each time and frequency point: denotes TF power in terms of decibels (dB). Final TF maps were computed as follows: . ERSP maps show mean log deviations from baseline power, averaged across participants for each testing time point. Investigated frequencies ranged from 3 to 55 Hz. The baseline was defined as the average of the segment [− 100: 500] ms for each epoch. Baseline correction was achieved by computing separately the mean power of each stimulus presentation (/a1/-/a2/-/a3/-/I/). This mean was subtracted from each time and frequency point to show variations in EEG activity.
Intertrial coherence (ITC), analogous to phase-locking value (PLV), allows the assessment of the strength of phase coherence or synchronization across trials in temporal and spectral domains53. The ITC computation uses only the phase of the complex values given by Morlet’s wavelet transform. To extract phase-locking information, ITC was computed according to Lachaux and his colleagues’ procedure54. ITC measures phase coupling across trials at all latencies and frequencies and is defined by: where θ represents the phase for a given frequency (f), time point (t), and trial (n). The obtained values are always defined between 0 and 1. Phase-locking values close to 1 indicate strong inter-trial phase-locking, thus representing evoked activity while scores closer to 0 indicate a high inter-trial phase variability54,55. See previously published articles from the same author for the original description of this method43,44.
Topographies
Past developmental literature showed that low frequencies are predominant in infants and that frequency boundaries are lower than in adults and shift with brain maturation. In fact, in infants and children, theta band varies between 3 and 5–6 Hz, alpha band varies between 6–9–10 Hz, and beta band includes frequency from 9 to 30 Hz50,51,56–60. On the other hand, few to no literature exists regarding exact time intervals of brain activity. The results found here and in our previous publications40,43,61,62 show theta and alpha activity after a post-stimulus delay, and beta activity early after stimuli onset. Thus, for selecting time intervals, ITC maps were created for each stimulus and testing time point (visit 1 and visit 2) to assess the time–frequency windows (TFW) in which EEG activity showed greater variation in synchronization across trials and testing time points. Based on these previous studies on infants EEG oscillations and the ITC maps, the 3 following TFWs were selected since they captured most of the activity: theta (3–5 Hz, 100–400 ms), alpha (5–10 Hz, 100–400 ms), and beta (10–30 Hz, 0–200 ms). We used ITC maps since it takes evoked responses into consideration, which means brain activity that is phase-locked to the stimulus onset. For each TFW of interest, the time–frequency spectrogram for each electrode available for each subject, and each stimulus were calculated. The power of each electrode was then averaged across all the participants for each TFW. An averaged topography including all the subjects was then computed for each stimulus (/a1/-/a2/-/a3/-/I/) based on the mean power of each TFW, for both ERSPs and ITCs. Topographic map inspection confirmed that the central region showed large brain activity. See previously published articles from the same author for the original description of this method43,44.
Statistical analyses
Descriptive statistics and preliminary analyses
Descriptive statistics and preliminary analyses were performed using SPSS Statistics, version 27 (IBM Corp., Armonk, NY, USA). Preliminary analyses were conducted to assure there were no differences between groups regarding annual family income (t(57) = 1.4, p = 0.17, d = 0.41). Differences between boys and girls regarding age at the three testing time points, for each group were also assessed (normocephalic group : visit 1: t(41) = − 1.1, p = 0.27, d = − 0.34, visit 2: t(41) = 1.1, p = 0.28, d = 0.33, visit 3: t(39) = − 0.52, p = 0.61, d = − 0.16, macrocephalic group : visit 1 : t(18) = − 0.21, p = 0.83, d = − 0.10, visit 2: t(18) = 0.09, p = 0.93, d = 0.04, visit 3: t(17) = − 0.78, p = 0.44, d = − 0.37). Since age the first visit varies between three months and eleven months and that neural responses change significantly during infancy du to brain maturation, this variable should be controlled in the main analysis. Moreover, when statistically assessing difference between groups regarding age at the first visit, results show a near significant difference with a medium effect size based on Cohen’s D (t(23,9) = − 1.3, p = 0.058, d = − 0.67). Although there were 3 missing participants for the FISQ and 15 missing participants for the EEG oddball task, no imputation was conducted since it has been shown that it is not necessary to use multiple imputations to handle missing data before applying mixed model analysis on longitudinal data63.
Linear mixed models
The main analyses were performed using SPSS Statistics, version 27 (IBM Corp., Armonk, NY, USA). Linear mixed models (LMM) analyses were chosen because they allow for unequal numbers of measurements, and the analysis uses all observations that are available for a given participant. Moreover, LMM allows the measuring time points to vary for different participants64,65. LMM analyses were conducted on ERSP values since they showed greater variability compared to ITC values.
To verify our first hypothesis, four general models were performed: Visit 1—FISQ; Visit 1—GAC; Visit 2—FISQ; Visit 2—GAC. In each model, the general pattern of responses across the standard trial (/a1/-/a2/-/a3/-/I/) was assessed and the association between the pattern of responses and intellectual abilities and adaptive functioning at preschool age was verified. Stimulus presentations, TFW, groups, and interactions between these variables were the fixed effects (4 presentations × 3 TFW × 2 groups), whereas FSIQ and GAC scores were the dependent variables. Age at the first visit (only for Visit 1 models) and sex were introduced sequentially as predictors to the models and a chi-square likelihood ratio was used to verify model fit improvement.
To assess our second hypothesis, two LMMs, one for each testing time point (visit 1 and visit 2), were conducted to assess the difference in the general pattern of responses across the standard trial (/a1/-/a2/-/a3/-/I/) between normocephalic and macrocephalic children. The models included stimulus presentations, TFW, and the group as the fixed effects (4 presentations × 3 TFW × 2 groups) and so were the interactions between these effects.
For all LMMs, intercept and slope were allowed to vary randomly. Restricted maximum likelihood (REML) was used since it tends to result in unbiased estimates of the variances and covariances66. The first-order autoregressive covariance structure (AR1) provided the best model fit for the Visit 1—FISQ and Visit 1—GAC models, whereas the heterogeneous first-order autoregressive covariance structure (ARH1) provided the best model fit for the Visit 2—FISQ and Visit 2—GAC models.
ANOVA
In relation to our second hypothesis, ANOVAs were conducted separately for each TFW, with each stimulus as the dependent variable and groups as the group variable to determine which stimulus (/a1/-/a2/-/a3/-/I/) varies the most between the two groups.
Results
Brain responses, cognitive abilities, and adaptive functioning
Linear mixed models
Visit 1 (3–10 months)—FISQ
The best model fit was found by including age at the first visit and sex as predictors [χ2 (3, N = 52) = 17.8, p < 0.001]. The results indicated that there was no significant relationship between the response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the first testing time point and FISQ scores measured at 4 years of age (F (1, 556.97) = 2.4, p = 0.12). No effect of age at the first testing time point, sex, groups or TFW were found.
Visit 1 (3–10 months)—GAC
The best model fit was found by including age at the first visit and sex as predictors [χ2 (3, N = 52) = 12.5, p < 0.01]. A significant interaction was found between the response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the first testing time point and GAC scores measured at 4 years of age (F (1, 575.9) = 6,90, p < 0.009), suggesting that infants with higher GAC scores showed a U-shaped pattern in response to the entire standard trial (/a1/-/a2/-/a3/-/I/). Infants with lower GAC scores tended to show a steady repetition suppression response between each presentation of the four stimuli, and therefore they showed no change detection response. No effect of age at the first testing time point, sex, groups or TFW were found.
Visit 2 (24 months)—FISQ
The best model fit was found by including sex as a predictor [χ2 (3, N = 59) = 8.9, p < 0.05]. The results indicated that there was no significant relationship between the response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the second testing time point and FISQ scores measured at 4 years of age (F (1, 523.89) = 2.96, p = 0.086). No effect of sex, groups nor TFW were found.
Visit 2 (24 months)—GAC
The best model fit was found by including sex as a predictor [χ2 (3, N = 59) = 18.1, p < 0.001]. No significant relationship between the response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the second testing time point and GAC scores measured at 4 years of age (F (1, 678) = 2.25, p = 0.13) was found. No effect of sex, groups nor TFW were found.
Brain responses and brain growth
Linear mixed models
Visit 1 (3–10 months)
The best model fit was found by including age at the first visit and sex as predictors [χ2 (3, N = 52) = 17.3, p < 0.001]. The results suggested that there was a significant difference between groups regarding response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the first testing time point (F (1, 598.8) = 8.47, p = 0.004). Responses of the normocephalic infants followed a more U-shaped pattern compared to macrocephalic infants who showed a more linear and steady response. No effect of age at the first testing time point, sex, or TFW were found.
Visit 2 (24 months)
The best model fit was found by including sex as a predictor [χ2 (3, N = 59) = 16.7, p < 0.001]. No significant difference between groups regarding response to the entire standard trial (/a1/-/a2/-/a3/-/I/) measured at the second testing time point (F (1, 693) = 1.80, p = 0.180) was found. This result suggests that macrocephalic children showed a pattern of response similar to normocephalic children when tested at two years of age.
ANOVA
Visit 1 (3–10 months)
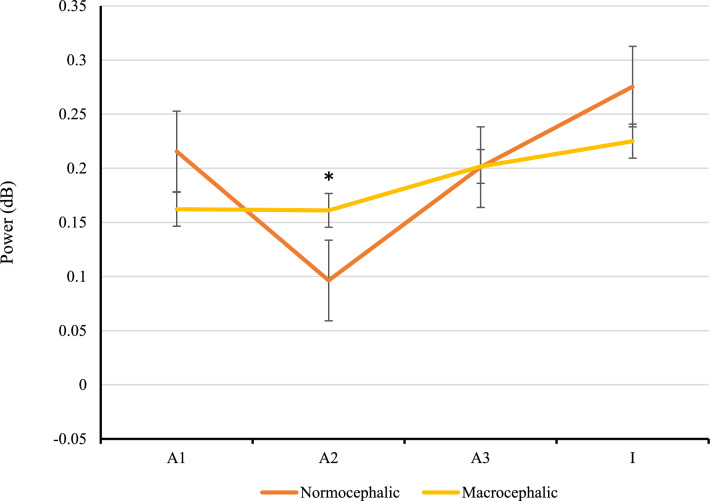
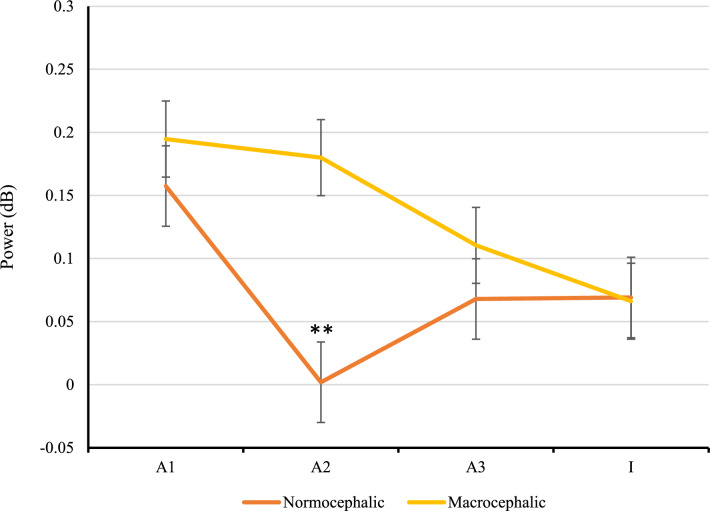
The results of the one-way analysis of variance indicated that there was a significant difference between groups regarding the response to the /a2/ in the theta frequency band (F (1, 109) = 7.73, p = 0.006) and in the beta frequency band (F (1, 50) = 13.35, p < 0.001). Normocephalic infants showed significantly less power on the second presentation of the stimulus /a/ compared to macrocephalic infants, who tended to show no repetition suppression between the first and the second presentation of the /a/ (Figs. 2 and 3).
Figure 2.
Averaged power (ERSP) of the theta TFW (3–5 Hz, 100–400 ms) for each group, at the first visit, in the central region. Normocephalic infants (orange line) showed a U-shaped pattern in response to the entire standard trial (/a1/-/a2/-/a3/-/I/) while macrocephalic infants (yellow line) showed a steady response between the first and the second presentation of the /a/. Error bars represent standard error. *p < 0.01.
Figure 3.
Averaged power (ERSP) of the beta TFW (10–30 Hz, 0–200 ms) for each group, at the first visit, in the central region. Normocephalic infants (orange line) showed a U-shaped pattern in response to the entire standard trial (/a1/-/a2/-/a3/-/I/) while macrocephalic infants (yellow line) showed a relatively steady response between the first and the second presentation of the /a/. Error bars represent standard error. **p < 0.001.
Visit 2 (24 months)
A significant difference between groups regarding the response to the /a2/ (F (1, 57) = 9.08, p = 0.004) in the alpha frequency band was found in the one-way analysis of variance. Again, normocephalic children showed significantly less power on the second presentation of the stimulus /a/ compared to macrocephalic children, who showed a less steep repetition suppression response between the first and the second presentation of the stimulus compared to the control group (Fig. 4). See supplementary material for the figures of the 3 TFW for each group at each testing time point.
Figure 4.
Averaged power (ERSP) of the alpha TFW (5–10 Hz, 100–400 ms) for each group, at the second visit, in the central region. Both normocephalic children (orange line) and macrocephalic children (yellow line) showed a U-shaped pattern in response to the entire standard trial (/a1/-/a2/-/a3/-/I/). Macrocephalic children displayed a less steep repetition suppression response between the first and the second presentation of the stimulus. Error bars represent standard error. *p < 0.01.
Discussion
This study aimed to investigate the relationship between repetition and change detection responses measured during infancy, and cognitive abilities and adaptive functioning in childhood. Our results indicated that when measured during the first year of life, repetition and change detection responses were not associated with cognitive abilities but predicted adaptive functioning at preschool age. Infants who displayed the expected U-shaped pattern in response to the entire standard trial (/a1/-/a2/-/a3/-/I/)43 were the ones who had the highest GAC scores (better adaptive functioning) at 4 years of age. On the other hand, no change detection response when presented with the /I/ was elicited in infants who had the lowest GAC scores. These results indicate that deviant responses should be analyzed in conjunction with the standard responses in neurodevelopmental studies. These findings are further in line with recent literature demonstrating that in children and adolescents with ASD, reduced neural responses to changes in stimuli input were associated with deficits in adaptive functioning67. Past studies have also suggested that in various clinical populations repetition and change detection responses were altered25–29,68–70 and that children with NDDs (ADHD, autism, patients with tics or language disorders) have significantly lower adaptive functioning scores compared to children without NDDs71. Based on past results and results of this study, it is possible to hypothesize that the capacity to flexibly adapt to life circumstances and the surrounding environment may be underlain by basic brain functions such as repetition and change detection responses. Moreover, the association found in our study was found regardless of the groups (normocephalic or macrocephalic), meaning that repetition and change detection responses could help identify children at risk of impaired adaptive functioning both in the general and clinical infant population. Regarding the absence of associations between repetition and change detection responses and cognitive abilities, one possible explanation could be that repetition suppression response is systematic in children with IQ scores within the normal range. In a study conducted by Orekhova et al.72, they demonstrated that children with autism in the normal IQ range showed repetition suppression between the first and the second presentation of auditory clicks while the ones with low IQ scores (within the limit and extremely low range) did not show repetition suppression. Moreover, studies comparing control groups with low IQ clinical groups found repetition suppression in the control group (IQ scores within the normal range) but no or altered repetition suppression response in clinical groups with low IQ scores25,27,28. Our results are thus in line with the literature since, for the most part, children in our sample displayed repetition suppression in response to the entire standard trial (/a1/-/a2/-/a3/-/I/) in the first year of life and most of the children had FISQ scores within the normal range at 4 years of age.
The second aim of this study was to assess whether brain growth is a factor contributing interindividual variability to repetition and change detection responses since it is developmental courses among young infants are well known to display a lot of variability. Our findings suggested that in the first year of life, macrocephalic infants differ from normocephalic infants in the way they respond to the entire standard trial (/a1/-/a2/-/a3/-/I/). Indeed, normocephalic infants showed the expected U-shape pattern but macrocephalic infants showed no repetition suppression or repetition enhancement between the first and the second presentation of the stimulus. A possible explanation could be that macrocephalic infants, compared to normocephalic infants, did not draw enough information from the first /a/ so they were not able to form a strong representation of the stimulus, thus explaining why they showed steady responses or repetition enhancement on the second presentation of the /a/. Indeed, it has been demonstrated that the direction of the repetition effect depends on the amount of information that can be drawn from the first presentation of the stimulus in order to establish a strong representation. If the representation needs to be strengthened, there is no repetition suppression, but rather repetition enhancement73,74. At the second visit, the results then suggested a normalized response in the macrocephalic children, responding in a U-shaped pattern as the normocephalic children. Nevertheless, a significant difference was found between the groups in response to the second presentation of the /a/, indicating that the U-shaped pattern was steeper in normocephalic children. In sum, the rate of brain growth was a factor of interindividual variability that influences repetition responses only during the first year of life but not anymore at two years of age. Moreover, our results also suggested that repetition and change detection responses at the second visit were not associated with cognitive abilities nor with adaptive functioning at preschool age, thus indicating that these neural responses lose their predictive power between the first and second year of life. These findings are in line with the ones of Knickmeyer et al.1 indicating that the first year of life represents a critical period for brain development since brain volume increases the most during this period. Based on their results, the authors suggested that there was an urgent need to identify children at risk for neurodevelopmental disorders within the first year of life to maximize the effects of interventions.
In this study, we were able to demonstrate that repetition and change detection responses have the potential to help identify children at risk for neurodevelopmental disorders in the first year of life. Our paradigm is sensitive to abnormal brain growth and can be linked to adaptive development in preschool years. Therefore, these cerebral responses meet the early prediction criteria of a diagnosis biomarker. Overall, we showed that these brain responses relate to typical development, they are sensitive to developmental change, they relate to a specific condition which may reflect an underlying neural mechanism. Nevertheless, more clinical populations should be evaluated with the task to quantify reliability, sensitivity, and specificity.
As future perspectives, since the effectiveness of a biomarker relies on its ability to stratify individuals into various diagnostic categories, in the future, the ability of the neuronal response to the entire standard trial to classify individuals according to their symptoms, level of functioning, cognitive impairment, or diagnosis should be established. Moreover, it would be relevant to relate the electrophysiological response to other measures beyond intellectual functioning and adaptive skills. This could, for example, include more specific cognitive functions such as executive functions, attention and memory, or questionnaires to measure autistic symptoms, ADHD symptoms, behavioral problems, or aspects of temperament.
Even though adding sex as a predictor improved the model fit significantly for all the models tested, no sex effect or interaction was found. This result is in line with a recent study showing no effect of sex on repetition suppression responses in infants under 12 months of age44. However, Clarke et al.75 have found differences in EEG maturation between boys and girls. The total power, in delta, theta, and alpha frequency bands, increased with age in boys but decreased in girls, suggesting a faster rate of change in girls76, possibly explaining why sex as a predictor fitted our data better but did not have an effect on repetition and change detection responses.
This study has some strengths as well as some limitations. We used a longitudinal design which allowed us to assess the development of the same individuals from the first months of life to 4 years of age, making it is possible to think that our findings highlight maturational effects. One limitation is the unequal group size, caused by the challenges of recruiting macrocephalic children and missing data, which can lead to unequal variance between groups. However, the linear mixed model analyses used in this study allowed to minimize the impact of unequal group size and missing data since it can deal easily with unbalanced longitudinal data sets66,77. Also, given the relatively small sample size, it limits the generalizability of the results. Finally, it is possible that ROIs and TFWs selection caused a circularity problem since data was first analyzed to select a subset, and then the same subset was reanalyzed to obtain the results78. However, ROIs and TFWs were selected both by data-driven approach and based on the literature.
Conclusion
Taken together, these findings demonstrated that only the repetition and change detection responses in the first year of life are predictive of adaptive functioning at 4 years of age. Further research should focus on testing this relationship in populations with various neurodevelopmental disorders to establish the sturdiness of these neural responses as biomarkers. Moreover, differences between macrocephalic and normocephalic infants in response to the oddball task have been observed only during the first year of life. When tested at two years of age, macrocephalic children responded in a very similar way as normocephalic children, suggesting that brain growth influences the neural response mostly in the early months of life.
Date availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Acknowledgements
The authors would like to thank all participating families. The authors would also like to thank the team at the Neurosciences of Early Development Laboratory, Marguerite Nolin for her help with data processing, and former team member Caroline Dupont, for assistance during implementation and data collection. Finally, we want to acknowledge the financial support of our funding sources. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) (DCO150GP to S.L.) and Kids Brain Heath Network (KBHN) (TDG12 to SL). Deguire was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) (2019–2022).
Author contributions
F.D. participated in data acquisition, analysis, and interpretation and wrote the manuscript. G.L.-A. and I.S.K. participated in data acquisition. V.C. concepted the oddball task. K.A. designed the scripts for EEG data analysis. S.L. is the director of the lab. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34669-9.
References
- 1.Knickmeyer RC, et al. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Publisher; 2013. [Google Scholar]
- 4.Cioni G, Inguaggiato E, Sgandurra G. Early intervention in neurodevelopmental disorders: Underlying neural mechanisms. Dev. Med. Child Neurol. 2016;58:61–66. doi: 10.1111/dmcn.13050. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Report on Disability 2011. World Health Organization; 2011. [PubMed] [Google Scholar]
- 6.FDA-NIH Biomarker Working Group. In BEST (Biomarkers, EndpointS, and other Tools) Resource. https://www.ncbi.nlm.nih.gov/books/NBK326791/ (Food and Drug Administration (US), National Institutes of Health (US), 2016). [PubMed]
- 7.Jeste SS, Frohlich J, Loo SK. Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Curr. Opin. Neurol. 2015;28:110. doi: 10.1097/WCO.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman LC, Varcin KJ. The promise of electroencephalography for advancing diagnosis and treatment in neurodevelopmental disorders. Biol. Psychiatry. 2018;3:7–9. doi: 10.1016/j.bpsc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MX. Analyzing Neural Time Series Data: Theory and Practice. MIT Press; 2014. [Google Scholar]
- 10.Ewen JB, Sweeney JA, Potter WZ. Conceptual, regulatory and strategic imperatives in the early days of EEG-based biomarker validation for neurodevelopmental disabilities. Front. Integr. Neurosci. 2019;13:45. doi: 10.3389/fnint.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. Resting state EEG abnormalities in autism spectrum disorders. J. Neurodev. Disord. 2013;5:1–14. doi: 10.1186/1866-1955-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: An endophenotype of autism spectrum disorder. PLoS One. 2012;7:e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Noordt S, Desjardins JA, Team B, Elsabbagh M. Inter-trial theta phase consistency during face processing in infants is associated with later emerging autism. Autism Res. 2022;15:834–846. doi: 10.1002/aur.2701. [DOI] [PubMed] [Google Scholar]
- 14.Jäncke L, Alahmadi N. Resting state EEG in children with learning disabilities: An independent component analysis approach. Clin. EEG Neurosci. 2016;47:24–36. doi: 10.1177/1550059415612622. [DOI] [PubMed] [Google Scholar]
- 15.Markovska-Simoska S, Pop-Jordanova N. Quantitative EEG in children and adults with attention deficit hyperactivity disorder: Comparison of absolute and relative power spectra and theta/beta ratio. Clin. EEG Neurosci. 2017;48:20–32. doi: 10.1177/1550059416643824. [DOI] [PubMed] [Google Scholar]
- 16.Buhle JT, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monastra VJ, et al. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology. 1999;13:424. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- 18.Rajamani KT, Wagner S, Grinevich V, Harony-Nicolas H. Oxytocin as a modulator of synaptic plasticity: Implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2018;10:17. doi: 10.3389/fnsyn.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Magee JC, Grienberger C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020;43:95–117. doi: 10.1146/annurev-neuro-090919-022842. [DOI] [PubMed] [Google Scholar]
- 21.Gotts SJ, Chow CC, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn. Neurosci. 2012;3:227–237. doi: 10.1080/17588928.2012.670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehaene-Lambertz G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370:292. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene-Lambertz G, Pena M. Electrophysiological evidence for automatic phonetic processing in neonates. NeuroReport. 2001;12:3155–3158. doi: 10.1097/00001756-200110080-00034. [DOI] [PubMed] [Google Scholar]
- 24.Basirat A, Dehaene S, Dehaene-Lambertz G. A hierarchy of cortical responses to sequence violations in three-month-old infants. Cognition. 2014;132:137–150. doi: 10.1016/j.cognition.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Côté V, et al. Distinct patterns of repetition suppression in Fragile X syndrome, down syndrome, tuberous sclerosis complex and mutations in SYNGAP1. Brain Res. 2021;1751:147205. doi: 10.1016/j.brainres.2020.147205. [DOI] [PubMed] [Google Scholar]
- 26.Guiraud JA, et al. Differential habituation to repeated sounds in infants at high risk for autism. NeuroReport. 2011;22:845–849. doi: 10.1097/WNR.0b013e32834c0bec. [DOI] [PubMed] [Google Scholar]
- 27.Knoth IS, et al. Auditory repetition suppression alterations in relation to cognitive functioning in fragile X syndrome: A combined EEG and machine learning approach. J. Neurodev. Disord. 2018;10:4. doi: 10.1186/s11689-018-9223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigoulot S, et al. Altered visual repetition suppression in fragile X syndrome: New evidence from ERPs and oscillatory activity. Int. J. Dev. Neurosci. 2017;59:52–59. doi: 10.1016/j.ijdevneu.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Shafer VL, Morr ML, Datta H, Kurtzberg D, Schwartz RG. Neurophysiological indexes of speech processing deficits in children with specific language impairment. J. Cogn. Neurosci. 2005;17:1168–1180. doi: 10.1162/0898929054475217. [DOI] [PubMed] [Google Scholar]
- 30.van Dyck LI, Morrow EM. Genetic control of postnatal human brain growth. Curr. Opin. Neurol. 2017;30:114. doi: 10.1097/WCO.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CA, Dagli A, Battaglia A. Genetic disorders associated with macrocephaly. Am. J. Med. Genet. A. 2008;146:2023–2037. doi: 10.1002/ajmg.a.32434. [DOI] [PubMed] [Google Scholar]
- 32.Tan AP, Mankad K, Gonçalves FG, Talenti G, Alexia E. Macrocephaly: Solving the diagnostic dilemma. Top. Magn. Reson. Imaging. 2018;27:197–217. doi: 10.1097/RMR.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 33.Tager-Flusberg H. Neurodevelopmental disorders. MIT Press; 1999. [Google Scholar]
- 34.Alper G, et al. Magnetic resonance imaging characteristics of benign macrocephaly in children. J. Child Neurol. 1999;14:678–682. doi: 10.1177/088307389901401010. [DOI] [PubMed] [Google Scholar]
- 35.Jeong G, Kim M, Han BH. Clinical features of macrocephaly at birth in Korea. Korean J. Pediatr. 2014;57:75. doi: 10.3345/kjp.2014.57.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muenchberger H, Assaad N, Joy P, Brunsdon R, Shores EA. Idiopathic macrocephaly in the infant: Long-term neurological and neuropsychological outcome. Childs Nerv. Syst. 2006;22:1242–1248. doi: 10.1007/s00381-006-0080-0. [DOI] [PubMed] [Google Scholar]
- 37.Nickel RE, Galtenstein JS. Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev. Med. Child Neurol. 1987;29:181–186. doi: 10.1111/j.1469-8749.1987.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 38.Sandler AD, Knudsen MW, Brown TT, Christian RM., Jr Neurodevelopmental dysfunction among nonreferred children with idiopathic megalencephaly. J. Pediatr. 1997;131:320–324. doi: 10.1016/S0022-3476(97)70176-8. [DOI] [PubMed] [Google Scholar]
- 39.Desch LW, Anderson SK, Snow JH. Relationship of head circumference to measures of school performance. Clin. Pediatr. 1990;29:389–392. doi: 10.1177/000992289002900705. [DOI] [PubMed] [Google Scholar]
- 40.López-Arango G, et al. Infant repetition effects and change detection: Are they related to adaptive skills? Eur. J. Neurosci. 2021;54:7193–7213. doi: 10.1111/ejn.15475. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D, Fancello GS, Cianchetti C. WPPSI-IV: Wechsler Preschool and Primary Scale of Intelligence. Pearson Education; 2014. [Google Scholar]
- 42.Oakland T, Harrison PL. Adaptive Behavior Assessment System-II: Clinical Use and Interpretation. Academic Press; 2011. [Google Scholar]
- 43.Deguire F, et al. Developmental course of the repetition effect and change detection responses from infancy through childhood: A longitudinal study. Cereb. Cortex. 2022;32:5467–5477. doi: 10.1093/cercor/bhac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deguire F, et al. The relationship between acute stress and EEG repetition suppression in infants. Psychoneuroendocrinology. 2019;104:203–209. doi: 10.1016/j.psyneuen.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Tucker DM. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalogr. Clin. Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-B. [DOI] [PubMed] [Google Scholar]
- 46.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Rigoulot S, et al. Peripherally presented emotional scenes: A spatiotemporal analysis of early ERP responses. Brain Topogr. 2008;20:216–223. doi: 10.1007/s10548-008-0050-9. [DOI] [PubMed] [Google Scholar]
- 48.Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. doi: 10.1111/1469-8986.3820343. [DOI] [PubMed] [Google Scholar]
- 49.Luck SJ. An Introduction to the Event-Related Potential Technique. MIT Press; 2014. [Google Scholar]
- 50.Orekhova E, Stroganova T, Posikera I, Elam M. EEG theta rhythm in infants and preschool children. Clin. Neurophysiol. 2006;117:1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Xie W, Mallin BM, Richards JE. Development of infant sustained attention and its relation to EEG oscillations: An EEG and cortical source analysis study. Dev. Sci. 2018;21:e12562. doi: 10.1111/desc.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999;3:151–162. doi: 10.1016/S1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 53.Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn. Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrmann, C., Grigutsch, M. & Busch, N. EEG oscillations and wavelet analysis (MIT Press, 2005).
- 56.Gilley PM, Uhler K, Watson K, Yoshinaga-Itano C. Spectral-temporal EEG dynamics of speech discrimination processing in infants during sleep. BMC Neurosci. 2017;18:1–17. doi: 10.1186/s12868-017-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orekhova EV, Stroganova TA, Posikera IN. Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. Int. J. Psychophysiol. 1999;32:151–172. doi: 10.1016/S0167-8760(99)00011-2. [DOI] [PubMed] [Google Scholar]
- 58.Saby JN, Marshall PJ. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;37:253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soto-Icaza P, Vargas L, Aboitiz F, Billeke P. Beta oscillations precede joint attention and correlate with mentalization in typical development and autism. Cortex. 2019;113:210–228. doi: 10.1016/j.cortex.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Stroganova TA, Orekhova EV. EEG and infant states. Infant EEG and Event-Related Potentials. 2007;251:280. [Google Scholar]
- 61.López-Arango G, et al. Impact of macrocephaly, as an isolated trait, on EEG signal as measured by spectral power and multiscale entropy during the first year of life. Dev. Neurosci. 2023 doi: 10.1159/000529722. [DOI] [PubMed] [Google Scholar]
- 62.López-Arango G, et al. Impact of brain overgrowth on sensorial learning processing during the first year of life. Front. Hum. Neurosci. 2022;16:928543. doi: 10.3389/fnhum.2022.928543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J. Clin. Epidemiol. 2013;66:1022–1028. doi: 10.1016/j.jclinepi.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Wiley; 2006. [Google Scholar]
- 65.West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Chapman and Hall/CRC; 2006. [Google Scholar]
- 66.West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval. Health Prof. 2009;32:207–228. doi: 10.1177/0163278709338554. [DOI] [PubMed] [Google Scholar]
- 67.Lassen J, et al. Reduced mismatch negativity in children and adolescents with autism spectrum disorder is associated with their impaired adaptive functioning. Autism Res. 2022;15:1469–1481. doi: 10.1002/aur.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen T-C, Hsieh MH, Lin Y-T, Chan P-YS, Cheng C-H. Mismatch negativity to different deviant changes in autism spectrum disorders: A meta-analysis. Clin. Neurophysiol. 2020;131:766–777. doi: 10.1016/j.clinph.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 69.Cheng C-H, Chan P-YS, Hsieh Y-W, Chen K-F. A meta-analysis of mismatch negativity in children with attention deficit-hyperactivity disorders. Neurosci. Lett. 2016;612:132–137. doi: 10.1016/j.neulet.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 70.Näätänen R, Sussman E, Salisbury D, Shafer V. Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topogr. 2014;27:451–466. doi: 10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ÅsbergJohnels J, et al. The relationship between intelligence and global adaptive functioning in young people with or without neurodevelopmental disorders. Psychiatry Res. 2021;303:114076. doi: 10.1016/j.psychres.2021.114076. [DOI] [PubMed] [Google Scholar]
- 72.Orekhova EV, et al. Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neurosci. Lett. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 73.Müller NG, Strumpf H, Scholz M, Baier B, Melloni L. Repetition suppression versus enhancement—It's quantity that matters. Cereb. Cortex. 2012;23:315–322. doi: 10.1093/cercor/bhs009. [DOI] [PubMed] [Google Scholar]
- 74.Turk-Browne NB, Scholl BJ, Chun MM. Babies and brains: Habituation in infant cognition and functional neuroimaging. Front. Hum. Neurosci. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol. 2001;112:806–814. doi: 10.1016/S1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 76.Harmony T, Marosi E, de León AED, Becker J, Fernández T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalogr. Clin. Neurophysiol. 1990;75:482–491. doi: 10.1016/0013-4694(90)90135-7. [DOI] [PubMed] [Google Scholar]
- 77.Shek DT, Ma C. Longitudinal data analyses using linear mixed models in SPSS: Concepts, procedures and illustrations. Sci. World J. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat. Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.