Abstract
Interactions between the nucleocapsid protein (NC) and reverse transcriptase of HIV-1 have been shown to promote the initiation of reverse transcription. We assayed the effect of NC on later events, using a strand transfer system with donor and acceptor HIV RNA templates and found that the presence of NC resulted in increased synthesis of full-length strand-transferred (FLST) DNA. This effect also occurred with mutated forms of NC that lacked both zinc fingers, or that contained a point mutation (histidine→cysteine) at amino acid 23. In contrast, NC-derived proteins containing only the proximal or distal zinc fingers, or lacking the N- and C-termini, were all unable to catalyze the synthesis of FLST DNA. Band-shift assays using both the mutated and wild-type forms of these proteins revealed that all the NC proteins promoted strand association between (–) strong-stop DNA [(–)ssDNA] and acceptor RNA. The zinc finger motifs were dispensable for full-length processive reverse transcription, and the N- and C-termini were required; however, all NC domains were dispensable for association of (–)ssDNA and acceptor RNA. This suggests that annealing is a less stringent reaction than DNA polymerization.
INTRODUCTION
Reverse transcription by human immunodeficiency virus type 1 (HIV-1) is a requirement for viral replication. In this process, the viral enzyme reverse transcriptase (RT) converts the single-stranded viral RNA genome into double-stranded DNA, which is translocated to the cell nucleus and integrated into the host genome (1). The initial product of reverse transcription is minus-strand strong-stop DNA [(–)ssDNA], transcription of which begins at the primer binding site (PBS) near the 5′ end of the viral RNA genome and stops at the end of the 5′ untranslated R region (2). This is followed by a strand transfer or template switching event, which involves the dissociation of RT from (–)ssDNA and its re-association at the 3′ end of the viral RNA; this is, in part, mediated by R region complementarity within both strands (1,2).
The factors controlling (–)ssDNA synthesis and the first template switch are not fully understood, but it is believed that the nucleocapsid protein (NC), a 7 kDa protein containing two zinc-binding domains, is of critical importance. In the context of the unprocessed Pr55Gag polyprotein, NC can direct incorporation of both primer tRNALys3 and viral genomic RNA into the budding virion, and may play a role in the assembly and maturation of the virus (3–8). In cell-free assays, purified NC protein is required for efficient placement of tRNALys3, and for the initiation and processivity of reverse transcription (9–12).
The template switch reaction is an important step in reverse transcription, since, with two copies of the viral genome, genetic recombination can occur. In this regard, HIV-1 NC promotes annealing of (–)ssDNA and 3′ R-containing RNA by up to 3000-fold, and reduces secondary structure inhibition of strand binding in the TAR region (13). Furthermore, NC is associated with decreased self-priming formed by fold-back transcription of secondary structure in TAR-region RNA, thereby increasing strand transfer efficiency, which may account for the in vivo absence of self-primed reverse transcription products in virions (14). This is consistent with studies showing increased RT pausing at the TAR site, with NC promoting the annealing between the hairpins of the acceptor and extended primer at this position, thereby facilitating the transfer event (15). Moreover, the presence of NC increases strand transfer by allowing more extended primers to transcribe from the donor and then acceptor templates (16).
Here, we report the effect of NC on strand transfer using a cell-free assay of reverse transcription. Additionally, we describe the binding of donor and acceptor species of RNA with (–)ssDNA, and the effects of mutations in NC proteins on this function.
MATERIALS AND METHODS
Donor and acceptor RNA synthesis
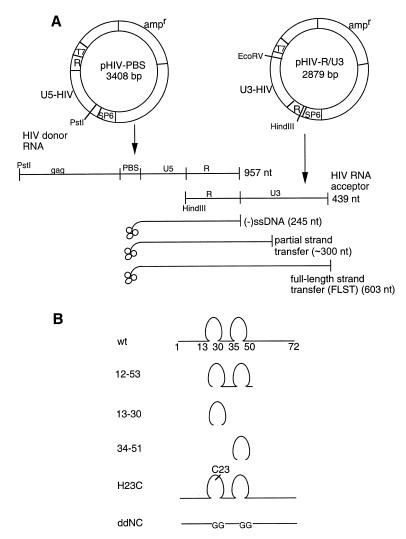
The plasmids pHIV-PBS and pHIV-R/U3 (17) were linearized with PstI and HindIII, respectively, then transcribed with T7 RNA polymerase using the Ambion T7 Megascript RNA kit according to the manufacturer’s instructions (Ambion, TX) (Fig. 1A). The donor RNA, containing HIV-1 gag sequences, the PBS, U5 and R regions, was 957 nt long. Because the donor RNA, when newly synthesized, partially contained T7 RNA polymerase promoter sequences, an oligonucleotide complementary to these sequences was first annealed to them, followed by digestion by RNase H to produce single-stranded RNA corresponding to HIV sequences (Gibco BRL, Mississauga, ON). Placental tRNALys3 (18) was annealed to the template at the PBS, and extension continued into R sequences that were also present on the acceptor RNA, which was 439 nt long (Fig. 1A). Initial transcription from the donor RNA template generated (–)ssDNA; template switching was evaluated by adding acceptor RNA. Thus, continued elongation of (–)ssDNA using acceptor RNA as a template generated a full-length strand transferred DNA (FLST DNA) of 603 nt. Partial strand transfer products were ≥300 nt long, while (–)ssDNA, the product of reverse transcription using the donor RNA template alone, was 245 nt in length. In addition, products of ~430 nt long were detected; these may represent transcription from the Ψ-PBS, a short sequence in acceptor RNA with a high degree of nucleotide sequence homology to the PBS (17). This system of evaluating strand transfer during HIV-1 reverse transcription has been previously characterized (17).
Figure 1.
(A) Generation of donor and acceptor RNA templates for in vitro strand transfer assay. (B). Schematic representation of wt (72 amino acid) and mutant NC peptides.
Reverse transcription
(–)ssDNA was generated in 9 µl reaction volumes; each tube contained 0.5 pmol tRNALys3 (18), 0.5 pmol RNA template, 0.2 mM of each dNTP, 10 mM dithiothreitol, 1.5 µl [α-32P]dCTP (3000 Ci/mmol), 1.5 µl [α-32P]dGTP (3000 Ci/mmol), 50 mM Tris–HCl (pH 7.2), 50 mM KCl and 5 mM MgCl2. Heat-annealing of tRNALys3 to the RNA template (85°C for 2 min, 55°C for 8 min, 37°C for 10 min) was followed by the addition of 0.5 pmol RT for a total volume of 10 µl. Reactions were incubated at 37°C for 45 min to generate (–)ssDNA. To examine synthesis of strand transferred DNA, 5 pmol of acceptor RNA template were added to each tube in a 10 µl cocktail with final concentrations of 10 mM dithiothreitol, 50 mM Tris–HCl (pH 7.2), 50 mM KCl and 5 mM MgCl2.
NCs were incubated for 1 h at 37°C in the strand transfer reaction to allow NC association with the (–)ssDNA and acceptor RNA. Polymerization was continued with the addition of 0.5 pmol of RT, followed by incubation for 45 min at 37°C. As a control, (–)ssDNA was heat-annealed to the acceptor RNA and extended to generate FLST DNA.
Nucleocapsid proteins
NCs, both wild-type (wt) and mutant (Fig. 1B), were synthesized by stepwise solid-phase as previously described (19,20). Amino acid sequences of NC were derived from HIV-1 LAV; wt NC was comprised of 72 amino acids. Mutant peptides included (i) the proximal zinc finger domain (amino acids 13–30); (ii) the distal zinc finger domain (amino acids 34–51); (iii) both zinc finger domains, but without the N- and C-terminal domains (amino acids 12–53); (iv) replacement of both zinc finger domains with diglycine peptides (ddNC); and (v) a histidine→cysteine point mutation in the proximal CCHC zinc-binding domain (H23C). Lyophilized proteins were resuspended in 50 mM Tris–HCl (pH 7.5) and stored at –80°C until use.
Band-shift assays
The binding of (–)ssDNA to acceptor RNA in the presence of NC was demonstrated by band-shift assays. A radioactively-labeled stock of (–)ssDNA was prepared as described above. Acceptor RNA was radioactively labeled by the incorporation of [α-32P]CTP during in vitro transcription with the T7 Megascript kit according to the manufacturer’s instructions (Ambion). Annealing of (–)ssDNA to acceptor RNA was in 50 mM Tris–HCl (pH 7.2), 50 mM KCl, 5 mM MgCl2 and 10 mM dithiothreitol. Reactions were incubated at 37°C for 1 h, at which time annealing was maximal (not shown), and run on 8% polyacrylamide gels, which were dried and exposed to Bio-Max film (Eastman Kodak, Rochester, NY). As a positive control, (–)ssDNA and acceptor RNA were heat-annealed as described above.
Analysis of reverse transcription products
Strand transfer reactions were stopped by the addition of stop solution (final concentration of 500 mM ammonium acetate, 10 mM EDTA, pH 8.0), extracted with phenol/chloroform, and precipitated with isopropanol overnight at –20°C. Reaction products were resuspended in formamide gel-loading buffer, boiled, cooled on ice, and run on 5% denaturing urea polyacrylamide gels (21). Products were visualized on Bio-Max film (Eastman Kodak) and analyzed by molecular imaging (Bio-Rad).
RESULTS
Figure 2A shows that with the addition of wt NC, there was an accumulation of FLST product at 603 nt (lanes 4–11). As with the reaction containing heat-annealed acceptor RNA (lane 3), this FLST product was in generally low abundance compared to the amount of (–)ssDNA, indicating a low degree of strand transfer. The percentage of FLST DNA relative to total DNA in each lane was determined by molecular image analysis (Fig. 2B). It was observed that the proportion of FLST DNA increased with higher concentrations of NC until a peak of 38% relative to total DNA was observed with 750 ng NC protein (23.5 nt/NC molecule) (Fig. 2A, lane 5, and B). Consistent with the effect of NC on (–)ssDNA synthesis (10), an overabundance of NC (1000 ng) was associated with decreased synthesis of FLST DNA (Fig. 2A, lane 4, and B). Concomitant with the appearance of FLST DNA, there was also a decrease in levels of (–)ssDNA, demonstrating the continued elongation of the latter using acceptor RNA as a template. The DNA products of ~260–280 nt may represent the result of fold-back transcription due to secondary structure (14), as evidenced by the presence of such bands in the absence of acceptor RNA (Fig. 2A, lane 2). In all cases, products of intermediate size (≥300 nt) were also detected; these may represent pausing by RT (9,22) as well as products primed from the Ψ-PBS (17).
Figure 2.
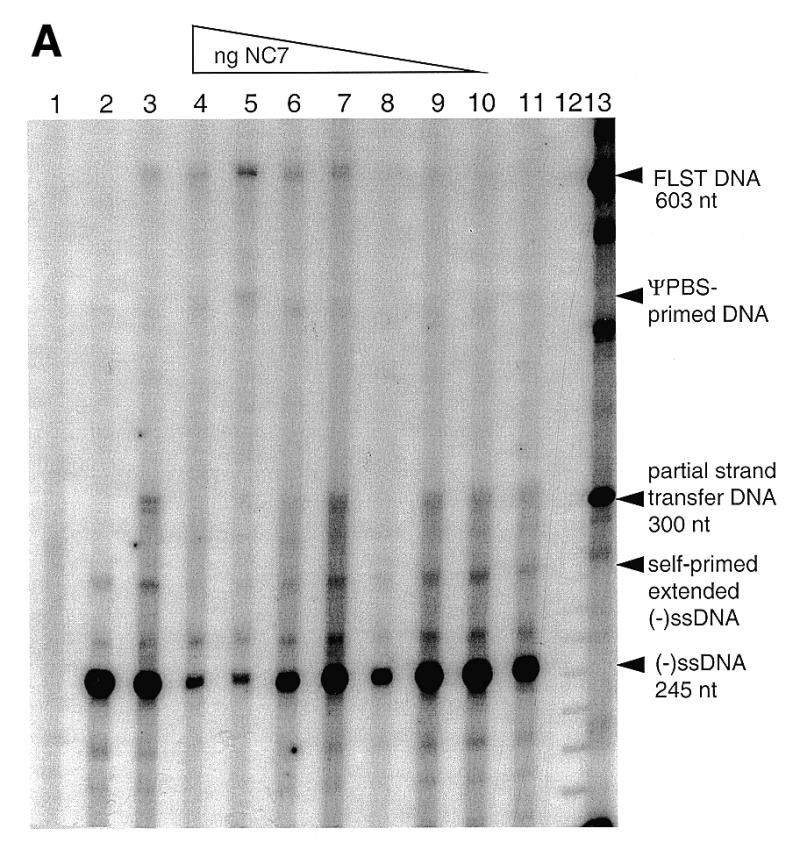
(A) wt NC promotes the synthesis of strand transferred DNA. Lane 1, negative control lacking donor RNA; lane 2, negative control lacking acceptor RNA; lane 3, heat-annealed (–)ssDNA and acceptor RNA; lanes 4–10, varying quantities of NC (1000, 750, 500, 250, 100, 50, 10 ng); lane 11, absence of NC; lane 12, 10 bp molecular weight marker; lane 13, 100 bp molecular weight marker. Products smaller than 245 indicate incomplete synthesis of (–)ssDNA. DNA products between 245 nt and ~270 nt are the result of self-primed DNA synthesis caused by fold-back of (–)ssDNA; this is seen in lane 2, the control lacking acceptor RNA. (B) Quantitation of FLST DNA relative to total DNA in the presence of wt NC protein.
The ability of mutant NC proteins to promote template-switching in reverse transcription was also studied (Fig. 3A and B). Again, it was found that increasing concentrations of wt NC promoted the synthesis of the FLST product in association with decreased accumulation of (–)ssDNA (Fig. 3A, lanes 2–4, and B). With the various mutant NC proteins, only the H23C (point mutation in the proximal zinc finger) and ddNC versions (replacement of the two zinc fingers by diglycine peptides) were associated with the appearance of significant amounts of FLST product (Fig. 3A, lanes 15–20, and B). With the H23C and ddNC mutant peptides, the percentage of FLST DNA relative to total DNA reached maxima of 11 and 16%, respectively (Fig. 3B). In contrast, the mutant peptide consisting of both zinc finger domains, but lacking the N- and C-terminal domains (12–53), was unable to promote the synthesis of FLST DNA (Fig. 3A, lanes 5–7). Similarly, the mutant peptides corresponding to the proximal (13–30) and distal (34–51) zinc finger domains were not associated with significant FLST DNA synthesis (Fig. 3A, lanes 8–13, and B). Self-primed extension of (–)ssDNA was again observed in the reactions lacking acceptor RNA (Fig. 3, lane 14) and lacking NC protein (Fig. 3, lane 1), and molecular image analysis revealed that the proportion of these DNA products did not change significantly upon the addition of NC (not shown). The donor RNA did not contain a complete TAR structure; an intact TAR is likely required to show a significant decrease in association with NC (14). As a negative control, histone, a DNA-binding protein, was not able to increase the synthesis of FLST DNA (Fig. 3A, lanes 21–23, and B). The absence of NC protein was associated with a minimal level of FLST DNA synthesis (Fig. 3A, lane 1, and B).
Figure 3.

(A) The effect of wt and mutant NC on the synthesis of strand transferred DNA. Lane 1, absence of NC. Varying concentrations of protein were used for each preparation of NC. Lanes 2, 5, 8, 11, 15, 18, 21 (100 ng); lanes 3, 6, 9, 12, 16, 19, 22 (500 ng); lanes 4, 7, 10, 13, 17, 20, 23 (1000 ng); lane 14, negative control lacking acceptor RNA. Histone also served as a negative control. Partial strand transfer DNA (ST DNA) indicates pausing in DNA synthesis. (B). Quantitation of FLST DNA relative to total DNA in the presence of wt NC and mutant NC peptides. 100 ng (open columns), 500 ng (hatched columns), 1000 ng (filled columns).
We next compared the use of heat-annealing versus NC to promote binding of (–)ssDNA and acceptor RNA. The synthesis of FLST DNA involves polymerization of DNA by RT, but also requires the association of (–)ssDNA and the acceptor RNA template. Since NC has been shown to promote the annealing of donor and acceptor templates (13,23,24), we wanted to determine whether the failure of mutant NC to promote FLST DNA synthesis was attributable to a deficiency at this step. Therefore, we generated (–)ssDNA and heat-annealed it to acceptor RNA that was radioactively labeled during its synthesis by in vitro transcription. A 5:1 ratio of (–)ssDNA to acceptor RNA was found to optimally promote the formation of a high-molecular weight complex in polyacrylamide gels (not shown). High concentrations of NC have often been associated with the formation of high-mobility aggregates (25); Figure 4A shows that the binding of (–)ssDNA and acceptor RNA formed such an aggregate when >10 ng of NC was employed (lanes 6–9). In contrast, with 10 ng of NC, we observed this complex at the same mobility as that generated by heat-annealing (Fig. 4A, lanes 3 and 5), indicating that efficient binding of (–)ssDNA and acceptor RNA had been promoted by NC. DNA of lower mobility than (–)ssDNA indicates incomplete reverse transcription during the batch preparation of labeled (–)ssDNA.
Figure 4.

(A) NC promotes the formation of a high-molecular weight complex of (–)ssDNA and acceptor RNA. Lane 1, (–)ssDNA alone; lane 2, acceptor RNA alone; lane 3, heat-annealed (–)ssDNA and acceptor RNA; lane 4, absence of NC; lanes 5–9, increasing amounts of NC (10, 50, 100, 250, 500 ng). (B) Determination of minimum amount of NC required to promote complex formation between (–)ssDNA and acceptor RNA in a band-shift assay. Lane 1, acceptor RNA alone; lane 2, (–)ssDNA alone; lane 3, heat-annealed (–)ssDNA and acceptor RNA; lane 4, absence of NC; lanes 5–10, increasing amounts of NC (2, 4, 6, 8, 10, 20 ng). Products smaller than (–)ssDNA indicate incomplete reverse transcription. High molecular-weight aggregates of acceptor RNA, (–)ssDNA, and large amounts of NC were unable to enter the gel.
In the absence of heat-annealing or NC, (–)ssDNA and RNA did not associate (Fig. 4A, lane 4). To determine the lower limits of NC which could mediate this binding, a lower range of NC concentrations (0–20 ng) was studied in band-shift assays (Fig. 4B, lanes 4–10), and it was found that as little as 4 ng of NC could promote the association of (–)ssDNA and acceptor RNA (Fig. 4B, lane 6). Once again, we observed the formation of high molecular weight aggregates that remained near the top of the gel (Fig. 4B, lanes 8–10). Therefore, we next used between 5 and 20 ng of mutant and wt NC proteins in the band-shift assays (Fig. 5). In contrast to the NC requirements for the synthesis of FLST DNA, we found that all mutant NC proteins (lanes 6–11 and 17–25) as well as wt (lanes 3–5) were able to promote the binding of (–)ssDNA and acceptor RNA. In the reactions containing NC peptides, the proportion of the binary complex relative to total DNA and RNA did not significantly increase with increasing concentrations of NC; this may reflect the fact that low concentrations of NC (5–20 ng) were used in these assays (Fig. 5). Thus, while mutations in NC may reduce synthesis of FLST DNA, the annealing between (–)ssDNA and acceptor RNA during the first strand transfer may not be as severely affected. Indeed, histone, which did not promote synthesis of FLST DNA, was also able to increase this binding (Fig. 5, lanes 12–14), suggesting non-specific interactions between protein and nucleic acids.
Figure 5.

Mutant and wt NC promote the binding of (–)ssDNA and acceptor RNA in a band-shift assay. Lane 1, heat-annealed (–)ssDNA and acceptor RNA; lane 2, absence of NC; lanes 3, 6, 9, 12, 17, 20, 23 (5 ng NC); lanes 4, 7, 10, 13, 18, 21, 24 (10 ng NC); lanes 5, 8, 11, 14, 19, 22, 25 (20 ng NC); lane 15, (–)ssDNA alone; lane 16, acceptor RNA alone. Products smaller than (–)ssDNA indicate incomplete reverse transcription. High molecular-weight aggregates of acceptor RNA, (–)ssDNA, and large amounts of NC7 were unable to enter the gel.
DISCUSSION
This study demonstrates that the effect of NC on strand transfer during reverse transcription may be separated into at least two steps: the annealing of (–)ssDNA to acceptor RNA, and processive synthesis by RT from the donor and then acceptor RNA templates. Less NC was required to promote association of (–)ssDNA and RNA than was needed for full-length synthesis of strand-transferred DNA, indicating that the requirement for NC in the former reaction may be less stringent than in the latter. In addition, all mutant NC peptides were associated with the binding of (–)ssDNA and acceptor RNA, but only two were able to increase synthesis of FLST DNA. These mutants were: (i) that which lacked both zinc fingers; (ii) that which contained a point mutation in the N-terminal zinc finger. These mutated peptides contain the same N- and C-terminal amino acids as wt NC, suggesting the importance of these domains in these reactions. In contrast, peptides consisting of the proximal zinc finger alone (13–30), the distal zinc finger alone (34–51), or both, but lacking the N- and C-termini (12–53), were all unable to catalyze synthesis of FLST DNA. Taken together, these results suggest that the domains of NC that are required for binding and elongation may be different, or that the binding reactions require less specificity. This helps to explain how histone, a DNA-binding protein, was able to promote formation of the binary complex but not the synthesis of full-length DNA.
Mechanisms involved in the initiation of reverse transcription may offer insights into the effect of NC on strand transfer. First, template switching in the presence of tRNALys3 is much more efficient than that which occurs with an oligonucleotide primer (17), and the interactions between tRNALys3 and template RNA change during the switch from initiation to elongation (26,27). It is conceivable that a similar conformational switch accompanies the transition from binding of (–)ssDNA and acceptor RNA to continued elongation; this may also involve specific interactions with tRNALys3. Such variation in conformation might explain why some mutant NC proteins were able to promote the binding reaction but not the full-length synthesis of viral DNA.
Mutations or deletions in the zinc finger domains of NC still allow annealing between tRNALys3 and viral RNA, but the fingers alone are insufficient in this regard (11,19,20,25,28). In contrast, the present findings show that the fingers alone are able to promote annealing of (–)ssDNA and acceptor RNA. This implies that sequence and structural differences in nucleic acid species may result in a requirement for different domains of NC for annealing. The complexes formed by these mutant peptides were not polymerization-competent, as shown by the lack of FLST DNA synthesis. Additional sequences, such as the N- and C-termini, may be required for the formation of a functional initiation or polymerization complex. This is supported by studies of single-base extensions of tRNALys3, in which there were no significant differences among reactions performed with wt NC, ddNC and H23C (11). The present findings are in agreement with these results, since we have shown that the same mutant peptides were able to promote synthesis of FLST DNA. In addition, we have demonstrated that the proximal or distal fingers of NC, alone, are able to confer annealing between (–)ssDNA and acceptor RNA but are incapable of catalyzing the synthesis of FLST DNA. Our data are also consistent with previous studies that showed that NC proteins, deleted of the zinc fingers, but conserving the N- and C-terminal domains, maintained viral RNA annealing activity (20). It has been shown that the basic residues that flank the proximal zinc finger are important for RNA binding and that they may cooperate with the zinc fingers in the packaging of the viral RNA genome (20,28). Nuclear magnetic resonance studies of NC have shown that the zinc finger domains are spatially proximate, with flexible, conformationally-independent N- and C-termini (29,30).
Western blot analysis has shown binding to RT by both wt NC and mutant NC lacking the N- and C-terminal domains (12–53), but not by NC lacking both zinc fingers (31). The present results show that such a mutated peptide can also promote the synthesis of FLST DNA. These observations underscore the structural complexity and probable transitional nature of the reverse transcription complex; in the presence of nucleic acids, different domains of NC may be important at different stages of reverse transcription. Further work will involve studying the effects of mutant NC proteins on RT processivity.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Dr Matthias Götte for critical reading of the manuscript and valuable suggestions. This research was performed by M.H. in partial fulfilment of the requirements for a Ph.D. degree, Faculty of Graduate Studies, McGill University, Montréal, Canada. M.H. was supported by a pre-doctoral fellowship from Health Canada. This work was supported by the Medical Research Council of Canada. We acknowledge ANRS and SIDACTION, French associations against AIDS, for their financial support.
REFERENCES
- 1.Götte M., Li,X. and Wainberg,M.A. (1999) Arch. Biochem. Biophys., 365, 199–210. [DOI] [PubMed] [Google Scholar]
- 2.Telesnitsky A. and Goff,S.P. (1993) In Skalka,A.M. and Goff,S.P. (eds), Reverse Transcriptase. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, Vol. 23, pp. 49–83.
- 3.Huang Y., Khorchid,A., Wang,J., Parniak,M.A., Darlix,J.-L., Wainberg,M.A. and Kleiman,L. (1997) J. Virol., 71, 4378–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick R.J., Chabot,D.J., Rein,A., Henderson,L.E. and Arthur,L.O. (1993) J. Virol., 67, 4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick R.J., Nigida,S.M.J., Bess,J.W.J., Arthur,L.O., Henderson,L.E. and Rein,A. (1990) J. Virol., 64, 3207–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson L. and Yu,X.-F. (1998) Virology, 251, 141–157. [DOI] [PubMed] [Google Scholar]
- 7.Ottmann M., Gabus,C. and Darlix,J.-L. (1995) J. Virol., 69, 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y.-x., Campbell,S., Harvin,D., Ehresmann,B., Ehresmann,C. and Rein,A. (1999) J. Virol., 73, 4251–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klarmann G.J., Schauber,C.A. and Preston,B.D. (1993) J. Biol. Chem., 268, 9793–9802. [PubMed] [Google Scholar]
- 10.Li X., Quan,Y., Arts,E.J., Li,Z., Preston,B.D., de Rocquigny,H., Roques,B.P., Darlix,J.-L., Kleiman,L., Parniak,M.A. and Wainberg,M.A. (1996) J. Virol., 70, 4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong L., Liang,C., Hsu,M., Kleiman,L., Petitjean,P., de Rocquigny,H., Roques,B.P. and Wainberg,M.A. (1998) J. Virol., 72, 9353–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan B. and Musier-Forsyth,K. (1997) Proc. Natl Acad. Sci. USA, 94, 13530–13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You J.C. and McHenry,C.S. (1994) J. Biol. Chem., 269, 31491–31495. [PubMed] [Google Scholar]
- 14.Guo J., Henderson,L.E., Bess,J., Kane,B. and Levin,J.G. (1997) J. Virol., 71, 5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.K., Palaniappan,C., Wu,W., Fay,P.J. and Bambara,R.A. (1997) J. Biol. Chem., 272, 16769–16777. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Rodríguez L., Tsuchihashi,Z., Fuentes,G.M., Bambara,R.A. and Fay,P.J. (1995) J. Biol. Chem., 270, 15005–15011. [DOI] [PubMed] [Google Scholar]
- 17.Arts E.J., Li,X., Gu,Z., Kleiman,L., Parniak,M.A. and Wainberg,M.A. (1994) J. Biol. Chem., 269, 14672–14680. [PubMed] [Google Scholar]
- 18.Jiang M., Mak,J., Ladha,A., Cohen,E., Klein,M., Rovinski,B. and Kleiman,L. (1993) J. Virol., 67, 3246–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rocquigny H., Ficheux,D., Gabus,C., Fournié-Zaluski,M.-C., Darlix,J.-L. and Roques,B.P. (1991) Biochem. Biophys. Res. Commun., 180, 1010–1018. [DOI] [PubMed] [Google Scholar]
- 20.de Rocquigny H., Gabus,C., Vincent,A., Fournié-Zaluski,M.-C., Roques,B. and Darlix,J.-L. (1992) Proc. Natl Acad. Sci. USA, 89, 6472–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2 Edn, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Abbotts J., Bebenek,K., Kunkel,T.A. and Wilson,S.H. (1993) J. Biol. Chem., 268, 10312–10323. [PubMed] [Google Scholar]
- 23.DeStefano J.J. (1996) J. Biol. Chem., 271, 16350–16356. [PubMed] [Google Scholar]
- 24.Tsuchihashi Z. and Brown,P.O. (1994) J. Virol., 68, 5863–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remy E., de Rocquigny,H., Petitjean,P., Muriaux,D., Theilleux,V., Paoletti,J. and Roques,B.P. (1998) J. Biol. Chem., 273, 4819–4822. [DOI] [PubMed] [Google Scholar]
- 26.Isel C., Ehresmann,C., Keith,G., Ehresmann,B. and Marquet,R. (1995) J. Mol. Biol., 247, 236–250. [DOI] [PubMed] [Google Scholar]
- 27.Isel C., Lanchy,J.-M., LeGrice,S.F.J., Ehresmann,C., Ehresmann,B. and Marquet,R. (1996) EMBO J., 15, 917–924. [PMC free article] [PubMed] [Google Scholar]
- 28.Lapadat-Topolsky M., Pernelle,C., Borie,C. and Darlix,J.-L. (1995) Nucleic Acids Res., 23, 2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morellet N., Jullian,N., de Rocquigny,H., Maigret,B., Darlix,J.-L. and Roques,B.P. (1992) EMBO J., 11, 3059–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morellet N., de Rocquigny,H., Mély,Y., Jullian,N., Déméné,H., Ottmann,M., Gérard,D., Darlix,J.-L., Fournie-Zaluski,M.C. and Roques,B.P. (1994) J. Mol. Biol., 235, 287–301. [DOI] [PubMed] [Google Scholar]
- 31.Druillennec S., Caneparo,A., de Rocquigny,H. and Roques,B.P. (1999) J. Biol. Chem., 274, 11283–11288. [DOI] [PubMed] [Google Scholar]