Abstract
Objective
To evaluate the prevalence of vitamin D deficiency and predictors for low vitamin D status in Korean adolescents living between latitudes 33° and 39°N.
Design
A descriptive cross-sectional study.
Setting
Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009.
Subjects
A total of 1510 healthy adolescents aged 12–18 years (806 male, mean age 14·7 years) participated. Possible predictors for low vitamin D status (log-transformed 25-hydroxyvitamin D (25(OH)D) concentrations) were evaluated.
Results
The prevalence of vitamin D deficiency (25(OH)D<20 ng/ml) was 89·1 % in spring, 53·7 % in summer, 63·9 % in autumn and 90·5 % in winter. Winter season, older age, higher education level reached, being female, being obese, a lack of vitamin D supplementation, lower milk consumption (0–<200 ml/d) and a lack of physical activity were unadjusted predictors (all P < 0·05). Multiple linear regression analysis showed that winter season (P < 0·001), higher education level (P < 0·001) and a lack of vitamin D supplementation (P = 0·012) were independent predictors for low vitamin D status. The modifying effect of season on the association between vitamin D supplement use and vitamin D status was significant (P < 0·001).
Conclusions
Vitamin D deficiency was highly prevalent in Korean adolescents, especially those in higher school grades. Vitamin D supplementation may contribute to maintain a better vitamin D status with lower seasonal variation. Further studies are required to determine optimal vitamin D intakes to maintain sufficient vitamin D status for Korean adolescents.
Keywords: Vitamin D deficiency, Prevalence, Risk factors, Adolescent, Korea
There is great interest in the role of vitamin D in multiple health outcomes. It is essential for skeletal health, and there has been increasing evidence linking vitamin D with benefits for non-skeletal outcomes such as type 2 diabetes mellitus, CVD, infectious/inflammatory disease, autoimmune disease and cancer( 1 ).
Although there has been much debate over the definition of vitamin D deficiency, most agree that a 25-hydroxyvitamin D (25(OH)D) concentration of <20 ng/ml is an indication of deficiency( 2 – 5 ). Using this definition, vitamin D deficiency is common in the USA( 6 – 9 ), New Zealand( 10 ), the UK( 11 ) and Beijing in China( 12 ). Recent data for the Korean population based on the Korea National Health and Nutrition Examination Survey (KNHANES) 2008( 13 ) showed that vitamin D insufficiency was most prevalent in those aged 20–29 years, followed by those aged 10–19 years. In the adult population, predictors for vitamin D insufficiency included being in younger age groups, having 25(OH)D concentrations checked in spring and winter, living in an urban area and undertaking indoor occupations( 13 ). However, the predictors for vitamin D insufficiency in the paediatric population were not included.
There has been a paucity of data on the importance of vitamin D status for health outcomes as well as on the important predictors for deficiency in Korean adolescents. The Dietary Reference Intake (DRI) of vitamin D for Korean children and adolescents was lowered from 10 μg/d in 2005 to 5 μg/d in 2010( 14 ), under the assumption that sunlight exposure could satisfy their vitamin D needs. The purposes of the present study were to: (i) evaluate the prevalence of vitamin D deficiency in Korean adolescents aged 12–18 years based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) in 2008–2009; and (ii) investigate the risk factors leading to low vitamin D status in Korean adolescents living between latitudes 33° and 39°N.
Materials and methods
Study participants
The present study is based on the data acquired during the second and third years (2008 and 2009) of KNHANES IV under permission of the Korea Centers for Disease Control and Prevention. KNHANES IV was a cross-sectional representative survey of the non-institutionalized population of Korea from 2007 to 2009. It consisted of a health interview survey, a nutrition survey and a health examination survey. KNHANES IV used a rolling sampling design with a complex, stratified and multistage probability sampling. Based on the 2005 National Census Registry for Korea, from among 264 186 primary sampling units, 200 were selected randomly. Then, twenty-three households were sampled from each unit to yield 4600 households. In 2008 and 2009, 25 250 individuals (12 528 and 12 722, respectively) were sampled; 19 386 (9308 and 10 078, respectively) of these individuals participated in health interviews and health examination surveys and 18 038 (8641 and 9397, respectively) participated in nutrition surveys. Among 1933 participants aged 12–18 years, 423 were excluded; 354 had missing 25(OH)D values and sixty-nine were on medications and/or had a chronic disease possibly affecting vitamin D absorption and metabolism (e.g. congenital heart disease, epilepsy, jaundice and/or liver disease, renal disease, thyroid disorder, malignancy, or a psychiatric or bone disease related to immobilization). Finally, 1510 participants (806 boys and 704 girls) aged 12–18 years were included in the present study. KNHANES IV was conducted according to the guidelines laid down in the Declaration of Helsinki. All participants in the survey signed an informed consent form. The institutional review board of the Korea Centers for Disease Control and Prevention approved the protocol.
Study variables
Factors were categorized to analyse the risk factors for vitamin D deficiency. Korea is located in a temperate region with four distinct seasons: spring (March to May), summer (June to August), autumn (September to November) and winter (December to February). The education levels of the study participants aged 12–18 years were categorized into 5th and 6th grades (elementary school, n 123), 7th to 9th grades (middle school, n 733), 10th to 12th grades (high school, n 546) and first collegiate year (n 57). This was based on the assumption that each group would have a different level of outdoor activity and sunlight exposure. BMI percentiles and BMI Z-scores were assigned on the basis of the 2007 Korean National Growth Charts( 15 ) and the participants were classified into three groups according to BMI percentile: normal weight (<85th percentile), overweight (85th–<95th percentile) and obese (≥95th percentile).
Any dietary vitamin D supplementation was indicated as ‘yes’ when the participants consumed any supplementation. This ‘yes’ group was subcategorized into two groups according to the amount of supplementation: ≥5 μg/d and 0–<5 μg/d. Milk consumption data were obtained from the diet, behaviour and nutrition section of the NHANES Sample Person Questionnaire and were categorized as 0–<200 ml/d, 200–<400 ml/d and ≥400 ml/d. Dietary and supplemental Ca intakes were compared with the DRI for Ca of Korean children and adolescents: 1000 mg/d for boys aged 12–14 years, 900 mg/d for girls aged 12–14 years, 900 mg/d for boys aged 15–18 years and 800 mg/d for girls aged 15–18 years( 14 ). Ca intake levels were categorized into two groups: ≥DRI and <DRI.
Participants were asked about their level of physical activity during a normal week. The definition of regular physical activity was based on the physical activity guideline provided by the US Department of Health and Human Services( 16 ) and output indicators of the Korea Youth Risk Behavior Web-based Survey( 17 ). Participants who performed moderate or vigorous physical activity for at least 60 min/d on 3d/week, and/or walking activity for at least 60 min/d on 5d/week, and/or muscular strength activity on more than 3d/week were assigned to the ‘regular physical activity’ group. Those participants who performed moderate or vigorous physical activity for at least 180 min/d on 3d/week were also assigned to the ‘regular physical activity’ group. Regular physical activity was recorded as ‘yes’, regardless of whether it took place indoors or outdoors.
The style of dress in Korea is westernized and generally conservative. When the weather gets warmer in May with temperatures greater than 17–18°C, the arms and legs are usually exposed in short-sleeved shirts and shorts or skirts. At the end of September when temperatures fall below 15–16°C, long-sleeved shirts and trousers are worn. The face and neck are usually exposed except during cold winter days when temperatures approach 0°C and scarves are used to cover up. In middle school and high school, uniforms are the norm, whereas the attire of elementary and college students is not regulated. However, there is little difference in the degree of skin exposure according to type of dress between the groups. Thus, the type of dress was not included as a possible predictor.
Measurement of serum 25-hydroxyvitamin D concentration
Blood samples were obtained by venepuncture, refrigerated immediately and transported to the central testing institute. Blood samples were analysed within 24 h after transportation. Serum 25(OH)D concentrations were measured using a gamma-counter (1470 Wizard; Perkin-Elmer, Turku, Finland) with a 25-hydroxyvitamin D 125I RIA kit (DiaSorin Inc., Stillwater, MN, USA). At serum 25(OH)D concentrations of 8·6, 22·7, 33·0 and 49·0 ng/ml (21·5, 56·8, 82·5 and 122·5 nmol/l), the inter-assay CV were 11·7 %, 10·5 %, 8·6 % and 12·5 %, respectively, and the intra-assay CV were 9·4 %, 8·2 %, 9·1 % and 11·0 %, respectively. KNHANES participates in the Vitamin D Standardization Program, so the measurement of 25(OH)D was standardized with the recently developed National Institute of Standards and Technology–Ghent University reference procedure( 18 ).
Statistical analysis
Statistical analyses were conducted using the SAS statistical software package version 9·2. Data were analysed using several SAS survey procedures employing appropriate KNHANES sample weights to account for the complex KNHANES sampling design. The procedures included unequal probabilities of selection, oversampling and non-response so that inferences could be made about the Korean adolescent participants. The log-transformed 25(OH)D concentrations were found to be distributed normally in a Q–Q plot. Participants’ characteristics were described using means and standard errors for continuous variables and numbers and percentages for categorical variables.
Participants’ characteristics were calculated by quartile categories of serum 25(OH)D concentration (<12, 12–<16, 16–<20 and ≥20 ng/ml). The Rao–Scott χ 2 test was used to analyse associations between categorical variables and quartiles of serum 25(OH)D concentration. We estimated means with their standard errors for age, height, BMI Z-score and serum alkaline phosphatase levels according to quartiles of serum 25(OH)D concentration, and examined these associations with a test for linear trend.
We performed unadjusted linear regression analyses to investigate the relationship between possible predictors and vitamin D status (log-transformed 25(OH)D concentrations). The assumptions of our linear regression models were evaluated using normal probability plots of the standardized residuals and scatter plots with standardized residuals v. standardized predicted values. The assumptions were satisfied. Because the effect of predictors on vitamin D status might vary depending on the season, the modifying effect of season on the association between vitamin D status and each predictor was evaluated using a test for interaction. Predictors with P < 0·2 in the univariate analyses and interaction effects with P < 0·05 were considered in the multiple linear regression analyses. Finally, multiple linear regression models were constructed for each season. Bonferroni-adjusted P values were reported for multiple linear regression models. For all analyses, P < 0·05 (two-sided) was considered statistically significant.
Results
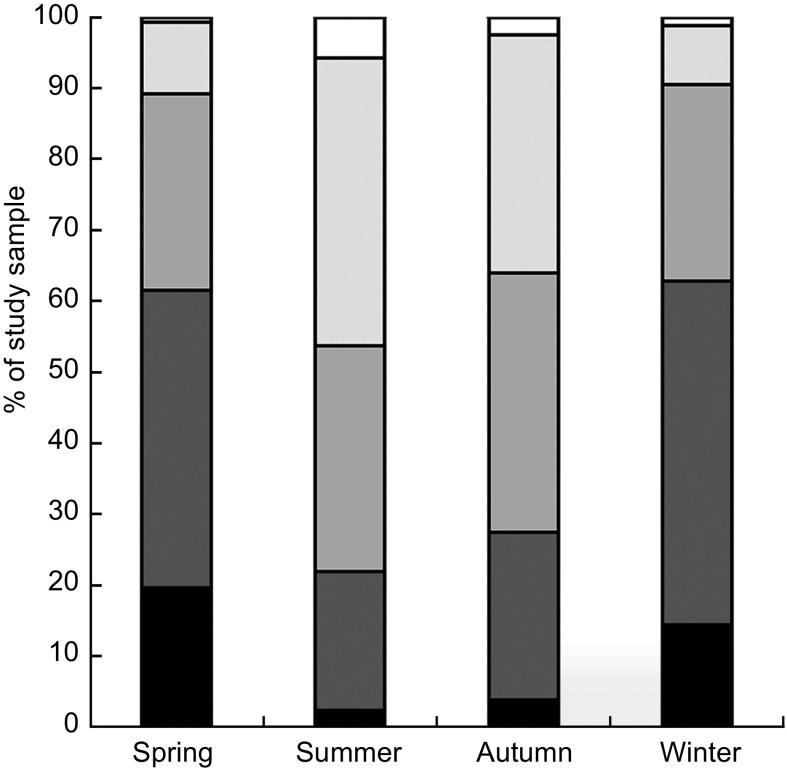
The study participants’ (53·4 % male) mean age was 14·7 (sd 1·9) years and their mean BMI Z-score was 0·025 (sd 1·05), with 12·2 % of them being overweight and 5·8 % obese. Figure 1 demonstrates the proportion of participants with serum 25(OH)D concentrations of <10, 10–<15, 15–<20, 20–<30 and ≥30 ng/ml (25, 25–<37·5, 37·5–<50, 50–<75 and ≥75 nmol/l) according to season. The prevalence of vitamin D deficiency, defined as a 25(OH)D level of <20 ng/ml, was 89·1 % in spring, 53·7 % in summer, 63·9 % in autumn and 90·5 % in winter.
Fig. 1.
Percentage of the study sample with serum 25-hydroxyvitamin D concentrations <10 ng/ml ( ), 10–<15 ng/ml (
), 10–<15 ng/ml ( ), 15–<20 ng/ml (
), 15–<20 ng/ml ( ), 20–<30 ng/ml (
), 20–<30 ng/ml ( ) and ≥30 ng/ml (
) and ≥30 ng/ml ( ) according to season: adolescents aged 12–18 years (n 1510), Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
) according to season: adolescents aged 12–18 years (n 1510), Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
Table 1 shows the associations between the quartile categories stratified according to serum 25(OH)D level and the participants’ characteristics. Across the 25(OH)D quartile categories, the participants in the highest 25(OH)D group were significantly younger (P < 0·001) and more physically active (P = 0·031) than those in the lowest 25(OH)D group. With increasing school grade, the serum 25(OH)D quartiles decreased significantly (P < 0·001). Significantly increasing trends for serum 25(OH)D were observed with increasing vitamin D supplement use (P = 0·028) and increasing milk consumption (P = 0·002). As the 25(OH)D quartiles decreased, the level of serum alkaline phosphatase increased (P < 0·001; Table 1).
Table 1.
Characteristics of the study sample according to quartile of serum 25-hydroxyvitamin D concentration: adolescents aged 12–18 years (n 1510), Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
| Quartile of 25-hydroxyvitamin D | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unweighted | <12 ng/ml | 12–<16 ng/ml | 16–<20 ng/ml | ≥20 ng/ml | |||||||||||||||||||||||||||
| n or Mean | % or se | n or Mean | % or se | n or Mean | % or se | n or Mean | % or se | n or Mean | % or se | P value | |||||||||||||||||||||
| Number | 1510 | 345 | 392 | 373 | 400 | ||||||||||||||||||||||||||
| Age (years), mean (se) | 1510 | 15·3 | 0·1 | 14·9 | 0·1 | 14·7 | 0·1 | 14·2 | 0·1 | <0·001‡ | |||||||||||||||||||||
| Sex, n (%) | 1510 | ||||||||||||||||||||||||||||||
| Males | 806 | 53·4 | 163 | 47·2 | 193 | 49·2 | 216 | 57·9 | 234 | 58·5 | 0·197 | ||||||||||||||||||||
| Females | 704 | 46·6 | 182 | 52·8 | 199 | 50·8 | 157 | 42·1 | 166 | 41·5 | |||||||||||||||||||||
| Season, n (%) | 1510 | ||||||||||||||||||||||||||||||
| Winter | 401 | 26·6 | 144 | 41·7 | 133 | 33·9 | 86 | 23·1 | 38 | 9·5 | <0·001 | ||||||||||||||||||||
| Spring | 322 | 21·3 | 128 | 37·1 | 92 | 23·5 | 67 | 18·0 | 35 | 8·8 | |||||||||||||||||||||
| Summer | 419 | 27·7 | 38 | 11·0 | 70 | 17·9 | 117 | 31·4 | 194 | 48·5 | |||||||||||||||||||||
| Autumn | 368 | 24·4 | 35 | 10·1 | 97 | 27·4 | 103 | 27·6 | 133 | 33·3 | |||||||||||||||||||||
| Height Z-score, mean (se) | 1507 | 0·58 | 0·06 | 0·55 | 0·05 | 0·72 | 0·05 | 0·60 | 0·05 | 0·393‡ | |||||||||||||||||||||
| BMI Z-score, mean (se) | 1506 | −0·03 | 0·06 | 0·09 | 0·06 | 0·10 | 0·05 | −0·05 | 0·05 | 0·815‡ | |||||||||||||||||||||
| Degree of obesity, n (%) | 1506 | ||||||||||||||||||||||||||||||
| Lean | 1234 | 81·9 | 289 | 84·3 | 307 | 78·3 | 300 | 80·4 | 338 | 84·9 | 0·084 | ||||||||||||||||||||
| Overweight | 184 | 12·2 | 32 | 9·3 | 54 | 13·8 | 47 | 12·6 | 51 | 12·8 | |||||||||||||||||||||
| Obese | 88 | 5·9 | 22 | 6·4 | 31 | 7·9 | 26 | 7·0 | 9 | 2·3 | |||||||||||||||||||||
| Education level reached, n (%) | 1459 | ||||||||||||||||||||||||||||||
| College student | 57 | 3·9 | 15 | 4·6 | 16 | 4·3 | 16 | 4·3 | 10 | 2·6 | <0·001 | ||||||||||||||||||||
| High-school student | 546 | 37·4 | 157 | 48·2 | 153 | 40·9 | 132 | 35·8 | 104 | 26·7 | |||||||||||||||||||||
| Middle-school student | 733 | 50·2 | 135 | 41·4 | 186 | 49·7 | 199 | 53·9 | 213 | 54·6 | |||||||||||||||||||||
| Elementary-school student | 123 | 8.4 | 19 | 5·8 | 19 | 5·1 | 22 | 6·0 | 63 | 16·2 | |||||||||||||||||||||
| Regular physical activity*, n (%) | 1496 | ||||||||||||||||||||||||||||||
| Yes | 853 | 57·0 | 158 | 48·5 | 222 | 57·1 | 230 | 62·0 | 243 | 61·7 | 0·031 | ||||||||||||||||||||
| No | 643 | 43·0 | 184 | 51·5 | 167 | 42·9 | 141 | 38·0 | 151 | 38·3 | |||||||||||||||||||||
| Ca intake (mg/d)†, n (%) | 1269 | ||||||||||||||||||||||||||||||
| ≥DRI | 123 | 9·7 | 25 | 8·7 | 28 | 9·3 | 34 | 10·9 | 36 | 10·6 | 0·681 | ||||||||||||||||||||
| <DRI | 1146 | 90·3 | 264 | 91·3 | 300 | 90·7 | 278 | 99·1 | 304 | 99·4 | |||||||||||||||||||||
| Vitamin D supplement use, n (%) | 1147 | ||||||||||||||||||||||||||||||
| Yes | 95 | 8·3 | 14 | 4·6 | 26 | 7·4 | 28 | 8·3 | 27 | 10·8 | 0·028 | ||||||||||||||||||||
| ≥5 μg/d | 35 | 3·1 | 3 | 1·0 | 12 | 3·4 | 12 | 3·6 | 8 | 3·2 | |||||||||||||||||||||
| 0–<5 μg/d | 60 | 5·2 | 11 | 3·6 | 14 | 4·0 | 16 | 4·7 | 19 | 7·6 | |||||||||||||||||||||
| No | 1052 | 91·7 | 275 | 90·8 | 301 | 85·3 | 281 | 83·4 | 195 | 78·3 | |||||||||||||||||||||
| Milk intake (ml/d), n (%) | 1219 | ||||||||||||||||||||||||||||||
| ≥400 ml/d | 92 | 7·5 | 19 | 6·9 | 12 | 3·8 | 29 | 9·6 | 32 | 9·8 | 0·002 | ||||||||||||||||||||
| 200–<400 ml/d | 364 | 29·9 | 75 | 27·2 | 78 | 24·7 | 102 | 33·8 | 109 | 33·5 | |||||||||||||||||||||
| 0–<200 ml/d | 763 | 62·6 | 182 | 65·9 | 226 | 71·5 | 171 | 55·6 | 184 | 56·6 | |||||||||||||||||||||
| Alkaline phosphatase (IU/l), mean (se) | 847 | 585 | 24·9 | 512 | 20·5 | 503 | 20·4 | 473 | 22·7 | <0·001‡ | |||||||||||||||||||||
DRI, Dietary Recommended Intake.
Data are presented as numbers and percentages or means with their standard errors, as indicated.
The Rao–Scott χ 2 test was used to analyse associations between quartiles of serum 25-hydroxyvitamin D and categorical variables.
*Regular physical activity: participants who performed moderate or vigorous physical activity for at least 60 min/d on 3 d/week, and/or walking activity for at least 60 mind on 5 d/week, and/or muscular strength activity on more than 3 d/week were assigned to the ‘regular physical activity’ group. Those participants who performed moderate or vigorous physical activity for at least 180 min/d on 3 d/week were also assigned to the ‘regular physical activity’ group.
†The recommended DRI is 1000 mg/d for boys aged 12–14 years, 900 mg/d for girls aged 12–14 years, 900 mg/d for boys aged 15–18 years and 800 mg/d for girls aged 15–18 years.
‡We estimated means with their standard errors for age, height, BMI Z-score and serum alkaline phosphatase levels according to the quartiles and examined these associations with a test for linear trend.
Table 2 shows the unadjusted predictors for low vitamin D status, with log-transformed 25(OH)D concentration as the dependent variable. Older age group (P < 0·001), female gender (P = 0·006) and a lack of physical activity (P = 0·002) were significantly associated with low vitamin D status. Obese participants had a lower vitamin D status than did those who were lean (P = 0·048) or overweight (P = 0·021). Participants who were not taking supplemental vitamin D had a lower vitamin D status than those who were taking 0–<5 μg/d (P = 0·043) and those who were taking ≥5 μg/d (P = 0·003). Participants who consumed ≥400 ml milk/d had a higher vitamin D status than those who consumed 0–<200 ml/d (P = 0·029). The interaction of season with the effect of vitamin D supplement use on vitamin D status was statistically significant (P = 0·040).
Table 2.
Results of unadjusted and multivariate-adjusted linear regression analyses with log-transformed 25-hydroxyvitamin D concentrations as the dependent variable (R 2 = 0·274): adolescents aged 12–18 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
| Unadjusted (n 1510) | Multivariate-adjusted (n 1056) | |||||||
|---|---|---|---|---|---|---|---|---|
| β | se | 95 % CI | P value | β | se | 95 % CI | P value | |
| Constant | – | – | – | – | 2·656 | 0·057 | 2·543, 2·769 | <0·001 |
| Age* | −0·041 | 0·005 | −0·052, −0·030 | <0·001 | – | – | – | – |
| Sex | ||||||||
| Males (v. females) | 0·058 | 0·021 | 0·017, 0·098 | 0·006 | 0·035 | 0·020 | −0·004, 0·075 | 0·080 |
| Season (v. winter) | <0·001 | <0·001 | ||||||
| Spring | −0·009 | 0·033 | −0·074, 0·055 | 0·774 | 0·010 | 0·034 | −0·056, 0·076 | 0·757 |
| Summer | 0·335 | 0·031 | 0·274, 0·397 | <0·001 | 0·314 | 0·036 | 0·243, 0·384 | <0·001 |
| Autumn | 0·277 | 0·030 | 0·218, 0·336 | <0·001 | 0·257 | 0·032 | 0·194, 0·320 | <0·001 |
| Degree of obesity (v. obese) | 0·043 | 0·100 | ||||||
| Lean | 0·072 | 0·036 | 0, 0·143 | 0·048 | 0·063 | 0·033 | −0·003, 0·128 | 0·060 |
| Overweight | 0·098 | 0·042 | 0·015, 0·182 | 0·021 | 0·075 | 0·038 | −0·002, 0·151 | 0·050 |
| Education level reached (v. elementary-school student) | <0·001 | <0·001 | ||||||
| College student | −0·228 | 0·060 | −0·346, −0·111 | <0·001 | −0·256 | 0·065 | −0·384, −0·127 | <0·001 |
| High-school student | −0·228 | 0·038 | −0·302, −0·153 | <0·001 | −0·202 | 0·040 | −0·280, −0·124 | <0·001 |
| Middle-school student | −0·111 | 0·038 | −0·185, −0·037 | 0·003 | −0·098 | 0·039 | −0·175, −0·020 | 0·014 |
| Regular physical activity† (v. yes) | ||||||||
| No | −0·067 | 0·021 | −0·109, −0·025 | 0·002 | −0·037 | 0·020 | −0·076, 0·002 | 0·060 |
| Vitamin D supplement use (v. no) | 0·006 | 0·012 | ||||||
| ≥5 μg/d | 0·146 | 0·049 | 0·049, 0·243 | 0·003 | 0·259 | 0·047 | 0·166, 0·352 | <0·001 |
| 0–<5 μg/d | 0·118 | 0·058 | 0·004, 0·233 | 0·043 | 0·249 | 0·087 | 0·077, 0·421 | 0·005 |
| Milk intake (v. ≥400 ml/d) | 0·058 | 0·591 | ||||||
| 0–<200 ml/d | −0·105 | 0·048 | −0·200, −0·011 | 0·029 | −0·035 | 0·034 | −0·101, 0·031 | 0·295 |
| 200–<400 ml/d | −0·309 | 0·051 | −0·138, 0·060 | 0·441 | −0·001 | 0·036 | −0·070, 0·071 | 0·978 |
| Interaction between season and vitamin D supplements (season × supplements)‡ | – | – | – | – | – | – | – | <0·001 |
| Ca intake (mg/d)§ (v. <DRI) | ||||||||
| ≥DRI | 0·006 | 0·031 | −0·066, 0·055 | 0·856 | – | – | – | – |
DRI, Dietary Recommended Intake.
*Age was excluded in multivariate-adjusted linear regression analysis because of collinearity with the education level reached.
†Regular physical activity: participants who performed moderate or vigorous physical activity for at least 60 min/d on 3 d/week, and/or walking activity for at least 60 min/d on 5 d/week, and/or muscular strength activity on more than 3 d/week were assigned to the ‘regular physical activity’ group. Those participants who performed moderate or vigorous physical activity for at least 180 min/d on 3 d/week were also assigned to the ‘regular physical activity’ group.
‡The modifying effect of season on the association between vitamin D supplement use and vitamin D status was significant in unadjusted and multivariate-adjusted linear regression analyses.
§The recommended DRI is 1000 mg/d for boys aged 12–14 years, 900 mg/d for girls aged 12–14 years, 900 mg/d for boys aged 15–18 years and 800 mg/d for girls aged 15–18 years.
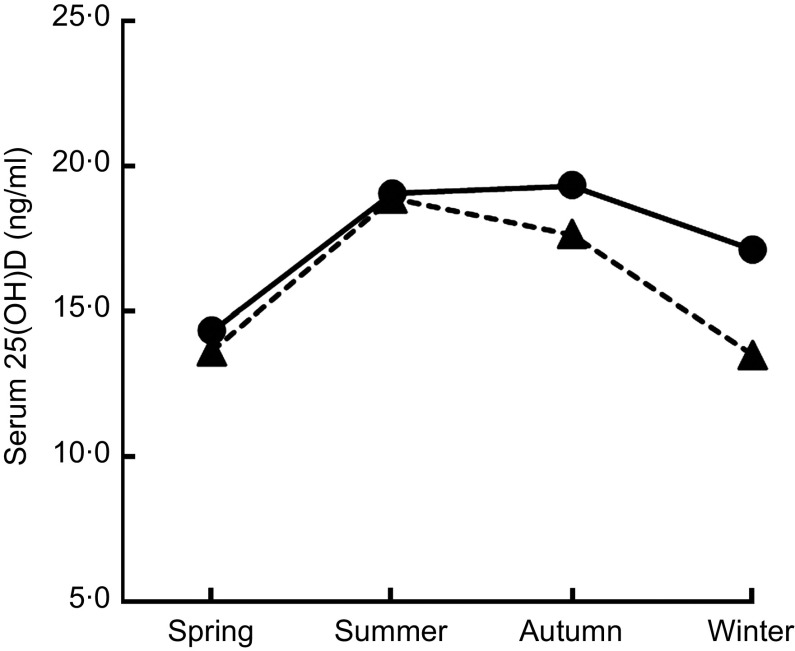
Multiple linear regression analysis showed that season (P < 0·001), education level (P < 0·001) and vitamin D supplement use (P = 0·012) were independent predictors for vitamin D status (Table 2). In addition, the modifying effect of season on the association between vitamin D supplement use and vitamin D status was significant (P < 0·001; Table 2, Fig. 2). Finally, multiple linear regression analyses were performed separately for each season (Table 3). During the winter, gender (P = 0·005), education level (P = 0·0 1 1), regular physical activity (P = 0·0 1 1) and vitamin D supplement use (P < 0·0 0 1) were significantly associated with vitamin D status.
Fig. 2.
The modifying effect of season on the association between vitamin D supplement use (– – ▴ – –, supplement use = no; —●—, supplement use = yes) and vitamin D status (measured as serum 25-hydroxyvitamin D (25(OH)D) concentration (ng/ml)) was significant (P < 0·001), after adjustments were made for other variables: adolescents aged 12–18 years (n 1510), Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
Table 3.
Results of multivariate-adjusted linear regression analyses with log-transformed 25-hydroxyvitamin D concentrations as the dependent variable separately for each season: adolescents aged 12–18 years (n 1056), Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009
| Spring (n 223) | Summer (n 256) | Autumn (n 264) | Winter (n 313) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | se | P value | β | se | P value | β | se | P value | β | se | P value | |
| Constant | 2·734 | 0·160 | <0·001 | 3·081 | 0·110 | <0·001 | 2·823 | 0·093 | <0·001 | 2·699 | 0·013 | <0·001 |
| Sex (v. females) | ||||||||||||
| Males | 0·017 | 0·047 | 0·723 | −0·006 | 0·043 | 0·890 | 0·060 | 0·039 | 0·125 | 0·071 | 0·024 | 0·005 |
| Degree of obesity (v. obese) | 0·554 | 0·144 | 0·315 | 0·882 | ||||||||
| Lean | 0·065 | 0·059 | 0·277 | 0·127 | 0·069 | 0·072 | 0·054 | 0·048 | 0·270 | 0·003 | 0·058 | 0·963 |
| Overweight | 0·074 | 0·088 | 0·406 | 0·122 | 0·077 | 0·119 | 0·072 | 0·050 | 0·157 | 0·019 | 0·064 | 0·765 |
| Education reached (v. elementary-school student) | 0·214 | <0·001 | 0·005 | 0·011 | ||||||||
| College student | −0·275 | 0·149 | 0·071 | −0·452 | 0·094 | <0·001 | −0·074 | 0·166 | 0·658 | −0·122 | 0·138 | 0·383 |
| High-school student | −0·172 | 0·124 | 0·114 | −0·300 | 0·093 | 0·002 | −0·214 | 0·065 | 0·020 | −0·184 | 0·060 | 0·004 |
| Middle-school student | −0·064 | 0·125 | 0·611 | −0·288 | 0·084 | 0·001 | −0·052 | 0·065 | 0·430 | −0·070 | 0·058 | 0·235 |
| Regular physical activity* (v. yes) | ||||||||||||
| No | −0·041 | 0·045 | 0·375 | −0·051 | 0·041 | 0·217 | −0·009 | 0·036 | 0·796 | −0·089 | 0·034 | 0·011 |
| Vitamin D supplement use (v. no) | 0·929 | 0·067 | 0·039 | <0·001 | ||||||||
| 0–<5 μg/d | 0·028 | 0·013 | 0·807 | 0·182 | 0·084 | 0·034 | 0·030 | 0·086 | 0·734 | 0·276 | 0·047 | <0·001 |
| ≥5 μg/d | −0·025 | 0·081 | 0·760 | 0·095 | 0·049 | 0·059 | 0·207 | 0·086 | 0·020 | 0·250 | 0·085 | 0·005 |
| Milk intake (v. ≥400 ml/d) | 0·335 | 0·726 | 0·924 | 0·836 | ||||||||
| 0–<200 ml/d | −0·106 | 0·076 | 0·168 | −0·035 | 0·038 | 0·363 | 0·014 | 0·055 | 0·690 | −0·060 | 0·071 | 0·399 |
| 200–<400 ml/d | −0·079 | 0·072 | 0·280 | −0·001 | 0·052 | 0·980 | 0·013 | 0·061 | 0·756 | 0·040 | 0·076 | 0·603 |
*Regular physical activity: participants who performed moderate or vigorous physical activity for at least 60 min/d on 3 d/week, and/or walking activity for at least 60 min/d on 5 d/week, and/or muscular strength activity on more than 3 d/week were assigned to the ‘regular physical activity’ group. Those participants who performed moderate or vigorous physical activity for at least 180 min/d on 3 d/week were also assigned to the ‘regular physical activity’ group.
Discussion
More than half of these Korean adolescents showed a serum 25(OH)D level of <20 ng/ml, even in summer and autumn. The prevalence of vitamin D deficiency reached about 90 % during winter and spring. There was a lower seasonal variation in serum 25(OH)D concentrations in participants who were taking vitamin D supplements than in those who were not. Winter season, higher education level and a lack of vitamin D supplementation were independent predictors for low vitamin D status in these Korean adolescents.
The concentrations of serum 25(OH)D were highest in summer and lowest in winter and spring. According to 2008–2009 data of the Korean Meteorological Administration, the shortest duration of sunshine is observed between November and March and between late June and July( 19 ). Despite the longest duration of sunshine being observed between April and May, the lowest level of serum 25(OH)D seen during winter had not yet recovered by spring. Additionally, the unexpectedly high prevalence of vitamin D deficiency in summer might be attributed to the short duration of sunshine between late June and July, because of the rainy season and heavy cloud cover at that time of year( 19 ). This high prevalence of vitamin D deficiency in Korean adolescents is consistent with previous observations including subjects of similar age groups living at latitudes around 37°N. For example, there was a 45 % prevalence in young women aged 16–22 years residing in California (38°N) between May and October( 20 ) and an 89·2 % prevalence among girls aged 15 (sd 0·4) years in Beijing (40°N) in winter( 12 ).
The age-related decline in the serum 25(OH)D level might be explained by unmeasured factors such as sunlight exposure, the change in fat mass and increased nutrient needs during growth( 21 ). Older age and higher education level were both associated with low vitamin D status. The level of schooling could affect vitamin D status through differences in sunlight exposure with differing amounts of outdoor activity. With increasing school grade, the total amount of time spent indoors in a classroom increases and participation in after-school outdoor activities decreases( 22 ). The prevalence of vitamin D deficiency throughout the year reached approximately 70 %, 80 % and 83 % in middle school, high school and college students, respectively. According to a recent report( 23 ), most American children in both southern (35°N) and northern (45°N) regions may not be getting adequate outdoor UVB exposures to satisfy their vitamin D needs all year. Although our study was limited because UVB exposure was not recorded in the KNHANES data, we assumed that decreased after-school daylight hours for participants enrolled in higher school grades might contribute to insufficient UVB exposure and low vitamin D status.
We hypothesized that vitamin D intake is an important predictor of low vitamin D status. Unfortunately, the KNHANES database does not detail total daily vitamin D intake in foods and supplements. Thus, we included two dietary factors in our analysis: vitamin D supplementation and milk consumption, although this is not enough to assess total daily vitamin D intake. Milk consumption was an unadjusted risk factor for low vitamin D status, but was not a multivariate-adjusted risk factor for low vitamin D status. This may be attributable to the fact that most Korean adolescents consume non-fortified milk, rather than fortified milk( 24 ). Milk intake in the average Korean household is very low and most Korean adolescents drink non-fortified milk that is provided in their school lunches( 24 ), which contains 0·25 μg of cholecalcidiol/100 ml( 25 ). However, vitamin D supplementation was an independent predictor for a better vitamin D status. The seasonal variation in serum 25(OH)D concentration was smaller in participants who were taking vitamin D supplements than in those who were not. Whiting et al. ( 26 ) recently determined the prevalence of meeting the DRI and the role of vitamin D supplement use among Canadians aged 6–79 years. Vitamin D supplement users had significantly higher 25(OH)D concentrations than did non-users, and no seasonal differences were found( 26 ). In Korean adolescents living between latitudes 33° and 39°N, the use of vitamin D supplements may contribute to maintain a better 25(OH)D status with lower seasonal variation.
In our study, 90 % of obese adolescents were vitamin D deficient, which is consistent with recent observations reporting high rates of vitamin D deficiency in obese individuals( 22 , 27 ). Despite a marginal statistical significance, obese adolescents independently had a lower vitamin D status than lean or overweight adolescents in our analysis. Recent studies reported an independent inverse relationship between serum 25(OH)D concentrations and adiposity measurements, including BMI percentiles, total fat mass and visceral adipose tissue, after adjustment for covariates( 9 , 21 ). Adipose tissue can sequester lipid-soluble vitamin D, leading to reduced bioavailability. However, whether adiposity per se lowers vitamin D levels is still controversial.
Whereas the estimated Adequate Intake for vitamin D was 5 μg/d in 1997, the vitamin D Estimated Average Requirement and RDA published by the US Institute of Medicine in 2010 were increased to 10 μg/d and 15 μg/d to give 25(OH)D concentrations of 16 ng/ml and 20 ng/ml, respectively( 3 ). In strong contrast to these recommendations, the Korean Nutrition Society lowered the DRI to 5 μg/d in 2010( 14 ), based on a small amount of evidence about the effect of vitamin D on health outcomes in Korean adolescents and the assumption that exposure to sunlight guarantees sufficient vitamin D status. However, the high prevalence of vitamin D deficiency raises the question of whether daylight outdoor hours and/or the current 2010 DRI of vitamin D are enough to maintain a sufficient vitamin D status in Korean adolescents.
The present study was limited by a lack of information about daylight hours, use of sunscreen in the summer season, total vitamin D intake and total body and/or visceral adiposity. Moreover, supporting variables related to outcomes of vitamin D deficiency such as serum intact parathyroid hormone levels or bone parameters were not measured. Although the Rural Development Administration food composition tables provide the vitamin D content of foods( 24 ), there is no comprehensive nutrient database or validated FFQ in Korea. Although our study cannot make any recommendation regarding current DRI for daily vitamin D intake in Korean adolescents, these limitations need to be addressed in further studies to investigate accurate assessment of total daily vitamin D intake and its relationship with 25(OH)D and parathyroid hormone concentrations and health outcomes.
Conclusion
Vitamin D deficiency was highly prevalent in Korean adolescents, especially those in higher school grades. More than half of them were vitamin D deficient, even in summer. Winter season, higher education level and a lack of vitamin D supplementation were independent risk factors for low vitamin D status. Vitamin D supplementation may contribute to maintain a better vitamin D status with lower seasonal variation. Further studies are required to determine optimal vitamin D intakes to maintain sufficient vitamin D status for Korean adolescents.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflicts of interest: All authors have no conflicts of interest. Authors’ contributions: Y.A.L., H.Y.K., S.W.Y. and C.H.S. were responsible for the conception and design of the study. Y.A.L., H.H., J.Y.K. and H.J.K. contributed to prepare the data set. Y.A.L. and H.H. performed the statistical analysis. Y.A.L. and C.H.S. drafted the manuscript. All authors played a role in interpretation of the results and approved the final manuscript.
References
- 1. Adams JS & Hewison M (2010) Update in vitamin D. J Clin Endocrinol Metab 95, 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holick MF & Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87, issue 4, 1080S–1086S. [DOI] [PubMed] [Google Scholar]
- 3. Ross AC, Manson JE, Abrams SA et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen CJ (2011) Clinical practice. Vitamin D insufficiency. N Engl J Med 364, 248–254. [DOI] [PubMed] [Google Scholar]
- 5. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 6. Kumar J, Muntner P, Kaskel FJ et al. (2009) Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124, e362–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon CM, DePeter KC, Feldman HA et al. (2004) Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158, 531–537. [DOI] [PubMed] [Google Scholar]
- 8. Weng FL, Shults J, Leonard MB et al. (2007) Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86, 150–158. [DOI] [PubMed] [Google Scholar]
- 9. Dong Y, Pollock N, Stallmann-Jorgensen IS et al. (2010) Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125, 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rockell JE, Green TJ, Skeaff CM et al. (2005) Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 years. J Nutr 135, 2602–2608. [DOI] [PubMed] [Google Scholar]
- 11. Absoud M, Cummins C, Lim MJ et al. (2011) Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One 6, e22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foo LH, Zhang Q, Zhu K et al. (2009) Relationship between vitamin D status, body composition and physical exercise in adolescent girls in Beijing. Osteoporos Int 20, 417–425. [DOI] [PubMed] [Google Scholar]
- 13. Choi HS, Oh HJ, Choi H et al. (2011) Vitamin D insufficiency in Korea – a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 96, 643–651. [DOI] [PubMed] [Google Scholar]
- 14. Ryoo E (2011) Adolescent nutrition: what do pediatricians do? Korean J Pediatr 54, 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moon JS, Lee SY, Nam CM et al. (2008) 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr 51, 1–25. [Google Scholar]
- 16. US Department of Health and Human Services (2008) 2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy! http://www.health.gov/paguidelines (accessed February 2013).
- 17. Korea Centers for Disease Control and Prevention (2009) The Statistics of 5th Korea Youth Risk Behavior Web-based Survey (KYRBWS) in 2009. http://yhs.cdc.go.kr/ (accessed March 2011).
- 18. Sempos CT, Vesper HW, Phinney KW et al. (2012) Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 243, 32–40. [DOI] [PubMed] [Google Scholar]
- 19. Korea Meteorological Administration (2012) 2008–2009 Climatic data. http://www.kma.go.kr/weather/climate/average_historic.jsp (accessed February 2013).
- 20. Kremer R, Campbell PP, Reinhardt T et al. (2009) Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson ML, Maalouf NM, Oden JD et al. (2012) Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 97, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park KW (2006) A study on adolescents’ daily leisure activities and use of cultural facilities. Culture Tourism Res 8, 7–24. [Google Scholar]
- 23. Godar DE, Pope SJ, Grant WB et al. (2012) Solar UV doses of young Americans and vitamin D3 production. Environ Health Perspect 120, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Rural Resources Development Institute (2006) Food composition table, 7th revision. http://lib.rda.go.kr/newlib/index.asp (accessed June 2012).
- 25. Kim JH (2007) Analysis of the awareness of the value and the consumption pattern on milk of elementary middle and high school students. J Korean Acad Fam Med 45, 23–33. [Google Scholar]
- 26. Whiting SJ, Langlois KA, Vatanparast H et al et al. (2011) The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 94, 128–135. [DOI] [PubMed] [Google Scholar]
- 27. Alemzadeh R, Kichler J, Babar G et al. (2008) Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 57, 183–191. [DOI] [PubMed] [Google Scholar]