Abstract
Maladjusted immune responses to the coronavirus disease 2019 (COVID-19), for example, cytokine release syndrome, may result in immunopathology and acute respiratory distress syndrome. Sphingosine-1-phosphate (S1P), a bioactive lipid mediator, and its S1P receptor (S1PR) are crucial in maintaining endothelial cell chemotaxis and barrier integrity. Apart from the S1P1 receptor-mediated mechanisms of sequestration of cytotoxic lymphocytes, including Th-17 and S1P1/2/3-mediated endothelial barrier functions, S1PR modulators may also attenuate cytokine release via activation of serine/threonine protein phosphatase 2A and enhance the pulmonary endothelial barrier via the c-Abl tyrosine kinase pathway. Chronic treatment with fingolimod (S1PR1,3,4,5 modulator) and siponimod (S1PR1,5 modulator) has demonstrated efficacy in reducing inflammatory disease activity and slowing down disease progression in multiple sclerosis. The decision to selectively suppress the immunity of a critically ill patient with COVID-19 remains a difficult choice. It has been suggested that treatment with fingolimod or siponimod may be appropriate to attenuate severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)–induced hyperinflammation in patients with COVID-19 since these patients are already monitored in an intensive care setting. Here, we review the use of S1PR modulators, fingolimod and siponimod, in regulating the inflammatory response to SARS-CoV-2 with the aim of understanding their potential rationale use in patients with COVID-19.
Keywords: fingolimod, siponimod, immune response, cytokine suppression
Introduction
Coronavirus disease 2019 (COVID-19) is a viral infection that primarily affects the respiratory tract. It is caused by the newly emergent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which was first recognized in Wuhan, China, in December 2019 (WHO 2020). It spread rapidly to several countries, and in March 2020, the World Health Organization declared COVID-19 as a pandemic. To date, more than 318 million (WHO 2022) cases of COVID-19 have been reported worldwide.
Although most people with COVID-19 develop mild or uncomplicated asymptomatic illness, ∼14% develop severe disease requiring hospitalization and oxygen support and 5% require admission to an intensive care unit (CDC 2021). In severe cases, COVID-19 can be complicated by acute respiratory distress syndrome (ARDS), which is the leading cause of death with a high mortality rate of ∼40% (Hasan and others 2020; Meyer and others 2021) with lower mortality rates in younger patients and higher mortality rates in older and more vulnerable patient populations (Ramasamy and Subbian 2021; Yeates and others 2021). Mortality risk is often associated with virus-induced hyperinflammation and an accompanying cytokine release syndrome or cytokine storm (Cron and others 2021).
The virus particles first invade the respiratory mucosa, infect other cells, and disrupt the vascular endothelial cell monolayer in the pulmonary microcirculation, triggering a series of immune responses (Guo and others 2020; Li and others 2020a). Secretion of large amounts of chemokines and cytokines (interleukin [IL]-1, IL-6, IL-8, IL-21, tumor necrosis factor beta, and monocyte chemoattractant protein-1) is promoted in infected cells in response to SARS-CoV-2 infection, with varying affection of the lung for different coronavirus variants (Hui and others 2022). These chemokines and cytokines, in turn, recruit lymphocytes and leukocytes at the site of infection.
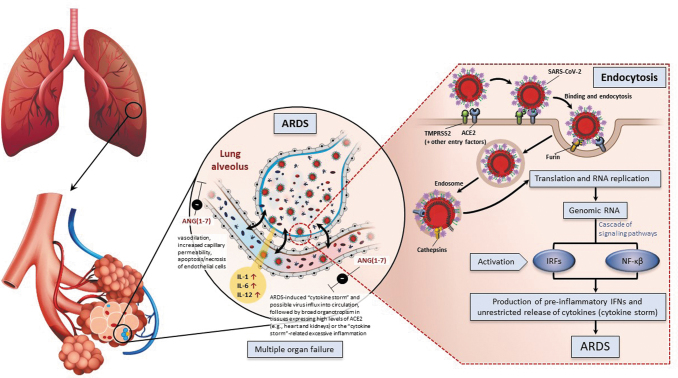
This accumulation of protein and inflammatory cell-filled fluid in the interstitial and alveolar compartments leads to pulmonary edema and ultimately respiratory failure. A subgroup of patients with severe COVID-19 might have a strong immune response to SARS-CoV-2, resulting in cytokine storm (Jorens and others 1992; Miller and others 1992, 1996; Chollet-Martin and others 1993; Donnelly and others 1993). Excessive immune response toward SARS-CoV-2 infection may play a key role in inducing ARDS and even organ failure (Fig. 1).
FIG. 1.
Innate immune response and adaptive immune responses of CoV during an infection (Bergmann and Silverman 2020; Hejrati and others 2021; Trougakos and others 2021). ACE, angiotensin-converting enzyme; ANG, angiotensin; ARDS, acute respiratory distress syndrome; CoV, coronaviruses; IL, interleukin; IRF, interferon regulatory factors; NF-κβ, nuclear factor kappa B.
The current management of COVID-19 aims at preventing severe manifestation and improving the prognosis of the patients. The National Institute of Health (NIH) recommends use of corticosteroids, anti-SARS-CoV-2 monoclonal antibodies, and antivirals alone or in combination based on the clinical presentation and oxygen requirement of the patients (NIH 2021). Glucocorticoids have been used during 2002–2004 in the management of SARS outbreak in patients who had severe immune response and were at a high risk of developing ARDS. Although glucocorticoids reduced inflammatory cytokines, an increased viral load and slower viral clearance is noted and as such should be used with caution (Li and others 2020b).
Several investigational drugs (baricitinib, tocilizumab, and anakinra) have been granted emergency use authorization for hospitalized COVID-19 patients (EMA 2021a, 2021b; US FDA 2022a). Considering the impact of COVID-19, numerous therapeutics are currently being evaluated in clinical trials for their safety and efficacy in treating COVID-19 and related complications. Remdesivir, an RNA-dependent RNA polymerase inhibitor, was one of the first U.S. Food and Drug Administration (FDA)-approved therapy for the treatment of COVID-19.
Remdesivir was initially approved for emergency use in hospitalized patients with severe COVID-19 and later, based on the clinical evidence (Rezagholizadeh and others 2021), approved for all patients (NIH 2022). Nirmatrelvir/ritonavir and molnupiravir have also received emergency use authorizations from the FDA (US FDA 2022b, 2022c).
Treatment strategies that are effective in regulating the amplified immune response, which characterizes the pathophysiology of ARDS, could potentially further improve the outcome of patients who have a severe pulmonary manifestation from COVID-19. Sphingosine-1-phosphate (S1P) is a signaling lysophospholipid that regulates multiple biological processes, including inflammatory response to viral infections; thus, targeting these pathways for modulation of hyperinflammatory responses has been considered in the past (Ebenezer and others 2016).
The S1P modulators such as fingolimod and siponimod are approved as chronic long-term disease-modifying treatments for multiple sclerosis to reduce disease activity and delay progression; they are not approved for use outside multiple sclerosis (US FDA 2012, 2019). In this article, we review the evidence for short-term use of S1P receptor (S1PR) modulators, fingolimod and siponimod, in regulating the inflammatory response to SARS-CoV-2 with an aim of understanding their potential rationale for complementary use in patients with COVID-19.
Pharmacological Evidence
Role of S1P in virus-induced hyperinflammation
The activation of leukocytes and endothelial cells is a critical step in the pathophysiology of acute lung injury (Grommes and Soehnlein 2011). The sphingolipids assume multiple roles in the regulation of neutrophil chemotaxis, neutrophil apoptosis, and endothelial and epithelial barrier functions (Table 1). Leukocyte transmigration and vascular permeability are frequently associated. The upregulation of neutral sphingomyelinase increases neutrophil chemotaxis and prolongs neutrophil survival, exerting a stronger lung injury.
Table 1.
Role of Sphingolipids and Sphingosine-1-Phosphate Receptor Modulators in Pathophysiology of Acute Lung Injury
| Mediators | NSMasea | ASMasea | S1P | S1PR1 | S1PR2 | S1PR3 |
|---|---|---|---|---|---|---|
| Physiological functions | ||||||
| Neutrophil chemotaxis |

|
|
||||
| Endothelial permeability |

|

|
||||
| Neutrophil apoptosis |

|

|
||||
| Epithelial permeability |

|

|

|

|
||
| Receptor modulators | ||||||
| Fingolimod | — | — | — | +++ | — | ++ |
| Siponimod | — | — | — | +++ | — | — |
From Grommes and Soehnlein (2011).
Catalyzes the breakdown of sphingomyelin to ceramide and phosphorylcholine.
+Indicates receptor affinity.
ASMase, acid sphingomyelinase; NSMase, neutral sphingomyelinase; S1P, sphingosine-1-phosphate; S1PR, S1P receptors.
Increased extracellular acid sphingomyelinase activity results in increased vascular permeability. The effects of S1P on neutrophils unfold in stages: an early effect on attenuation of chemotaxis and a late one on promoting neutrophil survival. The S1P exerts its pleotropic effects via signaling through a family of five specific G protein-coupled receptors (S1PR1–5), which are differentially expressed in cells and tissues (Hla and others 2008; Hla and Brinkmann 2011). The pattern of S1PR activation differentially regulates downstream signaling effector molecules.
The effect of S1P on the regulation of vascular permeability is bidirectional, with the barrier function being enhanced by S1PR1 but impaired by S1PR2 and S1PR3 (Brinkmann and Baumruker 2006; Swan and others 2010).
Role of S1P in barrier integrity
There is a wealth of observations indicating that S1P plays a key role in the control of endothelial/epithelial barrier integrity in the cardiovascular and pulmonary systems. Hence, it has been well established that the vascular endothelium and alveolar epithelium express S1PR-1, -2, and -3 (Garcia and others 2001; Brinkmann and Baumruker 2006; Brinkmann 2007; Hla and Brinkmann 2011) and that S1P bound to high-density lipoproteins is key for maintaining endothelial/epithelial barrier function (Kimura and others 2003; Camp and others 2016).
On the one hand, S1P1 signaling stabilizes adherens and tight junctions between endothelial and epithelial cells (McVerry and Garcia 2005) and causes local dilatation due to the persistent activation of endothelial nitric oxide synthase, release of nitric oxide, and its activation of the soluble guanylate cyclase (Wilkerson and others 2012). On the other hand, activation of S1P2 and/or S1P3 is associated with a disruption of adherens junctions and an increase in paracellular permeability (Sanchez and others 2003; McVerry and Garcia 2005; Singleton and others 2006).
Accordingly, the administration of a selective competitive S1P1 antagonist to mice produced a loss of capillary integrity (Sanna and others 2006) and potentiated histamine-induced vascular leakage, which could be prevented by co-administration of the S1P2-specific antagonist JTE-013 (Lee and others 2009). Taken together, these observations suggest that endothelial/epithelial barrier functions are the result of a delicate equilibrium between tissue-specific S1P1 and S1P2/3 tones. Thus, depending on the pre-existing balance of S1P1 and S1P2/3 tones in each specific organ, different outcomes could result from treatment with S1P modulators.
In the case of fingolimod, the approved daily 0.5-mg dosing in patients, known to achieve low nanomolar drug blood levels (Wu and others 2012) and retention of lymphocytes into lymphoid organs due to S1P1 downmodulation, is not expected to impact the S1P2-dependent tone in the cardiovascular system and is most unlikely able to displace S1P, at ≥100-fold higher concentration in the blood (Okajima 2002), from the vascular and endothelial S1P1 and S1P3 receptors. Hence, no major signs of altered barrier integrity could be observed in large-scale clinical trials with fingolimod, except for a few cases of macular edema (Calabresi and others 2014), revealing a local specificity at the level of S1P1 versus S1P2/3 tones (Brinkmann 2007).
Nevertheless, several preclinical studies have suggested that fingolimod could increase endothelial barrier function and prevent disruption of permeability barriers through the signaling of S1P1: The administration of fingolimod protected mice from vascular endothelial growth factor-induced vascular leakage (Sanchez and others 2003) and lipopolysaccharide-induced pulmonary edema (Peng and others 2004). In these models, a specific upregulation of endothelial S1P1 occurred as a consequence of the inflammation, rendering the endothelium more susceptible to S1P1-signaling and barrier enhancement by fingolimod (Igarashi and others 2003), with less pronounced downmodulation of the receptors by the drug.
At the same time, high concentrations or prolonged exposure of S1PR modulators may induce barrier disruption, vascular leak, and downregulation of S1P1 receptors (Shea and others 2010; Wang and others 2014; Knipe and others 2022). In other models, specific competitive S1P1 antagonists blocked S1P1-signaling, reduced endothelial barrier function, and caused leakage (Sanna and others 2006; Bigaud and others 2016).
Fingolimod and Siponimod: Potential Mechanism of Action in COVID-19
Short-term intervention with fingolimod or siponimod can attenuate maladjusted immune responses against SARS-CoV-2 via several difference mechanisms, S1P-mediated and non-S1P-mediated pathways, as described next.
S1PR-mediated mechanisms
The S1PR agonists play two major roles in the treatment of severe respiratory viral infections, namely suppressing cellular trafficking and downstream cytokine/chemokine production mediated through S1PR1 expressed in lymphocytes and lung tissues and maintenance of barrier functions by modulation of S1PR1–3 (Teijaro and others 2011; Walsh and others 2011, 2014). Taken together, this could curb early dysregulated innate immune responses that lead to cytokine storm in patients with COVID-19.
S1PR1 modulation and immune cell trafficking: S1PR1 agonists limit the migration of effector lymphocytes by sequestration in secondary lymphoid organs. In mouse infections with human influenza, fatal cytokine storm was aborted by treatment with the S1PR1 agonist. In this context, fingolimod and other S1PR modulators might largely spare T cells within the peripheral effector memory T cells (TEM) involved in defense against infection whereas the main therapeutic effect in multiple sclerosis remains on the central memory T cells (TCM) (Pinschewer and others 2000; Brinkmann and others 2010). Without vaccination, kinetics and titers of neutralizing antibodies were largely unaffected by S1PR modulators whereas concurrent fingolimod treatment in comparison to placebo decreased influenza vaccination-induced immune responses (Kappos and others 2015).
In patients with Middle East respiratory syndrome coronavirus, SARS-CoV, and swine flu (H1N1) influenza strong Th-17 responses have been observed (Josset and others 2013; Faure and others 2014). In a murine model, H1N1 was shown to cause acute lung injury through an IL-17–dependent pattern (Li and others 2012). Th-17 significantly contributes to the cytokine storm in pulmonary viral infection, including SARS-CoV-2, causing tissue damage and promoting pulmonary edema (Wu and Yang 2020). Fingolimod and siponimod acts as a functional antagonist of S1PR1 through internalization of the receptors.
S1PR1 is, among other tissues, expressed on lymphocytes. Both fingolimod and siponimod block the S1P-dependent chemotactic egress of lymphocytes from lymph nodes, thus reducing the number of lymphocytes in peripheral blood, including pro-inflammatory Th-17 cells (Sica and others 2019). Since the Th-17–mediated pathway is attenuated, it can be assumed that patients with Th-17–dominant immune profiles, such as COVID-19–associated ARDS, may benefit (Brinkmann 2009; Maggi and others 2012; Sica and others 2019; Wu and Yang 2020).
Non-S1PR–mediated mechanisms
Attenuated cytokine release via activation of serine/threonine protein phosphatase 2A (PP2A) and via Th-17-mediated pathway: Cytokine production involves the signaling pathways of mitogen-activated protein kinase, which is regulated by PP2A. Inhibition of PP2A has been shown to increase the production of IL-8 by respiratory epithelial cells, implying that PP2A is a key regulator of chemokine production during ARDS (Cornell and others 2009; Rahman and others 2015, 2016b). IL-8 concentrations correlate with the severity of lung lesions in patients with ARDS, and the blocking of IL-8 activity in animal models has been shown to be effective in modifying the pathophysiology of ARDS and improving mortality (Jorens and others 1992; Miller and others 1992, 1996; Chollet-Martin and others 1993; Donnelly and others 1993).
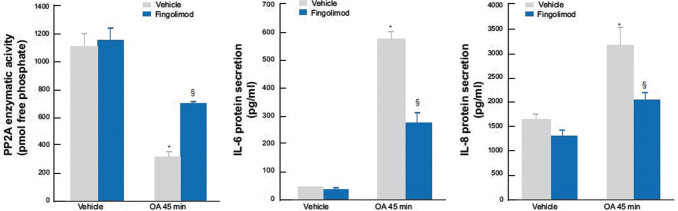
Fingolimod has been shown to increase PP2A activity in several preclinical studies (Rahman and others 2015, 2016a, 2016b; McHugh and others 2016). In addition, the activation of PP2A by fingolimod exceeds the okadaic acid-mediated inhibition of the basal activity of PP2A phosphatase and significantly suppresses IL-6 and IL-8 cytokine secretion in human alveolar epithelial cell lines (Fig. 2) (Rahman and others 2015, 2016a, 2016b). Downstream in the PPA2 mediated pathway, fingolimod also results in a 70% to 80% decrease in CXCL1 and CXCL2, two chemokines that play a key role in ARDS (McHugh and others 2016; Boro and Balaji 2017). In summary, fingolimod may offer therapeutic potential in ARDS as an activator of PP2A, thereby decreasing CXCL1 and CXCL2 and attenuating the Th-17 pathway.
FIG. 2.
Suppressed cytokine secretion by fingolimod via PP2A activation. The PP2A activator fingolimod overcomes OA-mediated inhibition of basal PP2A phosphatase activity and significantly represses IL-6 and IL-8 mRNA expression and cytokine secretion. A549 cells were treated for 6 h with 2.5 μM fingolimod before 45 min with 1 μM OA, compared with vehicle. IL-6 and IL-8 protein secretion measured at 24 h. *Denotes a significant effect of OA and §for fingolimod (P < 0.05). Data are mean + SEM values from three independent experiments. Figure adapted from Rahman and others (2015). mRNA, messenger RNA; OA, okadaic acid; PP2A, protein phosphatase 2A; SEM, standard error of mean. Reproduced under Creative Commons Attribution 4.0 International License from Rahman MM, Rumzhum NN, Morris JC, Clark AR, Verrills NM, Ammit AJ. Scientific Report 2015 May 18;5:10063; DOI: 10.1038/srep10063.
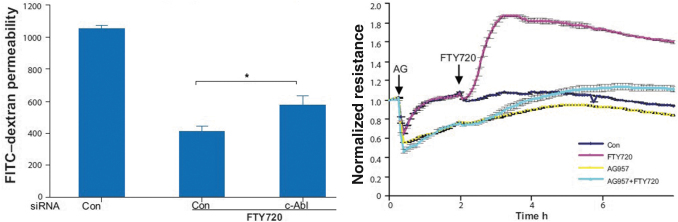
Enhancement of the pulmonary endothelial barrier via c-Abl tyrosine kinase pathway: Activation and disruption of the pulmonary endothelium ultimately leads to ARDS, in which increased barrier permeability results in leakage of protein-rich fluid from the vascular compartment to the interstitium and alveolar airspaces, leading to a life-threatening impairment of gas exchange. Another characteristic of acute ARDS-related inflammation is the significant and lasting increase in vascular permeability, which is associated with significant mortality. Fingolimod enhances pulmonary vascular endothelial barrier function by significantly increasing c-Abl kinase activity (Fig. 3) (Wang and others 2011).
FIG. 3.
Barrier enhancement by fingolimod via c-Abl tyrosine kinase. Left panel: HPAEC were transfected with 100 nM c-Abl or control siRNA and seeded onto Transwell inserts. After FTY720 stimulation (1 μM), FITC-dextran was added into the top chamber and incubated for 2 h. The fluorescent intensity of the bottom chamber was analyzed by fluorometry as per Methods. n = 4. *P < 0.031 versus control siRNA. Right panel: HPAEC were preincubated for 1 h with the c-Abl inhibitor, AG957 (20 μM), or vehicle control and then stimulated with FTY720 (1 μM) or vehicle. Data are representative of three independent experiments. Figure adapted from Wang and others (2011). AG957, c-Abl inhibitor; Con, control; FITC, fluorescein isothiocyanate; FTY720, fingolimod; HPAEC, human pulmonary artery endothelial cells; siRNA, small interfering RNA. Reproduced with permission of the © ERS 2022. European Respiratory Journal Jul 2011, 38 (1) 78-88; DOI: 10.1183/09031936.00047810. Copyright permission reference number is ERJPM076-2022-23.
It has been hypothesized that this action is mediated by lipid raft signaling, G-linked receptor coupling to downstream tyrosine phosphorylation events involving c-Abl and this pathway does not require adherens junction or tight junction protein complexes. Also, for fingolimod-analogs, a significant enhancement of endothelial cell barrier integrity has been shown (Camp and others 2016).
Short-Term Treatment with S1PR Modulators: Benefit-Risk Assessment for COVID-19–Associated ARDS
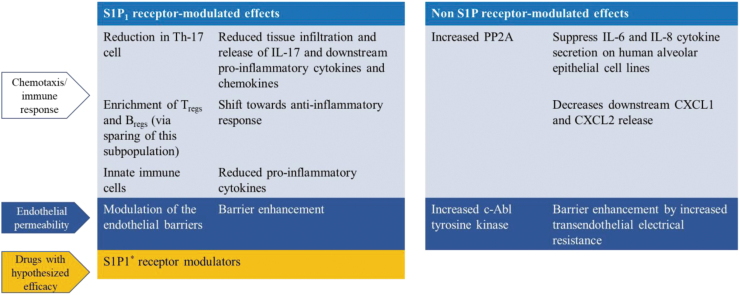
Studies have investigated the effects of fingolimod and other S1PR modulators in multiple indications (Stepanovska and Huwiler 2020). Several preclinical studies have suggested targeting S1P-mediated pathways for the treatment of acute lung injury and ARDS (Natarajan and others 2013; Ebenezer and others 2016). Fingolimod, a non-selective S1PR modulator, exerts its anti-inflammatory and barrier enhancement effects via both S1PR-mediated pathways and non-S1PR–mediated pathways (Fig. 4).
FIG 4.
Short-term exposure of S1PR modulators in COVID-19 ARDS: Summary of potential mechanisms. *Differential PK/PD profiles as described elsewhere. Bregs, regulatory B cell; IL, interleukin; PK/PD, pharmacokinetic/pharmacodynamic; PP2A, protein phosphatase 2A; S1P, sphingosine-1-phosphate; Th, helper T cell; Tregs, regulatory T cell.
The hypothesized beneficial effects of PP2A activation and decrease of Chemokine (C-X-C motif) ligand 1 (CLCX1) and Chemokine (C-X-C motif) ligand 2 (CXCL2) appear to be independent from S1PR modulation, and therefore, an unambiguous dose recommendation based on S1PR1 effects and absolute leukocyte count (ALC) reduction is not possible.
Fingolimod
The S1PR modulation is associated with certain well-characterized pharmacological effects on immune, cardiovascular, and pulmonary systems (Chun and Hartung 2010), and the onset of these pharmacological effects is of paramount importance while considering the management strategy in patients with COVID-19.
The desired reduction in circulating lymphocyte is evident within 4–6 h of first dose administration. Fingolimod results in reversible and selective sequestration of lymphocytes, sparing the effector memory immune response (Brinkmann 2009). Therefore, the risk of infections is relatively low in this short-term exposure as further reduction of circulating ALC should not persist too long.
The negative chronotropic effects of fingolimod on heart rate (HR) and the effects on cardiac conduction are transient and dose dependent. These effects are mostly evident on day 1 of dosing, with maximal effects observed within 2–4 h of first dose and are rarely observed beyond day 2 of dosing. With continuous dosing receptors are internalized resulting in desensitization to HR effects (Schmouder and others 2006). In the treatment setting, if necessary, patients could have continuous cardiac monitoring.
The early agonistic effects of fingolimod on S1PR 2/3 expressed by airway smooth muscle cells may induce airway hyper-responsiveness due to receptor modulation (Brinkmann and Baumruker 2006). Studies have also shown that fingolimod exhibits differential response patterns at the S1PR3 and has antagonistic properties under certain conditions (Sensken and others 2008; Jongsma and others 2009).
The interplay of physiological S1P and other ligands, such as fingolimod, at the receptor level is not clearly known. The minimal decreases in forced expiratory volume at 1 s and diffusing capacity for carbon monoxide has been observed with fingolimod at Month 1 (US FDA 2012). Short-term fingolimod treatment is unlikely to cause persistent respiratory symptoms after treatment discontinuation on day 3 (Brinkmann and Baumruker 2006; US FDA 2012).
Siponimod
The rapid oral dose titration achieves a robust reduction in ALC already within the first hours of treatment initiation and allows to reach therapeutic (2 mg) dose levels and exposures within the first 24 h (i.e., by day 2 of treatment) while keeping the expected effects on HR and atrioventricular conduction at treatment initiation manageable. Like fingolimod, the HR reduction shows a dose-dependent behavior; the most pronounced HR reduction is observed after the first dose (day 1), followed by smaller incremental decreases in HR on subsequent days of dosing.
Up titrating doses from 0.5 mg (day 1) to 2 mg (day 2 and onward) is not expected to result in pronounced cardiac effects as most of the S1P1 receptor internalization is achieved already on the 1st day of dosing. Siponimod does not have affinity toward S1PR 2/3 and, thus, unlikely to mediate any effect on increased vascular tone and barrier leakage (Gergely and others 2012). The short half-life of siponimod allows for fast washout with lymphocyte count recovery to normal values and re-establishing immune competence within 7 days of siponimod discontinuation (Glaenzel and others 2018).
Other S1PR modulators
Ponesimod and ozanimod are other orally active S1P1 modulator recently approved for treatment for relapsing-remitting multiple sclerosis (US FDA 2020, 2021). Like other S1P modulators, both follow a dose-dependent pharmacokinetics with rapid onset and reversible reduction of peripheral blood lymphocyte counts (Chun and others 2010; Piali and others 2011). Their rapid elimination and reversibility of reduction in peripheral blood lymphocytes count is believed to be beneficial for vaccination, serious infections, or during pregnancy planning (Kappos and others 2021). However, preclinical reports indicate that antibody response post-vaccination was blunted with ponesimod (Spiller and others 2021).
Summary
In a pandemic where people may die of ARDS, it has been suggested that S1P receptor modulators such as fingolimod and siponimod may be effective treatments for ARDS based on their described mechanisms of anti-inflammation and enhancement of barrier function. However, for patients critically ill with COVID-19, the decision to suppress immunity is a difficult one, and broader immunosuppression might be inadvisable. Beneficial anti-inflammatory effects should be weighed up against the potentially detrimental effects of inhibiting antiviral immunity, thereby delaying virus clearance and perpetuating illness.
An additional consideration is the possible reversal of beneficial effects after discontinuation of therapy. For example, within multiple sclerosis (MS), cases of post-fingolimod have been described. Finally, S1PR modulation and its effects on cardiac rhythm and conduction, possible risk of infections, and effects on pulmonary function in critically ill patients infected with SARS-CoV-2 need to be carefully evaluated.
Several trials of existing pharmaceuticals have been initiated, and additionally, the U.S. FDA has granted Emergency Use Authorization for some existing drugs (US FDA 2017). Recently, the U.S. FDA has authorized tixagevimab co-packaged with cilgavimab and administered together as a medication to prevent COVID-19 in some individuals aged 12 years and older (Evusheld 2021). This prevention medication is strictly for those who are not expected to have adequate immune responses to the vaccine or who have a severe allergy to the vaccine.
However, as COVID-19 variants continue to emerge, it is not yet known how effective this combination of monoclonal antibodies will be against each variant and vaccines continue to be the best defense available against the COVID-19 complications of the SARS-CoV-2 virus, including all its variants. In addition, various other treatments have received emergency use authorization (please refer to background section).
Studies of the SARS-CoV-2 vaccine responses in people living with MS have shown a potentially reduced antibody response among those who use certain disease-modifying therapies, including S1P modulators (siponimod, fingolimod, ponesimod, and ozanimod), alemtuzumab, and anti-CD20 monoclonal antibodies (ocrelizumab, ofatumumab, rituximab, and biosimilars) (Disanto and others 2021; Guerrieri and others 2021; Spiller and others 2021; Tallantyre and others 2022).
Evidence from recent individual patient case reports suggests that fingolimod treatment might be beneficial in reducing severe immune response to SARS-CoV-2. Patients with multiple sclerosis under fingolimod treatment infected with COVID-19 have reported mild to moderate outcomes. These patients, despite radiological evidence of severe COVID-19–related pneumonia, did not develop ARDS, and had rapid recovery (Barzegar and others 2020; Foerch and others 2020).
Thus, in the context of attenuating the SARS-CoV-2 induced hyperinflammation in patients with COVID-19, short-term therapy with fingolimod or siponimod has a rationale based on the known mechanisms of action. The potential side effects of S1PR modulation have been largely manageable in the context of the illness for which they were approved. Further, given that these patients are critically ill and already requiring treatment, monitoring for side effects would be possible within an intensive care setting (WHO 2020; CDC 2021).
Conclusion
The S1P receptor modulators, fingolimod and siponimod, have been proposed as a potential treatment option for COVID-19. However, in the absence of clinical evidence, further investigation should be considered with caution and only with continuous monitoring and after careful benefit-risk evaluation.
Acknowledgments
The authors thank Rohit Bhandari and Anuja Shah (Novartis Healthcare Ltd., Hyderabad, India) for providing medical writing support.
Author Disclosure Statement
T.H., G.G., and D.P.M. are full-time employees of Novartis Pharma AG, Basel, Switzerland. K.S.N., M.B., and O.P. are full-time employees of Novartis Institutes for Biomedical Research, Basel, Switzerland. M.d.M. and R.T. are full-time employees of Novartis Farma S.p.A., Origgio, Italy. V.B. and F.N. have no declaration of interest to declare. F.D. is an ex-employee of Novartis Pharma AG, Basel, Switzerland.
Funding Information
Novartis Pharma AG, Basel, Switzerland provided medical writing and editorial assistance for this article in accordance with Good Publication Practice (GPP3) guidelines.
References
- Barzegar M, Mirmosayyeb O, Nehzat N, Sarrafi R, Khorvash F, Maghzi AH, Shaygannejad V. 2020. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm 7(4):e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Silverman RH. 2020. COVID-19: coronavirus replication, pathogenesis, and therapeutic strategies. Cleve Clin J Med 87(6):321–327. [DOI] [PubMed] [Google Scholar]
- Bigaud M, Dincer Z, Bollbuck B, Dawson J, Beckmann N, Beerli C, Fishli-Cavelti G, Nahler M, Angst D, Janser P, Otto H, Rosner E, Hersperger R, Bruns C, Quancard J. 2016. Pathophysiological consequences of a break in S1P1-dependent homeostasis of vascular permeability revealed by S1P1 competitive antagonism. PLoS One 11(12):e0168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boro M, Balaji KN. 2017. CXCL1 and CXCL2 regulate NLRP3 inflammasome activation via G-protein–coupled receptor CXCR2. J Immunol 199(5):1660–1671. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. 2007. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115(1):84–105. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. 2009. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 158(5):1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Baumruker T. 2006. Pulmonary and vascular pharmacology of sphingosine 1-phosphate. Curr Opin Pharmacol 6(3):244–250. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9(11):883–897. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. 2014. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13(6):545–556. [DOI] [PubMed] [Google Scholar]
- Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, Garcia JG, Dudek SM. 2016. Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: methoxy-FTY720, fluoro-FTY720, and β-glucuronide-FTY720. Chem Phys Lipids 194:85–93. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2021. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (Last accessed November 11, 2021).
- Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. 1993. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun 61(11):4553–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP. 2010. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62(4):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell TT, Hinkovska-Galcheva V, Sun L, Cai Q, Hershenson MB, Vanway S, Shanley TP. 2009. Ceramide-dependent PP2A regulation of TNFalpha-induced IL-8 production in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol 296(5):L849–L856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron RQ, Caricchio R, Chatham WW. 2021. Calming the cytokine storm in COVID-19. Nat Med 27(10):1674–1675. [DOI] [PubMed] [Google Scholar]
- Disanto G, Sacco R, Bernasconi E, Martinetti G, Keller F, Gobbi C, Zecca C. 2021. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol 78(12):1529–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly SC, Haslett C, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Pollok AJ, Grant IS. 1993. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341(8846):643–647. [DOI] [PubMed] [Google Scholar]
- Ebenezer DL, Fu P, Natarajan V. 2016. Targeting sphingosine-1-phosphate signaling in lung diseases. Pharmacol Ther 168:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency). 2021a. EMA recommends approval for use of Kineret in adults with COVID-19. Available at https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19 (Last accessed January 19, 2022).
- EMA (European Medicines Agency). 2021b. EMA COVID 19 treatments: tocilizumab. Available at https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (Last accessed January 17, 2022).
- Evusheld. 2021. Evusheld (formerly AZD7442) long-acting antibody combination authorised for emergency use in the US for pre-exposure prophylaxis (prevention) of COVID-19. Available at https://www.astrazeneca.com/media-centre/press-releases/2021/evusheld-long-acting-antibody-combination-authorised-for-emergency-use-in-the-us-for-pre-exposure-prophylaxis-prevention-of-covid-19.html (Last accessed December 20, 2021).
- Faure E, Poissy J, Goffard A, Fournier C, Kipnis E, Titecat M, Bortolotti P, Martinez L, Dubucquoi S, Dessein R, Gosset P, Mathieu D, Guery B. 2014. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One 9(2):e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Friedauer L, Bauer B, Wolf T, Adam EH. 2020. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scleros Related Disord 42:102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. 2001. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108(5):689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, Pan S, Gray NS, Hinterding K, Cooke NG, Groenewegen A, Vitaliti A, Sing T, Luttringer O, Yang J, Gardin A, Wang N, Crumb WJ Jr., Saltzman M, Rosenberg M, Wallstrom E.. 2012. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol 167(5):1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaenzel U, Jin Y, Nufer R, Li W, Schroer K, Adam-Stitah S, Peter van Marle S, Legangneux E, Borell H, James AD, Meissner A, Camenisch G, Gardin A. 2018. Metabolism and disposition of siponimod, a novel selective S1P(1)/S1P(5) agonist, in healthy volunteers and in vitro identification of human cytochrome P450 enzymes involved in its oxidative metabolism. Drug Metab Dispos 46(7):1001–1013. [DOI] [PubMed] [Google Scholar]
- Grommes J, Soehnlein O. 2011. Contribution of neutrophils to acute lung injury. Mol Med 17(3–4):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri S, Lazzarin S, Zanetta C, Nozzolillo A, Filippi M, Moiola L. 2021. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J Neurol 269(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, Tan K-S, Wang D-Y, Yan Y. 2020. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Military Med Res 7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, Zaidi STR. 2020. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med 14(11):1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejrati A, Nurzadeh M, Roham M. 2021. Association of coronavirus pathogencity with the level of antioxidants and immune system. J Fam Med Prim Care 10(2):609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. 2011. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology 76(8 Suppl. 3):S3–S8. [DOI] [PubMed] [Google Scholar]
- Hla T, Venkataraman K, Michaud J. 2008. The vascular S1P gradient—Cellular sources and biological significance. Biochim Biophys Acta Mol Cell Biol Lipids 1781(9):477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KPY, Ho JCW, Cheung MC, Ng KC, Ching RHH, Lai KL, Kam TT, Gu H, Sit KY, Hsin MKY, Au TWK, Poon LLM, Peiris M, Nicholls JM, Chan MCW. 2022. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 603(7902):715–720. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas APV, Chen H, Michel T. 2003. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA 100(19):10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M, Van Unen J, Van Loenen P, Michel M, Peters S, Alewijnse A. 2009. Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P3). Br J Pharmacol 156(8):1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorens PG, Van Damme J, De Backer W, Bossaert L, De Jongh RF, Herman AG, Rampart M. 1992. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 4(6):592–597. [DOI] [PubMed] [Google Scholar]
- Josset L, Menachery VD, Gralinski LE, Agnihothram S, Sova P, Carter VS, Yount BL, Graham RL, Baric RS, Katze MG. 2013. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio 4(3):e00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Fox RJ, Burcklen M, Freedman MS, Havrdova EK, Hennessy B, Hohlfeld R, Lublin F, Montalban X, Pozzilli C, Scherz T, D'Ambrosio D, Linscheid P, Vaclavkova A, Pirozek-Lawniczek M, Kracker H, Sprenger T. 2021. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol 78(5):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Mehling M, Arroyo R, Izquierdo G, Selmaj K, Curovic-Perisic V, Keil A, Bijarnia M, Singh A, von Rosenstiel P. 2015. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 84(9):872–879. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. 2003. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscleros Thrombos Vascul Biol 23(7):1283–1288. [DOI] [PubMed] [Google Scholar]
- Knipe RS, Spinney JJ, Abe EA, Probst CK, Franklin A, Logue A, Giacona F, Drummond M, Griffith J, Brazee PL, Hariri LP, Montesi SB, Black KE, Hla T, Kuo A, Cartier A, Engelbrecht E, Christoffersen C, Shea BS, Tager AM, Medoff BD. 2022. Endothelial-specific loss of sphingosine-1-phosphate receptor 1 increases vascular permeability and exacerbates bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 66(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. 2009. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol 296(1):H33–H42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Zou Z, Yan Y, Wang W, Wang C, Chen Z, Xing L, Tang C, Ju X, Guo F, Deng J, Zhao Y, Yang P, Tang J, Wang H, Zhao Z, Yin Z, Cao B, Wang X, Jiang C. 2012. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res 22(3):528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. 2020a. Coronavirus infections and immune responses. J Med Virol 92(4):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, Liang Y. 2020b. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 34(6):1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Santarlasci V, Capone M, Rossi MC, Querci V, Mazzoni A, Cimaz R, De Palma R, Liotta F, Maggi E, Romagnani S, Cosmi L, Annunziato F. 2012. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur J Immunol 42(12):3180–3188. [DOI] [PubMed] [Google Scholar]
- McHugh WM, Russell WW, Fleszar AJ, Rodenhouse PE, Rietberg SP, Sun L, Shanley TP, Cornell TT. 2016. Protein phosphatase 2A activation attenuates inflammation in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 311(5):L903–L912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JGN. 2005. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signall 17(2):131–139. [DOI] [PubMed] [Google Scholar]
- Meyer NJ, Gattinoni L, Calfee CS. 2021. Acute respiratory distress syndrome. Lancet 398(10300):622–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EJ, Cohen AB, Matthay MA. 1996. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med 24(9):1448–1454. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA. 1992. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 146(2):427–432. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, Mathew B, Zhao Y, Wang L, Bittman R, Weichselbaum R, Berdyshev E, Garcia JGN. 2013. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respirat Cell Mol Biol 49(1):6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (National Institute of Health). 2021. COVID-19 Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/clinical-management-summary/ (Last accessed August 10, 2021).
- NIH (National Institute of Health). 2022. COVID-19 Treatment Guidelines. Remdesivir Drug Info. Available at https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/remdesivir/ (Last accessed November 14, 2021).
- Okajima F. 2002. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta 1582(1–3):132–137. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JGN. 2004. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respirat Crit Care Med 169(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- Piali L, Froidevaux S, Hess P, Nayler O, Bolli MH, Schlosser E, Kohl C, Steiner B, Clozel M. 2011. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther 337(2):547–556. [DOI] [PubMed] [Google Scholar]
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. 2000. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol 164(11):5761–5770. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Prünte L, Lebender LF, Patel BS, Gelissen I, Hansbro PM, Morris JC, Clark AR, Verrills NM, Ammit AJ. 2016a. The phosphorylated form of FTY720 activates PP2A, represses inflammation and is devoid of S1P agonism in A549 lung epithelial cells. Sci Rep 6:37297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Rumzhum NN, Hansbro PM, Morris JC, Clark AR, Verrills NM, Ammit AJ. 2016b, Activating protein phosphatase 2A (PP2A) enhances tristetraprolin (TTP) anti-inflammatory function in A549 lung epithelial cells. Cell Signal 28(4):325–334. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Rumzhum NN, Morris JC, Clark AR, Verrills NM, Ammit AJ. 2015, Basal protein phosphatase 2A activity restrains cytokine expression: role for MAPKs and tristetraprolin. Sci Rep 5:10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Subbian S. 2021. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin Microbiol Rev 34(3):e00299-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. 2021. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur J Pharmacol 897:173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik J-H, Wu M-T, Venkataraman K, Brinkmann V, Claffey K, Hla T. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 278(47):47281–47290. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. 2006. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2(8):434–441. [DOI] [PubMed] [Google Scholar]
- Schmouder R, Serra D, Wang Y, Kovarik JM, DiMarco J, Hunt TL, Bastien MC. 2006. FTY720: placebo-controlled study of the effect on cardiac rate and rhythm in healthy subjects. J Clin Pharmacol 46(8):895–904. [DOI] [PubMed] [Google Scholar]
- Sensken SC, Stäubert C, Keul P, Levkau B, Schöneberg T, Gräler MH. 2008. Selective activation of G alpha i mediated signalling of S1P3 by FTY720-phosphate. Cell Signal 20(6):1125–1133. [DOI] [PubMed] [Google Scholar]
- Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. 2010. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol 43(6):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica F, Centonze D, Buttari F. 2019. Fingolimod immune effects beyond its sequestration ability. Neurol Ther 8(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Ma SF, Garcia JG. 2006. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 281(45):34381–34393. [DOI] [PubMed] [Google Scholar]
- Spiller K, Aras R, DeGuzman M, Ramsburg E, Bhattacharya A. 2021. A short pause in ponesimod treatment completely restores the ability to mount post-vaccination antibody titers in mice; Available at https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1495953# (Last accessed December 20, 2021).
- Stepanovska B, Huwiler A. 2020. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res 154:104170. [DOI] [PubMed] [Google Scholar]
- Swan DJ, Kirby JA, Ali S. 2010. Vascular biology: the role of sphingosine 1-phosphate in both the resting state and inflammation. J Cell Mol Med 14(9):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J, Bramhall K, Chance R, Evangelou N, George K, Giovannoni G, Godkin A, Grant L, Harding KE, Hibbert A, Ingram G, Jones M, Kang AS, Loveless S, Moat SJ, Robertson NP, Schmierer K, Scurr MJ, Shah SN, Simmons J, Upcott M, Willis M, Jolles S, Dobson R. 2022. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol 91(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. 2011. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146(6):980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, Kastritis E, Pavlakis GN, Dimopoulos MA. 2021. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci 28(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA (U.S. Food and Drug Administration). 2012. GILENYA prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022527s008lbl.pdf (Last accessed November 21, 2021).
- US FDA (U.S. Food and Drug Administration). 2017. Emergency use authorization. Available at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (Last accessed November 21, 2021).
- US FDA (U.S. Food and Drug Administration). 2019. MAYZENT prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf (Last accessed December 20, 2021).
- US FDA (U.S. Food and Drug Administration). 2020. Ozonimod prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf (Last accessed January 17, 2022).
- US FDA (U.S. Food and Drug Administration). 2021. Ponesimod prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213498s000lbl.pdf (Last accessed December 20, 2021).
- US FDA (U.S. Food and Drug Administration). 2022a. Emergency use authorization: baricitinib and tocilizumab. Available at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (Last accessed January 17, 2022).
- US FDA. (U.S. Food and Drug Administration). 2022b. Emergency use authorization (Molnupiravir). Available at https://www.fda.gov/media/155053/download (Last accessed January 19, 2022).
- US FDA (U.S. Food and Drug Administration). 2022c. Emergency use authorization (PAXLOVID). Available at https://www.fda.gov/media/155049/download (Last accessed January 19, 2022).
- Walsh KB, Teijaro JR, Brock LG, Fremgen DM, Collins PL, Rosen H, Oldstone MB. 2014. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol 88(11):6281–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Rosen H, Oldstone MB. 2011. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol Res 51(1):15–25. [DOI] [PubMed] [Google Scholar]
- Wang L, Chiang ET, Simmons JT, Garcia JG, Dudek SM. 2011. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur Respir J 38(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sammani S, Moreno-Vinasco L, Letsiou E, Wang T, Camp SM, Bittman R, Garcia JG, Dudek SM. 2014. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Crit Care Med 42(3):e189–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Clinical management of COVID-19: Interim guidance 2020. Available at https://apps.who.int/iris/bitstream/handle/10665/330893/WHO-nCoV-Clinical-2020.3-eng.pdf?sequence=1&isAllowed=y (Last accessed November 11, 2021).
- WHO. 2022. WHO Coronavirus (COVID-19) Dashboard: Overview. Available at https://covid19.who.int/ (Last accessed January 17, 2022).
- Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM. 2012. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem 287(53):44645–44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Yang XO. 2020. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3):368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Mercier F, David OJ, Schmouder RL, Looby M. 2012. Population pharmacokinetics of fingolimod phosphate in healthy participants. J Clin Pharmacol 52(7):1054–1068. [DOI] [PubMed] [Google Scholar]
- Yeates EO, Nahmias J, Chinn J, Sullivan B, Stopenski S, Amin AN, Nguyen NT. 2021. Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure. PLoS One 16(6):e0253767. [DOI] [PMC free article] [PubMed] [Google Scholar]