Abstract
Objective
Traditional Inuit dietary patterns have been found to be beneficial for CVD but have not been investigated in relation to glucose intolerance. We examined the association between dietary patterns and type 2 diabetes mellitus (T2DM), impaired glucose tolerance (IGT) and impaired fasting glucose (IFG).
Design
Cross-sectional design with a priori derived dietary patterns from an FFQ resulted in five patterns: imported meat (n 196), traditional food (n 601), balanced diet (n 126), unhealthy diet (n 652) and standard diet (n 799).
Setting
Associations between dietary patterns and glucose-related outcomes were tested by linear and logistic regression analyses. Data included: dietary intake by FFQ, waist circumference, ethnicity, frequency of alcohol intake and smoking, physical activity, and oral glucose tolerance test results. Fasting participants and those without diagnosed T2DM were classified into normal glucose tolerance, IGT, IFG or T2DM. HOMA-IR (homeostatic model assessment–insulin resistance index) and HOMA-β (homeostatic model assessment of β-cell function) were calculated.
Subjects
Data included 2374 Inuit, aged 18+ years.
Results
Participants with a traditional dietary pattern had higher fasting plasma glucose (mean 5·73 (95 % CI 5·68, 5·78) mmol/l, P < 0·0001) and lowest HOMA-β (48·66 (95 % CI 46·86, 50·40), P < 0·0001). The traditional diet gave significantly higher odds for IFG and T2DM than the balanced diet, imported meat diet, standard diet and unhealthy diet.
Conclusions
Traditional food was positively associated with T2DM, IFG and fasting plasma glucose, and negatively associated with β-cell function, compared with a standard diet. The imported meat diet seemed the best in relation to glucose intolerance, with lowest fasting plasma glucose and lowest odds for IFG and T2DM.
Keywords: Type 2 diabetes mellitus, Inuit, Dietary patterns, Glucose intolerance
Inuit populations in the Arctic have gone through a nutritional transition from high consumption of traditional food, mostly consisting of marine mammals and fish, to a diet high in Western foods often with high contents of sugar and saturated fat( 1 ). At the same time, obesity has increased rapidly all over the Arctic followed by high prevalence of type 2 diabetes mellitus (T2DM) and impaired glucose tolerance (IGT)( 2 ).
The relationship between glucose intolerance and traditional food has been studied before. Former research conducted in Greenland found that fasting blood glucose was positively associated with consumption of seal( 3 ). Another study found that daily consumption of seal and salmon was associated with a lower prevalence of IGT and T2DM among Alaskan Natives( 4 ). These results are contradictory, but indicate that dietary factors may influence T2DM among the Inuit. The traditional diet is high in n-3 fatty acids due to a high content of marine mammals and fish. It has been shown that the fatty acid profile of the diet influences insulin sensitivity and insulin resistance( 5 ). Fish oil supplementation and consumption of fatty fish with high n-3 fatty acid content improved insulin sensitivity( 6 , 7 ). However, moderate fish oil supplementation showed no effect on insulin sensitivity or β-cell function in healthy individuals( 8 ). Furthermore, insulin sensitivity was not influenced by increasing the dietary n-3:n-6 ratio( 9 ). Other dietary factors influence the risk of T2DM as well. A dietary pattern characterized by red and processed meat, French fries, high-fat dairy products, refined grains and sweets resulted in a higher risk of T2DM( 10 ). A similar dietary pattern also with red and processed meat and refined grains was associated with an 18 % greater risk for T2DM( 11 ). Adherence to a diet-and-lifestyle recommendation index showed lower insulin levels among elderly Puerto Ricans( 12 ). Eilat-Adar et al. showed that a traditional dietary pattern among Alaskan Eskimos was associated with a more favourable cardiovascular profile( 13 ). However, dietary patterns, specifically a traditional Inuit dietary pattern, have not yet been evaluated in relation to T2DM. The aim of the present study was to investigate the association between a priori derived dietary patterns (predefined) and various glucose metabolism outcomes, insulin resistance and β-cell function, with a focus on the association between a traditional dietary pattern and T2DM and pre-diabetic stages among Inuit.
Methods
Data for the present cross-sectional study were collected from 2005 to 2010. In total, nine towns and thirteen villages all over Greenland were included. The study was approved by the ethical review committee for Greenland. Participants were informed by letter prior to data collection and oral and written information was given by the beginning of data collection. All participants signed informed consent.
Participants
Participants were selected through a random sampling of adults (aged 18+ years) with residence in Greenland. Greenland was divided into twelve regions. From each region we chose a number of villages and towns to be included in the study. In towns a random sample of 11–22 % was drawn from the central personal register. In villages, all adult inhabitants were invited to participate. The total sample selected from the central personal register consisted of 5009 individuals, both Inuit and Danes. The final sample included in the analyses was only of Inuit ethnicity. With a participation rate for Inuit of 68 %, the total study sample consisted of 3108 Inuit included in the final data set. Clinical information was available for 99 % of Inuit participants. Questionnaires were translated from Danish into Greenlandic and translated back into Danish in order to validate the translation. Interviews were conducted in Greenlandic or Danish according to the wish of the participant. Ethnicity was determined at enrolment based on the primary language of the participant and self-identification. Only one ethnicity was allowed for each participant. We also recorded the ethnicity of parents and grandparents of the participant, based on questionnaire information.
Confounders
We defined potential confounders from former research and tested crude associations by general linear models before confounders were added to the final model. The final model included sex, age, total energy intake (kJ/d), alcohol consumption, smoking, physical activity and ethnicity. The participants who were enrolled in the study as Inuit based on language or self-identification were further classified as partly Inuit or full Inuit characterized from history of the grandparents’ ethnicity: if one to three of the four grandparents were of Inuit heritage, the participant was defined as partly Inuit; if all four grandparents were Inuit, the participant was defined as having full Inuit ethnicity.
We obtained information about smoking status, alcohol consumption and physical activity by interview and questionnaires. Participants were characterized as having never smoked, previous smokers or current smokers. Regarding alcohol consumption, participants were grouped into daily drinkers, weekly drinkers, monthly drinkers and abstainers. We measured physical activity by a modified version of the International Physical Activity Questionnaire (IPAQ)( 14 ). From this questionnaire we estimated the weekly number of minutes engaged in physical activity. Physical activity (min/week) was log-transformed to achieve normal distribution.
Dietary assessment
Dietary data were collected by an FFQ which included twenty-five traditional and forty-three imported energy-contributing food items. Frequency of consumption (the past month for traditional food and the past 12 months for imported food), estimated portion sizes and seasonal variation were reported. Portion sizes were estimated from four different serving sizes illustrated by photographs. For traditional foods we additionally recorded seasonal length (the availability) and took that into consideration when calculating the annual consumption. Missing information on portion size was substituted by gender-specific medians. Missing information on season length was substituted by community-specific medians. From published food tables we calculated intakes of energy and macronutrients( 15 – 17 ). Individuals who reported energy intake lower than 3350 kJ/d (men) and 2100 kJ/d (women) or higher than 17 000 kJ/d (men) and 15 000 kJ/d (women) were excluded from the analyses. We calculated the energy contribution from various food groups to the total energy intake (E%), which were used to define dietary patterns in the following. The full version of the questionnaire can be viewed elsewhere( 18 ).
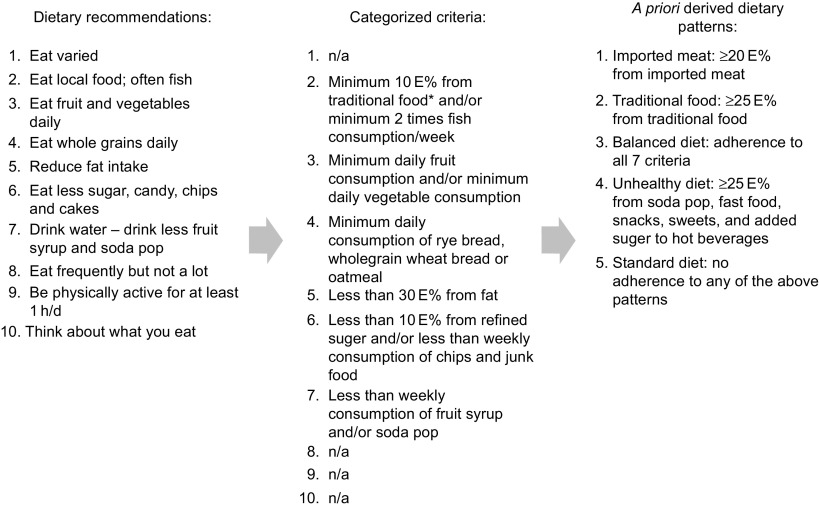
We identified five dietary patterns( 19 ). The standard diet was characterized by <25 E% from traditional food items (marine mammals and fish, caribou, musk ox), <20 E% from imported red meat and <25 E% from ‘unhealthy food items’ (fast food, snacks, sweets and soda pops), but yet not living up to the recommendations of the Nutritional Board of Greenland for a balanced diet. The balanced diet complied with at least seven of the nine recommendations by the Nutritional Board of Greenland. The imported meat diet had ≥20 E% from imported red meat (but <25 E% from traditional food items and <25 E% from unhealthy food items); the traditional diet had ≥25 E% from traditional food items (but <25 E% from unhealthy food items); the unhealthy diet had ≥25 E% from unhealthy food items. The process from dietary recommendations to the a priori derived dietary patterns is shown in Fig. 1. In a previous publication we developed the a priori dietary patterns and compared them with a data-driven approach (factor analysis/cluster analysis). We found that the two approaches resulted in overlapping dietary patterns, although still different from each other, since the traditional dietary pattern from the data-driven approach was very high in sugar and in the a priori approach it was more strictly defined based on the content of traditional food( 19 ). Our a priori derived dietary patterns were to a larger extent applicable to dietary interventions and to public health messages than the data-driven dietary patterns, and for this reason the present study uses the a priori dietary patterns.
Fig. 1.
The ten food-based dietary guidelines outlined by the Greenland Nutrition Board and the criteria upon which the a priori derived dietary patterns were based (*traditional food is defined as seal, whale, walrus, fish caught in open water, polar bear, musk ox, reindeer, wild fowls and berries; n/a, not applicable; E%, energy percentage)
Clinical measures
After minimum 8 h of fasting, the participants underwent a 2 h oral glucose tolerance test in which they received 246·5 ml (333·3 mg/ml) of glucose monohydrate equivalent to 75 g of glucose. Blood was drawn from the cubital vein at fasting state and 2 h after glucose intake. Plasma was separated, frozen at −20°C and transported to one central laboratory for measurement of plasma glucose using hexokinase/glucose-6-phosphate dehydrogenase determination on a Hitachi 912 Chemistry Analyser (Roche Diagnostics, Mannheim, Germany). Fasting insulin was measured using a two-site fluoroimmunometric assay for quantification of intact insulin in human serum (AutoDELFIA; Perkin Elmer, Waltham, MA, USA). Both glucose and insulin were analysed at Steno Diabetes Centre, Gentofte, Denmark. We defined impaired fasting glucose (IFG), IGT and T2DM according to the WHO criteria( 20 ): IFG was defined as fasting plasma glucose (FPG) >6·1 to 6·9 mmol/l; IGT as FPG <7·0 mmol/l and 2-h plasma glucose ≥7·8 to 11·1 mmol/l; finally T2DM was present when FPG ≥7·0 mmol/l or 2-h plasma glucose ≥11·1 mmol/l. The homeostatic model assessment–insulin resistance index (HOMA-IR) was used as a measure of insulin resistance and was calculated using the formula: HOMA-IR = [fasting insulin (pmol/l) × FPG (mmol/l)]/22·5. Higher values indicate insulin resistance. The homeostatic model assessment for β-cell function (HOMA-β) was used as a measure of β-cell function and was calculated using the formula: HOMA-β = [20 × fasting insulin (pmol/l)]/[FPG (mmol/l) − 3·5]. Higher values indicate good insulin secretion ability of the β cells. Waist circumference was measured on the standing participant midway between the rib and the iliac crest.
Statistical analyses
Data were analysed using the SPSS statistical software package version 19. Before analyses we excluded individuals with known T2DM, with missing data on physical activity, non-fasting individuals and individuals with unrealistic energy intake. A total of 784 participants were excluded and a further forty-seven participants were excluded in Table 3 due to missing values for 2-h plasma glucose measures. The baseline characteristics were tested by the χ 2 test (categorical variables) or general linear models (GLM for continuous variables). The association between dietary patterns and FPG, 2-h plasma glucose, fasting insulin, HOMA-IR and HOMA-β was tested by GLM with adjustment for sex, age, ethnicity (full or partial Inuit based on the ethnicity of the grandparents), waist circumference, total energy intake, physical activity and smoking status. Logistic regression analyses with forced entrance were used to analyse the association between dietary patterns and T2DM, IFG and IGT with the adjustments as above and furthermore we added the interaction between sex and dietary pattern. The association between T2DM and dietary patterns was limited to newly diagnosed cases. Energy intake (kJ/d) was log-transformed to achieve normal distribution. All clinical outcome variables were log-transformed to achieve normal distribution as well.
Table 3.
Mean values (and 95 % confidence intervals) of clinical parameters for the five dietary patterns among adult Inuit (n 2327, missing 47), Greenland, 2005–2010
| Imported meat | Traditional food | Balanced diet | Unhealthy diet | Standard diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n 192) | (n 591) | (n 123) | (n 636) | (n 785) | ||||||||||
| Mean | 95 % CI | P | Mean | 95 % CI | P | Mean | 95 % CI | P | Mean | 95 % CI | P | Mean | 95 % CI | |
| FPG (mmol/l) | 5·52 | 5·43, 5·59 | 0·3 | 5·73 | 5·68, 5·78 | 0·0001 | 5·61 | 5·50, 5·71 | 0·6 | 5·67 | 5·62, 5·71 | <0·0001 | 5·56 | 5·52, 5·59 |
| 2-h plasma glucose (mmol/l) | 5·47 | 5·22, 5·73 | 0·9 | 5·68 | 5·53, 5·84 | 0·03 | 5·55 | 5·22, 5·89 | 0·7 | 5·68 | 5·55, 5·84 | 0·03 | 5·46 | 5·34, 5·59 |
| Fasting insulin (pmol/l) | 39·6 | 37·1, 42·4 | 0·4 | 36·1 | 35·3, 38·1 | 0·04 | 37·1 | 34·1, 40·4 | 0·3 | 40·0 | 38·5, 41·5 | 0·2 | 38·7 | 37·6, 40·0 |
| HOMA-IR | 1·54 | 1·43, 1·65 | 0·3 | 1·44 | 1·38, 1·51 | 0·3 | 1·44 | 1·31, 1·58 | 0·5 | 1·56 | 1·51, 1·63 | 0·04 | 1·48 | 1·43, 1·54 |
| HOMA-β | 58·3 | 54·7, 62·1 | 0·2 | 48·7 | 46·9, 50·4 | <0·0001 | 52·2 | 48·1, 56·7 | 0·2 | 54·9 | 52·8, 56·8 | 0·4 | 55·8 | 54·1, 57·61 |
FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment–insulin resistance index; HOMA-β, homeostatic model assessment of β-cell function.
Analyses are adjusted for age, sex, ethnicity, waist circumference, physical activity, smoking and total energy intake. P values indicate the difference between the respective dietary pattern and the standard diet.
We checked the clinical variables for outliers and identified outliers using box plots. The HOMA-β variable was examined; we found negative values due to FPG ≤3·5 pmol/l, and these values were excluded as well. To include smoking in the model, we used dummy variables for the analyses in Table 3.
Results
A total of 3108 Inuit aged 18+ years (42 % men) participated (participation rate 68 %). The median age was 44 years (range 18–89 years). According to grandparents’ ethnicity, we characterized 13 % as having partly Inuit and 87 % as having full Inuit heritage. We excluded 352 participants due to unrealistic energy intake and more participants were excluded due to known T2DM (n 68) and not fasting (n 112) at the time of clinical examinations. Invalid physical activity data and outliers for HOMA-IR and HOMA-β values further reduced the study sample to a total of 2374 participants. In the initial analyses we found a significantly higher energy intake among men compared with women (mean 9890 (sd 3195) kJ/d v. 7987 (sd 2815) kJ/d, P < 0·0001) and men also had a higher intake of traditional food than women (22 E% v. 19 E%, P = 0·001). We found no significant difference in the intakes of imported meat and unhealthy sugar-dense foods, such as candy, cakes and soda pops (data not shown). However, we found that fruit and vegetables contributed more to the diet for women than for men (7 E% v. 4 E%, respectively, P < 0·0001; data not shown). The participation rate varied by sex and age; women participated in the data collection more often than men (data not shown).
Table 1 shows the baseline characteristics for men in each dietary pattern and Table 2 for women. The traditional dietary pattern was different from the other patterns with regard to ethnicity: the majority of the participants with a traditional pattern were Inuit with four Inuit grandparents. Smoking was very prevalent and all dietary patterns had a high percentage of active smokers. Alcohol consumption was not prevalent on a daily basis in any of the dietary patterns, although prevalent on a monthly basis. Due to the weak association between the dietary patterns and alcohol consumption, this confounder was not included in further analyses.
Table 1.
Behavioural and clinical characteristics of the five dietary patterns among Inuit men (n 987) aged 18+ years, Greenland, 2005–2010
| Imported meat | Traditional food | Balanced diet | Unhealthy diet | Standard diet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n 76) | (n 293) | (n 18) | (n 272) | (n 328) | |||||||
| Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | P value | |
| Age (years) | 43 | 11 | 51 | 13 | 48 | 16 | 40 | 15 | 46 | 14 | <0·0001 |
| Ethnicity (% fully Inuit) | 68 | – | 94 | – | 68 | – | 81 | – | 84 | – | <0·0001 |
| Waist circumference (cm) | 95 | 0·14 | 92 | 0·14 | 100 | 0·13 | 90 | 0·14 | 93 | 0·14 | <0·0001 |
| Physical activity (min/week) | 850 | 2·78 | 1131 | 3·52 | 1067 | 5·08 | 1031 | 4·91 | 1203 | 2·53 | 0·4 |
| Daily energy intake (kJ/d) | 9684 | 0·36 | 9560 | 0·41 | 9507 | 0·25 | 9536 | 0·42 | 8914 | 0·43 | 0·06 |
| Carbohydrate (E%) | 39 | – | 37 | – | 41 | – | 53 | – | 48 | – | <0·0001 |
| Protein (E%) | 22 | – | 25 | – | 27 | – | 17 | – | 19 | – | <0·0001 |
| Fat (E%) | 39 | – | 38 | – | 32 | – | 30 | – | 33 | – | <0·0001 |
| Saturated fat (g/d) | 38 | 14 | 29 | 13 | 29 | 13 | 27 | 11 | 28 | 12 | <0·0001 |
| n-3 Fatty acids (g/d) | 5 | 6 | 13 | 8 | 9 | 8 | 6 | 4 | 5 | 3 | <0·0001 |
| Fibre (g/d) | 20 | 8 | 17 | 7 | 25 | 6 | 17 | 8 | 23 | 10 | <0·0001 |
| Sugar (E%) | 12 | – | 11 | – | 6 | – | 26 | – | 13 | – | <0·0001 |
| Current smokers (%) | 63 | – | 67 | – | 44 | – | 68 | – | 64 | – | 0·3 |
| Previous smokers (%) | 20 | – | 21 | – | 33 | – | 19 | – | 22 | – | 0·6 |
| Daily drinkers (%) | 0 | – | 2 | – | 0 | – | 2 | – | 1 | – | 0·8 |
| Weekly drinkers (%) | 30 | – | 34 | – | 13 | – | 27 | – | 23 | – | 0·06 |
| Monthly drinkers (%) | 64 | – | 56 | – | 73 | – | 65 | – | 68 | – | 0·06 |
| Family history of diabetes (%) | 12 | – | 7 | – | 14 | – | 6 | – | 11 | – | 0·2 |
| IFG (%) | 5 | – | 24 | – | 28 | – | 14 | – | 12 | – | <0·0001 |
| IGT (%) | 7 | – | 5 | – | 11 | – | 2 | – | 3 | – | 0·03 |
| T2DM (%) | 10 | – | 16 | – | 17 | – | 10 | – | 9 | – | 0·06 |
E%, energy percentage; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus.
Table 2.
Behavioural and clinical characteristics of the five dietary patterns among Inuit women (n 1387) aged 18+ years, Greenland, 2005–2010
| Imported meat | Traditional food | Balanced diet | Unhealthy diet | Standard diet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n 120) | (n 308) | (n 108) | (n 380) | (n 471) | |||||||
| Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | P value | |
| Age (years) | 39 | 12 | 49 | 14 | 49 | 12 | 40 | 14 | 44 | 15 | <0·0001 |
| Ethnicity (% fully Inuit) | 82 | – | 93 | – | 85 | – | 86 | – | 88 | – | 0·02 |
| Waist circumference (cm) | 90 | 0·13 | 91 | 0·17 | 96 | 0·14 | 89 | 0·16 | 89 | 0·15 | <0·0001 |
| Physical activity (min/week) | 1187 | 2·68 | 1187 | 3·27 | 1510 | 1·96 | 1155 | 2·59 | 1180 | 2·89 | 0·3 |
| Daily energy intake (kJ/d) | 7869 | 0·37 | 7779 | 0·49 | 7358 | 0·44 | 7855 | 0·43 | 6967 | 0·45 | <0·0001 |
| Carbohydrate (E%) | 39 | – | 38 | – | 45 | – | 54 | – | 50 | – | <0·0001 |
| Protein (E%) | 22 | – | 26 | – | 24 | – | 17 | – | 19 | – | <0·0001 |
| Fat (E%) | 39 | – | 36 | – | 31 | – | 29 | – | 31 | – | <0·0001 |
| Saturated fat (g/d) | 31 | 11 | 25 | 13 | 22 | 11 | 23 | 11 | 21 | 9 | <0·0001 |
| n-3 Fatty acids (g/d) | 7 | 6 | 11 | 9 | 7 | 6 | 5 | 5 | 4 | 3 | <0·0001 |
| Fibre (g/d) | 18 | 7 | 15 | 7 | 26 | 11 | 15 | 7 | 20 | 8 | <0·0001 |
| Sugar (E%) | 11 | – | 11 | – | 5 | – | 27 | – | 12 | – | <0·0001 |
| Current smokers (%) | 75 | – | 70 | – | 52 | – | 77 | – | 65 | – | <0·0001 |
| Previous smokers (%) | 14 | – | 20 | – | 29 | – | 15 | – | 22 | – | 0·004 |
| Daily drinkers (%) | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – | 1·0 |
| Weekly drinkers (%) | 16 | – | 21 | – | 16 | – | 19 | – | 18 | – | 0·8 |
| Monthly drinkers (%) | 81 | – | 66 | – | 66 | – | 70 | – | 72 | – | 0·05 |
| Family history of diabetes (%) | 8 | – | 13 | – | 18 | – | 12 | – | 12 | – | 0·2 |
| IFG (%) | 7 | – | 17 | – | 13 | – | 8 | – | 11 | – | <0·001 |
| IGT (%) | 4 | – | 6 | – | 7 | – | 6 | – | 9 | – | 0·1 |
| T2DM (%) | 2 | – | 14 | – | 12 | – | 8 | – | 10 | – | 0·005 |
E%, energy percentage; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus.
Table 3 shows the mean values of clinical parameters according to dietary pattern. We found that FPG was significantly higher in the traditional pattern compared with the standard diet. Fasting insulin and HOMA-β were significantly lower in the traditional dietary pattern compared with the standard diet. Furthermore, insulin resistance was lower among participants with a traditional diet compared with a standard diet, although not significantly. Sex, age and waist circumference were associated with fasting glucose and insulin, 2-h plasma glucose, HOMA-IR and HOMA-β (data not shown). Physical activity and smoking were significantly associated with fasting insulin, 2-h plasma glucose, HOMA-IR and HOMA-β (data not shown). Energy intake was not significantly associated with any of the outcomes shown in Table 3. Ethnicity was significantly associated only with 2-h plasma glucose.
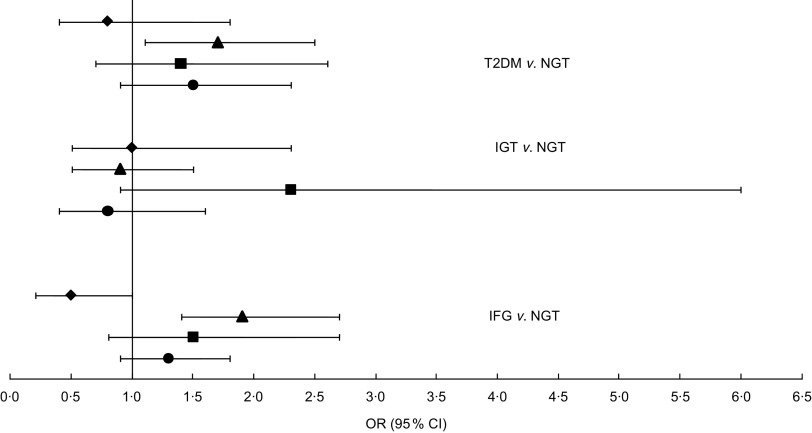
Figure 2 shows the association of each dietary pattern with T2DM, IFG and IGT as a forest plot with odds ratios and 95 % confidence intervals (where the standard diet is the reference; OR = 1·0). A total of 1581 participants were characterized as having normal glucose tolerance. The traditional dietary pattern showed significantly higher odds for IFG and T2DM, whereas no significant association was found for IGT. The balanced diet and the unhealthy diet also showed higher odds for IFG and T2DM, but neither was significant.
Fig. 2.
Odds ratios (with 95 % confidence intervals represented by horizontal lines) for type 2 diabetes mellitus (T2DM), impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), according to dietary pattern (⧫, imported meat; ▴, traditional food; ▪, balanced diet; ●, unhealthy diet), among 2374 Inuit aged 18+ years, Greenland, 2005–2010. The standard diet was set as reference (OR = 1·0); NGT, normal glucose tolerance. Analyses were adjusted for age, sex, waist circumference, ethnicity, physical activity, smoking and total energy intake. Number of participants included in each analysis: T2DM, n 1806; IGT, n 1703; IFG, n 1901; NGT, n 1581
Like the clinical outcomes shown in Table 3, age and waist circumference were associated with all glycaemic outcomes. Sex was associated only with IFG and IGT. The interaction between sex and dietary pattern was not significant for the associations with IFG and T2DM, but was significant for IGT; although, when we stratified the analyses on sex, the association was not statistically significant because there were only eighteen male participants with a balanced dietary pattern. The small number of cases in the balanced dietary pattern is the reason for the wide confidence interval for IGT in Fig. 2. Being female gave decreased odds compared with male (OR = 0·7, 95 % CI 0·5, 0·9) for IFG. Ethnicity was associated with IGT: being fully Inuit gave increased odds for having IGT (OR = 2·9, 95 % CI 1·2, 6·9). Ethnicity was almost significant for T2DM as well (P = 0·06) and showed the same tendency as IGT: OR = 1·8 (95 % CI 0·97, 3·36) for fully Inuit compared with partly Inuit (OR = 1·0). Smoking was the only confounder associated with IFG and physical activity was associated only with T2DM. These results are not shown.
Discussion
In the present study of associations between dietary patterns and glycaemic outcomes among adult Inuit, we found that a traditional diet, defined as the consumption of at least 25 E% of traditional food, was significantly associated with T2DM, higher fasting glucose and lower β-cell function. An unhealthy diet, defined by a high intake of added sugar and fast food, gave significantly higher insulin resistance compared with the standard diet, but this was not found for the traditional diet. Among the men 33 % and among the women 34 % were grouped in the standard diet, meaning that they do not adhere to any of the dietary guidelines outlined by the Greenland Nutrition Board.
Previously, it was found that FPG increased with the consumption of marine food( 3 ). Raised FPG could reflect impaired insulin secretion( 21 , 22 ). This corresponds well with our findings that the traditional dietary pattern was associated with significantly lower β-cell function. Furthermore, our study showed that in relation to T2DM or IFG, the traditional dietary pattern gave significantly higher odds for both disease outcomes. Previous research found that a dietary pattern high in sugar and processed meat increased the risk of T2DM( 23 ). The unhealthy diet with 26 E% and 27 E% from sugar (men and women, respectively) was significantly associated with higher FPG, higher 2-h plasma glucose and higher insulin resistance compared with the standard diet. High loads of sugar may increase the risk of insulin resistance irrespective of obesity level( 24 ).
In relation to our findings, it seems from Fig. 2 that the most advantageous dietary pattern is the imported meat diet, which indicated an almost significantly lower association with IFG, although not a significantly lower association with T2DM. Furthermore, we found that the unhealthy diet gave significantly lower odds for T2DM and IFG compared with the traditional diet. A follow-up study showed that an unhealthy dietary pattern high in junk food, sugar and red meat increased the risk of T2DM (OR = 1·56, 95 % CI 1·32, 1·93)( 10 ). Traditional food in Greenland is normally consumed by older generations and plays an important role especially in villages and smaller towns. In Greenland, populations living in small towns and villages live a more isolated life and some places have poor contact with health services and medical treatment. Co-morbidity in relation to poor glycaemic control is therefore a possible confounder that we do not have data for. Furthermore, we found women to have decreased odds for IFG, which is in accordance with previous research( 25 , 26 ). Traditional food in Greenland consists mainly of marine animals and results in a diet high in n-3 fatty acids. Our results showed that among both men and women the n-3 fatty acids intake was the highest in the traditional dietary pattern compared with the other patterns. Both human and animal models have shown that n-3 fatty acids can decrease the insulin resistance in muscle tissue( 27 , 28 ). Our results did not support this. Additionally, n-3 fatty acids may prevent T2DM, although not reversing the disease when first present( 27 ). Our data did not support this either, since traditional food gave higher odds for T2DM as well and the prevalence of both IFG and T2DM was high for both men and women eating the traditional diet. It is noteworthy that the imported meat dietary pattern had the lowest prevalence of IFG for both sexes and T2DM for men. Unknown lifestyle factors could play a role. Although we adjusted our analyses for many lifestyle factors known to be related to both diet and glucose metabolism outcomes, there is always a risk that unknown factors play a role.
Another explanation could be other dietary components that outweigh the benefits of n-3 fatty acids. Contaminants such as mercury and persistent organic pollutants accumulate in the top predator animals that constitute a major part of traditional food. Decreased β-cell function and impaired insulin action are both important early stages in the development of T2DM( 21 , 29 ). Contaminants could explain the lower β-cell function in the traditional dietary pattern. Another study found a significant association between persistent organic pollutants and low insulin secretion, although no associations were found between persistent organic pollutants and T2DM, IGT or insulin resistance( 30 ). However, the interpretation and conclusions drawn for HOMA indices should be cautious, since these indices have never been validated in Inuit populations.
Another factor is ethnicity. Inuit heritage was positively related to both the traditional pattern and T2DM. Although a significant residual remained for the association with T2DM, it cannot be excluded that genetic susceptibility to T2DM may confound the association. Regarding confounders, we chose to include factors that from former research were shown to be associated. We tested associations between our glycaemic outcomes and the predefined confounders. Increasing frequency of alcohol consumption has been shown to increase the risk of T2DM( 2 ). The risk of T2DM seems to be inversely related to smoking since current smokers compared with non-smokers have a lower risk of both T2DM and IGT among Inuit( 2 ), although that study was contrary to a large meta-analysis that found active smoking was associated with an increased risk of T2DM( 31 ). Anthropometric studies in Inuit populations have shown that BMI might be overestimating overweight and obesity( 32 ). Abdominal fat is a strong risk factor for various metabolic complications including T2DM compared with total fat mass( 33 , 34 ). We chose to use waist circumference as a measurement of abdominal fatness, which has been shown to correlate with incidence of insulin resistance, diabetes and pre-diabetes( 35 – 37 ).
A limitation in our study is regarding the FFQ, which was not validated. We excluded 352 participants as either under- or over-reporters based on their calculated energy intake. This proportion of misreporting could be related to the lack of validation of our FFQ, but to what extent this influences our results is unknown.
The energy percentage from sugar was similar in the traditional and the standard diets. High sugar intake cannot therefore explain the higher odds for IFG and T2DM in the association with traditional food. Further, the unhealthy diet was associated with higher FPG and gave higher odds for IFG and T2DM; however, this was not significant.
A priori derived dietary patterns show a realistic consumption of food instead of simple associations between single nutrients and disease outcomes. Foods are not consumed in isolation but as a mixed diet. Our approach to test dietary patterns takes the synergy between foods into consideration. One strength of our study is the large study sample, which gives statistical strength to the associations. In our study the response rate was 68 %, which is high for this kind of study and country. The study sample was randomly selected from the total population, which is a strength. However, in the final study sample younger men were under-represented and we tried to solve this bias by adjusting for both sex and age in all analyses. Another of the study's strengths is the fact that we conducted analyses only on newly identified cases of IFG, IGT and T2DM. In this way we avoided bias due to those participants already diagnosed with diabetes changing their lifestyle or diet as part of a treatment.
Conclusion
The present study does not argue for the consumption of traditional food for nutritional or health benefits. Due to increased problems of contamination, many Arctic areas have developed restrictions on the consumption of marine mammals( 38 ). On the contrary, it seems that imported meat is more advantageous in relation to IFG, T2DM, insulin secretion and fasting glucose. Nevertheless, to get the full understanding of T2DM in this population, we strongly recommend further research into the effects of ethnicity and the role of contaminants in relation to glucose intolerance.
Acknowledgements
Sources of funding: This study was funded by the Karen Elise Jensen Foundation; The Danish Agency for Science, Technology and Innovation (Danish Council for Independent Research); and the Commission for Scientific Research in Greenland. Conflicts of interest: C.J. has no conflict of interest. M.E.J. is employed by Steno Diabetes Centre A/S, a research hospital working in the Danish National Health Service and owned by Novo Nordisk A/S. M.E.J. and P.B. own shares in Novo Nordisk A/S. Author's contributions: C.J. analysed the data, performed the statistical analysis, wrote the paper and had primary responsibility for the final content. M.E.J. and P.B. designed and conducted the research, took part in data analysis and participated in writing the manuscript.
References
- 1. Hansen JC, Deutch B & Odland JØ (2008) Dietary transition and contaminants in the Arctic: emphasis on Greenland. Circumpolar Health Suppl 2008, 2, 6–96. [DOI] [PubMed] [Google Scholar]
- 2. Jørgensen ME, Bjerregaard P & Borch-Johnsen K (2002) Diabetes and impaired glucose tolerance among the Inuit population of Greenland. Diabetes Care 25, 1766–1771. [DOI] [PubMed] [Google Scholar]
- 3. Bjerregaard P, Pedersen HS & Mulvad G (2000) The associations of a marine diet with plasma lipids, blood glucose, blood pressure and obesity among the Inuit in Greenland. Eur J Clin Nutr 54, 732–737. [DOI] [PubMed] [Google Scholar]
- 4. Adler AI, Boyko EJ, Schraer CD et al. (1994) Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Diabetes Care 17, 1498–1501. [DOI] [PubMed] [Google Scholar]
- 5. Riccardi G, Giacco R & Rivellese AA (2004) Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23, 447–456. [DOI] [PubMed] [Google Scholar]
- 6. Ramel A, Martinez A, Kiely M et al. (2008) Beneficial effects of long-chain n-3 fatty acids included in a energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia 51, 1261–1268. [DOI] [PubMed] [Google Scholar]
- 7. Tsitouras PD, Gucciardo F, Salbe AD et al. (2008) High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res 40, 199–205. [DOI] [PubMed] [Google Scholar]
- 8. Giacco R, Cuomo V, Veesby B et al. (2007) Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr Metab Cardiovasc Dis 17, 572–580. [DOI] [PubMed] [Google Scholar]
- 9. Griffin MD, Sanders TAB, Davies IG et al. (2006) Effects of altering the ratio of dietary n6 to n3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45–70 y: the OPTILIP Study. Am J Clin Nutr 84, 1290–1298. [DOI] [PubMed] [Google Scholar]
- 10. van Dam RM, Rimm EB, Willet WC et al. (2002) Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med 136, 201–209. [DOI] [PubMed] [Google Scholar]
- 11. Nettleton JA, Steffen LM, Ni H et al. (2008) Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 31, 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B et al. (2011) Adherence Index based on the AHA 2006 Diet and Lifestyle Recommendations is associated with select caridovascular disease risk factors in older Puerto Ricans. J Nutr 141, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eilat-Adar S, Mete M, Nobmann ED et al. (2009) Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr 139, 2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. IPAQ Group (2005) The International Physical Activity Questionnaire. http://www.ipaq.ki.se (accessed May 2010).
- 15. National Food Institute (2007) Danish Food Composition Databank ed. 7.01. http://www.foodcomp.dk (accessed January 2008).
- 16. Centre for Iindigenous Peoples’ Nutrition and Environment (2005) Traditional Food Composition Nutribase. http://www.mcgill.ca/cine/resources/nutrient/ (accessed January 2008).
- 17. Health Canada (2007) Canada Nutrient File v.2007b. http://www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/index_e.html (accessed January 2008).
- 18. Bjerregaard P (2011) Inuit Health in Transition – Greenland survey 2005–2010. Population sample and survey methods. SIF Writings on Greenland vol. 19, 2nd ed. http://si-folkesundhed.dk/upload/inuit_health_in_transition_greenland_methods_5_2nd_revision.pdf (accessed October 2011).
- 19. Bjerregaard P & Jeppesen C (2010) Inuit dietary patterns in modern Greenland. Int J Circumpolar Health 69, 13–24. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization (2006) Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Geneva: WHO. [Google Scholar]
- 21. Weyer C, Bogardus C, Mott DM et al. (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faerch K, Vaag A, Holst JJ et al. (2008) Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 51, 853–861. [DOI] [PubMed] [Google Scholar]
- 23. Schulze MB, Hoffmann K, Manson JE et al. (2005) Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 82, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulze MB, Liu S, Rimm EB et al. (2004) Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 80, 348–356. [DOI] [PubMed] [Google Scholar]
- 25. Williams JW, Zimmet PZ, Shaw JE et al. (2003) Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter? Diabet Med 20, 915–920. [DOI] [PubMed] [Google Scholar]
- 26. Faerch K, Vaag A, Holst JJ et al. (2009) Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: The Inter99 Study. Diabetes Care 32, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taouis M, Dagou C, Ster C et al. (2002) n-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 282, E664–E671. [DOI] [PubMed] [Google Scholar]
- 28. Stettler R, Ith M, Acheson K et al. (2005) Interaction between dietary lipids and physical inactivity on insulin sensitivity and on intramyocellular lipids in healthy men. Diabetes Care 28, 1404–1409. [DOI] [PubMed] [Google Scholar]
- 29. Weyer C, Tataranni PA, Bogardus C et al. (2000) Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 24, 89–94. [DOI] [PubMed] [Google Scholar]
- 30. Jørgensen ME, Borch-Johnsen K & Bjerregaard P (2008) A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia 51, 1416–1422. [DOI] [PubMed] [Google Scholar]
- 31. Willi C, Bodenmann P, Ghali WA et al. (2007) Active smoking and the risk of type 2 diabetes. A systematic review and meta-analysis. JAMA 298, 2654–2664. [DOI] [PubMed] [Google Scholar]
- 32. Charbonneau-Roberts G, Saudny-Unterberger H, Kuhnlein HV et al. (2005) Body mass index may overestimate the prevalence of overweight and obesity among the Inuit. Int J Circumpolar Health 65, 163–169. [DOI] [PubMed] [Google Scholar]
- 33. Han TS, Feskens EJ, Lean ME et al. (1998) Associations of body composition with type 2 diabetes mellitus. Diabet Med 15, 129–135. [DOI] [PubMed] [Google Scholar]
- 34. Vasquez G, Durval S, Jacobs DRJ et al. (2007) Comparison of body mass index, waist circumference and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 29, 115–128. [DOI] [PubMed] [Google Scholar]
- 35. Charbonneau-Roberts G, Young TK & Egeland GM (2007) Inuit anthropometry and insulin resistance. Int J Circumpolar Health 66, 129–134. [DOI] [PubMed] [Google Scholar]
- 36. Schraer CD, Risica PM, Ebbeson SOE et al. (1999) Low fasting insulin levels in Eskimos compared to American Indians: are Eskimos less insulin resistant? Int J Circumpolar Health 58, 272–281. [PubMed] [Google Scholar]
- 37. Murphy NJ, Schraer CD, Thiele MC et al. (1995) Dietary change and obesity associated with glucose intolerance in Alaska natives. J Am Diet Assoc 95, 676–682. [DOI] [PubMed] [Google Scholar]
- 38. Jeppesen C, Bjerregaard P & Young TK (2011) Food-based dietary guidelines in circumpolar regions. Circumpolar Health Suppl 2011, 8, 4–40. [DOI] [PubMed] [Google Scholar]