Abstract
Objective
The most accurate method to estimate Na and K intakes is to determine 24 h urinary excretions of these minerals. However, collecting 24 h urine is burdensome. Therefore it was studied whether spot urine could be used to replace 24 h urine samples.
Design
Participants collected 24 h urine and kept one voiding sample separate. Na, K and creatinine concentrations were analysed in both 24 h and spot urine samples. Also 24 h excretions of Na and K were predicted from spot urine concentrations using the Tanaka and Danish methods.
Setting
In 2011 and 2012, urine samples were collected and brought to the study centre at Wageningen University, the Netherlands.
Subjects
Women (n 147) aged 19–26 years.
Results
According to p-aminobenzoic acid excretions, 127 urine collections were complete. Correlations of Na:creatinine, K:creatinine and Na:K between spot urine and 24 h urine were 0·68, 0·57 and 0·64, respectively. Mean 24 h Na excretion predicted with the Tanaka method was higher (difference 21·2 mmol/d, P<0·001) than the measured excretion of 131·6 mmol/d and mean 24 h Na excretion predicted with the Danish method was similar (difference 3·2 mmol/d, P=0·417) to the measured excretion. The mean 24 h K excretion predicted with the Tanaka method was higher (difference 13·6 mmol/d, P<0·001) than the measured excretion of 66·8 mmol/d. Bland–Altman plots showed large individual differences between predicted and measured 24 h Na and K excretions.
Conclusions
The ratios of Na:creatinine and K:creatinine in spot urine were reasonably well associated with their respective ratios in 24 h urine and appear to predict mean 24 h Na excretion of these young, Caucasian women.
Keywords: Urine, Sodium, Potassium, Monitoring, Prediction methods
A high intake of Na and a low intake of K are both associated with increased risks for elevated blood pressure, CVD and all-cause mortality( 1 , 2 ). Decreasing Na intake and increasing K intake could contribute to the prevention of hypertension( 3 ) and therefore has the potential to reduce risk of CVD. Although much research has been conducted on improving dietary intake of Na, people still have a higher Na intake than the recommended amount of 2 g/d( 4 , 5 ). High intakes are explained mainly by the contribution of salt from processed or restaurant-prepared foods( 5 ). For K, the majority of European women do not comply with the dietary recommendation of at least 3510 mg/d( 6 , 7 ). K intakes may be increased by consuming diets high in fresh fruits and vegetables( 6 ). These undesirable population intakes of Na and K indicate that new interventions are needed. Easy and reliable methods for monitoring and evaluating effects of these interventions are of interest.
To assess intakes of Na and K, self-reports of dietary intake or 24 h urine collections can be used( 8 , 9 ). Because self-reports are subjective and prone to errors, the most accurate method is to determine excretions of Na and K in 24 h urine samples. Unfortunately, collecting 24 h urine is a burdensome task, which might lead to incomplete collections( 10 , 11 ). Therefore, it would be much more convenient if Na and K concentrations in single urine samples, so-called spot urine samples, could be used as indicators of 24 h Na and K excretions.
The use of spot urine to replace 24 h urine collection has been studied for both Na and K in healthy populations, although most research has focused exclusively on Na( 10 – 16 ). Studies have shown good correlations between Na:creatinine and K:creatinine in spot urine and respective ratios in 24 h urine( 12 , 14 , 16 ). In addition, Brown and colleagues showed that β coefficients derived from regression analyses of spot urine samples v. 24 h urinary Na excretions can be used to predict 24 h excretions with reasonable accuracy( 15 ). These results indicate that spot urine has the potential to replace 24 h urine in epidemiological studies.
Also, methods to predict 24 h Na and K excretions from spot urine have been evaluated( 12 – 14 , 17 ). Tanaka and colleagues developed a method to predict both 24 h Na and K excretions from a spot urine sample and a Danish method was developed to predict only Na excretion( 12 , 13 ). With these methods, 24 h excretions are estimated from the Na:creatinine or K:creatinine ratios in spot urine and the predicted 24 h creatinine excretion. The predicted 24 h creatinine excretion is derived from an individual’s age, body weight and height. As these prediction methods have been developed in specific populations and include individuals’ characteristics, the methods might not perform similarly in different populations. To our knowledge, evaluation of these methods is limited to Asian and Danish populations.
Until now, there is still not enough evidence on the reliability of Na and K excretion from spot urine collections to replace 24 h urine collections for monitoring populations on adherence to a healthy diet( 16 ). Therefore, the associations between Na and K excretions in spot urine and 24 h urine in healthy, young, Caucasian women were investigated. We also studied the accuracy of predicting 24 h levels of urinary Na and K excretions from spot urine samples using the Tanaka and Danish methods. Based on literature, it was expected to find correlations in a range of 0·50 to 0·80 between Na concentrations in spot and 24 h urine( 16 ). Thereby, it was hypothesized that 24 h Na excretion can be predicted with reasonable accuracy from spot urine samples. It was assumed that the Danish( 13 ) method would perform better in young, Caucasian women than the Tanaka method( 12 ) because the Danish method was developed in a European population and includes gender-specific equations. For K, we did not have clear expectations since spot urine K has only been studied in a Japanese population( 12 ).
Methods
Participants
The participants were students taking a course on nutritional research methodology at Wageningen University. In May 2011 and in May 2012 respectively ninety-eight and ninety-seven students took part in the course, 160 of whom provided 24 h urine and spot urine collections. Because of the small number of men (n 13), only the data of the 147 women were used, of whom 138 reported birth date, height and weight.
Urine collection
Students were requested to collect 24 h urine in a 3-litre jug except for one spot urine sample, which the participants collected in a 500-ml jug. Collection of 24 h urine started after the first voiding in the morning and ended after the first voiding on the morning of the following day. Within these 24 h, participants were allowed to select any time for collection of spot urine except for the first morning urine, because of lower urinary Na and K excretions during the night( 18 ). Participants recorded the time of spot urine collection, whether the 24 h urine collection was incomplete and the use of specific medications or supplements that might interfere with the laboratory analysis. Urinary excretions of p-aminobenzoic acid (PABA) were used to check for completeness of 24 h urine collections( 19 ). For this purpose, participants took a tablet containing 80 mg PABA at three occasions, i.e. breakfast, lunch and dinner.
Chemical analysis
Participants brought both urine jugs to the laboratory of the Division of Human Nutrition of Wageningen University. The jugs were weighed and after homogenization of the contents, two 4-ml urine samples were taken from each jug that were immediately frozen and kept at −20°C. Thereafter, the empty jugs were weighed to calculate the net weight of the collected urine. Urinary Na and K were determined by an ion-selective electrode on a Synchron LX20 analyser (Beckman Coulter, Mijdrecht, the Netherlands). Urinary creatinine concentrations were determined at 520 nm on the Synchron LX20 by the modified Jaffé procedure using a commercial kit. PABA was determined with two different methods. In 2011, the colorimetric diazocoupling method( 19 ) was used to determine the total aromatic amines derived from PABA and its metabolites by means of UV–visible spectrophotometry at 540 nm (Jasco V-530 UV/VIS spectrophotometer; Jasco Inc., Easton, MD, USA). In 2012, HPLC( 20 ) was used to quantify the peak area of PABA. The HPLC system (Thermo Separation Products Spectra; Thermo Scientific Inc., Waltham, MA, USA) was equipped with a binary pump (model P2000), a solvent degasser (model SCM400), a temperature-controlled auto sampler (model AS3000), a UV–visible forward optical scanning detector (model UV3000), and control and integration software (Chromquest 5·0).
Check using p-aminobenzoic acid
The 24 h PABA excretions were calculated by adding the amount of PABA determined in the spot urine to the amount of PABA determined in the 3-litre jug. Seven women had PABA recoveries below the cut-off value of 85 % for colorimetric assay( 19 ) and three women had PABA recoveries below the cut-off value of 78 % for HPLC( 20 ). The data of these ten women and ten women who reported having incomplete urine collections were excluded from data analyses.
Predicting 24 h Na and K excretion
Both the Tanaka method and the Danish method were used to predict 24 h Na excretions and only the Tanaka method could predict K excretions( 12 , 13 ). The method of Tanaka was developed using urine collections of 591 Japanese participants aged 20–59 years, of whom 50 % were women( 12 ). Participants collected spot urine just before starting 24 h urine collection. Participants were interviewed to assess whether 24 h urine collections were complete. Na and K concentrations were determined by emission flame photometry and creatinine concentrations by the Jaffé method. The Danish method was developed using urine collections of 473 Danish participants aged 28–74 years, of whom 78 % were women( 13 ). Spot urine was collected on a separate day within two weeks of the 24 h urine collection. Complete 24 h urine collections with PABA recoveries of 78 % or more determined with HPLC were included. Na and creatinine concentrations were determined using Na and creatinine slides and Vitros Chemistry Products calibrator kits on a Vitros 250 chemistry system.
The prediction methods were developed in two steps. First, regression analysis was used to predict 24 h urinary creatinine excretions from age, sex, weight and height. Then a second regression analysis was performed to predict 24 h Na or K excretions. For this analysis, the 24 h urinary excretions were used as outcome measures and the Na:creatinine or K:creatinine, predicted 24 h urinary excretions of creatinine, age, weight and height as independent variables. The equations were transformed into the natural logarithm and the final model was obtained by using the exponential function. This led to the following equations(12,13):
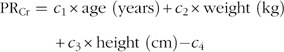 |
and
where PRCr=predicted 24 h creatinine excretion (mg/24 h), c 1 to c 6 are coefficients differing between methods and between minerals( 12 , 13 ), SUNa=spot urine Na (mmol/l), SUCr=spot urine creatinine (mg/l) and PRNa=predicted 24 h Na excretion (mmol/24 h). In the Tanaka method, spot urine Na is replaced by spot urine K to predict 24 h K excretion.
Data analyses
Data were analysed with the statistical software package IBM SPSS Statistics 20. From weight and height, BMI in kg/m2 was calculated. Pearson correlation coefficients between spot and 24 h urine were estimated for Na:creatinine, K:creatinine and Na:K in participants with complete urine collections (n 127). Predictions of 24 h Na and K excretion were only done for participants with complete data on age, weight and height, and complete urine collections (n 118). Student’s t test was used to test for mean differences between the measured and predicted 24 h Na and K excretions based on spot urine samples. Two-tailed probability levels for statistical tests were reported and P<0·05 was considered to be statistically significant. To evaluate individual differences Bland–Altman plots were made, in which the differences between the predicted and measured 24 h Na or K excretions were plotted against the average of the two methods( 21 ). Regression analyses were done to obtain equations estimating 24 h urinary Na and K excretions based on spot urine Na or K concentrations, spot urine creatinine concentrations, BMI and age.
Results
Participants were aged between 19 and 26 years (Table 1). The mean weight of the 24 h urine was 1820 (sd 645) g and the mean weight of the spot urine samples was 250 (sd 125) g. Five participants collected their spot urine samples in the morning (07.00 to 12.00 hours), 100 participants in the afternoon (12.00 to 18.00 hours) and thirty-four participants in the evening (18.00 to 24.00 hours). The remaining eight participants did not report the time of spot urine collection.
Table 1.
Descriptive characteristics of the participants; women (n 138*) aged 19–26 years, Wageningen, the Netherlands
| Characteristic | Mean | sd |
|---|---|---|
| Age (years) | 20·2 | 1·1 |
| Height (cm) | 171·3 | 6·7 |
| Weight (kg) | 63·1 | 7·8 |
| BMI (kg/m2) | 21·5 | 2·3 |
Number includes only those women with data on birth date, height and weight.
The correlations between Na:creatinine, K:creatinine and Na:K in spot urine and those in 24 h urine were 0·68, 0·57 and 0·64, respectively (for all correlations P<0·001).
The mean predicted and measured 24 h Na and K excretions are presented in Table 2. The mean 24 h Na excretion predicted with the Tanaka method was 21·2 mmol higher (P<0·001) than the measured 24 h Na excretion. The prediction of mean 24 h Na excretion with the Danish method was not significantly different from the measured excretion (P=0·417). The estimated mean 24 h excretion of K, only predicted with the Tanaka method, was 13·6 mmol lower (P<0·001) than the measured excretion. Finally, the measured 24 h creatinine was overestimated by the Tanaka method (P<0·001) and underestimated by the Danish method (P=0·004).
Table 2.
Comparison of 24 h levels of sodium, potassium and creatinine measured in 24 h urine and predicted from spot urine with the Tanaka( 12 ) or Danish( 13 ) prediction method; women (n 118) aged 19–26 years, Wageningen, the Netherlands
| Measured 24 h urine | Tanaka prediction method | Danish prediction method* | ||||
|---|---|---|---|---|---|---|
| Mean | sd | Mean or Mean difference or Correlation coefficient | sd or 95 % CI | Mean or Mean difference or Correlation coefficient | sd or 95 % CI | |
| Na (mmol/d)† | 131·8 | 50·9 | 153·1 | 37·4 | 135·0 | 16·6 |
| Mean difference (mmol/d)‡, measured – predicted | – | – | −21·2 | −28·4, −14·1 | −3·2 | −10·9, 4·6 |
| Correlation coefficient§, measured/predicted | – | – | 0·64 | – | 0·62 | – |
| K (mmol/d)† | 66·8 | 24·8 | 53·2 | 12·5 | – | – |
| Mean difference (mmol/d)‡, measured – predicted | – | – | 13·6 | 9·9, 17·3 | – | – |
| Correlation coefficient§, measured/predicted | – | – | 0·58 | – | – | – |
| Creatinine (mmol/d)† | 11·7 | 2·2 | 12·5 | 1·7 | 11·2 | 0·8 |
| Mean difference (mmol/d)‡, measured – predicted | – | – | −0·8 | −1·2, −0·5 | 0·5 | 0·2, 0·9 |
| Correlation coefficient§, measured/predicted | – | – | 0·49 | – | 0·53 | – |
The Danish method does not provide coefficients to predict K.
Data presented are means and standard deviations.
Data presented are mean differences with 95 % confidence intervals.
Data presented are correlation coefficients; all P<0·001 (two-tailed).
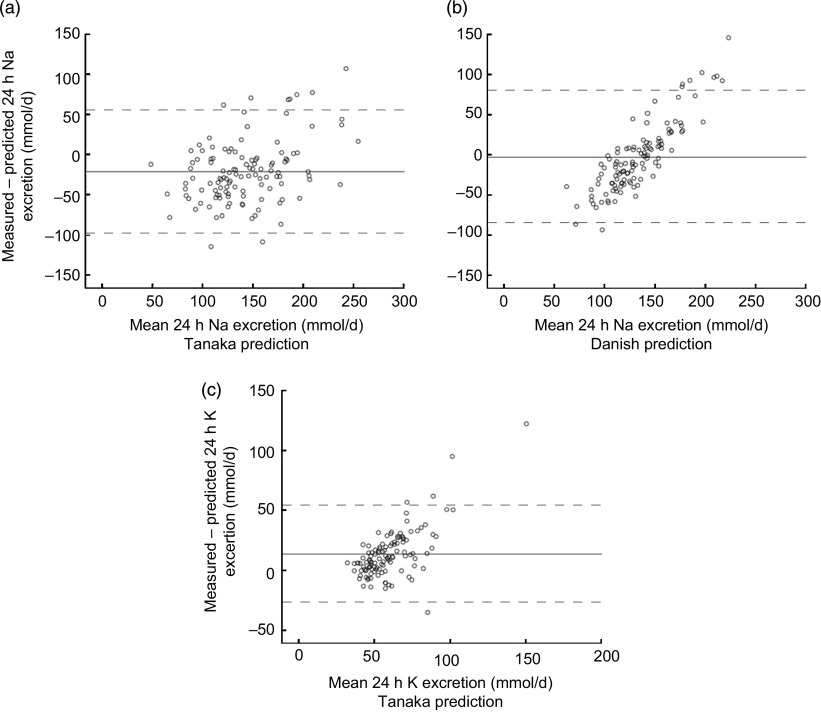
The Bland–Altman plots (Fig. 1) showed that the limits of agreement were −98 to 56 mmol/d for Na predicted with the Tanaka method and −87 to 80 mmol/d for Na predicted with the Danish method. For K, the limits of agreement were −26 to 53 mmol/d predicted with the Tanaka method. In addition, the plots showed an increasing underestimation of 24 h Na excretions predicted from spot urine samples with increasing measured 24 h Na excretions.
Fig. 1.
Bland–Altman plots showing differences between the measured and predicted 24 h sodium or potassium excretions plotted against the mean of the two methods: (a) sodium excretion and Tanaka prediction; (b) sodium excretion and Danish prediction; (c) potassium excretion and Tanaka prediction. ——— indicates the mean bias; — — — indicates the 95 % limits of agreement
Regression analyses provided equations for Na and K excretions (Table 3). The β for spot urine Na was 0·53 (95 % CI 0·34, 0·71) and the β for spot urine K was 0·54 (95 % CI 0·29, 0·78). Age did not significantly contribute to the regression model and was therefore excluded.
Table 3.
Results of the linear regression models of the association between 24 h sodium and potassium with spot urine sodium or potassium, spot urine creatinine and BMI; women (n 118) aged 19–26 years, Wageningen, the Netherlands
| Na* | K† | |||
|---|---|---|---|---|
| Variable | β | 95 % CI | β | 95 % CI |
| Intercept | 12·08 | −64·91, 89·06 | 47·76 | 0·09, 95·43 |
| Spot urine Na (mmol/l) | 0·53 | 0·34, 0·71 | N/A | N/A |
| Spot urine K (mmol/l) | N/A | N/A | 0·54 | 0·29, 0·78 |
| Spot urine creatinine (mmol/l) | −4·21 | −5·77, −2·65 | −2·67 | −3·92, −1·42 |
| BMI (kg/m2) | 5·23 | 1·67, 8·79 | 0·66 | −1·51, 2·83 |
N/A, not applicable.
Adjusted R 2=0·28.
Adjusted R 2=0·14.
Discussion
It was studied whether excretions of Na and K in spot urine samples could be used as indicators of 24 h urinary Na and K excretions, and thus of intake, in healthy, young, Caucasian women. The present study showed good correlations between Na:creatinine, K:creatinine and Na:K in spot urine and 24 h urine. The Danish method provided a reasonably accurate estimate of mean 24 h Na excretion from spot urine samples. However, the Tanaka method presented less accurate estimates for both Na and K. The Bland–Altman plots showed large limits of agreement, indicating substantial individual differences between the predicted and measured 24 h Na and K excretions. Regression analyses presented significant contributions of BMI, spot urine creatinine and spot urine Na or K to 24 h urinary excretions of Na or K.
The observed correlations of 0·68 for Na:creatinine and 0·57 for K:creatinine between spot and 24 h urine are comparable to those of 0·65 and 0·67 found by Tanaka and colleagues( 12 ). Thereby, the correlations are in the range of 0·50 to 0·80 as hypothesized( 16 ). The observed β coefficients of 0·53 and 0·54 for spot urine Na and K, respectively, are higher than the β of 0·34 for spot urine Na that Brown and colleagues found in women( 15 ). Based on these findings, we suggest that the order of individuals according to both ratios and levels of Na and K in spot urine is similar to the respective ratios in 24 h urine. Therefore, ratios of Na and K in spot urine can be useful to monitor intake and assess adherence to a healthy diet.
The use of spot urine to predict mean 24 h Na and K excretions of populations was also evaluated. The measured mean Na excretion of 3035 mg/d equals a salt intake of about 8·2 g/d when assuming a urinary Na excretion of 95 %( 10 ). Consequently the women in the present study exceeded the maximum recommended salt consumption of 5 g/d( 4 ); nevertheless their Na consumption is comparable to that of other Caucasian women( 5 ). The Tanaka method overestimated the mean Na excretion by 16 % and the Danish method more accurately estimated Na as compared with the measured excretion. These findings indicate that the Danish prediction method is the preferred method in the present study population.
The mean K intake was 3383 mg/d, when assumed that 77 % of K is excreted in urine( 22 ). The mean intake is thus slightly lower than the recommended intake level( 6 ), nevertheless the intake is comparable to that in European women( 7 ). With the Tanaka method a 20 % underestimation of mean 24 h K excretion was found, which is higher than the 8 % underestimation that Tanaka and colleagues found. These results indicate that it was not possible to accurately predict 24 h K excretion.
The results of the present study indicate that spot urine Na and K excretions have the potential to replace 24 h urine samples on a population level. First, the advantage of using Na:creatinine, K:creatinine and Na:K ratios to place individuals in order is that the data can be used without using regression or prediction models. However, ratios might be more difficult to interpret than levels. Second, developing a regression model on data of a part of the study population provides estimates of 24 h Na and K excretions and will have the best fit on those specific data. The disadvantage is that additional 24 h urine samples are needed from a subpopulation. Third, using the Tanaka or Danish prediction method provides 24 h Na or K estimates without collecting 24 h urine, but these methods may not provide the best estimate for all study populations.
The wide limits of agreement of the Bland–Altman plots indicate that spot urine cannot be used to accurately predict 24 h Na and K excretions of individuals( 21 ). These wide limits could be expected since spot urine represents Na and K excretions at one point in time. Excretions are influenced by circadian rhythms and are directly related to intake from meals and exercise; therefore, the excretions are not constant within 24 h( 14 , 23 ). Also other studies indicated that spot urine samples cannot be used to predict 24 h Na and K excretions of individuals( 12 , 13 , 23 ).
The Bland–Altman plots showed that with increasing levels of measured 24 h Na excretion both prediction methods had a tendency to increase underestimation. This excretion-related underestimation was also shown in the studies of Tanaka et al. and Toft et al.( 12 , 13 ), which indicates that for populations with higher mean 24 h excretions of Na, e.g. populations including men, the prediction methods might become less accurate.
The Danish prediction method gave more accurate results for estimating 24 h Na excretion than the Tanaka method. First of all, this might be due to the comparability of the mean Na excretions between the investigated populations. In the present population a mean excretion of 132 mmol/d was observed which was closer to the mean excretion of 139 mmol/d in the Danish women( 13 ) than to the 187 mmol/d in the Japanese population( 12 ). Second, the present study only included women. It is known that Na and K intakes( 24 ) and creatinine excretions( 25 ) are gender dependent and therefore the Danish method including gender-specific equations might have performed better than the Tanaka method that combined values for men and women.
The PABA concentrations in the urine samples were measured with two different methods: the colorimetric diazocoupling method and HPLC. The first method might lead to higher estimated PABA excretion by co-determination of aromatic enzymes, whereas the second method might lead to lower estimated PABA excretion because not all PABA metabolites in the urine are converted to PABA( 19 , 20 , 26 ). However, differences in estimation between the methods were taken into account by using the method-specific cut-off values for completeness of urine collection. It is expected that the use of two different methods for PABA analyses did not affect the results.
The strength of the present study is that it adds evidence to the limited number of studies investigating the association between spot and 24 h urine Na and K excretions in healthy participants( 16 ), especially as our study is the first in reporting findings in healthy, young, Caucasian women. However, the study also had some limitations. First, due to the small sample size it was not possible to develop and test regression models to predict 24 h Na and K excretions. Second, the study was conducted in generally healthy, young, Caucasian women and therefore the outcomes cannot be generalized to other populations. Populations with different age, body weight and height might provide a different accuracy of the prediction methods. Third, the effect of timing of spot urine collection was not evaluated. This might be of major importance as Na and K excretions vary considerably during the day( 23 ).
There is a growing body of literature on the use of spot urine collections to replace 24 h urine collections. This indicates that there is a need for methods that are less burdensome for participants. Although the spot urine method associates well with the 24 h method, it is not as accurate as the 24 h urine collection method to estimate daily Na and K excretions. Thus, the 24 h urine collection method remains the preferred tool. To reduce the burden of 24 h urine collection for participants, and at the same time increase the accuracy of estimating daily Na and K intakes, we suggest examining the association between multiple spot urine collections and 24 h urine collections in future studies.
Conclusion
In conclusion, the ratios Na:creatinine, K:creatinine and Na:K in spot urine samples were reasonably well associated with the respective ratios in 24 h urine samples in young, Caucasian women and may be useful to evaluate intake of these minerals as a replacement for 24 h urine collection. Also, spot urine samples can be used to predict mean 24 h Na excretion of this population with reasonable accuracy using the Danish prediction method.
Acknowledgements
Financial support: The work was supported by Wageningen University, Division of Human Nutrition, the Netherlands (no grant) and by the Netherlands Organization for Health Research and Development (ZonMW), The Hague (grant number 115100007). Both funders supported parts of the study design, conduct of the study, analysis of samples or data, interpretation of findings or the preparation of the manuscript. P.J.M.H. was funded by Wageningen University and the other authors by ZonMw. Conflict of interest: None. Authorship: E.J.C.H.v.H., P.J.M.H. and J.H.M.d.V. the designed research; E.J.C.H.v.H. conducted the research and analysed the data; all authors contributed to the research and wrote the paper; J.H.M.d.V. had primary responsibility for final content. All authors were involved in formulating research questions and all read and approved the final manuscript. Ethics of human subject participation: Ethical approval was not required. The researchers considered that the research did not fall under the remit of the Dutch Medical Research Act.
References
- 1. Yang Q, Liu T, Kuklina E et al. (2011) Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 171, 1183–1191. [DOI] [PubMed] [Google Scholar]
- 2. Rose G, Stamler J, Stamler R et al. (1988) Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geleijnse JM, Kok FJ & Grobbee DE (2003) Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 17, 471–480. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (2012) Guideline: Sodium Intake for Adults and Children. Geneva: WHO. [PubMed] [Google Scholar]
- 5. Brown IJ, Tzoulaki I, Candeias V et al. (2009) Salt intakes around the world: implications for public health. Int J Epidemiol 38, 791–813. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization (2012) Guideline: Potassium Intake for Adults and Children. Geneva: WHO. [PubMed] [Google Scholar]
- 7. Welch AA, Fransen H, Jenab M et al. (2009) Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr 63, Suppl. 4, S101–S121. [DOI] [PubMed] [Google Scholar]
- 8. Bentley B (2006) A review of methods to measure dietary sodium intake. J Cardiovasc Nurs 21, 63–67. [DOI] [PubMed] [Google Scholar]
- 9. Bingham SA, Cassidy A, Cole TJ et al. (1995) Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br J Nutr 73, 531–550. [DOI] [PubMed] [Google Scholar]
- 10. Bates C, Thurnham D & Bingham S (1997) Biochemical markers of nutrient intake. In Design Concepts in Nutritional Epidemiology, 1st ed., pp. 192–265 [BM Margetts and M Neslon, editors]. Oxford: Oxford University Press. [Google Scholar]
- 11. Ilich JZ, Blanuša M, Orlić ŽC et al. (2009) Comparison of calcium, magnesium, sodium, potassium, zinc, and creatinine concentration in 24-h and spot urine samples in women. Clin Chem Lab Med 47, 216–221. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T, Okamura T, Miura K et al. (2002) A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 16, 97–103. [DOI] [PubMed] [Google Scholar]
- 13. Toft U, Cerqueira C, Andreasen AH et al. (2013) Estimating salt intake in a Caucasian population: can spot urine substitute 24-hour urine samples? Eur J Prev Cardiol (Epublication ahead of print version). [DOI] [PubMed] [Google Scholar]
- 14. Mann SJ & Gerber LM (2010) Estimation of 24-hour sodium excretion from spot urine samples. J Clin Hypertens 12, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown IJ, Dyer AR, Chan Q et al. (2013) Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol 177, 1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji C, Sykes L, Paul C et al. (2012) Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica 32, 307–315. [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki T, Uezono K, Itoh K et al. (1991) Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Jpn J Public Health 38, 567–574. [PubMed] [Google Scholar]
- 18. Manchester RC (1933) The diurnal rhythm in water and mineral exchange. J Clin Invest 12, 995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bingham S & Cummings JH (1983) The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci (Lond) 64, 629–635. [DOI] [PubMed] [Google Scholar]
- 20. Jakobsen J, Ovesen L, Fagt S et al. (1997) Para-aminobenzoic acid used as a marker for completeness of 24 hour urine: assessment of control limits for a specific HPLC method. Eur J Clin Nutr 51, 514–519. [DOI] [PubMed] [Google Scholar]
- 21. Bland JM & Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310. [PubMed] [Google Scholar]
- 22. Holbrook JT, Patterson KY, Bodner JE et al. (1984) Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 40, 786–793. [DOI] [PubMed] [Google Scholar]
- 23. Wang CY, Cogswell ME, Loria CM et al. (2013) Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr 143, 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Lee L, Geelen A, Hooft van Huysduynen EJ et al. (2012) The Dutch Healthy Diet index (DHD-index): an instrument to measure adherence to the Dutch Guidelines for a Healthy Diet. Nutr J 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narayanan S & Appleton HD (1980) Creatinine: a review. Clin Chem 26, 1119–1126. [PubMed] [Google Scholar]
- 26. Johansson G, Bingham S & Vahter M (1999) A method to compensate for incomplete 24-hour urine collections in nutritional epidemiology studies. Public Health Nutr 2, 587–591. [DOI] [PubMed] [Google Scholar]