Abstract
Neoadjuvant chemotherapy is a therapeutic option for potentially resectable non‐small cell lung cancer (NSCLC). The role of neoadjuvant targeted therapy (NTT) remains less explored. This case highlights the use of neoadjuvant osimertinib in a case of advanced NSCLC. A 67‐year‐old woman had a left lower lobe lung mass measuring 5.0 × 5.1 × 7.0 cm with an enlarged subcarinal lymph node (LN) on her positron emission tomography scan. Following biopsy, a diagnosis of stage IIIB N2 (cT3N2M0) EGFR exon 19 deletion mutation‐positive lung adenocarcinoma was established. NTT using osimertinib 80 mg once daily was commenced. Subsequent re‐imaging at 3 months (ycT2bN2M0), 6 months (ycT1cN2M0) and 9 months showed tumour downstaging and resolution of the subcarinal LN (ycT1cN0M0). She underwent left lower lobectomy with systematic nodal dissection. All surgical specimens demonstrated no evidence of malignant cells (ypT0N0). Osimertinib could be the preferred NTT for potentially resectable NSCLC.
Keywords: neoadjuvant targeted therapy, non‐small cell lung cancer, Osimertinib, pathological complete response
The use of osimertinib as a safe and effective neoadjuvant targeted therapy for potentially resectable advance non‐small cell lung carcinoma.
INTRODUCTION
Osimertinib, a third‐generation Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitor (TKI), is a recognized first‐line treatment of metastatic EGFR‐mutant non‐small cell lung cancer (NSCLC). 1 However, the role of neoadjuvant EGFR‐TKI in NSCLC remains uncertain. We report a case of pathological complete response (pCR) defined as ‘the absence of residual viable tumour cells in all the pathological samples of the resected primary lung tumour and lymph nodes following neoadjuvant therapy (ypT0N0)’, with osimertinib in a patient with stage IIIB N2 EGFR‐mutant NSCLC.
CASE REPORT
A 67‐year‐old lady with bronchial asthma complained of worsening cough and mild wheezing for 4 months. Prior to this, her asthma had been well controlled on one puff of Budesonide/Formeterol (160 mcg/4.5 mcg) inhalation taken twice daily. There was no improvement despite optimizing her treatment. She has no other medical illness. She does not smoke and has no family history of malignancy. Physical examination of her respiratory system revealed no significant findings apart from occasional soft expiratory rhonchi heard over the left lower zone.
A computed tomography (CT) Thorax confirmed a left lower lobe lung lesion measuring 5.0 × 5.1 × 7.0 cm with an enlarged subcarinal lymph node (LN) (Figure 1A, B). A diagnosis of stage IIIB N2 (cT3N2Mx) EGFR exon 19 deletion mutation‐positive lung adenocarcinoma was established following endobronchial ultrasound‐guided fine needle aspiration (FNA) of the subcarinal LN and endoscopic‐endobronchial ultrasound‐guided FNA sampling of the left lower lobe mass (Figure 2A, B). The tumour cells also expressed 90% programmed death‐ligand 1(PD‐L1). Other molecular biomarker such as anaplastic lymphoma kinase (ALK) was negative. The patient's Eastern Cooperative Oncology Group (ECOG) performance status was 0 and she opted for curative surgical resection. A full body positron emission tomography‐computed tomography (PET‐CT) and a magnetic resonance imaging of the brain demonstrated absence of distant metastasis (cT3N2M0). Following discussion at the lung tumour board meeting, she was commenced on neoadjuvant targeted therapy (NTT) using osimertinib 80 mg once daily. Re‐imaging at 3 months (ycT2bN2M0) (Figure 1C, D) and 6 months demonstrated tumour downstaging (ycT1cN2M0) (stage IIIA) (Figure 1E, F). However, surgical resection was not possible because of the absence of a demarcation plane between the mass and descending aorta. At 9 months, the primary tumour had further shrunken in size to 2.0 × 2.2 × 1.8 cm and there was a resolution of the subcarinal LN (ycT1cN0M0) (Figure 1G, H). She underwent left lower lobectomy with systematic lymph node dissection (stations 11L, 4L, 7, 8 and 9 LNs). There was no evidence of residual malignant cells on histopathological examination of all surgical specimens (ypT0N0) (Figure 2C–F). Throughout her NTT, 6‐ and 12‐months postoperative period, she tolerated osimertinib well with only grade 1 skin reaction (dry skin) based on U.S. National Cancer Institute's Common Terminology Criteria for Adverse Events. 2 A 6‐ and 12‐months post resection PET‐CT demonstrated no recurrence. The patient was continued on osimertinib 80 mg once daily for 3 years post‐surgery.
FIGURE 1.
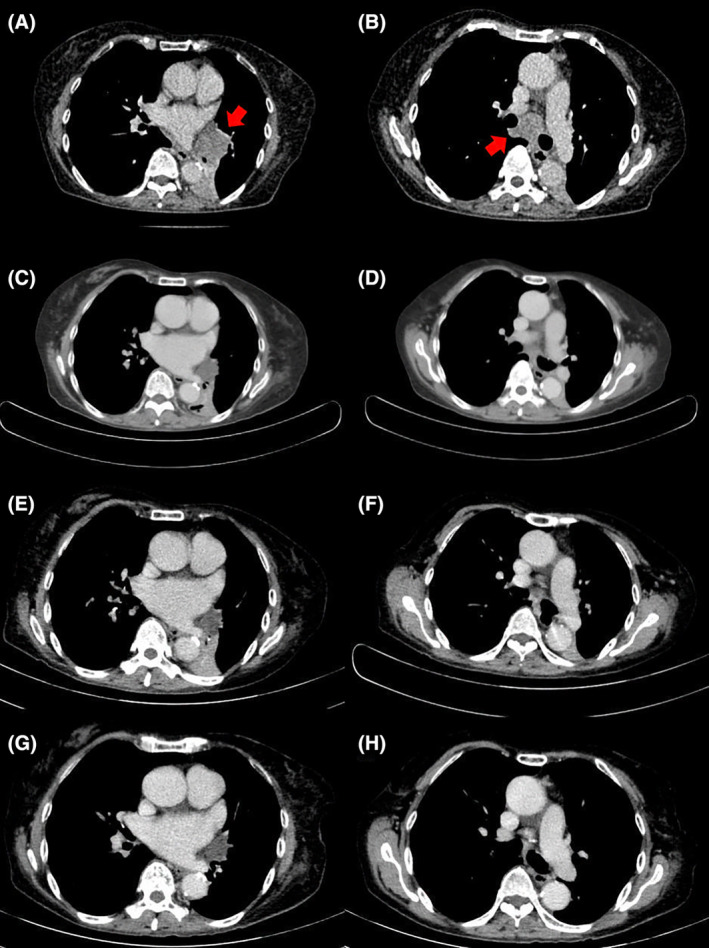
CT scan showing (A) Left lower lobe obstructing lung mass measuring 5.0 × 5.1 × 7.0 cm prior treatment (Red arrow) (B) Enlarged subcarinal lymph node 3.0 cm (LN) prior treatment (Red arrow) (C) Smaller left lower lobe mass measuring 2.4 × 3.1 × 4.0 cm after 3 months of treatment (D) Smaller subcarinal lymph node 1.9 cm after 3 months of treatment (E) Further reduction in left lower lobe obstructing lung mass measuring 2.8 × 2.1 × 2.0 cm at 6 months (F) Stable subcarinal lymph node (G) Left lower lobe mass measuring 2.2 × 1.8 cm (H) Resolved subcarinal lymph node.
FIGURE 2.
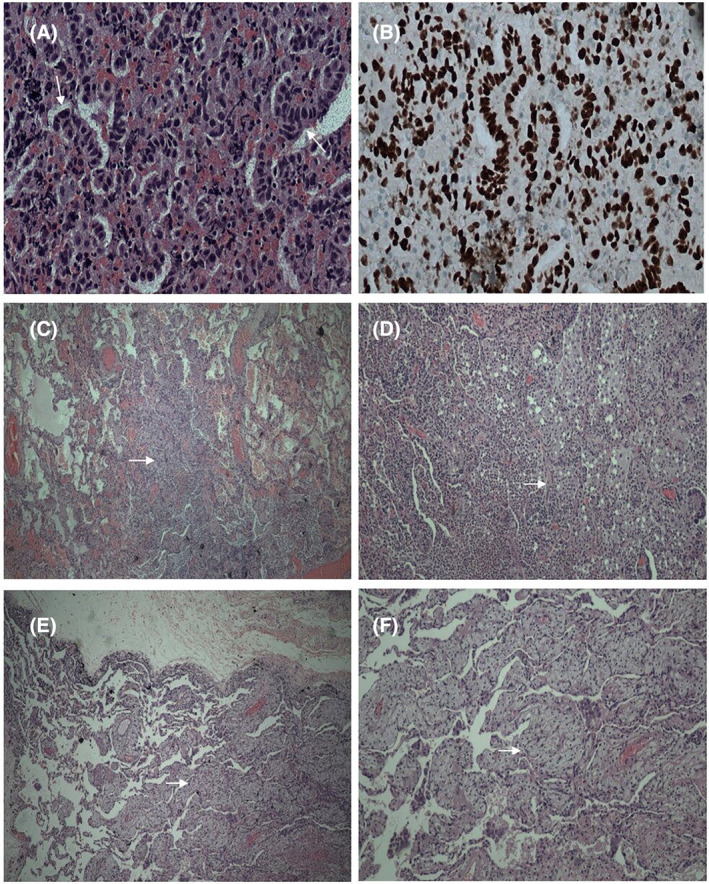
(A) Microscopic features of tissue clot coagulum from endoscopic needle aspirate showing malignant cells forming vague glandular architecture. The nuclei are enlarged, irregular and hyperchromatic (thin arrow) Haematoxylin & Eosin stain (H&E) ×400. (B) TTF‐1 immunohistochemical stain show positive staining in the nuclei H&E ×400. (C) Microscopical features of lung lobectomy specimen demonstrating no residual malignancy seen. Alveolar spaces are filled with mixed inflammatory cells (thin arrow) H&E ×100. (D) Neutrophilic aggregates fill the lung alveolar spaces H&E ×100. (E) Microscopical features of lung lobectomy specimen showing xanthogranulomatous like inflammation in the lung alveolar tissue (thin arrow) H&E ×100. (F) Sheets of foamy macrophages H&E ×200.
DISCUSSION
Neoadjuvant chemotherapy is a therapeutic option for cases of potentially resectable NSCLC. Recently, EGFR‐TKI‐NTT has also been explored because targeted therapy with EGFR‐TKIs results in better treatment outcomes and lower treatment‐related toxicity compared to platinum‐doublet chemotherapy in patients with metastatic NSCLC. 3 Although there is still a paucity of data on EGFR‐TKI‐NTT, some early reports supported its role. 3 , 4 , 5 , 6 In a case series of 13 patients, Hu et al has reported a 69.2% objective response rate, 100% complete resection rate and 100% pathologic downstaging rate with the use of osimertinib‐NTT. 7 When compared to conventional neoadjuvant chemotherapy, EGFR‐TKI‐NTT demonstrated higher objective response rate and median progression‐free survival. 4 However, in a pooled analysis of these early studies, NTT only resulted in a 14% downstaging and 0% pCR rates. 3 On the other hand, retrospective studies have reported pCR following neoadjuvant chemotherapy is associated with improved survival (HR for survival, 0.49%; 95% CI, 0.43–0.56). 8 But, the median rate of pCR following neoadjuvant chemotherapy is low (4%) (range, 0%–16%). 9 Interestingly, although previous neoadjuvant studies only involved patients up to stage IIIA disease, the phase II NEOS trial recruited patients with stage IIIb disease. 10 The preliminary results from the interim analysis of this study revealed that in a cohort of 28 patients, osimertinib is a potential agent for NTT with a 95% complete resection rate.
In our patient, osimertinib was used as NTT for stage IIIB (cT3N2M0) NSCLC with EGFR exon 19 deletion mutation. Generally, there is no consensus on the optimal duration of NTT, but it rarely exceeds 60 days based on previous studies. 4 This patient had an extended duration of NTT up to 9 months because complete surgical resection was not possible at 3 and 6 months, respectively, despite achieving a reasonable downstaging of the disease. Histopathological examination of the resected left lower lobe and the dissected LNs showed no evidence of malignant cells. Hence, confirming a pCR. Throughout the entire duration of her NTT and up to present, the patient only experienced mild side‐effect from osimertinib. This reinforces the better tolerated toxicity profile of EGFR‐TKI‐NTT compared to conventional cytotoxic chemotherapy. Nevertheless, although uncommon, it is important to monitor for potential serious effects of osimertinib when used for the long term, such as vision changes, interstitial lung disease, and arrythmias. Future studies are required to demonstrate the safety of osimertinib when used as EGFR‐TKI‐NTT.
In conclusion, this case provides some early insights on the efficacy and benefit of osimertinib‐NTT in pathologically downstaging and potentially achieving pCR in stage IIIB N2 NSCLC. The findings of this case report need to be confirmed by the outcome of the highly anticipated ongoing NeoADAURA (NCT04351555) clinical trial comparing three cycles (9 weeks) of neoadjuvant osimertinib with or without chemotherapy versus chemotherapy alone for EGFR‐mutated resectable (stage II‐IIIB N2) non‐squamous NSCLC. 11
AUTHOR CONTRIBUTIONS
Chun Ian Soo and Chong Kin Liam provided conceptualization and contributed equally for the case report. All authors have contributed substantially to the data acquisition and drafting of the original and revised manuscript and have read and approved the final version.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Soo CI, Ong DB‐L, Chin KK, Sia LC, Munusamy V, Ibrahim NH, et al. Pathological complete response in an advance stage IIIB non‐small cell lung cancer secondary to neoadjuvant osimertinib targeted therapy: A case report and review of literature. Respirology Case Reports. 2023;11:e01181. 10.1002/rcr2.1181
Associate Editor: David Lam
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non–small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. [DOI] [PubMed] [Google Scholar]
- 2. United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci). Common Terminology Criteria for Adverse Events (CTCAE). Ver. 5.0. Bethesda, MD. [cited 2023 Mar 1]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf
- 3. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT, et al. Neoadjuvant EGFR‐TKI therapy for EGFR‐mutant NSCLC: a systematic review and pooled analysis of five prospective clinical trials. Front Oncol. 2021;10:586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA‐N2 EGFR‐mutant non‐small‐cell lung cancer (EMERGING‐CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235–45. [DOI] [PubMed] [Google Scholar]
- 5. Belluomini L, Riva ST, Simbolo M, Nocini R, Trestini I, Avancini A, et al. Anticipating EGFR targeting in early stages of lung cancer: leave no stone unturned. Cell. 2021;10(10):2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu X, Zheng Y, Mai S, Tong Y, Yang L, Huang M, et al. Case report: an initially unresectable stage III pulmonary sarcomatoid carcinoma qith EGFR mutation achieving pathological complete response following neoadjuvant therapy with osimertinib plus chemotherapy. Front Oncol. 2022;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu Y, Ren S, Yang L, Tong Z, Wang R, Han W, et al. Osimertinib as neoadjuvant therapy for resectable non‐small cell lung cancer: a case series. Front Pharmacol. 2022;13:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waser NA, Adam A, Schweikert B, Vo L, McKenna M, Breckenridge M, et al. 1243P pathologic response as early endpoint for survival following neoadjuvant therapy (NEO‐AT) in resectable non‐small cell lung cancer (rNSCLC): systematic literature review and meta‐analysis. Ann Oncol. 2020;31:S806. [Google Scholar]
- 9. Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non‐small‐cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lyu C, Fang W, Ma H, Wang J, Jiao W, Wang R, et al. Osimertinib as neoadjuvant treatment for resectable stage II‐IIIB EGFR mutant lung adenocarcinoma (NEOS). J Clin Oncol. 2021;39(suppl 15):8524. [Google Scholar]
- 11. Tsuboi M, Weder W, Escriu C, Blakely C, He J, Dacic S, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR‐mutated resectable non‐small‐cell lung cancer: NeoADAURA. Future Oncol. 2021;17:4045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


