Abstract
Background:
Human milk diet, preferably mother's own milk (MOM) over donor milk (DM), is recommended for preterm infants. Expression of MOM in proximity to preterm infants, especially during or immediately after skin-to-skin contact (SSC), is associated with greater milk production. However, the correlation between SSC and MOM production during hospital admission in preterm infants has not yet been studied. Our study investigated the relationship between SSC and MOM production and consumption in preterm infants during the first postnatal month of life.
Materials and Methods:
This was a prospective cohort study. Mothers and their preterm infants born at <35 weeks by gestational age (GA) and eligible for SSC within the first 5 postnatal days were eligible for the study. Mothers were given a binder to document pumped breast milk volumes and SSC sessions. Pumped breast milk volumes, enteral feeding type and volume, and SSC duration and frequency were collected daily over the first 28 days of life, along with demographic, perinatal, and feeding data from electronic medical records (EMR).
Results:
Mean birth GA and weight were 30 ± 3 weeks and 1,443 ± 576 g, respectively. SSC duration was inversely correlated with GA and weight. The SSC duration was positively correlated with ingested MOM volume after correcting for birth GA. The SSC duration was predictive of increased volumes of pumped MOM.
Conclusion:
Our findings suggest that SSC duration is associated with improved MOM production and consumption. SSC can be a useful tool to increase MOM exposure and improve long-term health outcomes in preterm infants.
Keywords: skin to skin contact, breast feeding, premature infant, milk production
Introduction
Human milk diet, preferably mother's own milk (MOM) over donor milk (DM), is recommended for preterm infants.1 DM is often lower in fat, protein, and micronutrients compared to preterm MOM and has lower protective immunological components due to the pasteurization process.2 Colostrum, the initial milk produced by lactating mothers, contains almost double the protein and decreased fat compared to mature human milk. It contains large quantities of lactoferrin, immunoglobulin A, and white blood cells.3 Once human milk transitions to mature milk, there are significant day-to-day changes in the protein and fat content of milk. Preterm MOM differs from full-term MOM because it contains more protein, fat, free amino acids, and sodium.3 DM is typically pooled mature milk from term infants' mothers. When compared with the use of DM alone or with supplementation with MOM, preterm infants have improved growth with exclusive MOM unfortified diets.4
MOM has been shown to improve neurodevelopmental outcomes and reduce the risk of mortality, bronchopulmonary dysplasia, and necrotizing enterocolitis (NEC).2,3,5 Promoting skin-to-skin contact (SSC) in the neonatal intensive care unit (NICU) can be an effective method for increasing MOM production and subsequently increasing MOM consumption and improving the long-term health outcomes of preterm infants.
However, mothers of preterm infants in the NICU often produce inadequate amounts of breast milk due to many barriers, such as separation from their infants, delayed lactogenesis,1 maternal stress, and perinatal complications.6 In a report by the CDC, only ∼70% of preterm infants (born <37 weeks) received any breast milk during their hospital stay.7 The number of preterm infants receiving MOM at discharge is significantly lower.7 In a national Swedish study, only 13% of extremely preterm infants (born 22–27 weeks), 34% of very preterm infants (born 28–31 weeks), and 49% of moderately preterm infants (born 32–36 weeks) were exclusively breastfed at hospital discharge.8
SSC has been shown to be an important bonding tool for mothers with their preterm infants. SSC is described as placing an infant clothed in only a diaper and a hat in a prone or lateral position onto a caregiver's bare chest. Beneficial effects of SSC include decreased infant pain scores during procedures9 and improved infant–mother bonding.5,10–14 SSC has been shown to increase salivary oxytocin levels in mothers, fathers, and infants, which can help reduce stress15 and is associated with decreased parental cortisol levels.16
SSC also increases the likelihood of successful breastfeeding. SSC has been associated with increased overall rates of breastfeeding during hospital stay and up to 1 month after discharge.11,17,18 Infants receiving kangaroo mother care (defined as prolonged SSC, breastfeeding, and timely discharge home) have been associated with earlier initiation of breastfeeding than those receiving care in an incubator or warmer,19 as well as decreased time to full oral feeds and reduced feeding intolerance.20
Although multiple studies have shown that SSC can increase breastfeeding in healthy term infants, no study to date has shown a direct correlation between SSC duration and MOM production during NICU admission in preterm infants. This study aims to clarify the relationship between SSC and production and consumption of MOM in preterm infants during the first postnatal month, a crucial time for establishing MOM production and infant gut microbiome.
Methods
Study design and patient population
We performed a prospective cohort study of preterm infants born at <35 weeks' gestational age (GA) and admitted to the level III academic NICU at Tampa General Hospital between November 1, 2019, and December 30, 2020. Inclusion criteria were eligibility for SSC within the first 5 postnatal days. Our unit had a concurrent quality improvement project to encourage parents to start SSC during the first 5–7 days after birth, if medically qualified (Table 1). This criterion was used to reduce the variability in the studied population. The exclusion criteria were as follows: infants born to HIV-positive mothers, infants with unrepaired gastroschisis or omphaloceles, and infants who died or were discharged before 28 days of life. This study was approved by the Institutional Review Board of the University of South Florida.
Table 1.
Eligibility Criteria for Skin-to-Skin Contact
| Category | Criteria |
|---|---|
| Green (eligible for SSC) | Medically stable (no recent increases in ventilatory support, without rapidly rising serum bilirubin levels, low doses of dopamine with stable blood pressure, events at baseline) Stable respiratory status on nasal continuous positive airway pressure or intubated May be done simultaneously with twins if neither twin has suspected or proven infection |
| Yellow (may be eligible after discussion with medical team) | Infants during the first 24 hours of mechanical ventilation Apnea requiring significant stimulation Chronic persistent pulmonary hypertension Infants with umbilical artery catheters, peripheral arterial lines, or chest tubes Infants on phototherapy Infants requiring increased ventilatory support (increasing pressures, oxygen requirements, or reintubation) |
| Red (not eligible) | Acute persistent pulmonary hypertension Acute sepsis Unrepaired omphalocele/gastroschisis Severe hypotension requiring multiple continuous IV vasopressors ELBW infants (ELBW, defined as birth weight <1,000 g) in the first 72 hours of life Hypoxic ischemic encephalopathy requiring cooling |
ELBW, extremely low birth weight; SSC, skin-to-skin contact.
Skin to skin contact guidelines
Nursing staff were trained on proper SSC techniques through educational videos describing proper SSC. Infants were clothed in only a hat and diaper and placed prone onto the bare caregiver's chest for a minimum of 60 minutes. If caregivers were not using a kangaroo wrap, a warm blanket was provided to place over the infant. Caregivers were instructed that SSC could be terminated early at the parents' request or if the infant showed intolerance to SSC. Signs of intolerance included hypothermia despite appropriate warming techniques, an increase of FiO2 >10–15% over baseline, or an increase in episodes of apnea, bradycardia, or desaturation over the infant's baseline that were not resolved with normal interventions.
In addition, local Best Practice guidelines were revised and provided for consistency in practice (Table 1). The guidelines describe the criteria for determining an infant's eligibility for SSC via a color-coding system. The infants were evaluated daily for SSC eligibility by bedside nurses. Infants categorized “green” could have SSC without discussion with the medical team, infants categorized “red” were ineligible for SSC, and infants categorized “yellow” would require a discussion with the entire medical team on rounds to determine SSC eligibility for that day.
Data collection
Mothers of eligible infants were given a binder at the bedside to document their daily volumes (mL) of pumped MOM. They also documented the occurrence of SSC, duration of SSC, and the caregiver who performed SSC. The families were given detailed explanations of the binders by the research team and all bedside nurses were educated on proper documentation.
Data were collected from inpatient electronic medical records (EMR) of infants and mothers from both delivery and NICU admissions. The collected demographic and clinical information included birth weight (BW), GA, sex, ethnicity, race, maternal age, multiple gestation, type of pump used (hospital versus manual grade pump), prenatal and postnatal infections, and grade 3 or 4 intraventricular hemorrhage (IVH). Detailed information on enteral intake was recorded, including the type of feeding (MOM/DM/formula), feeding volume, and feeding readiness scores over the first 28 postnatal days. Other information recorded included times enteral and oral feeding was started, when infants took all feedings by mouth, and what the infant was primarily feeding upon discharge home.
Statistical analysis
Maternal and infant characteristics were summarized using descriptive statistics. Continuous variables were summarized as mean and standard deviation or median and interquartile range, and categorical variables were summarized as rates/percentages. Fisher's exact test was used to evaluate the associations between categorical variables. Differences in continuous variables were assessed using the Student's t test or the Mann–Whitney U test. The correlation between two continuous variables with non-normal distribution was assessed using a nonparametric Spearman's correlation test. The adjusted and unadjusted relationships between the dependent and independent variables were assessed using simple linear regression analysis. All p-values were two-tailed, and statistical significance was set at 5% for all comparisons.
For a simple linear regression model to detect multiple partial correlation coefficients of moderate size (0.36) between SSC duration and breast milk volume, we needed a sample size of 55 for a two-sided test with a 5% significance level and a power of 80.2%. With this sample size and repeated measures, we were able to detect smaller correlations. All statistical analyses were performed with IBM SPSS statistical software (IBM Corp. released 2020, IBM SPSS Statistics, Version 27.0; IBM Corp., Armonk, NY).
Results
Patient characteristics
A total of 100 preterm infants were born at <35 weeks of age during the study period. The final analysis included 56 patients who met our inclusion criteria. Of these, 46 completed the pumping logs. The mean GA and BW were 30 ± 3 weeks and 1,443 ± 576 g, respectively. The infants were also stratified by very low birth weight (VLBW, defined as BW <1,500 g, 57%) and extremely low birth weight (ELBW, defined as BW <1,000 g, 25%). The mean maternal age was 30 ± 7 years. Of the mothers, 45% were Caucasian, 32% were African American, and 39% were Hispanic. Of the mothers, 84% had access to a hospital-grade pump at home (Table 2).
Table 2.
Patient Demographics and Characteristics
| Characteristics | N = 56 |
|---|---|
| Gestational age (weeks) | 30 ± 3 |
| Minimum, maximum (weeks) | 24.0, 34.6 |
| Birthweight (g) | 1,443 ± 576 |
| Minimum, maximum (g) | 590, 2,820 |
| Maternal age (years) | 30 ± 7 |
| Vaginal delivery, n (%) | 33 (59) |
| Multiple gestation, n (%) | 16 (29) |
| Male, n (%) | 26 (46) |
| Apgar 1 minute | 6 ± 2 |
| Apgar 5 minutes | 8 ± 1 |
| Caucasian, n (%) | 25 (45) |
| African American, n (%) | 18 (32) |
| Hispanic ethnicity, n (%) | 22 (39) |
| English language, n (%) | 50 (89) |
| Electric pump, n (%) | 47 (84) |
| Antenatal corticosteroids, n (%) | 54 (96) |
| Maternal magnesium, n (%) | 38 (68) |
| Surfactant, n (%) | 18 (32) |
| Intraventricular hemorrhage, n (%) | 4 (7) |
| Age at full enteral feeds (days) | 7 ± 5 |
| Age at first breastfeeding (days) | 26 ± 27 |
| Age at full oral feedings (days) | 42 ± 35 |
| Length of hospital stay (days) | 51 ± 40 |
| Gestational age at discharge (weeks) | 38 ± 4 |
Skin to skin contact and perinatal data
There was a negative correlation between SSC duration and GA (r = −0.339, p = 0.005) and BW (r = −0.367, p = 0.011). Of the 56 infants who were eligible for SSC, there were 41 (73%) infants who received weekly SSC (defined as SSC conducted at least once weekly for 60 minutes during the first 28 days or until discharge) and 15 (27%) infants who did not receive weekly SSC. The weekly duration of SSC was widely distributed with median duration peaking at week 2 and decreasing at week 4 (Table 3).
Table 3.
Skin-to-Skin Contact Duration by Postnatal Week
| Total 28 days | Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|---|
| SSC duration (minutes), median (IQR) | 610 (335, 1,074) | 103 (0, 310) | 128 (0, 335) | 120 (0, 338) | 45 (0, 300) |
| Pumped MOM (mL), median (IQR) | 7,176 (3,844, 12,796) | 1,177 (384, 18,19) | 2,208 (947, 4,081) | 1,858 (412, 3,959) | 3,429 (758, 5,108) |
IQR, interquartile range; MOM, mother's own milk; SSC, skin-to-skin contact.
Skin to skin contact and feedings
Analysis of ingested MOM in the 56 patients who met the inclusion criteria showed a positive correlation between total SSC duration and total ingested MOM volume during the first 28 days when controlling for GA (r = 0.270, p = 0.047). With close to 30% of the study population were not receiving SSC at least once per week, we chose to analyze the frequency of SSC categorically (weekly versus nonweekly) in addition to analyzing the total duration of SSC over 28 days. Infants who had SSC at least once per week during the first postnatal month ingested higher volumes of MOM than infants who did not have regular SSC (mean = 4,132 mL versus 2,226 mL, p = 0.001) (Fig. 1).
FIG. 1.
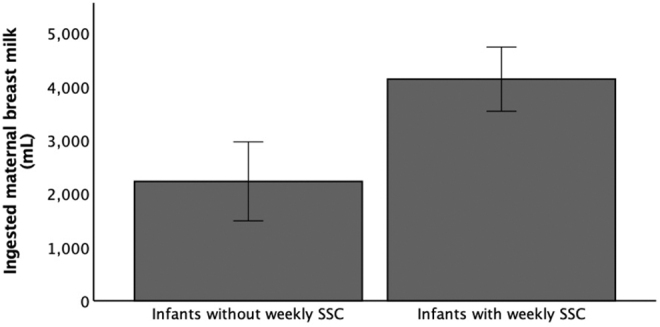
Ingested MOM volumes for infants without versus with weekly SSC. Infants who had regular SSC had higher ingested MOM volume than infants who did not have regular SSC (mean = 2,226 mL versus 4,132 mL, p = 0.001). MOM, mother's own milk; SSC, skin-to-skin contact.
The average age at full enteral feeding was 7 ± 5 days, age at first breastfeeding was 26 ± 27 days, and age at full oral feeds was 42 ± 35 days (Table 2).
The analysis of pumped MOM volumes was conducted in the 46 patients who had completed pumping logs in the provided binders. The daily pumped MOM volume increased over time as breast milk production was established. In these patients, the total SSC duration was not significantly correlated with the total pumped MOM volume (r = 0.266, p = 0.074) during the first 28 days of life.
However, weekly SSC duration was significantly correlated with weekly pumped MOM volume during weeks 3 (r = 0.385, p = 0.008) and 4 (r = 0.309, p = 0.036) (Fig. 2), but not during weeks 1 and 2 (r = 0.254, p = 0.088 and r = 0.032, p = 0.835, respectively). Regression analysis showed that the total SSC duration was a significant predictor of pumped milk volumes. For every minute of SSC, there was an associated increase of 2.5 ± 1.2 mL of pumped MOM (p = 0.049) (Table 4).
FIG. 2.
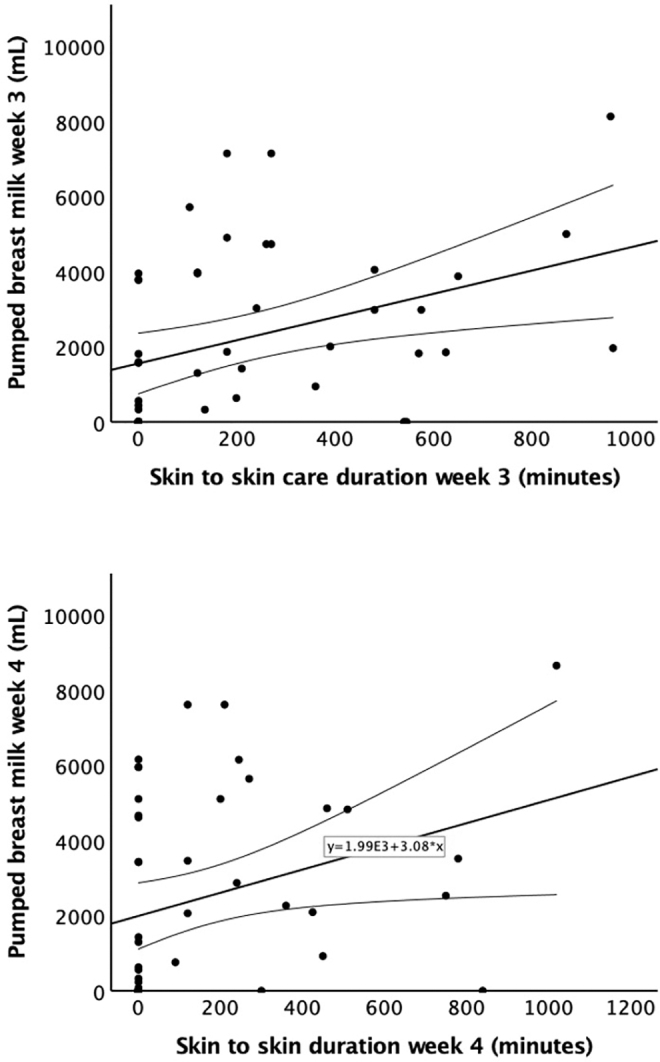
Weekly SSC duration versus pumped MOM volume. Weekly SSC duration was significantly correlated with pumped MOM volume during week 3 (r = 0.385, p = 0.008) and week 4 (r = 0.309, p = 0.036). MOM, mother's own milk; SSC, skin-to-skin contact.
Table 4.
Regression Analysis of Total Skin-to-Skin Contact Duration and Pumped Breast Milk Volumes
| Unstandardized coefficients |
Standardized coefficients |
t | Significance | ||
|---|---|---|---|---|---|
| B | Standard error | Beta | |||
| Constant | 6,546.934 | 1,378.804 | 4.478 | <0.001 | |
| SSC duration | 2.502 | 1.238 | 0.288 | 2.021 | 0.049 |
SSC, skin-to-skin contact.
Discussion
Mothers are often encouraged to perform SSC with their infants to improve their breast milk production. Our study showed that SSC duration was positively correlated with ingested MOM volume and that SSC duration was a positive predictor of pumped milk volume in preterm infant-mother dyads. For every minute of SSC, there was a potential increase of ∼1.3–3.7 mL in MOM production. This is equivalent to an increase of 2.6–7.5 oz with one 60-minute SSC session.
There are also cost benefits associated with SSC. DM cost ranges $3.00–5.00 USD per ounce.21 According to our study, a 1 hour SSC session would increase pumped MOM volume by 2.6–7.5 oz. For every hour of SSC conducted, the hospital could potentially save up to $37.50 USD compared to feeding with DM. If conducted only once per week, this would amount to savings of $150 USD per infant in a 1-month period. Our study also demonstrated that Infants who had SSC at least once per week during the first postnatal month ingested higher volumes of MOM than infants who did not have weekly SSC. Due to numerous benefits of MOM especially in preterm infant population, this cost saving will also reflect in long-term deduction of comorbidities during NICU admission.
The low frequency of SSC in our population is similar to findings in other centers. Despite the multitude of benefits, there are many barriers for mothers to perform SSC frequently in the NICU. A California perinatal quality improvement initiative showed that while clinicians value SSC, there was moderate variability in which infants were considered “stable” for SSC. The barriers identified in that study included having enough staff to assist with transfers from the incubator to the caregiver, getting staff internally motivated, and issues precluding visitation, especially in low-income families.22 According to a systematic review of the barriers and enablers of SSC for preterm infants, the top barriers identified by mothers were primarily resource-related, including issues with facilities, perceived negative attitudes from staff, lack of help, and low awareness of SSC.23
Furthermore, infants born to mothers with low socioeconomic status as well as mothers who spoke a language other than English have also been shown to experience lower rates of SSC in the NICU.24 We reduced the variabilities in determination of eligibility by standardizing the guidelines as outlined in Table 1. Our study duration was concurrent with a quality improvement project in the unit that trained NICU staff and caregivers to increase awareness of and encourage SSC during the first 5–7 days of life. However, our study did not control for factors such as socioeconomic status or language barriers.
We demonstrated that total SSC duration was predictive of total pumped MOM over first postnatal month. As we examined this relationship more granularly by week, we discovered that this positive association was present during week 3 and 4 but not during the first 2 weeks. The stages of lactation consist of the initial colostrum stage, the transitional milk stage, and the mature milk stage. While there is no formal definition, the period of transitional milk production is generally considered to start at 2–5 days postpartum and lasts until ∼14 days postpartum.25–27
Our study may suggest that SSC duration is correlated with the production of mature breast milk but not the production of colostrum or transitional milk. While colostrum and transitional milk are important for the initial immunologic development and rapid growth, mature milk is responsible for long-term nutrition and growth of the infant.28,29 Alternatively, our study may suggest that the positive effects of SSC on milk production in volume may not be observed until later in milk production. Perhaps, the effect of SSC on the early stages of lactation should be evaluated by other variables other than concurrent pumped milk volume, such as days to achieve full feeding volume.
We have overcome some of the challenges of conducting clinical studies during the Covid 19 pandemic. There was a significant restriction in parental visits which limited the ability to recruit extremely premature infants. The data on SSC duration and ingested MOM volumes were extracted from EMR; however, we relied on maternal documentation of pumped milk volumes. The exclusion criteria for participation in the study included criteria that indicated illness acuity of feeding intolerance. We chose to exclude patients with these characteristics (Table 1) because in addition to being indicative of medical instability precluding SSC, they were also possible confounders of breast milk consumption. Therefore, our study is applicable only to late preterm infants without significant illness acuity and without gastrointestinal illness.
This study is one of the first studies to examine the correlation between SSC and MOM production and consumption during the first month postpartum in preterm NICU infants. Our study showed that SSC duration correlates with MOM production and consumption. Possible directions for future studies include further investigation of the clinical and/or social barriers that hinder mothers and infants from performing and receiving SSC, especially in minority and underserved populations. Additional studies may include investigating the optimal frequency and duration of SSC in resource-rich countries, the timing of SSC in relationship to the different stages of milk production, and further exploration of the economic benefits of MOM as opposed to formula and DM feeding.
Data Access Statement
All relevant data are within the article.
Authors' Contributions
F.D. conceived of the study design and contributed to data collection, implementation of the study, and article preparation. A.S. contributed to article preparation and reviewing of data and references. A.K. contributed to study design, data analysis, and article editing. K.C. contributed to data collection and implementation of the study. A.L-J. supervised study design, data collection and analysis, and article editing. T.T.B.H. supervised study design, data collection and analysis, article preparation, and editing.
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Information
The study was supported by grant K23HL150300 from the National Heart, Lung, and Blood Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Meek JY, Noble L; Section on Breastfeeding. Policy statement: Breastfeeding and the use of human milk. Pediatrics 2022;150(1):e2022057988. [DOI] [PubMed] [Google Scholar]
- 2. Chetta KE, Schulz EV, Wagner CL. Outcomes improved with human milk intake in preterm and full-term infants. Semin Perinatol 2021;45(2):151384. [DOI] [PubMed] [Google Scholar]
- 3. Poulimeneas D, Bathrellou E, Antonogeorgos G, et al. Feeding the preterm infant: An overview of the evidence. Int J Food Sci Nutr 2021;72(1):4–13. [DOI] [PubMed] [Google Scholar]
- 4. Montjaux-Regis N, Cristini C, Arnaud C, et al. Improved growth of preterm infants receiving mother's own raw milk compared with pasteurized donor milk. Acta Paediatr 2011;100(12):1548–1554. [DOI] [PubMed] [Google Scholar]
- 5. Baley J; Committee on Fetus and Newborn. Skin-to-skin care for term and preterm infants in the neonatal ICU. Pediatrics 2015;136(3):596–599. [DOI] [PubMed] [Google Scholar]
- 6. Gupta S, Parikh T. Optimizing own mother's milk supply for NICU babies. J Neonatol 2020;34(1–2):83–87. [Google Scholar]
- 7. Chiang KV, Sharma AJ, Nelson JM, et al. Receipt of breast milk by gestational age—United States, 2017. MMWR Morb Mortal Wkly Rep 2019;68(22):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ericson J, Flacking R, Hellstrom-Westas L, et al. Changes in the prevalence of breast feeding in preterm infants discharged from neonatal units: A register study over 10 years. BMJ Open 2016;6(12):e012900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsson E, Ahlsen G, Eriksson M. Skin-to-skin contact reduces near-infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatr 2016;105(4):376–380. [DOI] [PubMed] [Google Scholar]
- 10. Butruille L, Blouin A, De Jonckheere J, et al. Impact of skin-to-skin contact on the autonomic nervous system in the preterm infant and his mother. Infant Behav Dev 2017;49:83–86. [DOI] [PubMed] [Google Scholar]
- 11. Gathwala G, Singh B, Singh J. Effect of Kangaroo Mother Care on physical growth, breastfeeding and its acceptability. Trop Doct 2010;40(4):199–202. [DOI] [PubMed] [Google Scholar]
- 12. Linner A, Westrup B, Lode-Kolz K, et al. Immediate parent-infant skin-to-skin study (IPISTOSS): Study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open 2020;10(7):e038938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorenz L, Dawson JA, Jones H, et al. Skin-to-skin care in preterm infants receiving respiratory support does not lead to physiological instability. Arch Dis Child Fetal Neonatal Ed 2017;102(4):F339–F344. [DOI] [PubMed] [Google Scholar]
- 14. Mehler K, Hucklenbruch-Rother E, Trautmann-Villalba P, et al. Delivery room skin-to-skin contact for preterm infants—A randomized clinical trial. Acta Paediatr 2020;109(3):518–526. [DOI] [PubMed] [Google Scholar]
- 15. Vittner D, McGrath J, Robinson J, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs 2018;20(1):54–62. [DOI] [PubMed] [Google Scholar]
- 16. Forde D, Fang ML, Miaskowski C. A systematic review of the effects of skin-to-skin contact on biomarkers of stress in preterm infants and parents. Adv Neonatal Care 2022;22(3):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayaraman D, Mukhopadhyay K, Bhalla AK, et al. Randomized controlled trial on effect of intermittent early versus late kangaroo mother care on human milk feeding in low-birth-weight neonates. J Hum Lact 2017;33(3):533–539. [DOI] [PubMed] [Google Scholar]
- 18. Renfrew MJ, Dyson L, McCormick F, et al. Breastfeeding promotion for infants in neonatal units: A systematic review. Child Care Health Dev 2010;36(2):165–178. [DOI] [PubMed] [Google Scholar]
- 19. Mekonnen AG, Yehualashet SS, Bayleyegn AD. The effects of kangaroo mother care on the time to breastfeeding initiation among preterm and LBW infants: A meta-analysis of published studies. Int Breastfeed J 2019;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandya D, Kartikeswar GAP, Patwardhan G, et al. Effect of early kangaroo mother care on time to full feeds in preterm infants—A prospective cohort study. Early Hum Dev 2021;154:105312. [DOI] [PubMed] [Google Scholar]
- 21. Trang S, Zupancic JAF, Unger S, et al. Cost-effectiveness of supplemental donor milk versus formula for very low birth weight infants. Pediatrics 2018;141(3):e20170737. [DOI] [PubMed] [Google Scholar]
- 22. Lee HC, Martin-Anderson S, Dudley RA. Clinician perspectives on barriers to and opportunities for skin-to-skin contact for premature infants in neonatal intensive care units. Breastfeed Med 2012;7(2):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidman G, Unnikrishnan S, Kenny E, et al. Barriers and enablers of kangaroo mother care practice: A systematic review. PLoS One 2015;10(5):e0125643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brignoni-Pérez E, Scala M, Feldman HM, et al. Disparities in kangaroo care for premature infants in the neonatal intensive care unit. J Dev Behav Pediatr 2022;43(5):e304–e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGuire MK, Seppo A, Goga A, et al. Best practices for human milk collection for COVID-19 research. Breastfeed Med 2021;16(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ongprasert K, Ruangsuriya J, Malasao R, et al. Macronutrient, immunoglobulin a and total antioxidant capacity profiles of human milk from 1 to 24 months: A cross-sectional study in Thailand. Int Breastfeed J 2020;15(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Zhang X, Mi L, et al. Comparative proteomic analysis of proteins in breast milk during different lactation periods. Nutrients 2022;14(17):3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ballard O, Morrow AL. Human milk composition: Nutrients and bioactive factors. Pediatr Clin North Am 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu X, Jackson RT, Khan SA, et al. Human milk nutrient composition in the United States: Current knowledge, challenges, and research needs. Curr Dev Nutr 2018;2(7):nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]


