Abstract
Objective
To assess the association between the consumption of sugar-sweetened carbonated beverages (SSCB) and obesity in children and adolescents from Navarra (Spain).
Design
We used a matched case–control study design. The exposure, SSCB consumption (1 serving: 200 ml), was measured with a previously validated FFQ. Anthropometrical measures were taken using standardized protocols. The outcome, obesity, was defined as BMI above the age- and sex-specific 97th percentile according to the Spanish reference charts. In the analysis we used conditional logistic regression. Potential confounders were controlled using a multivariable model.
Setting
Subjects were recruited in the paediatric departments of the Universidad de Navarra Clinic and the Navarra Hospital Complex, and in three primary health centres of Navarra. Controls were recruited when attending for a routine medical examination or vaccination.
Subjects
One hundred and seventy-four obese children and 174 individually sex- and age-matched controls, 52·87 % boys, with a mean age of 11·6 years. Exclusion criteria were dietary interventions, exposure to hormone treatment, development of secondary obesity due to endocrinopathy and serious intercurrent illness.
Results
Independently of other factors, high consumption of SSCB (>4 servings/week) was significantly associated with obesity (OR = 3·46; 95 % CI 1·24, 9·62; P = 0·01). Besides, each additional daily serving of SSCB was associated with a 69 % relative increase in the risk of obesity (OR = 1·69; 95 % CI 1·04, 2·73; P = 0·03).
Conclusions
We found a strong and significant association between SSCB consumption and obesity risk. Our results suggest a monotonic dose–response linear shape for this association in children and adolescents (P for trend = 0·02).
Keywords: Soft drinks, Soda, Children, Obesity
Obesity is a chronic disease that results from an imbalance of energy homeostasis. The mechanisms underlying this metabolic disorder reflect complex interactions of genetic, environmental and behavioural factors. Many studies suggest that obesity increases the risk of many other chronic diseases such as type 2 diabetes, metabolic syndrome or some types of cancer, and that becoming obese earlier in life clearly amplifies that risk. The increasing prevalence of obesity among children and adolescents has become an important public health problem and a priority issue for authorities( 1 , 2 ).
The worldwide study conducted in 2004 by the International Association for the Study of Obesity( 1 ) found among children aged 5–17 years that 10 % were overweight and 2–3 % were obese, according to the International Obesity Task Force criteria. In Europe the prevalence of overweight or obesity is over 20 % with many differences among countries. According to the International Association for the Study of Obesity, Spain shows one of the highest prevalences of obesity in Europe, with 29·5 % of girls and 32·3 % of boys being overweight or obese. These numbers are similar to those from the Aladino study, conducted in Spain between October 2010 and May 2011 and including more than 7600 children aged 6–9 years( 3 ). The latter cross-sectional study found a prevalence of 26·2 % for overweight and a prevalence of 18·3 % for obesity among Spanish children.
The consumption of soft drinks has increased globally in parallel with the obesity epidemic. According to the Beverage Marketing Corporation, the US liquid refreshment beverage market grew by 1·0 % in 2012 with the production of 29·8 billion gallons, and carbonated soft drinks remain by far the biggest liquid refreshment beverage category( 4 ). Something similar happened in Spain. According to the latest data published by the Refreshment Beverages Association (ANFABRA), liquid refreshment beverage production increased by 1·4 % in 2007 with the production of 4400 million litres just in Spain( 5 ). Although sugar-free beverage production has increased, nowadays it represents only 25 % of the total production. The consumption of soft drinks in Spain increased by 41·5 % from 1991 to 2001 and is still on the rise( 6 ). According to the Dietary Intake National Survey conducted in 2008 in Madrid among children aged 5 to 12 years, the mean consumption of sugar-sweetened beverages was 55·7 ml/d (1·88 US fl oz/d). The consumption tends to increase with age, being twice as high among preadolescents compared with children aged 5 years (92·6 v. 45·2 ml/d (3·13 v. 1·53 US fl oz/d))( 7 ).
Many studies suggest that the obesity epidemic is strongly related to an increase in soft drink consumption( 8 – 10 ). The term ‘soft drink’ includes sodas and other sugar-sweetened beverages such as fruit-flavoured juices, lemonade and iced tea. The term ‘soda’ refers specifically to sugar-sweetened carbonated beverages (SSCB). Depending on the flavour and the brand, one soda provides 176 to 218 kJ (42 to 52 kcal) and 10·6 to 12·9 g of added sugar per 100 ml (3·38 US fl oz), which means 2·2–2·7 teaspoons of sugar per 100 ml (7·2–9 teaspoons of sugar in a regular can of 330 ml). This added sugar appears in the form of high-fructose corn syrup (45 % glucose and 55 % fructose) in the USA and in the form of sucrose (50 % glucose, 50 % fructose) in Europe. It has been suggested that the change in body fatness might be mediated principally by higher actual energy intake due to the amount of rapidly absorbable sugars and the lower satiety associated with liquid carbohydrates compared with carbohydrates consumed in solid form( 11 – 13 ). In addition, according to some authors, fructose, which is digested, absorbed and metabolized differently from glucose, is much more likely than glucose to be the culprit of the deleterious effects of added sugars in inducing weight gain and other metabolic traits. Fructose acutely increases thermogenesis, TAG levels and lipogenesis, as well as blood pressure, and raises uric acid, which has been defined as an important and independent predictor of obesity and metabolic syndrome. However, fructose does not enhance insulin secretion or leptin production, both important factors in food intake and body weight regulation( 14 – 18 ).
The aim of the present study was to assess the association between SSCB consumption and obesity in a matched case–control study conducted in Navarra (Spain).
Experimental methods
Participants
The study population was 348 children and adolescents (174 cases and 174 matched controls) aged 5·5–18·8 years (mean and median age: 11·6 years) enrolled in the GENOI (Grupo Navarro de Estudio de la Obesidad Infantil) case–control study, started in 2001( 19 ). Participants were recruited from the paediatric departments at the Navarra Hospital Complex and the University of Navarra Clinic and from three primary health centres of Navarra (Spain). Cases (n 174) were children/adolescents with BMI above the age- and sex-specific 97th percentile according to the Spanish BMI reference charts( 20 ). Exclusion criteria were dietary intervention, exposure to hormone treatment, development of secondary obesity due to endocrinopathy and serious intercurrent illness. Controls (n 174) were healthy children/adolescents with BMI below the 97th percentile of the same reference charts, recruited when they attended the primary care centres for a routine medical examination or vaccination. Controls were individually matched to cases by sex and age (±6 months).
Anthropometric measures were collected in a medical environment by trained personnel using standard procedures. Height was measured to the nearest centimetre and weight to the nearest 100 g with a digital balance (TBF-300A Body Composition Analyser/Scale; TANITA, Tokyo, Japan). Body fat mass percentage was determined by bioelectrical impedance (TBF-300A Body Composition Analyser/Scale; TANITA).
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the correspondent ethics committee. Written informed consent was obtained from participants older than 12 years and the parents of younger participants.
Exposure assessment
A trained researcher conducted individual interviews with each participant and his/her parents according to standardized protocols. Information about SSCB consumption was obtained from a previously validated semi-quantitative FFQ( 21 , 22 ). For each item in the questionnaire an average portion size was defined and each participant and his/her parents were asked how often they had consumed them throughout the previous year. One serving of SSCB was estimated as 200 ml (6·76 US fl oz). The FFQ offered nine possible response categories ranging from ‘never or almost never’ to ‘six or more times a day’. From this information, four different categories were defined for SSCB consumption: (i) never or almost never, (ii) <1 serving/week, (iii) 1–4 servings/week and (iv) >4 servings/week. The total consumption per day was calculated for each participant from the information in the FFQ.
Statistical analysis
Student's t test and the χ 2 test were used when comparing means and proportions. A P value ≤0·05 was set as the statistical significance level for all statistical analyses.
Conditional multivariable logistic regression models were used to assess the association between SSCB consumption and obesity among children and adolescents. Cases and controls were matched by sex and age. We calculated the conditional (matched) odds ratio and its 95 % confidence interval for obesity associated with each category of SSCB consumption. Additionally, the consumption of SSCB was introduced in the model as a continuous variable and we calculated the odds ratio and 95 % confidence interval for obesity associated with each additional daily serving of SSCB (200 ml, 6·76 US fl oz). Potential confounders were taken into account in the multivariable model.
The association between SSCB consumption and continuous variables, such as BMI or body fat mass percentage, was assessed by a multivariable-adjusted linear regression model. We used the first category (never or almost never consumption) as the reference category for all analyses.
Besides the information about SSCB consumption, the FFQ included 118 other food items, grouped in the following categories: dairy products, eggs, meat and fish, vegetables, fruits, legumes and cereals, fats and oils, bakery, beverages and other foods not included in the previous categories. The beverage section of the FFQ included not only SSCB, but also other soft drinks such as fruit juices, fruit-flavoured juices, non-caloric or diet beverages and water. Total energy intake was estimated from the data in the FFQ.
Many different foods and dietary patterns have been suggested to be associated with obesity. We calculated the adherence to the Mediterranean dietary pattern according to the index described by Trichopoulou et al. In our study, the score ranged from 0 to 8 because alcohol intake was excluded from the index( 23 ). Nowadays, the Mediterranean dietary pattern is being displaced by a Westernized pattern, characterized by higher fast-food consumption. The indicator of fast-food consumption in our study was estimated as the sum of three items of the FFQ: pizza, hamburgers and sausages( 10 ). In order to adjust by potential confounders, we introduced this indicator and other sugar-sweetened beverages, such as fruit-flavoured juices, in the multivariable model.
The interviews also included a previously validated physical activity questionnaire containing seventeen activities and ten possible response categories for frequency, ranging from ‘never’ to ‘eleven hours or more per week’( 24 ). Based on published studies, a multiple of resting metabolic rate was assigned to each activity (MET-h). We defined an activity metabolic equivalent index and calculated the physical activity developed by each participant (MET-h/week). Sedentary behaviour was estimated as the sum of hours spent watching television and in front of the computer. Physical activity and sedentary behaviour were introduced separately and as continuous variables in the multivariable model to control for their potential confounding effect.
We used the residual method (linear regression model) to calculate the age-adjusted BMI. An ANCOVA was used to calculate the sex- and age-adjusted mean and 95 % confidence interval of BMI across categories of SSCB consumption.
Results
Table 1 shows the main baseline characteristics of both case and control groups. As expected, cases and controls were very different in anthropometric indices. Cases showed a significantly higher BMI Z-score and body fat mass percentage than controls (both P < 0·01). We found an unexpected significantly higher total energy intake in controls than in cases (P < 0·01), which might be related to higher energy expenditure in controls and higher prevalence of sedentary lifestyles in cases. In fact, we found significantly higher physical activity in controls (P < 0·01). The sedentary behaviour was included separately in the multivariable analysis. Cases reported more hours per week watching television or using a computer, but this difference was not statistically significant.
Table 1.
Baseline main characteristics of the participants: obese children (cases) and individually sex- and age-matched controls aged 5·5–18·8 years (mean age 11·6 years), Navarra (Spain)
| Controls (n 174) | Cases (n 174) | ||||
|---|---|---|---|---|---|
| Mean or n | sd or % | Mean or n | sd or % | P | |
| Sex (male) | 92 | 52·87 | 92 | 52·87 | 1 |
| Age (years) | 11·60 | 2·71 | 11·50 | 2·62 | 0·73 |
| BMI (kg/m2) | 19·03 | 2·80 | 27·78 | 4·67 | <0·01 |
| BMI Z-score | 0·20 | 0·89 | 3·82 | 1·65 | <0·01 |
| Body fat mass percentage | 18·19 | 8·09 | 35·39 | 7·25 | <0·01 |
| SSCB (servings/d)* | 0·23 | 0·46 | 0·45 | 1·01 | <0·01 |
| Never or almost never | 68 | 39·08 | 56 | 32·18 | |
| <1 serving/week | 35 | 20·11 | 28 | 16·09 | |
| 1–4 servings/week | 57 | 32·76 | 64 | 36·78 | |
| >4 servings/week | 14 | 8·05 | 26 | 14·94 | |
| Fruit-flavoured juice (servings/d)* | 0·82 | 1·64 | 0·73 | 1·70 | 0·56 |
| Energy intake (kJ/d) | 13 414 | 2880 | 11 911 | 381 | <0·01 |
| Physical activity (MET-h/week) | 36 | 21 | 20 | 12 | <0·01 |
| TV or PC use (h/week) | 16·21 | 10·79 | 18·00 | 10·64 | 0·12 |
| Adherence to the MDP (0–8 points)† | 3·09 | 1·52 | 3·14 | 1·54 | 0·77 |
| Fast food (servings/d)‡ | 0·26 | 0·22 | 0·31 | 0·27 | 0·06 |
| Commercial bakery items (servings/d) | 1·81 | 1·30 | 1·72 | 1·37 | 0·51 |
SSCB, sugar-sweetened carbonated beverage; MET, metabolic equivalent of task; TV, television; PC, personal computer; MDP, Mediterranean dietary pattern.
*1 SSCB serving = 200 ml (6·76 US fl oz).
†Adaptation of the index described by Trichopoulou et al.( 22 ). The consumption of alcohol was excluded in this modified index.
‡Fast-food consumption indicator was calculated as the sum of hamburger, pizza and sausage consumption.
As also shown in Table 1, the estimated daily consumption of SSCB, calculated from the FFQ, was significantly higher in cases than controls (P < 0·01); on the contrary, cases reported lower consumption of fruit-flavoured juices but this difference was not statistically significant.
We found a similar and low adherence to the Mediterranean dietary pattern in both groups. Cases exhibited a higher consumption of fast food but a lower consumption of commercial bakery items than controls, although these differences were not statistically significant.
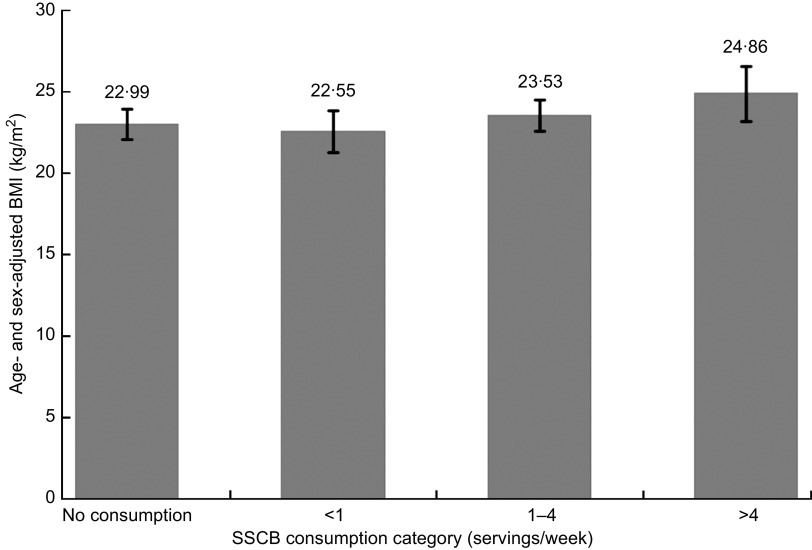
Figure 1 shows the mean sex- and age-adjusted BMI values across categories of SSCB consumption. Higher categories of SSCB consumption were not statistically different from the lowest category regarding average BMI, but we did find a significant trend (P for trend = 0·043) supporting a direct association between higher SSCB consumption and higher sex- and age-adjusted BMI.
Fig. 1.
Age- and sex-adjusted BMI in each category of sugar-sweetened carbonated beverage (SSCB) consumption (1 SSCB serving = 200 ml (6·76 US fl oz)) among 174 obese children (cases) and 174 individually sex- and age-matched controls aged 5·5–18·8 years (mean age 11·6 years), Navarra (Spain). Values are means with their 95 % confidence intervals represented by vertical bars; P for trend = 0·043
We calculated the odds ratio and its 95 % confidence interval for obesity associated with each category of SSCB consumption. As shown in Table 2, independently of other factors, the point estimate of the odds ratio was above the null value for all categories of SSCB consumption, but it was not significant for the intermediate categories (<1 or 1–4 servings/week). Nevertheless, we found a direct, strong and significant association for the highest category of SSCB consumption (>4 servings/week) and obesity (OR = 2·25; 95 % CI 1·12, 5·19; P = 0·02), which became stronger when confounders were controlled for in the multivariable model (OR = 3·46; 95 % CI 1·24, 9·62; P = 0·01).
Table 2.
Odds ratios and 95 % confidence intervals for obesity according to SSCB consumption among 174 obese children (cases) and 174 individually sex- and age-matched controls aged 5·5–18·8 years (mean age 11·6 years), Navarra (Spain). ‘Never or almost never consumption’ was considered as the reference category (1 SSCB serving = 200 ml (6·76 US fl oz))
| OR | 95 % CI | P | |
|---|---|---|---|
| Model 1 | |||
| For each + 1 daily serving consumption | 1·56 | 1·09, 2·25 | 0·01 |
| Consumption category | |||
| Never or almost never | 1·00 | – | |
| <1 serving/week | 0·94 | 0·51, 1·73 | 0·85 |
| 1–4 servings/week | 1·40 | 0·86, 2·29 | 0·17 |
| >4 servings/week | 2·25 | 1·12, 5·19 | 0·02 |
| Model 2 | |||
| For each + 1 daily serving consumption | 1·69 | 1·04, 2·73 | 0·03 |
| Consumption category | |||
| Never or almost never | 1·00 | – | |
| <1 serving/week | 1·11 | 0·46, 2·69 | 0·80 |
| 1–4 servings/week | 1·52 | 0·75, 3·08 | 0·24 |
| >4 servings/week | 3·46 | 1·24, 9·62 | 0·01 |
SSCB, sugar-sweetened carbonated beverage.
Model 1: adjusted for sex and age (matching variables).
Model 2: adjusted for sex, age, total energy intake, physical activity, sedentary behaviour (time spent watching television or using a computer), fast-food consumption (pizza, hamburgers and sausages) and other sugar-sweetened beverage consumption (fruit-flavoured juices).
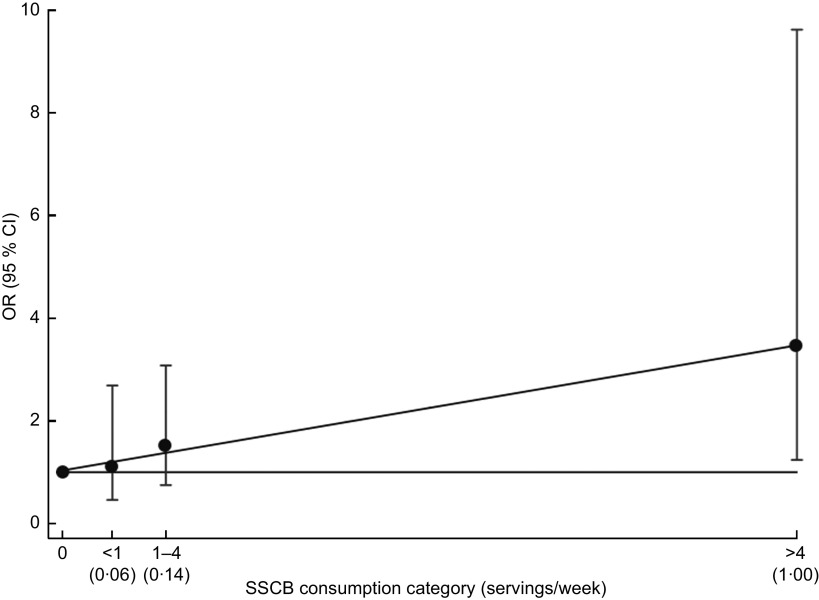
Figure 2 shows the linear trend for obesity and SSCB consumption. We represented the previously defined four categories of consumption and used the same reference category. We used a continuous scale and each category is represented according to the median value of SSCB consumption within that category. We drew a fitting line that showed a significant linear trend (P for trend = 0·02), suggesting a monotonic dose–response effect of SSCB consumption on obesity among children and adolescents.
Fig. 2.
Odds ratio (•) and 95 % confidence interval (represented by vertical bar) for obesity in each category of sugar-sweetened carbonated beverage (SSCB) consumption (1 SSCB serving = 200 ml (6·76 US fl oz)) among 174 obese children (cases) and 174 individually sex- and age-matched controls aged 5·5–18·8 years (mean age 11·6 years), Navarra (Spain). The linear trend is represented using the median consumption (servings/d) for each category in the x-axis; P for trend = 0·02
When we assessed SSCB consumption as a continuous exposure (Table 2), we found a significant association for obesity and each additional daily serving of SSCB (OR = 1·56; 95 % CI 1·09, 2·25; P = 0·01), which remained significant in the multivariable model (OR = 1·69; 95 % CI 1·04, 2·73; P = 0·03) after adjusting for age, sex, total energy intake, physical activity, sedentary behaviour, fast-food and fruit-flavoured juice consumption.
In an ancillary analysis, we assessed age-adjusted BMI as the dependent variable (data not shown). We used a logarithmic transformation in order to achieve a normal distribution of the data. The adjusted multivariable linear regression model showed a significant association between age-adjusted BMI and each daily serving of SSCB consumption (β = +0·03 kg/m2; 95 % CI +0·001, +0·06 kg/m2; P = 0·038). When we studied the different categories of SSCB consumption, the adjusted multivariable linear regression model showed a significant association for age-adjusted BMI and SSCB consumption of >4 servings/week v. the category of reference (β = +0·07 kg/m2; 95 % CI +0·003, +0·15 kg/m2; P = 0·042).
The assessment of the association between SSCB consumption and body fat mass percentage is shown in Table 3. We found a direct but non-significant association for the intermediate v. the lowest category of SSCB consumption and body fat mass percentage (β = +2·82 in the crude model and β = +2·15 in the adjusted model). Nevertheless, our results showed a direct and significant association between body fat mass percentage and the highest v. the lowest category of SSCB consumption (β = +5·14; 95 % CI +0·98, +9·30; P = 0·01) that remained significant in the multivariable-adjusted model (β = +4·80; 95 % CI +1·04, +8·56; P = 0·01). We also found a significant and direct association between each daily extra serving of SSCB and body fat mass percentage in both the crude (β = +1·83; 95 % CI +0·32, +3·35; P = 0·02) and the adjusted model (β = +1·47; 95 % CI +0·11, +2·82; P = 0·03).
Table 3.
Beta coefficients and 95 % confidence intervals for body fat mass percentage according to SSCB consumption among 174 obese children (cases) and 174 individually sex- and age-matched controls aged 5·5–18·8 years (mean age 11·6 years), Navarra (Spain). ‘Never or almost never consumption’ was considered as the reference category (1 SSCB serving = 200 ml (6·76 US fl oz))
| β | 95 % CI | P | |
|---|---|---|---|
| Model 1 | |||
| For each + 1 daily serving consumption | +1·83 | +0·32, +3·35 | 0·02 |
| Consumption category | |||
| Never or almost never | 0·00 | – | |
| <1 serving/week | −0·06 | −3·46, +3·34 | 0·97 |
| 1–4 servings/week | +2·82 | −0·07, +5·72 | 0·06 |
| >4 servings/week | +5·14 | +0·98, +9·30 | 0·01 |
| Model 2 | |||
| For each + 1 daily serving consumption | +1·47 | +0·11, +2·82 | 0·03 |
| Consumption category | |||
| Never or almost never | 0·00 | – | |
| <1 serving/week | −0·06 | −3·23, +3·11 | 0·97 |
| 1–4 servings/week | +2·15 | −0·56, +4·87 | 0·12 |
| >4 servings/week | +4·80 | +1·04, +8·56 | 0·01 |
SSCB, sugar-sweetened carbonated beverage.
Model 1: adjusted for sex and age (matching variables).
Model 2: adjusted for sex, age, total energy intake, physical activity, sedentary behaviour (time spent watching television or using a computer), fast-food consumption (pizza, hamburgers and sausages) and other sugar-sweetened beverage consumption (fruit-flavoured juices).
Discussion
The present case–control study suggests a monotonic, linear dose–response direct association between SSCB consumption and obesity among children and adolescents (P for trend = 0·02). We found that, independently of other factors, each daily serving of SSCB was significantly associated with a higher risk of obesity (P = 0·03). We also found a strong and significant association for obesity and the highest category of SSBC consumption (>4 servings/week) v. the category of reference (never or almost never; P = 0·01).
The inclusion of BMI as the dependent variable in the multivariable analysis may provide useful information, especially for clinicians. Our results showed, independently of other factors, a direct, monotonic and significant association between each extra daily serving of SSCB and age-adjusted BMI (P = 0·038), as well as a significant difference in age-adjusted BMI between the highest v. the lowest category of SSCB consumption (P = 0·042).
According to our results, the consumption of >4 servings SSCB/week was significantly associated with a 4·80 % increment in body fat mass percentage (P = 0·012), independently of other factors. Our results also suggest that, independently of other factors, each extra daily serving of SSCB was significantly associated with a 1·47 % increment in body fat mass percentage (P = 0·03). These results agree with the published literature, which considers that the association between SSCB and obesity may be explained by increments in body fat percentage( 11 – 13 ).
The association between soft drinks and obesity is controversial. The interpretation of published studies finding no association is difficult because of small sample sizes, short duration of follow-up, some method-related issues and possible uncontrolled confounding( 25 – 27 ). Two systematic reviews published in 2006 and 2007 found sufficient evidence to recommend a population-level reduction in soft drink consumption( 28 , 29 ). A recent systematic review published in 2013 found twenty-one cohort studies in children which assessed the effect of sugar intake on body fatness( 30 ). In most of these cohorts sugar-sweetened beverages were the major source of sugar intake. The latter systematic review found fifteen studies that showed a positive association and four that showed null associations; two of these studies reported fruit juice as the main sugar exposure. The quantitative meta-analysis of these cohorts showed a significantly increased risk of being overweight associated with higher consumption of sugar-sweetened beverages. That study also found five intervention trials in children that assessed the effect of advising to reduce dietary sugars on children's BMI. The meta-analysis of these trials found no association, but the authors suggested significant heterogeneity and poor compliance with the intervention trial as possible explanations for the inconsistent results.
Two recently published intervention trials in children found that the replacement of sugar-sweetened beverages by non-caloric beverages reduced weight gain( 31 , 32 ). One of these studies was conducted among overweight and obese children. The experimental group received a 1-year intervention designed to reduce the consumption of sugar-sweetened beverages. The increase in BMI was smaller in the intervention group after 1-year intervention, but not after 2-year follow-up. In any case, the role of non-caloric beverages in weight change is still debated. High-glycaemic-index foods seem to stimulate the consumption of other such foods. It has been suggested that sugar-sweetened beverages promote weight gain by increasing energy intake because of both the amount of added sugar and the low satiety associated with liquid carbohydrates. Nevertheless, non-caloric beverages have been associated with weight gain and the underlying mechanism still remains unclear( 33 – 35 ). Soft drink consumption, even of non-caloric soft drinks, is considered an unhealthy habit and it has been suggested that unhealthy habits tend to cluster( 36 , 37 ). It is also believed that individuals who consume non-caloric soft drinks seem to eat more because they have the sensation of having reduced their total energy intake. Linked to these two theories, some studies suggest that the palatability of both sugar-sweetened and non-caloric soft drinks increases the subjective feeling of hunger and consequently energy intake and weight gain( 11 , 12 ).
Despite the direct association suggested by the point estimates, we could not find a significant association between obesity and intermediate categories of SSCB consumption. In our opinion, this might be because of small sample size, but also because of the inherent limitations of a semi-quantitative FFQ. Overall, our results agree with the published literature and current knowledge about SSCB consumption and obesity.
Many studies support that physical activity has a protective role against obesity in children by increasing energy expenditure( 19 ). In our study, we found a significant negative association between physical activity and obesity in agreement with those studies (P < 0·01 in the multivariable model).
The Mediterranean dietary pattern has been suggested as a protective factor for obesity and CVD among adults but also among adolescents( 38 – 40 ). In our study, both cases and controls showed similar and low adherence to the Mediterranean dietary pattern (P = 0·77). Thus, this result does not allow us to affirm that the Mediterranean dietary pattern had a protective role against obesity among children. In the same way, both fast food and commercial bakery items have been suggested as risk factors for obesity and CVD( 10 , 41 ). We did not find any statistically significant difference in consumption of either commercial bakery items or fast food between cases and controls. Although the difference between groups in fast-food consumption was close to the significance level when comparing means, it became much lower in the multivariable model (P = 0·2).
Television watching has been defined as a proxy for sedentary behaviour in children. It has been suggested that television watching could even modify the association between some single nucleotide polymorphisms and obesity risk( 42 ). The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study found that excessive television watching was associated with higher consumption of energy-dense foods and drinks, enhancing the idea that unhealthy habits tend to cluster( 37 ). Although obese children reported more hours per week watching television or in front of a computer screen, we did not find any significant difference in sedentary behaviours between cases and controls (P = 0·74 in the multivariable model).
We found an unexpected inverse association between obesity and total energy intake. In our sample, cases reported significantly lower energy intake than controls. However, controls reported significantly higher physical activity and consequently a higher total energy expenditure that may explain this difference. The change in body fatness associated with higher sugar-sweetened beverage consumption seems to be mediated by higher energy intakes. According to this hypothesis, some authors defend that including total energy intake in the multivariable model reduces the actual strength of the association( 28 ). In our study, the introduction of total energy intake in the multivariable model did not affect the association between SSCB consumption and obesity found in the crude model. Although this may be due to the high energy intake referred by non-obese children, it may also suggest more complex mechanisms underlying the association between SSBC consumption and obesity.
Nevertheless, our study is not exempt of limitations. The main limitation of a case–control study is the misreporting of food habits, which usually becomes a differential information bias that reduces, or even inverts, the measures of association. Some studies defend that under-reporting is directly associated with children's BMI or with parents’ appreciation of the child's status and that it is more common when referring to non-desirable exposures. This social desirability bias may be an explanation for the difference in energy intake reported by cases and controls in our study. However, a differential information bias in our study would result in a reduction in the real strength of the measures of association( 43 ).
As explained in the Experimental methods section, when assessing the exposure, we used an FFQ in order to obtain information about food and beverage consumption during the previous year. Nevertheless, we cannot completely avoid a potential reverse causality bias, because the assessed dietary variables might be causes as well as consequences of the outcome. Attending to our data, this potential bias may also be an explanation for the results obtained in some dietary variables with unexpected results (total energy intake, adherence to the Mediterranean dietary pattern or commercial bakery items consumption) and for the relatively high BMI found among occasional consumers of SSCB (<1 serving/week). However, in our opinion, this potential bias would not affect the main results of our study, because the potential effect of this bias would be in the opposite direction to our findings.
In addition, we cannot deny the existence of potential residual confounding due to other factors, already suggested as potential confounders, but not included in our model because of a lack of information, such as parental educational level.
The association between SSCB consumption and obesity is supported by many well-designed studies. The high intake of energy in the form of added sugars, particularly the high quantity of sucrose (or high-fructose corn syrup in USA), seems to explain, at least partially, this association. However, the association between obesity and non-caloric beverages reported by some studies reflects that the underlying mechanisms may be more complex and multifactorial. In addition, the association between obesity and other sugar-sweetened beverages such as fruit-flavoured juices still remains unclear. The published literature about this issue is not conclusive. In our opinion, due the extended consumption of this kind of beverage among children and adolescents, deeper studies are needed to clarify this possible association of other sugared beverages, different from SSCB, with obesity risk.
Conclusion
In conclusion, our study suggests that SSCB consumption might be associated with an increment in BMI and body fat mass percentage. According to our results, a high consumption of SSCB, >4 servings/week, was strongly associated with obesity in childhood and adolescence (OR = 3·46; P = 0·01). In addition, each additional daily serving of SSCB (200 ml, 6·76 US fl oz) was associated with a 69 % relatively increased risk of obesity among children and adolescents (P = 0·03).
According to current knowledge, children and adolescents should be encouraged to reduce sugar-sweetened beverage consumption, particularly SSCB, and to increase the consumption of water as the main source of liquid( 44 ). It seems reasonable to conclude that the strategies designed to reduce the high prevalence of overweight and obesity should include education on beverage consumption in order to modify unhealthy habits of beverage consumption among children and adolescents.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflicts of interest: All authors have denied any financial or other relationship that might lead to a conflict of interest. Ethics: This study was approved by the Institutional Review Board of the Navarra University. Authors’ contributions: All authors have participated in the elaboration of the present study, including the collection of data, statistical analyses and interpretation of the results, and drafting and editing of the present paper. All the authors have seen and approved the contents of the submitted manuscript.
GENOI (Grupo Navarro de Estudio de la Obesidad Infantil) Members: C Azcona-SanJulian, JA Martínez, M Chueca, M Oyarzabal, A Patiño, R Pelach and MJ Moreno-Aliaga.
References
- 1. International Association for the Study of Obesity (2013) World map of obesity. http://www.iaso.org/resources/world-map-obesity/?map=children (accessed April 2013).
- 2. Daniels SR (2006) The consequences of childhood overweight and obesity. Future Child 16, 47–67. [DOI] [PubMed] [Google Scholar]
- 3. Estudio Aladino (2010–2011) Estrategia NAOS. Ministerio de Sanidad, Política Social e Igualdad. http://www.naos.aesan.msssi.gob.es/naos/ficheros/investigacion/ALADINO.pdf (accessed April 2013).
- 4. Beverage Marketing Corporation (2013) New Report. http://www.beveragemarketing.com/news.asp (accessed April 2013).
- 5. Asociación de Bebidas Refrescantes (2013) Memoria 2012. http://www.refrescantes.es/page_view.php?PageID=ESP_Sector&ContentID=228 (accessed April 2013).
- 6. Comité de Nutrición de la Asociación Española de Pediatría (2003) Consumption of fruit juices and beverages by Spanish children and teenagers: health implications of their poor use and abuse. An Pediatr (Barc) 58, 584–593. [PubMed] [Google Scholar]
- 7. Agencia Española de Seguridad Alimentaria y Nutrición (2009–2010) Encuesta nacional de ingesta dietética. http://www.aesan.msc.es/AESAN/web/evaluacion_riesgos/subseccion/otras_encuestas.shtml (accessed April 2013).
- 8. Mozaffarian D, Hao T, Rimm EB et al. (2011) Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 364, 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan A, Malik VS, Hao T et al. (2013) Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 37, 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bes-Rastrollo M, Sanchez-Villegas A, Gomez-Gracia E et al. (2006) Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study. Am J Clin Nutr 83, 362–370. [DOI] [PubMed] [Google Scholar]
- 11. DiMeglio DP & Mattes RD (2000) Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes (Lond) 24, 794–800. [DOI] [PubMed] [Google Scholar]
- 12. Schulze MB, Manson JE, Ludwig DS et al. (2004) Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292, 927–934. [DOI] [PubMed] [Google Scholar]
- 13. Ludwig DDS (2002) The glycemic index – physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287, 2414–2423. [DOI] [PubMed] [Google Scholar]
- 14. Sievenpiper JL, de Souza RJ, Mirrahimi A et al et al. (2012) Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 156, 291–304. [DOI] [PubMed] [Google Scholar]
- 15. Livesey G & Taylor R (2008) Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 88, 1419–1437. [DOI] [PubMed] [Google Scholar]
- 16. Johnson RJ, Perez-Pozo SE, Sautin YY et al et al. (2009) Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 30, 96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silbernagel G, Machann J, Unmuth S et al et al. (2011) Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr 106, 79–86. [DOI] [PubMed] [Google Scholar]
- 18. Bray GA (2010) Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol 21, 51–57. [DOI] [PubMed] [Google Scholar]
- 19. Ochoa MC, Moreno-Aliaga MJ, Martinez-Gonzalez MA et al et al. (2007) Predictor factors for childhood obesity in a Spanish case–control study. Nutrition 23, 379–384. [DOI] [PubMed] [Google Scholar]
- 20. Sobradillo B (2004) Curvas y Tablas de Crecimiento (Estudios Longitudinal y Transversal). Bilbao: Fundación Faustino Orbegozo Eizaguirre. [Google Scholar]
- 21. Martin-Moreno JM, Boyle P, Gorgojo L et al et al. (1993) Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 22, 512–519. [DOI] [PubMed] [Google Scholar]
- 22. de la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M et al et al. (2010) Reproducibility of an FFQ validated in Spain. Public Health Nutr 13, 1364–1372. [DOI] [PubMed] [Google Scholar]
- 23. Trichopoulou A, Costacou T, Bamia C et al et al. (2003) Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348, 2599–2608. [DOI] [PubMed] [Google Scholar]
- 24. Ainsworth BE, Haskell WL, Leon AS et al et al. (1993) Compendium of physical activities – classification of energy costs of human physical activities. Med Sci Sports Exerc 25, 71–80. [DOI] [PubMed] [Google Scholar]
- 25. Ruxton CHS, Gardner EJ & McNulty HM (2010) Is sugar consumption detrimental to health? A review of the evidence 1995–2006. Crit Rev Food Sci Nutr 50, 1–19. [DOI] [PubMed] [Google Scholar]
- 26. Van Baak MA & Astrup A (2009) Consumption of sugars and body weight. Obes Rev 10, 9–23. [DOI] [PubMed] [Google Scholar]
- 27. Mattes RD, Shikany JM, Kaiser KA et al et al. (2011) Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev 12, 346–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vartanian LR, Schwartz MB & Brownell KD (2007) Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 97, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malik VS, Schulze MB & Hu FB (2006) Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 84, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Te Morenga L, Mallard S & Mann J (2013) Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 346, e7492. [DOI] [PubMed] [Google Scholar]
- 31. de Ruyter JC, Olthof MR, Seidell JC et al et al. (2012) A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 367, 1397–1406. [DOI] [PubMed] [Google Scholar]
- 32. Ebbeling CB, Feldman HA, Chomitz VR et al et al. (2012) A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 367, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forshee RA & Storey ML (2003) Total beverage consumption and beverage choices among children and adolescents. Int J Food Sci Nutr 54, 297–307. [DOI] [PubMed] [Google Scholar]
- 34. Black RM, Tanaka P, Leiter LA et al et al. (1991) Soft drinks with aspartame – effect on subjective hunger, food selection, and food-intake of young-adult males. Physiol Behav 49, 803–810. [DOI] [PubMed] [Google Scholar]
- 35. Black RM, Leiter LA & Anderson GH (1993) Consuming aspartame with and without taste. Differential-effects on appetite and food-intake of young-adult males. Physiol Behav 53, 459–466. [DOI] [PubMed] [Google Scholar]
- 36. Schuit AJ, van Loon AJ, Tijhuis M et al et al. (2002) Clustering of lifestyle risk factors in a general adult population. Prev Med 35, 219–224. [DOI] [PubMed] [Google Scholar]
- 37. Rey-Lopez JP, Vicente-Rodriguez G, Repasy J et al et al. (2011) Food and drink intake during television viewing in adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Public Health Nutr 14, 1563–1569. [DOI] [PubMed] [Google Scholar]
- 38. Estruch R, Ros E, Salas-Salvado J et al et al. (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368, 1279–1290. [DOI] [PubMed] [Google Scholar]
- 39. Bibiloni M, Martinez E, Llull R et al et al. (2011) Metabolic syndrome in adolescents in the Balearic Islands, a Mediterranean region. Nutr Metab Cardiovasc Dis 21, 446–454. [DOI] [PubMed] [Google Scholar]
- 40. Beunza JJ, Toledo E, Hu FB et al et al. (2010) Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr 92, 1484–1493. [DOI] [PubMed] [Google Scholar]
- 41. Sayon-Orea C, Bes-Rastrollo M, Basterra-Gortari FJ et al et al. (2013) Consumption of fried foods and weight gain in a Mediterranean cohort: The SUN project. Nutr Metab Cardiovasc Dis 23, 144–150. [DOI] [PubMed] [Google Scholar]
- 42. Ochoa MC, Moreno-Aliaga MJ, Martinez-Gonzalez MA et al et al. (2006) TV watching modifies obesity risk linked to the 27Glu polymorphism of the ADRB2 gene in girls. Int J Pediatr Obes 1, 83–88. [DOI] [PubMed] [Google Scholar]
- 43. Boernhorst C, Huybrechts I, Hebestreit A et al et al. (2013) Diet–obesity associations in children: approaches to counteract attenuation caused by misreporting. Public Health Nutr 16, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muckelbauer R, Libuda L, Clausen K et al et al. (2009) Promotion and provision of drinking water in schools for overweight prevention: randomized, controlled cluster trial. Pediatrics 123, e661–e667. [DOI] [PubMed] [Google Scholar]