Abstract
Aims:
Disrupted affective processes are core features of psychosis; yet emotion reactivity and emotion regulation impairments have not been fully characterized in individuals at clinical high-risk for developing psychosis (CHR) or adolescents diagnosed with a psychotic disorder (AOP). Characterizing these impairments may provide a fuller understanding of factors contributing to psychosis risk and psychosis onset. Using cross-sectional and longitudinal data, we evaluated (1) group-level effects of emotion reactivity and regulation, (2) stability of group-level effects over time and age, (3) relationships between emotion reactivity and regulation, and (4) associations between these measures and psychosocial functioning and clinical symptomatology.
Methods:
Eighty-seven participants (CHR = 32, TD = 42, AOP = 13; 12–25 years, 1–5 visits) completed the Emotion Reactivity Scale, Difficulties in Emotion Regulation Scale, and Emotion Regulation Questionnaire. We assessed psychotic symptoms with the Structured Interview for Prodromal Syndromes and measured real-world functioning with the Global Functioning: Social and Role Scales. We used analysis of variance to assess Aim 1 and linear mixed models to address Aims 2–4.
Results:
CHR and AOP endorsed experiencing heightened levels of emotion reactivity and greater difficulty utilizing emotion regulation strategies compared to TD. These impairments were stable across time and adolescent development. Greater levels of emotion reactivity were associated with greater emotion regulation impairments. Greater impairments in emotion regulation were associated with lower social functioning and greater negative symptom severity.
Conclusion:
Therapeutic interventions designed to reduce emotion reactivity and improve one’s ability to utilize emotion regulation strategies may be effective in reducing clinical symptomatology and improving real-world functioning in CHR and AOP.
Keywords: affective, negative symptoms, prodromal, schizophrenia-spectrum, social functioning
1 |. INTRODUCTION
Disrupted affective processes are core features of psychosis (Roff & Knight, 1978; Walker & Davis, 1993). Greater affective dysfunction in psychosis is associated with impaired daily functioning, reduced quality of life, increased likelihood of relapse, and greater cognitive impairments (Booij et al., 2018; Chapman et al., 2020; Fialko et al., 2006; Kimhy et al., 2012; Myin-Germeys et al., 2001). Detailed characterization of emotion reactivity and regulation deficits in individuals across the psychosis spectrum will aid in the development of optimal intervention techniques to reduce clinical symptoms and improve real-world functioning.
Emotion reactivity is the extent to which an individual experiences emotions, that is, how intense, fast, and long one experiences emotions in response to stimuli (Nock et al., 2008). Heightened stress-sensitivity – increased emotion reactivity to stressful situations – is observed across the psychosis spectrum, including individuals at clinical high-risk for developing psychosis (CHR; Booij et al., 2018; DeVylder et al., 2013, 2016; Myin-Germeys et al., 2001; Reininghaus et al., 2016). However, broader aspects of emotion reactivity have not been assessed in CHR or adolescents diagnosed with a psychotic disorder (AOP). Comparing CHR individuals to an-age matched group with an established psychotic disorder diagnosis (i.e., the AOP group) will provide insights about whether emotion reactivity is elevated as a function of psychotic symptom severity and to what extent the emotion processing abnormalities are present prior to the onset of a full-blown psychotic disorder.
It is also unknown to what extent emotion reactivity in psychosis is associated with difficulties in emotion regulation, that is, the ability to cope with or alter one’s emotions in response to an emotionally eliciting event. Heightened levels of emotion reactivity may predispose an individual to experience difficulties in emotion regulation (Campos et al., 1989; Davidson, 2003; Izard, 1990; Porges et al., 1994; Thompson, 1994); furthermore, these two processes may influence real-world functioning in different ways. Also, given that emotion reactivity and regulation are associated with distinct neurobiological pathways (Dugré et al., 2019; Frank et al., 2014; Fusar-Poli et al., 2009; Gyurak et al., 2011; Kohn et al., 2014; Lee et al., 2012; Phillips et al., 2008; Pozzi et al., 2021; Taylor et al., 2012), identifying the extent to which CHR and/or AOP individuals experience elevated emotion reactivity and/or difficulty engaging in effective emotion regulation strategies may inform us about possible mechanisms underlying psychosis onset.
The most commonly used self-report measure of emotion regulation is the Emotion Regulation Questionnaire (Gross & John, 2003), based on Gross’ model of emotion regulation (Gross, 1998a, 1998b). This model posits that emotion regulation consists of the modulation of emotional arousal through (1) cognitive reappraisal, the cognitive effort required to change the emotional impact of a situation, and (2) expressive suppression, the inhibition of emotionally expressive behaviours. In adults with schizophrenia, increased engagement in cognitive reappraisal is associated with better social functioning, while increased engagement in expressive suppression is associated with poorer clinical outcomes (Badcock et al., 2011; Chapman et al., 2020; Gross & John, 2003; Henry et al., 2008; Kimhy et al., 2012; Perry et al., 2011). Recent evidence finds that, compared to healthy controls, individuals across the psychosis spectrum engage in less cognitive reappraisal, but do not differ in reported levels of expressive suppression (Chapman et al., 2020). Furthermore, though this study examined individuals across the psychosis spectrum (i.e., psychotic-like experiences, CHR individuals, and individuals with a psychotic disorder diagnosis), the comparisons were conducted in three separate studies and were not able to directly compare whether the level of emotion regulation impairments varied as a function of psychotic symptom severity.
While Gross’s model focuses on controlling one’s level of arousal in an emotion-eliciting situation, a model by Gratz and Roemer expands upon that definition of emotion regulation to include the awareness, understanding, and acceptance of emotions, as well as the ability to act appropriately regardless of one’s emotional state (Gratz & Roemer, 2004). The Difficulties in Emotion Regulation Scale (DERS, Gratz & Roemer, 2004) assesses emotion regulation within this expanded model context. In adults, responses to the DERS shared unique variance with anxiety symptoms, after accounting for the two specific emotion regulation strategies of the ERQ (Bardeen & Fergus, 2014). Though the ERQ is important for capturing variability in affective disturbances across the psychosis spectrum (Badcock et al., 2011; Chapman et al., 2020; Henry et al., 2008; Kimhy et al., 2012; Perry et al., 2011), the DERS may capture additional insights about how emotion regulation impairments and how they are related to symptoms and functioning in CHR and AOP.
Psychosis often develops during the transition from adolescence to adulthood, when affective processes are still developing (Blakemore, 2008, 2012; Blakemore et al., 2010). There is evidence that emotion reactivity levels and engagement in emotion regulation strategies change across normative adolescent development (Claes et al., 2014; Gullone et al., 2010; Teixeira et al., 2015). In adolescents 12–20 years old, older ages were associated with higher emotion reactivity levels (Claes et al., 2014). Another study of youth 9–15 years old found that younger participants reported engaging in more expressive suppression, as well as more cognitive reappraisal (Gullone et al., 2010). Consistent with this work, another study found that 9th graders endorsed engaging in more cognitive reappraisal, as well as more expressive suppression in comparison to older counterparts (Teixeira et al., 2015). Characterization of age-associated disruptions in emotion reactivity and regulation in adolescents and young adults experiencing psychosis-spectrum symptoms is important for understanding factors contributing to the development of affective disturbances in adults with psychosis.
Using cross-sectional data, we examined group differences (CHR vs. AOP vs. typically developing adolescents and young adults, TD) in self-reported emotion reactivity levels and emotion regulation strategies. With longitudinal data, we assessed the stability of these differences across time and age, as well as the relationship between emotion reactivity and regulation. We also used the longitudinal data to assess how emotion reactivity and emotion regulation measures were related to clinical symptomatology and real-world functioning in adolescents across the psychosis-spectrum. We hypothesized that, in comparison to TD, CHR and AOP would endorse overall higher emotion reactivity levels and greater impairments in emotion regulation. Based on previous literature (Claes et al., 2014; Gullone et al., 2010; Teixeira et al., 2015), we predicted that, regardless of group status, on average, older adolescents would report heightened levels of emotion reactivity, less engagement in cognitive reappraisal and more engagement in expressive suppression. We expected that heightened emotion reactivity levels would be associated with greater impairments in emotion regulation. Finally, we expected impaired emotion reactivity and regulation to be associated with greater severity in clinical symptoms and/or lower social and role functioning.
2 |. METHODS
2.1 |. Participants
The final sample consisted of 87 unique individuals (12–25 years, 1–5 visits). The sample was derived from two naturalistic studies of CHR and AOP conducted at the University of Pittsburgh; thus, the length of time in between visits varied (mean length of time between visits: 280.5 days, range: 79–1081 days). Please see Table S1 for the number of visits for each participant. The sample consisted of individuals at clinical high-risk for developing psychosis (CHR, N = 32 unique participants), adolescents with a psychotic disorder diagnosis (AOP, N = 13 unique participants), and demographically comparable, typically-developing adolescents and young adults (TD, N = 42 unique participants). Based on the Structured Interview for Psychosis-Risk Syndromes (SIPS, McGlashan et al., 2014), CHR participants met criteria for one of three conditions: (1) attenuated/subthreshold psychotic symptoms; (2) transient, recent-onset psychotic symptoms; or (3) a substantial, recent drop in functioning in conjunction with schizotypal personality disorder diagnosis or a first-degree relative with a psychotic disorder. AOP participants were 12–18 years old and met DSM-IV criteria for a schizophrenia-spectrum diagnosis (i.e., schizophrenia, schizoaffective disorder, or schizophreniform disorder), based on information gathered during a Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2009). TD participants were recruited from the community and excluded from participation if they displayed evidence of any major mental disorder or psychosis-risk syndrome, based on information gathered during clinical interviews. Participants were excluded if they had a neurological disorder that affected performance on study tasks, insufficient fluency in English, an estimated IQ of <70, or if they endorsed substance or alcohol abuse and/or dependence within the past 6 months.
All participants underwent a verbal and written informed consent process. Subjects <18 years provided written assent, while their parent or guardian completed written consent. The University of Pittsburgh Institutional Review Board approved all procedures.
2.2 |. Clinical measures
A master’s-level trained clinician assessed participants on the SIPS positive, negative, disorganized and general symptoms scales. Individual symptoms are rated from zero (absence of symptoms) to six (psychotic level of symptoms). This measure has shown excellent inter-relatability (Meyer et al., 2005; Miller et al., 2003). We used the sum of the positive and negative SIPS symptom scores as separate dimensional psychotic symptoms measures.
2.3 |. Global functioning: Social and roles scales
Real-world functioning was measured using the Global Functioning: Social Scale (GFS) and Global Functioning: Role Scale (GFR). These scales were specifically developed for younger individuals (Cornblatt et al., 2007) and measure the quality and quantity of personal relationships (GFS) or one’s performance in daily school or work roles (GFR). Functioning is measured on a 10-point scale, with 1 representing ‘extreme dysfunction’ and 10 representing ‘superior functioning’.
2.4 |. Cognition
A Full Scale IQ estimate was derived from two subtests (Vocabulary and Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence as a measure of general intellectual functioning (Wechsler, 2011).
2.5 |. Emotion reactivity
Emotion reactivity was evaluated using the Emotion Reactivity Scale (ERS; Nock et al., 2008). The ERS is a 21-item self-report questionnaire that measures how one experiences emotions in response to sensory input. A higher ERS score reflects higher reactivity to emotive stimuli. The total score consists of three subscales: sensitivity, arousal, and persistence. Table S2 includes subscale definitions, examples, and score ranges for the subscales.
2.6 |. Emotion regulation
Emotion regulation was evaluated using two questionnaires: the ERQ (Gross & John, 2003) and DERS (Gratz & Roemer, 2004). The ERQ consists of two subscales: Cognitive Reappraisal (6 items) and Expressive Suppression (4 items). Items rated from 1 (strongly disagree) to 7 (strongly agree). The DERS consists of 36-items that measure engagement in specific emotion regulation strategies. Items are rated on from 1 (almost never) to 5 (almost always). The DERS consists of six subscales: Nonacceptance of Emotional Responses, Difficulties in Engaging in Goal Directed Behaviour, Impulse Control Difficulties, Lack of Emotional Awareness, Limited Access to Emotion Regulation Strategies, and Lack of Emotional Clarity. Please see Tables S3 and S4 for questionnaire details.
3 |. STATISTICAL ANALYSES
We used R version 4.0.0 (R Core Team, 2020) to conduct all statistical analyses. To compare demographic variables between groups, we performed univariate analyses of variance (ANOVA) for continuous variables and χ2 tests for categorical variables. This demographic comparison was restricted to baseline, cross-sectional data. We used the baseline, cross-sectional data to first examine group differences (CHR vs. AOP vs. TD) on emotion reactivity and emotion regulation strategies. Here, each independent observation (participant) had data from one visit. In this cross-sectional analysis, we conducted univariate ANOVAs with each affective measure as the dependent variable (total ERS, total DERS, ERQ Cognitive Reappraisal, and ERQ Expressive Suppression scores) and group status as the independent variable. We conducted simple effects comparisons on significant interactions or group effects using emmeans (Lenth, 2020). For all models, we included sex and age as covariates. We also conducted post-hoc analyses of emotion reactivity and regulation subscales.
To examine the effects of age and maturation on group differences, we used the longitudinal data to conduct separate linear mixed models for each affective measure. We examined the fixed effects of age, group, and visit, as well as the interaction between these variables. To account for the non-independence of longitudinal data (multiple visits), participant was included as a random effect (intercept).
Using these fixed and random effects, we built linear mixed models to assess the relationship between emotion reactivity (predictor) and emotion regulation (dependent variable) and the relationship between the affective measures and total positive and negative symptoms and/or social and role functioning measures.
To ensure that possible confounds were not driving results, we re-ran all analyses covarying for IQ, antipsychotic medication status, and parental socioeconomic status (SES).
4 |. RESULTS
Participant information is reported in Table 1. All three groups were matched on age, sex and race. In comparison to TD, CHR and AOP had lower SES levels, lower IQ, and higher levels of clinical symptomatology.
TABLE 1.
Demographic and clinical information on samples examined. The three groups were matched on age, sex and race
| TD (N = 42) | CHR (N = 32) | AOP (N = 13) | p | |
|---|---|---|---|---|
| Mean age, years (SD) | 17.8 (2.8) | 18.5 (2.9) | 17.2 (1.7) | 0.31 |
| Age range, years | 13–23 | 13–25 | 13–20 | 0.31 |
| Sex: Female/Male | 21/21 | 15/17 | 8/5 | 0.67 |
| Race: Caucasian/African American/Asian/Multiple | 36/2/3/1 | 26/4/1/1 | 9/4/0/0 | 0.24 |
| Mean SES (SD) | 49.4 (10.9) | 39.8 (12.0) | 38.5 (9.5) | 1.3e-03 |
| Mean Intelligence Quotient (SD) | 111.4 (9.2) | 105.4 (10.9) | 103.4 (13.0) | 0.02 |
| Mean of total positive symptoms (SD) | 0.1 (0.4) | 11.5 (3.8) | 19.0 (3.5) | 8.6e-40 |
| Mean of total negative symptoms (SD) | 0.3 (0.8) | 12.8 (6.0) | 12.1 (6.2) | 5.0e-21 |
| N prescribed antipsychotic medication | 0 | 8 | 8 | NA |
Note: In comparison to typically developing adolescents and adults (TD), the clinical high risk for developing psychosis (CHR) and adolescent-onset psychosis (AOP) groups had significantly lower intelligence quotient (IQ), parental socioeconomic status (SES), and endorsed higher levels of total positive and negative symptoms.
4.1 |. CHR and AOP youth report elevated emotion reactivity levels and greater emotion regulation impairment in comparison to TD
On the ERS, there was a significant group effect on overall self-reported emotion reactivity (F = 27.5, p < .001, q = <0.001, Table 2, Figure 1). In comparison to TD, CHR (t = 7.0, p < .001) and AOP (t = 4.4, p < .001) reported experiencing elevated emotion reactivity. Differences were observed across all ERS subscales (Table S5, Figure S1A).
TABLE 2.
Group effects [typically developing adolescents and adults (TD) vs. individuals at clinical high risk for developing psychosis (CHR) vs. adolescent-onset psychosis (AOP)] on emotion reactivity (ERS total score), emotion regulation (DERS total score), cognitive reappraisal (ERQ cognitive reappraisal) and expressive suppression (ERQ expressive suppression
| Measure | Degrees of freedom | F | p | q | Pairwise comparison |
|---|---|---|---|---|---|
| ERS total score | 2,80 | 27.5 | 8.1e-10 | 1.6e-09 | TD < CHR TD < AOP |
| DERS total score | 2,81 | 44.3 | 9.9e-14 | 4.0e-13 | TD < CHR TD < AOP |
| ERQ cognitive reappraisal | 2,79 | 9.2 | 2.5e-04 | 3.3e-04 | TD > CHR |
| ERQ expressive suppression | 2,79 | 4.1 | 0.02 | 0.02 | TD < AOP |
Abbreviations: DERS, Difficulties in Emotion Regulation Scale; ERQ, Emotion Regulation Questionnaire; ERS, Emotion Reactivity Scale.
FIGURE 1.
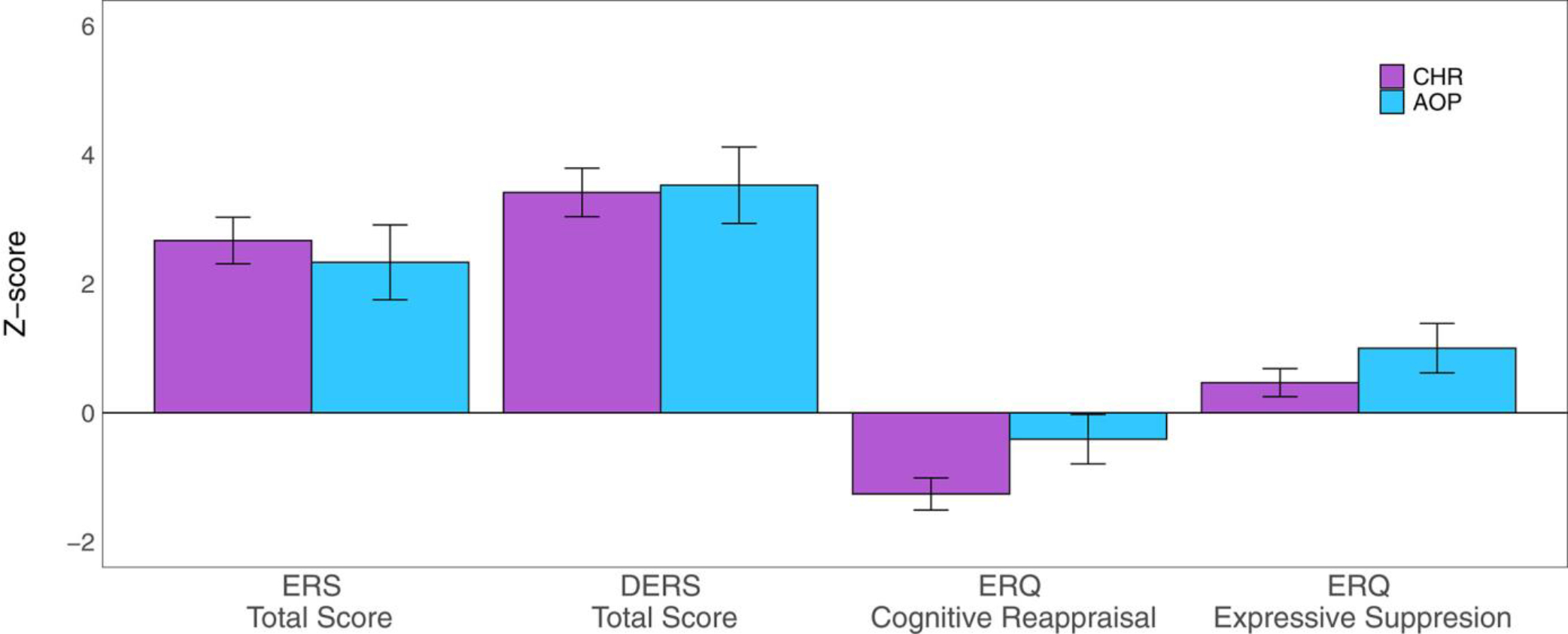
Mean Z-score and standard error plots of emotion reactivity total score on the Emotion Reactivity Scale (ERS total score), emotion regulation total score on the Difficulties in Emotion Regulation Scale (DERS total score), Cognitive Reappraisal on the Emotion Regulation Questionnaire (ERQ cognitive reappraisal), and Expressive Suppression on the Emotion Regulation Questionnaire (ERQ expressive suppression) for clinical high risk youth (CHR, purple) and adolescents with a psychosis disorder (AOP, blue), in reference to typically developing youth (TD) mean and standard deviation. CHR and AOP youths endorsed significantly higher levels of emotion reactivity, and impaired use of emotion regulation strategies on the DERS, less engagement in cognitive reappraisal, and more engagement in expressive suppression compared to TD youth
On the ERQ, a significant group effect was observed for cognitive reappraisal (F = 9.2, p < .001, q < .001) and expressive suppression (F = 4.1, p = .02, q = 0.02,Table 2). In comparison to TD, CHR reported less engagement in cognitive reappraisal (t = −4.3, p < .001); however, there was not a statistically significant difference between AOP and TD (t = −0. 96, p = .34). In comparison to CHR, AOP reported more engagement in cognitive reappraisal (t = 2.0, p = .04). Compared to TD, AOP were more likely to engage in emotionally expressive behaviour (t = 2.9, p < .001). However, there was not a statistically significant difference between CHR and TD (t = 1.7, p = .08) in expressive suppression. There was also a significant group effect on total DERS score (F = 44.3, p < .001, q < 0.001, Table 2). In comparison to TD, CHR (t = 6.4, p < .001) and AOP (t = 8.5, p < .001) endorsed overall greater emotion regulation impairments. Impairments were present across all DERS subscales (Table S6, Figure S1B).
For most measures, these results remained significant when SES, IQ, and antipsychotic medication status were added to the model (Tables S7 and S8).
4.2 |. Levels of emotion reactivity and regulation are consistent across adolescent development
In the longitudinal data, we found that, for all groups, emotion reactivity and regulation impairments remained consistent and stable across age (Figure 2, Table S9) and visit (Figure S2, Table S10). There were no interactions between group and age or group and visit on any affective measure (Table S11).
FIGURE 2.
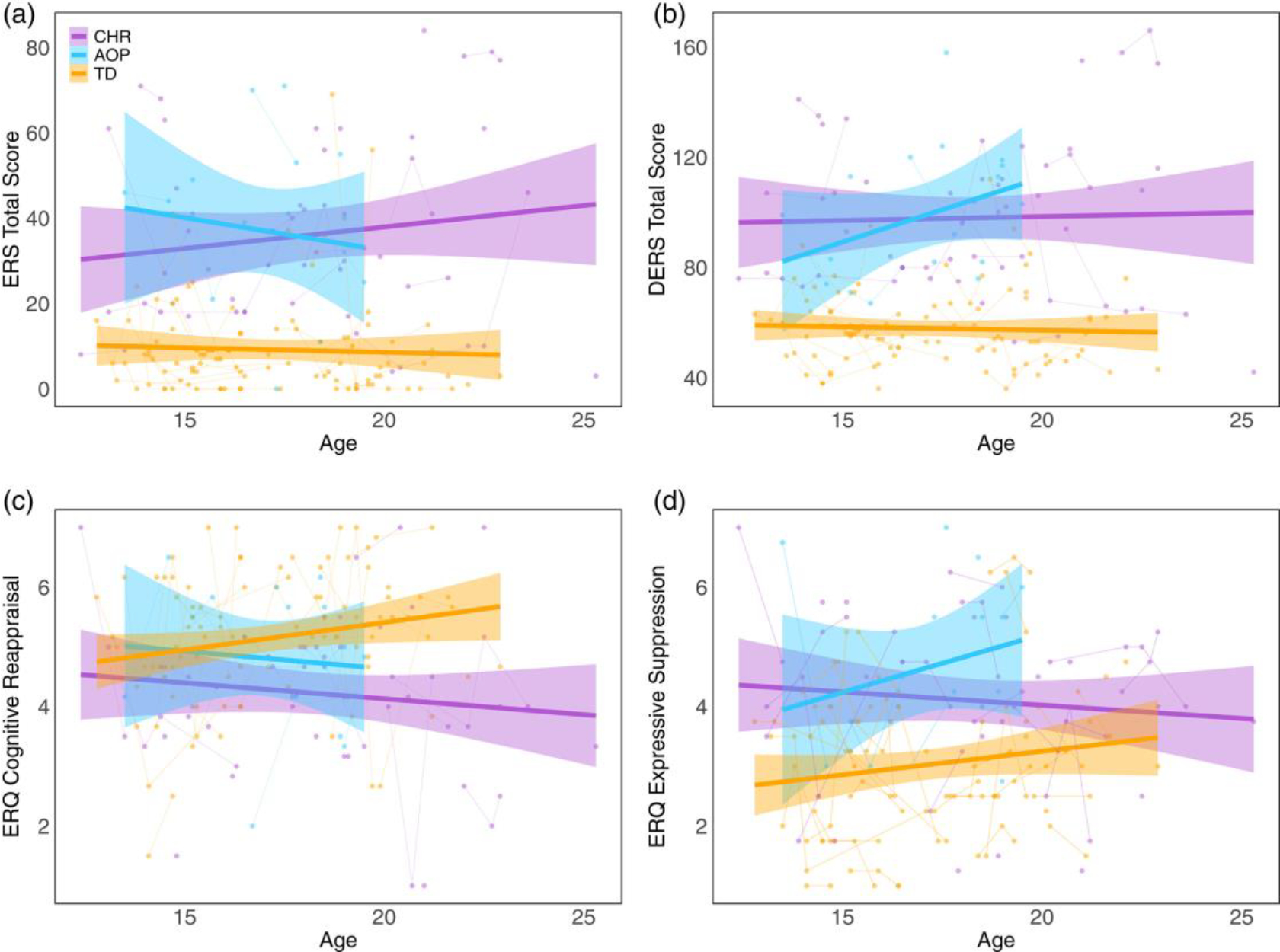
Relationship between age and emotion reactivity (ERS total score), emotion regulation (DERS total score), cognitive reappraisal (ERQ cognitive reappraisal), and expressive suppression (ERQ expressive suppression) in clinical high-risk for developing psychosis (CHR, purple), adolescent-onset psychosis (AOP, blue), and typically developing (TD, orange) across development. Over time, levels of (a) global emotion reactivity, (b) overall impairment in emotion regulation via the DERS, and (c) engagement in cognitive reappraisal and (d) expressive suppression remain stable across development in CHR, AOP, and TD. DERS, Difficulties in Emotion Regulation Scale; ERQ, Emotion Regulation Questionnaire; ERS, Emotion Reactivity Scale
4.3 |. Emotion reactivity is associated with greater impairment in engaging in emotion regulation
Across all groups, there was a significant effect of ERS Total Score on emotion regulation, with higher emotion reactivity associated with greater impairment on the DERS (R2 = 0.16) and less reported engagement in Cognitive Reappraisal (R2 = 0.05) (Table 3). However, there was no significant effect of ERS Total Score on ERQ Expressive Suppression (R2 < 0.01); please see Table S12 and Figure S3 for details. After correction for multiple comparisons, there were no significant interactions of age, group, or visit on the relationship between emotion reactivity and emotion regulation measures (Table S13). Please see Supplemental Text for reports of trend-level interactions.
TABLE 3.
Relationship between emotion reactivity (ERS total score) and emotion regulation (DERS total sore), cognitive reappraisal (ERQ cognitive reappraisal) and expressive suppression (ERQ expressive suppression) in typically developing youth (TD), clinical high-risk for developing psychosis (CHR) and adolescents-onset psychosis (AOP) independently
| TD | CHR | AOP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Measure | Beta | SE | t | p | Beta | SE | t | p | Beta | SE | t | p |
| DERS total score | 0.59 | 0.13 | 4.51 | 6.3e-05 | 0.73 | 0.12 | 6.13 | 1.5e-06 | 0.75 | 0.21 | 3.63 | 5.5e-03 |
| ERQ cognitive reappraisal | −0.18 | 0.16 | −1.16 | 0.26 | −0.01 | 0.19 | −0.08 | 0.94 | −0.83 | 0.26 | −3.19 | 0.01 |
| ERQ expressive suppression | 0.41 | 0.14 | 2.94 | 5.7e-03 | −0.37 | 0.19 | −1.93 | 0.06 | −0.42 | 0.26 | −1.65 | 0.14 |
Abbreviations: DERS, Difficulties in Emotion Regulation Scale; ERQ, Emotion Regulation Questionnaire; ERS, Emotion Reactivity Scale.
4.4 |. Greater emotion regulation impairment was associated with greater negative symptom severity and lower social functioning in CHR and AOP
In CHR and AOP, greater negative symptom severity was associated with less cognitive reappraisal (β = −0.27, t = −2.4, p = .02, q = 0.09, Figure 3) and greater overall impairment on the DERS (β = 0. 31, t = 2.4, p = .02, q = 0.09, Figure 3). There was also a relationship between lower social functioning and greater impairment on the DERS (β = −0.30, t = −2.1, p = .04, q = 0.31, Figure 3c). See Tables S14 and S15 for complete results.
FIGURE 3.
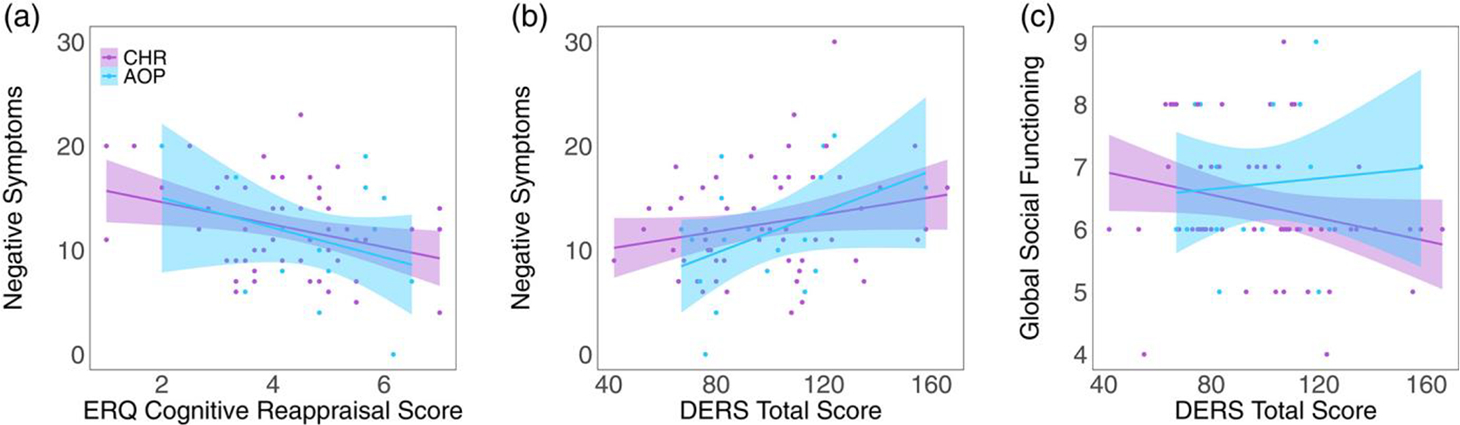
Relationships between the affective measure and real-world functioning and psychotic symptoms in clinical high-risk for developing psychosis (CHR, purple) and adolescent-onset psychosis (AOP, blue) over time (1–5 visits). Within the combined CHR and AOP groups, (a) greater impairments in emotion regulation (DERS total score) are associated with lower social functioning (global social functioning). (b) Greater emotion regulation impairments (DERS total score) are associated with greater severity in negative symptoms. (c) More engagement in cognitive reappraisal (ERQ cognitive reappraisal) is associated with less severity in negative symptoms. DERS, Difficulties in Emotion Regulation Scale; ERQ, Emotion Regulation Questionnaire; ERS, Emotion Reactivity Scale
5 |. DISCUSSION
We found elevated emotion reactivity levels and emotion regulation impairments in individuals at clinical high risk for developing psychosis (CHR) and adolescents with a psychotic disorder (AOP) in comparison to typically developing adolescents and adults (TD). These effects were stable across age and time. Emotion reactivity levels accounted for a small, yet statistically significant portion of the variance in emotion regulation (5–16%). Finally, greater emotion regulation impairments are associated with lower social functioning and greater severity of negative symptoms. These findings provide a detailed characterization of heightened emotion reactivity and impaired emotion regulation in adolescents and young adults across the psychosis spectrum.
5.1 |. Global emotion reactivity levels are elevated in CHR and AOP
In comparison to TD participants, we found that emotion reactivity levels were similarly elevated in CHR and AOP and comparable to emotion reactivity levels endorsed by adolescents and young adults experiencing diverse psychopathologies (Nock et al., 2008). All assessed aspects of emotion reactivity were elevated in these two groups. There is an extensive amount of literature linking stress sensitivity to psychotic symptoms (Booij et al., 2018; DeVylder et al., 2013, 2016; Myin-Germeys et al., 2001; Myin-Germeys & van Os, 2007; Reininghaus et al., 2016); our work expands upon this evidence and shows that other aspects of emotion reactivity are also elevated in the psychosis spectrum. In the future, it may be important to incorporate techniques from evidence-based interventions known to reduce emotion reactivity (e.g., Dialectical Behavioural Therapy or DBT, Linehan, 2014, 2018) as therapeutic strategies for CHR and AOP. In DBT, individuals are taught specific techniques to reduce emotional arousal (e.g., holding an ice cube or engaging in intense exercise), and shorten the persistence of emotional arousal (e.g., participating in distracting activities). Finally, this work also adds to the large body of evidence showing that elevated emotion reactivity is a transdiagnostic feature of many psychiatric disorders (Bylsma et al., 2008, 2011; Carthy et al., 2010; McLaughlin et al., 2010).
5.2 |. CHR and AOP report impairments in utilizing emotion regulation strategies
Similar to previous work in adults with psychosis (Badcock et al., 2011; Chapman et al., 2020; Henry et al., 2008; Kimhy et al., 2012; Perry et al., 2011), CHR and AOP reported increased difficulty engaging in effective emotion regulation strategies. This study builds additional support to the existing literature that emotion regulation abnormalities are present in individuals at CHR (Chapman et al., 2020; Gruber et al., 2018; Kimhy et al., 2016; Lincoln et al., 2018). Furthermore, heightened levels of emotion reactivity and impairments in emotion regulation reported by CHR and AOP are similar in magnitude to those observed in adolescents diagnosed with other psychiatric disorders (Becerra et al., 2013; Zhang et al., 2018). Taken together, these findings underscore the importance of emotion regulation as a transdiagnostic risk factor for many psychiatric disorders. Although it is well-recognized that emotion regulation impairments are transdiagnostic risk factors of internalizing and externalizing disorders (e.g., Drabick et al., 2010; Heleniak et al., 2016; Malhi et al., 2017; Weissman et al., 2019), emotion dysregulation is not commonly evaluated as a possible risk factor for later transition to a psychotic disorder in CHR. Given that the emotion regulation impairments in CHR were not diminished when we covaried for cognitive abilities (Table S8), which have been identified as significant predictors of psychosis conversion (Kim et al., 2011; Riecher-Rössler et al., 2009; Seidman et al., 2010, 2016), it is possible that emotion regulation impairments may account for unique and additional variance in our ability to predict conversion to psychosis. Indeed, other affective processes (e.g., emotion recognition) have been shown to be significant predictors of conversion to psychosis (Allott et al., 2014; Corcoran et al., 2015).
It should also be noted that emotion reactivity and emotion regulation did not vary as a function of psychosis symptom severity. Recent behavioural and electrophysiological evidence supports our findings as other studies find that individuals with established psychotic disorders and CHR individuals exhibit similar levels of neural and behavioural disruption in emotion regulation (Chapman et al., 2020; Kim et al., 2021). Similar findings have been observed in social cognition impairments (Addington et al., 2008; Piskulic et al., 2016). In conjunction with findings indicating that elevated emotion reactivity and emotion regulation impairments are present in many psychiatric disorders (Bylsma et al., 2008, 2011; Carthy et al., 2010; Drabick et al., 2010; Heleniak et al., 2016; Malhi et al., 2017; McLaughlin et al., 2010; Weissman et al., 2019), it is likely that emotion processing impairments are indicative of more general psychopathology.
5.3 |. Emotion reactivity levels and emotion regulation abilities are stable across time and age
Though some studies have found that adolescence is a time of significant increases in emotion reactivity and changes in emotion regulation abilities (Claes et al., 2014; Gullone et al., 2010; Teixeira et al., 2015), we found that average emotion reactivity and emotion regulation levels were similar from adolescence through adulthood, and across multiple visits. Our results suggest that emotion reactivity and emotion regulation are stable, trait-like features in adolescents and young adults across the psychosis spectrum. Alternatively, reported age-associated effect sizes are often small to medium, so we may have been underpowered to detect an age effect.
5.4 |. Emotion reactivity and emotion regulation are related, yet distinct processes in CHR, AOP and TD
We found that in all groups, there was a small, yet distinct relationship between emotion reactivity and emotion regulation. Individuals with elevated levels of emotion reactivity had greater impairment in their ability to regulate emotions. Given that emotion reactivity levels accounted for a small, yet statistically significant portion of the variance in emotion regulation (5–16%), our findings add to previous works showing that emotion regulation and reactivity are distinct, yet related constructs (Salsman & Linehan, 2012; Veilleux et al., 2014; Zelkowitz & Cole, 2016). Emotion reactivity and regulation have known neural underpinnings. Emotion reactivity is associated with increased activation in the amygdala, visual and temporal cortices, and thalamus (Fusar-Poli et al., 2009; Guyer et al., 2008; Pfeifer et al., 2011; Pozzi et al., 2021), while emotion regulation is associated with activation of the dorso- and ventrolateral prefrontal cortices and the anterior cingulate cortex (Frank et al., 2014; Hare et al., 2005; Kohn et al., 2014; Ochsner et al., 2002; Pozzi et al., 2021). Thus, the variation unique to these processes may be driven by these distinct neural systems. Given that connectivity between regions underlying both processes is disrupted across the psychosis spectrum (e.g., amygdala-prefrontal connectivity, Anticevic et al., 2011, 2013; Gee et al., 2012; Jalbrzikowski et al., 2019; Liu et al., 2014; Modinos et al., 2010), it is plausible that connectivity between regions from the two networks underlies the shared processes.
5.5 |. Emotion regulation impairments are related to social functioning and negative symptoms in CHR and AOP
Greater levels of emotion regulation impairments are associated with lower social functioning and greater negative symptom severity in CHR and AOP. This is consistent with findings in adults with established psychosis (Henry et al., 2008; Kimhy et al., 2012; Perry et al., 2011). Because there are strong ties between social functioning and negative symptoms in adults with a psychotic disorder diagnosis (Kalin et al., 2015; Robertson et al., 2014), in the future it will be important to examine emotion regulation as a possible moderator or mediator of this relationship. Furthermore, we identified relationships between clinical symptomatology/psychosocial functioning and emotion regulation, but not emotion reactivity. Thus, interventions targeting emotion regulation improvement may have the strongest impact on symptoms and functioning in CHR and AOP populations.
In follow-up studies, it will be informative to further probe the observed relationship between emotion regulation and social functioning. In a related body of literature, researchers found that individuals with a schizophrenia diagnosis, as well as CHR individuals, exhibit substantial emotion awareness (alexithymia) deficits (Kimhy et al., 2012, 2016). Furthermore, alexithymia deficits impact psychotic symptom severity during daily functioning (Kimhy, 2020). Thus, it is possible that a relationship between better emotion regulation and higher social functioning exists because individuals who can accurately identify and label their emotions have better emotion regulation abilities, which then influences functioning. Future studies should examine alexithymia deficits as a possible mediator or moderator of the relationship between emotion regulation and social functioning.
5.6 |. Limitations
There were several limitations to our study. We were not able to assess the extent to which affective disruptions predicted conversion to psychosis because only two CHR participants transitioned to a psychotic disorder at follow-up. Given the relevance of affective processes to the development of psychosis (Allott et al., 2014; Corcoran et al., 2015), it will be important to assess how emotion reactivity levels and emotion regulation abilities improve the ability to predict who will develop a psychotic disorder. Also, we are limited by our sample size. The individual group samples were modest and our findings should be replicated in an independent sample. Furthermore, though we employed a longitudinal design, we did not have enough power to assess within-subject change by including a term for a random slope. Instead, consistent with previous literature (Taylor et al., 2016), having multiple measurements on the same individual likely reduces phenotypic measurement error and increases statistical power for group-level inferences. In the future, it will be important to assess within-subject change in emotion reactivity and emotion regulation in CHR and AOP. Also, we did not directly assess substance use, which is related to emotion regulation capabilities (Fox et al., 2008; Gottfredson & Hussong, 2013; Weiss et al., 2017). Future studies should investigate the relationships between substance use, emotion regulation abilities, and psychotic symptoms. Finally, it will also be important to see how self-report emotion reactivity and regulation measures compare to ‘in-the-moment’ assessments (e.g., Niendam et al., 2018) or laboratory experiments (Gruber et al., 2018; Lincoln et al., 2018).
6 |. CONCLUSIONS AND FUTURE DIRECTIONS
We find that there are global, elevated emotion reactivity levels and overall emotion regulation impairments in CHR and AOP, these impairments are stable across time and age, and impairments in emotion regulation are related to clinical symptoms and social functioning in psychosis-spectrum youth. In the future, to better understand neural mechanisms underlying emotion regulation, we plan to examine how neural circuitry associated with the cognitive control of emotion and affective processes in schizophrenia (e.g., Jimenez et al., 2019; Tully et al., 2014) relates to behavioural indicators of emotion regulation in psychosis spectrum youth.
Supplementary Material
ACKNOWLEDGEMENTS
The project described was supported by the National Institutes of Health through grants K01MH112774 (Maria Jalbrzikowski) and K23MH097040 (Peter Bachman). We thank the faculty and staff of the WPH Psychosis Recruitment and Assessment Core and WPH At Risk and Assessment Core for their assistance in diagnostic and clinical symptomatology assessments.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Addington J, Penn D, Woods SW, Addington D, & Perkins DO (2008). Social functioning in individuals at clinical high risk for psychosis. Schizophrenia Research, 99(1–3), 119–124. 10.1016/j.schres.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott KA, Schäfer MR, Thompson A, Nelson B, Bendall S, Bartholomeusz CF, Yuen HP, McGorry PD, Schlögelhofer M, Bechdolf A, & Amminger GP (2014). Emotion recognition as a predictor of transition to a psychotic disorder in ultra-high risk participants. Schizophrenia Research, 153(1–3), 25–31. 10.1016/j.schres.2014.01.037 [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, & Glahn DC (2013). Global prefrontal and Fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biological Psychiatry, 73(6), 565–573. 10.1016/j.biopsych.2012.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Corlett PR, & Barch DM (2011). Negative and nonemotional interference with visual working memory in schizophrenia. Biological Psychiatry, 70(12), 1159–1168. 10.1016/j.biopsych.2011.07.010 [DOI] [PubMed] [Google Scholar]
- Badcock JC, Paulik G, & Maybery MT (2011). The role of emotion regulation in auditory hallucinations. Psychiatry Research, 185(3), 303–308. 10.1016/j.psychres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Bardeen JR, & Fergus TA (2014). An examination of the incremental contribution of emotion regulation difficulties to health anxiety beyond specific emotion regulation strategies. Journal of Anxiety Disorders, 28(4), 394–401. 10.1016/j.janxdis.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Becerra R, Cruise K, Murray G, Bassett D, Harms C, Allan A, & Hood S (2013). Emotion regulation in bipolar disorder: Are emotion regulation abilities less compromised in euthymic bipolar disorder than unipolar depressive or anxiety disorders? Open Journal of Psychiatry, 03(04), 1–7. 10.4236/ojpsych.2013.34A001 [DOI] [Google Scholar]
- Blakemore S-J (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J (2012). Development of the social brain in adolescence. Journal of the Royal Society of Medicine, 105(3), 111–116. 10.1258/jrsm.2011.110221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij SH, Snippe E, Jeronimus BF, Wichers M, & Wigman JTW (2018). Affective reactivity to daily life stress: Relationship to positive psychotic and depressive symptoms in a general population sample. Journal of Affective Disorders, 225, 474–481. 10.1016/j.jad.2017.08.051 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, & Rottenberg J (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. 10.1016/j.cpr.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Taylor-Clift A, & Rottenberg J (2011). Emotional reactivity to daily events in major and minor depression. Journal of Abnormal Psychology, 120(1), 155–167. 10.1037/a0021662 [DOI] [PubMed] [Google Scholar]
- Campos JJ, Campos RG, & Barrett KC (1989). Emergent themes in the study of emotional development and emotion regulation. Developmental Psychology, 25(3), 394–402. 10.1037/0012-1649.25.3.394 [DOI] [Google Scholar]
- Carthy T, Horesh N, Apter A, & Gross JJ (2010). Patterns of emotional reactivity and regulation in children with anxiety disorders. Journal of Psychopathology and Behavioral Assessment, 32(1), 23–36. 10.1007/s10862-009-9167-8 [DOI] [Google Scholar]
- Chapman HC, Visser KF, Mittal VA, Gibb BE, Coles ME, & Strauss GP (2020). Emotion regulation across the psychosis continuum. Development and Psychopathology, 32(1), 219–227. 10.1017/S0954579418001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Smits D, & Bijttebier P (2014). The Dutch version of the emotion reactivity scale: Validation and relation with various behaviors in a sample of high school students. European Journal of Psychological Assessment, 30(1), 73–79. 10.1027/1015-5759/a000171 [DOI] [Google Scholar]
- Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, Gur RC, & Javitt DC (2015). Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: A neurodevelopmental perspective. Psychological Medicine, 45(14), 2959–2973. 10.1017/S0033291715000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, & Cannon TD (2007). Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin, 33(3), 688–702. 10.1093/schbul/sbm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ (2003). Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology, 40(5), 655–665. 10.1111/1469-8986.00067 [DOI] [PubMed] [Google Scholar]
- DeVylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, & Corcoran CM (2013). Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychological Medicine, 43(2), 259–268. 10.1017/S0033291712001262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVylder JE, Koyanagi A, Unick J, Oh H, Nam B, & Stickley A (2016). Stress sensitivity and psychotic experiences in 39 low- and middle-income countries. Schizophrenia Bulletin, 42(6), 1353–1362. 10.1093/schbul/sbw044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabick DAG, Ollendick TH, & Bubier JL (2010). Co-occurrence of ODD and anxiety: Shared risk processes and evidence for a dual-pathway model: CO-OCCURRENCE OF ODD AND ANXIETY. Clinical Psychology: Science and Practice, 17(4), 307–318. 10.1111/j.1468-2850.2010.01222.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugré JR, Bitar N, Dumais A, & Potvin S (2019). Limbic hyperactivity in response to emotionally neutral stimuli in schizophrenia: A neuroimaging meta-analysis of the Hypervigilant mind. American Journal of Psychiatry, 176(12), 1021–1029. 10.1176/appi.ajp.2019.19030247 [DOI] [PubMed] [Google Scholar]
- Fialko L, Freeman D, Bebbington PE, Kuipers E, Garety PA, Dunn G, & Fowler D (2006). Understanding suicidal ideation in psychosis: Findings from the psychological prevention of relapse in psychosis (PRP) trial. Acta Psychiatrica Scandinavica, 114(3), 177–186. 10.1111/j.1600-0447.2006.00849.x [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, & Williams J (2009). Structured clinical Interview for DSM-IV axis I disorders clinician version SCID-I. American Psychiatric Association. [Google Scholar]
- Fox HC, Hong KA, & Sinha R (2008). Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors, 33(2), 388–394. 10.1016/j.addbeh.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, & Sabatinelli D (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews, 45, 202–211. 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, & Politi P (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34(6), 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Karlsgodt KH, van Erp TGM, Bearden CE, Lieberman MD, Belger A, … Cannon TD (2012). Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: A preliminary study. Schizophrenia Research, 134(1), 1–9. 10.1016/j.schres.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson NC, & Hussong AM (2013). Drinking to dampen affect variability: Findings from a college student sample. Journal of Studies on Alcohol and Drugs, 74(4), 576–583. 10.15288/jsad.2013.74.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. [Google Scholar]
- Gross JJ (1998a). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224–237. 10.1037/0022-3514.74.1.224 [DOI] [PubMed] [Google Scholar]
- Gross JJ (1998b). The emerging field of emotion regulation: An Integrative review. Review of General Psychiatry, 29. [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gruber J, Strauss GP, Dombrecht L, & Mittal VA (2018). Neuroleptic-free youth at ultrahigh risk for psychosis evidence diminished emotion reactivity that is predicted by depression and anxiety. Schizophrenia Research, 193, 428–434. 10.1016/j.schres.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Gullone E, Hughes EK, King NJ, & Tonge B (2010). The normative development of emotion regulation strategy use in children and adolescents: A 2-year follow-up study: A longitudinal study of two specific emotion regulation strategies. Journal of Child Psychology and Psychiatry, 51(5), 567–574. 10.1111/j.1469-7610.2009.02183.x [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, & Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. 10.1162/jocn.2008.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, & Etkin A (2011). Explicit and implicit emotion regulation: A dual-process framework. Cognition & Emotion, 25(3), 400–412. 10.1080/02699931.2010.544160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, & Casey BJ (2005). Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry, 57(6), 624–632. 10.1016/j.biopsych.2004.12.038 [DOI] [PubMed] [Google Scholar]
- Heleniak C, Jenness JL, Vander Stoep A, McCauley E, & McLaughlin KA (2016). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40(3), 394–415. 10.1007/s10608-015-9735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Rendell PG, Green MJ, McDonald S, & O’Donnell M (2008). Emotion regulation in schizophrenia: Affective, social, and clinical correlates of suppression and reappraisal. Journal of Abnormal Psychology, 117(2), 473–478. 10.1037/0021-843X.117.2.473 [DOI] [PubMed] [Google Scholar]
- Izard CE (1990). Facial expressions and the regulation of emotions. Journal of Personality and Social Psychology, 58(3), 487–498. 10.1037/0022-3514.58.3.487 [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Murty VP, Tervo-Clemmens B, Foran W, & Luna B (2019). Age-associated deviations of amygdala functional connectivity in youths with psychosis Spectrum disorders: Relevance to psychotic symptoms. American Journal of Psychiatry, 176(3), 196–207. 10.1176/appi.ajp.2018.18040443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AM, Riedel P, Lee J, Reavis EA, & Green MF (2019). Linking resting-state networks and social cognition in schizophrenia and bipolar disorder. Human Brain Mapping, 40(16), 4703–4715. 10.1002/hbm.24731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin M, Kaplan S, Gould F, Pinkham AE, Penn DL, & Harvey PD (2015). Social cognition, social competence, negative symptoms and social outcomes: Inter-relationships in people with schizophrenia. Journal of Psychiatric Research, 68, 254–260. 10.1016/j.jpsychires.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Jang JH, Kim E, Shim G, Park HY, Hong KS, & Kwon JS (2011). Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophrenia Research, 130(1–3), 170–175. 10.1016/j.schres.2011.04.023 [DOI] [PubMed] [Google Scholar]
- Kim M, Hwang WJ, Park J, Kim T, Oh S, & Kwon JS (2021). Neurophysiological correlate of emotion regulation by cognitive reappraisal and its association with psychotic symptoms in early psychosis. Schizophrenia Bulletin, 47(1), 87–96. 10.1093/schbul/sbaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D (2020). The impact of emotion awareness and regulation on psychotic symptoms during daily functioning. NPJ Schizophrenia, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Gill KE, Brucato G, Vakhrusheva J, Arndt L, Gross JJ, & Girgis RR (2016). The impact of emotion awareness and regulation on social functioning in individuals at clinical high risk for psychosis. Psychological Medicine, 46(14), 2907–2918. 10.1017/S0033291716000490 [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, & Gross JJ (2012). Emotion awareness and regulation in individuals with schizophrenia: Implications for social functioning. Psychiatry Research, 200(2–3), 193–201. 10.1016/j.psychres.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U (2014). Neural network of cognitive emotion regulation – An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, & Davidson RJ (2012). Amygdala–prefrontal coupling underlies individual differences in emotion regulation. NeuroImage, 62(3), 1575–1581. 10.1016/j.neuroimage.2012.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Sundag J, Schlier B, & Karow A (2018). The relevance of emotion regulation in explaining why social exclusion triggers paranoia in individuals at clinical high risk of psychosis. Schizophrenia Bulletin, 44(4), 757–767. 10.1093/schbul/sbx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan. (2014). DBT? Skills training manual (2nd ed.). Guilford Publications. [Google Scholar]
- Linehan. (2018). Cognitive-behavioral treatment of borderline personality disorder. Guilford Publications. [Google Scholar]
- Liu H, Tang Y, Womer F, Fan G, Lu T, Driesen N, Ren L, Wang Y, He Y, Blumberg HP, Xu K, & Wang F (2014). Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophrenia Bulletin, 40(2), 469–477. 10.1093/schbul/sbt044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Byrow Y, Outhred T, Das P, & Fritz K (2017). Irritability and internalizing symptoms: Modeling the mediating role of emotion regulation. Journal of Affective Disorders, 211, 144–149. 10.1016/j.jad.2016.12.021 [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Walsh BC & Woods SW (2014). Structured interview for psychosis-risk syndromes. 47. [Google Scholar]
- McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, & Koenen KC (2010). Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depression and Anxiety, 27(12), 1087–1094. 10.1002/da.20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, Niendam TA, Loewy RL, Ventura J, & Cannon TD (2005). The psychosis Prodrome in adolescent patients viewed through the lens of DSM-IV. Journal of Child and Adolescent Psychopharmacology, 15(3), 434–451. 10.1089/cap.2005.15.434 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, … Woods SW (2003). Prodromal assessment with the Structured Interview for prodromal Syndromes and the scale of prodromal symptoms: Predictive validity, Interrater reliability, and training to reliability. Schizophrenia Bulletin, 29(4), 703–715. 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, & Aleman A (2010). Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophrenia Research, 118(1–3), 88–97. 10.1016/j.schres.2010.01.030 [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, & van Os J (2007). Stress-reactivity in psychosis: Evidence for an affective pathway to psychosis. Clinical Psychology Review, 27(4), 409–424. 10.1016/j.cpr.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, & Delespaul PA (2001). Emotional reactivity to daily life stress in psychosis. Archives of General Psychiatry, 58(12), 1137–1144. 10.1001/archpsyc.58.12.1137 [DOI] [PubMed] [Google Scholar]
- Niendam TA, Tully LM, Iosif A-M, Kumar D, Nye KE, Denton JC, … Pierce KM (2018). Enhancing early psychosis treatment using smartphone technology: A longitudinal feasibility and validity study. Journal of Psychiatric Research, 96, 239–246. 10.1016/j.jpsychires.2017.10.017 [DOI] [PubMed] [Google Scholar]
- Nock MK, Wedig MM, Holmberg EB, & Hooley JM (2008). The emotion reactivity scale: Development, evaluation, and relation to self-injurious thoughts and behaviors. Behavior Therapy, 39(2), 107–116. 10.1016/j.beth.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JDE (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Perry Y, Henry JD, & Grisham JR (2011). The habitual use of emotion regulation strategies in schizophrenia: Emotion regulation in schizophrenia. British Journal of Clinical Psychology, 50(2), 217–222. 10.1111/j.2044-8260.2010.02001.x [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, & Dapretto M (2011). Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69(5), 1029–1036. 10.1016/j.neuron.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Liu L, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Bearden CE, Mathalon DH, & Addington J (2016). Social cognition over time in individuals at clinical high risk for psychosis: Findings from the NAPLS-2 cohort. Schizophrenia Research, 171(1–3), 176–181. 10.1016/j.schres.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, & Maiti AK (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2/3), 167–186. 10.2307/1166144 [DOI] [PubMed] [Google Scholar]
- Pozzi E, Vijayakumar N, Rakesh D, & Whittle S (2021). Neural correlates of emotion regulation in adolescents and emerging adults: A meta-analytic study. Biological Psychiatry, 89(2), 194–204. 10.1016/j.biopsych.2020.08.006 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Reininghaus U, Kempton MJ, Valmaggia L, Craig TKJ, Garety P, Onyejiaka A, Gayer-Anderson C, So SH, Hubbard K, Beards S, Dazzan P, Pariante C, Mondelli V, Fisher HL, Mills JG, Viechtbauer W, McGuire P, van Os J, Murray RM, … Morgan C (2016). Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: An experience sampling study. Schizophrenia Bulletin, 42(3), 712–722. 10.1093/schbul/sbv190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler A, Pflueger MO, Aston J, Borgwardt SJ, Brewer WJ, Gschwandtner U, & Stieglitz R-D (2009). Efficacy of using cognitive status in predicting psychosis: A 7-year follow-up. Biological Psychiatry, 66(11), 1023–1030. 10.1016/j.biopsych.2009.07.020 [DOI] [PubMed] [Google Scholar]
- Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, & Harvey PD (2014). Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophrenia Research, 160(1–3), 136–141. 10.1016/j.schres.2014.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff JD, & Knight RA (1978). Young adult schizophrenics: Prediction of outcome and antecedent childhood factors. Journal of Consulting and Clinical Psychology, 46(5), 947–952. 10.1037/0022-006X.46.5.947 [DOI] [PubMed] [Google Scholar]
- Lenth Rusell. (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. Retrieved from https://CRAN.R-project.org/package=emmeans
- Salsman NL, & Linehan MM (2012). An investigation of the relationships among negative affect, difficulties in emotion regulation, and features of borderline personality disorder. Journal of Psychopathology and Behavioral Assessment, 34(2), 260–267. 10.1007/s10862-012-9275-8 [DOI] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, … North American Prodrome Longitudinal Study (NAPLS) Group. (2010). Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Archives of General Psychiatry, 67(6), 578–588. 10.1001/archgenpsychiatry.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, & Woods SW (2016). Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the north American prodrome longitudinal study. JAMA Psychiatry, 73(12), 1239–1248. 10.1001/jamapsychiatry.2016.2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Simpkin AJ, Haycock PC, Dudbridge F, & Zuccolo L (2016). Exploration of a polygenic risk score for alcohol consumption: A longitudinal analysis from the ALSPAC cohort. PLoS One, 11(11), e0167360. 10.1371/journal.pone.0167360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, & Johnson TD (2012). Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biological Psychiatry, 71(2), 136–145. 10.1016/j.biopsych.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A, Silva E, Tavares D, & Freire T (2015). Portuguese validation of the emotion regulation questionnaire for children and adolescents (ERQCA): Relations with self-esteem and life satisfaction. Child Indicators Research, 8(3), 605–621. 10.1007/s12187-014-9266-2 [DOI] [Google Scholar]
- Thompson RA (1994). Emotion regulation: A theme in search of definition. Society for Research in Child Development 29. [PubMed] [Google Scholar]
- Tully LM, Lincoln SH, & Hooker CI (2014). Lateral prefrontal cortex activity during cognitive control of emotion predicts response to social stress in schizophrenia. NeuroImage: Clinical, 6, 43–53. 10.1016/j.nicl.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux JC, Skinner KD, Reese ED, & Shaver JA (2014). Negative affect intensity influences drinking to cope through facets of emotion dysregulation. Personality and Individual Differences, 59, 96–101. 10.1016/j.paid.2013.11.012 [DOI] [Google Scholar]
- Walker F, & Davis M (1993). Of Schizophrenia: Of emotion. American Journal of Psychiatry, 7, 1654–1660. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler abbreviated scale of intelligence: WASI-II (2nd. ed.). Pearson. [Google Scholar]
- Weiss NH, Bold KW, Sullivan TP, Armeli S, & Tennen H (2017). Testing bidirectional associations among emotion regulation strategies and substance use: A daily diary study: Emotion regulation and substance use. Addiction, 112(4), 695–704. 10.1111/add.13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, & McLaughlin KA (2019). Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Development and Psychopathology, 31(3), 899–915. 10.1017/S0954579419000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowitz RL, & Cole DA (2016). Measures of emotion reactivity and emotion regulation: Convergent and discriminant validity. Personality and Individual Differences, 102, 123–132. 10.1016/j.paid.2016.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Opmeer EM, van der Meer L, Aleman A, Ćurčić-Blake B, & Ruhé HG (2018). Altered frontal-amygdala effective connectivity during effortful emotion regulation in bipolar disorder. Bipolar Disorders, 20(4), 349–358. 10.1111/bdi.12611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


