Abstract
Background
Mortality and morbidity due to neonatal sepsis and necrotising enterocolitis (NEC) remain high despite the use of potent antimicrobial agents. Agents that modulate inflammation may improve outcomes. Pentoxifylline (PTX), a phosphodiesterase inhibitor, is one such agent. This is an update of a review first published in 2003 and updated in 2011 and 2015.
Objectives
To assess the effectiveness and safety of intravenous PTX as an adjunct to antibiotic therapy on mortality and morbidity in neonates with suspected or confirmed sepsis and neonates with NEC.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, and trial registries in July 2022. We also searched the reference lists of identified clinical trials and handsearched conference abstracts.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs assessing the efficacy of PTX with antibiotics (any dose or duration) for treatment of suspected or confirmed sepsis or NEC in neonates. We included three comparisons: (1) PTX with antibiotics compared to placebo or no intervention with antibiotics; (2) PTX with antibiotics compared to PTX with antibiotics and adjunct treatments such as immunoglobulin M‐enriched intravenous immunoglobulin (IgM‐enriched IVIG); (3) PTX with antibiotics compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics.
Data collection and analysis
We reported typical risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) for continuous outcomes derived from a fixed‐effect model of meta‐analysis. We calculated the number needed to treat for an additional beneficial outcome (NNTB) if there was a statistically significant reduction in RD.
Main results
We identified no new studies for this update. We included six RCTs (416 neonates). All of the included studies examined neonates with sepsis; we identified no studies on neonates with NEC. Four of the six trials had high risk of bias for at least one risk of bias domain.
Comparison 1: PTX with antibiotics compared to placebo with antibiotics, or antibiotics alone, in neonates with sepsis may reduce all‐cause mortality during hospital stay (typical RR 0.57, 95% CI 0.35 to 0.93; typical RD −0.08, 95% CI −0.14 to −0.01; NNTB 13, 95% CI 7 to 100; 6 studies, 416 participants, low‐certainty evidence) and may decrease length of hospital stay (LOS) (MD −7.74, 95% CI −11.72 to −3.76; 2 studies, 157 participants, low‐certainty evidence). The evidence is very uncertain that PTX with antibiotics compared to placebo or no intervention results in any change in chronic lung disease (CLD) (RR 1.50, 95% CI 0.45 to 5.05; 1 study, 120 participants, very low‐certainty evidence), severe intraventricular haemorrhage (sIVH) (RR 0.75, 95% CI 0.28 to 2.03; 1 study, 120 participants, very low‐certainty evidence), periventricular leukomalacia (PVL) (RR 0.50, 95% CI 0.10 to 2.63; 1 study, 120 participants, very low‐certainty evidence), NEC (RR 0.56, 95% CI 0.29 to 1.06; 6 studies, 405 participants, very low‐certainty evidence), or retinopathy of prematurity (ROP) (RR 0.40, 95% CI 0.08 to 1.98; 1 study, 120 participants, very low‐certainty evidence) in neonates with sepsis.
Comparison 2: the evidence is very uncertain that PTX with antibiotics compared to PTX with antibiotics and IgM‐enriched IVIG has any effect on mortality (RR 0.71, 95% CI 0.24 to 2.10; 102 participants, 1 study, very low‐certainty evidence) or development of NEC in neonates with sepsis (RR 1.33, 95% CI 0.31 to 5.66; 1 study, 102 participants, very low‐certainty evidence). The outcomes of CLD, sIVH, PVL, LOS, and ROP were not reported.
Comparison 3: the evidence is very uncertain that PTX with antibiotics compared to IgM‐enriched IVIG with antibiotics has any effect on mortality (RR 1.25, 95% CI 0.36 to 4.39; 102 participants, 1 study, very low‐certainty evidence) or development of NEC (RR 1.33, 95% CI 0.31 to 5.66; 102 participants, 1 study, very low‐certainty evidence) in neonates with sepsis. The outcomes of CLD, sIVH, PVL, LOS, and ROP were not reported.
All of the included studies evaluated adverse effects due to PTX, but none were reported in the intervention group in any of the comparisons.
Authors' conclusions
Low‐certainty evidence suggests that adjunct PTX therapy in neonatal sepsis may decrease mortality and length of hospital stay without any adverse effects. The evidence is very uncertain if PTX with antibiotics compared to PTX with antibiotics and IgM‐enriched IVIG, or PTX with antibiotics compared to IgM‐enriched IVIG with antibiotics, has any effect on mortality or development of NEC. We encourage researchers to undertake well‐designed multicentre trials to confirm or refute the effectiveness and safety of pentoxifylline in reducing mortality and morbidity in neonates with sepsis or NEC.
Keywords: Humans; Infant, Newborn; Anti-Bacterial Agents; Anti-Bacterial Agents/adverse effects; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/drug therapy; Immunoglobulin M; Immunoglobulins, Intravenous; Immunoglobulins, Intravenous/therapeutic use; Infant, Premature; Lung Diseases; Neonatal Sepsis; Neonatal Sepsis/drug therapy; Pentoxifylline; Pentoxifylline/adverse effects; Retinopathy of Prematurity; Sepsis; Sepsis/drug therapy
Plain language summary
Pentoxifylline for treatment of sepsis and necrotising enterocolitis in neonates
Review question
What are the benefits and risks of pentoxifylline (PTX) for treating infection and a gut condition (necrotising enterocolitis, NEC) in newborns up to 28 days of age?
Key messages
• For treating infection in the newborn, PTX with antibiotics was effective in decreasing death and duration of hospital stay without adverse effects. However, our confidence in this finding is low due to the small number of studies, all of which were of low quality.
• We did not find any studies for the use of PTX in treating severe bowel disorder (NEC)
• We need better and larger studies to fully understand if PTX is beneficial without risks for treatment of infection and NEC in newborns.
What is the problem?
Bacterial or fungal infection of the blood and NEC is a condition with digestive tract injury and infection seen in premature babies. Infection and NEC are treated with antibiotics, but still some babies die and suffer from complications.
How can we improve the treatment for infection or NEC in the newborn?
In addition to antibiotics, changing the body's response to infection (inflammation) may decrease deaths and complications. Pentoxifylline alters the body's response to infection or NEC and may have beneficial effects.
What did we want to find out?
We wanted to know if the use of PTX in addition to antibiotics can decrease deaths and complications such as lung disease, eye disease, duration of stay in the hospital, and time on the breathing machine. We also wanted to know if the use of PTX is safe without adverse effects.
What did we do?
We searched for studies that investigated whether:
• PTX with antibiotics compared to placebo (dummy treatment) with antibiotics or antibiotics alone;
• PTX with antibiotics compared to PTX with antibiotics and other drugs such as immunoglobulin; or
• PTX with antibiotics compared to other drugs such as immunoglobulin with antibiotics
was effective in decreasing deaths or other complications without adverse effects in newborns with infection or NEC. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found six eligible studies (416 newborn participants) that evaluated PTX with antibiotics in newborns with infection.
Main results
1. We found that PTX in combination with antibiotics may decrease deaths and duration of hospital stay in newborns with infection.
It is unclear if pentoxifylline treatment has any effect on lung disease, eye disease, gut injury, or brain injury as a result of infection.
The identified studies did not report any adverse effects due to PTX.
No completed studies looked at PTX treatment in newborns with NEC.
2. When PTX was compared to other drugs such as immunoglobulin in addition to PTX, it was unclear if treatment affected deaths or gut injury. Effects of treatment on lung, eye, or brain injury were not studied.
3. When PTX was compared to other drugs such as immunoglobulin, it was unclear if treatment affected deaths or gut injury. Effects of treatment on lung, eye, gut, or brain injury were not studied.
What are the limitations of the evidence?
We have low confidence in our finding that PTX in addition to antibiotics decreases death and duration of hospital stay in newborns with infection.
Three main factors reduced our confidence in the evidence. Firstly, the six identified studies were small with few participants. Secondly, four of the six studies were poorly conducted. Consequently, the results of further research could differ from the results of this review.
We have very low confidence in the effects of:
• PTX compared to other drugs such as immunoglobulin in addition to PTX; or
• PTX compared to other drugs such as immunoglobulin
on deaths and gut injury because only two studies were available with few participants, and the studies were poorly conducted. The effects of these treatments on lung disease, eye disease, or brain injury as a result of infection were not studied.
How up‐to‐date is this evidence?
The evidence is current to July 2022.
Summary of findings
Summary of findings 1. Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for neonatal sepsis.
| Pentoxifylline with antibiotics (any dose or duration) compared to placebo with antibiotics or antibiotics alone for neonatal sepsis | |||||
|
Patient or population: neonates with sepsis Settings: neonatal intensive care unit Intervention: PTX Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | PTX | ||||
|
All‐cause mortality during hospital stay |
36/207 | 20/209 | RR 0.57 (0.35, 0.93) | 416 participants (6 studies) | ⊕⊕⊝⊝
Lowa |
|
Chronic lung disease |
4/60 | 6/60 | RR 1.50 (0.45, 5.05) | 120 participants (1 study) | ⊕⊝⊝⊝ Very lowb |
| Severe intraventricular haemorrhage | 8/60 | 6/60 | RR 0.75 (0.28, 2.03) | 120 participants (1 study) | ⊕⊝⊝⊝
Very lowb |
|
Periventricular leukomalacia |
4/60 | 2/60 | RR 0.50 (0.10, 2.63) | 120 participants (1 study) | ⊕⊝⊝⊝
Very lowb |
| Length of hospital stay (LOS) in days | The mean LOS ranged across control groups from 33.8 to 38.3 days. | The mean LOS in the intervention groups was 22.6 to 31.3 days. | Mean difference −7.74, 95% CI −11.72, −3.76 |
157 participants (2 studies) | ⊕⊕⊝⊝ Lowc |
| Necrotising enterocolitis, any Bell stage | 13/203 | 23/202 | RR 0.56 (0.29, 1.06) | 405 participants (6 studies) | ⊕⊝⊝⊝ Very lowd |
|
Retinopathy of prematurity, any stage |
5/60 | 2/60 | RR 0.40 (0.08, 1.98) | 120 participants (1 study) | ⊕⊝⊝⊝
Very lowb |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTX: pentoxifylline; RR: risk ratio | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded to low because four studies had a high risk of bias, and the summary estimate was imprecise with wide CIs.
bDowngraded to very low because there was only one included study and due to imprecision of the summary estimate.
cDowngraded to low because there were only two included studies, one of which had a high risk of bias.
dDowngraded to very low because three studies had a high risk of bias, and the summary estimate was imprecise with wide CIs.
Summary of findings 2. Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and immunoglobulin M‐enriched intravenous immunoglobulin for neonatal sepsis.
| Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and immunoglobulin M‐enriched intravenous immunoglobulin for neonatal sepsis | ||||||
|
Patient or population: neonates with sepsis Settings: neonatal intensive care unit Intervention: PTX Comparison: PTX with IgM‐enriched IVIG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PTX with IgM‐enriched IVIG | PTX | |||||
| All‐cause mortality during hospital stay | 7/51 | 5/51 | RR 0.71 (0.24, 2.10) | 102 participants (1 study) | ⊕⊝⊝⊝
Very lowa |
|
| Chronic lung disease in survivors | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Severe intraventricular haemorrhage | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Periventricular leukomalacia | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Length of hospital stay in days for survivors to discharge | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Necrotising enterocolitis, any Bell stage | 3/51 | 4/51 | RR 1.33 (0.31, 5.66) | 102 participants (1 study) | ⊕⊝⊝⊝
Very lowa |
|
| Retinopathy of prematurity, any stage | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IgM: immunoglobulin M; IVIG: intravenous immunoglobulin; PTX: pentoxifylline; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded to very low because there was only one included study and due to imprecision of the summary estimate.
Summary of findings 3. Pentoxifylline with antibiotics (any dose or duration) compared to immunoglobulin M‐enriched intravenous immunoglobulin with antibiotics for neonatal sepsis.
| Pentoxifylline with antibiotics (any dose or duration) compared to immunoglobulin M‐enriched intravenous immunoglobulin with antibiotics for neonatal sepsis | ||||||
|
Patient or population: neonates with sepsis Settings: neonatal intensive care unit Intervention: PTX Comparison: IgM‐enriched IVIG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IgM‐enriched IVIG | PTX | |||||
| All‐cause mortality during hospital stay | 4/51 | 5/51 | RR 1.25 (0.36, 4.39) | 102 participants (1 study) | ⊕⊝⊝⊝
Very lowa |
|
| Chronic lung diseasein survivors | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Severe intraventricular haemorrhage | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Periventricular leukomalacia | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| Length of hospital stay in days for survivors to discharge | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
|
Necrotising enterocolitis, any Bell stage |
3/51 | 4/51 | RR 1.33 (0.31, 5.66) | 102 participants (1 study) | ⊕⊝⊝⊝
Very lowa |
|
| Retinopathy of prematurity, any stage | ‐ | ‐ | ‐ | ‐ | ‐ | None reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IgM: immunoglobulin M; IVIG: intravenous immunoglobulin; PTX: pentoxifylline; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded to very low because there was only one included study and due to imprecision of the summary estimate.
Background
Description of the condition
Neonatal sepsis is the most common cause of neonatal deaths worldwide (Lawn 2006). The incidence of neonatal sepsis in high‐income countries is reported to be between 1 and 4 cases per 1000 live births (Stoll 2004a), but in low‐ and middle‐income countries, it is significantly higher (6.5 to 38 per 1000 live births) (Zaidi 2005). The incidence of neonatal sepsis is inversely proportional to gestational age and birthweight (Kaufman 2004). Early‐onset sepsis (sepsis in infants less than 72 hours of life) occurs in 1.5% to 1.9% of very low‐birthweight (VLBW) infants (birthweight 401 g to 1500 g) (Stoll 2005). In a cohort of 6956 VLBW infants admitted to National Institute of Child Health and Human Development Neonatal Research Network hospitals during 1998 to 2000, 21% had one or more blood culture‐proven late‐onset sepsis (onset after 72 hours of life) (Stoll 2002). Mortality of infants with late‐onset sepsis was 18% (36% for those infected with gram‐negative organisms), and morbidities (including patent ductus arteriosus, prolonged ventilation, prolonged need for intravascular access, bronchopulmonary dysplasia, necrotising enterocolitis (NEC), and length of hospital stay) were significantly higher in infected infants. Sepsis significantly affects long‐term neurodevelopmental outcomes, either by direct infection of the central nervous system or as a result of inflammatory injury. In a large cohort study of 6093 extremely low‐birthweight (ELBW) infants (birthweight less than 1000 g), infected infants had a significantly higher incidence of adverse neurodevelopmental outcomes at follow‐up, characterised by cerebral palsy, low scores on Bayley Scales of Infant Development, and vision impairment when compared with uninfected infants (Stoll 2004b).
NEC occurs in about 1% to 5% of infants admitted to the neonatal intensive care unit. The most consistent risk factors are prematurity and low birthweight (Lin 2006). The pathogenesis of NEC is not entirely clear. Gastrointestinal immaturity, enteral feeding (especially formula feeding), presence of bacteria, and inflammation all play a part in the development of NEC (Lin 2006). Tumour necrosis factor alpha (TNF‐α) and platelet‐activating factor are the most important among pro‐inflammatory cytokines to have been implicated in the development of NEC (Caplan 1990a; Caplan 1990b). The pivotal role of TNF‐α in NEC is supported by the fact that, in an animal model, monoclonal antibody to TNF‐α reduced incidence of NEC from 80% to 17% (Halpern 2006). Mortality from NEC is high (15% to 30%), and 20% to 40% of infants with NEC undergo surgery. Infants who had NEC have delayed neurodevelopmental outcomes at 18 to 22 months corrected age (Lin 2006; Stoll 2004b).
Mortality and morbidity due to sepsis and NEC remain high despite the use of potent antimicrobial agents (Stoll 2002; Stoll 2005). Increased use of antimicrobials has led to a global emergence of antibiotic resistance (Levy 1998). Adjunct therapies may be important in increasing the efficacy of antimicrobial agents. Excessive or uncontrolled inflammatory response may be responsible for the multi‐organ dysfunction and systemic inflammatory response seen in sepsis. The balance of pro‐ and anti‐inflammatory cytokines may determine the severity and ultimate outcome in sepsis syndromes and NEC (Edelson 1999; Harris 2005; Ng 2003). Evidence has also shown that inflammation plays an important role in cerebral and pulmonary injury (Adams‐Chapman 2006; Speer 1999), especially in the preterm neonate. When used in conjunction with antibiotics, immunomodulating agents may help to re‐establish the balance between pro‐ and anti‐inflammatory responses and may influence clinical outcome in neonatal sepsis and NEC.
Description of the intervention
Pentoxifylline, a xanthine derivative, is a phosphodiesterase inhibitor that suppresses TNF‐α production by adenyl cyclase activation and increased cellular cyclic adenosine 3',5'‐monophosphate concentration. Pentoxifylline has attracted increased interest since the discovery that inhibition of tumour necrosis factor gene transcription reduces mortality from sepsis. TNF‐α increases peroxidation of arachidonic acid, activates polymorphonuclear leukocytes, increases eicosanoids, and increases its own production, thereby amplifying the inflammatory response (Vilcek 1991). Inhibition of TNF‐α production by pentoxifylline negates this response and thereby may improve outcome. Pentoxifylline also has beneficial effects on endothelial cell function and coagulation in sepsis (Boldt 1996; Wang 1996).
How the intervention might work
Pentoxifylline has been shown to have beneficial effects in humans and animal models of sepsis and NEC. In sepsis, pentoxifylline has been shown to improve haemodynamics (including renal blood flow) and to prevent transition from a hyperdynamic to a hypodynamic response (Bacher 1997; Krysztopik 1996; Yang 1999; Zeni 1996). Pentoxifylline also ameliorates inflammatory lung injury after endotoxaemia (Michetti 2003). In adults and neonates, pentoxifylline has been shown to decrease serum levels TNF‐α, interleukin‐1, and interleukin‐10, but not interleukin‐6 or interleukin‐8 (Bienvenu 1995; Zeni 1996). In a rat model of NEC, pentoxifylline reduced the incidence and severity of NEC (Travadi 2006). To date, no significant adverse effects have been reported in either animal or human studies.
Why it is important to do this review
The potential beneficial effects of pentoxifylline make it a promising agent for the treatment of sepsis and NEC in neonates. We performed this systematic review to determine the efficacy and safety of pentoxifylline as an adjunct to antibiotics in the treatment of sepsis or NEC in neonates.
Objectives
To assess the effectiveness and safety of intravenous pentoxifylline as an adjunct to antibiotic therapy on mortality and morbidity in neonates with suspected or confirmed sepsis and neonates with necrotising enterocolitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised controlled trials.
Types of participants
We included neonates (less than 28 days old, at any gestational age or birthweight) with confirmed or suspected sepsis or neonates with NEC (Bell's stage 2 or 3) on antibiotics (Bell 1978).
We defined confirmed sepsis as clinical signs and symptoms consistent with infection and microbiologically proven with a positive blood culture, cerebrospinal fluid culture, urine culture (obtained by a suprapubic tap), or culture from a normally sterile site (e.g. pleural fluid, peritoneal fluid, or autopsy specimens) for bacteria or fungi.
We defined suspected sepsis as clinical signs and symptoms consistent with sepsis without isolation of a causative organism.
We defined NEC as an acute gastrointestinal disorder that manifests clinically with systemic signs (temperature instability, apnoea, bradycardia, lethargy, hypotension, metabolic acidosis, hyponatraemia, thrombocytopenia, disseminated intravascular coagulation), intestinal signs (feed intolerance, gastrointestinal bleeding, abdominal tenderness, abdominal wall cellulitis, abdominal distension), radiological features (non‐specific intestinal dilation and ileus in stage I, by pneumatosis intestinalis and air in the portal tree in stage II, or pneumoperitoneum in stage III), and pathologically by intestinal necrosis (Bell 1978; Walsh 1986).
Types of interventions
The intervention was intravenous pentoxifylline at any dosage or duration used as adjunct to antibiotics to treat suspected or confirmed neonatal sepsis or NEC.
We included the following comparisons.
Pentoxifylline with antibiotics (any dose or duration) compared to placebo with antibiotics or antibiotics alone.
Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as immunoglobulin M‐enriched intravenous immunoglobulin (IgM‐enriched IVIG).
Pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics.
Types of outcome measures
Primary outcomes
All‐cause mortality during hospital stay.
Secondary outcomes
Neurological outcome at two years of age or more (neurodevelopmental outcome assessed by a validated test) (Bayley 2005; Jacobs 2013).
Chronic lung disease in survivors (chronic lung disease defined as oxygen requirement at 36 weeks' postmenstrual age) (Jobe 2001; NIH 1979).
Adverse outcomes directly attributable to pentoxifylline: thrombocytopenia (platelet count less than 100 x 109/L), increased gastric residue (gastric aspirate greater than 10% of oral feed), vomiting, cholestatic jaundice requiring therapy.
Severe intraventricular haemorrhage, grades III and IV (Papile 1978).
Periventricular leukomalacia (defined as necrosis of white matter in a characteristic distribution, i.e. in the white matter dorsal and lateral to the external angles of lateral ventricles involving particularly the centrum semiovale and optic and acoustic radiations and diagnosed by neuroimaging) (Volpe 1995).
Duration of assisted ventilation through an endotracheal tube (days).
Length of hospital stay in days for survivors to discharge.
Necrotising enterocolitis (infants with sepsis only): a) NEC any Bell stage and b) NEC (definite NEC and perforated NEC, Bell's stage 2 or 3) (Bell 1978; Walsh 1986).
Retinopathy of prematurity (post hoc analysis): a) ROP any stage, b) ROP stages III and IV (ICROP 1984).
Search methods for identification of studies
Cochrane Neonatal Information Specialist M Fiander wrote new search strategies to increase sensitivity. Given these changes, no date limits were applied to the searches.
Electronic searches
We searched the following databases in July 2022. We conducted searches without language or publication type limits. We applied no date limit to searches for trials, but limited searches for systematic reviews from 2020 forward.
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies (CRS) (Issue 7, 2022)
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions (1946 to 29 July 2022)
Embase via Ovid (1974 to 29 July 2022)
CINAHL (EBSCOhost) (Cumulative Index to Nursing and Allied Health Literature) (1985 to 31 July 2022)
Search strategies are shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4.
Searching other resources
We searched the following trial registries on 29 July 2022.
ISRCTN registry (www.isrctn.com) (formerly controlledtrials.com)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal)
US National Library of Medicine ClinicalTrials.gov (www.clinicaltrials.gov)
Australian New Zealand Clinical Trials Registry (ANZCTR) (anzctr.org.au/)
Search strategies are shown in Appendix 5.
We searched the following conferences or conference sources up to 2020 or 2022, depending upon availability.
Pediatric Academic Societies (PAS) conference up to 2022
European Academy of Paediatric Societies (EAPS) up to 2020
BIOSIS from January 1992 to July 2021 (we did not have access after July 2021)
Note: for the previous version of this review (Pammi 2015), Abstracts2View was searched; however, this site has not been available since 2014.
We contacted authors who published in the field for possible unpublished studies and identified one ongoing study (ACTRN12606000257561). We additionally searched the reference lists of identified clinical trials and review authors' personal files up to July 2022
Data collection and analysis
We employed the standard methods of Cochrane Neonatal Review Group guidelines in creating this update.
Selection of studies
We managed search results in EndNote (EndNote). We used Covidence for screening (Covidence), and recorded results in sufficient detail to create a PRISMA flow diagram (Liberati 2009). We conducted title/abstract review independently. We independently assessed the full‐text versions of potentially relevant studies identified during title/abstract screening. At both stages, we resolved any disagreements by discussion. We documented the reasons for exclusion or any studies excluded at the full‐text stage; for details see Characteristics of excluded studies.
Data extraction and management
We designed forms for trial inclusion/exclusion, data extraction, and for requesting additional information from authors of the original reports. We performed data extraction independently using specifically designed paper forms and compared for any differences, which we then resolved by discussion.
Assessment of risk of bias in included studies
We independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool for the following domains (Higgins 2011).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion. See Appendix 6 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used the standard methods of Cochrane Neonatal. We performed statistical analyses using Cochrane Review Manager 5 software (Review Manager 2020). We analysed categorical data using risk ratio (RR), risk difference (RD), and the number needed to treat for an additional beneficial outcome (NNTB). We analysed continuous data using mean difference (MD). We reported the 95% confidence interval (CI) on all estimates.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials, and an infant was considered only once in the analysis. If in future updates we find cluster‐randomised trials, the participating neonatal unit or section of a neonatal unit or hospital will be the unit of analysis. We will analyse any cluster‐randomised trials using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or from a study with a similar population, as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If we use ICCs from a similar trial or from a study with a similar population, we will report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will only combine the results from both if there is little heterogeneity between the study designs, and interaction between the effect of the intervention and the choice of randomisation unit is considered to be unlikely. We will acknowledge any possible heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate possible effects of the randomisation unit.
Dealing with missing data
Where feasible, we performed analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we analysed all participants in the treatment group to which they had been randomised, regardless of the actual treatment received. When we identified important missing data (in the outcomes) or unclear data, we contacted the original investigators to request the missing data.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using the I2 statistic to determine the appropriateness of pooling data and performing meta‐analysis. We deferred meta‐analysis if heterogeneity was high (greater than 75%). We used the following cut‐offs to report on the degree of heterogeneity:
less than 25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 74%: moderate heterogeneity; and
greater than 75%: high heterogeneity.
If we detected statistical heterogeneity, we explored the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses. We used a fixed‐effect model for meta‐analysis.
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes against the reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. We planned to document studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes in the 'Characteristics of included studies' tables. We planned to use funnel plots to screen for publication bias if there were a sufficient number of studies (> 10) reporting the same outcome. If publication bias was suggested by a significant asymmetry of the funnel plot on visual assessment, we would incorporate this in our assessment of the certainty of the evidence.
Data synthesis
We performed the meta‐analysis using Review Manager 5 software (Review Manager 2020). For estimates of typical RR and RD, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We performed all meta‐analyses using the fixed‐effect model.
We analysed all neonates including subgroups defined in Criteria for considering studies for this review on an intention‐to‐treat basis, irrespective of whether they survived to complete their allocated treatment.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
-
Gestational age:
preterm neonates (born before 37 completed weeks' gestation);
term neonates (born at or after 37 completed weeks of gestation).
-
Time of onset of sepsis:
early‐onset sepsis (sepsis in the first 72 hours of life);
late‐onset sepsis (sepsis after the first 72 hours of life).
-
Suspected or confirmed sepsis:
neonates with suspected sepsis (clinical signs and symptoms consistent with sepsis without isolation of causative organism treated with antibiotics);
neonates with confirmed sepsis;
neonates with confirmed gram‐negative sepsis;
neonates with confirmed fungal sepsis.
Sensitivity analysis
If we identified substantial heterogeneity, we would conduct sensitivity analysis to determine if the findings were affected by inclusion of only those trials considered to have used adequate methodology with a low risk of bias (selection and performance bias). We planned to report results of sensitivity analyses for our primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes.
All‐cause mortality before hospital discharge
Chronic lung disease
Severe intraventricular haemorrhage
Periventricular leukomalacia
Length of hospital stay
Necrotising enterocolitis, any Bell stage
Retinopathy of prematurity, any stage
We used GRADEpro GDT software to create three summary of findings tables to report the certainty of the evidence for the following three comparisons (GRADEpro GDT).
Comparison 1: pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention with antibiotics (Table 1).
Comparison 2: pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as IgM‐enriched IVIG (Table 2).
Comparison 3: pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics (Table 3).
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
For details of studies, see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search identified 565 references (499 from databases and 66 from trial registries). After removal of 136 duplicates, 429 references were available for title/abstract screening. We reviewed 13 full texts, of which we excluded 4 studies, assessed 1 study as awaiting classification; and identified 2 ongoing studies (Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies). We included six studies in the review (Adel 2010; Akdag 2014; Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). For details of the study selection process, see Figure 1.
1.
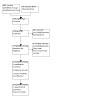
PRISMA flow diagram illustrating study selection process.
Included studies
Adel 2010 quasi‐randomised 37 neonates with sepsis based on the day of admission to the neonatal unit to intravenous (IV) pentoxifylline (5 mg/kg/h for 6 hours for 6 consecutive days) or placebo (equal volume of normal saline for 6 consecutive days) as adjuncts to antibiotics. Seventeen out of 37 received pentoxifylline, and 20 out of 37 received placebo. The study reported the outcomes of mortality, length of hospital stay, multi‐organ dysfunction, coagulation profiles including platelet count and C‐reactive protein, shock, and NEC. Of the 37 neonates with suspected sepsis, 6 infants were culture‐negative but included in the analysis.
Akdag 2014 randomised 204 neonates with sepsis to 4 groups: pentoxifylline (6 mg/kg IV over 4 hours for 3 consecutive days), pentoxifylline and IgM‐enriched IVIG (pentaglobin, 250 mg/kg over 4 hours for 3 consecutive days), IgM‐enriched IVIG (pentaglobin), or placebo (normal saline). The study reported the outcomes of mortality, NEC, oliguria/anuria, hepatic failure, disseminated intravascular coagulation, pulmonary haemorrhage, and laboratory parameters of inflammation (white blood cell count, C‐reactive protein, interleukin‐6, TNF‐α, and neutrophil CD64). Length of hospital stay was reported in days (mean) but did not have standard deviations to use in meta‐analysis.
Ali 2006 randomised 50 preterm neonates with culture‐proven sepsis (less than 37 weeks' gestational age) to pentoxifylline intravenously (5 mg/kg/h for 6 hours for 3 consecutive days, n = 25) as an adjunct to antibiotics, or to a control group that received antibiotics alone (n = 25). The study reported the outcomes of mortality, development of NEC, length of hospital stay, duration of ventilation, and adverse effects. Duration of ventilation and length of hospital stay were reported in days (mean) but did not have standard deviations to use in meta‐analysis.
Lauterbach 1996 randomised 40 preterm neonates (less than 36 weeks' gestation) with suspected late‐onset sepsis to receive either pentoxifylline or placebo as adjunct to antibiotics. In 4 out of 20 neonates in the treatment group and 7 out of 20 neonates in the placebo group sepsis was not confirmed, and these neonates were excluded from analysis. Outcomes were thus reported in only the 29 neonates with confirmed sepsis (16 in treatment and 13 in placebo group). Of these neonates, confirmed gram‐negative sepsis occurred in 10 in the pentoxifylline group and 10 in the placebo group. The following outcomes were reported: i) plasma TNF‐α levels, ii) mortality during hospital stay, iii) NEC, and iv) adverse effects (adverse effects due to pentoxifylline were not reported specifically). Lauterbach 1999 randomised 100 preterm neonates (less than 36 weeks' gestation) with suspected late‐onset sepsis to receive either pentoxifylline or placebo as adjunct to antibiotics. In 10 out of 50 neonates in the treatment group and 12 out of 50 neonates in the placebo group sepsis was not confirmed, and these neonates were excluded from analysis. Outcomes were thus reported for only the 78 neonates with confirmed sepsis (40 in the pentoxifylline and 38 in the placebo group). Of these neonates, confirmed gram‐negative sepsis occurred in 15 in the pentoxifylline group and 14 in the placebo group. The following outcomes were reported: i) plasma levels of TNF‐α, interleukin‐6, and interleukin‐1, ii) mortality during hospital stay, iii) NEC, and iv) adverse effects (adverse effects due to pentoxifylline were not reported specifically).
Shabaan 2015 randomised 120 preterm neonates to intravenous pentoxifylline at 5 mg/kg/h for 6 hours on 6 successive days or normal saline (placebo). Of these neonates, 78 had confirmed sepsis and 52 had confirmed gram‐negative sepsis. The primary outcome was death before hospital discharge; secondary outcomes were length of hospital stay, duration of respiratory support, duration of antibiotic use, short‐term morbidity (chronic lung disease, NEC, intraventricular haemorrhage, periventricular leukomalacia, and retinopathy of prematurity), TNF‐α concentrations, C‐reactive protein concentrations, and adverse effects of pentoxifylline.
For further details, see Characteristics of included studies.
Excluded studies
We excluded four studies at the full‐text stage.
Non‐randomised study from Turkey evaluating intravenous pentoxifylline as an adjunct to antibiotic therapy on mortality and morbidity in VLBW preterm neonates with nosocomial sepsis (n = 18) (Hamilcikan 2017a).
Non‐randomised study from Turkey comparing pentoxifylline and pentaglobin used as adjuncts to antibiotics in VLBW preterm neonates with nosocomial sepsis (Hamilcikan 2017b). This study may have included the same participants as Hamilcikan 2017a.
Seventeen preterm infants with sepsis were given pentoxifylline and compared with a historical control group of 13 septic infants who did not receive pentoxifylline. The study reported mortality and adverse effects. We excluded the study as it was neither randomised nor quasi‐randomised (Lauterbach 1994).
Non‐randomised study including 20 neonates with sepsis; the first 13 infants received pentoxifylline, and the next 7 participants constituted the control group. The study reported outcomes of mortality, leukocyte count, serum C‐reactive protein level, TNF‐α, and interleukin‐6 levels (Selim 2004).
For further details, see Characteristics of excluded studies.
Studies awaiting classification
Sareno 2013 reported a randomised controlled trial where preterm infants ≤ 1500 g with suspected infection admitted to the neonatal intensive care unit were randomised to receive either pentoxifylline at a dose of 6 mg/kg/h or placebo. The primary outcome measured was occurrence of all‐cause mortality between groups; this was analysed on an intention‐to‐treat basis. Secondary outcomes measured included mortality from sepsis, adverse drug reactions, and length of hospital stay. The report is published as a conference abstract, and we are awaiting missing data from authors.
For further details, see Characteristics of studies awaiting classification.
Ongoing studies
ACTRN12606000257561 is a pilot study to randomise 80 preterm (less than 32 weeks' gestation) neonates with stage 2 or 3 NEC to intravenous pentoxifylline or an equal volume of placebo at 5 mg/kg/h for 12 hours a day (60 mg/kg/day) for 2 consecutive days, followed by infusion for 6 hours a day (30 mg/kg/day) for the next 4 consecutive days. The primary outcome is to assess the efficacy and safety of pentoxifylline in preventing the progression of NEC or death, or both. Secondary outcomes are reduction in plasma TNF‐α levels, duration of hospital stay, duration of total parenteral nutrition support, and time to full enteral feeds. This study did not enrol patients, but has metamorphosed into the PROTECT trial (ACTRN12616000405415), with changes to study participants and objectives; we are awaiting communication with the principal investigator.
ACTRN12616000405415 is an international multicentre trial that plans to enrol approximately 1800 preterm neonates (born < 29 weeks' gestational age). The primary aim is to evaluate the effect of treatment with intravenous pentoxifylline compared to placebo, starting within six hours from blood culture taken for suspected late‐onset sepsis or NEC. After 48 hours, treatment will cease if diagnosis is refuted, or will continue for 4 days if diagnosis is proven. The primary outcome to measure effectiveness is survival without disability at 18 to 24 months of age (corrected for gestation).
For further details, see Characteristics of ongoing studies.
Risk of bias in included studies
See: Characteristics of included studies (Figure 2; Figure 3)
2.

Risk of bias summary of the included studies in seven domains.
3.

Risk of bias of the included studies in seven domains.
Allocation
Three trials had high risk of selection bias due to either lack of randomisation details or absence of concealment of allocation (Adel 2010; Ali 2006; Lauterbach 1996).
Blinding
All included trials except two, Adel 2010; Ali 2006, reported blinding of the intervention. None of the included trials reported clearly on blinding of outcome assessment, hence detection bias could be an issue.
Incomplete outcome data
Two trials had high risk of attrition bias, as participants were excluded from the final analysis (Lauterbach 1996; Lauterbach 1999).
Selective reporting
Two trials reported outcomes only in neonates with culture‐proven sepsis (Lauterbach 1996; Lauterbach 1999).
Other potential sources of bias
Two trials had unclear risk for other bias due to lack of methodological details of the study (Lauterbach 1996; Lauterbach 1999).
The number of risk of bias domains increased to seven compared to the previous version of the review (Pammi 2015); this has not impacted our conclusions regarding risk of bias in the included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo with antibiotics or antibiotics alone
Six randomised controlled trials where pentoxifylline was used for the treatment of neonatal sepsis were eligible for inclusion in the review (Adel 2010; Akdag 2014; Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015), and reported the following outcomes.
Primary outcome
All‐cause mortality during hospital stay
All six studies reported on all‐cause mortality in infants with sepsis (Adel 2010; Akdag 2014; Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). Pentoxifylline used as an adjunct to antibiotics in neonates with sepsis may reduce all‐cause mortality during hospital stay compared to placebo or no intervention (typical risk ratio (RR) 0.57, 95% confidence interval (CI) 0.35 to 0.93; typical risk difference (RD) −0.08, 95% CI −0.14 to −0.01; number needed to treat for an additional beneficial outcome (NNTB) 13, 95% CI 7 to 100; 6 studies, 416 participants, low‐certainty evidence, Analysis 1.1). There was mild heterogeneity (I2 = 30% for RR) among the six trials for this outcome.
1.1. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 1: All‐cause mortality during hospital stay
Subgroup analyses
1. Gestational age
a. Preterm neonates (born before 37 completed weeks' gestation)
Four trials reported outcomes for the subgroup of preterm infants (Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). Pentoxifylline used as an adjunct to antibiotics in preterm neonates with sepsis may reduce all‐cause mortality during hospital stay (typical RR 0.38, 95% CI 0.20 to 0.71; typical RD −0.13, 95% CI −0.21 to −0.05; NNTB 8, 95% CI 5 to 20; 4 studies, 277 participants, low‐certainty evidence, Analysis 1.1). There was no heterogeneity (I2 = 0) among the four trials for this outcome.
b. Term neonates (born at or after 37 completed weeks of gestation). No data were available for subgroup analysis.
2. Time of onset of sepsis
a. Early‐onset sepsis (sepsis in the first 72 hours of life). No data were available for subgroup analysis.
b. Late‐onset sepsis (sepsis after the first 72 hours of life)
Three studies reported on all‐cause mortality in infants with late‐onset sepsis (Lauterbach 1996; Lauterbach 1999; Shabaan 2015). Pentoxifylline used as an adjunct to antibiotics in neonates with sepsis may reduce all‐cause mortality during hospital stay in neonates with late‐onset sepsis (typical RR 0.42, 95% CI 0.19 to 0.95; typical RD −0.12, 95% CI −0.23 to −0.02; NNTB 8, 95% CI 4 to 50; 3 studies, 169 participants, low‐certainty evidence, Analysis 1.1). There was no heterogeneity (I2 = 0 for RR) among the three trials for this outcome.
3. Suspected or confirmed sepsis
a. Neonates with suspected sepsis (clinical signs and symptoms consistent with sepsis without isolation of causative organism treated with antibiotics). No data were available for subgroup analysis.
b. Neonates with confirmed sepsis
Four studies reported on all‐cause mortality in infants with confirmed sepsis (Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). Pentoxifylline used as an adjunct to antibiotics in neonates with confirmed sepsis may reduce all‐cause mortality during hospital stay (typical RR 0.37, 95% CI 0.19 to 0.73; typical RD −0.14, 95% CI −0.23 to −0.05; NNTB 7, 95% CI 4 to 20; 4 studies, 235 participants, low‐certainty evidence, Analysis 1.1). There was no heterogeneity (I2 = 0% for RR) among the four trials for this outcome.
c. Neonates with confirmed gram‐negative sepsis
Four studies reported on all‐cause mortality in infants with confirmed sepsis (Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). Pentoxifylline used as an adjunct to antibiotics in neonates with confirmed gram‐negative sepsis may decrease all‐cause mortality during hospital stay (typical RR 0.33, 95% CI 0.16 to 0.72; typical RD −0.19, 95% CI −0.31 to −0.07; NNTB 5, 95% CI 3 to 14; 4 studies, 143 participants, low‐certainty evidence, Analysis 1.1). There was no heterogeneity (I2 = 0% for RR) among the four studies for this outcome.
d. Neonates with confirmed fungal sepsis. No data were available for subgroup analysis.
Secondary outcomes
1. Neurological outcome at two years of age or more
No study reported this outcome.
2. Chronic lung disease
One study reported this outcome (Shabaan 2015). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics in neonates with sepsis affects risk of chronic lung disease compared to antibiotics alone (RR 1.50, 95% CI 0.45 to 5.05; RD 0.03, 95% CI −0.07 to 0.13; 1 study, 120 participants, very low‐certainty evidence, Analysis 1.2).
1.2. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 2: Chronic lung disease
3. Adverse outcomes
No study reported this outcome.
4. Severe intraventricular haemorrhage
One study reported this outcome (Shabaan 2015). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics in neonates with sepsis affects risk of severe intraventricular haemorrhage compared to antibiotics alone (RR 0.75, 95% CI 0.28 to 2.03; RD −0.03, 95% CI −0.15 to 0.08; 1 study, 120 participants, very low‐certainty evidence, Analysis 1.3).
1.3. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 3: Severe intraventricular haemorrhage (grade 3 and 4)
5. Periventricular leukomalacia
One study reported this outcome (Shabaan 2015). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics in neonates with sepsis affects risk of periventricular leukomalacia compared to antibiotics alone (RR 0.50, 95% CI 0.10 to 2.63; RD −0.03, 95% CI −0.11 to 0.04; 1 study, 120 participants, very low‐certainty evidence, Analysis 1.4).
1.4. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 4: Periventricular leukomalacia
6. Duration of assisted ventilation through an endotracheal tube (days)
No study reported this outcome.
7. Length of hospital stay
Two studies reported this outcome (Adel 2010; Shabaan 2015). The evidence suggests that pentoxifylline used as an adjunct to antibiotics in neonates with sepsis may decrease length of hospital stay (the estimated mean difference for the outcome was MD −7.74, 95% CI −11.72 to −3.76; 2 studies, 157 participants, low‐certainty evidence, Analysis 1.5). There was no heterogeneity (I2 = 0 for RR) between the two trials for this outcome.
1.5. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 5: Length of hospital stay
8. Outcome for neonates with sepsis only: NEC, any Bell stage
Six studies reported this outcome (Adel 2010; Akdag 2014; Ali 2006; Lauterbach 1996; Lauterbach 1999; Shabaan 2015). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics in neonates with sepsis has any effect on the risk of developing NEC compared to antibiotics alone (typical RR 0.56, 95% CI 0.29 to 1.06; typical RD −0.05, 95% CI −0.11 to 0.00; 6 studies, 405 participants, very low‐certainty evidence, Analysis 1.6). There was mild heterogeneity (I2 = 21% for RR) among the three trials for this outcome.
1.6. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 6: Necrotising enterocolitis, any Bell stage
9. Retinopathy of prematurity, any stage
One study reported this outcome (Shabaan 2015). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics in neonates with sepsis affects retinopathy of prematurity compared to antibiotics alone (RR 0.40, 95% CI 0.08 to 1.98; RD −0.05, 95% CI −0.13 to 0.03; 1 study, 120 participants, very low‐certainty evidence, Analysis 1.7).
1.7. Analysis.

Comparison 1: Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis, Outcome 7: Retinopathy of prematurity, any stage
Comparison 2. Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as IgM‐enriched IVIG
One study was eligible for inclusion in this comparison (Akdag 2014).
Primary outcome
All‐cause mortality during hospital stay
One study reported this outcome (Akdag 2014). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to pentoxifylline plus IgM‐enriched IVIG in neonates with sepsis affects all‐cause mortality during hospital stay (RR 0.71, 95% CI 0.24 to 2.10; RD −0.04, 95% CI −0.16 to 0.09; 1 study, 102 participants, very low‐certainty evidence, Analysis 2.1).
2.1. Analysis.

Comparison 2: Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as IgM‐enriched IVIG, Outcome 1: All‐cause mortality
Secondary outcomes
The following outcomes were not reported on in the included study (Akdag 2014).
Neurological outcome at two years of age or more
Chronic lung disease in survivors (chronic lung disease defined as oxygen requirement at 36 weeks' postmenstrual age)
Adverse outcomes
Severe intraventricular haemorrhage
Periventricular leukomalacia
Duration of assisted ventilation through an endotracheal tube (days)
Length of hospital stay in days for survivors to discharge
8. Necrotising enterocolitis, any Bell stage
One study reported this outcome (Akdag 2014). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to pentoxifylline plus IgM‐enriched IVIG in neonates with sepsis affects risk of NEC (RR 1.33, 95% CI 0.31 to 5.66; RD 0.02, 95% CI −0.08 to 0.12; 1 study, 102 participants, very low‐certainty evidence, Analysis 2.2).
2.2. Analysis.

Comparison 2: Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as IgM‐enriched IVIG, Outcome 2: Necrotising enterocolitis, any Bell stage
9. Retinopathy of prematurity, any stage
No study reported this outcome.
Comparison 3. Pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics
One study was eligible for inclusion in this comparison (Akdag 2014).
Primary outcome
All‐cause mortality during hospital stay
One study reported this outcome (Akdag 2014). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to IgM‐enriched IVIG in neonates with sepsis affects all‐cause mortality during hospital stay (RR 1.25, 95% CI 0.36 to 4.39; RD 0.02, 95% CI −0.09 to 0.13; 1 study, 102 participants, very low‐certainty evidence, Analysis 3.1).
3.1. Analysis.

Comparison 3: Pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics, Outcome 1: All‐cause mortality
Secondary outcomes
The following outcomes were not reported on in the included study (Akdag 2014).
Neurological outcome at two years of age or more
Chronic lung disease in survivors (chronic lung disease defined as oxygen requirement at 36 weeks' postmenstrual age)
Adverse outcomes
Severe intraventricular haemorrhage
Periventricular leukomalacia
Duration of assisted ventilation through an endotracheal tube (days)
Length of hospital stay in days for survivors to discharge
8. Necrotising enterocolitis, any Bell stage
One study reported this outcome (Akdag 2014). The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to IgM‐enriched IVIG in neonates with sepsis affects risk of NEC (RR 1.33, 95% CI 0.31 to 5.66; RD 0.02, 95% CI −0.08 to 0.12; 1 study, 102 participants, very low‐certainty evidence, Analysis 3.2).
3.2. Analysis.

Comparison 3: Pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics, Outcome 2: Necrotising enterocolitis, any Bell stage
9. Retinopathy of prematurity, any stage
No study reported this outcome.
II. Pentoxifylline for neonates with NEC
Lack of eligible randomised or quasi‐randomised trials precluded comparisons of pentoxifylline for the treatment of NEC.
Discussion
Summary of main results
We identified six randomised controlled trials that reported our prespecified neonatal outcomes. The included trials randomised 416 newborn infants with sepsis to pentoxifylline, placebo, or IVIG. Pentoxifylline used as an adjunct to antibiotics in neonates with sepsis may reduce all‐cause mortality during hospital stay compared to placebo or no intervention (typical RR 0.57, 95% CI 0.35 to 0.93; typical RD −0.08, 95% CI −0.14 to −0.01; NNTB 13, 95% CI 7 to 100; 6 studies, 416 participants, low‐certainty evidence). In subgroup analyses of all‐cause mortality during hospital stay, low‐certainty evidence suggests that pentoxifylline therapy may decrease neonatal deaths due to confirmed sepsis, confirmed gram‐negative sepsis, in late‐onset sepsis, and in preterm infants. The evidence suggests that pentoxifylline used as an adjunct to antibiotics in neonates with sepsis may decrease length of hospital stay (the estimated mean difference for the outcome was −7.74 days, 95% CI −11.72 to −3.76; 2 studies, 157 participants, low‐certainty evidence). Based on one to three trials with high risk of bias, the evidence is very uncertain that pentoxifylline therapy has any effect on chronic lung disease, severe intraventricular haemorrhage, periventricular leukomalacia, development of NEC, or retinopathy of prematurity.
The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to pentoxifylline plus IgM‐enriched IVIG in neonates with sepsis affects all‐cause mortality during hospital stay or development of NEC (1 study, 102 participants, very low‐certainty evidence).
The evidence is very uncertain whether pentoxifylline used as an adjunct to antibiotics compared to IgM‐enriched IVIG in neonates with sepsis affects all‐cause mortality during hospital stay or development of NEC (1 study, 102 participants, very low‐certainty evidence).
Adverse outcomes directly attributable to pentoxifylline (thrombocytopenia (platelet count less than 100 x 109/L), increased gastric residue (gastric aspirate greater than 10% of oral feed), vomiting, cholestatic jaundice requiring therapy) were not reported in the included trials.
Overall completeness and applicability of evidence
The six randomised controlled trials were performed in neonatal intensive care units in Poland, Egypt, Turkey, and India. Four of these studies suffered from high risk of bias, namely selection, detection, and attrition biases. However, all of the included trials evaluated neonates with sepsis, including preterm infants and infants with proven sepsis. The small sample sizes and low certainty of the evidence decrease the generalisability and applicability of the evidence.
Quality of the evidence
We rated four studies as at high risk of bias, and two studies as at low risk of bias. Using the GRADE approach, we downgraded the certainty of the evidence from six studies for the outcome all‐cause mortality during hospital stay to low. Reasons for downgrading included high risk of bias in the included studies and the imprecision of the summary estimate. We also downgraded the certainty of the evidence to low for the outcome length of hospital stay, because the evidence came from only two studies, one of which had a high risk of bias. We downgraded the certainty of the evidence for the outcomes of chronic lung disease, severe intraventricular haemorrhage, periventricular leukomalacia, and retinopathy of prematurity to very low because only a few studies reported these data, and the summary estimate was imprecise with large CIs. Only one study reported the comparisons of pentoxifylline with IVIG alone or with pentoxifylline plus IVIG, and the summary estimate was imprecise.
Potential biases in the review process
We strove to decrease biases in the review process by following standard Cochrane methodology, which included a sensitive literature search without date limits; despite this, we did not identify any new studies for inclusion in this update. We did not find trials that evaluated pentoxifylline in neonates with NEC, and it is likely that any such trials were not done rather than missed by our search strategy. The included studies did not report relevant secondary outcomes. Our post hoc analysis of retinopathy of prematurity as an additional outcome did not change our conclusions. We pursued the investigators of published randomised controlled trials for additional data and missing information with limited success.
Agreements and disagreements with other studies or reviews
Harris and colleagues reviewed the use of pentoxifylline in preterm neonates who had sepsis (two randomised controlled trials (included in this review), one quasi‐randomised study (included in this review), and two observational studies) or NEC (one case series) (Harris 2010). The review authors also included cohort and observational studies and did not synthesise data into a meta‐analysis. Harris and colleagues discuss a decrease in mortality similar to this review and improvement in other neonatal outcomes including lung disease and inflammatory markers. The review authors acknowledged that the included studies were small with significant methodological limitations, hence better‐quality evidence is needed.
Peng 2022 conducted a meta‐analysis using a random‐effects model of seven studies (one observational study (Selim 2004), and the six randomised controlled trials included in our review). Peng and colleagues reported that pentoxifylline decreased length of hospital stay and metabolic acidosis but not mortality (Peng 2022).
Authors' conclusions
Implications for practice.
Evidence from six small studies suggests that pentoxifylline therapy as an adjunct to antibiotics compared to placebo or no intervention in neonatal sepsis may decrease mortality (low‐certainty evidence) and length of hospital stay (low‐certainty evidence) without any adverse effects, but evidence is very uncertain on the development of necrotising enterocolitis (very low‐certainty evidence). The evidence is very uncertain if pentoxifylline with antibiotics compared to pentoxifylline with antibiotics and immunoglobulin M‐enriched intravenous immunoglobulin (IgM‐enriched IVIG) or pentoxifylline with antibiotics compared to IgM‐enriched IVIG with antibiotics affects mortality or development of necrotising enterocolitis (very low‐certainty evidence). We encourage researchers to undertake large, well‐designed multicentre trials to confirm or refute the effectiveness and safety of pentoxifylline in reducing mortality and morbidity in neonates with sepsis or necrotising enterocolitis.
Implications for research.
Researchers should be encouraged to undertake adequately powered, well‐designed, multicentre randomised controlled trials to confirm or refute the role of pentoxifylline in the treatment of neonatal sepsis and necrotising enterocolitis. Trials should report on clinically important comorbidities of sepsis (e.g. chronic lung disease, periventricular leukomalacia, and duration of assisted ventilation, among others) and long‐term neurological outcomes. Researchers may consider comparing pentoxifylline with other adjunctive modalities that decrease inflammatory injury in the treatment of neonatal sepsis and necrotising enterocolitis.
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2023 | New search has been performed | We updated the search in July 2022. No new trials were included, but we found one ongoing trial (ACTRN12616000405415), and excluded two trials (Hamilcikan 2017a; Hamilcikan 2017b). One outcome, retinopathy of prematurity, was included post hoc. |
| 20 June 2023 | New citation required but conclusions have not changed | The data analyses and conclusions remain the same. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 29 June 2014 | New citation required but conclusions have not changed | No changes to conclusions |
| 29 June 2014 | New search has been performed | We updated the search in May 2014 and added two new studies (Shabaan 2015 and Akdag 2014). We revised the review by adding a summary of findings table and changing the ongoing study (ZTB 2009) to an included study (Akdag 2014). We used the GRADE approach to rate the quality of evidence as high, moderate, low, or very low. |
| 8 July 2011 | New citation required but conclusions have not changed | No change to conclusions |
| 8 July 2011 | New search has been performed | This updates the review 'Pentoxyfilline for treatment of sepsis and necrotizing enterocolitis in neonates' published in the Cochrane Database of Systematic Reviews (Haque 2003). Search updated 8 July 2011. We included two new trials and added one ongoing study to the review. |
| 7 December 2010 | Amended | Contact details updated. |
| 21 February 2008 | Amended | Converted to new review format |
| 11 September 2007 | New search has been performed | This updates the review 'Pentoxifylline for neonatal sepsis' published in the Cochrane Database of Systematic Reviews, Issue 2, 2003 (Haque 2003). The updated search did not identify any new trials. Two ongoing trials using pentoxifylline in the treatment of necrotising enterocolitis were identified. The title and the review have been modified to include pentoxifylline treatment for necrotising enterocolitis, in view of emerging evidence for potential benefits of the use of pentoxifylline for this condition. |
| 28 January 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Information Specialist Michelle Fiander for writing and running the search strategies for this review.
We thank Cochrane Neonatal Group's Roger Soll and William McGuire, Co‐ordinating Editors; and Fiona Russell, Michelle Fiander, and Jane Cracknell, Managing Editors, for editorial support of this review.
We thank Sven M Schulzke from University Children's Hospital Basel (UKBB), and Siree Kampfen, Department of Neonatology, University Children's Hospital (UKBB), University of Basel, for peer review and constructive comments.
We also thank Lisa Winer, copy editor, for her work.
Appendices
Appendix 1. Cochrane CRS strategy
| Cochrane CRS | ||
| 31‐Jul‐22 | ||
| 1 | MESH DESCRIPTOR Pentoxifylline AND CENTRAL:TARGET | 3094 |
| 2 | (agapurin or artal or azupentat or azutrenat or bl 191 or bl191 or c‐vex or carpental or cental or ceretal or claudicat retard or "eht 0201" or eht0201 or elorgan or erytral or fixoten or flexital or harin or harine or hemovas or ikomio or ipentol or kentadin or oxopurin 400 sr or oxpentifylline or oxpentiphylline or oxypentifylline or penphylline or pentong or pentopak or pentox* or pentyllin or perencal or perental or peridane or pexal or pexol or platof or ralofect or ralofekt or relofekt or rentylin or tarontal or thrental or torental or torestal or trenfyl or trenlin or trental or trepal‐400 or vazofen):ti,ab,kw AND CENTRAL:TARGET | 1400 |
| 3 | #1 OR #2 | 3917 |
| 4 | MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL AND CENTRAL:TARGET | 7724 |
| 5 | (Phosphodiesteras* adj2 Inhibit*):ti,ab,kw AND CENTRAL:TARGET | 1967 |
| 6 | #5 OR #4 | 8922 |
| 7 | MESH DESCRIPTOR enterocolitis EXPLODE ALL AND CENTRAL:TARGET | 473 |
| 8 | MESH DESCRIPTOR sepsis EXPLODE ALL AND CENTRAL:TARGET | 5041 |
| 9 | (sepsis* or septic* or Pyemia* or Pyohemi* or Pyaemi* or ((blood* or bloodstream*) adj2 (infect* or poison*))):ti,ab,kw AND CENTRAL:TARGET | 14931 |
| 10 | #9 OR #8 OR #7 | 17148 |
| 11 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 17912 |
| 12 | MESH DESCRIPTOR Intensive Care, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 363 |
| 13 | MESH DESCRIPTOR Intensive Care Units, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 903 |
| 14 | MESH DESCRIPTOR Gestational Age EXPLODE ALL AND CENTRAL:TARGET | 2928 |
| 15 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs):ti,ab,kw AND CENTRAL:TARGET | 77904 |
| 16 | #11 OR #12 OR #13 OR #14 OR #15 | 81312 |
| 17 | #3 AND #16 | 103 |
| 18 | #6 AND #10 AND #16 | 17 |
| 19 | #18 OR #17 | 108 |
Appendix 2. MEDLINE strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to July 29, 2022 | ||
| # | Searches | Results |
| 1 | pentoxifylline/ [MeSH/EMTREE] | 4320 |
| 2 | (agapurin or artal or azupentat or azutrenat or bl 191 or bl191 or c‐vex or carpental or cental or ceretal or claudicat retard or "eht 0201" or eht0201 or elorgan or erytral or fixoten or flexital or harin or harine or hemovas or ikomio or ipentol or kentadin or oxopurin 400 sr or oxpentifylline or oxpentiphylline or oxypentifylline or penphylline or pentong or pentopak or pentox* or pentyllin or perencal or perental or peridane or pexal or pexol or platof or ralofect or ralofekt or relofekt or rentylin or tarontal or thrental or torental or torestal or trenfyl or trenlin or trental or trepal‐400 or vazofen).ti,ab,kw,kf. | 7236 |
| 3 | or/1‐2 [Pentoxifylline] | 7879 |
| 4 | exp Phosphodiesterase Inhibitors/ | 88829 |
| 5 | (Phosphodiesteras* adj2 Inhibit*).ti,ab,kw,kf. | 13372 |
| 6 | or/4‐5 [Drug category] | 93687 |
| 7 | exp sepsis/ | 136863 |
| 8 | exp enterocolitis/ [includes necrotising] | 14504 |
| 9 | (sepsis* or septic* or Pyemia* or Pyohemi* or Pyaemi* or ((blood* or bloodstream*) adj2 (infect* or poison*))).ti,ab,kw,kf. | 197502 |
| 10 | (enterocoliti* or colienteriti* or NEC).ti,ab,kw,kf. | 22328 |
| 11 | or/7‐10 [NEC or Sepsis] | 285286 |
| 12 | exp Infant, Newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ or Gestational Age/ | 707018 |
| 13 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1004443 |
| 14 | or/12‐13 [Filter: Neonatal Population 04‐2022‐MEDLINE] | 1313879 |
| 15 | randomized controlled trial.pt. | 574087 |
| 16 | controlled clinical trial.pt. | 94966 |
| 17 | randomized.ti,ab. | 619035 |
| 18 | placebo.ti,ab. | 236764 |
| 19 | drug therapy.fs. | 2516603 |
| 20 | randomly.ti,ab. | 389079 |
| 21 | trial.ti,ab. | 709192 |
| 22 | groups.ti,ab. | 2415024 |
| 23 | or/15‐22 [Cochrane HSSS‐SM Filter; Box 6.4.a Cochrane Handbook] | 5488787 |
| 24 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1058014 |
| 25 | (control* adj2 (group? or random* or trial? or study)).ti,ab,kw,kf. | 1053768 |
| 26 | or/24‐25 [Additional terms to increase sensitivity] | 1636420 |
| 27 | exp animals/ not humans/ | 5033285 |
| 28 | (or/23,26) not 27 [RCT Filter: Medline] | 5031488 |
| 29 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ [/ finds same as.pt. syntax] | 281523 |
| 30 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 278904 |
| 31 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 35286 |
| 32 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 36171 |
| 33 | (hand search* or handsearch*).ti,ab,kf,kw. | 10608 |
| 34 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 32709 |
| 35 | meta‐analysis as topic/ or network meta‐analysis/ | 25398 |
| 36 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 243644 |
| 37 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 297414 |
| 38 | (cochrane or systematic review?).jw. | 19418 |
| 39 | or/29‐38 [SR filter‐Medline; based on CADTH https://searchfilters.cadth.ca] | 562706 |
| 40 | 3 and 14 [Pentoxifylline AND Neontal Terms] | 197 |
| 41 | 6 and 11 and 14 [Drug Cateory AND NEC/Sepsis AND Neonatal Terms] | 118 |
| 42 | or/40‐41 [Results before filters] | 270 |
| 43 | 42 and 28 [RCT Results] | 130 |
| 44 | 42 and 39 and 202*.yr. [Systematic reviews 2020 forward] | 1 |
| 45 | or/43‐44 [All Results] | 130 |
Appendix 3. Embase strategy
| Embase 1974 to 2022 July 29 | ||
| # | Searches | Results |
| 1 | pentoxifylline/ [MeSH/EMTREE] | 14466 |
| 2 | (agapurin or artal or azupentat or azutrenat or bl 191 or bl191 or c‐vex or carpental or cental or ceretal or claudicat retard or "eht 0201" or eht0201 or elorgan or erytral or fixoten or flexital or harin or harine or hemovas or ikomio or ipentol or kentadin or oxopurin 400 sr or oxpentifylline or oxpentiphylline or oxypentifylline or penphylline or pentong or pentopak or pentox* or pentyllin or perencal or perental or peridane or pexal or pexol or platof or ralofect or ralofekt or relofekt or rentylin or tarontal or thrental or torental or torestal or trenfyl or trenlin or trental or trepal‐400 or vazofen).ti,ab,kw,kf,du,dy,tn. | 18581 |
| 3 | or/1‐2 [Pentox] | 18581 |
| 4 | exp phosphodiesterase inhibitor/ [EMTREE] | 190909 |
| 5 | (Phosphodiesteras* adj2 Inhibit*).ti,ab,kw,kf. | 17888 |
| 6 | or/4‐5 [Drug Category] | 194223 |
| 7 | enterocolitis/ or necrotizing enterocolitis/ | 20263 |
| 8 | exp sepsis/ | 309684 |
| 9 | (sepsis* or septic* or Pyemia* or Pyohemi* or Pyaemi* or ((blood* or bloodstream*) adj2 (infect* or poison*))).ti,ab,kw,kf. | 287232 |
| 10 | (enterocoliti* or colienteriti* or NEC).ti,ab,kw,kf. | 29444 |
| 11 | or/7‐10 [NEC or Sepsis] | 444609 |
| 12 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ or gestational age/ | 751844 |
| 13 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1184279 |
| 14 | or/12‐13 [Filter: Neonatal Population 03‐2022‐OVID EMBASE] | 1443014 |
| 15 | Randomized controlled trial/ or Controlled clinical study/ | 910289 |
| 16 | random$.ti,ab,kw. | 1820756 |
| 17 | Randomization/ | 94488 |
| 18 | placebo.ti,ab,kw. | 344497 |
| 19 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 258905 |
| 20 | double blind procedure/ | 197173 |
| 21 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 414061 |
| 22 | parallel group$1.ti,ab. | 29761 |
| 23 | (crossover or cross over).ti,ab. | 117344 |
| 24 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 384521 |
| 25 | (open adj label).ti,ab. | 98477 |
| 26 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1485045 |
| 27 | (control* adj2 (group? or random*)).ti,ab,kw,kf. | 1209091 |
| 28 | or/15‐27 [ Terms based on Cochrane Central strategy‐ How Central is Created] | 3113755 |
| 29 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 23905129 |
| 30 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 30852508 |
| 31 | 30 not 29 [Animal Exclusion‐https://community‐cochrane‐org.ezproxy.uvm.edu/sites/default/files/uploads/inline‐files/Embase%20animal%20filter.pdf] | 6947379 |
| 32 | 28 not 31 [Filter: RCT‐EMBASE] | 2676548 |
| 33 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ [EMTREE] | 518355 |
| 34 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 337771 |
| 35 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 49513 |
| 36 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 44044 |
| 37 | (hand search* or handsearch*).ti,ab,kw. | 12873 |
| 38 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 43117 |
| 39 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 310131 |
| 40 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 373572 |
| 41 | (cochrane or systematic review?).jn,jx. | 30454 |
| 42 | (overview adj2 reviews).ti. | 118 |
| 43 | or/33‐42 [SR Filter: EMBASE based on CADTH filter: https://searchfilters.cadth.ca] | 798026 |
| 44 | 3 and 14 [Pentoxifylline AND Neonatal Terms] | 478 |
| 45 | 6 and 11 and 14 [Drug Category AND Sepsis/NEC AND Neonatal Terms] | 712 |
| 46 | or/44‐45 [Results before filters] | 1153 |
| 47 | 46 and 32 [RCT Results] | 160 |
| 48 | 46 and 43 [Systematic Review Results] | 78 |
| 49 | or/47‐48 [All results] | 206 |
Appendix 4. CINAHL strategy
| CINAHL Complete; EBSCOHost | ||
| July‐31‐2022 | ||
| Advanced Search; Search mode: Boolean/Phrase | ||
| # | Query | Results |
| 1 | (MH "Pentoxifylline") | 518 |
| 2 | TI ( (agapurin or artal or azupentat or azutrenat or bl 191 or bl191 or c‐vex or carpental or cental or ceretal or claudicat retard or "eht 0201" or eht0201 or elorgan or erytral or fixoten or flexital or harin or harine or hemovas or ikomio or ipentol or kentadin or oxopurin 400 sr or oxpentifylline or oxpentiphylline or oxypentifylline or penphylline or pentong or pentopak or pentox* or pentyllin or perencal or perental or peridane or pexal or pexol or platof or ralofect or ralofekt or relofekt or rentylin or tarontal or thrental or torental or torestal or trenfyl or trenlin or trental or trepal‐400 or vazofen) ) OR AB ( (agapurin or artal or azupentat or azutrenat or bl 191 or bl191 or c‐vex or carpental or cental or ceretal or claudicat retard or "eht 0201" or eht0201 or elorgan or erytral or fixoten or flexital or harin or harine or hemovas or ikomio or ipentol or kentadin or oxopurin 400 sr or oxpentifylline or oxpentiphylline or oxypentifylline or penphylline or pentong or pentopak or pentox* or pentyllin or perencal or perental or peridane or pexal or pexol or platof or ralofect or ralofekt or relofekt or rentylin or tarontal or thrental or torental or torestal or trenfyl or trenlin or trental or trepal‐400 or vazofen) ) | 638 |
| 3 | S1 OR S2 | 797 |
| 4 | (MH "Phosphodiesterase Inhibitors+") | 3,586 |
| 5 | TI (Phosphodiesteras* N2 Inhibit*) OR AB (Phosphodiesteras* N2 Inhibit*) | 1,619 |
| 6 | S4 OR S5 | 4,310 |
| 7 | (MH "Sepsis+") OR (MH "Neonatal Sepsis") | 30,959 |
| 8 | (MH "Enterocolitis+") OR (MH "Enterocolitis, Necrotizing") | 3,647 |
| 9 | TI ( (sepsis* or septic* or Pyemia* or Pyohemi* or Pyaemi* or ((blood* or bloodstream*) N2 (infect* or poison*))) ) OR AB ( (sepsis* or septic* or Pyemia* or Pyohemi* or Pyaemi* or ((blood* or bloodstream*) N2 (infect* or poison*))) ) | 44,927 |
| 10 | TI ( (enterocoliti* or colienteriti* or NEC) ) OR AB ( (enterocoliti* or colienteriti* or NEC) ) | 4,397 |
| 11 | S7 OR S8 OR S9 OR S10 | 60,120 |
| 12 | (MH "Infant, Newborn+") OR (MH "Infant, Large for Gestational Age") OR (MH "Infant, Low Birth Weight+") OR (MH "Infant, Postmature") OR (MH "Infant, Premature") OR (MH "Intensive Care, Neonatal+") OR (MH "Intensive Care Units, Neonatal") OR (MH "Gestational Age") | 171,731 |
| 13 | TI ((babe or babes or baby* or babies or gestational age# or infant# or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born# or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term# or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs) OR AB (babe or babes or baby* or babies or gestational age# or infant# or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born# or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term# or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs) | 257,845 |
| 14 | S12 OR S13 | 321,168 |
| 15 | (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Randomized Controlled Trials+") OR (MH "Double‐Blind Studies") | 171,721 |
| 16 | (MH "Clinical Trials+") | 341,694 |
| 17 | TI (randomi#ed or randomly) OR AB (randomi#ed or randomly) OR AB (randomi#ed or randomly) OR AB (randomi#ed or randomly) | 359,579 |
| 18 | AB randomly | 104,951 |
| 19 | AB placebo | 64,957 |
| 20 | AB (trial) | 331,183 |
| 21 | AB groups | 876,324 |
| 22 | TI (quasirandom* or quasi‐random*) OR AB (quasirandom* or quasi‐random*) | 2,195 |
| 23 | S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 | 1,283,162 |
| 24 | (MH "Animal Studies") | 148,280 |
| 25 | (MH "Human") | 2,584,163 |
| 26 | S24 NOT S25 | 123,454 |
| 27 | S23 NOT S26 | 1,244,112 |
| 28 | (MH "Systematic Review") | 112,201 |
| 29 | (MH "Meta Analysis") | 64,235 |
| 30 | ( TI ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) OR ( AB ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) | 138,072 |
| 31 | ( TI ((integrative N3 (review* or overview*)) or (collaborative N3 (review* or overview*)) or (pool* N3 analy*)) ) OR ( AB ((integrative N3 (review* or overview*)) or (collaborative N3 (review* or overview*)) or (pool* N3 analy*)) ) | 18,428 |
| 32 | ( TI (data synthes* or data extraction* or data abstraction*) ) OR ( AB (data synthes* or data extraction* or data abstraction*) ) | 13,853 |
| 33 | AB (hand search* or handsearch*) | 4,877 |
| 34 | AB (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*) | 9,417 |
| 35 | ( TI met analy* or metanaly* or meta regression* or metaregression*) ) OR ( AB met analy* or metanaly* or meta regression* or metaregression*) ) | 4,805 |
| 36 | AB (medline or cochrane or pubmed or medlars or embase OR CINAHL) | 115,502 |
| 37 | S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 244,269 |
| 38 | S4 AND S14 | 167 |
| 39 | S6 AND S11 AND S14 | 6 |
| 40 | S38 OR S39 | 170 |
| 41 | S40 AND S27 | 55 |
| 42 | ( S40 AND S37 ) AND DT 202* | 1 |
| 43 | S41 OR S42 | 55 |
Appendix 5. Trial registry strategies
| Date | Source | Terms | |
| July‐31‐2022 | Clinicaltrials.gov | Pentoxifylline (Other terms) AND age Child (birth to17 ) | 19 |
| July‐31‐2022 | Clinicaltrials.gov | Pentoxifylline (Other terms) AND neonatal [Condition or disease] | 5 |
| July‐31‐2022 | Clinicaltrials.gov | Pentoxifylline (Other terms) AND sepsis [Condition or disease] | 3 |
| July‐31‐2022 | Clinicaltrials.gov | Pentoxifylline (Other terms) AND enterocolitis [Condition or disease] | 1 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Title) AND Neonatal (Condition) | 6 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Title] AND Neonate (Condition) | 0 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Title] AND Neonatal (Condition) | 6 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Title] AND Sepsis [Condition] | 6 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Title] AND Enterocolitis [Condition] | 3 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Intervention) AND Neonatal (Condition) | 5 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Intervention] AND Sepsis [Condition] | 5 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Intervention] AND Enterocolitis [Condition] | 3 |
| July‐31‐2022 | ICTRP | Pentoxifylline (Intervention] AND Neonate (Title) | 0 |
| July‐31‐2022 | ISRCTN | Pentoxifylline (Text word] AND Neonatal [condition | 0 |
| July‐31‐2022 | ISRCTN | Pentoxifylline (Intervention] AND Neonatal [condition | 0 |
| July‐31‐2022 | ISRCTN | Pentoxifylline (Intervention] AND Sepsis [condition] | 0 |
| July‐31‐2022 | ISRCTN | Pentoxifylline (Intervention] AND Enterocolitis [condition] | 0 |
| July‐31‐2022 | ISRCTN | Pentoxifylline [Text search] found 18 but none in neonate or child population | 0 |
| August‐18‐2022 | ANZCTR | Pentoxifylline [Description of intervention /exposure | 4 |
| Total | 66 | ||
Appendix 6. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. We assessed blinding separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared the prespecified outcomes versus outcomes reported in the published results. If the study protocol was not published in advance, we contacted the study authors to gain access to it. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would be expected to have been reported); or
unclear risk.
7. Other sources of bias. Did the study appear to be free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, or if the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Pentoxifylline with antibiotics (any dose or duration) compared to placebo or no intervention for the treatment of neonatal sepsis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality during hospital stay | 6 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.32, 0.57] |
| 1.1.1 All neonates | 6 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.93] |
| 1.1.2 Preterm neonates | 4 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.20, 0.71] |
| 1.1.3 Late‐onset sepsis | 3 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.95] |
| 1.1.4 Confirmed sepsis | 4 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.19, 0.73] |
| 1.1.5 Neonates with confirmed gram negative sepsis | 4 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.16, 0.72] |
| 1.2 Chronic lung disease | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.45, 5.05] |
| 1.3 Severe intraventricular haemorrhage (grade 3 and 4) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.03] |
| 1.4 Periventricular leukomalacia | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.10, 2.63] |
| 1.5 Length of hospital stay | 2 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐7.74 [‐11.72, ‐3.76] |
| 1.6 Necrotising enterocolitis, any Bell stage | 6 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.29, 1.06] |
| 1.7 Retinopathy of prematurity, any stage | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 1.98] |
Comparison 2. Pentoxifylline with antibiotics (any dose or duration) compared to pentoxifylline with antibiotics and adjunct treatments such as IgM‐enriched IVIG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.10] |
| 2.2 Necrotising enterocolitis, any Bell stage | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.66] |
Comparison 3. Pentoxifylline with antibiotics (any dose or duration) compared to adjunct treatments such as IgM‐enriched IVIG with antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.39] |
| 3.2 Necrotising enterocolitis, any Bell stage | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adel 2010.
| Study characteristics | ||
| Methods | Single‐centre, quasi‐randomised trial where neonates with suspected sepsis were randomised to pentoxifylline if they were admitted on Tuesday or Thursday and to placebo if admitted on Monday or Wednesday Period of study: December 2007 and August 2008 No concealment of allocation Blinding of intervention: yes Blinding of outcome assessment: unclear Completeness of follow‐up: yes |
|
| Participants | Single centre, at Ain Shams University, Cairo, Egypt Total participants: 37 PTX group: n = 17 (gestational age 35.94 ± 4.04 weeks, bwt 2.47 ± 0.89 kg) Control group: n = 20 (gestational age 36.05 ± 3.12 weeks, bwt 2.21 ± 0.59 kg) Neonates with suspected sepsis with maternal and clinical risk factors Maternal risk factors: fever ≥ 38 °C or premature rupture of membranes > 36 hours, or both Neonatal risk factors: elevated C‐reactive protein and abnormalities of complete blood count, deterioration of respiratory and cardiac functions, feeding intolerance, abdominal distension, temperature instability, lethargy or irritability, and hepatosplenomegaly The intervention and placebo groups did not differ significantly in gestational age, birthweight, or Apgar scores. |
|
| Interventions | Pentoxifylline (5 mg/kg/h for 6 hours for 6 consecutive days) or placebo (equal volume of normal saline for 6 consecutive days) as an adjunct to antibiotics | |
| Outcomes | Mortality, length of hospital stay, multi‐organ dysfunction, coagulation profiles including platelet count and C‐reactive protein, shock, and NEC | |
| Notes | 6 of 37 neonates with sepsis were culture‐negative, and outcomes were not reported separately for this group. Funding sources or declarations of interest were not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear |
| Allocation concealment (selection bias) | High risk | No allocation concealment due to quasi‐randomisation by day of the week |
| Blinding (performance bias and detection bias) All outcomes | High risk | Test drug and placebo dispensed in similar syringes, but participants were quasi‐randomised based on the day of admission. Hence, the efficacy of the blinding unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all infants reported. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Akdag 2014.
| Study characteristics | ||
| Methods | Prospective, double‐blind, controlled study, conducted in the neonatal intensive care unit of Zekai Tahir Burak Maternity Teaching Hospital, Ankara, Turkey Period of study: August 2009 to October 2010 4‐arm study comparing pentoxifylline, IgM‐enriched IVIG, and both against placebo |
|
| Participants | Newborn infants with sepsis Exclusion criteria were major congenital abnormalities, intraventricular haemorrhage (grade 3 or 4), symptoms of a congenital infection, and inborn errors of metabolism Total number of participants: 204 in 4 groups Placebo group: (n = 51, median and range of bwt 1410 g (620 g to 4300 g), gestational age 31 (25 to 40) weeks) Pentoxifylline group (n = 51, median and range of bwt 1490 g (620 g to 4580 g), gestational age 31 (24 to 42) weeks) Pentaglobin group (n = 51, median and range of bwt 1320 g (620 g to 3860 g), gestational age 30 (24 to 41) weeks) Pentoxifylline + pentaglobin group (n = 51, median and range of bwt 1330 g (540 g to 4100 g), gestational age 30 (24 to 40) weeks) 89/204 had positive blood cultures, and 13 had positive urine cultures. |
|
| Interventions | Pentoxifylline (6 mg/kg IV over 4 hours for 3 consecutive days), pentoxifylline + IgM‐enriched IVIG (pentaglobin, 250 mg/kg over 4 hours for 3 consecutive days), IgM‐enriched IVIG (pentaglobin for 3 consecutive days), or placebo (normal saline for 3 consecutive days) | |
| Outcomes | Mortality, NEC, oliguria/anuria, hepatic failure, disseminated intravascular coagulation, pulmonary haemorrhage, and lab parameters (white blood cell count, C‐reactive protein, interleukin‐6, TNF‐α, and neutrophil CD64) | |
| Notes | The authors state that there were no funding or conflicts of interest. Length of hospital stay was reported in days (mean), but did not have standard deviations to use in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | None reported |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes containing the randomisation arm. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The infusions were covered with plastic covers, and infusion vials were identical for the different arms. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The infusions were covered with plastic covers, and infusion vials were identical for the different arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None noted |
Ali 2006.
| Study characteristics | ||
| Methods | Single‐centre trial at Sher‐i‐Kashmir Institute of Medical Sciences (SKIMS), Srinagar, India Period of study: October 2004 to November 2005 Randomisation details not reported. Concealment of allocation: unclear Blinding of intervention: no Blinding of outcome assessment: unclear Completeness of follow‐up: yes |
|
| Participants | 50 premature infants with culture‐proven sepsis (< 37 weeks' gestational age); 25 in PTX group and 25 in control group Inclusion criteria: culture‐proven sepsis, gestation < 37 weeks, clinical signs of sepsis including cardiovascular and respiratory dysfunction, and written consent Exclusion criteria: intraventricular haemorrhage, congenital infection, and culture negativity Gestational age in both the treatment and placebo groups ranged from 32 to 37 weeks. Birthweight ranged from 950 g to 2580 g in the treatment group, and 1000 g to 2650 g in the control group. 42/50 of enroled infants had gram‐negative sepsis. |
|
| Interventions | Pentoxifylline intravenously (5 mg/kg/h for 6 hours for 3 consecutive days) in conjunction with antibiotics or control group who received antibiotics First‐line antibiotics were amoxicillin + clavulanic acid and amikacin |
|
| Outcomes | Mortality, development of NEC, length of hospital stay, duration of ventilation, and adverse effects (hypertension, irritability, deterioration of vital signs) were reported. | |
| Notes | Although mean length of hospital stay and duration of ventilation were reported, standard deviations were not, and so not included in meta‐analyses. Funding sources and declarations of interest were not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | None reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all participants reported. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Lauterbach 1996.
| Study characteristics | ||
| Methods | Single‐centre, randomised, placebo‐controlled trial No details of randomisation given. Blinding of intervention: yes Blinding of outcome: yes Completeness of follow‐up: no 11/40 participants were excluded from analysis. |
|
| Participants | Neonatal Unit, Jagiellonian University Hospital, Poland Neonates < 36 weeks' gestation with clinically suspected sepsis after the first week of life Criteria for sepsis: at least 2 of the following: feed intolerance, abdominal distention, lethargy, irritability, temperature instability, hyperbilirubinaemia, hepatosplenomegaly Exclusions: major congenital malformation, grades III and IV intraventricular haemorrhage, and congenital infections Mean gestational age was 31.5 weeks in treatment group and 32.3 weeks in placebo group. Mean birthweight was 1.75 kg in treatment group and 1.86 kg in placebo group. Period of study: 1 March to 30 July 1994 Total participants: 40; 20 in each group Only infants with positive blood culture were analysed. Treatment group 16/20 (4 negative culture), placebo group 13/20 (7 negative culture). |
|
| Interventions | Pentoxifylline (Trental; Boehring‐Hoscht) 5 mg/kg/h for 6 hours, repeated on 2nd and 3rd day (n = 20) Placebo: equal volume of normal saline for 6 hours, repeated on 2nd and 3rd day (n = 20) Amoxicillin + clavulanate (Augmentin) and amikacin were used as first‐line antibiotics in both groups. All enroled neonates received a single dose of immunoglobulin intravenously on the first day of therapy. |
|
| Outcomes | Reported outcomes were:
|
|
| Notes | Funded by the Polish State Committee for Research (grant nos 4 P05E03408 and 0070/S4) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not known |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention ‐ yes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/40 participants were excluded from analyses. |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Not clear |
Lauterbach 1999.
| Study characteristics | ||
| Methods | 2‐centre study: Neonatal Unit, Medical College Jagiellonian, University of Kraków, Poland and Intensive Therapy Unit at Polish Mother Memorial Hospital, Lodz, Poland Randomised, placebo‐controlled study Concealment of randomisation: yes Blinding of intervention: yes Blinding of outcome assessment: unclear Completeness of follow‐up: no 22/100 were excluded. |
|
| Participants | Preterm infants < 36 weeks of gestation, after first week of life with suspected sepsis Criteria for sepsis: at least 3 of the following: feeding intolerance, abdominal distention, temperature instability, disordered peripheral circulation (as described by paleness, peripheral cyanosis, mottled skin, and capillary refill time > 3 seconds), lethargy, irritability, and hepatosplenomegaly Positive blood culture required for confirmation of sepsis. Period of study: 1 January 1995 to 30 July 1996 Exclusions: congenital malformations, congenital infections, and grades III and IV intraventricular haemorrhage Total recruitment: 100 10/50 from the treatment group and 12/50 from placebo group excluded as sepsis was not confirmed. |
|
| Interventions | Pentoxifylline (Pentilin; KRKA Slovenia) 5 mg/kg/h for 6 hours for 6 successive days (n = 50) Placebo: equal volume of normal saline for 6 hours for 6 successive days (n = 50) First‐line antibiotics were amoxicillin + clavulanate (Augmentin) and amikacin and were comparable between groups. |
|
| Outcomes | Reported outcomes were:
|
|
| Notes | Funding sources and declarations of interest were not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate.The code was held by the representative of Pharmaceutical Inc.‐KRKA‐Slovenia |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22/100 were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Not clear |
Shabaan 2015.
| Study characteristics | ||
| Methods | Double‐blind randomised controlled trial Neonatal Intensive Care Unit of Mansoura University Children’s Hospital, Mansoura, Egypt Period of study: June 2011 to May 2013 |
|
| Participants | 120 preterm infants were enroled in the trial. 60 in the PTX group with a mean ± SD of bwt (grams) 1404 ± 417; gestational age (weeks) 30.2 ± 2.5 60 in the placebo arm with a mean ± SD of bwt (grams) 1370 ± 471; gestational age (weeks) 30.1 ± 2.2 Inclusion criteria: appropriate for gestational age, preterm infants with suspected or confirmed LOS (sepsis recognised after 72 hours from birth) Exclusion criteria: preterm infants with major congenital malformations, chromosomal anomalies, inborn errors of metabolism, and clinical or laboratory evidence of a congenital infection |
|
| Interventions | Intravenous pentoxifylline 5 mg/kg/h for 6 hours on 6 successive days or normal saline (placebo) same volume over 6 hours on 6 successive days First‐line antibiotics were ampicillin/sulbactam and gentamicin and were comparable between groups. |
|
| Outcomes | The primary outcome was death before hospital discharge. Secondary outcomes were length of hospital stay, duration of respiratory support, duration of antibiotics use, short‐term morbidity (chronic lung disease, NEC, severe intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity), TNF‐α concentrations, C‐reactive protein levels, and adverse effects of pentoxifylline. |
|
| Notes | Published article in 2015. Abstract was in 2014. Funding sources and declarations of interest were not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated numbers. |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer‐generated numbers and the randomisation code was held by the representative nurse practitioner assigned for drugs preparation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind controlled trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
bwt: birthweight; IgM: immunoglobulin M; IV: intravenous; IVIG: intravenous immunoglobulin; LOS: late‐onset sepsis; NEC: necrotising enterocolitis; PTX: pentoxifylline; SD: standard deviation; TNF‐α: tumour necrosis factor alpha
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hamilcikan 2017a | Not a randomised or quasi‐randomised trial |
| Hamilcikan 2017b | Not a randomised or quasi‐randomised trial |
| Lauterbach 1994 | Not a randomised or quasi‐randomised trial |
| Selim 2004 | Not a randomised or quasi‐randomised trial |
Characteristics of studies awaiting classification [ordered by study ID]
Sareno 2013.
| Methods | Randomised controlled trial where participants were randomised to receive either pentoxifylline at a dose of 6 mg/kg/h or placebo |
| Participants | Preterm infants ≤ 1500 g with suspected infection admitted to the neonatal intensive care unit. Infants with major congenital malformations, congenital infections, and severe haemorrhage were excluded from the study. |
| Interventions | Pentoxifylline at a dose of 6 mg/kg/h or placebo. Pentoxifylline was administered as a 6 mL infusion for 6 hours for 6 days. The control group received normal saline in the same manner as the pentoxifylline infusion. Infants, parents, and physicians (outcome assessors) were blinded to the treatment assignments. |
| Outcomes | The primary outcome measured in the study was the occurrence of all‐cause mortality between groups. Secondary outcomes measured include mortality from sepsis, adverse drug reactions, and length of hospital stay. The primary outcome was analysed on an intention‐to‐treat basis. |
| Notes | Published as an abstract. We contacted the author for more details, but as of yet have received no response. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12606000257561.
| Study name | Safety and efficacy of pentoxifylline as a treatment for preventing the progression of NEC in preterm neonates |
| Methods | Randomised, placebo‐controlled trial |
| Participants | Premature neonates < 32 weeks' gestation with stage 2 or 3 NEC |
| Interventions | Pentoxifylline at 5 mg/kg/h for 12 hours for 2 days followed by infusion for 6 hours a day for the next 4 days OR equal volume of placebo in controls |
| Outcomes | Primary: efficacy and safety of pentoxifylline in preventing the progression of NEC or death, or both Secondary: reduction in plasma tumour necrosis factor alpha levels, extent of bowel resection at surgery, duration of hospital stay and total parenteral nutrition support, and duration to full enteral feeds |
| Starting date | Late 2014 |
| Contact information | Sanjay Patole, email: Sanjay.Patole@health.wa.gov.au |
| Notes | This trial has not recruited any patients till data (as of March 2023). |
ACTRN12616000405415.
| Study name | Can pentoxifylline improve long‐term outcomes in preterm infants with late‐onset sepsis or necrotising enterocolitis? A pragmatic, randomised, placebo‐controlled trial |
| Methods | Randomised controlled trial |
| Participants | Preterm infants born at < 29 weeks' gestation |
| Interventions | Ideally within 6 hours (no later than 12 hours) of onset of suspected LOS or NEC, patient will receive pentoxifylline intravenous infusion 1 mL/kg/h for 12 hours/day (60 mg/kg/day) for 2 days. If the diagnosis of NEC or sepsis diagnosis is confirmed, this will be followed by 1 mL/kg/h for 6 hours/day (30 mg/kg/day) on days 3 to 6. If the diagnosis of NEC or LOS is not confirmed, the intervention will be discontinued at that time. Placebo is normal saline. Within 6 hours of onset of suspected LOS or NEC, patient will receive normal saline (NaCl 0.9%) intravenous infusion 1 mL/kg/h for 12 hours/day for 2 days. If the diagnosis of NEC or sepsis diagnosis is confirmed, this will be followed by 1 mL/kg/h for 6 hours/day (30 mg/kg/day) on days 3 to 6. If the diagnosis of NEC or LOS is not confirmed, the intervention will be discontinued at that time. |
| Outcomes | The primary outcome is survival without any disability at 24 months (corrected for gestation) in infants treated with intravenous pentoxifylline or placebo for LOS or NEC. Secondary outcomes are survival until discharge home and at 24 months (+/− 6 months), any disability at 24 months (+/− 6 months), disability by different grades and types will be assessed based on the BSID III and/or ASQ score and/or short health questionnaire administered to access cerebral palsy, deafness and blindness, composite secondary outcome looking at i) surgery for proven NEC and ii) progression of NEC stage II to stage IIIA/B as per Modified Bell's Staging Criteria for NEC, duration of parenteral nutrition, brain injury is defined as grade 3 and 4 intraventricular haemorrhage (on either side of the head), duration of mechanical ventilation in days, chronic lung disease, severe retinopathy of prematurity, length of hospital stay, plasma cytokine levels, magnetic resonance imaging at 38 to 42 weeks' corrected gestational age, and time to full enteral feeds. |
| Starting date | 2016 |
| Contact information | Prof Karen Simmer AO, email: karen.simmer@health.wa.gov.au |
| Notes | Currently recruiting, 597 of 1800 already recruited |
ASQ: Ages & Stages Questionnaires; BSID: Bayley Scales of Infant Development; LOS: length of stay; NEC: necrotising enterocolitis
Differences between protocol and review
The secondary outcome of NEC was modified as a) NEC (any Bell stage) and b) Bell's stage 2 or 3
Retinopathy of prematurity a) ROP any stage, b) ROP stage III‐IV were added as a secondary outcome.
We updated the risk of bias assessment to include seven categories.
Contributions of authors
Mohan Pammi:
Updated the review
Assisted in writing the protocol and review
Independently assessed study methodology and extracted data from eligible studies
Entered and checked data in Review Manager 5 for the review updates
Assisted in contacting authors for more information on published articles and in tracing unpublished articles
Khalid Haque:
Assessed methodology
Extracted relevant data from eligible studies
Contacted study authors for more data on published and unpublished trials
Wrote the text of the review
Assisted in updating the review
Sources of support
Internal sources
-
Epsom & St. Helier NHS Trust, UK
Administrative support for previous versions of this review
-
National Perinatal Epidemiology Unit, Headington, Oxford, UK
Administrative support for previous versions of this review
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families
Declarations of interest
Mohan Pammi is an Associate Editor with the Cochrane Neonatal Group, but has taken no part in the editorial processes for this review, and otherwise declares no conflict of interest.
Khalid Haque declares that he has no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adel 2010 {published data only}
- Adel M, Awad HA, Abdel-Naim AB, Al-Aziz MM. Effects of pentoxifylline on coagulation profile and disseminated intravascular coagulation incidence in Egyptian septic neonates. Journal of Clinical Pharmacy and Therapeutics 2010;35(3):257-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Akdag 2014 {published data only}
- Akdag A, Dilmen U, Haque K, Dilli D, Erdeve O, Goekmen T. Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis. American Journal of Perinatology 2014;31(10):905-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ali 2006 {published data only}
- Ali W, Ahmed P, Bhat MA, Mushtaq AB, Mushtaq S. Pentoxifylline in treatment of sepsis of premature infants. JK Practitioner. A Journal of Current Clinical Medicine Surgery 2006;13(4):204-7. [Google Scholar]
Lauterbach 1996 {published data only}
- Lauterbach R, Zembala M. Pentoxifylline reduces plasma tumour necrosis factor-alpha concentration in premature infants with sepsis. European Journal of Pediatrics 1996;155(5):404-9. [DOI: 10.1007/BF01955273] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lauterbach 1999 {published data only}
- Lauterbach R, Pawlik D, Danuta K, Wieslaw K, Ewah K, Marek Z. Effect of immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo controlled, double-blind trial. Critical Care Medicine 1999;27(4):807-14. [DOI: 10.1097/00003246-199904000-00042] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shabaan 2015 {published and unpublished data}
- Shabaan AE, Nasef N, Shouman B, Nour I, Mesbah A, Abdel-Hady H. Pentoxifylline therapy for late-onset sepsis in preterm infants: a randomized controlled trial. Pediatric Infectious Disease Journal 2015;34(6):e143-8. [DOI: 10.1097/INF.0000000000000698] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hamilcikan 2017a {published data only (unpublished sought but not used)}
- Hamilçıkan Ş, Can E, Büke Ö, Erol M, Gayret ÖB. Pentoxifylline treatment of very low birth weight neonates with nosocomial sepsis. American Journal of Perinatology 2017;34(08):795-800. [DOI: 10.1055/s-0037-1598596] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamilcikan 2017b {published data only (unpublished sought but not used)}
- Hamilcikan S, Can E, Buke O, Polat C, Ozcan E. Pentoxifylline and pentaglobin adjuvant therapies for neonatal nosocomial sepsis in neonates less than 1500g weight. Journal of the Pakistan Medical Association 2017;67(10):1482-6. [PMID: ] [PubMed] [Google Scholar]
Lauterbach 1994 {published data only}
- Lauterbach R, Pawlik D, Tomaszcyk B, Cholewa B. Pentoxifylline treatment of sepsis of premature infants: preliminary clinical observations. European Journal of Pediatrics 1994;153(9):672-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Selim 2004 {published data only}
- Selim K, Hüseyin C, Ibrahim KH, Hasan BU, Kazim U, Hüseyin K. Effect of pentoxifylline on tumor necrosis factor-alpha and interleukin-6 levels in neonatal sepsis. Medical Journal of Malaysia 2004;59(3):391-4. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Sareno 2013 {published data only (unpublished sought but not used)}
- Sareno JC, Mantaring JB. Pentoxifylline therapy among preterm neonates < 1,500 g in reducing mortality from neonatal sepsis: a double-blind, randomized placebo-controlled trial. Critical Care 2013;17(Suppl 4):P14. [DOI: 10.1186/cc12914] [DOI] [Google Scholar]
References to ongoing studies
ACTRN12606000257561 {published data only}
- ACTRN12606000257561. Safety and efficacy of pentoxifylline as a treatment for preventing the progression of necrotising enterocolitis in preterm neonates – a randomised, placebo controlled pilot trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1403&isReview=true (first received 27 June 2006).
ACTRN12616000405415 {published data only}
- ACTRN12616000405415. Intravenous pentoxifylline as adjunct therapy to improve long-term disability in preterm infants [Can Pentoxifylline improve long-term outcomes in preterm infants with late-onset sepsis or necrotising enterocolitis? A pragmatic, randomised, placebo-controlled trial]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000405415 (first received 30 March 2022).
Additional references
Adams‐Chapman 2006
- Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bacher 1997
- Bacher A, Mayer N, Klimscha W, Oismüller C, Steltzer H, Hammerle A. Effects of pentoxifylline on haemodynamics and oxygenation in septic and non-septic patients. Critical Care Medicine 1997;25(5):795-800. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 2005
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2005. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotising enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bienvenu 1995
- Bienvenu J, Doche C, Gutowski MC, Lenoble M, Lepape A, Perdrix JP. Production of pro-inflammatory cytokines and cytokines involved in the TH1/TH2 balance is modulated by pentoxifylline. Journal of Cardiovascular Pharmacology 1995;25 Suppl 2:S80-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boldt 1996
- Boldt J, Müller M, Heyn S, Welters I, Hempelmann G. Influence of long term continuous intravenous administration of pentoxifylline on endothelial related coagulation in critically ill patients. Critical Care Medicine 1996;24(6):940-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caplan 1990a
- Caplan MS, Hsueh W. Necrotizing enterocolitis: role of platelet activating factor, endotoxin, and tumor necrosis factor. Journal of Pediatrics 1990;117(1 Pt 2):S47-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caplan 1990b
- Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. Journal of Pediatrics 1990;116(6):960-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed before July 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Edelson 1999
- Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 1999;103(4 Pt 1):766-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote. Version EndNote X9. Philadelphia, PA: Clarivate, 2013 (accessed before July 2023).
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 December 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Halpern 2006
- Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. American Journal of Physiology. Gastrointestinal and Liver Physiology 2006;290(4):G757-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2005
- Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing enterocolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. Journal of Pediatrics 2005;147(4):462-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2010
- Harris E, Schulzke SM, Patole SK. Pentoxifylline in preterm neonates. Pediatric Drugs 2010;12(5):301-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
ICROP 1984
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaufman 2004
- Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clinical Microbiology Reviews 2004;17(3):638-80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krysztopik 1996
- Krysztopik RJ, Bentley FR, Spain DA, Wilson MA, Garrison RN. Free radical scavenging by lazaroids improves renal blood flow during sepsis. Surgery 1996;120(4):657-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lawn 2006
- Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. International Journal of Epidemiology 2006;35(3):706-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Levy 1998
- Levy SB. Antimicrobial resistance: bacteria on the defence. Resistance stems from misguided efforts to try to sterilize our environment. BMJ 1998;317(7159):612-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2006
- Lin PW, Stoll BJ. Necrotizing enterocolitis. Lancet 2006;368(9543):1271-83. [PMID: 17027734]17027734 [Google Scholar]
Michetti 2003
- Michetti C, Coimbra R, Hoyt DB, Loomis W, Junger W, Wolf P. Pentoxifylline reduces acute lung injury in chronic endotoxemia. Journal of Surgical Research 2003;115(1):92-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2003
- Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Archives of Disease in Childhood. Fetal Neonatal Ed 2003;88(3):F209-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health Report of Workshop on Bronchopulmonary Dysplasia. Report of Workshop on Bronchopulmonary Dysplasia. In: NIH Publication No. 80-1660. Washington, DC: National Institutes of Health, 1979. NIH, 1979. [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peng 2022
- Peng P, Xia Y. Influency of pentoxifylline treatment for neonatal sepsis: a meta-analysis of randomised controlled trials. Hongkong Journal of Emergency Medicine 2022;29:121-8. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Speer 1999
- Speer CP. Inflammatory mechanisms in neonatal chronic lung disease. European Journal of Pediatrics 1999;158 Suppl 1:S18-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2004a
- Stoll BJ. Infections of the neonatal infant. In: Behrman RE, Kliegman RM, Jenson HB, editors(s). Nelson Textbook of Pediatrics. 17th edition. Philadelphia: Saunders, 2004:623-40. [Google Scholar]
Stoll 2004b
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292(19):2357-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2005
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatric Infectious Disease Journal 2005;24(7):635-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Travadi 2006
- Travadi J, Patole S, Charles A, Dvorak B, Doherty D, Simmer K. Pentoxifylline reduces the incidence and severity of necrotizing enterocolitis in a neonatal rat model. Pediatric Research 2006;60(2):185-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vilcek 1991
- Vilcek J, Lee TH. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. Journal of Biological Chemistry 1991;266(12):7313-6. [PMID: ] [PubMed] [Google Scholar]
Volpe 1995
- Volpe JJ. Neurology of the Newborn. 3rd edition. Philadelphia, London: WB Saunders, 1995. [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1996
- Wang P, Wood TJ, Ba ZF, Chaudry IH. Pentoxifylline maintains vascular endothelial cell function during hyperdynamic sepsis. Surgery 1996;120(2):367-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 1999
- Yang S, Zhou M, Koo DJ, Chaudry IH, Wang P. Pentoxifylline prevents the transition from hyperdynamic to hypodynamic response during sepsis. American Journal of Physiology 1999;277(3 Pt 2):H1036-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2005
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldman DA. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeni 1996
- Zeni F, Pain P, Vindimian M, Gay JP, Gery P, Bertrand M, et al. Effects of pentoxifylline on circulating cytokine concentrations and haemodynamics in patients with septic shock: results from a double blind, randomized, placebo controlled study. Critical Care Medicine 1996;24(2):207-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haque 2003
- Haque K, Mohan P. Pentoxifylline for neonatal sepsis. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD004205. [DOI: 10.1002/14651858.CD004205] [DOI] [PubMed] [Google Scholar]
Haque 2011
- Haque KN, Pammi M. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD004205. [DOI: 10.1002/14651858.CD004205.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pammi 2015
- Pammi M, Haque KN. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD004205. [DOI: 10.1002/14651858.CD004205.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]


