Abstract
Objective
To combine evidence from randomized controlled trials to assess the effect of Fe-fortified foods on mean Hb concentration in children (<10 years).
Design
We conducted a meta-analysis of randomized, controlled, Fe-fortified feeding trials that evaluated Hb concentration. The weighted mean difference was calculated for net changes in Hb by using random-effects models. Meta-regression and covariate analyses were performed to explore the influence of confounders on the net pooled effect.
Setting
Trials were identified through a systematic search of PubMed, the Cochrane Library and secondary references.
Subjects
Eighteen studies covering 5142 participants were identified. The duration of feeding of fortified foods ranged from 6 to 12 months in these studies.
Results
Eighteen studies were included and evaluated in the meta-analysis. The overall pooled estimate of Hb concentration showed a significant increase in the fortification group compared with the control group (weighted mean difference = 5·09 g/l; 95 % CI 3·23, 6·95 g/l; I 2 = 90 %, τ 2 = 18·37, P < 0·0001). Meta-regression analysis indicated that duration of feeding was positively related to the effect size (regression coefficient = 0·368; 95 % CI 0·005, 0·731; P < 0·05). The net pooled effect size after removing the confounders was 4·74 (95 % CI 3·08, 6·40) g/l.
Conclusions
We observed an association between intake of Fe-fortified foods and Hb concentration in children aged <10 years. Fe-fortified foods could be an effective strategy for reducing Fe-deficiency anaemia in children.
Keywords: Meta-analysis, Iron fortification, Meta-regression, Covariate meta-analysis
Deficiencies of micronutrients are a major public health problem in developing countries. Fe-deficiency anaemia is a worldwide public health problem; global prevalence is estimated at 24·8 % and the highest prevalence occurs in sub-Saharan Africa and south central Asia( 1 ). In the case of breast-fed children aged ≥6 months, complementary food is expected to meet the requirements for almost all micronutrients( 2 ). Although modest information exists on the nutritional status of schoolchildren, studies from several developing countries have demonstrated a high prevalence of micronutrient deficiencies in this age group( 3 – 5 ). Undernutrition in general and Fe deficiency in particular among schoolchildren can lead to anaemia and negatively affect growth( 6 ), motor and cognitive development( 7 ) and immune function( 8 ). All of these can adversely affect academic performance( 9 ). However, on account of suboptimal feeding practices in terms of quantity and quality, children often tend to receive Fe much below their daily requirement. Therefore an alternative method of providing Fe for this vulnerable segment of the population is required. Among the various strategies, Fe fortification of foods has been suggested as a cost-effective, long-term, population-based strategy with better compliance to improve Fe status and to prevent Fe deficiency worldwide( 10 , 11 ). Despite several studies during the last 25 years, Fe deficiency still continues as a significant public health problem( 12 ) and its prevention is essential, especially in developing countries( 13 ). The prevalence of Fe deficiency in developed countries has declined substantially in the past 15–20 years due to the introduction of fortified foods( 14 ) and other public health programmes such as nutritional advice, emphasis on breast-feeding, education and hygiene( 15 – 18 ).
In India, limited studies have evaluated the effect of Fe-fortified iodized common salt on Hb levels. The results suggested progressive increase in net Hb concentration in the range of 10–30 g/l after 1 year in rural communities and 5 g/l in urban schoolchildren( 19 ). Thus, there is evidence that children might benefit more from Fe fortification like their counterparts in developed countries.
An array of Fe fortificants suitable for different food vehicles is available. However, the extent of the effect of Fe-fortified foods depends on several factors, like the amount of endogenous Fe in the diet, the amount of Fe fortification, the bioavailability of the Fe fortificant, the food matrix, the frequency of consumption of the fortified food and its duration of feeding, and the Fe status of the individual, as well as the overall nutritional status of the target population( 12 , 20 ). The efficacy of Fe fortification strategies is generally evaluated by longitudinal, targeted or population-based, randomized controlled trials (RCT) carried out in a fixed time frame. Fe fortification is considered efficacious when it significantly improves the biomarkers of Fe status and reduces the prevalence of Fe-deficiency anaemia in a population by almost 10 %( 21 , 22 ). However, conducting an efficacy trial is costly, invasive and logistically demanding.
In recent years robust study design and meta-analysis have advanced significantly and are readily available( 23 ). In the present study, we aimed to systematically review the current literature and to perform a meta-analysis to estimate the effect of Fe-fortified foods on Hb concentration in children. Since we expected heterogeneity among studies, we also explored whether factors such as age, duration of the study and levels of fortification could predict the effects on Hb concentration in children.
Methods
Literature search
The steps in this process were conducted according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines for meta-analysis( 24 ). We searched both PubMed and the Cochrane Library databases from 1990 up to December 2010, and also reviews and the reference lists of the articles, using the keyword ‘food fortification’ paired with ‘iron’ or ‘hemoglobin’ or ‘dual fortification’ or ‘triple fortification’ or ‘multiple micronutrient fortification’ and ‘fortification trial’.
Selection criteria
The search was regardless of language and publication status. These studies included multiple intervention groups with other micronutrients that were administered simultaneously; the outcome measure was the effect of Fe fortification on Hb concentration only. The studies were limited to publications where RCT evaluated Fe fortification among children aged <10 years for its effect on Hb concentration.
Data extraction and quality assessment
The title and abstracts of the studies identified in the web search were read and irrelevant studies were excluded. Full texts of the remaining studies were retrieved. To avoid publication bias, only peer-reviewed published studies were included. The extraction of data consisted of obtaining sample size, age, duration of intervention, levels of fortification and mean change and standard deviation of Hb concentration in the intervention and control groups. The search, data extraction and quality assessment were completed independently by two content experts according to the inclusion criteria and confirmed by using recommended criteria for RCT( 25 , 26 ). Concealment of allocation was classified as ‘adequate’, ‘unclear’, ‘inadequate’ or ‘not used’, based on randomization, blinding and reporting of withdrawals. Blinding was classified as ‘double blinding’, ‘single blinding’, ‘no blinding’ or ‘unclear’. In designs employing two or more different intervention groups (different levels of fortification or administration regimens) and a single control group, the sample size of the control group was equally allotted to the number of intervention groups while retaining the same mean value for the change and its standard deviation. In reporting such designs, each intervention subgroup was analysed separately. Thus, some studies contributed more than one intervention component with a single control group for the statistical analysis and resulted in a greater number of trials than the number of studies included.
Statistical analysis
Our primary outcome was the mean change in Hb concentration on account of consumption of Fe-fortified foods. The effect size, which is the difference in means between the Fe-fortified and the control groups, is referred to as the weighted mean difference (WMD) and was calculated for the selected trials. Once an effect size was estimated for each trial, the overall effect of these results was assessed by the Q statistic, that measures the extent of inconsistency among studies. The Q test was computed under the assumption of homogeneity among the effect sizes and the statistic follows the χ 2 distribution with k−1 degrees of freedom, k being the number of studies. Another strategy for quantifying the heterogeneity in a meta-analysis consists of estimating the variance (τ 2) between studies. The parameter I 2 quantifies the extent of heterogeneity from a collection of effect sizes, which is interpreted as approximately the percentage of total variation in study estimates due to heterogeneity rather than sampling error. The overall WMD of these results was assessed for sampling error (homogeneous, τ 2 = 0). A fixed-effects meta-analysis was applied to obtain the pooled effect size with 95 % confidence interval or else a random-effects meta-analysis was performed (heterogeneous, τ 2 > 0)( 27 , 28 ).
The heterogeneity of results was represented in the form of a forest plot. Typically, for each study, there is a blob in the middle of the 95 % confidence interval that represents the single best mean estimate of the Hb concentration found in that study. The pooled or combined result of the WMD in Hb is represented by a diamond, the width of which is the 95 % confidence interval for the pooled data. A vertical line is displayed to indicate no effect and to differentiate between the studies that favour the intervention group or the control group. The forest plot also describes the χ 2 (Q-test statistic), τ 2, df, I 2, Z and P value. An I 2 value of more than 50 % is considered to indicate significant heterogeneity between the trials( 29 ). Publication bias was assessed with the funnel plot and Egger regression test. This is equivalent to a weighted, linear, ordinary least squares regression model with standard error as a covariate( 30 ).
If heterogeneity existed (I 2 > 50 %), a meta-regression approach was used to test the study heterogeneity by relating study characteristics. The confounders were identified and a covariate meta-analysis was performed to estimate the net pooled effect size, after removing the effect of covariates (confounders).
Statistical analyses were performed with Review Manager (RevMan) software version 5·1, IBM SPSS version 19·0 and Comprehensive Meta-Analysis (CMA) software trial version (www.meta-analysis.com).
Results
Search results
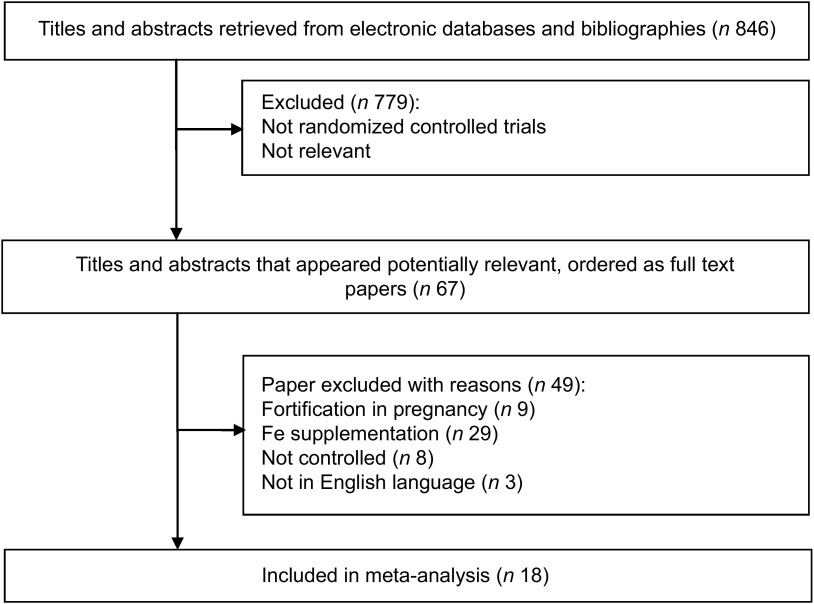
A total of 846 articles were identified, of which 779 were excluded because they were not RCT or their interventions were not relevant to the purpose of the current analysis. Sixty-seven potentially relevant articles were selected for full text evaluation, out of which eighteen relevant articles were submitted to meta-analysis after employing the inclusion and exclusion criteria (Fig. 1).
Fig. 1.
Flow diagram for inclusion in the present meta-analysis of randomized controlled trials assessing the effect of iron-fortified foods on mean Hb concentration in children (<10 years)
Study characteristics and data quality
Characteristics of the eighteen studies included in the analysis( 31 – 48 ) are shown in Table 1. All of these were RCT, out of which six were double blind, two were cluster randomized trials and the remaining ten were randomized trials. Of the ten RCT, all had similar Hb concentrations in intervention and control groups at baseline. A total of 5142 children of average age 6 months to 9·5 years, with levels of Fe fortification such that daily Fe intake through fortified food ranged between 3·5 and 12·7 mg per child, with intervention duration ranging between 6 and 24 months, were studied. These studies have been carried out over the past 20 years. Included in the analysis were five studies each from Brazil and India; two each from Vietnam and South Africa; and one each from Indonesia, Kenya, Korea and the USA.
Table 1.
Summary of trials assessing the effect of iron-fortified foods on mean Hb concentration in children (<10 years)
| Reference | Year | Study duration (months) | Average age (years) | Level of fortification (mg/child per d) | Food vehicle | Fe compound | Country | Initial sample size | Study design |
|---|---|---|---|---|---|---|---|---|---|
| Bradley et al.( 31 ) | 1993 | 6 | 0·6 | 12·7 and 7·4 | Milk | Ferrous sulfate | USA | 347 | B/A, DB |
| de Oliveira et al.( 32 ) | 1996 | 6 | 3·5 | 10 | Water | Ferrous sulfate | Brazil | 88 | RCT, B/A |
| Giorgini et al.( 33 ) | 2001 | 6 | 3·5 | 4 | Sweet roll | Iron bis-glycinate | Brazil | 92 | B/A |
| Sari et al.( 34 ) | 2001 | 3 | 5 | 4·5 | Candies | Elemental ferrous | Indonesia | 117 | DB, PC |
| van Stuijvenberg et al.( 35 ) | 2001 | 12 | 8·5 | 5 | Biscuits | Ferrous fumarate | South Africa | 108 | B/A |
| de Almeida et al.( 36 ) | 2003 | 4 | 3·5 | 4 | Orange juice | Ferrous sulfate·7H2O | Brazil | 50 | B/A |
| Beinner et al.( 37 ) | 2005 | 4 | 2·7 | 12 | Water | Ferrous sulfate·7H2O | Brazil | 160 | B/A |
| Moretti et al.( 38 ) | 2006 | 3·5 | 9·5 | 5 | Rice | Ferric pyrophosphate | India | 184 | RCT, DB |
| van Stuijvenberg et al.( 39 ) | 2006 | 7·5 | 8·5 | 3·7 | Sliced bread | Ferrous bis-glycinate | South Africa | 160 | RCT |
| Zimmermann et al.( 40 ) | 2006 | 4 | 7 | 10 | Rice | Micronized ferric pyrophosphate | India | 134 | DB, RCT |
| Andang'o et al.( 41 ) | 2007 | 5 | 4·5 | 5·9 | Maize | NaFeEDTA | Kenya | 516 | RCT |
| Le Huong et al.( 42 ) | 2007 | 6 | 7 | 10·7 | Noodles | NaFeEDTA | Vietnam | 425 | RCT |
| Moreira-Araújo et al.( 43 ) | 2007 | 9 | 3·5 | 10 | Snacks | Ferrous fumarate | Brazil | 380 | B/A |
| Varma et al.( 44 ) | 2007 | 6 | 4·3 | 14 | Khichidi | Ferrous fumarate | India | 516 | CRT, DB |
| Rim et al.( 45 ) | 2008 | 6 | 0·8 | 10 | Rice | Ferrous sulfate | Korea | 234 | RCT |
| Nga et al.( 46 ) | 2009 | 4 | 7 | 6 | Biscuits | Ferrous fumarate | Vietnam | 510 | DB, RCT |
| Osei et al.( 47 ) | 2010 | 8 | 8 | 10 | Micronutrient premix | NaFeEDTA | India | 499 | CRT, PC |
| Sazawal et al.( 48 ) | 2010 | 24 | 2 | 9·6 | Milk | Ferrous sulfate | India | 633 | RCT |
| Mean | 6·5 | 4·7 | 8·3 | ||||||
| sd | 4·2 | 3·0 | 3·4 |
B/A, before-and-after study; DB, double blind; RCT, randomized controlled trial; PC, placebo controlled; CRT, cluster randomized trial.
All eighteen studies evaluated the effect of various levels of fortification on Hb concentration. Four studies of multiple interventions with multiple micronutrients were included and only those groups that received Fe were considered( 31 , 38 , 44 , 46 ). Of these four included studies, each one had more than one trial. The trials were either based on different levels of intervention of food fortification conducted on two occasions (before and after) or they were compared with placebo. In each trial the number of participants, mean and standard deviation of Hb concentration were estimated for conducting meta-analysis.
Effects of Fe fortification on Hb concentration
The meta-analysis results indicated that the mean change in Hb concentration was significantly higher in the Fe-fortified group than in the control group (n 5142; WMD = 5·09 g/l, 95 % CI 3·23, 6·95 g/l; P < 0·00001), as depicted on the forest plot (Fig. 2). There was significant heterogeneity for the mean Hb concentration reported among the included trials. All statistical tests of heterogeneity – such as the Q statistic (χ 2 = 226·03, df = 23), which was more than df; τ 2 greater than zero (τ 2 = 18·37); and I 2 greater than 50 % (I 2 = 90 %) – were higher than the expected value, indicating heterogeneity among the studies. Meta-regression analysis was performed to detect the source of heterogeneity and indicated that the duration of the intake of fortified food was positively related to the effect size (regression coefficient = 0·368, 95 % CI 0·005, 0·731; P < 0·05). The significant differences in the extent of improvement in Hb levels as reported in the forest plot (Fig. 2) are perhaps due to different time periods of the feeding regimens of Fe-fortified foods to the children. Increased duration of receiving fortified foods might have resulted in higher levels of Hb.
Fig. 2.
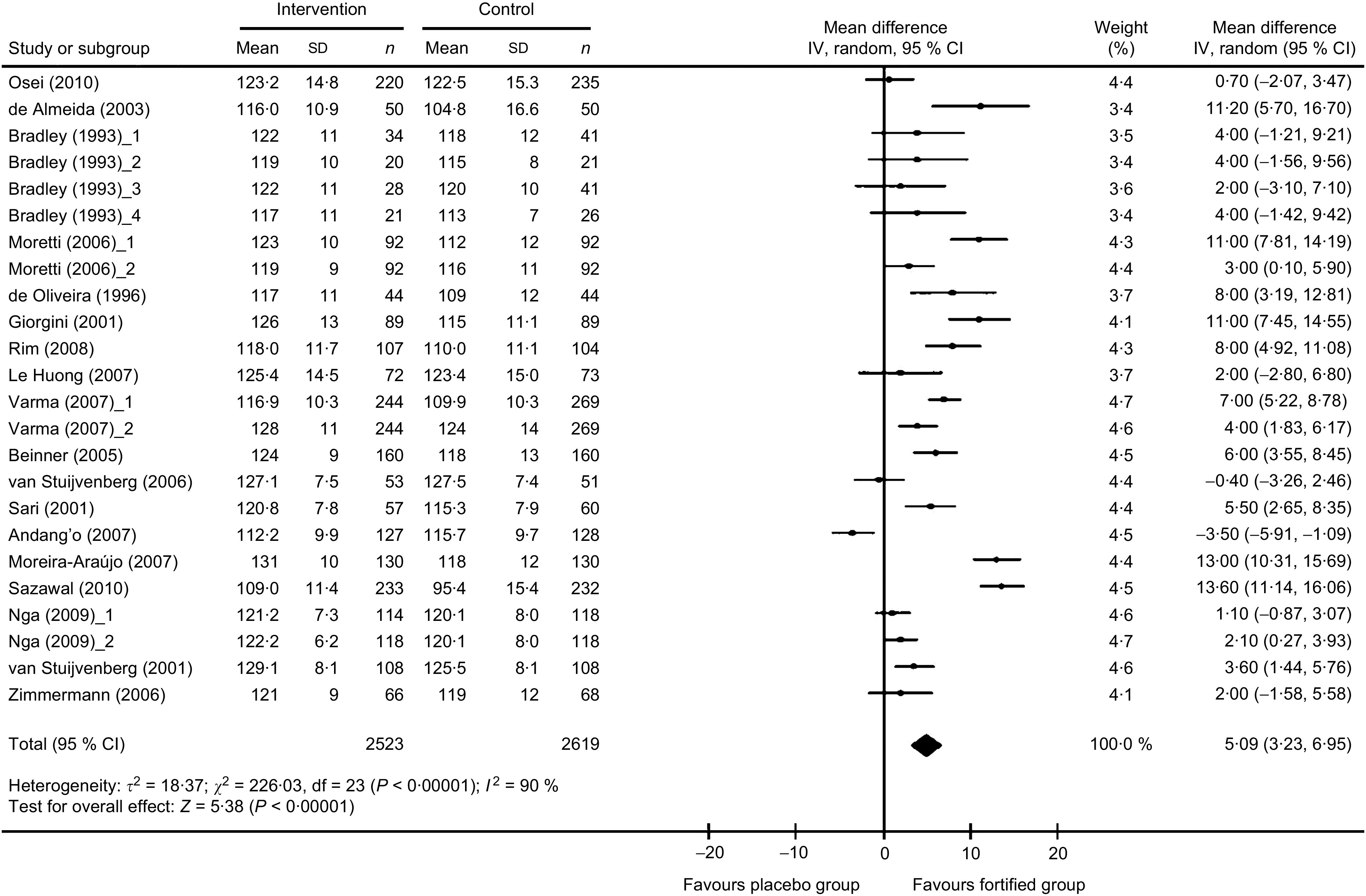
Forest plot: effect of iron fortification on mean difference in Hb concentration in comparison with no intervention or placebo control in children (<10 years). Random-effects meta-analysis of weighted mean difference (WMD; and 95 % CI) on Hb concentration with iron-fortified food intervention compared with control group. The sizes of data markers indicate the weight of each study in the analysis. Horizontal lines represent 95 % CI. Blob indicates best estimate and diamond indicates the summary estimate of the WMD
Covariate meta-analysis was performed to eliminate the effect of the confounder, duration of the study. Upon removal of the confounders the net effect of fortification on Hb concentration in children was found to be 4·74 (95 % CI 3·08, 6·40) g/l, as compared with the calculated pooled effect size of 5·09 (95 % CI 3·23, 6·95) g/l.
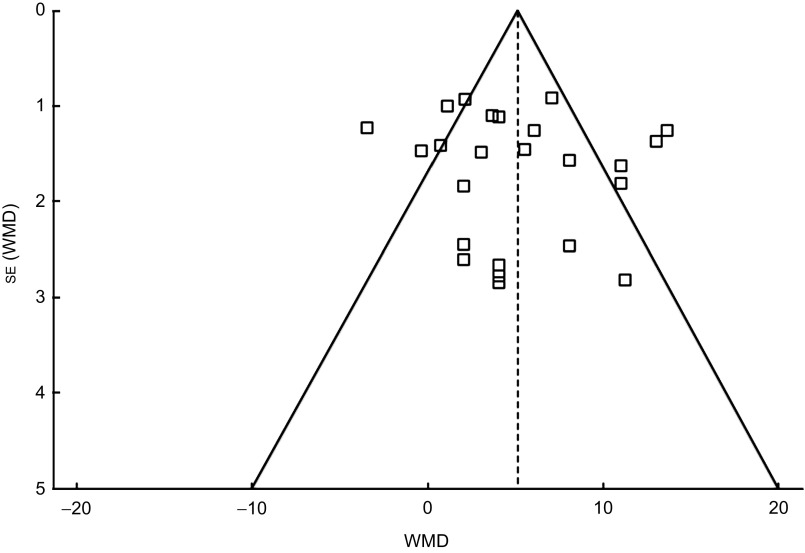
Publication bias
The funnel plot (Fig. 3) was symmetrical, indicating the probable absence of publication bias which was confirmed using Egger's weighted regression method (Egger test, P = 0·6276).
Fig. 3.
Funnel plot of all individual studies in the meta-analysis. Studies that evaluated the effect of iron fortification on Hb concentration in children (<10 years) were plotted with their weighted mean difference (WMD) on the x-axis and the corresponding standard error of the WMD along the y-axis
Discussion
The Fe fortification intervention varied across trials; hence, results should be interpreted accordingly. The present meta-analysis of eighteen studies consisting of twenty-four trials found that Fe fortification was significantly associated with increased Hb concentration in intervention children compared with the controls. However, there was heterogeneity in the results across the trials.
The present study adopted sequential statistical methods to verify that implementation of fortified foods with Fe improves Hb concentration in the child beneficiaries. The presence of heterogeneity is an important attribute of meta-analysis that can influence the results and was tested by the Q statistic, τ 2 and I 2, with the results represented in the form of a forest plot. The results of each trial included in the analysis showed the mean difference in Hb concentration (5·09 g/l) favours the intervention group, suggesting that the Fe fortification improves the mean Hb level of children. We found that the value of Q is more than the degrees of freedom, indicating heterogeneity and suggesting that the variation in the mean changes of Hb between intervention and control groups is due to systematic underlying differences( 28 , 49 ). One of the limitations of this statistic could be due to the inclusion of studies with n < 30( 31 ) and this might have contributed to the heterogeneity. Similarly, the second measure of heterogeneity, τ 2, also indicated that the variance of WMD was more than zero, which confirms that there existed heterogeneity among the trials. A third measure of heterogeneity, the I 2 statistic, which is a derivative of Q, was 90 %, also suggesting heterogeneity among the selected trials( 50 , 51 ).
Since there was heterogeneity among the trials, fixed-effects meta-analysis could not be performed and we applied the random-effects meta-analysis. The random-effects meta-analysis showed a significant impact of Fe fortification on Hb concentration among the child beneficiaries and provides evidence to suggest that food fortification with Fe is an ideal strategy to correct Fe-deficiency anaemia among children <10 years of age.
Further, to understand the true effect of food fortification with Fe on Hb concentration, meta-regression analysis was performed to explain the influence of confounders such as age, duration of intervention and levels of fortification( 52 ). We observed that the duration of the study is an effective confounder. The covariate meta-analysis showed that the net effect was 4·74 g/l after removing the effect of confounders. Yet another critical step in meta-analysis is the publication bias which can lead to inflated estimates of efficacy. We observed that there was heterogeneity among trials as some of the trials did not fit into the funnel. However, Egger's regression test suggested that there was no publication bias (P = 0·6276).
One concern is related to the unresolved issue regarding an interaction between Fe and infection. As per the review by Oppenheinmer( 53 ), most adverse effects of Fe supplementation have been reported following the use of parenteral Fe in geographical areas where malaria is endemic. Subsequently, the results of a trial conducted in Pemba, Zanzibar showed an increase in risk of hospitalization and mortality after Fe supplementation among Fe-replete children in a malaria-endemic setting( 54 ). A very similar trial in malaria-free areas in Nepal found no such adverse effect( 55 ). However, no adverse effects were reported in the studies covered in present paper where Fe-fortified foods were involved.
There is emerging evidence to suggest that reports of RCT from certain countries mostly have statistically positive results on micronutrients( 56 ). This may be due to the fact that in those countries, anaemia is largely due to Fe deficiency (single nutrient) rather than the multifactorial aetiology reported from developing countries. However, the present study suggests the possibility of a positive effect of Fe fortification on Hb in children. This phenomenon needs to be investigated further. There is a need to conduct more trials in developing countries so as to investigate whether Fe fortification improves Hb concentration and also to assess other confounders, such as intakes of protein, energy and folic acid, which could contribute in improving Hb levels.
Conclusion
This present study suggests that consumption of Fe-fortified foods significantly increases the Hb concentration in children aged <10 years. Further research efforts should concentrate on higher quality and more rigorous randomized trials with longer follow-up to resolve the uncertainty regarding the safety and clinical effectiveness.
Acknowledgements
Source of funding: The authors gratefully acknowledge the financial and fellowship support extended by the National Institute of Nutrition (NIN), Indian Council of Medical Research, Hyderabad, India, for carrying out the study. Conflicts of interest: There are no conflicts of interest. Ethics: Ethical approval was not required for the present study. Authors’ contributions: R.A. contributed in the data collection, analysis and manuscript preparation. M.V.V.R. developed the study protocol, secured funds, supervised the study and guided manuscript preparation. K.M.N. contributed to development of the study protocol and manuscript writing. Acknowledgements: The authors thank Dr B. Sesikeran (Director, NIN) for his encouragement, and also Mr K. Venkaiah and Dr N. Bala Krishna (Biostatistics Division, NIN) for their insightful inputs.
References
- 1. World Health Organization (2008) Worldwide Prevalence of Anaemia 1993–2005. Geneva: WHO. [Google Scholar]
- 2. Brown K, Dewey K & Allen L (1998) Complementary Feeding of Young Children in Developing Countries. A Review of Current Scientific Knowledge. Geneva: WHO. [Google Scholar]
- 3. Le HT, Brouwer ID, Verhoef H et al. (2007) Anemia and intestinal parasite infection in school children in rural Vietnam. Asia Pac J Clin Nutr 16, 716–723. [PubMed] [Google Scholar]
- 4. Neumann CG, Bwibo NO, Murphy SP et al. (2003) Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. J Nutr 133, 11 Suppl. 2, 3941S–3949S. [DOI] [PubMed] [Google Scholar]
- 5. Sivakumar B, Nair KM, Sreeramulu D et al. (2006) Effect of micronutrient supplement on health and nutritional status of school children: biochemical status. Nutrition 22, 1 Suppl., S15–S25. [DOI] [PubMed] [Google Scholar]
- 6. Lawless JW, Latham MC, Stephenson LS et al. (1994) Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr 124, 645–654. [DOI] [PubMed] [Google Scholar]
- 7. Black MM (2003) Micronutrient deficiencies and cognitive functioning. J Nutr 133, 11 Suppl. 2, S3927–S3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thurnham DI (1997) Micronutrient and immune function: some recent developments. J Clin Pathol 50, 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popkin BM & Lim-Ybanez M (1982) Nutrition and school achievement. Soc Sci Med 16, 53–61. [DOI] [PubMed] [Google Scholar]
- 10. Allen L, de Benoist B, Dary O et al. (2006) Guidelines on Food Fortification with Micronutrients, 2nd ed. Geneva: WHO and FAO. [Google Scholar]
- 11. Horton S (2006) The economics of food fortification. J Nutr 136, 1068–1071. [DOI] [PubMed] [Google Scholar]
- 12. Nelson M, White J & Rhodes C (1993) Haemoglobin, ferritin and iron intakes in British children aged 12–14 years: a preliminary investigation. Br J Nutr 70, 147–155. [DOI] [PubMed] [Google Scholar]
- 13. Nelson M, Bakaliou F & Trivedi A (1994) Iron deficiency anaemia and physical performance in adolescent girls from different ethnic backgrounds. Br J Nutr 72, 427–433. [DOI] [PubMed] [Google Scholar]
- 14. Spanjerberg MQI & Jansen EHJM (2000) Iron Deficiency and Overload in Relation to Nutrition. RIVM Report no. 650250004. Bilthoven: RIVM. [Google Scholar]
- 15. Bhutta ZA, Ahmed T, Black RE et al. (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. [DOI] [PubMed] [Google Scholar]
- 16. Oski FA (1993) Iron deficiency in infancy and childhood. N Engl J Med 329, 190–193. [DOI] [PubMed] [Google Scholar]
- 17. Wharton BA (1999) Iron deficiency in children: detection and prevention. Br J Haematol 106, 270–280. [DOI] [PubMed] [Google Scholar]
- 18. Hallberg L (2001) Perspective on nutritional iron deficiency. Annu Rev Nutr 21, 1–21. [DOI] [PubMed] [Google Scholar]
- 19. Nair KM (2001) Alternate strategies for improving iron nutrition: lessons from recent research. Br J Nutr 85, Suppl. 2, S187–S191. [PubMed] [Google Scholar]
- 20. Hurrell R (2007) Linking the bioavailability of iron compounds to the efficacy of iron-fortified foods. Int J Vitam Nutr Res 77, 166–173. [DOI] [PubMed] [Google Scholar]
- 21. Mei Z, Cogswell ME, Parvata I et al. (2005) Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr 135, 1974–1980. [DOI] [PubMed] [Google Scholar]
- 22. Hurrell R, Ranum P, de Pee S et al. (2010) Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull 31, Suppl. 1, S7–S21. [DOI] [PubMed] [Google Scholar]
- 23. Hedeker D & Gibbons RD (2006) Longitudinal Data Analysis. Hoboken, NJ: Wiley. [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarke M & Oxman AD (editors) (2001) Assessment of study quality. In Cochrane Reviewers Handbook 4.1.1. Oxford: Cochrane Collaboration. [Google Scholar]
- 26. Juni P, Altman DG & Egger M (2001) Assessing the quality of randomized controlled trials. In Systematic Reviews in Health Care: Meta-analysis in Context, 2nd ed., pp. 87–108 [M Egger, G Davey Smith and DG Altman, editors]. London: BMJ Books. [Google Scholar]
- 27. DerSimonian R & Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- 28. Borenstein M, Hedges LV, Julian PT et al. (2009) Introduction to Meta-Analysis. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bradley CK, Hillman L, Sherman AR et al. (1993) Evaluation of two iron-fortified, milk-based formulas during infancy. Pediatrics 91, 908–914. [PubMed] [Google Scholar]
- 32. de Oliveira JE, Scheid MM, Desai ID et al. (1996) Iron fortification of domestic drinking water to prevent anemia among low socioeconomic families in Brazil. Int J Food Sci Nutr 47, 213–219. [DOI] [PubMed] [Google Scholar]
- 33. Giorgini E, Fisberg M, De Paula RA et al. (2001) The use of sweet rolls fortified with iron bis-glycinate chelate in the prevention of iron deficiency anemia in preschool children. Arch Latinoam Nutr 51, 1 Suppl. 1, 48–53. [PubMed] [Google Scholar]
- 34. Sari M, Bloem MW, de Pee S et al. (2001) Effect of iron-fortified candies on the iron status of children aged 4–6 y in East Jakarta, Indonesia. Am J Clin Nutr 73, 1034–1039. [DOI] [PubMed] [Google Scholar]
- 35. van Stuijvenberg ME, Dhansay MA, Smuts CM et al. (2001) Long-term evaluation of a micronutrient-fortified biscuit used for addressing micronutrient deficiencies in primary school children. Public Health Nutr 4, 1201–1209. [DOI] [PubMed] [Google Scholar]
- 36. de Almeida CAN, Crott GCI, Ricco RG et al. (2003) Control of iron deficiency anemia in Brazilian preschool children using iron-fortified orange juice. Nutr Res 23, 27–33. [Google Scholar]
- 37. Beinner MA, Lamounier JA & Tomaz C (2005) Effect of iron-fortified drinking water of daycare facilities on the hemoglobin status of young children. J Am Coll Nutr 24, 107–114. [DOI] [PubMed] [Google Scholar]
- 38. Moretti D, Zimmermann MB, Muthayya S et al. (2006) Extruded rice fortified with micronized ground ferric pyrophosphate reduces iron deficiency in Indian schoolchildren: a double blind randomized controlled trial. Am J Clin Nutr 84, 822–829. [DOI] [PubMed] [Google Scholar]
- 39. van Stuijvenberg ME, Smuts CM, Wolmarans P et al. (2006) The efficacy of ferrous bis-glycinate and electrolytic iron as fortificants in bread in iron-deficient school children. Br J Nutr 95, 532–538. [DOI] [PubMed] [Google Scholar]
- 40. Zimmermann MB, Muthayya S, Moretti D et al. (2006) Iron fortification reduces blood lead levels in children in Bangalore, India. Pediatrics 11, 72014–72021. [DOI] [PubMed] [Google Scholar]
- 41. Andang'o PE, Osendarp SJ, Ayah R et al. (2007) Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomized controlled trial. Lancet 369, 1799–1806. [DOI] [PubMed] [Google Scholar]
- 42. Le Huong T, Brouwer ID, Nguyen KC et al. (2007) The effect of iron fortification and de-worming on anaemia and iron status of Vietnamese schoolchildren. Br J Nutr 97, 955–962. [DOI] [PubMed] [Google Scholar]
- 43. Moreira-Araújo RSR, Araújo MAM & Arêas JAG (2007) Fortified food made by the extrusion of a mixture of chickpea, corn and bovine lung controls iron-deficiency anaemia in preschool children. Food Chem 107, 158–164. [Google Scholar]
- 44. Varma JL, Das S, Sankar R et al. (2007) Community-level micronutrient fortification of a food supplement in India: a controlled trial in preschool children aged 36–66 mo. Am J Clin Nutr 85, 1127–1133. [DOI] [PubMed] [Google Scholar]
- 45. Rim H, Kim S, Sim B et al. (2008) Effect of iron fortification of nursery complementary food on iron status of infants in the DPR Korea. Asia Pac J Clin Nutr 17, 264–269. [PubMed] [Google Scholar]
- 46. Nga TT, Winichagoon P, Dijkhuizen MA et al. (2009) Multi-micronutrient-fortified decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. J Nutr 139, 1013–1019. [DOI] [PubMed] [Google Scholar]
- 47. Osei AK, Rosenberg IH, Houser RF et al. (2010) Community-level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. J Nutr 140, 1146–1154. [DOI] [PubMed] [Google Scholar]
- 48. Sazawal S, Dhingra U, Dhingra P et al. (2010) Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PLoS One 5, e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sterling TD, Rosenbaum WI & Weinkam JJ (1995) Publication decisions revisited: the effect of the outcome of statistical tests on the decision to publish and vice versa. Am Stat 49, 108–112. [Google Scholar]
- 50. Cook DJ, Guyatt GH, Ryan G et al. (1993) Should unpublished data be included in meta-analysis? Current convictions and controversies. JAMA 269, 2749–2753. [PubMed] [Google Scholar]
- 51. Moher D, Pham B, Klassen TP et al. (1998) Does the language of publication of reports of randomized trials influence the estimates of intervention effectiveness reported in meta-analyses? Presented at Sixth International Cochrane Colloquium, Baltimore, MD, USA, 22–26 October 1998.
- 52. Egger M, Zellwerger-Zahner T, Schneider M et al. (1997) Language bias in randomized controlled trials published in English and German. Lancet 350, 326–329. [DOI] [PubMed] [Google Scholar]
- 53. Oppenheimer SJ (1989) Iron and infection: the clinical evidence. Acta Paediatr Scand Suppl 361, 53–62. [DOI] [PubMed] [Google Scholar]
- 54. Sazawal S, Black RE, Ramsan M et al. (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomized, placebo-controlled trial. Lancet 367, 133–143. [DOI] [PubMed] [Google Scholar]
- 55. Tielsch JM, Khatry SK, Stoltzfus RJ et al. (2006) Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomized, placebo-controlled trial. Lancet 367, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vickers A, Goyal N, Harlan R et al. (1998) Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 19, 1159–1166. [DOI] [PubMed] [Google Scholar]