Abstract
Background
The basis for qualification for venom immunotherapy (VIT) is the fulfilment of both the clinical and immunological criteria. Diagnostic tests that confirm the immunological criterion of an IgE-mediated sensitization include skin prick tests (SPT), intradermal tests (IDT), and serum specific IgE (sIgE) for the culprit venom.
Objective
This study aimed to assess the usefulness of SPT as the immunological marker in the diagnosis of insect venom sensitization in children with history of systemic reaction (SR) to insect sting evaluated by means of I-IV-grades Mueller's scale. There are no such studies in children.
Methods
This cross-sectional study sample consisted of 416 children aged 3–18 years (mean age 10.6 ± 3.8), 76% males, all with the history of a systemic reaction (SR) after a Hymenoptera sting (48% of grade III/IV according to Mueller scale), diagnosed between 1999 and 2019 in the tertiary referral centre. The standard diagnostic tests were used. Specificity, sensitivity, and positive and negative predictive values were computed to assess the diagnostic properties of the clinical tests to distinguish between mild and severe SR. To assess the relative value of an individual test in predicting the qualification to VIT we incorporated the Shapley value (SV).
Results
Positive SPT results were found in up to no more than 3% of children; among them less than 1% had only positive SPT and were negative for sIgE and IDT. Approximately 85% of the children had detectable venom sIgE, followed by positive IDT (75%). Almost 70% of children had positive both sIgE and IDT results. In children with grade III/IV reaction, about 80% of children had positive results of both of these tests. sIgE and IDT had sensitivity >0.80, whereas SPT had high specificity (>0.97) in differentiating between mild and severe SR. Relative value of diagnostic tests in predicting qualification to VIT varied between venoms. Bee venom IDT had higher SV (0.052) than sIgE (0.041). In contrast, wasp venom sIgE had higher SV (0.075) than IDT (0.035).
Conclusion
SPTs are not an useful immunological marker of venom sensitization in children, and eliminating SPT does not result in a loss of diagnostic accuracy. Limiting diagnostics to venom sIgE and IDT would shorten the procedure and reduce costs. Future studies are needed to determine if venom sIgE as the first line diagnostic test, with IDT added only if the venom sIgE is undetectable, is an optimal diagnostic process.
Keywords: Insect venom allergy, Skin prick test, Intradermal test, Specific IgE, Venom immunotherapy
Graphical abstract
Data from a 20-year cross-sectional study of 416 children aged 3 to 18 (76% of boys) with a history of systemic reaction after a Hymenoptera sting (48% of grade III/IV reactions), aimed at the critical assessment of the usefulness of standard diagnostic tests (SPT, IDT, sIgE) in qualification for venom immunotherapy. Positive SPT results were found in up to no more than 3% of children, positive sIgE were present in up to 85%, followed by positive IDT (up to 76%). The percentage of children who were qualified for bee venom VIT with a positive any test result exceeded 70% for each type of test and was the highest for SPT. The percentage of children who were qualified for wasp venom VIT with a positive any test result ranged between 33% to 59%. No child was qualified for VIT based solely on the positive results of SPT regardless the kind of venom. SPTs are not an useful immunological marker of venom sensitization in children, and eliminating SPT does not result in a loss of diagnostic accuracy.
Introduction
According to the European Anaphylaxis Registry, insect venom is the most common cause of anaphylaxis in adolescents and one of the leading causes of anaphylaxis in children.1 Clinical history of systemic reaction (SR) to insect sting is of key importance for undertaking allergy diagnosis.2, 3, 4 If venom-induced clinical symptoms meet anaphylaxis criteria according to either one of its current definitions of the National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN) (2006) modified by the World Allergy Organization (WAO) (2019),5,6 diagnostic evaluation is required to confirm an IgE-dependent sensitization and venom immunotherapy (VIT) is indicated.2, 3, 4 Standard diagnostic methods for insect venom anaphylaxis include skin prick tests (SPT), intradermal tests (IDT), and analysis of serum specific IgE (sIgE) for venom extract. For additional diagnostic insight, measuring sIgE for venom components (component resolved diagnosis, CRD) may increase analytic sensitivity, detection of cross-reactivity and determination of individual sensitization profiles.4,7, 8, 9 The basophil activation test (BAT), increasingly used, may also be helpful in some doubtful cases.10
Skin testing is laborious and time-consuming, and might be perceived as painful. A critical evaluation of current approach to IDT in venom tested adults has been recently published by Quirt et al.11 The validity of the shortening IDT procedure in children, by limiting it to concentrations of venom from 0.01 mcg/ml to 1.0 mcg/ml, to reduce pain and costs, was previously documented,12 and applied by other authors.13 In this study, we critically evaluated the utility of the standard SPT testing in the diagnosis of children with history of SR to insect sting. There are no studies critically assessing the usefulness of SPT in the diagnosis of venom allergy in children and its application to initiation of VIT.
Methods
Sample description
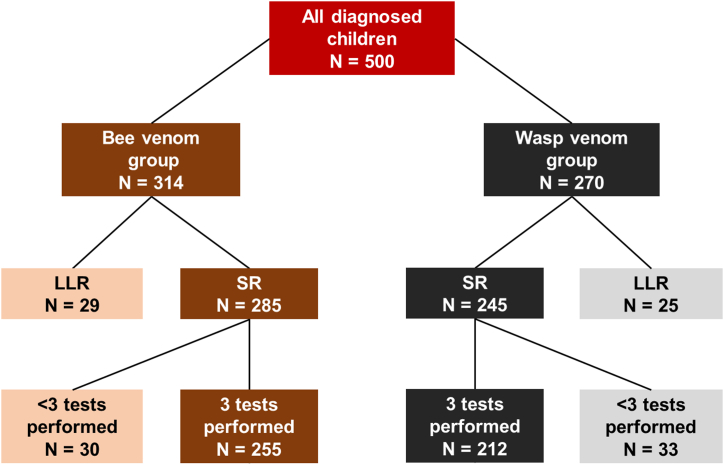
In the years 1999–2019, 500 children were admitted for one-day in-hospital diagnostic procedures due to a SR after an insect sting or due to large local reaction after an insect sting exclusively in the head or neck region. From this population, a group of 416 children, who developed SR grades I-IV according to Mueller's classification14 and had all 3 standard diagnostic tests (sIgE, IDT and SPT) performed, was selected. Details of the inclusion and exclusion criteria for this analysis are summarized in Fig. 1. The data were obtained from the tertiary referral pediatric center in southeastern Poland.
Fig. 1.
The chart of inclusion and exclusion criteria for the analysis
All the participants over 16 years and caregivers of those younger than 16 provided their written consents prior to any diagnostic procedures. The study was conducted in accordance with the principles of Declaration of Helsinki and was approved by local Bioethical Committee.
Diagnostic approach
Eligibility for VIT was evaluated using the criteria of the European Academy of Allergy and Clinical Immunology (EAACI) (2005, 2019), the American Academy of Asthma, Allergy and Immunology (AAAAI), and the American College of Asthma, Allergy and Immunology (ACAAI) (2017).2, 3, 4,7,14, 15, 16 The first visit was dedicated to completion of a detailed medical history using complete source records, collection of blood samples, and skin tests. All patients were tested for wasp and bee venom serum sIgE, and in selected cases, for hornet venom sIgE. Due to lack of a hornet venom diagnostic extract in Poland, the children suspected of hornet anaphylaxis, had their SPT and IDT performed with wasp venom and they were included in the wasp venom tested group during further analysis. The skin tests included SPT and IDT with either bee or wasp venom, based on patient's history. For children in whom the specific insect could not be identified on the basis of history, or those suspected of having SR after being stung by insects of both species, skin tests were performed with the venom of both species. In final analysis only those children who had a complete set of three tests (sIgE, SPT, IDT) for the venom of a given species were included. Children with negative results of all tests were scheduled for the follow-up visit in 3–6 months to repeat the complete series of diagnostic procedures. In such cases our analysis included only the results of the first set of diagnostic tests.
Due to the history of SR, all patients regardless of tests results, during the first visit, were trained in the treatment of anaphylaxis and provided with emergency medication, including self-administered adrenaline, per EAACI guidelines.17
Venom-sIgE was evaluated using ImmunoCAP® (Thermofisher Scientific, Sweden). Values equal to at least 0.35 kUa/l were regarded as detectable. SPT was performed with a commercial venom extract 100 mcg/ml (Pharmalgen Wasp Venom and Pharmalgen Bee Venom, ALK-Abelló A/S, Denmark); the results were regarded positive when wheal diameters caused by histamine and the specific venom were greater than or equal to 3 mm after 15 min. IDT were performed with venom concentrations of 0.0001 mcg/ml to 1.0 mcg/ml, during time period from 1999 to 2017, then starting from 2017 the venom concentrations were limited to 0.01 mcg/ml to 1.0 mcg, based on the results of study by Cichocka-Jarosz et al.12 Results of IDT were regarded as positive when wheal diameter for any venom concentration was equal or greater than 5 mm after 15 min.14 Skin puncture was performed by means of allergy lancets (Heinz Herenz Medizinalbedarf GmbH, Germany), a new sterile lancet was used for each puncture. Each child had 3 SPTs placed (histamine control, saline, and venom) and 3 or more IDTs.
Statistical analysis
Distributions of analyzed variables were presented as counts and percentages. Relationships between qualitative variables were analyzed using Fisher-Freeman-Halton Chi-square test. Differences between proportions of positive tests were performed using McNemar test for dependent samples proportions with continuity correction. Sensitivity, specificity, positive and negative predictive values were computed to assess the diagnostic properties of the clinical tests to distinguish between mild (grades I-II) and severe (grades III-IV) SRs.
To assess the relative value of particular diagnostic test or severity of reaction in predicting the qualification to VIT, we incorporated the Shapley value (SV), which is a solution concept originated from cooperative game theory.18 The SV was originally used for linear regression and R-squared as a measure of fit. To estimate the SV for a set of predictors it is necessary to estimate the R-squared for so called null model including none of the predictors (the prediction is based solely on the knowledge of distribution of dependent variable), then for the models including only 1 of each predictors, then for the models including each combination of 2 of the predictors, and more, reaching finally the model including all predictors. We used logistic regression model to predict qualification to VIT which is a binary variable, and as a measure of fit of the model we used the percent of correct classification of dependent variable categories, which was an input to calculate the SV. Therefore, in this paper, SV reflects the weighted average improvement in percent of correct classification, which particular diagnostic test or severity of reaction provide while predicting qualification to VIT. Therefore theoretically SV ranges from 0 to 1. However, as there is no computational possibility to estimate logistic regression model with no predictors, we assumed the percent of correct classification for the null model to be equal to the percentage of the children qualified to VIT for particular kind of venom. With such assumption the maximum of SV may not exceed the difference between 1 and this value. We used four variables to predict the qualification to VIT: severity of SR differentiating severe from mild reactions, and results of sIgE, IDT and SPT tests (indicating positive result of particular test).
Statistical analysis was performed using IBM SPSS Statistics 26 for Windows.
Results
Characteristics of study group
The children were in the age group 3–18 years, three-fourths of the sample were male. About one-half of the children had the history of grade III-IV SR to the sting. In total, 364 children or their parents were able to identify the culprit insect (Table 1). Out of 255 children tested for bee venom hypersensitivity (bee venom group) with 3 diagnostic tests (ie, SPT, IDT, and sIgE), 158 children (62%) were qualified for VIT based on the clinical criterion (systemic reaction grade II-IV in Mueller's classification) and the immunological criterion (positive result of at least 1 test confirming an IgE-dependent venom sensitization). In the group of 212 children tested by means of analogous procedures with wasp venom (wasp venom group), 106 children (50%) were qualified for VIT (Table 2). Among above mentioned children, 51 were tested with both venoms, out of which 2 were qualified for VIT with wasp venom and 5 were qualified for VIT with both venoms.
Table 1.
Demographic and clinical characteristics of the study groups divided according to the type of venom tested
| Bee venom | Wasp venom | Total | |
|---|---|---|---|
| No | 255 (100%) | 212 (100%) | 416 (100%) |
| Age | 10.5 ± 3.7 | 10.9 ± 3.8 | 10.6 ± 3.8 |
| Gender (male) | 189 (74%) | 164 (77%) | 315 (75.7%) |
| Mueller's grade | |||
| I | 60 (23%) | 57 (27%) | 92 (22%) |
| II | 77 (30%) | 64 (30%) | 123 (30%) |
| III | 68 (27%) | 52 (25%) | 117 (28%) |
| IV | 50 (20%) | 39 (18%) | 84 (20%) |
| Culprit insect recognized | |||
| Bee | 202 (79%) | 0 (0%) | 202 (49%) |
| Wasp | 0 (0%) | 154 (73%) | 154 (37%) |
| Bee and wasp | 6 (2%) | 6 (3%) | 6 (1%) |
| Hornet | 0 (0%) | 2 (1%) | 2 (1%) |
| Not established | 47 (19) | 50 (23%) | 52 (12%) |
Table 2.
Qualification for venom immunotherapy (VIT) with respect to the results of diagnostic tests
| Bee venom | p | Wasp venom | p | |
|---|---|---|---|---|
| Total | 158 (62%) | – | 106 (50%) | – |
| Severity | <0.001 | <0.001 | ||
| I & II Mueller's grade | 46 (34%) | 21 (17%) | ||
| III & IV Mueller's grade | 112 (95%) | 85 (93%) | ||
| sIgE | <0.001 | <0.001 | ||
| negative | 1 (3%) | 1 (3%) | ||
| positive | 157 (70%) | 105 (59%) | ||
| SPT | 0.046 | 1.000 | ||
| negative | 151 (61%) | 105 (50%) | ||
| positive | 7 (100%) | 1 (33%) | ||
| IDT | <0.001 | <0.001 | ||
| negative | 19 (28%) | 14 (28%) | ||
| positive | 139 (74%) | 92 (57%) | ||
| Number of positive tests | <0.001 | <0.001 | ||
| 0 | 0 (0%) | 0 (0%) | ||
| 1 | 19 (39%) | 15 (31%) | ||
| 2 | 133 (76%) | 90 (62%) | ||
| 3 | 6 (100%) | 1 (50%) | ||
| Distribution of positive results | <0.001∗ | <0.001∗ | ||
| None | 0 (0%) | 0 (0%) | ||
| sIgE only | 18 (43%) | 14 (44%) | ||
| SPT only | 0 (0%) | 0 (0.0%) | ||
| IDT only | 1 (14%) | 1 (7%) | ||
| sIgE & SPT | 1 (100%) | 0 (0%) | ||
| sIgE & IDT | 132 (75) | 90 (62%) | ||
| SPT & IDT | 0 (0%) | 0 (0%) | ||
| sIgE & SPT & IDT | 6 (100%) | 1 (50%) |
Numbers represent absolute count and relative percentage of the children qualified to VIT within a particular venom category as function of positive results in different test types, their cumulative number and their possible configurations respectively. ∗ - test performed for categories with at least 10 cases.
Abbreviations: IDT, intradermal test; sIgE, specific IgE; SPT, skin prick test; VIT, venom immunotherapy
Standard diagnostic methods
In both venom groups SPT was positive in no more than 3% of the children; however the percentage of children with positive results of only SPT was less than 1% (Fig. 2A and B). Positive results of sIgE and intradermal tests (IDT) were observed in about 85% and 75% of the children, respectively. About 68% of children have positive results of both sIgE and IDT, regardless of kind of venom (Fig. 2A and B). The proportion of positive sIgE was significantly higher than the proportion of positive IDT (p < 0.001 for bee venom group, p = 0.020 for wasp venom group), and both were significantly higher than the proportion of positive SPT (p < 0.001 for both comparisons), regardless of the kind of venom (Fig. 2A and B). No side effects were observed when performing both SPT and IDT.
Fig. 2.
A)Diagnostic tests' results in the study sample in bee venom group. B) Diagnostic tests' results in the study sample in wasp venom group. Abbreviations: IDT, intradermal test; sIgE, specific IgE; SPT, skin prick test; VIT, venom immunotherapy
In children with III/IV Mueller's grade these numbers markedly increased — in bee venom-tested children sIgE was positive in 94%, and IDT was positive in 86%. In wasp venom-tested children sIgE was positive in 92%, and IDT was positive in 81%. Summarizing in grade III/IV reactors, 80% of bee venom-tested and 79% of wasp venom-tested children had positive results of both of these tests, meanwhile, SPT was positive in only 5% of bee venom-tested and in 1% of wasp venom-tested children (Fig. 2A and B).
Among Mueller's grade III/IV reactors, 6 children tested for bee venom allergy (5%) and 6 children tested for wasp venom allergy (7%) had negative results in all 3 tests. In further follow-up evaluation all test results remained negative.
Predictive properties of tests and its usefulness for qualification to VIT
In the bee venom group, 94% of children with Mueller's grade IV after sting, 96% of children with grade III, 48% with grade II and 15% of children with Mueller's grade I, were qualified for bee venom VIT. The percentage of children who were qualified for bee venom VIT with a positive test result exceeded 70% for each type of test and was the highest for SPT. Out of the 7 children with positive bee venom SPT results, 86% (n = 6) had a positive also sIgE and IDT results. The remaining 14% (n = 1) had a positive result of sIgE only. All of them were qualified to VIT with bee venom.
No child was qualified for VIT based solely on the positive results of SPT with bee venom. In contrast, the fraction of the patients qualified for bee venom VIT with negative SPT result for bee venom was over two times higher than that of children with negative results of IDT and over 20 times higher than that of children with negative results of sIgE. The greater number of positive tests, the higher the percentage of children qualified for bee venom VIT (Table 2). In the sample of children qualified for bee venom VIT, positive results of sIgE, IDT, and SPT were observed in 99%, 88% and 4%, respectively.
In the wasp venom group, 95% of children with Mueller's grade IV after sting, 92% of children with grade III, 30% with grade II and 4% of children with Mueller's grade I, were qualified for VIT. The percentage of children who were qualified for wasp venom VIT, based on their positive SPT results was over 1.5 times lower than those who were qualified with positive results of sIgE or IDT. There were only 3 children with positive results of SPT to wasp venom, 67% (n = 2) of them had positive SPT concurrent with positive results of sIgE and IDT, while in case of 33% (n = 1) positive SPT was the only positive test. However this particular patient was not eligible for VIT since he was classified as Mueller's grade I.
No child was qualified for the wasp venom VIT based solely on the positive SPT. The fraction of children qualified for wasp venom VIT with a negative result of SPT to wasp venom was almost 1.5 times higher than that of children with negative results of IDT and over 15 times higher than that of children with negative results of sIgE. In the sample of patients with 2 or 3 positive wasp venom tests, there was almost twice as many children qualified for wasp venom VIT compared to the children with a single positive test (Table 2). The children qualified for wasp VIT showed positive results of sIgE, IDT, and SPT in 99%, 87%, and 1%, respectively.
The highest sensitivity (above 0.9) in discrimination between mild (Mueller's grades I/II) and severe (Mueller's grades III/IV) SRs after a sting in both species of venom was obtained for sIgE, and it was close to zero for SPT. On the contrary, the specificity was almost 1 for SPT, and did not exceed 0.36 for the other types of tests. The positive predictive value (PPV) for sIgE and IDT in both species of venom was about 0.5 and was lower than the PPV for SPT in both venoms. Negative predictive value (NPV) was the highest for sIgE (approx. 0.8) and the lowest (approx. 0.55) for SPT. Accuracy for all tests in both insect species ranged between 0.5 and 0.6 (Table 3).
Table 3.
Properties of different diagnostic tests in discrimination between I/II vs III/IV Muller grades for wasp and bee venom
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
|---|---|---|---|---|---|
| Bee venom | |||||
| sIgE | 0.94 | 0.18 | 0.50 | 0.77 | 0.53 |
| IDT | 0.86 | 0.36 | 0.54 | 0.75 | 0.59 |
| SPT | 0.05 | 0.99 | 0.86 | 0.55 | 0.56 |
| Wasp venom | |||||
| sIgE | 0.92 | 0.21 | 0.47 | 0.79 | 0.52 |
| IDT | 0.81 | 0.27 | 0.46 | 0.66 | 0.50 |
| SPT | 0.01 | 0.98 | 0.33 | 0.57 | 0.57 |
Relative value of intradermal testing as compared to detection of serum sIgE
The highest SV was estimated for the clinical manifestation of the allergic reaction according to Mueller's grade both in case of bee (0.086) and wasp (0.29) venoms. The SV for skin and blood tests differed between 2 venoms. For bee venom, higher SV was calculated for IDT (0.052) than for sIgE (0.041). In contrast, for wasp venom tests, higher SV was calculated for sIgE (0.075) than for IDT (0.035). The SV for SPT was equal to 0 for both kinds of venoms, indicating that, the results of SPT did not increase the quality of prediction of the patient's eligibility to initiate VIT (Table 4).
Table 4.
Percentage of correctly classified cases by logistic regression models including tests as a predictors of qualification to VIT for particular venom
| Bee venom | Wasp venom | |
|---|---|---|
| No predictors | 0.62 | 0.50 |
| 1 predictor | ||
| Mueller's grade | 0.80 | 0.87 |
| sIgE | 0.73 | 0.65 |
| IDT | 0.73 | 0.60 |
| SPT | 0.62 | 0.50 |
| 2 predictors | ||
| Mueller's grade & sIgE | 0.73 | 0.90 |
| Mueller's grade & IDT | 0.75 | 0.87 |
| Mueller's grade & SPT | 0.80 | 0.87 |
| sIgE & IDT | 0.73 | 0.66 |
| sIgE & SPT | 0.73 | 0.65 |
| IDT & SPT | 0.74 | 0.60 |
| 3 predictors | ||
| Mueller's grade & sIgE & IDT | 0.80 | 0.90 |
| Mueller's grade & sIgE & SPT | 0.73 | 0.90 |
| Mueller's grade & IDT & SPT | 0.75 | 0.87 |
| sIgE & IDT & SPT | 0.73 | 0.66 |
| 4 predictors | ||
| Mueller's grade & sIgE & IDT & SPT | 0.80 | 0.90 |
Abbreviations: IDT, intradermal test; sIgE, specific IgE; SPT, skin prick test; VIT, venom immunotherapy
Discussion
Diagnosis of allergy to insect venom in the light of current guidelines
Diagnostics of venom allergy is recommended only in these children in whom the severity of systemic symptoms after a sting justifies treatment with culprit insect VIT.2, 3, 4,7 Our study included only children with SR. In children with SR of I/II grades, diagnostic process was limited to the patients in whom a high risk of subsequent stings was associated with the proximity of the bee hives or with decreased quality of life due to high anxiety.
The WAO guidelines recommend SPT as the first level of diagnostic procedure in case of suspected type I, immediate, IgE-mediated venom allergy.19 The test is regarded as safe, of high enough sensitivity and good specificity when performed and interpreted correctly, while IDT, regarded as more sensitive than SPT, is usually done as the second step.2,14,19, 20, 21 However, it appears that sensitivity of venom SPT is rather low, reaching up to 64%, while combination of SPT with IDT reaches sensitivity about 94%; therefore, it is recommended to perform both tests sequentially.19, 20, 21, 22 In spite of the above results, SPT were commonly used as the diagnostic test in pediatric and adult centres performing Hymenoptera venom allergy (HVA) diagnostics in some European countries.23, 24, 25
The results presented in current paper suggest that SPT sensitivity in diagnostics of IgE-dependent HVA in children may be substantially over-estimated. In this study, the number of positive SPT results (3%) was over twenty times lower than the number of positive sIgE or IDT (over 70), regardless the kind of venom. An isolated positive SPT result occurred in less than 1% of children.
The skin tests, both SPT and IDT, were recognized by WAO as secure procedures.26 In line with the WAO Statement, systemic allergic reactions to venom skin tests were rare, and near-fatal or fatal reactions were exceedingly rare.27 Also in this study none of the children had side effects due to skin testing with any kind of venom. Even more, the simultaneous intradermal testing with different venoms was safe and efficient.28 In Strohmeier's study, 472 (98.7%) patients tolerated the simultaneous intradermal testing (0.02 ml of venom in concentrations from 0.001 up to 1.0 μg/ml of honey bee and wasp venom), without any side-effects.28 Despite a low risk of SRs, many scientific institutions both US and European, recommend to perform a graduated approach for venom skin testing.14,20, 21, 22,24,26
In doubtful cases, such as double sensitization or negative results of standard diagnostic tests, in a patient with confirmed history of SR, more advanced procedures of CRD with the evaluation of sIgE to certain venom components or basophil activation test (BAT) is recommended.2,4,7,8,10,14,21,24,29 The BAT should be carried out in highly specialized laboratories for diagnostic purposes, only in specific situations. Its role as a diagnostic tool in patients with mast cell disorders and negative venom-specific IgE and skin test results remains controversial.24 According to Korosec et al, BAT allowed to confirm Hymenoptera venom allergy in 81% of patients suspected of HVA with negative sIgE results and in 60% of patients who are negative for both sIgE and IDT with insect venom.30,31 The current manuscript does not address diagnostics by means of CRD and BAT.
In the era of personalized medicine, it is necessary to consider limiting procedures that are not relevant to clinical decision making.9,32, 33, 34 In earlier work by Cichocka-Jarosz et al, authors suggested exclusion of the 2 lowest venom concentrations (lower than 0.01 μg/ml) during IDT in children,12 while Quirt et al in the studies of IDT in adult patients, proposed reduction of the venom testing to 1 concentration equal to 1 μg/ml.11 Although a possibility of the single-step venom IDT is very attractive as safe and time-sparing, we would have some concern regarding its technical accuracy, possibility of false-positive results in case of a too deep puncture of the skin or false-negative results in case of a too superficial one, and difficulty of re-evaluation in case of a single intradermal puncture. The role of the proper technique during skin testing is also emphasized by other authors.35
Park et al, proposed an updated order of the validation tests based on results of their prospective study.36 The initial step should include the sIgE assessment. In case of negative results in a patient with clinical signs suggestive of a systemic allergic reaction, IDT should be performed.36 In Park's results, there was significant discordance between positive IDT and sIgE levels evaluated by ImmunoCAP® allergy panel; however, according to author's statement, they were complementary. It was especially pronounced in Vespide venoms. More than 47% of the case patients would have different venom immunotherapy prescriptions if ImmunoCAP® and IDT had been performed during initial diagnosis versus IDT alone.36 However, Park's results varied from these from our study indicating the clinically relevant difference between children and adults with respect to HVA diagnostics. In this study results of sIgE and IDT were concordant in about 79% of tested children, what suggested that most of diagnosed young patients might be qualified to VIT based on the results of one diagnostic tool in case of conclusive clinical indications for treatment.
The order of diagnostic procedures proposed by Park et al might be considered also in paediatric patients. Taking into account that in this study about 85% of children have positive venom serum sIgE, only about 15% of patients would need to be checked with another type of test (to exclude false-negative results). Since qualifying a child for VIT treatment requires a certain severity of symptoms after a sting and confirmation by any test of the IgE-mediated mechanism of this reaction, the use of such a procedure would save children (especially younger ones) from several painful skin punctures. In recent times, such a tendency is also observed in the management of allergy to inhaled and food allergens, due to the greater availability and less demanding (blood collection), though highly specialized procedure.37 It is currently the matter of debate, whether molecular allergy diagnosis will replace screenings by skin prick test in the future.38 For example, the ImmunoCAP test might be the preferred as a single test for possible allergy to nuts, wheat, other specific foods, and anaphylaxis of any cause, while SPT might be considered as the preferred test for latex allergy.37
Predictive properties of tests and its usefulness for qualification to VIT
Of all the diagnosed children, 50% in wasp venom group and 62% in bee venom group, were qualified for VIT. Among grade III/IV reactors over 90% of patients were qualified for VIT, regardless of kind of venom. The low percentage of children qualified for VIT with the history of grade I/II reaction, resulted from the fact that according to the guidelines they do not have an absolute indications for immunotherapy.2, 3, 4 After education in the field of prevention, clarification of clinical indications, and contraindications to VIT, as well as provision of intervention drugs, patients were instructed to return for re-evaluation in case of subsequent reaction to an insect sting. In the sample of children qualified for bee venom VIT, positive results of sIgE and IDT were observed in more than 85% of children whereas positive SPT in less than 5%. SPT result did not influence the decision to undertake VIT.
Sensitivity of analysed tests in discriminating between mild and severe SRs, was proportional to positivity of these tests in our sample. On the other hand, their specificity was proportional to percentage of negative tests results, being rather low for sIgE and IDT and very high for SPT. Both these facts resulted in accuracy about 0.5 for all the tests, suggesting that precision of predicting severity of reaction based on tests scores is slightly higher that predicting it just by chance. Similarly to adults the results of skin testing and levels of venom-specific IgE do not predict the degree of future systemic reactivity of untreated or treated patients.14,24,39
It should be also underlined that diagnostic procedures do not differ between children and adults,2,4,14,24 though the skin reactivity seems to be lower in pediatric patients.12 There is a concept that higher skin reactivity in adults is reflected by a lower concentration of venom extract yielding positive IDT results and greater wheal diameter. Hypothetically this may result from higher mast cell concentration in the forearm skin in adults; however, there is no literature about mast cell distribution in children's skin.40
Relative value of intradermal testing as compared to detection of sIgE
Coefficients such as sensitivity and specificity, estimate the value of a particular diagnostic test based solely on its results, therefore we considered necessary to estimate diagnostic value of certain test using method which takes into account the relationship between evaluated test and the other tests used in the same settings. We decided to use the Shapley value for its simplicity in computation and interpretation. Using this coefficient SPT demonstrated the most limited value (SV equaled 0) as a diagnostic tool in the approach to HVA children, both for wasp and bee venom. This meant that SPT results did not increase percentage of correct classifications, when predicting qualification to VIT, based on results of all performed diagnostics tests and severity of SR measured by means of Mueller's classification. This was consistent with the rate of SPT positive results in our study sample of children with SR to insect stings, which did not exceed 3%.
As concerns the other diagnostic tests, which positive results occurred several times more frequently than those of SPT, SV indicated that IDT and sIgE differ between venoms in terms of their relative value in determining qualification to VIT. In bee venom group, IDT was the test with greater impact on qualification to VIT than the sIgE, whereas in wasp venom group it was opposite. However in wasp venom sample the difference between SV values for both tests was greater than in bee venom group. Also SV estimated for severity of reaction is much smaller for bee than for wasp venom group. These differences originate from the fact that in bee venom group the percentage of children qualified to VIT (we assumed this value as the fit of the null model) is greater than in wasp venom group, and the possible improvement in correct classification when adding other predictors to the model is lower. Additionally in bee venom group there exist a predictor which was not included in SV analysis, namely vicinity to beehives, which diminishes impact of other factors in prediction of qualification to bee venom VIT.
Limitations and strengths of the study
Our retrospective study has some limitations. While wasp venom allergy is highly prevalent in Europe, our centre provides medical service to areas with a high prevalence of beekeeping; hence, a higher proportion of children of beekeepers would have been enrolled in the study than in the general population. We did not differentiate subspecies within Vespula species, assuming that Vespula germanica and Vespula vulgaris are the most common in the countries with the same geographic location as Poland. The distinction between them does not affect the choice of VIT preparation, which is universal for Vespula venom. So far, the problem of Polistes is marginal in Poland, which is a problem in most European countries and results from the sporadic occurrence of allergy to the venom of this insect, unlike in the Mediterranean countries.41 The fact of the lack of hornet venom for diagnosis and treatment may also be considered a limitation. However, strong, though individually variable, cross-reactivity occurs between the venoms of different Vespinae species (Vespula, Dolichovespula, Vespa). In Central Europe, anaphylaxis after European hornet stings is nearly always due to cross-reactivity with Vespula venom.42, 43, 44 For this reason immunotherapy with Vespula venom is justified in case of hornet venom anaphylaxis,45 though in patients with ascertained Vespa crabro-induced allergic reactions a specific Vespa crabro VIT, where available, would be more adequate, at least concerning the safety profile.46
The strengths of this study include a large cross-sectional pediatric sample, a uniform methodology, the use of data originating from a single reference centre over twenty consecutive years. Additionally, we rigorously followed methodology of the qualification for VIT process according to the guidelines, and applied advanced statistical analysis methods to verify the process.
In conclusion, SPT is a redundant immunological diagnostic marker in children and adolescents with insect venom allergy, since it does not significantly affects the decision to introduce VIT. Modified diagnostic approach in children meeting the clinical criterion of VIT may start from measuring venom serum sIgE test and following with IDT only in the event of its negative result. This would shorten the procedure and reduce costs, and possibly also improve the patient's quality of life.
Abbreviations
AAAAI, American Academy of Asthma, Allergy and Immunology; ACAAI, American College of Asthma, Allergy and Immunology; BAT, basophil activation test; CRD, component resolved diagnosis; EAACI, European Academy of Allergy and Clinical Immunology; HVA, Hymenoptera venom allergy; IDT, intradermal test; NIAID/FAAN, National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network; NPV, negative predictive value; PPV, positive predictive value; sIgE, specific IgE; SPT, skin prick test; SR, systemic reaction; SV, Shapley value; VIT, venom immunotherapy; WAO, World Allergy Organization.
Acknowledgements
We thank all parents and their children for their support in providing data.
Funding
No funding resources were engaged.
Data availability statement
The data that support the findings of this study are not publicly available due to the fact that their containing information could compromise the privacy of research participants but are available from the corresponding author upon a reasonable request (email address: ewa.cichocka-jarosz@uj.edu.pl).
Author contributions
E.C-J, P.B. contributed to conceptualization , E.C-J., U.J-W, N.M., B.K., Z.M-D contributed to data acquisition, P.B. contributed to statistical analysis, E.C-J., P.B, A.N-W., U.J-W. contributed to draft preparation, E.C-J., U.J-W., P.B., A.N-W., N.M., B.K., Z.M-D., G.L. contributed to review of the final draft. E.C-J., A.N-W., G.L. contributed to supervision, E.C-J. takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics statement
All the participants over 16 years and caregivers of the younger children provided their written consents prior to any diagnostic procedures. The study was conducted in accordance with the principles of Declaration of Helsinki. The use of the data was approved by the Bioethics Committee of the Jagiellonian University. Clinical trial Bioethics Committee number: KBET No 67/L/2007.
Submission declaration
All authors approved final version of manuscript. Manuscript has been neither published, nor stays under consideration to be published elsewhere.
Potential competing interests
The authors report no competing interests.
References
- 1.Grabenhenrich L.B., Dölle S., Moneret-Vautrin A., et al. Anaphylaxis in children and adolescents: the European anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Sturm G.J., Varga E.M., Roberts G., et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–764. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 3.Alvaro-Lozano M., Akdis C.A., Akdis M., et al. EAACI allergen immunotherapy user's guide. Pediatr Allergy Immunol. 2020;31(Suppl 25):S1–S101. doi: 10.1111/pai.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden D.B.K. Update on insect sting anaphylaxis. Curr Allergy Asthma Rep. 2021;21:16. doi: 10.1007/s11882-021-00998-w. [DOI] [PubMed] [Google Scholar]
- 5.Turner P.J., Worm M., Ansotegui I.J., et al. WAO Anaphylaxis Committee Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019;12 doi: 10.1016/j.waojou.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardona V., Ansotegui I.J., Ebisawa M., et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm G.J., Arzt-Gradwohl L., Varga E.M. Medical algorithms: diagnosis and treatment of hymenoptera venom allergy. Allergy. 2019;74:2016–2018. doi: 10.1111/all.13817. [DOI] [PubMed] [Google Scholar]
- 8.Bilò M.B., Corsi A., Pravettoni V., et al. Development of a model care pathway for the management of Hymenoptera venom allergy: evidence-based key interventions and indicators. Clin Transl Allergy. 2020;4:8. doi: 10.1186/s13601-020-00312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blank S., Grosch J., Ollert M., Bilò M.B. Precision medicine in hymenoptera venom allergy: diagnostics, biomarkers, and therapy of different endotypes and phenotypes. Front Immunol. 2020;22 doi: 10.3389/fimmu.2020.579409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos A.F., Alpan O., Hoffmann H.J. Basophil activation test: mechanisms and considerations for use in clinical trials and clinical practice. Allergy. 2021;76:2420–2432. doi: 10.1111/all.14747. [DOI] [PubMed] [Google Scholar]
- 11.Quirt J.A., Wen X., Kim J., Herrero A.J., Kim H.L. Venom allergy testing: is a graded approach necessary? Ann Allergy Asthma Immunol. 2016;116:49–51. doi: 10.1016/j.anai.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Cichocka-Jarosz E., Stobiecki M., Brzyski P., et al. Simplification of intradermal skin testing in Hymenoptera venom allergic children. Ann Allergy Asthma Immunol. 2017;118:326–332. doi: 10.1016/j.anai.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Schrautzer C., Arzt-Gradwohl L., Bokanovic D., et al. A safe and efficient 7-week immunotherapy protocol with aluminum hydroxide adsorbed vespid venom. Allergy. 2020;75:678–680. doi: 10.1111/all.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biló B.M., Rueff F., Mosbech H., Bonifazi F., Oude-Elberink J.N. EAACI interest group on insect venom hypersensitivity. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339–1349. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 15.Bonifazi F., Jutel M., Biló B.M., Birnbaum J., Muller U. EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005;60:1459–1470. doi: 10.1111/j.1398-9995.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 16.Abrams E.M., Golden D.B.K. Approach to patients with stinging insect allergy. Med Clin. 2020;104:129–143. doi: 10.1016/j.mcna.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bilò M.B., Cichocka-Jarosz E., Pumphrey R., et al. Self-medication of anaphylactic reactions due to hymenoptera stings-an EAACI task force consensus statement. Allergy. 2016;71:931–943. doi: 10.1111/all.12908. [DOI] [PubMed] [Google Scholar]
- 18.Shapley L.S. A value for n-person games. Contrib Theory Games. 1953;2(28):307–317. [Google Scholar]
- 19.Ansotegui I.J., Melioli G., Canonica G.W., et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2019.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden D.B., Demain J., Freeman T., et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Golden D.B.K. Insect sting allergy: new guidelines from the European and USA consensus groups: algorithms and recommendations. Curr Opin Allergy Clin Immunol. 2019;19:456–461. doi: 10.1097/ACI.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 22.Bilò M.B., Tontini C., Martini M., Corsi A., Agolini S., Antonicelli L. Clinical aspects of hymenoptera venom allergy and venom immunotherapy. Eur Ann Allergy Clin Immunol. 2019;51:244–258. doi: 10.23822/EurAnnACI.1764-1489.113. [DOI] [PubMed] [Google Scholar]
- 23.Cichocka-Jarosz E., Brzyski P., Jedynak-Wąsowicz U., Nittner-Marszalska M. Immunotherapy section of polish society of allergology working group. Current practices in diagnosis of hymenoptera venom allergy in Poland. Adv Dermatol Allergol. 2021;38:222–225. doi: 10.5114/ada.2021.106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilò M.B., Pravettoni V., Bignardi D., et al. Hymenoptera venom allergy: management of children and adults in clinical practice. J Investig Allergol Clin Immunol. 2019;29:180–205. doi: 10.18176/jiaci.0310. [DOI] [PubMed] [Google Scholar]
- 25.Diwakar L., Ewan P., Huber P.A., Clark A., Nasser S., Krishna M.T. The impact of national guidelines on venom immunotherapy practice in the United Kingdom. Clin Exp Allergy. 2016;46:749–753. doi: 10.1111/cea.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalski M.L., Ansotegui I., Aberer W., et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ J. 2016;9:33. doi: 10.1186/s40413-016-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sellaturay P., Nasser S., Ewan P. The incidence and features of systemic reactions to skin prick tests. Ann Allergy Asthma Immunol. 2015;115:229–233. doi: 10.1016/j.anai.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Strohmeier B., Aberer W., Bokanovic D., Komericki P., Sturm G.J. Simultaneous intradermal testing with hymenoptera venoms is safe and more efficient than sequential testing. Allergy. 2013;68:542–544. doi: 10.1111/all.12123. [DOI] [PubMed] [Google Scholar]
- 29.Cabrera C.M., Palacios-Cañas A., Joyanes-Romo J.B., Urra J.M., Mur P. Basophil activation test as alternative method to CAP-inhibition in patients with double sensitization to vespid venoms. Mol Immunol. 2022;149:59–65. doi: 10.1016/j.molimm.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Korosec P., Erzen R., Silar M., Bajrovic N., Kopac P., Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009;39:1730–1737. doi: 10.1111/j.1365-2222.2009.03347.x. [DOI] [PubMed] [Google Scholar]
- 31.Korošec P., Šilar M., Eržen R., et al. Clinical routine utility of basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol. 2013;161:363–368. doi: 10.1159/000348500. [DOI] [PubMed] [Google Scholar]
- 32.Jakob T., Rafei-Shamsabadi D., Spillner E., Müller S. Diagnostics in Hymenoptera venom allergy: current concepts and developments with special focus on molecular allergy diagnostics. Allergo J Int. 2017;26:93–105. doi: 10.1007/s40629-017-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiener M., Graessel A., Ollert M., Schmidt-Weber C.B., Blank S. Allergen-specific immunotherapy of Hymenoptera venom alle–gy - also a matter of diagnosis. Hum Vaccines Immunother. 2017;13:2467–2481. doi: 10.1080/21645515.2017.1334745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adkinson N.F., Jr., Hamilton R.G. Clinical history-driven diagnosis of allergic Diseases: utilizing in vitro IgE testing. J Allergy Clin Immunol Pract. 2015;3:871–876. doi: 10.1016/j.jaip.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Fatteh S., Rekkerth D.J., Hadley J.A. Skin prick/puncture testing in North America: a call for standards and consistency. Allergy Asthma Clin Immunol. 2014;10:44. doi: 10.1186/1710-1492-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H.J., Brooks D.I., Chavarria C.S., Wu R.L., Mikita C.P., Beakes D.E. Combining discordant serum IgE and skin testing improves diagnostic and therapeutic accuracy for hymenoptera venom hypersensitivity immunotherapy. J Allergy Clin Immunol Pract. 2022;10:837–843.e3. doi: 10.1016/j.jaip.2021.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths R.L.M., El-Shanawany T., Jolles S.R.A., et al. Comparison of the performance of skin prick, ImmunoCAP, and ISAC tests in the diagnosis of patients with allergy. Int Arch Allergy Immunol. 2017;172:215–223. doi: 10.1159/000464326. [DOI] [PubMed] [Google Scholar]
- 38.Jensen-Jarolim E., Jensen A.N., Canonica G.W. Debates in allergy medicine: molecular allergy diagnosis with ISAC will replace screenings by skin prick test in the future. World Allergy Organ J. 2017;10:33. doi: 10.1186/s40413-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollstein M.M., Matzke S.S., Lorbeer L., et al. Intracutaneous skin tests and serum IgE levels cannot predict the grade of anaphylaxis in patients with insect venom allergies. J Asthma Allergy. 2022;15:907–918. doi: 10.2147/JAA.S367272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssens A.S., Heide R., den Hollander J.C., Mulder P.G.M., Tank B., Oranje A.P. Mast cell distribution in normal adult skin. J Clin Pathol. 2005;58:285e289. doi: 10.1136/jcp.2004.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosch J., Lesur A., Kler S., et al. Allergen content of therapeutic preparations for allergen-specific immunotherapy of European paper wasp venom allergy. Toxins. 2022;14:284. doi: 10.3390/toxins14040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmer W. Kreuzreaktionen zwischen den Giften von Hymenopteren unterschiedlicher Familien, Gattungen und Arten [Cross reactions between Hymenoptera venoms from different families, genera and species] Hautarzt. 2014;65:775–779. doi: 10.1007/s00105-014-2776-5. [DOI] [PubMed] [Google Scholar]
- 43.Erzen R., Koren A., Selb J., et al. Clinical, serological and basophil response to a wasp sting in patients with European hornet sting anaphylaxis. Clin Exp Allergy. 2021;51:1641–1644. doi: 10.1111/cea.13998. [DOI] [PubMed] [Google Scholar]
- 44.Ludman S.W., Boyle R.J. Stinging insect allergy: current perspectives on venom immunotherapy. J Asthma Allergy. 2015;8:75–86. doi: 10.2147/JAA.S62288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Vázquez V., Armisén M., Gómez-Rial J., Lamas-Vázquez B., Vidal C. Immunotherapy with Vespula venom for Vespa velutina nigrithorax anaphylaxis: preliminary clinical and immunological results. Clin Exp Allergy. 2022;52:345–347. doi: 10.1111/cea.14039. [DOI] [PubMed] [Google Scholar]
- 46.Macchia D., Cortellini G., Mauro M., et al. Vespa crabro immunotherapy versus Vespula-venom immunotherapy in Vespa crabro allergy: a comparison study in field re-stings. World Allergy Organ J. 2018;11(1):3. doi: 10.1186/s40413-018-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to the fact that their containing information could compromise the privacy of research participants but are available from the corresponding author upon a reasonable request (email address: ewa.cichocka-jarosz@uj.edu.pl).