Abstract
Purpose:
Although participation of adolescents and young adults (AYAs) in cancer clinical trials (CCTs, i.e., cancer-directed treatment studies) is low, their decision-making perspectives are not well understood, especially following recent diagnosis.
Methods:
Semistructured interviews with younger AYAs (15–21 years old) eligible for a CCT were to be held within 60 days of beginning treatment at Children's Hospital Los Angeles, an academic pediatric hospital. Using grounded theory methods, key themes regarding CCT participation, barriers, and facilitators were identified from interview transcripts. Thematic saturation was confirmed.
Results:
Of nine participants, three were <18 years old, four Hispanic, six male, six diagnosed with leukemia, eight enrolled in a CCT, and eight also enrolled in ancillary studies. Four overarching themes emerged: (1) Initial Consent encompassed the first discussion of CCT with patients reflecting positive and negative effects of timing, decisional role, and the emotional impact following cancer diagnosis; (2) Informing Participation involved decision-making processes, specific knowledge, comprehension, and external influences; (3) Participant Relationships emphasized the importance of communication and relationships with providers and parents; and (4) Patient Determinants centered on motives from different perspectives, pre-conceived attitudes, and understanding of CCTs.
Conclusion:
Recommendations for improving CCT participation among younger AYAs include separating the diagnosis/treatment and CCT discussions, assigning AYAs a meaningful decisional role, having ongoing provider conversations, designing trials to minimize burden, and developing age-appropriate decision aids.
Keywords: adolescent and young adult, clinical trial enrollment, qualitative
Introduction
Each year, in the United States, almost 90,000 adolescents and young adults (AYAs, 15–39 years of age) are diagnosed with cancer, over eight times the number of children younger than 15 years. However, many studies have documented that a much smaller proportion of AYAs than children participate in cancer clinical trials (CCTs), generally 10% or less for AYAs compared with 40%–60% for children.1,2 Poor AYA participation in CCTs hinders efforts to improve survival for high-risk subgroups, for advance supportive care, to investigate cancer and host biology, and to access novel therapies in this at-risk population.3,4
Reasons for low AYA enrollment onto CCTs are complex and operate at the national, institutional, provider, and patient levels.1 Recent data indicate that proportional AYA enrollment onto cooperative group CCTs is significantly higher at National Cancer Institute (NCI)-designated comprehensive cancer centers (NCI-CCC) than community-based sites.5,6 However, even within NCI-CCCs, AYA enrollment differs according to whether AYAs are treated in a pediatric or adult setting due to barriers to care for AYAs.7 We recently conducted a prospective observational cohort study of 152 consecutive AYAs diagnosed with cancer at either Children's Hospital Los Angeles (CHLA) or its adult-focused affiliate, the USC Norris Cancer Hospital, to compare AYA enrollment and barriers by setting. CCT enrollment was low at both sites (11.8% and 7.1%, respectively).
However, in the adult hospital, the major barrier was lack of available CCTs at the institutional level (only 16.7% of AYAs had an available CCT), whereas in the pediatric setting, the major barrier was poor uptake at the patient level (44.1% of AYAs had an available CCT).8 Those results suggested that in the pediatric setting, patient-level factors may be particularly important for successful enrollment of younger AYAs. Although the choice to participate in an available CCT also reflects both provider and institutional influences, patient-level factors, including the AYAs' understanding, emotional status, relationships with providers, decision-making role, and psychosocial support, are central.
To date, research into such factors has been relatively limited.9 In studies where the AYA patient's views have been solicited, most engaged patients well into the treatment process, with more time passed since diagnosis. At that point, AYAs' opinions about CCT enrollment may be confounded by their treatment experience, thus less reflective of concerns of newly diagnosed patients.10–13
To gain insight into how the above factors influence younger AYAs, we conducted a qualitative study to identify key themes in facilitators and barriers influencing participation in CCTs. We focused specifically on AYAs who were newly diagnosed with cancer and eligible for an available CCT.
Methods
Study design, setting, and participants
This qualitative study utilized a grounded theory approach and was conducted at CHLA, a quaternary care children's hospital, from March to August 2018. Eligible patients were 15–21 years of age at diagnosis of their first cancer, English speaking, and eligible for enrollment onto at least one CCT (defined as a cancer-directed treatment study) activated at CHLA. Patients were to be approached within 60 days of initiating treatment. Each participant's birth date, sex, race, ethnicity, cancer type, and date of diagnosis were collected from the medical record. Enrollment status (yes/no) onto the CCT and/or any other active ancillary studies were confirmed with the Clinical Research Support Office. The Institutional Review Board approved this study (IRB ID CHLA-17-00446). Before participation, written informed consent from the participant or parent/guardian and assent for minors were obtained. Participants received a $50 gift card after interview completion.
Interviews
Semistructured cognitive interviews were conducted using a guide informed by extant literature and clinical experience (Supplementary File). Open-ended questions addressed general knowledge about clinical trials, the participant's CCT decision, family/friend support, and motives. In-person or telephone interviews were conducted by S.M.T., per patient preference. Interviews lasted 30–45 minutes. Interviews were audio-recorded, de-identified, and transcribed verbatim by an external company.14
Data analysis
Interview transcripts were subjected to thematic analysis.15 Using an inductive approach, themes were developed and manually coded as they arose during participant interviews by E.M.M. and A.A.; J.B. used transcript samples to validate the coding process.16 Each theme was organized around barriers, facilitators, and connections across themes and subthemes for participants. Data saturation was confirmed once new data discovery became redundant and the consensus was evident across respondent views.17–20 This was achieved with nine interviews; no additional participant was recruited.
Results
Participants and CCT enrollment patterns
Of 10 eligible patients approached for this study, 9 were interviewed and 1 declined to participate (Table 1). Three participants were <18 years old, four were Hispanic/Latino, six were male, and six were diagnosed with acute lymphoblastic leukemia (ALL). Six interviews were conducted within 60 days of diagnosis (mean for all interviews 91 days, median 42 days, and range 3–301 days). Of the 9 participants, 8 enrolled in a therapeutic CCT (ALL, 6; germ cell tumor, 2). Seven of these eight also enrolled in one or more concurrent nontherapeutic studies (Table 2).
Table 1.
Characteristics of Study Participants (N = 9)
| Variable | Category | Count |
|---|---|---|
| Gender | Male | 6 |
| Female | 2 | |
| Transgender female | 1 | |
| Race | White | 5 |
| Ethnicity | Hispanic | 4 |
| Age at interview, years | 16–17 | 3 |
| 18–20 | 6 | |
| Time from diagnosis to interview, days | ≤60 | 6 |
| >60 | 3 | |
| Diagnosis | ALL (high risk B cell) | 6 |
| Germ cell tumor (testicular) | 2 | |
| Acute promyelocytic leukemia | 1 | |
| Therapeutic CCT | Eligible to participate | 9 |
| Enrolled | 8 |
ALL, acute lymphoblastic leukemia; CCT, cancer clinical trial.
Table 2.
Characteristics of Studies in Which Participants Were Enrolled
| Category | Short title | Sponsor | Design | Phase | Brief summary |
|---|---|---|---|---|---|
| CCT | AALL1131 | Children's Oncology Group | Therapeutic randomized control trial | Phase 3 | Multistrata study for patients with newly diagnosed high-risk pre-B ALL. Randomization after initial course of induction chemotherapy based on response to chemotherapy |
| AGCT1531 | Children's Oncology Group | Active surveillance and therapeutic randomized control trial | Phase 3 | Study of active surveillance for patients with newly diagnosed low-risk germ cell tumors and a randomized control trial for patients with standard-risk germ cell tumors | |
| AAML1331 | Children's Oncology Group | Therapeutic nonrandomized control trial | Phase 3 | Study of patients with newly diagnosed standard and high-risk acute promyelocytic leukemia to determine standard of care treatment | |
| Biology, specimen procurement, or registry | Extra Bone Marrow | Investigator initiated | Observational cohort | N/A | Mechanism for collecting extra bone marrow for anticipated biology studies in patients with suspected leukemia |
| ALL08B1 | Children's Oncology Group | Observational cohort | N/A | International tumor registry to develop a risk-based classification system with patients with newly diagnosed ALL | |
| ACCRN07 | Children's Oncology Group | Observational cohort | N/A | International tumor registry study for Children's Oncology Group to collect information about children with cancer | |
| Quality of life or supportive care | IDEAL | Investigator initiated | Interventional cohort (nonrandomized) | N/A | Pilot study of exercise and dietary intervention to improve treatment response and reduce toxicity during Induction phase of treatment for ALL |
| Ped-PRO-CTCAE | Investigator initiated | Observational cohort | N/A | Development of patient-reported outcome measure | |
| Chemotherapy adherence and drug level monitoring | ALL Thiopurines | Investigator initiated | Observational cohort | N/A | Assessment of reported adherence to 6-mercaptopurine, drug levels, and outcomes |
CCT, cancer clinical trial; ALL, acute lymphoblastic leukemia; N/A, not applicable.
Themes arising from interviews
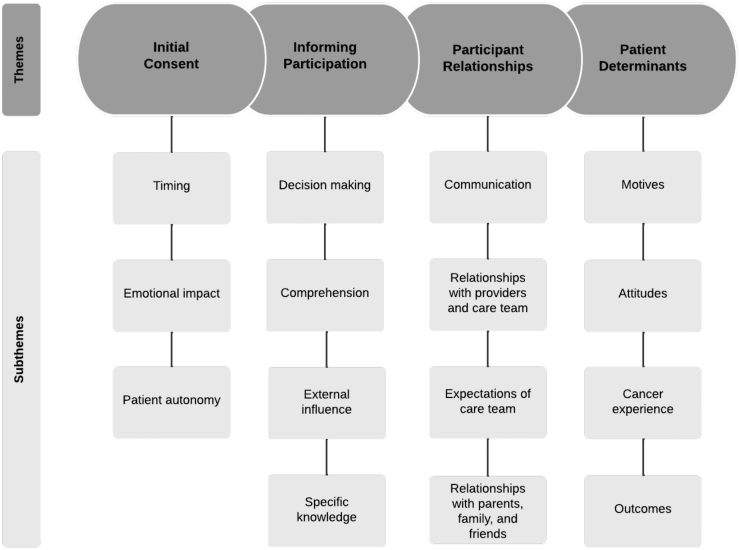
Four overarching themes were identified as contributing to patient decision-making about CCT participation: initial consent, informing participation, participant relationships, and patient determinants. In the discussion that follows, we describe these four themes, their subthemes in terms of barriers and facilitators, and connections between the themes and subthemes. There is substantial overlap between subthemes as many could serve as facilitators and barriers based on context. The themes and subthemes are depicted in Figure 1 and representative quotes are shown in Table 3.
FIG. 1.
Themes and subthemes of barriers and facilitators to clinical trial uptake among adolescents.
Table 3.
Representative Quotes Supporting Themes and Subthemes of Barriers and Facilitators to Clinical Trial Uptake Among Adolescents
| Theme | Subtheme | Barriers | Facilitators |
|---|---|---|---|
| Initial consent | Timing | “I feel like it would have been a lot more helpful if I would have gotten… two, three more days just to get a little more used to… my medicine and how it worked. Cause I think it took me, like, a week to get… stable on the heavy chemo.” ID 10 | “I think it was good…timing because I had a blood cancer…for some people it doesn't go away…as fast sometimes…but it was in that prime state when I was not at my worst but going through treatment but still not doing good…” ID 02 |
| Emotional impact | “Well, it wasn't so much the trial that was a little overwhelming, so much as… the diagnosis because those were within minutes of each other.” ID 18 | “So, while I was told that I had cancer for the first time, I wasn't exactly thrilled, but I wasn't completely panicked either. I knew where I was, and I knew that the hospital was going to do its best to try to take care of me.” ID 18 | |
| Patient autonomy | “They actually go to my dad first cause they didn't really know my age or anything. Most of the people assumed that I was under age, maybe like 17.” ID 10 | “I assessed it with those who brought the trial to me, certain doctors, and staff. I didn't really discuss it outside of that just simply for me because I was quick to make up my mind.” ID 15 | |
| Informing participation | Decision-making | “I just chose to participate in the ones that didn't seem like too much because…I don't want to be in, like, five and then be stuck at the hospital all day long. But it was pretty easy for me to say yes to the couple I did. Those were the first ones that approached me…I'm always down to help somebody else in my situation in the future.” ID 02 | |
| Comprehension | “Yeah, I just can't remember anything because it's so much information and so little time.” ID 14 | “They gave us the pamphlets or the papers that described everything, they went over everything about what the study was about, how they were gonna do the study, what it's for, why they're doing it. And then they asked if I would like to participate, and then I said yes.” ID 20 | |
| External influence | “My parents…don't really know anything about it and they're kind of more closed off as far as medicine…as soon as they ask me about the study, they told me. They told me I couldn't do it.” ID 10 | “Oh, well, I can't really make decisions by myself, so I guess it's easier when they, uh, push you towards one side.” ID 19 | |
| Specific knowledge | “As far as I was made aware… the doctor had told me that the trial chemical… is suspected to be less harmful to patients but at the same time, they were also attempting to measure whether or not it was as effective as the standard treatment.” ID 18 | ||
| Participant relationships | Communication | “I think it was a little confusing, especially for me. I was kind of, like, in and out of cons-, consciousness and my dad kind of just hearing that, oh okay, clinical trials, or studies, and then this is your treatment. I think he kind of was a little concerned about it as to, like, maybe he thought that what the medicine I was getting was probably going to be tested, or something like that, as far as I don't know how he thinks.” ID 10 | “I was asked to participate by one of the doctors who was in charge of my care, and I think that this is pretty effective because he's already looking over me and other reasons, so I think it was handled well.” ID 20 |
| Relationships with providers and care team | “That's questionable, actually, because I've never really been, you know, in a hospital room with me being the patient and all. But, I've seen like more worried faces…but I feel safe here because they say it's the best hospital or whatever.” ID 04 | “Everybody's been pretty good though. Yeah, like explaining and being…nice and they know how to explain everything, questions” ID 14 | |
| Expectations of care team | “…In regards to doctors, I think they feel an obligation towards attempting to improve the medical field… And I think that's why…they are very hoping that patients…would sign onto that, but at the same time, they understand…when a patient would say no.” ID 18 | ||
| Relationships with parents, family, and friends | “Yeah, that's the thing that… my dad handled… because he couldn't read English and speak English very well, and my mom wasn't there. So I think that's more of the reason why… I think he did ask a couple questions but he was still kind of uncomfortable with the idea.” ID 10 | “…They talked to my parents in a different room than me, mainly because I'm, you know, I've only been here for a week and my first day being here they had to, like, pull my parents aside and then come back inside and tell me.” ID 04 | |
| Patient determinants | Motives | “I decided to participate cause I think it would help the researchers and also the people find a cure, or try to find a cure” ID 12 | |
| Attitudes | “I've actually had, you know, a little bit of doubt about them…a lot of things go across your mind… and I get sort of a weird vibe out of it.” ID 04 | “I generally don't see them as anything but good…they're not really invasive. They contribute to more of an understanding of what you're fighting. So, I don't really see any downsides to them.” ID 15 | |
| Cancer experience | “Well, I just felt, um, sad.” ID 12 | “[At] the age of 16 and someone tells you, oh guess what, you might die if you don't do this, it just pushes you forward and makes you not want to give up, you know.” ID 04 | |
| Outcomes | “They tell me everything's just going to be okay. This thing…is actually helping you. And I know that it, and I know it's helping because the minute I got in this place I looked dead inside. My skin was, um, I don't remember, pale a little. And now, like, I've got some of my color back. I feel more energetic. ID 04 |
Initial consent
The initial consent theme refers to CCT discussion with patients. The first discussion of CCT with patients set the stage for patient receptivity to enrollment. Initial consent was conjoined with discussions about diagnosis and cancer treatment in this sample. Important subthemes were timing and patient autonomy, with connection to the emotional impact of a recent cancer diagnosis.
Barriers
Several participants discussed timing as a key barrier, citing feeling overwhelmed at the time of CCT discussion due to concurrent discussions about diagnosis and treatment. A 20-year-old Hispanic female said, “I feel like it would have been a lot more helpful if I would have gotten… two, three more days just to get a little more used to… my medicine and how it worked. Cause I think it took me, like, a week to get… stable on the heavy chemo” (ID 10, interviewed more than 60 days following diagnosis). Prominent barriers involving patient autonomy included personal difficulty making decisions and being infantilized by staff (an assumption that patients younger than 18 years were not able to make their own decisions).
Facilitators
Participants also cited timing as a facilitator, in that they realized considering CCT participation was necessary for treatment planning. One 18-year-old Hispanic male described his recollection of his initial CCT discussion:
“It wasn't so much the trial that was a little overwhelming, so much as the diagnosis because those were within minutes of each other. While I was told that I had cancer for the first time, I wasn't exactly thrilled, but I wasn't completely panicked either. I knew where I was, and I knew that the hospital was going to do its best to try to take care of me. And in regards to the trial itself, I thought of it as sort of an opportunity” (ID 18, interviewed within 60 days following diagnosis).
Patient autonomy was also mentioned as a facilitator through control over decision-making and the ability to retain information.
Connecting subthemes
Emotional impact was frequently cited as influencing initial consent and was a connecting subtheme to timing, patient autonomy, and understanding and comprehension. Emotional impact included feeling overwhelmed and confused, while discussing CCT options, leading to difficulty listening and comprehending information.
Informing participation
The informing participation theme refers to how decisions are made by the participant, involvement of their family and other support systems, and their ability to retain information about the CCT. Important subthemes were the decision-making process, specific knowledge, comprehension, and external influences.
Barriers
The primary barrier to informing participation was the decision-making process, which included feeling overwhelmed, understanding of the CCT, and the role of external influences. Participants mentioned that regardless of their age, parents are almost always approached first about a decision.
Unlike parents, AYAs are not empowered to ask questions. Almost all participants, even those older than 18 years, stated that ultimately their parents make the treatment decisions. A 20-year-old Hispanic female explained that “Staff go to my dad first ‘cause they didn't really know my age or anything. Most of the people assumed that I was underage, maybe like 17” (ID 10, interviewed more than 60 days following diagnosis). Comprehension of the CCT was a barrier to uptake, as indicated by a 20-year-old Hispanic male who said, “I couldn't listen to some things that they were telling me, and I'm still kind of confused” (ID 12, interviewed more than 60 days following diagnosis).
Facilitators
The primary facilitators to Informing Participation were decision-making and specific knowledge about the CCT. Frequently mentioned facilitators to decision-making included CCTs that were more convenient and less burdensome, positive relationship with their provider (e.g., trusting, open lines of communication, relaxed approach), the role of parents (e.g., ultimate decision makers, help patient with logistical needs, ask questions), and the role of the larger family (e.g., support system, any first-hand or relevant experience). For example, a 17-year-old non-Hispanic white male said, “I chose to participate in the ones that did [not] seem like too much because I was already stuck at the hospital all day long… It was pretty easy for me to say yes to the couple I did” (ID 02, interviewed more than 60 days following diagnosis).
Specific knowledge and comprehension of their CCT also served as facilitators, in addition to a barrier as described above. Some participants in a CCT were able to recall the purpose of the study, their randomization assignment, and the drug names. Despite reporting comprehension of the study as a facilitator to enrollment, participants were unable to recall specific details of their CCT. Some participants recalled specific details of ancillary/nontherapeutic or supportive care studies, which they confused as their CCT. For example, a 20-year-old non-Hispanic white female tried to describe her CCT, but instead recalled her supportive care study, saying, “[It] was going to be an MRI scan and that's all I remember”; and when asked about whether she remembered details of the treatment study, the same participant said, “No” (ID 14, interviewed within 60 days following diagnosis).
Indeed, eight of the nine participants in this study were enrolled on a CCT and could not recall specific details of that CCT, although they reported details specific to one or more ancillary/nontherapeutic or supportive care studies in which they were also enrolled. An 18-year-old non-Hispanic white transgender female indicated, “None of the trials that I'm a part of really have any risk of adverse effects” (ID 15, interviewed within 60 days following diagnosis), while a 19-year-old non-Hispanic white male said, “Something bad would happen if I didn't participate,” in response to being asked if there were any possible side effects (ID 20, interviewed within 60 days following diagnosis).
Connecting subthemes
Frequent connecting subthemes for informing participation included decision-making, comprehension, and external influence. Decision-making was frequently cited as a connecting subtheme to communication, patient autonomy, and comprehension and influenced how a patient made their decision to enroll in a CCT. Comprehension of specific details regarding the CT was closely connected to communication and patient autonomy. Several participants cited being given too much information at one time regarding their diagnosis, treatment, and CCT options. One participant mentioned, “It can be really overwhelming for people to hear and have to consent, and all of these things within the first few days of a diagnosis, which was somewhat of my experience” (ID 15, interviewed within 60 days following diagnosis).
Participant relationships
The participant relationships theme refers to important individuals in the cancer care experience and how information is conveyed to or from those individuals. Important subthemes were communication and the relationship with their care team.
Barriers
Poor communication with the care team was frequently cited as a barrier leading to a lack of understanding. When asked about their recollection of CCT discussions, one participant said, “You guys give too much information” (ID 14, interviewed within 60 days following diagnosis). Relationship with the care team resulted in barriers to CCT enrollment, including clinician demeanor and not seeing their care team members frequently.
Facilitators
First, effective communication between the patient and their care team served as a facilitator by increasing transparency, improving the ability to ask questions, and allowing for recommendations to be made by the patient. Some participants suggested separating the CCT discussions from initial diagnosis/treatment and having ongoing conversations with the care team to foster communication and strengthen the relationship.
Second, facilitators to building a relationship with their care team included frequent communication, trust in their care team, knowledge of their expertise, and feeling safe. A participant explained that they were “…Informed of the differences between the trial and the standard treatment… and knowing that, I willingly signed onto the trial” (ID 18, interviewed within 60 days following diagnosis). External influence was another connection closely tied to communication, feelings of obligation, expectations of the care team, and the role of family and friends. One non-Hispanic white male who was 17 indicated it is easier to make decisions when your family “pushes you towards one side” (ID 19, interviewed within 60 days following diagnosis).
However, a 20-year-old Hispanic female stated that because she appears young and is treated at a children's hospital, staff assume she is not able to make her own decisions and discuss them with her parent first (ID 10, interviewed more than 60 days following diagnosis). Another participant, a 20-year-old non-Hispanic white female, explained that her parents are not amenable to medicine and medical decision-making and immediately told her she could not participate in the CCT, despite the patient having done her own research about the CCT and expressing interest (ID 14, interviewed within 60 days following diagnosis).
Connecting subthemes
Within the Participant Relationships theme, the subtheme of communication was connected to parents making the ultimate decision, decision-making process, timing, comprehension, and external influences. One participant remembered, “[My providers] talked to my parents in a different room than me, mainly because I've only been here for a week and my first day being here they had to, like, pull my parents aside and then come back inside and tell me” (ID 04, interviewed within 60 days following diagnosis).
Patient determinants
The patient determinants theme refers to how the participant understands the intentions of others, and overall thoughts regarding the cancer care journey and CCT participation. This included subthemes of motives, attitudes, the cancer experience, and outcomes of CCT participation.
Barriers
Participants described the subtheme of motives of different parties as a barrier to CCT enrollment. Motives were related to attitudes, including negative feelings. One participant said, “I get sort of a weird vibe out of it,” and acknowledged his limited understanding (ID 04, interviewed within 60 days following diagnosis). Barriers stemming from the cancer experience included negative attitudes leading to negative feelings (e.g., inability to trust their provider or care team, leading to doubt or fear) and the needs of others. For example, a participant indicated that if a new type of treatment was adopted too quickly after a CCT and “Immediately replaced the standard [of care] and there's something that hasn't been [studied] enough, there could potentially be side effects that were [unexpected]… and that could have severe implications [for future patients]” (ID 18, interviewed within 60 days following diagnosis).
Facilitators
For facilitators of CCT uptake, subthemes included motives of the patient (e.g., incentives, the belief that something negative would happen if they did not participate) or provider (e.g., desire to advance medicine, improve survival for future patients) and general attitudes of helping to improve care or treatment were discussed. For example, one participant indicated that she enrolled in the CCT because she “wanted to make it easier for others… seeing the younger children around here makes me feel sympathetic I guess, and I would rather they have a less painful experience while they try to get through this, so I would willingly sign onto that, so that, you know, the medicine could improve” (ID 18, interviewed within 60 days following diagnosis).
Another participant explained that, “You get [an incentive] out of it if you join,” in reference to receiving a Fitbit for participating in a physical activity study (ID 19, interviewed within 60 days following diagnosis). Facilitators of positive outcomes of CCT participation were positive attitudes related to helping others (altruism). Specifically, the ability to help improve treatment for others, the importance of racial and ethnic diversity and representativeness in CCTs, and the belief that more people should participate in CCTs, if they are not too burdensome. One participant indicated, “I generally don't see [CCTs] as anything but good…they're not really invasive, they contribute to more of an understanding of what you're fighting. So, I don't really see any downsides to them” (ID 15, interviewed within 60 days following diagnosis).
Connecting subthemes
In the theme of Patient Determinants, notable connections included those subthemes of understanding and attitudes regarding CCT. Attitudes were also closely connected to motives, the cancer experience, external influences, and neutral feelings toward CCTs. The overall cancer experience and outcomes were considered Patient Determinants, both of which included barriers and facilitators to CCT uptake.
Discussion
We identified four major themes with addressable facilitators and barriers that play a role in younger AYA decision-making about CCT participation. Although previous studies indicate that patient-level barriers contribute substantially to AYA nonparticipation, few have explored them directly. In a recent systematic review of barriers and facilitators to AYA CCT enrollment, only 3 of 13 studies involved patient interviews, with a fourth recently published.9,12 In contrast to those, our study provides new information derived from younger, more ethnically diverse AYAs interviewed soon after diagnosis and concurrently enrolled on ancillary studies beyond disease-focused CCTs. This approach helps ensure our results are generalizable and reflect the experience of newly diagnosed patients. Overall, our results highlight the need for better approaches to inform, involve, and support young AYAs in meaningful decision-making about participating in CCTs and related studies.
Some of the most prominent findings in our study involve the related themes of Initial Consent and Informing Participation. Specifically, these include the deleterious effects of “information overload” intertwined with emotional distress caused when providers discuss CCT options along with the new diagnosis of cancer. This finding likely reflects the early time point of our interviews and generally aligns with other studies among AYAs and providers in the United States and United Kingdom.10,12,13,21 To overcome these barriers, our participants endorse involving the AYA patient in the decision-making process, promoting their autonomy, and allowing more time to process information and emotions.9
At the same time, AYAs express mixed preferences about their involvement in CCT decision-making. Some prefer to leave decisions to their caregivers or health care professionals, while others prefer a more active role and feel their engagement is minimal, even when caregivers view the AYAs role as substantial.10,22,23 These unique features of AYA CCT participation were voiced in our study, along with frustration when CCT discussions occurred with parents before or apart from the AYA.
In addition, our study found that participants frequently conflated the CCT with other supportive care or biology studies for which they were offered enrollment. One previous study has reported such “study confusion” and underscores the difficulties some AYAs experience in rapidly assimilating large amounts of information.24 Although “study confusion” may not have served as a barrier to CCT enrollment here, it probably reflects the overall suboptimal knowledge AYAs have about informed consent and the CCT. Having a poor understanding of what CCTs mean was identified as a barrier for AYA enrollment in one previous study.12 There is a need for research assessing the accuracy of AYAs' specific knowledge about CCTs on which they enroll, including risk for side effects, as well as the impact of having multiple study opportunities presented all at once, as commonly done.
Participant relationships emphasized the importance of communication, patient's relationship with their care team, expectations of the care team, and relationships with parents, family, and friends, which have been substantiated in prior studies among AYAs. Indeed, poor communication and parental preference have been reported as barriers, while improving patient-provider relationships and building trust may help act as facilitators to CCT uptake.7,12,23,25 Furthermore, it has been shown that AYAs who do not have the support of family and their health system during the enrollment process can act as a barrier to CCT uptake.12
In regard to Patient Determinants, participants in this study and others have described how misinformation or misconceptions about CCTs can act as a barrier.12,13,24 Previous studies have also described the importance of potential benefits of participating for the patient, including improved disease control, a desire to be altruistic to fellow survivors, and the benefit of helping to advance science.10–13,21,23 AYAs reported positive outcomes of CCT participation due to altruism (helping to improve treatment for others), the importance of racial and ethnic diversity and representativeness in CCTs, and the belief that more people should participate in CCTs (if they are not too burdensome). The findings among other studies regarding facilitators, barriers, and resulting recommendations underscore the importance of a patient- and family-centered, individualized approach regarding the CCT decision-making process, comprehension, external influences, and specific knowledge regarding CCTs.
Strengths of this study include the use of in-depth interviewing to obtain a detailed understanding of knowledge, perceptions, motivations, and emotions of AYAs soon after diagnosis.15 Compared with similar studies where AYAs were interviewed 1–12 years post-treatment, our sample was ethnically more diverse, younger, and generally interviewed within weeks of diagnosis (median of 42 days), features that reduce recall bias and increase generalizability.10–13 Three of our participants were not able to be interviewed until after 60 days and it is possible their recollection of specific details and impressions was less complete than it would have been otherwise.
Nonetheless, their timing was still substantially earlier than most previous studies and their clinical characteristics and perspectives were similar to participants interviewed at earlier time points. It should be noted that, although our eligibility criteria required participants to be English speaking, this should not have biased our sample as virtually all Hispanic children and adolescents at our center have English fluency from the time they enter elementary school. As non-English speakers were not eligible, our results may not be representative of those populations.
Limitations of this study include relatively few disease-focused CCTs being available at the time of this study, resulting in a smaller, less heterogeneous pool of potential participants than anticipated. Nevertheless, there was sufficient redundancy within participant responses and themes to ensure data saturation was achieved. A related limitation is that the therapeutic CCT on which most participants were enrolled was a frontline ALL study where randomization was delayed until after the first month of induction therapy, which could have led to participants having fewer strong impressions about CCTs than if they had undergone up-front randomization.
On the other hand, a high proportion of participants also enrolled in biology, registry, quality–of-life, supportive care, and/or treatment adherence studies. Although views about ancillary studies were not elucidated fully, they are reflected in our results. Finally, this study was conducted at a single, large quaternary care children's hospital in a densely populated urban setting. Due to potential differences in baseline AYA perspectives and how AYAs are approached for research participation, this could possibly impact the generalizability of our results.
Pragmatic recommendations arising from our four major themes are summarized in Table 4. The first group of recommendations would improve the initial consenting process by separating discussion of a new diagnosis from the CCT, delivering technical information more effectively, and delaying presentation of ancillary studies, if possible. The second group would expand the AYA's decisional role through more direct communication, by promoting meaningful involvement, and by building autonomy. The third group would enhance communication with AYAs in ways that deliver information and also build trust. A final recommendation involves supporting, where appropriate and with honesty and integrity, AYAs who may be inclined by altruism to participate in CCTs, as many are. Future research should be focused on developing decision aids and tailored interventions that will improve meaningful AYA decision-making regarding participation in CCTs and related studies.
Table 4.
Recommendations for Addressing Barriers and Incorporating Facilitators Identified in Thematic Analysis
| Theme | Barriers | Facilitators | Combined recommendations |
|---|---|---|---|
| Initial consent | • Information overload • Feeling overwhelmed • Infantilization by staff |
• Understanding CCT participation necessary for treatment planning • Viewing CCT as an opportunity |
• Allow a few days to buffer impact of new diagnosis before requiring decision on CCT, if clinically feasible and appropriate • Deliver medical information in two or more shorter conversations • Provide supplemental handouts about the CCT • Clearly delineate CCT from other studies that must be presented shortly after diagnosis • Delay presentation of ancillary studies after first week after CCT decision, if feasible • Direct CCT conversations primarily toward AYA • Avoid circumventing AYA through parental discussions • Recruit parents to be allies in encouraging AYA to ask questions, express values and preferences, and play decision-making role for CCT and care • Encourage all bedside care providers to build AYA autonomy • Over time, build institutional culture of empowering AYAs in all aspects of decision-making • Develop provider communication skills that build rapport with AYAs • Utilize open communication style with AYA that encourages questions and dialog • Be forthcoming with information to AYA • Answer AYA's questions directly • Convey sense of hope and encouragement to AYA • Visit inpatient AYAs liberally and informally, perhaps outside of formal rounds if feasible • Engage parent/guardian, siblings, and peers in degree and manner desired by AYA • Endorse CCT as potential avenue for altruism toward future AYAs through research, if appropriate |
| Informing participation | • Not empowered to ask questions • Perceiving that parents make the decision anyway • Poor comprehension of the CCT |
• Positive relationship with providers (trust, open lines of communication, relaxed approach) • Parental support (help with logistical needs, assistance in asking questions) • Understanding purpose and details of the CCT • Distinguishing between CCT and ancillary studies |
|
| Participant relationships | • Poor communication with team • Negative clinician demeanor • Not seeing providers frequently enough |
• Transparency • Encouragement of questions • Separate CCT presentation from initial diagnosis discussion • Frequent communication with team • Trust in care team • Feeling safe and cared for by team • Support of family and friends |
|
| Patient determinants | • Feeling uncomfortable or distrustful about CCTs in general • Inability to trust provider leading to doubt or fear |
• Perceived direct benefit from CCT • Favorable attitude toward improving cancer care and helping future patients • Belief in improving racial and ethnic diversity in CCTs • Material incentives for participating |
AYA, adolescent and young adult.
Supplementary Material
Acknowledgments
We honor and thank the cancer patients who participated in this study.
Authors' Contributions
E.M.M.: conceptualization, methodology, formal analysis, data curation, investigation, writing—original draft, writing—reviewing and editing, and visualization; S.M.T.: conceptualization, methodology, data curation, investigation, writing—reviewing and editing, and funding acquisition. J.B.: conceptualization, methodology, formal analysis, validation, data curation, and writing—reviewing and editing. C.Y.O.: conceptualization and writing—reviewing and editing. K.M.: conceptualization and writing—reviewing and editing. A.A.: data curation and writing—reviewing and editing. J.M.: conceptualization, funding acquisition, and writing—reviewing and editing. D.R.F.: conceptualization, methodology, resources, writing—reviewing and editing, supervision, project administration, and funding acquisition.
Author Disclosure Statement
The listed authors fulfill all authorship requirements as stated in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals and have no competing financial interests that exist.
Funding Information
This study was supported partly by the National Institutes of Health, National Center for Advancing Translational Science (NCATS) through the Southern California Clinical and Translational Science Institute (UL1TR001855, Stefanie M. Thomas). Funding was also provided by the National Institutes of Health, National Cancer Institute (5T32CA009492-34, Erin M. Mobley and Carol Y. Ochoa; F99CA264294, Carol Y. Ochoa), and the National Institutes of Health, National Institute on Aging internal career development award at the University of Florida College of Medicine Jacksonville through the JAX-ASCENT Junior Scholar Award (5R33AG056540, Erin M. Mobley).
Supplementary Material
References
- 1. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. 2015;3(2):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer facts & figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger JM, Cook E, Tai E, Bleyer A. Role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrari A MM, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50:1101–4. [DOI] [PubMed] [Google Scholar]
- 5. Roth ME, O'Mara AM, Seibel NL, et al. Low enrollment of adolescents and young adults onto cancer trials: insights from the community clinical oncology program. J Oncol Pract. 2016;12(4):e388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth ME, Unger JM, O'Mara AM, et al. Enrollment of adolescents and young adults onto SWOG cancer research network clinical trials: a comparative analysis by treatment site and era. Cancer Med. 2020;9(6):2146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children's hospital. J Pediatr Hematol Oncol. 2007;29(12):811–4. [DOI] [PubMed] [Google Scholar]
- 8. Thomas SM, Malvar J, Tran HH, et al. A prospective comparison of cancer clinical trial availability and enrollment among adolescents/young adults treated at an adult cancer hospital or affiliated children's hospital. Cancer. 2018;124(20):4064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siembida EJ, Loomans-Kropp HA, Trivedi N, et al. Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: identifying opportunities for intervention. Cancer. 2020;126(5):949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barakat LP, Schwartz LA, Reilly A, et al. A qualitative study of phase III cancer clinical trial enrollment decision-making: perspectives from adolescents, young adults, caregivers, and providers. J Adolesc Young Adult Oncol. 2014;3(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bell JAH, Forcina V, Mitchell L, et al. Perceptions of and decision making about clinical trials in adolescent and young adults with Cancer: a qualitative analysis. BMC Cancer. 2018;18(1):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abrahão R, Alvarez EM, Waters AR, et al. A qualitative study of barriers and facilitators to adolescents and young adults' participation in cancer clinical trials: oncologist and patient perspectives. Pediatr Blood Cancer. 2022;69(4):e29479. [DOI] [PubMed] [Google Scholar]
- 13. Pearce S, Brownsdon A, Fern L, et al. The perceptions of teenagers, young adults and professionals in the participation of bone cancer clinical trials. Eur J Cancer Care (Engl). 2018;27(6):e12476. [DOI] [PubMed] [Google Scholar]
- 14. Alpha Dog Transcription. Alpha Dog Transcription. 2021. Accessed August 27, 2021 from: https://alphadogtranscriptions.com/
- 15. Spencer J, Ritchie J, O'Connor W. Analysis: practices, principles, and processes. In: Ritchie J, Lewis J (Eds). Qualitative research practice: a guide for social scientists and researchers. London: Sage; 2003. [Google Scholar]
- 16. Armstrong D, Gosling A, Weinman J, TM. The place of inter-rater reliability in qualitative research: an empirical study. Sociology. 1997;31(3):597–606. [Google Scholar]
- 17. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guest G, Namey E, Chen M. A simple method to assess and report thematic saturation in qualitative research. PLoS One. 2020;15(5):e0232076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Given LM. 100 Questions (and answers) about qualitative research. Thousand Oaks, CA: Sage; 2016. [Google Scholar]
- 20. Sandelowski M. Theoretical saturation. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- 21. Lavender V, Gibson F, Brownsdon A, et al. Health professional perceptions of communicating with adolescents and young adults about bone cancer clinical trial participation. Support Care Cancer. 2019;27(2):467–75. [DOI] [PubMed] [Google Scholar]
- 22. Hart RI, Cameron DA, Cowie FJ, et al. The challenges of making informed decisions about treatment and trial participation following a cancer diagnosis: a qualitative study involving adolescents and young adults with cancer and their caregivers. BMC Health Serv Res. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hendricks-Ferguson VL, Cherven BO, Burns DS, et al. Recruitment strategies and rates of a multi-site behavioral intervention for adolescents and young adults with cancer. J Pediatr Health Care. 2013;27(6):434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mobley EM, Foster KJ, Terry WW. Identifying and understanding the gaps in care experienced by adolescent and young adult cancer patients at the University of Iowa Hospitals and Clinics. J Adolesc Young Adult Oncol. 2018;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118(14):3614–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.