Abstract
Background
Early Childhood Caries is a chronic disease of childhood and salivary parameters are considered as one of the prime etiological factors of Early Childhood Caries.
Aim
To develop a systematic review based on the relation between physical and chemical properties of saliva and Early childhood caries by comparing children with and without Early childhood caries.
Methods
PubMed, Cochrane, Lilacs, Embase, Scopus, and additional manual search was done up to April 2021 to identify the original cross-sectional observational studies published in English. The risk of bias and quality of the included papers were assessed based on New castle Ottawa guidelines.
Results
From a total of 1709 identified studies, only 22 articles were included in this systematic review and 10 studies were qualified for meta-analysis. Eight studies were classified as ‘‘moderate risk of bias’’ and fourteen studies were classified as ‘‘high risk of bias’’.
Conclusion
There was a significant difference in physical and chemical properties of saliva in children with and without Early childhood caries. Since wide disparity were evident in available studies, further studies are needed to arrive to a definitive conclusion.
1. Introduction
Saliva is a complex body fluid and maintains the health of the oral cavity with its organic and inorganic constituents. The various functions of saliva include lubrication, serving as an ion reservoir, cleansing action, digestion of carbohydrates, antimicrobial action, buffering capacity, pellicle formation and maintaining water balance.1 Saliva contains various hormones, antibodies, growth factors, enzymes and microbes similar to serum. Importantly, saliva is used as a diagnostic tool due to its ease of availability, low cost, minimal risk of cross contamination and is more economical in terms of storage as compared to serum.2 Salivary characteristics and its components have been considered as a predisposing factor for the development of Early Childhood Caries (ECC) in children.3,4 ECC is a complex disease of childhood in children below 6 years of age and affects the newly erupted immature teeth, leading to the development of hypoplastic defects.5
The physical properties of saliva, namely its pH and buffering capacity neutralizes the acid produced by the cariogenic bacteria, whereas, the flow rate and viscosity provide a flushing effect and helps in eliminating bacteria and food debris from the tooth surfaces. Saliva acts as a source of calcium and phosphate and plays an imperative role in remineralization of incipient carious lesions. These inorganic ions of saliva improve post-eruptive maturation of enamel by influencing the precipitation or dissolution of Hydroxy Apatite Crystals (HAP).6, 7, 8 Salivary proteins and peptides have several anti-bacterial properties and help to prevent development of dental caries. Salivary amylase, an important salivary enzyme, is available in abundance in the oral cavity and helps in modulating the bacterial activity, and promotes clearance of bacteria, thereby, reducing the incidence of caries.9 Salivary immunoglobulin, namely secretory IgA acts as a first line of defence and protects the oral mucosa from bacterial adhesion by neutralizing the bacterial toxins and enzymes.10,11 Saliva and Gingival Crevicular Fluid (GCF) contains several antimicrobial peptides, namely, α, β defensins, histatins and human cathelicidin LL-37, which possess both bactericidal and/or bacteriostatic activities against oral pathogens.12, 13, 14 Histatins 1, 3 and 5 are natural antimicrobial peptides present in the saliva and it destabilizes the bacterial cell membrane. One of the important functions of histatin is the inhibition of matrix metallo proteinases (MMP) and promoting wound healing.15 LL-37 is present both in saliva and GCF and studies have reported that LL-37 has antimicrobial activity against Streptococci and Lactobacillus.14 Carbonic Anhydrase (CA) VI, a salivary isoenzyme maintains the physiologic pH of saliva and its pH alteration capacity is based on its concentration in saliva and not based on its activity.16, 17, 18 Studies identified that the concentration of CA VI was higher in caries active children, the possible explanation could be that this isoenzyme adheres to the enamel pellicle and biofilm, regulates the PH and neutralizes the acids produced by oral pathogens.19,20 Salivary lysozymes are an antimicrobial enzyme with a molecular weight of 14.7 kDa. It degrades the bacterial cell wall by activation of bacterial autolysins and bacterial aggregation.21, 22, 23, 24 A study done by Lertsirivorakul et al. reported that the salivary lysozyme levels were found to be higher in children with Severe ECC (S ECC), explaining its possible association with ECC.25
To date, several cross-sectional and longitudinal studies have been published, providing insight into the salivary components and its role in ECC. However, it is difficult to interpret whether the ECC is associated only with increase/decrease of individual parameter. Because of different parameters assessed in each study under different circumstances, the results and conclusions obtained in many studies are sometimes conflicting and may be difficult to compare and interpret. Due to the presence of wide range of salivary factors and its role in ECC, this systematic review was undertaken to answer the following important questions:
What is the role of the physical and chemical properties of saliva in association with ECC?
Are there variations in the physical and chemical properties of saliva in children with and without ECC?
Furthermore, meta-analysis of selected parameters was done to find out a possible relationship between salivary factors and ECC. We hypothesize that there is a difference in the physical and chemical properties of saliva in children with and without ECC.
2. Materials and methods
The present systematic review was carried out according to the protocols of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analysis) GUIDELINES.26
2.1. Search strategies
The literature search was performed using broad MeSH terms and keywords and covered data up to April 2021. The data were obtained using the MEDLINE (PubMed) search engine, Cochrane, Lilacs, Embase, Scopus as well as an additional manual search. The MeSH terms and the key words used were (children) AND (physicochemical properties) OR (inorganic ions) OR (saliva) OR (biochemical parameters) OR (salivary proteins) OR (salivary peptides) OR (salivary enzymes) AND (with and without early childhood caries) OR (caries free and caries active children). The studies which were identified through the electronic search using the cited keywords but did not evaluate the relation between physical and chemical properties of saliva and ECC were excluded from this systematic review as they were considered to have a different research focus. To identify grey literature, www.opengrey.eu and Google Scholar were also searched for any unpublished material. We hand searched several key journals with the help of an experienced librarian to identify articles that could have been missed from the electronic data base search. The reference lists of the included articles were also checked to identify any potential article which could have been missed in the electronic search. The reference lists of the retrieved articles were also checked for additional studies.
2.2. Selection criteria
This systematic review and meta-analysis included cross-sectional observational studies which evaluated the relation between salivary physical and chemical properties and ECC by comparing children with and without ECC. The selected literature covers the data up to April 2021. Studies included all healthy children regardless of race, gender, socioeconomic status from birth to six years of age. Children without intake of any medication were selected, as medication could affect the salivary composition. Articles on genetic studies, reviews, case reports, abstracts, letters and conference proceedings were excluded. Articles published in English were only considered to include in the systematic review during electronic and hand search. The search identified a total of 1709 studies to be included in the present systematic review. Following the removal of 1083 duplicates, 626 records were screened based on the title, abstract, and keywords. Of these, 576 records were eliminated due to different outcome variable. The remaining 50 papers were assessed completely. The reason for exclusion of 28 articles at this stage were due to reasons such as different age groups, treatment comparison, genetic studies, different sample selection and comparison with rampant caries. After a full text review, 22 studies were included in the present systematic review. Fig. 1 depicts the PRISMA flow diagram of study identification process.
Fig. 1.
PRISMA Search Flow chart.
2.3. Inclusion criteria
Criteria for inclusion was based on PECO strategy, as described below.
P (participants): Healthy children less than 6 years of age.
E (Exposure) - Children with ECC.
C (comparison): Children without ECC.
O (outcome): Saliva, physicochemical properties, inorganic ions, salivary proteins, salivary peptides, salivary enzymes.
2.4. Data extraction and quality assessment of included studies
Data extraction was done by two independent reviewers. DR and PR assessed all the articles independently with respect to the inclusion and exclusion criteria. Any conflicts were resolved by a third reviewer RG, and required input was provided. The data extraction was done using electronic Excel spread sheet (Excel 10, Microsoft Corp., Redwood City, Calif., USA). Studies that required more information were obtained through communication with the author through Research Gate. After removing duplicates, the titles and abstracts of the records were screened based on the eligibility criteria to decide upon the inclusion for further full-text reading. Quality assessment was performed based on New Castle Ottawa guidelines for cross-sectional studies.27 The New Castle Ottawa checklist was used by both the reviewers for coding the data and these reviewers were calibrated for inter-examiner agreement (Kappa- 0.9). Studies were categorized as having low, moderate, and high methodological quality, according to the number of stars allotted to each study. Studies were considered to have low risk of bias if the studies were allotted with over 7 stars, moderate risk if between 5 and 7 stars, and high risk if under 5 stars. Review Manager 2012 statistical software (Revman version 5.3, London, UK) was used to enter the data for meta-analysis and the mean difference was plotted in Forest plot. The mean difference of individual salivary parameters with 95% confidence interval (CI) were calculated and pooled in meta-analysis. Chi-square and I2 test were done to assess the clinical heterogenecity of the studies. An I2 value between 50% and 100% was considered to have statistical heterogenecity. Random effect generalized linear models with 95% CI were used to estimate effect size. Review Manager 2012 statistical software (Revman version 5.3, London, UK) was used to evaluate publication bias. The publication bias was examined visually using funnel plot and Egger's test was used to assess the degree of asymmetry.
3. Results
3.1. Study selection
A total of 22 studies were identified for inclusion in the review. The electronic search provided a total of 1702 articles, and manual search provided 7 articles; hence there were a total of 1709 articles. Of these 1709 articles, 1083 articles were excluded after identification of duplicates. Out of 626 articles, 576 articles were excluded after reading the title, abstracts, and full texts as they did not meet the inclusion criteria. The full texts of the included 22 articles were reviewed in detail. Fig. 1 depicts the flow chart of complete data collection and search process.
3.2. Characteristics of included studies
Information regarding the study characteristics is given in Table 1. This systematic review and meta-analysis included the studies that discusses the physical and chemical properties of saliva. In addition, other parameters evaluated in the studies include total antioxidant capacity, salivary fluoride, microbial profile and analysis of bacterial protein. Sample size notably varies between all the studies. Children less than 6 years were included in the study and only one study has evaluated children in 2 age groups, but the data of children between 3 and 5 years were included in the review.28 Eight studies have evaluated salivary pH, 6 studies have assessed flow rate, 8 studies have estimated buffering capacity, 2 studies have evaluated salivary viscosity, 5 studies have measured the salivary calcium, 6 studies have assessed salivary phosphorus, 2 studies estimated alkaline phosphatase and 11 studies looked into salivary proteins and peptides.
Table 1.
Characteristics of included studies.
| Author/Year Study | group (n) | Age (Months/years) | Parameters evaluated | Other parameters | Data of outcome variable (p value) | Results |
|---|---|---|---|---|---|---|
| de Farias DG, 2003 | 40 Children 20-ECC 20-caries-free children |
12–47 months | Salivary antibodies, Salivary amylase and salivary proteins | – | IgA - p < 0.05* IgG - p < 0.05* IgM – p > 0.05 Total proteins - p > 0.05 Amylase (U/L) - p > 0.05 |
Salivary IgA and IgG were higher in children with ECC. No significant difference in IgM, proteins and salivary amylase between the groups |
| Shahrabi M, 2008 | 75 Children 25-caries free 25- Moderate caries 25- Severe caries |
3–5 Years | Salivary Calcium, Phosphate Alkaline Phosphatase |
– | Salivary calcium – p value – 0.9 Salivary phosphate – p value – 0.2 Alkaline phosphatase – p value – 0.07 |
Salivary phosphate and alkaline phosphatase were found to be higher in caries free children though it was not statistically significant |
| Bagherian A, 2008 | 90 Children caries free- 45 ECC-45 |
36–70 months | SIgA, IgG | – | SIgA – p value – 0.01* SIgG – p value – 0.04* |
Salivary SIgA and IgG were significantly higher in children with ECC |
| Shifa et al., 2008 | 20 Children caries free- 10 ECC-10 |
3–6 years | sIgA | – | sIgA – p value – 0.76 | No significant difference in sIgA level between the groups |
| Sharaf AA, 2010 | 90 children ECC-60 caries -free-30 |
36–71 months | Salivary flow rate Buffering capacity |
Bacterial counts (Mutans Streptococci and Lactobacilli) | Salivary flow rate – p value – 0.06 Salivary Buffering capacity – p value – 0.75 |
No statistically significant difference in the salivary buffering capacity and salivary flow rate between the groups. |
| Martínez-Pabón MC, 2010 | 201 Children ECC- 143 Caries-free- 58 |
2–5 years | Salivary flow rate pH Buffering capacity |
Bacterial counts | Salivary flow rate – p value – 0.12 Salivary pH - p value – 0.61 Buffering capacity- p value – 0.70 |
No significant difference in salivary pH, buffering capacity and flow rate between ECC and non ECC group |
| Bhalla S, 2010 | 100 Children caries free- 50 ECC-50 |
4–6 years | Salivary flow rate, pH mean protein concentration, and the electrophoretic profile of salivary proteins |
– | Salivary flow rate - p > 0.05 Salivary pH - p > 0.05 Mean protein - p > 0.05 Proline rich protein – p value – 0.02 * Amylase – p value – 1.00 Glyco protein – p value – 0.02* |
A significant inverse correlation between the mean protein concentration and the whole salivary flow rate. Proline rich proteins were found to be higher in caries-free children and glycoprotein was found to be higher in children with ECC. |
| Bagherian A, 2012 | 90 Children caries free- 45 ECC-45 |
36–70 months | Salivary pH, Buffering capacity, Calcium, Phosphate sIgA |
– | Salivary pH- p value −0.002* Salivary Buffering capacity – p value - 0.002* sIgA- p value – 0.015* Salivary calcium – p value - 0.84 Salivary Phosphate – p value – 0.34 |
Salivary pH and Buffering capacity were high in caries free children. No significant difference in salivary calcium and phosphate level between the groups sIgA concentration was significantly higher among the ECC group |
| Kaur et al., 2012 | 60 Children caries free- 30 ECC-30 |
4–6 years | Flow rate pH Buffering capacity Viscosity, Calcium Phosphate Alkaline phosphatase |
– | Flow rate - p < 0.001* pH- p < 0.001* Buffering capacity- p < 0.001* Viscosity - p < 0.001* Calcium - p > 0.05 Phosphate- p < 0.001* Alkaline phosphatase - p < 0.05 |
PH, Buffering capacity, alkaline phosphatase were found to be high in caries -free children. Salivary viscosity, calcium and phosphorus were found to be higher in children with ECC |
| Jolly et al., 2014 | 30 children ECC-15 caries-free-15 |
3–6 years | Salivary calcium and phosphorus | – | Salivary calcium in stimulated saliva p value – 0.05 Salivary calcium in unstimulated saliva – p value −0.02* Salivary phosphorus in stimulated saliva – 0.01* Salivary phosphorus in unstimulated saliva- p value - 0.8 |
Salivary calcium and inorganic phosphorus were found to be higher in caries free children. |
| Jayaraj D,2015 | 100 Children caries free- 50 ECC-50 |
Under 6 years of age | alivary flow rate, Salivary pH and Buffering capacity | – | Salivary flow rate – p value – 0.77 Salivary pH- p value - 0.24 Buffering capacity – p value – 0.30 |
No significant difference was evident in salivary pH and buffering capacity between the groups. |
| Muchandi S, 2015 | 50 Children caries free- 25 SECC-25 |
3–5 years | Salivary pH | TAC | Salivary pH- p value - <0.0001* | Salivary pH was higher in caries- free group |
| Jurczak A, 2015 | 82 Children 41- ECC 41-caries-free children |
ECC- 5 ± 2.5 Caries-free-5 ± 1.5 |
histatin-5 β-defensin-2 |
Bacterial Profile | Histatin −5 – p value – 0.0002* β-defensin-2- p value - 0.04* |
Significant increase in the concentration of histatin-5 and β-defensin-2 ECC group The increase in the level of histatin-5 and β-defensin-2 is positively correlated with the progression of the disease. |
| Lertsirivorakul et al., 2015 | 64 Children caries free- 32 S ECC-32 |
4–6 years | Salivary Flow rate Total protein Salivary lysozyme |
– | Salivary flow rate – p value - 0.93 Total protein – p value - 0.98 Lysozyme- p value -< 0.001* |
No significant difference in salivary flow rate and mean protein concentration was evident between the groups. Salivary lysozyme values were found to be increased in children with S-ECC |
| Colombo et al., 2016 | 57 Children caries free- 19 ECC-17 S-ECC- 21 |
36–60 months | Total salivary IgA levels | Microbial culture Detection of salivary IgA antibody reactive with S. mutans GbpB |
Salivary IgA – p value – 0.125 | No significant difference in the salivary IgA level between caries-free, ECC and S ECC group Children with severe early childhood caries and high levels of mutans streptococci have reduced salivary IgA response to S. mutans GbpB |
| Colombo et al., 2016 | 83 Children caries free- 29 ECC-25 S-ECC- 29 |
36–60 months | Salivary concentrations of cathelicidin LL-37, human β-defensin 2 (Hbd-2), human β-defensin 3 (Hbd-3) and human-histatin 5 (HTN-5) |
– | LL-37 – p value - 0.007* hBD-2 – p value - 0.01* hBD-3 – p value - 0.10 HTN-5 – p value - 0.68 |
Weak correlation of antimicrobial peptides among CF, ECC and S-ECC groups. |
| Makawi Y, 2017 | 120 Children Divided into high caries and low caries group |
3–5 years 13–15 years |
pH Buffering capacity |
Carbonic anhydrase | pH – p value - < 0.001* Buffering capacity -p value - < 0.001* |
Significant difference in pH and Buffering capacity between both the groups |
| Villavicencio et al., 2018 | 124 children ECC-69 CARIES-FREE-55 |
3–4 years | Buffering capacity | CFU, Plaque index | Buffering capacity – p value – 0.3 | Though it was not statistically significant, buffering capacity was found to be higher in caries- free group |
| Bachtiar EW,2018 | 32 Children Caries free-16 ECC-16 |
3–5 Years | Salivary viscosity Salivary protein profile |
– | Not mentioned | Salivary viscosity was higher in children with ECC |
| Jayakaran TG, 2020 | 86 Children caries free-43 ECC-43 |
3–6 years | salivary peptide HNP1 | – | Salivary peptide HNP1 p value - < 0.001* | Statistically significant difference in salivary peptide HNP1 in children with and without ECC. A decrease in salivary peptide HNP1 was observed in children with ECC. |
| Abbas MJ,2020 | 77 children Caries free- 39 ECC- 38 |
37–72 months | Salivary flow rate, pH, Buffering capacity, phosphate | Salivary Fluoride | Salivary pH- p value – 0.9 Buffering capacity – p value −0.71 Salivary flow rate – p value – 0.32 Salivary phosphate – p value – 0.29 |
No significant difference in salivary flow rate, pH, buffering capacity and phosphate between the groups |
| Aruna S, 2020 | 18 Children caries free-9 ECC = 9 |
3–6 Years | Salivary Calcium, Phosphorus | – | Not mentioned | Salivary Calcium and phosphorus were higher in caries-free group than in children with ECC |
3.3. Risk of bias assessment
Information regarding quality assessment of included study is explained in Table 2. New Castle Ottawa guidelines for cross-sectional studies has been used to assess the quality of included study. Ascertainment of exposure was not considered for quality assessment, as all the included studies were cross-sectional, so exposure could not be identified. Eight studies were found to have moderate risk of bias and 14 studies were found to have high risk of bias and low level of evidence.
Table 2.
Quality assessment and Risk of Bias evaluation.
| Criteria | de Farias, 2003 | Shahrabi M, 2008 | Bagherian A, 2008 | Shifa S et al., 2008 | Sharif AA, 2010 | Martínez-Pabón MC, 2010 | Bhalla S, 2010 | Bagherian A, 2012 | Kaur A,2012 | Jolly LR et al., 2014 |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection (Maximum 5 stars) 1. Representativeness of the Sample |
||||||||||
| a.Truly representative of the average in the target population. * (all subjects or random sampling) | ||||||||||
| b.Somewhat representative of the average in the target group. * (non-random sampling) | ||||||||||
| c.Selected group of users/convenience sample | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| d.No description of sample strategy | ||||||||||
| 2.Sample size: | ||||||||||
| a.Justified and satisfactory * (including sample size calculation). | ||||||||||
| b.Not justified. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3.Non-respondents: | ||||||||||
| Comparability between respondents and non-respondents characteristics is established, and the response rate is satisfactory. | ||||||||||
| a.The response rate is unsatisfactory, or the comparability between respondents and non-respondents is unsatisfactory. | ||||||||||
| b.No description of the response rate or the characteristics of the responders and the non-responders | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4.Ascertainment of the exposure (risk factor) | ||||||||||
| a.Validated measurement tool** | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| b.Non-validated measurement tool, but the tool is available or described* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| c.No description of the measurement tool | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5.Comparability: (Maximum 2 stars) The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled. |
||||||||||
| a.The study controls for the most important factor * | * | * | * | * | * | * | * | * | * | * |
| b.The study control for any additional factor* | * | * | ||||||||
| 6.Outcome: (Maximum 3 stars) Assessment of outcome: |
||||||||||
| a.Independent blind assessment** | ||||||||||
| b.Unblinded assessment ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| c.Self-report* | ||||||||||
| d.No description | ||||||||||
| 7.Statistical test: | ||||||||||
| a.Statistical test used to analyse the data clearly described, appropriate and measures of association presented including confidence intervals and probability level (p value).* | * | * | * | * | * | * | * | * | * | * |
| b.Statistical test not appropriate, not described or incomplete. |
| Criteria | Jayaraj D, 2015 | Muchandi S, 2015 | Jurczak A, 2015 | Lertsirivorakul J et al., 2015 | Colombo NH et al., 2016 | Colombo NH et al., 2016 | Makawi Y,2017 | Villavicencio J, 2018 | Jayakaran TG, 2020 | Bachtiar EW, 2018 | Abbas MJ, 2020 | Aruna S, 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection (Maximum 5 stars) 1. Representativeness of the Sample |
||||||||||||
| a.Truly representative of the average in the target population. * (all subjects or random sampling) | NA | |||||||||||
| b.Somewhat representative of the average in the target group. * (non-random sampling) | NA | |||||||||||
| c.Selected group of users/convenience sample | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA |
| d.No description of sample strategy | NA | |||||||||||
| 2.Sample size: | ||||||||||||
| a.Justified and satisfactory * (including sample size calculation). | * | * | * | * | NA | |||||||
| b.Not justified. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ||||
| 3.Non-respondents: | ||||||||||||
| a.Comparability between respondents and non-respondents characteristics is established, and the response rate is satisfactory. | * | * | ||||||||||
| b.The response rate is unsatisfactory, or the comparability between respondents and non-respondents is unsatisfactory. | ||||||||||||
| c.No description of the response rate or the characteristics of the responders and the non-responders | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 4.Ascertainment of the exposure (risk factor) | ||||||||||||
| a.Validated measurement tool** | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| b.Non-validated measurement tool, but the tool is available or described.* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| c.No description of the measurement tool | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5.Comparability: (Maximum 2 stars) The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled. |
||||||||||||
| a.The study controls for the most important factor * | * | * | * | * | * | * | * | * | * | * | * | * |
| b.The study control for any additional factor* | * | * | * | * | ||||||||
| 6.Outcome: (Maximum 3 stars) Assessment of outcome: |
||||||||||||
| a.Independent blind assessment** | ||||||||||||
| b.Unblinded assessment ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| c.Self report* | ||||||||||||
| d.No description | ||||||||||||
| 7.Statistical test: | ||||||||||||
| a.Statistical test used to analyse the data clearly described, appropriate and measures of association presented including confidence intervals and probability level (p value).* | * | * | * | * | * | * | * | * | * | * | * | * |
| b.Statistical test not appropriate, not described or incomplete. |
NA – Not applicable.
✓- Tick mark is given where stars cannot be given.
3.4. Description of meta analysis results
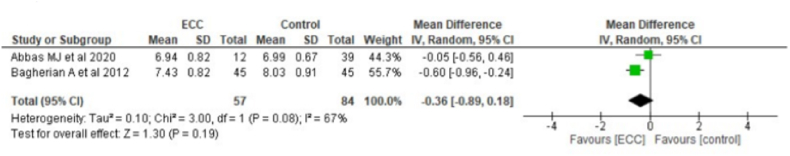
Fig. 2a shows relationship between ECC and salivary pH levels from 2 studies containing 57 children with ECC and 84 caries-free children. Pooled data indicated that children with ECC had a low salivary pH level than controls (MD = 0.36, 95% CI: 0.89, 0.18). Fig. 2b depicts the relationship between SECC and salivary pH levels from 2 studies containing 51 children with SECC and 64 caries-free children. Pooled data showed that children with SECC had a low salivary pH (MD = 0.58, 95% CI:1.52,0.36).
Fig. 2a.
Relationship between ECC and PH.
Fig. 2b.
Relationship between severe ECC and PH.
Fig. 3 shows relationship between ECC and salivary flow rate from 3 studies containing 93 children with ECC and 90 caries-free children. Pooled data indicated that children with ECC had a low salivary flow than caries-free children (MD = 0.41, 95% CI:0.73, 0.09).
Fig. 3.
Relationship between Salivary flow and ECC.
Fig. 4 shows relationship between ECC and salivary calcium from 4 studies containing 99 children with ECC and 99 caries free children. Forest plot shows a heterogenicity with I2 value of 82%. (MD = 0.53, 95% CI: 1.68, 0.63). Forest plot depicts that calcium levels are low in children with ECC as compared to caries-free children. Outlier was detected in one study due to wide variation in calcium levels between ECC and caries-free group.
Fig. 4.
Relationship between salivary calcium and ECC
Fig. 5 shows relationship between ECC and salivary phosphate, and depicts that salivary phosphate level was found to be high in children ECC as compared to caries free children. Outlier was detected in one study due to wide variation in phosphate levels between ECC and caries-free group.
Fig. 5.
Relationship between salivary phosphate and ECC.
Fig. 6a represents the relationship between ECC and salivary IgA from 2 studies. The forest plot shows that salivary IgA was found to be high in children with ECC. Fig. 6b depicts the relationship between IgG and ECC and the IgG values were found to be high in children with ECC as compared to caries free children.
Fig. 6a.
Relationship between salivary IgA and ECC
Fig. 6b.
Relationship between IgG and ECC
3.5. Publication bias
Fig. 7a, Fig. 8, Fig. 9, Fig. 10, Fig. 11 shows a Symmetrical funnel plot distribution for all the studies evaluated pH, salivary flow rate, buffering capacity, calcium and phosphate. Asymmetrical distribution was evident in the funnel plot for the studies that had evaluated the pH of S ECC children and IgA levels. (Fig. 7b, Fig. 12). Publication bias in the aforementioned studies could be due to a smaller sample size or only the positive findings of the studies have been reported. .
Fig. 7a.
Funnel plot for pH of ECC and Caries free children.
Fig. 8.
Funnel plot for Salivary flow rate of ECC and Caries free children.
Fig. 9.
Funnel plot for Salivary calcium of ECC and Caries free children.
Fig. 10.
Funnel plot for Salivary phosphate of ECC and Caries free children.
Fig. 11.
Funnel plot for Buffering capacity of ECC and Caries free children.
Fig. 7b.
Funnel plot for pH of SECC and Caries free children.
Fig. 12.
Funnel plot for Salivary IgA of ECC and Caries free children.
4. Discussion
The studies selected for this systematic review and meta-analysis were those that best satisfied the minimum criteria to be able to evaluate the role of physical and chemical properties of saliva and ECC. Although several studies related to ECC can be found on the scientific databases, only 22 studies were considered suitable for systematic review. Among the included studies, few studies found no significant differences, while others found a significant difference in the evaluated physical and chemical properties and ECC. This systematic review did not include other types of studies such as longitudinal studies, or comparison of physical and chemical properties before and after treatment, in order to maintain the homogeneity with the study design. There was no publication bias evident from the screened studies.
4.1. Salivary pH and buffering capacity
The pH and buffering action of saliva can alter the low plaque pH, thus preventing demineralization of enamel. Hence pH and buffering capacity of saliva plays a vital role in preventing the initiation of dental caries. Salivary pH and buffering capacity in children with and without ECC have been studied extensively with diversified results. Since salivary pH and buffering capacity can be considered as a potential tool for caries risk assessment in children, studies are still being carried out due to its potential importance.
Studies have reported that pH and buffering capacity of saliva were high in caries free children as compared to caries active children28, 29, 30, 31, 32, 33 The Ph required for enamel dissolution to initiate dental caries should fall below critical pH, hence higher salivary pH in caries free children might be making the caries initiation difficult. Similarly, high buffering capacity of saliva neutralizes the acid produced by micro-organism and hence less chance of caries initiation.
On the contrary, studies also have reported that there was no significant difference in pH and buffering capacity of saliva in children with and without ECC.34, 35, 36, 37, 38, 39 The authors justify that, pH and buffering capacity solely cannot be considered as a risk factor for ECC. Importance should be given to diet, microbial flora, salivary protein, as these factors could dominate pH and buffering capacity.
4.2. Salivary flowrate
Salivary flushing is essential for maintenance of oral health and clearance of microorganisms and food components. The salivary flow rate is low in young children, hence, evaluating the salivary flow rate in young children helps us to identify its role in ECC.
Some studies have concluded that low salivary flow rate is a vital indicative of an increased risk of caries in children29,31,32 However, other studies demonstrates that salivary flow rate is not a contributing factor in caries rate in children.34,37,38 Tenovuo et al. had stated that, there exists a threshold limit of salivary flow rate and it is specific for each individual. So, considering the normal flow rate would be ideal for population level than while screening for individual patients. Similarly, studies state that, it is important to establish a reference value for salivary flow rate in children, because the salivary flow rate of children coincides with the reference value of hyposalivation in adults.41 So, it is necessary to develop a reference value of salivary flow rate in children. In addition, seasonal temperature can affect the flow rate of saliva42, 43, 44 There is no evidence that the above-mentioned studies have taken these points into consideration while conducting the studies. Hence, salivary flow rate is an important parameter for caries activity, and in future, it is important to take the above points into consideration while evaluating this parameter in children.
4.3. Salivary viscosity
Few studies have evaluated the salivary viscosity of children with and without ECC, and stated that salivary viscosity was higher in children with ECC and caries -free children have low viscous and watery saliva.33,40,45 This highly viscous saliva is less effective in oral clearance and could be a contributing factor for ECC.
4.4. Inorganic ions of saliva
4.4.1. Salivary calcium, phosphate and alkaline phosphatase
Saliva acts as a source of calcium and phosphate and plays an imperative role in remineralization of incipient carious lesion. These inorganic ions of saliva improve post-eruptive maturation of enamel by influencing the precipitation or dissolution of Hydroxy Apatite Crystals (HAP).46,47 Saliva maintains calcium and phosphate in supersaturated state and helps in neutralizing acids. Alkaline phosphatase is a non-specific enzyme and it maintains the level of calcium and phosphate to sustain the demineralization and remineralization process.
With regard to the role of salivary calcium and phosphorus levels in ECC, conflicting reports have been published. A study by Aruna et al. reported an increased salivary calcium and phosphorus levels in caries-free children, as opposed to children with ECC.48 However, the results of this study should be evaluated with caution as it is a pilot study. Contrary to this, Turtola et al. and Elizarova and Petrovich reported an increase in salivary calcium in children with increased caries activity49,50 Kaur et al. and Mahajana et al. reported an increase in salivary phosphate level in caries active children than the caries free children.33,51 Jolly et al. evaluated salivary calcium and phosphorus and found an increase in salivary calcium levels in caries free children and no difference in salivary phosphorus between ECC and caries free children.52 Similarly, Gandhy and Damle reported an increase in inorganic phosphate level in children with rampant caries.53 The increase in salivary calcium levels in caries active children could be due to release of calcium from demineralized tooth, thereby increasing salivary calcium levels. On the other hand, few studies insisted that there was no difference in salivary calcium and phosphate level in caries-free and caries-active children.28,36,54,55 One of the possible explanations for no difference in calcium in both the groups could be due to the fact that, saliva is a blood filtrate and the unaltered level of calcium in children with ECC might be due to the regulatory role of parathyroid hormone (PTH), maintaining its level homogeneously in both ECC and caries free children.56,57
Kaur et al. and Shahrabi et al. estimated the level of salivary alkaline phosphatase between caries-free and caries-active children and reported a higher alkaline phosphatase activity in caries-free children.33,54 The above-mentioned studies also reported a higher level of calcium and phosphorus in caries-free children and the reason could be due to higher alkaline phosphatase activity, or vice versa.
4.5. Salivary proteins and peptides
Out of the 22 included studies, 11 studies have assessed salivary proteins, enzymes and immunoglobulins. These salivary proteins and peptides possess an important function of resistance of oral mucosa to infection. Salivary immunoglobulins provide a host immune response and adaptive immunity against oral pathogens. In addition, it also enhances the activity of other salivary enzymes.58
Few studies have assessed the salivary immunoglobulins, namely IgA, IgG, IgM and sIgA and compared in children with and without ECC. De Farias et al., Bagherain et al. (2008 & 2012) has stated that the salivary IgA and IgG levels were significantly increased in children with ECC.29,59,60 The long duration of carious process would have lodged numerous micro organisms and that could have stimulated immune response with secondary increase in immunoglobulin levels. Contrary to this, Shifa et al. and Colombo NH et al. found no correlation in salivary IgA levels between children with and without ECC.61,62
Bachtiar EW and Bhalla et al. et al. assessed the electrophoretic profile of salivary protein using SDS page and stated that Proline Rich Protein was found to be higher in caries free children, explaining its protective role.37,45 In addition, Bachtiar et al. found a decreased frequency of occurrence of cysteine and albumin in children with ECC.45
Lertsirivorakul et al. evaluated activity of lysozyme in children with and without ECC and the author noticed an increased lysozyme activity in children with SECC.25 Moslemi et al. analysed the salivary lysozyme levels in children before and after dental treatment, and found an increased lysozyme activity in caries free children. The author justified that it provides an antimicrobial effect, and it has an important role in dental caries prevention.63 As only 2 studies had been conducted on salivary lysozymes in Iran and Thai children, and the available results are contradictory, further research is mandatory to explore the role of salivary lysozymes in the near future.
Colombo et al. estimated the levels of Antimicrobial Peptides (AMP) in a group of children with ECC, SECC and caries -free. The author found no significant difference in antimicrobial peptides between the group. The author further added, though there was no significant difference in individual peptide, salivary hBD-2 or HTN-5 are positively correlated with level of Mutans Streptococci.64 Jayakaran et al. estimated the level of AMP namely salivary peptide HNP1 and stated that, the salivary peptide HNP1 was found to be low in children with ECC as compared to caries free children.65 In contrast, Jurczak et al. found an increased level of AMP in children with ECC as compared to children with mild demineralization.66 However, direct comparison cannot be done on the above 3 studies as one study compared the salivary lysozyme and caries progression, one study has assessed salivary peptide level and ECC, and one study compared the salivary lysozyme and level of Mutans Streptococci and Lactobacilli. Since wide disparity is evident in the available studies on AMP, further research is needed to explore its role in ECC.
5. Limitations
Though the results of the aforementioned studies regarding salivary physical and chemical properties and its relationship with ECC looks promising, there is a wide disparity in the study results. Several other etiological factors, namely diet, virulence of microorganisms and genetic patterns of salivary proteins and micro-organisms could have accounted for this difference. Similarly, all the included studies were cross-sectional, so in future, it is important to conduct a prospective cohort with adequate follow-up of the same children at different age groups to arrive at a definitive conclusion. As ECC is considered as a multifactorial etiology, a single variable cannot be considered as a predictor of ECC, since many confounding factors accounts for the variation. For E.g.: Systemic fluoride intake during the tooth eruption is considered as a confounding factor and many studies haven't accounted systemic fluoride while selecting the study sample and this parameter also varies between population in different studies. Hence a properly designed longitudinal study with adequate follow up of the same children along with consideration of the above-mentioned factors would give us a broader view on the role of physical and chemical properties of saliva in children.
6. Conclusion
With the light of available evidence, following conclusions can be drawn:
-
1.
Physical and chemical properties of saliva play an important role in prevention of dental caries in children
-
2.
Physical and chemical properties vary between children with and without ECClista
-
3.
Further studies are needed with long term follow-ups of similar group children belonging to different age groups to find out the difference in physical and chemical properties in primary and permanent dentition
-
4.
These parameters can be utilized in chair side salivary tests to evaluate the caries risk status of children
Declaration of competing interest
The authors have no funding to disclose.
References
- 1.Edgar M., Dawes C., O'Mullane D., editors. Saliva and Oral Health. third ed. British Dental Association; London: 2004. pp. 1–2. [Google Scholar]
- 2.Hicks J., Garcia-Godoy F., Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1) J Clin Pediatr Dent. 2003;28:47–52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 3.Stookey G.K. The effect of saliva on dental caries. J Am Dent Assoc. 2008;139 doi: 10.14219/jada.archive.2008.0347. 11S-7S. [DOI] [PubMed] [Google Scholar]
- 4.Lenander-Lumikari M., Loimaranta V. Saliva and dental caries. J Adv Dev Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 5.Javaid M.A., Ahmed A.S., Durand R., Tran S.D. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res. 2016;6:67–76. doi: 10.1016/j.jobcr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitts N.B., Zero D.T., Marsh P.D., et al. Dental caries. Nat Rev Dis Prim. 2017;3(1):1–6. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 7.Dawes C. Salivary flow JADA patterns and the health of hard and soft oral tissues. J Am Dent Assoc. 2008;139:18S–24S. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 8.Shellis R.P. A microcomputer program to evaluate the saturation of complex solutions with respect to biominerals. J Bioinform. 1988;4:373–379. doi: 10.1093/bioinformatics/4.3.373. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen A.M., Belstrom D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent. 2019;80:S3–S12. doi: 10.1016/j.jdent.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann NY Acad Sci. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 11.Russell M.W., Hajishengallis G., Childers N.K., et al. Secretory immunity in defense against cariogenic Mutans Streptococci. Caries Res. 1999;33:4–15. doi: 10.1159/000016490. [DOI] [PubMed] [Google Scholar]
- 12.Joly S., Maze C., McCray P.B., Guthmiller J.M. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorr S.U. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 14.Oudhoff M.J., Bolscher J.G., Nazmi K., et al. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. Faseb J. 2008;22:3805–3812. doi: 10.1096/fj.08-112003. [DOI] [PubMed] [Google Scholar]
- 15.Kivela J., Parkkila S., Parkkila A.K., Leinonen J., Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI. J Physiol. 1999;520:315–320. doi: 10.1111/j.1469-7793.1999.t01-1-00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkkila S., Parkkila A.K., Vierjoki T., Stahlberg T., Rajaniemi H. Competitive time-resolved immunofluorometric assay for quantifying carbonic anhydrase VI in saliva. Clin Chem. 1993;39:2154–2157. [PubMed] [Google Scholar]
- 17.Kivela J., Parkkila S., Waheed A., Parkkila A.K., Sly W.S., Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem. 1997;43:2318–2322. [PubMed] [Google Scholar]
- 18.Frasseto F., Parisotto T.M., Peres R.C., Marques M.R., Line S.R., Nobre Dos Santos M. Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res. 2012;46:194–200. doi: 10.1159/000337275. [DOI] [PubMed] [Google Scholar]
- 19.Leinonen J., Kivela J., Parkkila S., Parkkila A.K., Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res. 1999;33:185–190. doi: 10.1159/000016515. [DOI] [PubMed] [Google Scholar]
- 20.de‐Sousa E.T., Lima‐Holanda A.T., Nobre‐dos‐Santos M. Carbonic anhydrase VI activity in saliva and biofilm can predict early childhood caries: a preliminary study. Int J Paediatr Dent. 2021;31:361–371. doi: 10.1111/ipd.12717. [DOI] [PubMed] [Google Scholar]
- 21.Laible N.J., Germaine G.R. Bactericidal activity of human lysozyme, muramidase- inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect Immun. 1985;48:720–728. doi: 10.1128/iai.48.3.720-728.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollock J.J., Iacono V.J., Bicker H.G., et al. The binding, aggregation and lytic properties of lysozyme. Microbial aspects of dental caries. 1976;2:325–352. [Google Scholar]
- 23.Roger V., Tenovuo J., Lenander-Lumikari M., Söderling E., Vilja P. Lysozyme and Lactoperoxidase inhibit the adherence of Streptococcus mutans NCTC 10449 (serotype c) to saliva-treated hydroxyapatite in vitro. Caries Res. 1994;28:421–428. doi: 10.1159/000262015. [DOI] [PubMed] [Google Scholar]
- 24.Twetman S., Lindqvest L., Sund M.-L. Effect of human lysozyme on 2-deoxyglucose uptake by Streptococcus mutans and other oral micro-organisms. J Dent Res. 1986;20:223–229. doi: 10.1159/000260939. [DOI] [PubMed] [Google Scholar]
- 25.Lertsirivorakul J., Petsongkram B., Chaiyarit P., Klaynongsruang S., PitipHat W. Salivary lysozyme in relation to dental caries among Thai preschoolers. J Clin Pediatr Dent. 2015;39:343–347. doi: 10.17796/1053-4628-39.4.343. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Modesti P.A., Reboldi G., Cappuccio F.P. Newcastle-Ottawa Quality Assessment Scale (adapted for cross sectional studies) PLoS One. 2016:1–2. [Google Scholar]
- 28.Makawi Y., El-Masry E., El-Din H.M. Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. J Unexplored Med Data. 2017;2:9–15. [Google Scholar]
- 29.Bagherian A., Asadikaram G. Comparison of some salivary characteristics between children with and without early childhood caries. Indian J Dent Res. 2012 1;23:628–632. doi: 10.4103/0970-9290.107380. [DOI] [PubMed] [Google Scholar]
- 30.Animireddy D., Bekkem V.T., Vallala P., Kotha S.B., Ankireddy S., Mohammad N. Evaluation of pH, buffering capacity, viscosity and flow rate levels of saliva in caries-free, minimal caries and nursing caries children: an in vivo study. Contemp Clin Dent. 2014;5:324–328. doi: 10.4103/0976-237X.137931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muchandi S., Walimbe H., Bijle M.N., Nankar M., Chaturvedi S., Karekar P. Comparative evaluation and correlation of salivary total antioxidant capacity and salivary pH in caries-free and severe early childhood caries children. J Contemp Dent Pract. 2015;16:234–237. doi: 10.5005/jp-journals-10024-1667. [DOI] [PubMed] [Google Scholar]
- 32.Kuriakose S., Sundaresan C., Mathai V., Khosla E., Gaffoor F.M. A comparative study of salivary buffering capacity, flow rate, resting pH, and salivary Immunoglobulin A in children with rampant caries and caries-resistant children. J Indian Soc Pedod Prev Dent. 2013;31:69–73. doi: 10.4103/0970-4388.115697. [DOI] [PubMed] [Google Scholar]
- 33.Kaur A., Kwatra K.S., Kamboj P. Evaluation of non-microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J Indian Soc Pedod Prev Dent. 2012;30:212–217. doi: 10.4103/0970-4388.105013. [DOI] [PubMed] [Google Scholar]
- 34.Sharaf A.A., El Meligy O.S., Tallab H.Y. Salivary characteristics in a sample of preschool children with severe early childhood caries (S-ECC) J King Abdulaziz Univ - Mar Sci. 2010;17:41–58. [Google Scholar]
- 35.Martínez-Pabón M.C., Ramírez-Puerta B.S., Escobar-Paucar G.M., Franco-Cortés Á.M. Physicochemical salivary properties, Lactobacillus, Mutans Streptococci counts and early childhood caries in preschool children of Colombia. Acta Odontol Latinoam. 2010;23:249–256. [PubMed] [Google Scholar]
- 36.Abbas M.J., Al-Hadithi H.K., Mahmood M.A., Hussein H.M. Comparison of some salivary characteristics in Iraqi children with early childhood caries (ECC) and children without early childhood caries. Clin Cosmet Invest Dent. 2020;12:541–550. doi: 10.2147/CCIDE.S275963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhalla S., Tandon S., Satyamoorthy K. Salivary proteins and early childhood caries: a gel electrophoretic analysis. Contemp Clin Dent. 2010;1:17–22. doi: 10.4103/0976-237X.62515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayaraj D., Ganesan S. Salivary pH and buffering capacity as risk markers for early childhood caries: a clinical study. Int J Clin Pediatr Dent. 2015;8:167–171. doi: 10.5005/jp-journals-10005-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villavicencio J., Arango M.C., Ordonez A., Contreras A., Villegas L.M. Early childhood caries, salivary and microbiological aspects among 3-to 4-year-old children in Cali, Colombia. Eur Arch Paediatr Dent. 2018;19:347–352. doi: 10.1007/s40368-018-0365-5. [DOI] [PubMed] [Google Scholar]
- 40.Tenovuo J. Salivary parameters of relevance for assessing caries activity in individuals and populations. Community Dent Oral Epidemiol. 1997;25:82–86. doi: 10.1111/j.1600-0528.1997.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 41.Bretz W a, do Valle E.V., Jacobson J.J., et al. Unstimulated salivary flow rates of young children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:541–545. doi: 10.1067/moe.2001.114004. [DOI] [PubMed] [Google Scholar]
- 42.Shannon I.L. Climatological effects on human parotid gland function. Arch Oral Biol. 1966;11:451–453. doi: 10.1016/0003-9969(66)90109-9. [DOI] [PubMed] [Google Scholar]
- 43.Mandel I.D. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- 44.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 45.Bachtiar E.W., Hermawan I.Y., Bachtiar B.M. Analysis of salivary protein profiles and its viscosity in early childhood caries (A cross-sectional study) J Clin Diagn Res. 2018;12:28–30. [Google Scholar]
- 46.Jenkins G.N. fourth ed. Blackwell Scientific Pub; Oxford: 1978. Physiology and Biochemistry of the Mouth; pp. 284–359p. [Google Scholar]
- 47.Mandel ID. The functions of saliva. J Dent Res. 66:987, 623-627. [DOI] [PubMed]
- 48.Aruna S., Meenakshi B., Rama K.V., Valarmathi S. Salivary levels of calcium and phosphorus in children with and without early childhood caries: a pilot study. SRM J Res Dent Sci. 2020;11(2):72–75. [Google Scholar]
- 49.Turtola L. Dental caries and its prevention. Proc Finn Dent Soc. 1978;74:36–37. [PubMed] [Google Scholar]
- 50.Elizarora V.M., Petrovich I.U. Ionized calcium in the saliva of children with multiple caries. Stomatologia. 1997;76:6–8. [PubMed] [Google Scholar]
- 51.Mahajan S., Suneja B., Kaur P. An attempt to correlate biochemical parameters in saliva with dental carries in children of two different age groups: a comparative study. Int J Oral Health Sci. 2017;7:96–100. [Google Scholar]
- 52.Jolly L.R., Shetty A. Calcium and inorganic phosphorous levels in stimulated and unstimulated saliva in early childhood caries-A comparative study. J Academy Dent Edu. 2014;1:5–11. [Google Scholar]
- 53.Gandhy M., Damle G. Relation of salivary inorganic phosphorous and alkaline phosphatase to the dental caries status in children. J Indian Soc Pedod Prev Dent. 2002;21:135–138. [PubMed] [Google Scholar]
- 54.Shahrabi M., Nikfarjam J., Alikhani A., Akhoundi N., Ashtiani M., Seraj B. A comparison of salivary calcium, phosphate, and alkaline phosphatase in children with severe, moderate caries, and caries free in Tehran's kindergartens. J Indian Soc Pedod Prev Dent. 2008;26:74–77. doi: 10.4103/0970-4388.41621. [DOI] [PubMed] [Google Scholar]
- 55.Vijayaprasad K.E., Ravichandra K.S., Vasa A.A., Suzan S. Relation of salivary calcium, phosphorus and alkaline phosphatase with the incidence of dental caries in children. J Indian Soc Pedod Prev Dent. 2010;28:156–161. doi: 10.4103/0970-4388.73789. [DOI] [PubMed] [Google Scholar]
- 56.Walsh L.J. Preventive dentistry for the general dental practitioner. Aust Dent J. 2000;45:76–82. doi: 10.1111/j.1834-7819.2000.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 57.Vasudevan D.M., Sreekumari S., Vaidyanathan K. sixth ed. Jaypee Brothers Medical Publishers Pvt. Ltd.; 2011. Textbook of Biochemistry for Medical Students; pp. 413–415p. [Google Scholar]
- 58.Law V., Seow W.K., Townsend G. Factors influencing oral colonization of mutans Streptococci in young children. Aust Dent J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 59.De Farias D.G., Bezerra A.C. Salivary antibodies, amylase and protein from children with early childhood caries. Clin Oral Invest. 2003;7:154–157. doi: 10.1007/s00784-003-0222-7. [DOI] [PubMed] [Google Scholar]
- 60.Bagherian A., Jafarzadeh A., Rezaeian M., Ahmadi S., Rezayati M. Comparison of the salivary immunoglobulin concentration levels between children with early childhood caries and caries-free children. Iran J Immunol. 2008;5:217–221. [PubMed] [Google Scholar]
- 61.Shifa S., Muthu M.S., Amarlal D., Prabhu V.R. Quantitative assessment of IgA levels in the unstimulated whole saliva of caries-free and caries-active children. J Indian Soc Pedod Prev Dent. 2008;26:158–161. doi: 10.4103/0970-4388.44031. [DOI] [PubMed] [Google Scholar]
- 62.Colombo N.H., Pereira J.A., Da Silva M.E., et al. Relationship between the IgA antibody response against Streptococcus mutans GbpB and severity of dental caries in childhood. Arch Oral Biol. 2016;67 doi: 10.1016/j.archoralbio.2016.03.006. 22-2. [DOI] [PubMed] [Google Scholar]
- 63.Moslemi M., Sattari M., Kooshki F., et al. Relationship of salivary lactoferrin and lysozyme concentrations with early childhood caries. J Dent Res Dent Clin Dent Prospects. 2015;9:109–114. doi: 10.15171/joddd.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colombo N.H., Ribas L.F., Pereira J.A., et al. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch Oral Biol. 2016;69:40–46. doi: 10.1016/j.archoralbio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Jayakaran T.G., Rekha C.V., Annamalai S., Baghkomeh P.N. Salivary peptide human neutrophil defensin1–3 and its relationship with early childhood caries. Dent Res J. 2020;17:459–464. [PMC free article] [PubMed] [Google Scholar]
- 66.Jurczak A., Kościelniak D., Papież M., Vyhouskaya P., Krzyściak W. A study on β-defensin-2 and histatin-5 as a diagnostic marker of early childhood caries progression. J Biol Res. 2015;48:1–9. doi: 10.1186/s40659-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]