Key Points
Question
Do Panax notoginseng saponins (Xuesaitong soft capsules) improve function in patients with ischemic stroke?
Findings
In this multicenter randomized clinical trial of 3072 patients with ischemic stroke, the proportion of patients achieving functional independence at 3 months with P notoginseng saponins was 89% vs 82% with placebo; the difference was statistically significant.
Meaning
These findings suggest that Panax notoginseng saponins enhance functional independence in patients with ischemic stroke.
This randomized clinical trial examined whether the use of Panax notoginseng saponins was safe and efficacious for improving functional independence in patients with ischemic stroke.
Abstract
Importance
Preclinical and clinical studies have suggested the neuroprotective effect of Panax notoginseng saponins (Xuesaitong soft capsules). However, robust evidence in patients with ischemic stroke is lacking.
Objective
To assess the efficacy and safety of Xuesaitong soft capsules in patients with ischemic stroke.
Design, Setting, and Participants
This multicenter, double-blind, placebo-controlled randomized clinical trial was conducted at 67 tertiary health centers in China from July 1, 2018, to June 30, 2020. Included patients were aged 18 to 75 years with a diagnosis of ischemic stroke and a National Institutes of Health Stroke Scale score between 4 and 15.
Interventions
Eligible patients were randomly assigned within 14 days after symptom onset to receive either treatment with Xuesaitong soft capsules (120 mg orally twice daily) or placebo (120 mg orally twice daily) for 3 months.
Main Outcomes and Measures
The primary outcome was functional independence at 3 months, defined as a modified Rankin Scale score of 0 to 2.
Results
Among 3072 eligible patients with ischemic stroke who were randomized, 2966 (96.5%) were included in the modified intention-to-treat cohort (median [IQR] age, 62 [55-68] years; 1982 male [66.8%]). The number of patients who achieved functional independence at 3 months was 1328 (89.3%) in the Xuesaitong group and 1218 (82.4%) in the control group (odds ratio, 1.95; 95% CI, 1.56-2.44; P < .001). In the safety cohort, serious adverse events occurred in 15 of 1488 patients (1.0%) in the Xuesaitong group and 16 of 1482 (1.1%) in the control group (P = .85).
Conclusions and Relevance
In this randomized clinical trial, Xuesaitong soft capsules significantly increased the likelihood of functional independence at 3 months in patients with ischemic stroke, indicating that this may be a safe and effective alternative therapy to improve prognosis in this population.
Trial Registration
Chinese Clinical Trial Registry Identifier: ChiCTR1800016363
Introduction
Stroke, characterized by high morbidity, disability, and mortality, is the leading cause of death in China, contributing substantially to the global burden of diseases.1 Ischemic stroke accounts for approximately 70% of all strokes. Currently, recanalization therapy via intravenous thrombolysis and mechanical thrombectomy has allowed a great breakthrough in improving the neurologic prognosis of patients with ischemic stroke.2,3 However, recanalization therapy benefits a limited number of patients because of the strict time window and high operational requirements.4 Therefore, the discovery and development of an effective and safe alternative therapy are needed to further improve the prognosis of patients with ischemic stroke.
Panax notoginseng saponins, the bioactive ingredients of P notoginseng, a well-known and highly prized plant used in Chinese medicine, are a class of dammarane tetracyclic triterpene compounds, with the main active components being ginsenoside Rb1, Rd, Rg1, Re, and notoginsenoside R1. Panax notoginseng saponins have been used for the treatment of ischemic diseases since antiquity, and Xuesaitong soft capsules, prepared from P notoginseng saponins, were licensed in 1999 by the National Medical Products Administration in China for the treatment of ischemic stroke. An experimental study5 found that Xuesaitong administration during the acute phase of ischemic stroke significantly reduced the infarct volume and mitigated neurologic impairment in mice models with middle cerebral artery occlusion, suggesting that Xuesaitong soft capsules may be beneficial for stroke recovery. Proposed neuroprotective mechanisms of Xuesaitong soft capsules include antioxidation, anti-inflammation, antiapoptosis, and enhanced angiogenesis.6,7,8,9 A recent systematic review10 with meta-analysis (17 trials; 1942 patients), with a moderate certainty level of evidence, suggested that Xuesaitong soft capsules were significantly associated with favorable clinical outcomes in patients with ischemic stroke. However, there is still an absence of prospective outcome data of large confirmatory trials supporting this evidence. Therefore, this double-blind, placebo-controlled, multicenter study was conducted to determine the efficacy and safety of Xuesaitong soft capsules in addition to standard care in patients with ischemic stroke.
Methods
Trial Design and Patient Population
This double-blind, placebo-controlled randomized clinical trial had an estimated sample size of 3000 patients and was conducted at 67 tertiary health centers in China from July 1, 2018, to June 30, 2020 (ChiCTR1800016363). The study protocol and statistical analysis plan are available in Supplement 1. The trial was approved by the ethics committee of each participating center. The trial organization is given in the eAppendix in Supplement 2. Enrolled patients or their legally authorized representatives provided written informed consent for participation in the trial. The trial was designed and conducted by members of the steering committee and was monitored by an independent data safety monitoring board. The trial was conducted in accordance with the principles of the Declaration of Helsinki11 and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized trials.
Patients were eligible for inclusion in the trial if they were between the ages of 18 and 75 years, had a clinical diagnosis of ischemic stroke within 14 days of symptom onset, had a prestroke score of 0 or 1 on the modified Rankin scale (mRS), and had a score of 4 to 15 on the National Institutes of Health Stroke Scale (NIHSS) at randomization. The main exclusion criteria included intracranial hemorrhage or other nonischemic brain disease, aneurysm, acute coronary syndrome, and any contraindications to use P notoginseng saponins. Details of the inclusion and exclusion criteria are provided in the trial protocol in Supplement 1.
Randomization
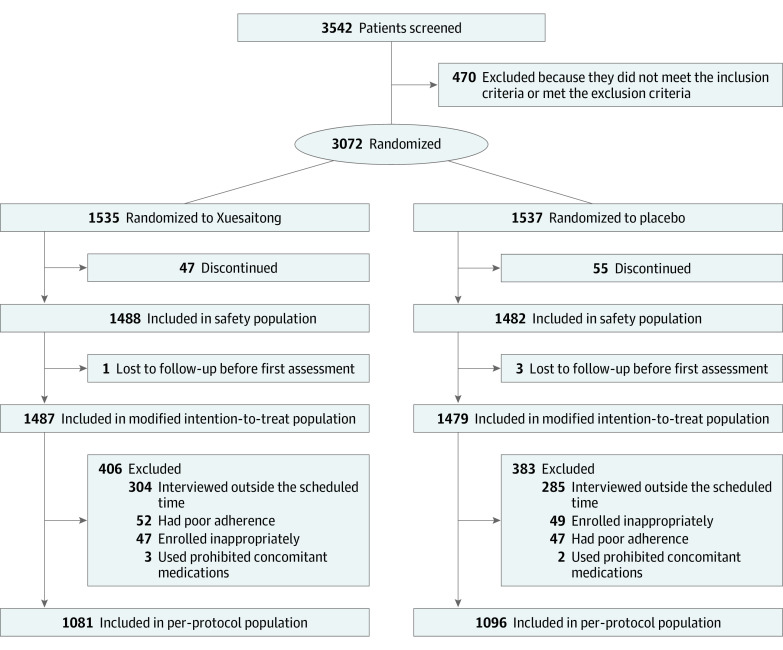
A total of 3542 patients were screened for eligibility, of whom 470 were excluded because they did not meet the inclusion criteria or met exclusion criteria. For patients eligible for randomization, each center assigned randomization codes in strict sequential order. Randomization codes were generated by an automated centralized randomization system. Patients were randomly assigned in a 1:1 ratio to receive Xuesaitong soft capsules (Xuesaitong group) or placebo (control group). Patients randomly assigned to the Xuesaitong group received an oral administration of Xuesaitong (60 mg per capsule; 2 capsules [120 mg] taken twice daily), whereas the patients in the control group received an oral administration of Xuesaitong placebo (60 mg per capsule; 2 capsules [120 mg] taken twice daily). The treatment continued for 3 months.
Outcomes
The primary efficacy outcome was the proportion of patients with functional independence (mRS ≤2) at 3 months after randomization. The mRS is an ordinal scale that ranges from 0 to 6, with 0 to 1 indicating no disability, 2 to 5 indicating a disability (increasing from 2 to 5), and 6 indicating death. Outcomes were obtained by means of structured interviews conducted by investigators certified to assess the mRS scores, who were unaware of the treatment assignments. The secondary efficacy outcomes were as follows: (1) rate of recurrent stroke (ischemic stroke and intracerebral hemorrhage) at 3 months and 12 months; (2) proportion of patients with functional independence at 12 months; (3) proportion of patients with no or minimal disability, defined as an mRS score of 1 or lower, at 3 months and 12 months; (4) NIHSS score change from baseline to 3 months; (5) rate of composite cerebrovascular events (ischemic stroke, intracerebral hemorrhage, myocardial infarction, and vascular death) at 3 months and 12 months; (6) EuroQoL Group 5-Dimension (EQ-5D) score at 3 months and 12 months; (7) Barthel Index change from baseline to 3 months and 12 months; and (8) platelet counts and coagulation indicators (prothrombin time, activated partial thromboplastin time) at 3 months. The primary safety outcome was serious adverse events within 3 months. Other secondary safety outcomes included symptomatic intracranial hemorrhage, all-cause mortality, and adverse events.
Sample Size Calculation
Referring to data from the eligible population in the China National Stroke Registry, assuming that the proportion of patients in the control group with functional independence at 3 months is 45% and the proportion in the Xuesaitong group is relatively increased by 12%, with a significance level of 0.05 and a power of 0.80, 1342 patients were required in each group. Accordingly, considering an attrition rate of 10%, 1492 patients were required in each group. Rounded to an integer, the trial was expected to enroll 3000 patients.
Statistical Analysis
After randomization, all patients who received at least 1 record of treatment and efficacy evaluation were included in the modified intention-to-treat cohort, which was used to analyze the efficacy outcomes of the trial. Additionally, an analysis of the efficacy data was also performed in the per-protocol cohort, which included patients who completed the treatment prescribed in the protocol without major protocol deviations. All patients who received drug treatment were included in the safety cohort for safety outcomes evaluation.
Baseline characteristics were compared between the 2 groups. Descriptive statistics were presented as medians (IQRs) for continuous variables and numbers (percentages) for categorical variables. Intergroup comparisons were conducted by means of a Wilcoxon rank sum test for continuous variables and a χ2 test for categorical variables. Efficacy and safety outcomes were also compared. The χ2 test or Fisher exact test was used to compare the differences of the binary outcomes between the groups, including partial efficacy outcomes (functional independence, no or minimal disability, recurrent stroke, and composite cerebrovascular events) and all safety outcomes. For functional independence and no or minimal disability, binary logistic regression analyses were conducted to calculate odds ratios (ORs) with 95% CIs. For recurrent stroke and composite cerebrovascular events, Cox proportional hazards regression models were established to calculate hazard ratios and 95% CIs. For other efficacy outcomes, including changes in NIHSS score, EQ-5D score, changes in Barthel Index, platelet counts, and coagulation indicators, the t test or Wilcoxon rank sum test was used for intergroup comparison. Additionally, subgroup analyses were predefined based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (large artery atherosclerosis, cardioembolism, small vessel occlusion, other determined cause, or undetermined cause),12 age (<65 or ≥65 years), sex (male or female), diabetes (yes or no), and hypertension (yes or no). The subgroup analyses were performed for the primary outcome using logistic regression with interaction terms between treatment and subgroup.
Statistical significance was set at P ≤ .05 (2-sided). All statistical analyses were prespecified and performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
A total of 3072 eligible patients were randomly assigned to receive Xuesaitong (n = 1535) or placebo (n = 1537). Among the randomized patients, 2970 were included in the safety cohort, 2966 in the modified intention-to-treat cohort, and 2177 in the per-protocol cohort (Figure 1). The baseline demographic and clinical characteristics in the modified intention-to-treat cohort are presented in Table 1. In the modified intention-to-treat cohort, the median age was 62 (IQR, 55-68) years; 1982 patients (66.8%) were men and 984 (33.2%) were women; the median NIHSS score at baseline was 5 (IQR, 4-7); and 263 patients (8.9%) received intravenous thrombolysis and 18 (0.6%) received mechanical thrombectomy.
Figure 1. Participant Flow Diagram.
Table 1. Baseline Characteristics of the Study Participantsa.
| Characteristic | Xuesaitong group (n = 1487) | Control group (n = 1479) |
|---|---|---|
| Age, median (IQR), y | 62 (55-68) | 62 (55-68) |
| Sex | ||
| Male | 957 (64.4) | 1025 (69.3) |
| Female | 530 (35.6) | 454 (30.7) |
| BMI, median (IQR) | 24.5 (22.6-26.6) | 24.5 (22.5-26.6) |
| Heart rate, median (IQR) | 75 (69-80) | 76 (69-80) |
| SBP, median (IQR), mm Hg | 142 (132-156) | 141 (132-157) |
| DBP, median (IQR), mm Hg | 84 (78-92) | 85 (78-93) |
| NIHSS score, median (IQR) | 5 (4-7) | 5 (4-7) |
| EQ-5D score, median (IQR) | 75 (60-86) | 75 (60-90) |
| Barthel Index, median (IQR) | 75 (55-90) | 75 (55-90) |
| Intravenous thrombolysis | 140 (9.4) | 123 (8.3) |
| Mechanical thrombectomy | 12 (0.8) | 6 (0.4) |
| Medical and personal history | ||
| Hypertension | 835 (56.2) | 821 (55.5) |
| Diabetes | 356 (23.9) | 361 (24.4) |
| Hyperlipidemia | 73 (4.9) | 79 (5.3) |
| Current smoking | 356 (23.9) | 385 (26.0) |
| Current drinking | 191 (12.8) | 181 (12.2) |
| Laboratory test results, median (IQR) | ||
| Glucose, mg/dL | 102.70 (90.09-136.94) | 104.50 (90.09-135.14) |
| Total cholesterol, mg/dL | 173.75 (146.72-200.77) | 173.75 (146.72-200.77) |
| Fibrinogen, mg/dL | 98.64 (81.63-115.65) | 98.64 (81.63-115.65) |
| Platelet counts, ×103/μL | 212 (173-252) | 211 (173-250) |
| PT, s | 11.2 (10.5-12.3) | 11.3 (10.5-12.3) |
| APTT, s | 28.1 (24.5-32.4) | 28.0 (24.5-32.3) |
| TOAST classification | ||
| Large artery atherosclerosis | 789 (53.1) | 788 (53.3) |
| Cardioembolism | 15 (1.0) | 21 (1.4) |
| Small vessel occlusion | 618 (41.6) | 611 (41.3) |
| Other determined cause | 23 (1.5) | 16 (1.1) |
| Undetermined cause | 42 (2.8) | 43 (2.9) |
Abbreviations: APTT, activated partial thromboplastin time; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DBP, diastolic blood pressure; EQ-5D, EuroQoL Group 5-Dimension; NIHSS, National Institutes of Health Stroke Scale; PT, prothrombin time; TOAST, Trial of Org 10172 in Acute Stroke Treatment; SBP, systolic blood pressure.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; total cholesterol to millimoles per liter, multiply by 0.0259; fibrinogen to grams per liter, multiply by 0.01; and platelet counts to ×109/L, multiply by 1.
Data are presented as number (percentage) of patients unless otherwise indicated.
Efficacy Outcomes
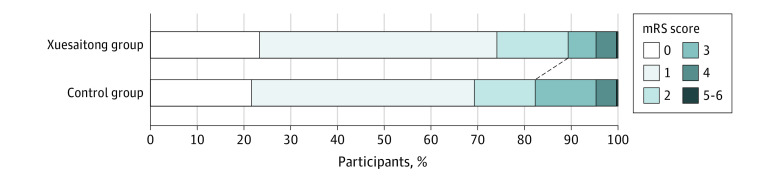
The results of the efficacy outcomes in the modified intention-to-treat cohort are presented in Table 2 and eTable 1 in Supplement 2. The proportion of patients with functional independence at 3 months was 89.3% (n = 1328) in the Xuesaitong group and 82.4% (n = 1218) in the control group (OR, 1.95; 95% CI, 1.56-2.44; P < .001) (Figure 2; eTable 2 in Supplement 2). Similar results were observed in the per-protocol cohort, with 973 patients (90.0%) in the Xuesaitong group and 913 (83.3%) in the control group functionally independent at 3 months (OR, 1.98; 95% CI, 1.51-2.60; P < .001) (eTables 3 and 4 and eFigure 1 in Supplement 2).
Table 2. Efficacy Outcomes at 3 Months in the Modified Intention-to-Treat Cohorta.
| Outcome | Xuesaitong group (n = 1487) | Control group (n = 1479) | OR or HR (95% CI) | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| Functional independenceb | 1328 (89.3) | 1218 (82.4) | 1.95 (1.56 to 2.44) | <.001 |
| Secondary outcomes | ||||
| Recurrent stroke | 14 (0.9) | 17 (1.1) | 0.82 (0.40 to 1.66) | .58 |
| No or minimal disability | 1102 (74.1) | 1025 (69.3) | 1.27 (1.08 to 1.49) | .004 |
| NIHSS score change from baseline to 3 mo, median (IQR)c | −4 (−5 to −3) | −4 (−5 to −3) | NA | .02 |
| Composite cerebrovascular events | 15 (1.0) | 18 (1.2) | 0.83 (0.42 to 1.65) | .59 |
| EQ-5D score, median (IQR)d | 90 (80 to 95) | 90 (80 to 95) | NA | .03 |
| Barthel Index change from baseline to 3 mo, median (IQR)e | 15 (5 to 35) | 15 (5 to 30) | NA | .006 |
| Platelet counts and coagulation indicators, median (IQR)f | ||||
| Platelet counts, ×103/μL | 216 (175 to 256) | 215 (177 to 258) | NA | .94 |
| PT, s | 11.4 (10.7 to 12.4) | 11.3 (10.6 to 12.4) | NA | .71 |
| APTT, s | 28.5 (24.5 to 32.9) | 27.9 (24.7 to 33.1) | NA | .72 |
Abbreviations: APTT, activated partial thromboplastin time; EQ-5D, EuroQoL Group 5-Dimension; HR, hazard ratio; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; PT, prothrombin time; OR, odds ratio.
SI conversion factors: To convert platelet counts to ×109/L, multiply by 1.
Data are presented as number (percentage) of patients unless otherwise indicated.
Twenty-eight patients (1.9%) in the Xuesaitong group and 26 patients (1.8%) in the control group were lost to the 3-month follow-up; the last observed follow-up data were used to replace the 3-month follow-up data based on the last-observation-carried-forward approach.
Data of NIHSS score change from baseline to 3 months were missing in 59 patients.
Data of EQ-5D score at 3 months were missing in 57 patients.
Data of Barthel Index change from baseline to 3 months were missing in 57 patients.
Data of platelet counts, PT, and APTT at 3 months were missing in 353, 402, and 414 patients, respectively. The number of missing data were similar between the 2 groups.
Figure 2. Distribution of the Modified Rankin Scale (mRS) Scores at 3 Months.
Regarding the secondary efficacy outcome, the numbers of patients with no or minimal disability were 1102 (74.1%) in the Xuesaitong group compared with 1025 (69.3%) in the control group at 3 months (OR, 1.27; 95% CI, 1.08-1.49; P = .004) and 1243 (85.6%) in the Xuesaitong group compared with 1191 (82.3%) in the control group at 12 months (OR, 1.27; 95% CI, 1.05-1.53; P = .01). The median NIHSS score change from baseline to 3 months was higher in the Xuesaitong group than that in the control group (−4 [IQR, −5 to −3] vs −4 [IQR, −5 to −3]; P = .02), suggesting that Xuesaitong was associated with greater improvements in neurologic deficits. The median EQ-5D score at 3 months was 90 (IQR, 80-95) in the Xuesaitong group and 90 (IQR, 80-95) in the control group (P = .03). This phenomenon of higher EQ-5D score of the Xuesaitong group was also observed at 12 months, with a median score of 95 (IQR, 90-98) in the Xuesaitong group and 94 (IQR, 90-96) in the control group (P = .009). Additionally, the median Barthel Index change from baseline to 3 months was higher in the Xuesaitong group (15 [IQR, 5-35] vs 15 [IQR, 5-30]; P = .006). Recurrent stroke at 3 and 12 months, functional independence at 12 months, composite cerebrovascular events at 3 months and 12 months, Barthel Index change from baseline to 12 months, and platelet counts and coagulation indicators at 3 months were not significantly different between the 2 groups. Additionally, there were no significant differences in preventive medications and status between the 2 groups at 1 month (eTable 5 in Supplement 2).
Prespecified subgroup analyses are shown in Figure 3. The treatment effects for the primary outcome remained consistent in almost all prespecified subgroups, including those based on the TOAST classification, age, sex, diabetes, and hypertension. All of the variables showed a direction of effect in favor of Xuesaitong. Similar results of subgroup analyses were obtained in the per-protocol cohort (eFigure 2 in Supplement 2).
Figure 3. Subgroup Analyses.
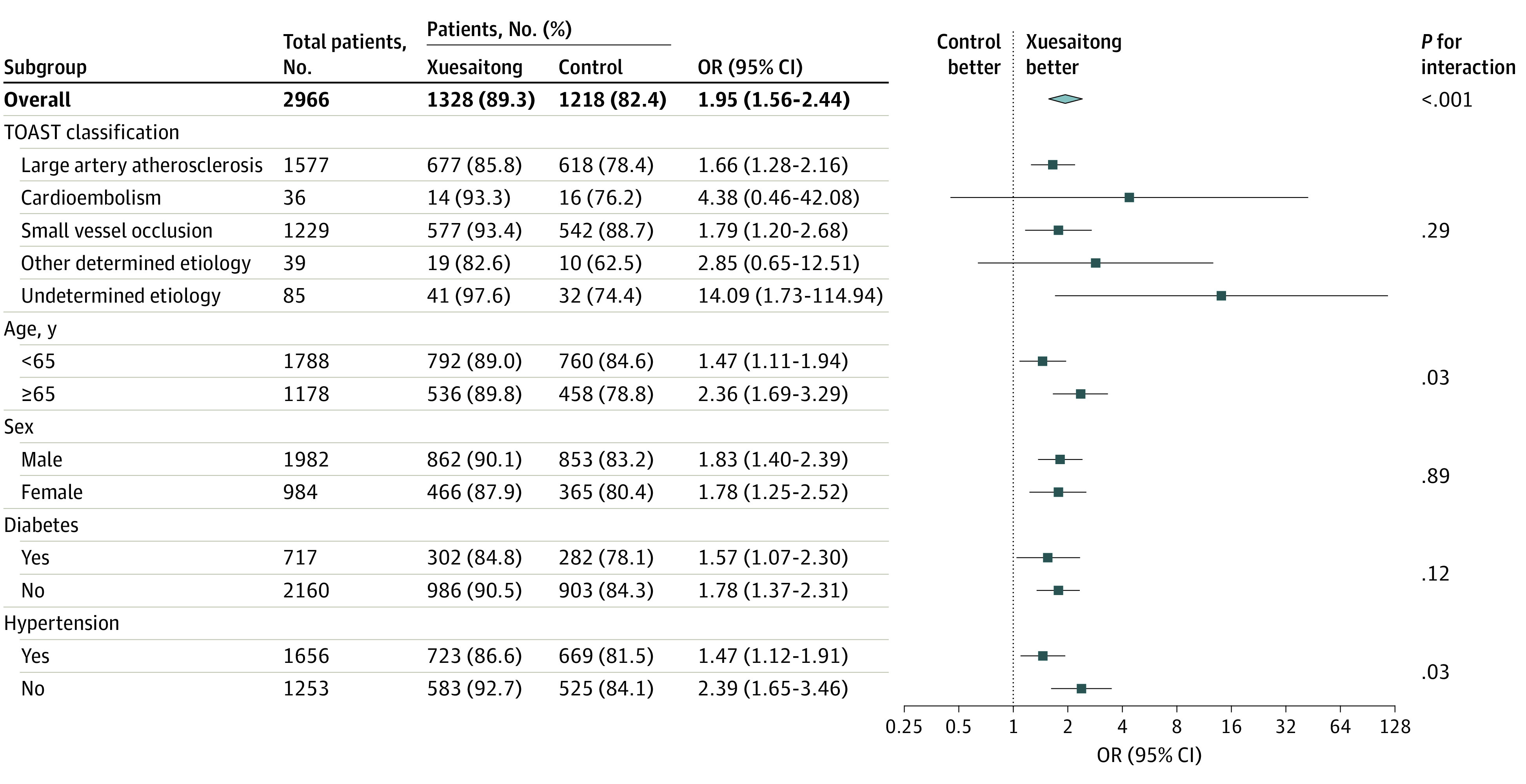
OR indicates odds ratio; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Safety Outcomes
There was no significant difference in safety outcomes between the 2 groups in the safety cohort. The incidence of serious adverse events within 3 months was 1.0% (15 of 1488 patients) in the Xuesaitong group and 1.1% (16 of 1482 patients) in the control group (P = .85). Results of the other secondary safety outcomes, including symptomatic intracranial hemorrhage (0.1% vs 0; P = .32), all-cause mortality (0.1% vs 0.1%; P = .56), and adverse events (2.8% vs 3.6%; P = .20), were similar in the 2 groups.
Discussion
In this randomized clinical trial of patients who had ischemic stroke within 14 days of symptom onset, we found that the proportion of patients with functional independence at 3 months in the Xuesaitong group was higher than that in the placebo group. Additionally, both groups had similar safety outcomes, including serious adverse events within 3 months of treatment. In sum, our findings provide supportive clinical evidence of the application of Xuesaitong in the treatment of patients with ischemic stroke.
A recently published systematic review and meta-analysis10 suggested that Xuesaitong was superior to conventional or other drug treatments in improving the symptoms of patients with stroke and that long-term use of Xuesaitong contributed to the recovery of neurologic function and quality of life in patients with ischemic stroke. However, restricted by the clinical heterogeneity and low quality of the included studies, high-quality evidence from a large-scale, well-designed, controlled randomized clinical trial is required to further confirm its clinical efficacy and safety. To date and to the best of our knowledge, this study is the largest randomized clinical trial to investigate the efficacy and safety of Xuesaitong in the treatment of ischemic stroke. The results suggest that Xuesaitong could provide neuroprotection for patients with ischemic stroke.
Research on neuroprotection for stroke has been ongoing for many years.13 However, many once-promising neuroprotective agents failed to provide the desired neuroprotective benefits against stroke in clinical trials.14 In an experimental study,15 for instance, researchers found that NA-1 (also known as nerinetide or Tat-NR2B9c) could inhibit excitotoxic cell death and subsequent brain damage. Additionally, experiments with rodents and nonhuman primates as focal cerebral ischemia models also implied that NA-1 treatment could reduce infarct volume and alleviate neurologic impairments after ischemia occurs.16,17,18 However, a recently published multicenter, double-blind, controlled randomized clinical trial19 did not support the strong neuroprotective effect of NA-1 found in the laboratory, revealing that NA-1 did not increase the proportion of ischemic stroke cases with favorable outcomes following mechanical thrombectomy compared with those receiving placebo. Natalizumab is a humanized monoclonal antibody to cell adhesion glycoprotein α4 integrin, which acts as an anti-inflammatory by inhibiting the migration of mononuclear leukocytes through the endothelium into the brain parenchyma.20,21 Although α4 integrin inhibition has been demonstrated in preclinical studies to provide neuroprotective benefits in models of focal cerebral ischemia,22,23 the results from a clinical trial did not support the efficacy of natalizumab in treating patients with ischemic stroke.24 Furthermore, translation from preclinical to clinical studies has been difficult in other neuroprotective agents, such as albumin, uric acid, and magnesium sulfate.25,26,27,28,29,30 The potential neuroprotective agents mentioned above shared a common feature: single-agent therapy. Brain tissue damage following cerebral ischemia develops from the interaction of complex pathophysiologic processes in time and space, involving numerous injury mechanisms and molecular events.31 On the basis of this finding, a single neuroprotective agent targeting a single injury mechanism or molecular event will not provide the expected efficacy, whereas multiagent therapy might produce clinically effective neuroprotection.32,33
Panax notoginseng saponins are composed of multiple active components, which is perhaps the main difference with other neuroprotective agents. Panax notoginseng saponins contain approximately 20 different saponins, among which ginsenoside Rb1, Rd, Rg1, Re, and notoginsenoside R1 are the top 5 saponins, accounting for 90% of the total P notoginseng saponins.34 Different saponins act with different neuroprotective mechanisms. For example, a recent study35 found that ginsenoside Rb1 inhibits astrocyte activation and promotes the transfer of astrocyte mitochondria to neurons to combat ischemic stroke. Ginsenoside Rg1 alleviates cerebral ischemia-reperfusion injury by inhibiting oxidative stress, calcium overload, and neuroinflammation.36 Notoginsenoside R1 ameliorates blood-brain barrier permeability after ischemic stroke by intervening in tight junction degradation and redistribution.37 We deduce that the positive results of this trial may be attributed to the synergistic neuroprotective effects of different active components of P notoginseng saponins on ischemic brain tissue.
Limitations
Our trial has some limitations. First, we expected to include patients with mild to moderate ischemic stroke with baseline NIHSS scores ranging from 4 to 15, but most of the included patients had mild stroke (median baseline NIHSS score of 5 in both groups), resulting in a higher-than-expected proportion of patients functionally independent at 3 months. Second, despite the study being based on a large sample population, there was a statistically significant difference in sex between the 2 groups at baseline. However, considering that women with ischemic stroke are less likely to have favorable outcomes at 3 months in China,38 the better outcome in the Xuesaitong group (with a higher proportion of women) seems to emphasize the strong neuroprotective effects of the agent. Additionally, the patient population in this trial differed from those in other stroke trials, mainly in the predominance of Han Chinese, lower age, higher proportion of men, and lower proportion of cardioembolism; thus, the generalizability of the results may be limited. Furthermore, most patients with ischemic stroke in this trial did not receive intravenous thrombolysis or mechanical thrombectomy. However, given the increasing popularity of recanalization therapy, further research on the efficacy and safety of neuroprotective agents based on successful recanalization is warranted.
Conclusions
In this randomized clinical trial, Xuesaitong soft capsules were associated with increased functional independence at 3 months among patients with ischemic stroke within 14 days of onset, without increasing the risk of safety events. These findings indicate that Xuesaitong soft capsules may be a safe and effective alternative therapy to improve prognosis in patients with ischemic stroke.
Trial Protocol and Statistical Analysis Plan
eTable 1. Efficacy Outcomes at 12 Months in the Modified Intention-to-Treat Cohort
eTable 2. Distribution of the Modified Rankin Scale at 3 Months in the Modified Intention-to-Treat Cohort
eTable 3. Efficacy Outcomes at 3 Months in the Per-Protocol Cohort
eTable 4. Efficacy Outcomes at 12 Months in the Per-Protocol Cohort
eTable 5. Medications and Status at 1 Month in the Modified Intention-to-Treat Cohort
eFigure 1. Distribution of the Modified Rankin Scale at 3 Months in the Per-Protocol Cohort
eFigure 2. Subgroup Analyses in the Per-Protocol Cohort
eAppendix. Trial Organization
Data Sharing Statement
References
- 1.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251-272. doi: 10.1016/S0140-6736(15)00551-6 [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Dirks M, Niessen LW, van Wijngaarden JD, et al. ; PRomoting ACute Thrombolysis in Ischemic StrokE (PRACTISE) Investigators . Promoting thrombolysis in acute ischemic stroke. Stroke. 2011;42(5):1325-1330. doi: 10.1161/STROKEAHA.110.596940 [DOI] [PubMed] [Google Scholar]
- 5.Li F, Zhao H, Han Z, et al. Xuesaitong may protect against ischemic stroke by modulating microglial phenotypes and inhibiting neuronal cell apoptosis via the STAT3 signaling pathway. CNS Neurol Disord Drug Targets. 2019;18(2):115-123. doi: 10.2174/1871527317666181114140340 [DOI] [PubMed] [Google Scholar]
- 6.Zhou N, Tang Y, Keep RF, Ma X, Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21(10):1189-1195. doi: 10.1016/j.phymed.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi X, Yu W, Liu L, et al. Panax notoginseng saponins administration modulates pro- /anti-inflammatory factor expression and improves neurologic outcome following permanent MCAO in rats. Metab Brain Dis. 2017;32(1):221-233. doi: 10.1007/s11011-016-9901-3 [DOI] [PubMed] [Google Scholar]
- 8.Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 2009;121(3):412-418. doi: 10.1016/j.jep.2008.10.042 [DOI] [PubMed] [Google Scholar]
- 9.Hui Z, Sha DJ, Wang SL, et al. Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement Altern Med. 2017;17(1):70. doi: 10.1186/s12906-017-1579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, Chen Z, Li X, et al. The efficacy and safety of the Xuesaitong soft capsule in the treatment of patients with ischemic stroke: systematic review and meta-analysis. Ann Palliat Med. 2022;11(8):2695-2708. doi: 10.21037/apm-22-748 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST (Trial of Org 10172 in Acute Stroke Treatment). Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 13.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 Experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467-477. doi: 10.1002/ana.20741 [DOI] [PubMed] [Google Scholar]
- 14.Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. doi: 10.1016/j.expneurol.2020.113518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarts M, Liu Y, Liu L, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298(5594):846-850. doi: 10.1126/science.1072873 [DOI] [PubMed] [Google Scholar]
- 16.Sun HS, Doucette TA, Liu Y, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39(9):2544-2553. doi: 10.1161/STROKEAHA.107.506048 [DOI] [PubMed] [Google Scholar]
- 17.Bråtane BT, Cui H, Cook DJ, Bouley J, Tymianski M, Fisher M. Neuroprotection by freezing ischemic penumbra evolution without cerebral blood flow augmentation with a postsynaptic density-95 protein inhibitor. Stroke. 2011;42(11):3265-3270. doi: 10.1161/STROKEAHA.111.618801 [DOI] [PubMed] [Google Scholar]
- 18.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483(7388):213-217. doi: 10.1038/nature10841 [DOI] [PubMed] [Google Scholar]
- 19.Hill MD, Goyal M, Menon BK, et al. ; ESCAPE-NA1 Investigators . Efficacy and Safety of Nerinetide for the Treatment of Acute Ischaemic Stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878-887. doi: 10.1016/S0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 20.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336-1342. doi: 10.1212/01.WNL.0000158329.30470.D0 [DOI] [PubMed] [Google Scholar]
- 21.Rudick RA, Sandrock A. Natalizumab: alpha 4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother. 2004;4(4):571-580. doi: 10.1586/14737175.4.4.571 [DOI] [PubMed] [Google Scholar]
- 22.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32(1):206-211. doi: 10.1161/01.STR.32.1.206 [DOI] [PubMed] [Google Scholar]
- 23.Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32(1):199-205. doi: 10.1161/01.STR.32.1.199 [DOI] [PubMed] [Google Scholar]
- 24.Elkins J, Veltkamp R, Montaner J, et al. Safety and Efficacy of Natalizumab in Patients With Acute Ischaemic Stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16(3):217-226. doi: 10.1016/S1474-4422(16)30357-X [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg MD, Palesch YY, Hill MD, et al. ; ALIAS and Neurological Emergencies Treatment Trials (NETT) Investigators . High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12(11):1049-1058. doi: 10.1016/S1474-4422(13)70223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Xiao Z. Albumin therapy for acute ischemic stroke: a meta-analysis. Neurol Sci. 2021;42(7):2713-2719. doi: 10.1007/s10072-021-05244-9 [DOI] [PubMed] [Google Scholar]
- 27.Aliena-Valero A, Baixauli-Martín J, Castelló-Ruiz M, Torregrosa G, Hervás D, Salom JB. Effect of uric acid in animal models of ischemic stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2021;41(4):707-722. doi: 10.1177/0271678X20967459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamorro A, Amaro S, Castellanos M, et al. ; URICO-ICTUS Investigators . Safety and Efficacy of Uric Acid in Patients With Acute Stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453-460. doi: 10.1016/S1474-4422(14)70054-7 [DOI] [PubMed] [Google Scholar]
- 29.Saver JL, Starkman S, Eckstein M, et al. ; FAST-MAG Investigators and Coordinators . Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372(6):528-536. doi: 10.1056/NEJMoa1408827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avgerinos KI, Chatzisotiriou A, Haidich AB, Tsapas A, Lioutas VA. Intravenous magnesium sulfate in acute stroke. Stroke. 2019;50(4):931-938. doi: 10.1161/STROKEAHA.118.021916 [DOI] [PubMed] [Google Scholar]
- 31.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391-397. doi: 10.1016/S0166-2236(99)01401-0 [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, Wu C, Dornbos D III, et al. Multiphase adjuvant neuroprotection: a novel paradigm for improving acute ischemic stroke outcomes. Brain Circ. 2020;6(1):11-18. doi: 10.4103/bc.bc_58_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong XY, Liu L, Yang QW. Refocusing neuroprotection in cerebral reperfusion era: new challenges and strategies. Front Neurol. 2018;9:249. doi: 10.3389/fneur.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu J, Xu N, Zhang J, Geng X, Zhang R. Panax notoginseng saponins and their applications in nervous system disorders: a narrative review. Ann Transl Med. 2020;8(22):1525. doi: 10.21037/atm-20-6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni XC, Wang HF, Cai YY, et al. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022;54:102363. doi: 10.1016/j.redox.2022.102363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y, Li X, Yang L, et al. Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J Ginseng Res. 2022;46(4):515-525. doi: 10.1016/j.jgr.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Li Y, Han Y, et al. Notoginsenoside R1 intervenes degradation and redistribution of tight junctions to ameliorate blood-brain barrier permeability by Caveolin-1/MMP2/9 pathway after acute ischemic stroke. Phytomedicine. 2021;90:153660. doi: 10.1016/j.phymed.2021.153660 [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Wang CJ, Gu HQ, et al. Sex differences in short-term and long-term outcomes among patients with acute ischemic stroke in China. Stroke. 2022;53(7):2268-2275. doi: 10.1161/STROKEAHA.121.037121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Efficacy Outcomes at 12 Months in the Modified Intention-to-Treat Cohort
eTable 2. Distribution of the Modified Rankin Scale at 3 Months in the Modified Intention-to-Treat Cohort
eTable 3. Efficacy Outcomes at 3 Months in the Per-Protocol Cohort
eTable 4. Efficacy Outcomes at 12 Months in the Per-Protocol Cohort
eTable 5. Medications and Status at 1 Month in the Modified Intention-to-Treat Cohort
eFigure 1. Distribution of the Modified Rankin Scale at 3 Months in the Per-Protocol Cohort
eFigure 2. Subgroup Analyses in the Per-Protocol Cohort
eAppendix. Trial Organization
Data Sharing Statement