Abstract
Contemporary orthodontics entails using advanced materials and devices, simplifying the process of tooth movement. It is well documented that orthodontic materials are subjected to various fluctuations and stresses in the oral environment, such as salivary pH, dietary habits, temperature changes, and masticatory loads. These changes reduce bonding materials’ longevity, plasticize resin polymers, and reduce elastic properties. In addition, the corrosion of orthodontic appliances in the oral environment has concerned clinicians for some time. This is focused on two principal issues: whether corrosion products are absorbed into the body and cause either localized or systemic effects, and the results of corrosion on the physical properties and the clinical performance of orthodontic appliances. Recently, another major concern is the potential release of bisphenol-A from materials containing polymers such as thermoplastic aligners and resins, which is known to induce xenoestrogenicity and cytotoxicity when the tissue level exceeds the daily recommended intake. However, most of these findings are based on in vitro studies that suffer from serious drawbacks such as failure to replicate the exact oral environment and process during orthodontic treatment. Therefore, developing clinically relevant methods should be the goal of future research related to the aging of orthodontic materials. The purpose of this review is to outline the impact of the oral environment on contemporary orthodontic materials.
Keywords: Allergy, aligners, archwire, bracket, corrosion, cytotoxicity, elastics, friction, oral environment, orthodontic materials
Introduction
The orthodontic treatment combines biological and material sciences for the esthetic and functional alignment of teeth and jaws.[1] The adverse changes in orthodontic material inside the oral cavity are a significant concern for an efficient treatment process. The collective materials used during orthodontic treatment may include metal alloys, elastics, springs, dental cement and composite resin, and thermoplastic aligners. Due to different structural properties, each material may undergo various reactive changes intraorally.[2] Major factors affecting the integrity of materials inside the oral cavity are the salivary pH, oral microflora and their products, complex three-dimensional multiaxial loading, dietary habits, and the material's surface integrity.[2,3,4,5] Extensive research on orthodontic materials simulating the oral condition has shown extraneous results due to dynamic changes inside the oral cavity. In addition, changes in the material's properties have affected the biomechanics of treatment. The retrieval analysis is commonly used to study the alteration and degradation of the material in the oral cavity. However, it is performed from the materials already used in different oral conditions, and their effects are analyzed through different analytical methods.[6,7]
Corrosive effects are one of the most significant risks for orthodontic alloys intraorally, degrading the material's biological and mechanical properties.[8] Saliva acts as an electrolyte medium, and saliva's pH has been a major factor accelerating the corrosive phenomenon. The normal pH range of saliva is 6.8–7.2.[9] Food and oral hygiene products, habits like smoking and alcohol consumption, and long-term medications for systemic disease can alter the pH of saliva. Therefore, it can affect the frictional property and strength of alloys. Different types of corrosion are seen, such as pitting, crevice, intergranular, galvanic, and stress corrosion.[10] In the oral cavity, the leaching of ions from alloy can affect the potential properties of the material and sensitivity reactions on the host.[2] As a result, the intraoral alterations impact material durability, and the life expectancy of the material may differ from what is anticipated. In this study, we aimed to review the frequently used orthodontic materials in daily practice and their change in properties intraorally and their effect on treatment.
Orthodontic brackets
Brackets are the principal component of fixed treatment that transfers force from activated wire to teeth, resulting in desired tooth movement. Frictional resistance, binding, and notching are the major clinical challenges associated with sliding mechanics.[11] Due to frictional resistance, almost 12%–60% of the applied force is lost.[12] Therefore, higher orthodontic forces are required to overcome the higher levels of frictional resistance, resulting in iatrogenic damage such as root resorption and anchorage loss. According to Kusy and Whitley,[13] the variables that influence static friction during the sliding of an archwire are surface roughness, hardness, wire stiffness, geometry, fluid media, and surface chemistry. These variables are influenced by hostile oral environmental changes such as pH fluctuation, temperature change, corrosion attack, and microbial and enzymatic degradation of materials in the oral cavity.[8,10]
Manufacturing defects and heavy mechanical force applied on the bracket surface during treatment cause roughness and lead to debris and plaque accumulation.[14] Bacterial aggregation and the release of enzymatic products have been shown to affect the physical properties of the metal surface.[15] Saliva as a fluid medium also affects the frictional property. At low load levels, saliva acts as a lubricant, but saliva may increase friction at high loads. When archwires bind against the bevel surface of the bracket under a high load, saliva might be forced out from the contact areas, therefore allowing no lubrication between the archwire and the bracket, ultimately resulting in increased frictional resistance.[16]
Resistance induced by bracket archwire ligation also affects the frictional property, mainly depending on surface roughness and ligation force.[17] By virtue of its design, the self-ligating brackets may have retention areas that promote biofilm adherence compared to conventional brackets with steel wire ligation. Elastomeric ligation has more biofilm retention areas and is generally not used in patients with poor oral hygiene.[18] Since orthodontic therapy may continue for a long time, the brackets may be delaminated by the wear and corrosion process. It may be caused by the mechanical force of the archwire and hostility of the electrolytic environment of saliva that is variable from the start of treatment. Delamination also elicits increased bacterial adherence and friction.[19]
Corrosion of brackets can cause the leaching of metal ions, and most commonly, iron, chromium, and nickel are released into the body, and these elements have harmful effects. Nickel is the most predominant metal ion released over time, leading to potential adverse effects such as hypersensitivity reactions.[20] Stainless steel and titanium brackets have passivating oxide layers to resist corrosion. However, the rough surface of titanium leads to plaque accumulation and aggravates corrosion. Fluoride mouthwashes also affect the titanium brackets, and the presence of fluoride ions interacts with titanium, forming hydrated titanium oxides and salts, thus inducing pitting corrosion. Saliva containing 452.5 ppm fluoride ions at pH 4.2 promotes corrosion of titanium alloys.[21] It has been shown that metal ions leach out more in recycled brackets than new ones. During recycling, the brackets are heat treated at 300°C–500°C, which causes intergranular corrosion due to the removal of chromium carbide at grain boundaries.[22,23]
Another common problem during treatment is debonding brackets due to decreased bond strength.[24] The bond failure depends upon the bonding material type, and the time the bracket can withstand the harsh intraoral conditions. Composite adhesives weaken with aging, and long-time exposure to saliva might decrease the bond strength and the fluctuations in oral environment is an aggravating factor.[25] Viscoelastic properties and aging of composite adhesives, along with heavy mechanical forces and enzymatic degradation, cause bond failure.[26] Cigarette smoking also affects the bond strength, where low shear bond strength was seen in metallic brackets than in ceramic brackets in an in vitro study.[27]
The evolution of tooth-colored ceramic and plastic brackets reduced the corrosion effects compared to metallic brackets. Monocrystalline and polycrystalline alumina brackets are mostly used. A major concern is increased fracture incidence and the complicated debonding process of ceramic brackets.[28] Mono and polycrystalline groups have similar mechanical properties, except for elastic index. Monocrystalline brackets have a higher elastic index when compared to polycrystalline brackets. Crack propagation is irregular due to the crystal orientation of the monocrystalline structure.[29] Plastic brackets get more easily stained and discolored with intraoral use compared to ceramic brackets.[30]
Orthodontic wires
Archwires made of different materials are used for tooth movement, and every material reacts differently intraorally. Archwires should provide an ideal force delivery, and it may depend on frictional property, biocompatibility, fracture, and corrosion resistance.[31] Wire dimension, material types, and various intraoral conditions affect their physical and mechanical properties. The most common wires are stainless steel, nickel-titanium (NiTi), beta-titanium, and cobalt-chromium. The recent development of esthetic composite and coated wires has enhanced the esthetics, particularly in adult patients.[32,33]
Stainless steel archwires are commonly used in sliding mechanics due to their decreased frictional coefficient.[34] These wires are placed for months during space closure, so examining the mechanical properties of these working archwires is essential. Metal in an aqueous solution is thermodynamically unstable if its propensity to change from solid state to ionic form is linked with a decrease in energy. Factors influencing the direction of energy are surface morphology and phase of the metal structure, solution composition, galvanic coupling between dissimilar metals, pH, and temperature variation.[34,35] These potential factors hasten the aging process, leading to surface roughness and debris accumulation, fracture, and changes in the desired frictional properties of the wire. During sliding mechanics, there is an increase in friction between the stainless steel archwires and brackets, which corresponds to the amount of debris and surface roughness on archwires.[4,36,37] There is a magnitude of force loss of 20.8% (1.48 N) due to friction caused by debris, and this is an important factor when force is calculated for space closure. Therefore, archwires should be cleaned at every appointment to remove plaque and debris.[37]
NiTi wires are commonly used for their super elasticity and shape memory features. During activation of these wires in the bracket slot of angulated or rotated teeth, complex loading and masticatory forces along with intraoral aging cause decreased fracture resistance. Wire fracture is commonly seen in the midspan of the mandibular premolar and first molar regions, exposing the wire to masticatory loads.[38] Ligation and notching of wire surface also act as nuclei for degradation. There is reduced grain size at the compressed location and stress-induced martensitic transformation. Work-hardened martensitic NiTi wires might also cause a brittle fracture. Microstructural changes are also observed in galvanic couples between two dissimilar metals.[38]
Oxygen and hydrogen may cause either oxidation or reduction in the oral cavity. The oxidation environment is the least corrosive condition. Different chemicals such as mouthwash cause pitting corrosion on wires and increase the frictional property.[10] The oxide film on the wire surface is removed by the acidic nature of mouthwash and this accelerates corrosion. The point at which the oxide film of an alloy is released and dissolution of alloy begins is called the breakdown potential of an alloy.[39] The lower the breakdown potential, the higher the risk of corrosion. The breakdown potential of stainless steel wire is 400 mv, NiTi wires ranges from 300 to 750 mv, epoxy-coated NiTi is 1800 mv, and that of titanium wire is 2000 mv. Though most inert, titanium alloys are susceptible to corrosion in environments with low pH and fluoride levels.[35] Formation of Na2 TiF6 complex in acidic fluoride-containing solution decrease the corrosion resistance of titanium(Ti)-containing alloys. Passive TiO2 film on Ti-containing wires has shown to be destroyed in 0.5% sodium fluoride contained in artificial saliva.[21] Therefore, this Protective passive layer should have high breakdown potential and lower anodic current density. Furthermore, the metal ions released from the corrosion products may stain the enamel surface and reduce the material's biocompatibility.[40]
Orthodontic elastics
Low cost, high flexibility, and relatively low force have made the elastomers a commonly used material for ligation and traction mechanics, rotation correction, the separation between teeth, and torque expression.[41] Natural latex rubbers are most frequently used. Due to latex sensitivity in a few patients, synthetic elastomers were introduced into the market.[42] Synthetic elastomers like polyurethane polyesters have better strength and resistance to abrasion when compared to natural rubber. Due to local environmental changes, the efficiency of the elastic materials was decreased, and immediate force decay and breakage of elastics were the most common effects.[43] Force degradation of 65%–75% was seen within 48 h of wear, which decreased the efficiency of its usage.[44] When compared to latex elastics, non-latex elastics showed a large decrease in force within 1 h and continued to show significant loss within 24 h. There was an initial force decay of 27.32% in non-latex elastics when compared to 14.60% in latex elastics. Furthermore, after 24 h, the latex elastics had less force decay of 19.92% than non-latex elastics which showed 39.23%.[45,46] The degradation of latex elastics with a larger diameter was slower than that of ones with a smaller diameter due to more flexibility.[47]
There was a marked decrease in force within the first 4 h, and pH had no significant correlation with force decay.[48] Different color elastic materials were introduced for esthetic consideration and enhanced patient compliance. Pigments added in elastomers affected the mechanical properties, leading to more force degradation.[49] There was more significant force decay in colored E-chain after 24 h and 21 days, compared to nonpigmented ones.[50] Elastomers are biodegradable in the oral environment by hydrolysis of secondary bonds, which results in relaxation. Intermittent stress of elastomers, pH, oxygen content, and temperature changes are the main factors that cause the relaxation of elastomers. The aging of elastomers is characterized by changes in surface roughness and mechanical properties of the material. Initially, absorption of oral fluids and bacterial flora on the surface of elastics leads to the hydrolysis process. When interacting with oxygen and ozone content, superficial cracks are formed in elastomers due to the oxidation process. Force degradation and elastic chain displacement are unaffected by the daily use of 0.05% sodium fluoride, which is most frequently used as a mouthwash adjunct during orthodontic therapy. Also, the chemical nature of different beverages had no force degradation effect.[51]
NiTi coil springs
Due to its low continuous force delivery, the use of nickel-titanium (NiTi) coil springs is one of the most common orthodontic traction methods.[52] Several studies and retrieval analyses have been conducted to study force delivery in different oral environmental conditions in vitro.[53–56] NiTi coil spring was not affected by environmental conditions like water, coke, or turmeric solution.[57] However, temperature changes affected the force delivery properties due to modifications in the crystal structure of the alloy, but there was no clinical significance. When maintained in distilled water at 37°C, there were no changes in force decay.[57] After 4 weeks, force decay was 12.12% for in vitro springs and 11.57% for clinical springs. There was a 7% additional decrease in force between 4 and 8 weeks of use, but it stabilized after that.[55] This agrees with an in vitro study by Angolkar et al.[53] who reported that 8%–20% force decreased in 28 days among different metal alloy coil springs. Intrinsic force loss of spring material and a large decrease in spring length between space closure caused higher force decay of 48% over 22 weeks.[58] There was little force decay in any configuration when using artificial saliva, dry saliva, or mouth rinses with chlorhexidine and NaF.[59,60] To ensure more cost-effective and efficient treatment, the force decay properties of NiTi coil springs in diverse intraoral conditions must be comprehended. The various adverse effects on orthodontic materials are shown in Figure 1.
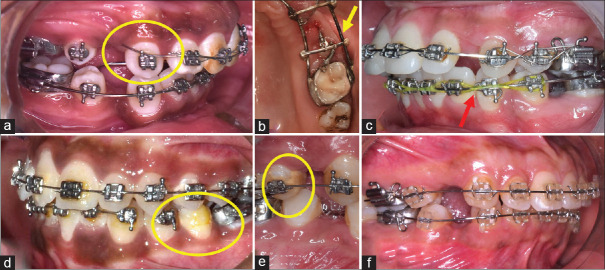
Figure 1.
Adverse effects on orthodontic materials. (a) Wire (stainless steel) breakage in a patient that was reported after 6 months. (b) Corrosion effect on stainless steel wire framework due to loss of passivating effect after soldering. (c) Discoloration and force decay of elastomeric chain in a patient, reported after 8 weeks. (d) Bond failure and stress-induced breakage of NiTi archwire. (e) Debris accumulation leads to the friction of the archwire and bracket interface and decalcification of enamel, possibly resulting in bond failure. (f) Clear brackets stained due to dietary habits. NiTi = nickel-titanium
Clear aligners
Recently, aligners have been a preferred treatment over conventional fixed mechanotherapy due to esthetic impact and other advantages like patient comfort, periodontium care, decreased in-office time, and emergency visits.[61] There are more than 27 diverse brands available; Invisalign is the most commonly used.[62] They are thermoplastic polymers with either crystalline or amorphous structure. The mechanical properties of these polymers are influenced by their structural properties like molecular and crystal structures.[63] The molecular orientation that depends on the processing methods and conditions determines their properties. Aligners are highly viscoelastic materials and react markedly to changes in temperature, humidity, elastic deformation, and manufacturing process.[64] The magnitude of changes in temperature and water-absorbing properties in a simulated intraoral environment differed in different aligners.[65] Hygroscopic expansion intraorally may affect the shape and orthodontic force applied to teeth.[66] Mechanical changes of retrieved aligners after 2 weeks of use were investigated and no significant difference was found, except decreased elastic modulus and hastened stress relaxation effects after clinical use. There was no change in the creep strain of Invisalign after 2 weeks.[67]
Bradley et al.[68] compared the mechanical properties of new and retrieved aligners intraorally present for at least 29 days. Elastic modulus and hardness decreased, making the material more brittle and less resistant to creep behavior. However, no chemical differences were found. Some trace elements like aluminum, nickel, zinc, and tin were detected in Invisalign material during its use.[67,69] They are significantly decreased after clinical use, but trace amounts can cause an allergic reaction. Surface morphology viewed by scanning electron microscope showed abrasion, delamination, and adsorption of integuments and calcified biofilm deposits.[67,70]
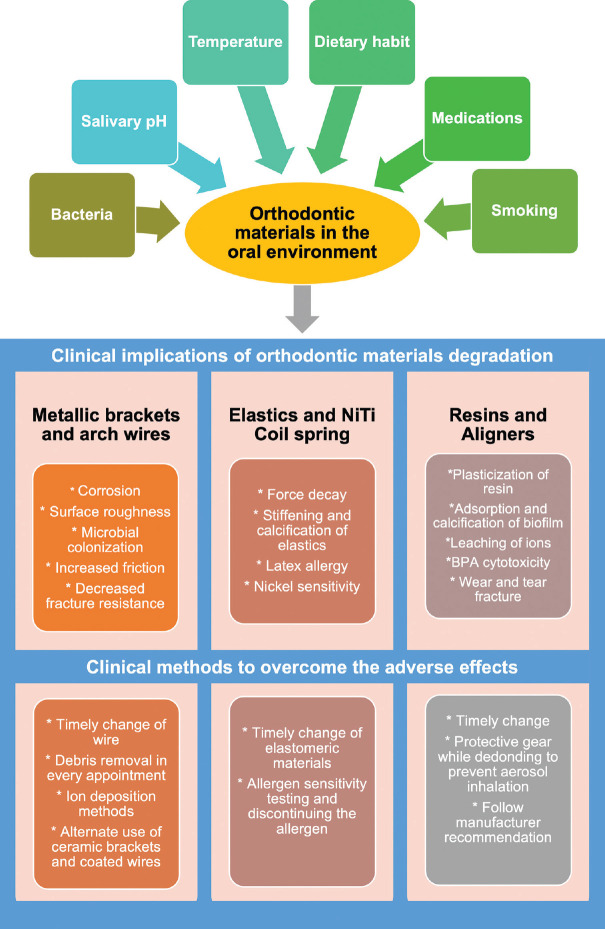
Aligners inside the oral cavity undergo wear and cracks, potentially releasing bisphenol-A (BPA). BPA is a principal constituent in manufacturing polymers and dental resins.[71] Its synthetic organic compound, which acts on estrogen, androgen, and thyroid receptors, interferes with normal hormonal functions.[72] BPA toxicity can cause precocious puberty, promote the growth of hormone-dependent tumors, influence metabolic disorders such as polycystic ovarian syndrome, disrupt glycemic control, and increase insulin resistance in type 2 diabetic patients.[73] According to the European Food Safety Authority (EFSA) 2015, the normal threshold value for BPA intake is 4 μg/kg body weight/day, which is well below the level released from aligners.[72] However, according to the EFSA re-evaluation draft in 2021, a total daily intake of 0.04 ng BPA/kg body weight/day was established due to their effects on immune system.[74] BPA-activated estrogen receptor-β (Erβ) first targets the oral keratinocytes, which shows BPA can diffuse easily through oral mucosal tissues.[75] Many studies reported that BPA release is below toxic levels, but in a clinical situation, its release is an additive mechanism particularly when the patient constantly changes aligners. An overview of the effect of the oral environment on orthodontic materials with clinical implications and methods to overcome are depicted in Figure 2.
Figure 2.
Depiction of factors influencing the oral environment, their subsequent effect on orthodontic material, and steps to overcome the adverse effects
Conclusion
A nonspecific aging pattern, including the calcification of absorbed ion complexes and proteinaceous debris, is anticipated when orthodontic materials are exposed to the oral cavity. This could influence the morphologic, structural, and compositional traits, including the mechanical properties of orthodontic alloys and polymers. Moreover, the performance and physical characteristics of orthodontic materials in the oral cavity may differ from those of their as-received or in vitro-aged equivalents. Therefore, clinicians should be aware of the limitations brought on by aging of materials and should keep track of the outcomes to achieve efficient and predictable treatment results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Eliades T. Orthodontic material applications over the past century: Evolution of research methods to address clinical queries. Am J Orthod Dentofacial Orthop. 2015;147:S224–31. doi: 10.1016/j.ajodo.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Eliades T, Bourauel C. Intraoral aging of orthodontic materials: The picture we miss and its clinical relevance. Am J Orthod Dentofacial Orthop. 2005;127:403–12. doi: 10.1016/j.ajodo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Izquierdo PP, de Biasi RS, Elias CN, Nojima LI. Martensitic transformation of austenitic stainless steel orthodontic wires during intraoral exposure. Am J Orthod Dentofacial Orthop. 2010;138:714.e1–5. doi: 10.1016/j.ajodo.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Normando D, de Araujo AM, Marques Ida S, Barroso Tavares Dias CG, Miguel JA. Archwire cleaning after intraoral ageing: The effects on debris, roughness, and friction. Eur J Orthod. 2013;35:223–9. doi: 10.1093/ejo/cjr104. [DOI] [PubMed] [Google Scholar]
- 5.Bakdach WMM, Haiba M, Hadad R. Changes in surface morphology, chemical and mechanical properties of clear aligners during intraoral usage: A systematic review and meta-analysis. Int Orthod. 2022;20:100610. doi: 10.1016/j.ortho.2022.100610. [DOI] [PubMed] [Google Scholar]
- 6.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22:317–26. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Eliades T, Papadopulos JS, Eliades G, Silikas N, Watts DC. Multi-technique characterization of retrieved bone cement from revised total hip arthroplasties. J Mater Sci Mater Med. 2003;14:967–72. doi: 10.1023/a:1026350616079. [DOI] [PubMed] [Google Scholar]
- 8.Sifakakis I, Eliades T. Adverse reactions to orthodontic materials. Aust Dent J. 2017;62:20–8. doi: 10.1111/adj.12473. [DOI] [PubMed] [Google Scholar]
- 9.Kubala E, Strzelecka P, Grzegocka M, Lietz-Kijak D, Gronwald H, Skomro P, et al. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. BioMed Res Int 2018. 2018 doi: 10.1155/2018/6572381. 6572381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi TP, Upadhayay SN. An overview of orthodontic material degradation in oral cavity. Indian J Dent Res. 2010;21:275–84. doi: 10.4103/0970-9290.66648. [DOI] [PubMed] [Google Scholar]
- 11.Burrow SJ. Friction and resistance to sliding in orthodontics: A critical review. Am J Orthod Dentofacial Orthop. 2009;135:442–7. doi: 10.1016/j.ajodo.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Kusy RP, Whitley JQ. Friction between different wire-bracket configurations and materials. Semin Orthod. 1997;3:166–77. doi: 10.1016/s1073-8746(97)80067-9. [DOI] [PubMed] [Google Scholar]
- 13.Kusy RP, Whitley JQ, Ambrose WW, Newman JG. Evaluation of titanium brackets for orthodontic treatment: Part I. The passive configuration. Am J Orthod Dentofacial Orthop. 1998;114:558–72. doi: 10.1016/s0889-5406(98)70176-3. [DOI] [PubMed] [Google Scholar]
- 14.Daems J, Celis JP, Willems G. Morphological characterization of as-received and in vivo orthodontic stainless steel archwires. Eur J Orthod. 2009;31:260–5. doi: 10.1093/ejo/cjn104. [DOI] [PubMed] [Google Scholar]
- 15.Chang CJ, Lee TM, Liu JK. Effect of bracket bevel design and oral environmental factors on frictional resistance. Angle Orthod. 2013;83:956–65. doi: 10.2319/101612-808.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusy RP, Whitley JQ. Influence of fluid media on the frictional coefficients in orthodontic sliding. Semin Orthod. 2003;9:281–9. [Google Scholar]
- 17.Cury SE, Aliaga-Del Castillo A, Pinzan A, Sakoda KL, Bellini-Pereira SA, Janson G. Orthodontic brackets friction changes after clinical use: A systematic review. J Clin Exp Dent. 2019;11:e482–90. doi: 10.4317/jced.55676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcez AS, Suzuki SS, Ribeiro MS, Mada EY, Freitas AZ, Suzuki H. Biofilm retention by 3 methods of ligation on orthodontic brackets: A microbiologic and optical coherence tomography analysis. Am J Orthod Dentofacial Orthop. 2011;140:e193–8. doi: 10.1016/j.ajodo.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Houb-Dine A, Bahije L, Oualalou Y, Benyahia H, Zaoui F. Topographic and chemical surface modifications to metal brackets after a period in the mouth. Int Orthod. 2017;15:515–28. doi: 10.1016/j.ortho.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Grimsdottir MR, Gjerdet NR, Hensten-Pettersen A. Composition and in vitro corrosion of orthodontic appliances. Am J Orthod Dentofacial Orthop. 1992;101:525–32. doi: 10.1016/0889-5406(92)70127-V. [DOI] [PubMed] [Google Scholar]
- 21.Lee TH, Wang CC, Huang TK, Chen LK, Chou MY, Huang HH. Corrosion resistance of titanium-containing dental orthodontic wires in fluoride-containing artificial saliva. J Alloys Compd. 2009;488:482–9. [Google Scholar]
- 22.Huang TH, Ding SJ, Min Y, Kao CT. Metal Ion Release from New and Recycled Stainless Steel Brackets. Eur J Orthod. 2004;26:171–7. doi: 10.1093/ejo/26.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Doomen RA, Nedeljkovic I, Kuitert RB, Kleverlaan CJ, Aydin B. Corrosion of orthodontic brackets: Qualitative and quantitative surface analysis. Angle Orthod. 2022;92:661–8. doi: 10.2319/072321-584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirhashemi AH, Hosseini MH, Chiniforoush N, Soudi A, Moradi M. Shear bond strength of rebonded ceramic brackets using four different methods of adhesive removal. J Dent (Tehran) 2018;15:54–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Oesterle LJ, Shellhart WC. Effect of aging on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop. 2008;133:716–20. doi: 10.1016/j.ajodo.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Szczesio-Wlodarczyk A, Sokolowski J, Kleczewska J, Bociong K. Ageing of dental composites based on methacrylate resins—A critical review of the causes and method of assessment. Polymers (Basel) 2020;12:882. doi: 10.3390/polym12040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omar H, Haggag S, Ghoneima A. The effect of cigarette smoke on the shear bond strength of metallic and ceramic orthodontic brackets: An in vitro study. Int Orthod. 2020;18:121–6. doi: 10.1016/j.ortho.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Karamouzos A, Athanasiou AE, Papadopoulos MA. Clinical characteristics and properties of ceramic brackets: A comprehensive review. Am J Orthod Dentofacial Orthop. 1997;112:34–40. doi: 10.1016/s0889-5406(97)70271-3. [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulou E, Polychronis G, Konstantonis D, Sifakakis I, Zinelis S, Eliades T. A study of the mechanical properties of as-received and intraorally exposed single-crystal and polycrystalline orthodontic ceramic brackets. Eur J Orthod. 2020;42:72–7. doi: 10.1093/ejo/cjz024. [DOI] [PubMed] [Google Scholar]
- 30.Filho HL, Maia LH, Araújo MV, Eliast CN, Ruellas AC. Colour stability of aesthetic brackets: Ceramic and plastic. Aust Orthod J. 2013;29:13–20. [PubMed] [Google Scholar]
- 31.Castro SM, Ponces MJ, Lopes JD, Vasconcelos M, Pollmann MCF. Orthodontic wires and its corrosion—The specific case of stainless steel and beta-titanium. J Dent Sci. 2015;10:1–7. [Google Scholar]
- 32.Kusy RP. Orthodontic biomaterials: From the past to the present. Angle Orthod. 2002;72:501–12. doi: 10.1043/0003-3219(2002)072<0501:OBFTPT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Batista DM, Faccini M, Valarelli FP, Cançado RH, Oliveira RC, Oliveira RCG, et al. Attractiveness of different esthetic orthodontic wires. Dental Press J Orthod. 2020;25:27–32. doi: 10.1590/2177-6709.25.6.027-032.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prashant PS, Nandan H, Gopalakrishnan M. Friction in orthodontics. J Pharm Bioallied Sci. 2015;7(Suppl 2):S334–8. doi: 10.4103/0975-7406.163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Al Shayeb RA, Alhaija ESA, Al-Khateeb S, Rashaid AHB. The effect of intraoral aging of the working stainless steel archwire on the rate of premolar extraction space closure: A randomized clinical trial. Clin Oral Investig. 2022;26:3011–20. doi: 10.1007/s00784-021-04283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marques IS, Araújo AM, Gurgel JA, Normando D. Debris, roughness and friction of stainless steel archwires following clinical use. Angle Orthod. 2010;80:521–7. doi: 10.2319/081109-457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinelis S, Eliades T, Pandis N, Eliades G, Bourauel C. Why do nickel-titanium archwires fracture intraorally? Fractographic analysis and failure mechanism of in-vivo fractured wires. Am J Orthod Dentofacial Orthop. 2007;132:84–9. doi: 10.1016/j.ajodo.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 39.Kao CT, Huang TH. Variations in surface characteristics and corrosion behaviour of metal brackets and wires in different electrolyte solutions. Eur J Orthod. 2010;32:555–60. doi: 10.1093/ejo/cjp146. [DOI] [PubMed] [Google Scholar]
- 40.Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: Implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222–37. doi: 10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Singh V, Pokharel P, Pariekh K, Roy D, Singla A, Biswas K. Elastics in orthodontics: A review. Health Renaiss. 1970;0:49–56. [Google Scholar]
- 42.Leite LP, Bell RA. Adverse hypersensitivity reactions in orthodontics. Semin Orthod. 2004;10:P240–3. [Google Scholar]
- 43.Beattie S, Monaghan P. An in vitro study simulating effects of daily diet and patient elastic band change compliance on orthodontic latex elastics. Angle Orthod. 2004;74:234–9. doi: 10.1043/0003-3219(2004)074<0234:AIVSSE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Kamisetty SK, Nimagadda C, Begam MP, Nalamotu R, Srivastav T, GS S. Elasticity in Elastics-An in-vitro study. J Int Oral Health. 2014;6:96–105. [PMC free article] [PubMed] [Google Scholar]
- 45.Notaroberto DFC, Martins MME, Goldner MTA, Mendes AM, Quintão CCA. Force decay evaluation of latex and nonlatex orthodontic intraoral elastics: In vivo study. Dent Press J Orthod. 2018;23:42–7. doi: 10.1590/2177-6709.23.6.042-047.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pithon MM, Mendes JL, da Silva CA, Lacerda Dos Santos R, Coqueiro RD. Force decay of latex and non-latex intermaxillary elastics: A clinical study. Eur J Orthod. 2016;38:39–43. doi: 10.1093/ejo/cjv005. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Lv C, Yan F, Feng J. Force degradation of orthodontic latex elastics analyzed in vivo and in vitro. Am J Orthod Dentofacial Orthop. 2020;157:313–9. doi: 10.1016/j.ajodo.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Sauget PS, Stewart KT, Katona TR. The effect of pH levels on nonlatex vs latex interarch elastics. Angle Orthod. 2011;81:1070–4. doi: 10.2319/011811-34.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam TV, Freer TJ, Brockhurst PJ, Podlich HM. Strength decay of orthodontic elastomeric ligatures. J Orthod. 2002;29:37–43. doi: 10.1093/ortho/29.1.37. [DOI] [PubMed] [Google Scholar]
- 50.Andhare P, Datana S, Agarwal SS, Chopra SS. Comparison of in vivo and in vitro force decay of elastomeric chains/modules: A systematic review and meta analysis. J World Fed Orthod. 2021;10:155–62. doi: 10.1016/j.ejwf.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Al-Hussain ZAA, Nahidh M. Carbonated soft drinks and orthodontics: Review of literature. Turk J Orthod. 2021;34:136–42. doi: 10.5152/TurkJOrthod.2020.20107. [DOI] [PubMed] [Google Scholar]
- 52.Fang S, Zhong Y, Li M, Luo J, Khadka N, Jiang C, et al. Comparing two methods of orthodontics space closure: A randomized clinical trial. Int J Clin Exp Med. 2017;10:14667–72. [Google Scholar]
- 53.Angolkar PV, Arnold JV, Nanda RS, Duncanson MG., Jr Force degradation of closed coil springs: An in vitro evaluation. Am J Orthod Dentofacial Orthop. 1992;102:127–33. doi: 10.1016/0889-5406(92)70024-5. [DOI] [PubMed] [Google Scholar]
- 54.Santos AC, Tortamano A, Naccarato SR, Dominguez-Rodriguez GC, Vigorito JW. An in vitro comparison of the force decay generated by different commercially available elastomeric chains and NiTi closed coil springs. Braz Oral Res. 2007;21:51–7. doi: 10.1590/s1806-83242007000100009. [DOI] [PubMed] [Google Scholar]
- 55.Cox C, Nguyen T, Koroluk L, Ko CC. In-vivo force decay of nickel-titanium closed-coil springs. Am J Orthod Dentofac Orthop. 2014;145:505–13. doi: 10.1016/j.ajodo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oshagh M, Khajeh F, Heidari S, Torkan S, Fattahi HR. The effect of different environmental factors on force degradation of three common systems of orthodontic space closure. Dent Res J (Isfahan) 2015;12:50–6. doi: 10.4103/1735-3327.150331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nattrass C, Ireland AJ, Sherriff M. The effect of environmental factors on elastomeric chain and nickel titanium coil springs. Eur J Orthod. 1998;20:169–76. doi: 10.1093/ejo/20.2.169. [DOI] [PubMed] [Google Scholar]
- 58.Nightingale C, Jones SP. A clinical investigation of force delivery systems for orthodontic space closure. J Orthod. 2003;30:229–36. doi: 10.1093/ortho/30.3.229. [DOI] [PubMed] [Google Scholar]
- 59.Al-Jumaili K, Ali A. Evaluation and comparison of the effect of artificial saliva and mouthwash solution on force degradation of different types of orthodontic traction aids (comparative in vitro study) Al-Rafidain Dent J. 2011;11:52–62. [Google Scholar]
- 60.Mirhashemi AH, Khameneh NH, Shahpoorzadeh K, Shahroudi AS. Comparison of force decay pattern in orthodontic elastomeric chains and NiTi closed coil springs, affected by five different mouthwashes: An in vitro study. Dentistry 3000. 2021;9:95–106. [Google Scholar]
- 61.Robertson L, Kaur H, Fagundes NCF, Romanyk D, Major P, Flores Mir C. Effectiveness of clear aligner therapy for orthodontic treatment: A systematic review. Orthod Craniofac Res. 2020;23:133–42. doi: 10.1111/ocr.12353. [DOI] [PubMed] [Google Scholar]
- 62.Weir T. Clear aligners in orthodontic treatment. Aust Dent J. 2017;62:58–62. doi: 10.1111/adj.12480. [DOI] [PubMed] [Google Scholar]
- 63.Condo’ R, Pazzini L, Cerroni L, Pasquantonio G, Lagana’ G, Pecora A, et al. Mechanical properties of “two generations” of teeth aligners: Change analysis during oral permanence. Dent Mater J. 2018;37:835–42. doi: 10.4012/dmj.2017-323. [DOI] [PubMed] [Google Scholar]
- 64.Elkholy F, Schmidt S, Schmidt F, Amirkhani M, Lapatki BG. Force decay of polyethylene terephthalate glycol aligner materials during simulation of typical clinical loading/unloading scenarios. J Orofac Orthop. 2021 doi: 10.1007/s00056-021-00364-5. doi: 10.1007/s00056-021-00364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu JH, Kwon JS, Jiang HB, Cha JY, Kim KM. Effects of thermoforming on the physical and mechanical properties of thermoplastic materials for transparent orthodontic aligners. Korean J Orthod. 2018;48:316–25. doi: 10.4041/kjod.2018.48.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryokawa H, Miyazaki Y, Fujishima A, Miyazaki T, Maki K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod Waves. 2006;65:64–72. [Google Scholar]
- 67.Fang D, Zhang N, Chen H, Bai Y. Dynamic stress relaxation of orthodontic thermoplastic materials in a simulated oral environment. Dent Mater J. 2013;32:946–51. doi: 10.4012/dmj.2013-131. [DOI] [PubMed] [Google Scholar]
- 68.Gerard Bradley T, Teske L, Eliades G, Zinelis S, Eliades T. Do the mechanical and chemical properties of InvisalignTM appliances change after use? A retrieval analysis. Eur J Orthod. 2016;38:27–31. doi: 10.1093/ejo/cjv003. [DOI] [PubMed] [Google Scholar]
- 69.Chen S, Li S, Fang D, Bai Y. Quantification of metal trace elements in orthodontic polymeric aligners and retainers by inductively coupled plasma mass spectrometry (ICP-MS) Int J Clin Exp Med. 2016;9:16273–82. [Google Scholar]
- 70.Charavet C, Gourdain Z, Graveline L, Lupi L. Cleaning and disinfection protocols for clear orthodontic aligners: A systematic review. Healthcare (Basel) 2022;10:340. doi: 10.3390/healthcare10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katras S, Ma D, al Dayeh A, Tipton D. Bisphenol A release from orthodontic clear aligners: An in-vitro study. Recent Prog Mater. 2021;3:034. [Google Scholar]
- 72.Francisco I, Paula AB, Ribeiro M, Marques F, Travassos R, Nunes C, et al. The biological effects of 3d resins used in orthodontics: A systematic review. Bioengineering (Basel) 2022;9:15. doi: 10.3390/bioengineering9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.EFSA Panel on Food contact materials, enzymes, flavourings, and processing aids CEF. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs – Part 1: Exposure assessment. EFSA J. 2015;13:3978. [Google Scholar]
- 74.EFSA CEP Panel updates from November 2021 to April. 2022. [Last assessed on 2022 Aug 05]. Available from: https://www.foodpackagingforum.org/news/efsa-cep-panel-updates-from-november-2021-to-april-2022 .
- 75.Gayrard V, Lacroix MZ, Collet SH, Viguié C, Bousquet-Melou A, Toutain PL, et al. High bioavailability of Bisphenol A from sublingual exposure. Environ Health Perspect. 2013;121:951–6. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]