Key Points
Question
In patients with acute ischemic stroke due to large vessel occlusion selected to receive endovascular thrombectomy (EVT), does recent exposure to vitamin K antagonists (VKAs) increase the risk of symptomatic intracranial hemorrhage?
Findings
In this retrospective, registry-based study that included 32 715 patients with acute ischemic stroke treated with EVT, symptomatic intracranial hemorrhage occurred in 6.8% of patients with recent use of a VKA and in 6.4% of patients with no preceding use of an oral anticoagulant, a difference that was not statistically significant after multivariable adjustment. However, the subgroup of patients with recent use of a VKA and a presenting international normalized ratio greater than 1.7 had a significantly increased risk of symptomatic intracranial hemorrhage (8.3%) compared with those taking no oral anticoagulant.
Meaning
Among patients with acute ischemic stroke selected to undergo EVT, recent use of a VKA was not significantly associated with an increased risk of symptomatic intracranial hemorrhage overall, although the risk was significantly increased among the subgroup of patients with recent VKA use and an elevated international normalized ratio at the time of presentation.
Abstract
Importance
Use of oral vitamin K antagonists (VKAs) may place patients undergoing endovascular thrombectomy (EVT) for acute ischemic stroke caused by large vessel occlusion at increased risk of complications.
Objective
To determine the association between recent use of a VKA and outcomes among patients selected to undergo EVT in clinical practice.
Design, Setting, and Participants
Retrospective, observational cohort study based on the American Heart Association’s Get With the Guidelines–Stroke Program between October 2015 and March 2020. From 594 participating hospitals in the US, 32 715 patients with acute ischemic stroke selected to undergo EVT within 6 hours of time last known to be well were included.
Exposure
VKA use within the 7 days prior to hospital arrival.
Main Outcome and Measures
The primary end point was symptomatic intracranial hemorrhage (sICH). Secondary end points included life-threatening systemic hemorrhage, another serious complication, any complications of reperfusion therapy, in-hospital mortality, and in-hospital mortality or discharge to hospice.
Results
Of 32 715 patients (median age, 72 years; 50.7% female), 3087 (9.4%) had used a VKA (median international normalized ratio [INR], 1.5 [IQR, 1.2-1.9]) and 29 628 had not used a VKA prior to hospital presentation. Overall, prior VKA use was not significantly associated with an increased risk of sICH (211/3087 patients [6.8%] taking a VKA compared with 1904/29 628 patients [6.4%] not taking a VKA; adjusted odds ratio [OR], 1.12 [95% CI, 0.94-1.35]; adjusted risk difference, 0.69% [95% CI, −0.39% to 1.77%]). Among 830 patients taking a VKA with an INR greater than 1.7, sICH risk was significantly higher than in those not taking a VKA (8.3% vs 6.4%; adjusted OR, 1.88 [95% CI, 1.33-2.65]; adjusted risk difference, 4.03% [95% CI, 1.53%-6.53%]), while those with an INR of 1.7 or lower (n = 1585) had no significant difference in the risk of sICH (6.7% vs 6.4%; adjusted OR, 1.24 [95% CI, 0.87-1.76]; adjusted risk difference, 1.13% [95% CI, −0.79% to 3.04%]). Of 5 prespecified secondary end points, none showed a significant difference across VKA-exposed vs VKA-unexposed groups.
Conclusions and Relevance
Among patients with acute ischemic stroke selected to receive EVT, VKA use within the preceding 7 days was not associated with a significantly increased risk of sICH overall. However, recent VKA use with a presenting INR greater than 1.7 was associated with a significantly increased risk of sICH compared with no use of anticoagulants.
This retrospective cohort study assesses the association between recent use of oral vitamin K antagonists and symptomatic intracranial hemorrhage among patients with acute ischemic stroke undergoing endovascular thrombectomy.
Introduction
Endovascular thrombectomy (EVT) is the mainstay of treatment for patients with acute ischemic stroke due to large vessel occlusion.1 This was demonstrated in 10 randomized clinical trials that showed that EVT was effective and associated with a low risk of complications.2,3,4,5,6,7,8,9,10,11 However, the risk of symptomatic intracranial hemorrhage (sICH) in the setting of recent oral vitamin K antagonist (VKA) administration has not been well delineated among patients selected for EVT in routine practice.
Although the deployment of EVT in patients exposed to VKAs did not raise safety concerns in the randomized trials comparing this therapy with best medical management, such patients constituted only a small proportion of enrollees. Despite this lack of evidence, the 2019 acute stroke management guidelines do not identify VKA use or the international normalized ratio (INR; a laboratory index of the anticoagulant effect of VKAs) as decision-driving parameters.12 Investigating the safety of EVT in the setting of VKA use in clinical practice is desirable because these patients may have more comorbidities than clinical trial participants, therapeutic anticoagulation may create procedural challenges, and a larger data set may be capable of detecting smaller effects.
Several prior studies13,14,15 addressed this question, with conflicting results and estimates of the rate of sICH after EVT from 2.9%14 to 12.5%.15 Owing to the small number of patients in previous studies, there was a limited opportunity for multivariable adjustment, which is critical in evaluating outcomes in observational data. The present study addresses this knowledge gap by using population-based data from the American Heart Association’s Get With the Guidelines–Stroke (GWTG-Stroke) registry. The objective of this study was to determine whether recent use of a VKA was associated with an increased risk of sICH in patients selected for treatment with EVT.
Methods
Study Design
This was a retrospective, observational cohort study using data from the GWTG-Stroke registry in the US between October 2015 and March 2020. The study period was chosen to coincide with the widespread adoption of EVT across the US after publication of the seminal EVT trials in 2015. Given that data were collected for quality improvement purposes, rather than primarily for research, data sharing agreements require an application process in order for other researchers to access the data. The requirement for informed consent was waived by the institutional review board of the Duke University School of Medicine (Durham, North Carolina). Each participating site receives either (1) human subjects research approval to enroll patients with a waiver of written informed consent under the Common Rule (45 CFR §46) or (2) a waiver of authorization and exemption from subsequent review by their institutional review board.
Study Setting
GWTG-Stroke is a nationwide registry and quality improvement initiative under the auspices of the American Heart Association/American Stroke Association. The full details of registry stewardship, data collection, and data utilization processes have been published in-depth previously.16 To summarize, trained hospital personnel use a patient management tool to collect data on consecutive patients with acute ischemic stroke admitted to each participating hospital. Standardized data available in the registry include demographics, medical history, medication exposures prior to admission, diagnostic testing (including cerebral imaging), acute therapies, in-hospital treatment, and complications. There is a high degree of accuracy and concordance between data entered and medical record review.17 IQVIA (Parsippany, New Jersey) serves as the data collection and coordination center. The Duke Clinical Research Institute (Durham, North Carolina) serves as the data analysis center and has an agreement to analyze the aggregate deidentified data for research purposes.
Study Population
This study included patients with acute ischemic stroke selected to undergo EVT and presenting to GWTG-Stroke hospitals between October 1, 2015, and March 31, 2020. Patients were excluded if they were taking any non-VKA anticoagulant or combination of anticoagulants (a direct oral anticoagulant [dabigatran, rivaroxaban, apixaban, or edoxaban], argatroban, desirudin, fondaparinux, low-molecular-weight heparin, lepirudin, unfractionated heparin, or another specified or unspecified anticoagulant). Patients were also excluded if they developed stroke symptoms in the hospital, were transferred out, received EVT at an outside hospital, or were missing information on discharge status. To restrict the study to patients who met guideline-based practice for EVT treatment throughout the duration of the study period, we also excluded patients presenting greater than 6 hours from time last known to be well or who had a presenting National Institutes of Health Stroke Scale (NIHSS) score of less than 6 or missing score data.
Exposures
The primary exposure was recent use of a VKA prior to stroke vs no VKA use. Use of a VKA was defined as documentation within the medical chart that a patient had taken “warfarin,” “warfarin sodium,” “Coumadin,” or “Jantoven” within the 7 days prior to hospital arrival. We also assessed the association between INR (dichotomized as ≤1.7 and >1.7) and study end points. The threshold of 1.7 was chosen a priori to connote a clinically significant anticoagulant effect because this is the value above which the thrombolytic therapy is contraindicated.12 We then identified concomitant receipt of intravenous tissue plasminogen activator (tPA) as an additional exposure stratified across subgroups based on the INR threshold of 1.7.
End Points
The primary end point was sICH, defined as evidence of new intracranial hemorrhage on computed tomography within 36 hours of EVT, supported by a physician’s notation indicating an accompanying clinical deterioration that was due to the hemorrhage. This definition has previously been used in GWTG-Stroke analyses.18 It closely approximates the European Cooperative Acute Stroke Study (ECASS) III19 definition of sICH except that it does not mandate a 4-point increase in the NIHSS score as part of the definition of clinical deterioration. Our secondary end points included life-threatening or serious systemic hemorrhage (defined as any hemorrhage within 36 hours of EVT necessitating ≥3 units of packed red blood cells), other serious complications that required additional medical interventions or prolonged length of stay, any complications of reperfusion therapy (ie, any of the first 3 end points), in-hospital mortality, and a composite of in-hospital mortality or discharge to hospice. Exploratory end points included ambulatory status at discharge (restricted to the subgroup of patients who were ambulatory prior to the current event), freedom from disability (modified Rankin Scale score of 0-1 [vs 2-6] at discharge), functional independence (modified Rankin Scale score of 0-2 [vs 3-6] at discharge), and discharge location (home, inpatient rehabilitation facility, or skilled nursing facility). All patients who died in the hospital were assigned a modified Rankin Scale score of 6. Types of hemorrhagic complications on brain imaging were also reported.
Statistical Analysis
This was a prespecified analysis based on an existing nationwide stroke registry. Descriptive statistics, including medians with IQRs for continuous variables and counts with percentages for categorical variables, were used. The descriptive statistics presented in the text are the raw, unweighted statistics (weighted estimates are included in eTables 1 and 2 in Supplement 1). A propensity score overlap weighting analysis was used to determine the independent association between VKA use and study end points. To create propensity scores, a multivariable logistic regression model was created with VKA use vs no VKA use as the dependent variable. Model covariates were chosen a priori (and documented in the prespecified statistical analysis plan) and included key demographic, clinical, and hospital-level factors expected to be associated with stroke outcome. Patient characteristics included age, biological sex, race and ethnicity, insurance status, medical history (smoking, diabetes, coronary artery disease/myocardial infarction, dyslipidemia, atrial fibrillation, prior stroke, prior transient ischemic attack, heart failure, hypertension, peripheral vascular disease, carotid stenosis, prosthetic cardiac valve, and kidney failure), medications prior to stroke (antiplatelets, antihypertensives, lipid-lowering agents, diabetes medications), intravenous tPA use at either the treating hospital or an outside hospital, initial NIHSS score, time last known to be well to EVT initiation time, systolic blood pressure, and serum glucose. We chose to include race and ethnicity in this study because of known inequities in stroke care across racial and ethnic groups.20 Within the GWTG-Stroke registry, data on race and ethnicity are ascertained by staff from various sources, including patient self-designation recorded by administrative personnel during the registration process or on a nursing intake form. The patient management tool within GWTG-Stroke supports a multiple selection option that includes both racial and ethnic categories. Hospital characteristics included stroke center certification status (primary stroke center vs comprehensive stroke center vs no formal certification), academic status, annual ischemic stroke volume, annual EVT volume, number of hospital beds, geographic region, and rural/urban status.
Overlap weighting21,22,23 is a propensity score weighting method that was applied to facilitate exact balance of covariates between patients who were and were not exposed to VKAs. This method assigns to each patient a weight that is proportional to their probability of receiving the alternative treatment option given their baseline characteristics. Odds ratios (ORs) with corresponding 95% CIs were calculated for the primary, secondary, and exploratory end points using a multivariable logistic regression model with generalized estimating equations to account for within-hospital clustering on the propensity-weighted population. Additionally, adjusted risk differences were calculated from the model by assuming an identity link function. Using similar statistical methodology, the analysis was replicated in patients stratified into those who did and did not receive intravenous tPA. The role of presenting INR was addressed in 3 ways. First, the primary analysis was replicated in patients with VKA use dichotomized into those with an INR of 1.7 or lower vs greater than 1.7, comparing end points in each of those subgroups with patients with no VKA use. Second, we explored the association between VKA (with INRs ≤1.7 and >1.7) and receipt of intravenous tPA on the end points of interest. Third, among patients taking a VKA, separate logistic regression models were created to examine the adjusted association between a continuous measure of INR and each binary end point. We used the Stone and Koo additive spline method24 to fit INR as a spline with 4 knots and depicted this graphically.
In general, patient- and hospital-level data were either complete or had small missing rates. However, INR was missing in 21.7% of patients taking VKAs. INR was not included as an adjustment covariate in the multivariable models. Furthermore, those with missing INR were excluded from subgroup analyses (INR ≤1.7 vs INR >1.7). Hospital-level data were complete for all hospital-level characteristics except for number of hospital beds, which had a missing rate of 2.1%. For patient-level variables with missing values, imputation was used, with imputed values obtained by the fully conditional specification method. Patients with missing data on hospital-level variables and/or end points were not imputed and were excluded from the models.
A 2-sided α = .05 was used to connote significance for all statistical tests. The point estimates and corresponding 95% CIs for the secondary and exploratory end points should be considered as hypothesis generating only. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 4.0.2 (R Foundation). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)25 checklist is included as an eAppendix in Supplement 1.
Results
Baseline Characteristics
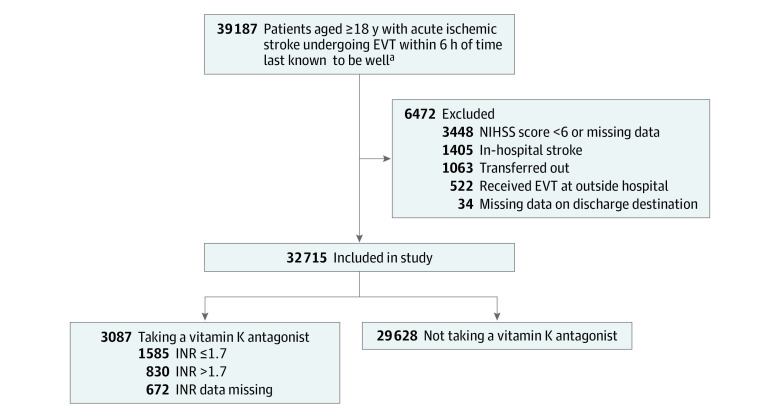
A total of 32 715 patients with acute ischemic stroke (median age, 72 years [IQR, 60-82 years]; 50.7% female) selected to undergo EVT at 594 GWTG-Stroke hospitals between October 1, 2015, and March 31, 2020, were included in the study. Figure 1 presents the flow of the study population. In total, 29 628 patients (90.6%) were not taking a VKA prior to experiencing a stroke and 3087 patients (9.4%) were taking a VKA. Table 1 summarizes key clinical and demographic characteristics (hospital-level characteristics are summarized in eTable 3 in Supplement 1). Those taking a VKA were older (median, 78 vs 71 years), less likely to receive intravenous tPA (42.5% vs 78.6%) or have concomitant antiplatelet use (28.1% vs 39.5%), and more likely to have atrial fibrillation (82% vs 25.5%), coronary artery disease (32.5% vs 22.2%), heart failure (26.2% vs 11.3%), and prior stroke (26.2% vs 16.3%). Stroke severity, measured by the NIHSS, was slightly higher in the VKA group (median NIHSS score of 18 [IQR, 14-23] vs 17 [IQR, 13-22]). In contrast, onset-to-EVT times (median, 209 [IQR, 154-265] minutes vs 205 [IQR, 152-268] minutes), and the distribution of sites of arterial occlusion did not differ substantially between the groups. Baseline characteristics of patients in the study registry taking a VKA and potentially eligible for EVT (NIHSS score ≥6 and presenting within 6 hours of time last known to be well), divided into those selected and not selected for EVT, are presented in eTable 4 in Supplement 1.
Figure 1. Flow of Patients Included in the Study.
EVT indicates endovascular therapy; INR, international normalized ratio; NIHSS, National Institutes of Health Stroke Scale. Trained hospital personnel use a patient management tool to collect data on consecutive patients with acute ischemic stroke admitted to each participating hospital.
aPatients with recent use of vitamin K antagonist or no use of any anticoagulant prior to stroke.
Table 1. Baseline Characteristics of the Study Populationa.
| Characteristics | Use of vitamin K antagonists (n = 3087)b | No use of vitamin K antagonists (n = 29 628) |
|---|---|---|
| Age, median (IQR), y | 78 (69-85) | 71 (60-81) |
| Sex, No. (%) | ||
| Female | 1655 (53.6) | 14 937 (50.4) |
| Male | 1432 (46.4) | 14 691 (49.6) |
| Race and ethnicity, No. (%)c | ||
| Asian | 87 (2.8) | 934 (3.2) |
| Non-Hispanic Black | 372 (12.1) | 4496 (15.2) |
| Hispanic | 194 (6.3) | 2111 (7.1) |
| Non-Hispanic White | 2249 (72.9) | 20 188 (68.1) |
| Otherd | 185 (6.0) | 1899 (6.4) |
| Health insurance status, No. (%)e | ||
| Medicare | 1602 (52.8) | 12 494 (44.1) |
| Private | 1112 (36.6) | 11 481 (40.5) |
| Medicaid | 248 (8.2) | 2892 (10.2) |
| Self-pay/no insurance | 66 (2.2) | 1372 (4.8) |
| Not documented | 8 (0.3) | 118 (0.4) |
| Admission year, No. (%) | ||
| 2015 | 119 (3.9) | 976 (3.3) |
| 2016 | 582 (18.9) | 4790 (16.2) |
| 2017 | 755 (24.5) | 6363 (21.5) |
| 2018 | 783 (25.4) | 7625 (25.7) |
| 2019 | 763 (24.7) | 8711 (29.4) |
| 2020 | 85 (2.8) | 1163 (3.9) |
| Medical history, No. (%) | ||
| Atrial fibrillation/flutter | 2530 (82.0) | 7561 (25.5) |
| Hypertension | 2404 (77.9) | 20 696 (69.9) |
| Dyslipidemia | 1579 (51.1) | 12 418 (41.9) |
| Coronary artery disease/prior myocardial infarction | 1002 (32.5) | 6566 (22.2) |
| Diabetes | 860 (27.9) | 7212 (24.3) |
| Heart failure | 809 (26.2) | 3350 (11.3) |
| Prior stroke | 809 (26.2) | 4841 (16.3) |
| Prosthetic heart valve | 347 (11.2) | 358 (1.2) |
| Chronic kidney disease | 316 (10.2) | 1998 (6.7) |
| Smoking | 274 (8.9) | 5176 (17.5) |
| Prior transient ischemic attack | 226 (7.3) | 1406 (4.7) |
| Peripheral vascular disease | 177 (5.7) | 936 (3.2) |
| Carotid stenosis | 94 (3.0) | 762 (2.6) |
| Medications prior to admission, No. (%) | ||
| Any antiplatelet medication | 867 (28.1) | 11 701 (39.5) |
| Single antiplatelet medication | 815 (26.4) | 10 072 (34.0) |
| Dual antiplatelet medication | 49 (1.6) | 1601 (5.4) |
| Antihypertensive agent | 2324 (84.5) | 15 562 (59.0) |
| Lipid-lowering agent | 1715 (55.6) | 11 295 (38.1) |
| Oral hypoglycemic agent | 571 (22.4) | 4238 (16.9) |
| Key clinical characteristics | ||
| Body mass index, median (IQR)f | 27.4 (23.7-32.0) | 27.7 (24.2-32.3) |
| Systolic blood pressure, median (IQR), mm Hg | 148 (130-167) | 148 (131-168) |
| Diastolic blood pressure, median (IQR), mm Hg | 82 (71-94) | 82 (71-95) |
| Heart rate, median (IQR), /min | 82 (70-96) | 80 (70-93) |
| Serum glucose, median (IQR), mg/dL | 123 (105-151) | 123 (105-153) |
| International normalized ratio | ||
| Median (IQR) | 1.5 (1.2-1.9) | 1.1 (1.0-1.1) |
| >1.7, No./total (%) | 830/2415 (34.4) | 257/21 456 (1.2) |
| Serum creatinine, median (IQR), mg/dL | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) |
| Ambulatory status, No. (%) | ||
| Prior to admission | ||
| Able to ambulate independently | 2186 (93.1) | 21 440 (95.2) |
| With assistance from another person | 114 (4.9) | 713 (3.2) |
| Unable to ambulate | 47 (2.0) | 379 (1.7) |
| At admission | ||
| Able to ambulate independently | 116 (7.0) | 1531 (9.9) |
| With assistance from person | 277 (16.7) | 2814 (18.1) |
| Unable to ambulate | 1269 (76.4) | 11 187 (72.0) |
| NIHSS score, median (IQR)g | 18 (14-23) | 17 (13-22) |
| Key treatment characteristics | ||
| Arrival via emergency medical services, No. (%) | 1761 (57.0) | 17 122 (57.8) |
| Off-hours arrival, No. (%) | 1745 (56.5) | 16 513 (55.7) |
| Onset-to-EVT time, median (IQR), min | 209 (154-265) | 205 (152-268) |
| Door-to-EVT time, median (IQR), min | 77 (46-112) | 77 (46-111) |
| Intravenous tPA, No. (%) | 1313 (42.5) | 23 290 (78.6) |
| Onset-to-needle time, median (IQR), min | 111 (85-151) | 104 (77-146) |
| Door-to-needle time, median (IQR), min | 48 (37-61) | 38 (28-50) |
| Onset-to-arrival time, median (IQR), min | 120 (54-193) | 115 (53-193) |
| Large vessel occlusion, No. (%) | 2038 (94.7) | 20 672 (93.8) |
| Site of occlusion, No. (%) | ||
| Middle cerebral artery | 1762 (86.5) | 17 508 (84.7) |
| Internal carotid artery | 357 (17.5) | 3936 (19.0) |
| Other cerebral artery branch | 73 (3.6) | 760 (3.7) |
| Basilar artery | 56 (2.7) | 812 (3.9) |
| Vertebral artery | 10 (0.5) | 248 (1.2) |
Abbreviations: EVT, endovascular thrombectomy; tPA, tissue plasminogen activator.
Hospital-level characteristics of the study population are available in eTable 1 in Supplement 1.
Vitamin K antagonist exposure was defined as chart documentation that a dose was ingested within the preceding 7 days.
Race and ethnicity were ascertained from various sources including self-designation by administrative personnel during the registration process or on nursing intake forms.
Other includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, multiracial, and any other non-Black or non-White racial categories.
When patients were eligible for both Medicaid and Medicare, they were assigned to Medicaid for the purposes of this analysis.
Calculated as weight in kilograms divided by height in meters squared.
The National Institutes of Health Stroke Scale (NIHSS) is a measure of stroke severity ranging from 0 to 42. It is scored by clinical personnel during the acute stroke assessment process. Higher scores indicate more severe stroke symptoms.
Primary End Point
Table 2 presents unadjusted and adjusted estimates of each end point according to VKA vs no-VKA status. Of 3087 patients taking a VKA, 211 (6.8%) experienced an sICH. Of 29 628 patients not taking a VKA, 1904 (6.4%) developed an sICH. After risk adjustment, the difference was not statistically significant between the VKA and no-VKA groups (adjusted OR, 1.12 [95% CI, 0.94-1.35]; adjusted risk difference, 0.69% [95% CI, −0.39% to 1.77%]).
Table 2. End Points According to Treatment Status.
| End points | No./total (%) | Adjusted odds ratio (95% CI)b | Adjusted absolute risk difference (95% CI) | |
|---|---|---|---|---|
| Use of vitamin K antagonists (n = 3087)a | No use of vitamin K antagonists (n = 29 628) | |||
| Primary end point | ||||
| Symptomatic intracranial hemorrhage <36 h after endovascular thrombectomy | 211/3087 (6.8) | 1904/29 628 (6.4) | 1.12 (0.94-1.35) | 0.69 (−0.39 to 1.77) |
| Secondary end points | ||||
| Life-threatening, serious systemic hemorrhage <36 h after endovascular thrombectomy | 36/3087 (1.2) | 301/29 628 (1.0) | 1.42 (0.91-2.23) | 0.38 (−0.14 to 0.91) |
| Other serious complicationc | 156/3087 (5.1) | 1483/29 628 (5.0) | 1.07 (0.89-1.28) | 0.31 (−0.57 to 1.19) |
| Any complications of reperfusion therapyd | 395/3087 (12.8) | 3610/29 628 (12.2) | 1.12 (0.98-1.29) | 1.24 (−0.25 to 2.72) |
| In-hospital mortality | 501/3087 (16.2) | 3869/29 628 (13.1) | 1.01 (0.89-1.14) | 0.09 (−1.64 to 1.82) |
| In-hospital mortality or discharge to hospice | 837/3087 (27.1) | 6107/29 628 (20.6) | 1.04 (0.93-1.16) | 0.73 (−1.45 to 2.92) |
| Exploratory end points | ||||
| Able to ambulate independently | 627/2133 (29.4) | 7533/20 908 (36.0) | 1.11 (0.99-1.25) | 2.15 (−0.25 to 4.56) |
| Discharge modified Rankin Scale score | ||||
| 0-1 (freedom from disability) | 290/2452 (11.8) | 3717/22 886 (16.2) | 1.27 (1.08-1.50) | 2.36 (0.68 to 4.03) |
| 0-2 (functional independence) | 458/2452 (18.7) | 5405/22 886 (23.6) | 1.29 (1.13-1.47) | 3.73 (1.79 to 5.67) |
| Discharge to home | 643/3087 (20.8) | 8332/29 628 (28.1) | 1.08 (0.97-1.21) | 1.30 (−0.59 to 3.19) |
| Discharge to hospice | 342/3087 (11.1) | 2275/29 628 (7.7) | 1.05 (0.90-1.23) | 0.48 (−1.04 to 2.00) |
| Discharge to inpatient rehabilitation facility | 918/3072 (29.9) | 9382/29 481 (31.8) | 1.09 (0.98-1.22) | 1.77 (−0.41 to 3.95) |
| Discharge to skilled nursing facility | 677/3072 (22.0) | 5689/29 481 (19.3) | 0.84 (0.75-0.93) | −3.27 (−5.16 to −1.39) |
Vitamin K antagonist exposure was defined as chart documentation that a dose was ingested within the preceding 7 days.
Adjusted odds ratios were computed from a logistic regression model with generalized estimating equations. The independent variable is vitamin K antagonist exposure (vs no vitamin K antagonist exposure). Overlap weighting was applied to control for baseline imbalances between groups using propensity scores derived from a multivariable logistic regression model with key demographic, clinical, and hospital-level covariates.
Other serious complication describes any complication that necessitated additional medical intervention or a prolonged length of stay and that was unexpected or out of proportion to a patient’s expected course and documented as complications of endovascular thrombectomy specifically. These include rapid development of malignant edema, angioedema, or recurrent stroke. These were ascertained by trained abstractors at each site.
Any complications of reperfusion therapy include (1) symptomatic intracranial hemorrhage <36 hours; (2) life-threatening, serious, systemic hemorrhage <36 hours; and (3) other serious complication (described above). These were ascertained by trained abstractors at each site.
Secondary End Points
The rate of life-threatening systemic hemorrhage was 1.2% in the VKA group compared with 1.0% in the no-VKA group (adjusted OR, 1.42 [95% CI, 0.91-2.23]; adjusted risk difference, 0.38% [95% CI, −0.14% to 0.91%]). The rates of in-hospital mortality or discharge to hospice were 27.1% in the VKA group vs 20.6% in the no-VKA group (adjusted OR, 1.04 [95% CI, 0.93-1.16]; adjusted risk difference, 0.73% [95% CI, −1.45% to 2.92%]). In unadjusted analyses, there was a numerically higher rate of a hemorrhagic complication on brain imaging in those taking a VKA compared with those not taking a VKA (20.4% vs 18.8%) (eTable 5 in Supplement 1). These hemorrhagic complications comprised parenchymal hematoma type 2 (3.6% vs 2.9%), intraventricular hemorrhage (2.8% vs 2.3%), subarachnoid hemorrhage (6.5% vs 6.2%), or a hemorrhage remote to the site of the primary infarct (0.8% vs 0.7%).
Exploratory End Points
After risk adjustment, patients taking a VKA prior to EVT were significantly more likely to have a modified Rankin Scale score of 0 to 1 (11.8% vs 16.2%; adjusted OR, 1.27 [95% CI, 1.08-1.50]; adjusted risk difference, 2.36% [95% CI, 0.68%-4.03%]) and a modified Rankin Scale score of 0 to 2 (18.7% vs 23.6%; adjusted OR, 1.29 [95% CI, 1.13-1.47]; adjusted risk difference, 3.73% [95% CI, 1.79%-5.67%]). The 2 groups did not significantly differ with respect to the odds of being ambulatory at discharge or being discharged to home, hospice, or an inpatient rehabilitation facility.
INR and Study End Points
Of 2415 patients with a documented admission INR, 1585 had an INR of 1.7 or lower (median INR, 1.3 [IQR, 1.1-1.5]) and 830 had an INR greater than 1.7 (median INR, 2.1 [IQR, 1.9-2.5]). Baseline characteristics were broadly similar across these subgroups, although there was a higher rate of intravenous thrombolysis use in patients with an INR of 1.7 or lower than in those with an INR greater than 1.7 (59.2% vs 8.1%) (eTable 6 in Supplement 1). When compared with patients not taking a VKA, those taking a VKA who had an INR greater than 1.7 had significantly higher odds of sICH (8.3% vs 6.4%; adjusted OR, 1.88 [95% CI, 1.33-2.65]; adjusted risk difference, 4.03% [95% CI, 1.53%-6.53%]) (Table 3). In contrast, those taking a VKA who had an INR of 1.7 or lower had no significant difference in the odds of sICH (6.7% vs 6.4%; adjusted OR, 1.24 [95% CI, 0.87-1.76]; adjusted risk difference, 1.13% [95% CI, −0.79% to 3.04%]) compared with patients not taking a VKA. When modeling INR as a continuous variable (Figure 2), the odds of sICH were lowest in the 1 to 1.2 range of INR values. There was a gentle inflection in the 1.2 to 1.4 range before a plateau was seen in the 2.2 to 2.6 range of INR values.
Table 3. Risk of Symptomatic Intracranial Hemorrhage (Primary Study End Point) Stratified by VKA Use, Admission INR, and Intravenous tPA Exposurea.
| VKA use, No./total (%)b | No VKA use, No./total (%) | VKA use vs no VKA use | VKA use with INR ≤1.7, No./total (%) | VKA use with INR >1.7, No./total (%) | VKA use with INR ≤1.7 vs no VKA use | VKA use with INR >1.7 vs no VKA use | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)c | Adjusted absolute risk difference (95% CI) | Adjusted OR (95% CI)c | Adjusted absolute risk difference (95% CI) | Adjusted OR (95% CI)c | Adjusted absolute risk difference (95% CI) | |||||
| All patients | 211/3087 (6.8) | 1904/29 628 (6.4) | 1.12 (0.94-1.35) | 0.69 (−0.39 to 1.77) | 106/1585 (6.7) | 69/830 (8.3) | 1.24 (0.87-1.76) | 1.13 (−0.79 to 3.04) | 1.88 (1.33-2.65) | 4.03 (1.53 to 6.53) |
| Patients treated with intravenous tPA | 93/1313 (7.1) | 1588/23 290 (6.8) | 0.92 (0.73-1.17) | −0.55 (−2.12 to 1.02) | 66/939 (7.0) | 11/67 (16.4) | 1.06 (0.66-1.72) | 0.43 (−3.04 to 3.90) | 2.00 (1.01-3.95) | 6.43 (−1.51 to 14.38) |
| Patients not treated with intravenous tPA | 118/1774 (6.7) | 316/6338 (5.0) | 1.37 (1.02-1.84) | 1.64 (0.05 to 3.24) | 40/646 (6.2) | 58/763 (7.6) | 1.33 (0.86-2.05) | 1.48 (−0.92 to 3.89) | 1.58 (1.08-2.30) | 2.60 (0.30 to 4.89) |
Abbreviations: OR, odds ratio; INR, international normalized ratio; tPA, tissue plasminogen activator; VKA, vitamin K antagonist.
Within 36 hours of endovascular thrombectomy.
VKA exposure was defined as chart documentation that a dose was ingested within the preceding 7 days.
All adjusted odds ratios were computed from a logistic regression model with generalized estimating equations. The independent variable is VKA exposure (vs no VKA exposure). Overlap weighting was applied to control for baseline imbalances between groups using propensity scores derived from a multivariable logistic regression model with key demographic, clinical, and hospital-level covariates.
Figure 2. Unadjusted and Adjusted Association of Presentation INR With sICH and Life-threatening Systemic Hemorrhage.
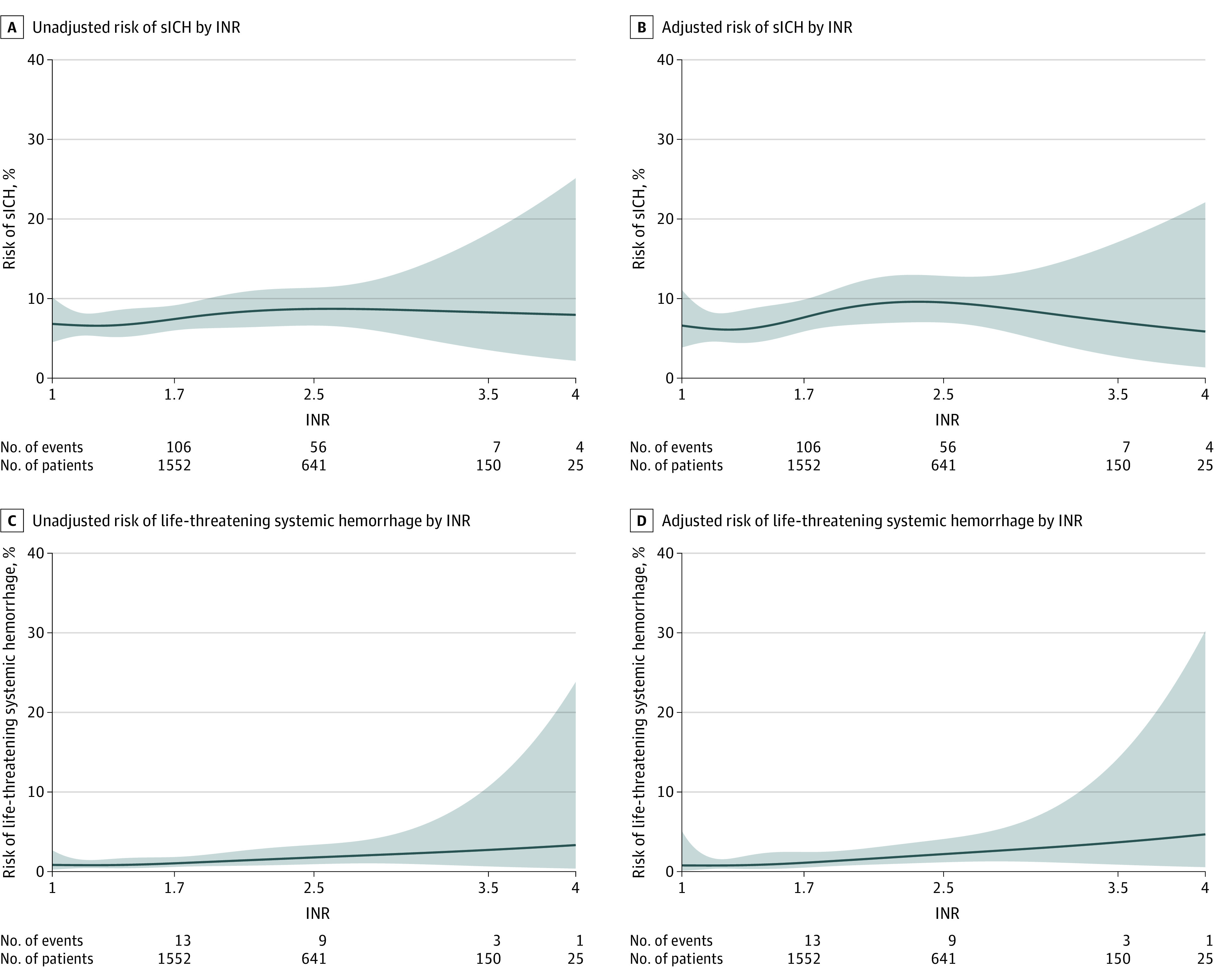
sICH indicates symptomatic intracranial hemorrhage. This figure includes only patients taking a vitamin K antagonist and with international normalized ratio (INR) data recorded at presentation (n = 2415). Logistic regression modeling was conducted to examine the unadjusted and adjusted relationship between INR and the end point in question. Propensity scores calculated using overlap weighting were used for adjustment. The Stone and Koo additive spline method with 4 knots was fit to generate the plots. Under each graph, the numbers of patients and events are shown for each outcome for a given INR range. For example, there were 106 sICHs occurring for 1552 patients with an INR of 1.0 to 1.7 and 56 sICHs occurring for 641 patients with an INR of 1.8 to 2.5. Because of clinical relevance, each graph starts at INR = 1.
There was a significantly higher rate of life-threatening systemic hemorrhage in those in the VKA group with an INR greater than 1.7 compared with the no-VKA group (1.6% vs 1.0%; adjusted OR, 3.2 [95% CI, 1.31-7.86]; adjusted risk difference, 1.26% [95% CI, −0.07% to 2.58%]) but not in those with an admission INR of 1.7 or lower (0.9% vs 1.0%; adjusted OR, 1.50 [95% CI, 0.61-3.65]; adjusted risk difference, 0.29% [95% CI, −0.41% to 0.98%]) (eTable 7 in Supplement 1). Despite the significantly higher risk of sICH in patients with an INR greater than 1.7, after risk adjustment there were no statistically significant differences in in-hospital mortality compared with the no-VKA group (17.7% vs 13.1%; adjusted OR, 1.13 [95% CI, 0.89-1.42]; adjusted risk difference, 1.72% [95% CI, −1.73% to 5.17%]). With respect to the exploratory end points, there was no statistically significant difference for those with VKA use and an INR greater than 1.7 compared with the no-VKA group in terms of the ability to ambulate at discharge (27.4% vs 36.0%; adjusted OR, 0.95 [95% CI, 0.77-1.16]; adjusted risk difference, −1.05% [95% CI, −4.92% to 2.81%]), functional independence (modified Rankin Scale score of 0-2, 16.5% vs 23.6%; adjusted OR, 1.13 [95% CI, 0.88-1.46]; adjusted risk difference, 1.60% [95% CI, −1.80% to 5.01%]), or freedom from disability (modified Rankin Scale score of 0-1, 10.5% vs 16.2%; adjusted OR, 1.15 [95% CI, 0.81-1.63]; adjusted risk difference, 1.18% [95% CI, −1.85% to 4.20%]).
INR, tPA Administration, and sICH
In patients receiving intravenous tPA, there was no statistically significant difference in the odds of sICH among those with VKA use vs no VKA use (7.1% vs 6.8%; adjusted OR, 0.92 [95% CI, 0.73-1.17]; adjusted risk difference, −0.55% [95% CI, −2.12% to 1.02%]) (Table 3). In patients not treated with intravenous tPA, there were significantly higher odds of sICH in those with VKA use vs no VKA use (6.7% vs 5.0%; adjusted OR, 1.37 [95% CI, 1.02 to 1.84]; adjusted risk difference, 1.64% [95% CI, 0.05%-3.24%]). Among those treated with intravenous tPA, patients with recent VKA use who had a presenting INR greater than 1.7 had significantly higher odds of sICH compared with those with no VKA use (16.4% vs 6.8%; adjusted OR, 2.00 [95% CI, 1.01-3.95]; adjusted risk difference, 6.43% [95% CI, −1.51% to 14.38%]). Among patients not treated with intravenous tPA, patients taking a VKA who had a presenting INR greater than 1.7 also had significantly higher odds of sICH compared with those with no VKA use (7.6% vs 5.0%; adjusted OR, 1.58 [95% CI, 1.08-2.30]; adjusted risk difference, 2.60% [95% CI, 0.30%-4.89%]). In contrast, in patients exposed to a VKA who had a presenting INR of 1.7 or lower and were treated with intravenous tPA, there was no statistically significant difference in the odds of sICH compared with those not taking a VKA (7.0% vs 6.8%; adjusted OR, 1.06 [95% CI, 0.66-1.72]; adjusted risk difference, 0.43% [95% CI, −3.04% to 3.90%]). Similarly, in patients exposed to a VKA who had a presenting INR of 1.7 or lower and were not treated with intravenous tPA, there no was statistically significant difference in the odds of sICH (6.2% vs 5.0%; adjusted OR, 1.33 [95% CI, 0.86-2.05]; adjusted risk difference, 1.48% [95% CI, −0.92% to 3.89%]). Other study end points disaggregated by VKA treatment, admission INR, and intravenous tPA exposure are presented in eTables 8, 9, 10, and 11 in Supplement 1.
Discussion
In this large US-based study of patients with acute ischemic stroke selected to undergo EVT, encompassing a diverse population from both academic and community settings,26 recent use of a VKA was not significantly associated with increased risk of sICH overall. However, the risk of sICH was increased among the subgroup of patients with recent VKA use and an elevated INR at the time of presentation.
This study is noteworthy in the context of several prior conflicting publications.13,14,15,27,28,29,30,31 Two notable studies used the ECASS II32 definition of sICH, which encompasses any new hemorrhage observed on computed tomography and clinical deterioration, regardless of whether the hemorrhage is perceived to have caused the deterioration. The first study, from the German Stroke Registry–Endovascular Treatment,15 reported outcomes in 479 patients taking a VKA from a subset of academic hospitals in Germany. This study did not find an association between VKA use and sICH, although the rate of sICH was high in each group (12% in the VKA group and 15% in the no-VKA group). The second study, based on the BEYOND-SWIFT registry,13 reported significantly increased odds of sICH in patients taking VKAs (adjusted OR, 2.55 [95% CI, 1.35-4.84]), which persisted in a sensitivity analysis including only those with a therapeutic INR. A study based on the MR CLEAN Registry,31 using the Heidelberg classification system for sICH33 (a strictly radioanatomic classification that does not account for neurological worsening attributable to the sICH as the definition used in the present study does), reported 23 ICH events among 404 patients undergoing EVT while taking a VKA and did not find a significant association between VKA use and sICH (adjusted OR, 0.74 [95% CI, 0.44-1.26]).
There are several reasons posited for an association between anticoagulation and outcomes in patients undergoing EVT, especially among those with an elevated INR. In general, patients who are prescribed VKA are often older and have a higher burden of atrial fibrillation and other high-risk cardioembolic substrates than other patients, and these comorbidities may influence outcomes. Friable, infarcted, newly reperfused cerebral tissue may be more prone to hemorrhage in the setting of anticoagulation. Anticoagulation may also enhance bleeding risk at the femoral access site or at other extracranial locations (this was borne out in this analysis, which demonstrated significantly higher odds of systemic hemorrhage in those with an admission INR >1.7 but not in those with an admission INR ≤1.7). Furthermore, thrombi may be qualitatively different in patients treated with anticoagulation.34 In addition, anticoagulation with VKAs precludes the use of intravenous tPA when a patient’s INR is within a therapeutic or supratherapeutic range (ie, an INR of ≥1.712). There is increasing evidence that intravenous tPA may be of appropriately low risk in patients with acute ischemic stroke and recent VKA use with a presenting INR less than 1.735,36 and in the setting of recent direct oral anticoagulant use.37,38,39,40 The low risk of sICH associated with tPA use in patients with recent use of VKA and an INR of 1.7 or lower may reflect clinician selection of patients in clinical practice who are otherwise at very low hemorrhagic risk.
Conversely, anticoagulation may positively impact procedural success by reducing the rates of preprocedural thrombus propagation or vessel reocclusion after successful thrombectomy. For this reason, deliberate anticoagulation (with intravenous heparin) has been permitted at the proceduralist’s discretion in several trials of EVT,2,41,42,43 whereas it was not explicitly forbidden in others.3,4,5,6,44,45 Post hoc analyses of the TREVO 246 and Multi MERCI47 trials suggest that the use of periprocedural heparin was associated with a better functional status without increasing the odds of sICH. In this study, the actual proportions of patients who were free of disability (modified Rankin Scale score of 0-1) or functionally independent (modified Rankin Scale score of 0-2) at discharge were lower in those taking a VKA than in those not taking a VKA, but after risk adjustment, the odds of being free of disability or functionally independent at discharge were significantly higher in those taking a VKA. These directionality changes from the unadjusted to adjusted point estimates in each association may reflect the higher degree of baseline comorbidity and stroke severity in patients taking a VKA. However, this study included only patients selected to receive EVT in clinical practice (and not the entire population of VKA-treated patients potentially eligible for EVT), which can introduce index event bias or collider bias. The observation of better functional outcomes associated with recent VKA use prior to EVT in this study may thus be susceptible to bias introduced by the study design and therefore should be interpreted with caution.
Limitations
This study has several limitations. First, data quality is strongly dependent on the quality of documentation at each site. However, data in the GWTG-Stroke registry has been shown to have a high degree of fidelity with medical chart review.17 Second, this study lacked data on significant radiographic and technical factors that may influence procedural success, including the Alberta Stroke Program Early CT Score (ASPECTS; a measure of the degree of established infarction on noncontrast computed tomography), mode of access (femoral vs radial), use of general anesthesia as opposed to conscious sedation, the exact thrombectomy technique, number of passes used, and recanalization status. Third, the analysis was restricted to patients taking VKAs, and it is not known if the results can be extrapolated to patients taking direct oral anticoagulants. Fourth, recent use of a VKA was defined as within the preceding 7 days, and the precise timing of last VKA intake is not known. This limitation is partially overcome by the incorporation of admission INR into the analyses, which allowed determination of whether anticoagulation was at a therapeutic level (with the caveat that this information was missing in approximately 22% of patients). Fifth, the analysis was restricted to patients presenting within 6 hours of time last known to be well, and it is not known if the results apply to those treated with EVT in the 6- to 24-hour time window. Sixth, this study was confined to US hospitals voluntarily participating in and patients enrolled in the GWTG-Stroke registry, and generalizability to other hospitals is uncertain. Seventh, several of the reported differences in sICH risk across groups were statistically significant but of small magnitude. Eighth, although the analysis controlled for a large number of potential covariates associated with the odds of sICH and functional outcomes, it remains possible that there is residual confounding across groups. Thus, the exploratory findings of associated improvements in functional outcomes in those treated with a VKA should be interpreted with caution. Ninth, the study population included only patients taking a VKA who had a large vessel occlusion and were selected for EVT. Thus, the findings may not be generalizable to all patients taking VKAs who develop a stroke associated with large vessel occlusion.
Conclusions
Among patients with acute ischemic stroke selected to receive EVT, recent VKA use within the preceding 7 days was not associated with a significantly increased sICH risk overall. However, recent VKA use with a presenting INR greater than 1.7 was associated with a significantly increased risk of sICH compared with no use of anticoagulants.
eAppendix. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist
eTable 1. Weighted Means/Proportions and Standardized Differences for the Association of EVT With Study Endpoints by Taking a VKA vs Not Taking a VKA
eTable 2. Weighted Means/Proportions and Standardized Differences for the Association of EVT With Study Endpoints by Abnormal INR Values for Patients Taking a VKA vs Not Taking a VKA
eTable 3. Hospital-Level Characteristics of the Study Population
eTable 4. Baseline Characteristics of Those Patients in the Study Registry Taking a VKA and Potentially Eligible for EVT Stratified by Those Who Were and Were Not Treated With EVT
eTable 5. Radiographic Endpoints According to Treatment Status
eTable 6. Baseline Characteristics of the Study Population Taking a VKA and With Documented INR According to Presenting INR
eTable 7. Endpoints According to VKA Treatment Status and Admission INR
eTable 8. Endpoints According to VKA Treatment Status in Patients Treated With IV tPA
eTable 9. Endpoints According to VKA Treatment Status in Patients Not Treated With IV tPA
eTable 10. Endpoints According to VKA Treatment Status and Admission INR in Patients Treated With IV tPA
eTable 11. Endpoints According to VKA Treatment Status and Admission INR in Patients Not Treated With IV tPA
Data Sharing Statement
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE Investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir KW, Ford GA, Messow CM, et al. ; PISTE Investigators . Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38-44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 13.Meinel TR, Kniepert JU, Seiffge DJ, et al. Endovascular stroke treatment and risk of intracranial hemorrhage in anticoagulated patients. Stroke. 2020;51(3):892-898. doi: 10.1161/STROKEAHA.119.026606 [DOI] [PubMed] [Google Scholar]
- 14.Koge J, Tanaka K, Yamagami H, et al. Mechanical thrombectomy for stroke patients anticoagulated with direct oral anticoagulants versus warfarin. J Neurol Sci. 2021;427:117545. doi: 10.1016/j.jns.2021.117545 [DOI] [PubMed] [Google Scholar]
- 15.Küpper C, Feil K, Wollenweber FA, et al. ; GSR Investigators . Endovascular stroke treatment in orally anticoagulated patients: an analysis from the German Stroke Registry–Endovascular Treatment. J Neurol. 2021;268(5):1762-1769. doi: 10.1007/s00415-020-10369-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonarow GC, Reeves MJ, Smith EE, et al. ; GWTG-Stroke Steering Committee and Investigators . Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With the Guidelines–Stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 17.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With the Guidelines–Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163(3):392-398. doi: 10.1016/j.ahj.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43(9):2293-2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 20.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121(13):1492-1501. doi: 10.1161/CIRCULATIONAHA.109.881490 [DOI] [PubMed] [Google Scholar]
- 21.Li F, Morgan L, Zaslavsky A. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390-400. doi: 10.1080/01621459.2016.1260466 [DOI] [Google Scholar]
- 22.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188(1):250-257. doi: 10.1093/aje/kwy201 [DOI] [PubMed] [Google Scholar]
- 23.Thomas L, Li F, Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA. 2020;323(5):466-467. doi: 10.1001/jama.2019.21558 [DOI] [PubMed] [Google Scholar]
- 24.Stone C, Koo C. Additive Splines in Statistics. American Statistical Association; 1986:45-48. [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 26.Reeves MJ, Fonarow GC, Smith EE, et al. Representativeness of the Get With the Guidelines–Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43(1):44-49. doi: 10.1161/STROKEAHA.111.626978 [DOI] [PubMed] [Google Scholar]
- 27.Zapata-Wainberg G, Ximénez-Carrillo Á, Trillo S, et al. ; Madrid Stroke Network . Mechanical thrombectomy in orally anticoagulated patients with acute ischemic stroke. J Neurointerv Surg. 2018;10(9):834-838. doi: 10.1136/neurintsurg-2017-013504 [DOI] [PubMed] [Google Scholar]
- 28.L’Allinec V, Sibon I, Mazighi M, et al. ; Endovascular Treatment in Ischemic Stroke Investigators . MT in anticoagulated patients: direct oral anticoagulants versus vitamin K antagonists. Neurology. 2020;94(8):e842-e850. doi: 10.1212/WNL.0000000000008873 [DOI] [PubMed] [Google Scholar]
- 29.Rebello LC, Haussen DC, Belagaje S, Anderson A, Frankel M, Nogueira RG. Endovascular treatment for acute ischemic stroke in the setting of anticoagulation. Stroke. 2015;46(12):3536-3539. doi: 10.1161/STROKEAHA.115.011285 [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Araque ME, Chavarría-Miranda A, Gómez-Vicente B, et al. Oral anticoagulation and risk of symptomatic hemorrhagic transformation in stroke patients treated with mechanical thrombectomy: data from the Nordictus registry. Front Neurol. 2020;11:594251. doi: 10.3389/fneur.2020.594251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldhoorn RB, van de Graaf RA, van Rees JM, et al. ; MR CLEAN Registry Investigators—Group Authors . Endovascular treatment for acute ischemic stroke in patients on oral anticoagulants: results from the MR CLEAN Registry. Stroke. 2020;51(6):1781-1789. doi: 10.1161/STROKEAHA.119.028675 [DOI] [PubMed] [Google Scholar]
- 32.Hacke W, Kaste M, Fieschi C, et al. ; Second European-Australasian Acute Stroke Study Investigators . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352(9136):1245-1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 33.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Hang Y, Cao Y, et al. association between prior anticoagulation and thrombus composition in mechanical thrombectomy patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2022;31(4):106347. doi: 10.1016/j.jstrokecerebrovasdis.2022.106347 [DOI] [PubMed] [Google Scholar]
- 35.Xian Y, Liang L, Smith EE, et al. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA. 2012;307(24):2600-2608. doi: 10.1001/jama.2012.6756 [DOI] [PubMed] [Google Scholar]
- 36.Mazya MV, Lees KR, Markus R, et al. ; Safe Implementation of Thrombolysis in Stroke Investigators . Safety of intravenous thrombolysis for ischemic stroke in patients treated with warfarin. Ann Neurol. 2013;74(2):266-274. doi: 10.1002/ana.23924 [DOI] [PubMed] [Google Scholar]
- 37.Kam W, Holmes DN, Hernandez AF, et al. Association of recent use of non-vitamin k antagonist oral anticoagulants with intracranial hemorrhage among patients with acute ischemic stroke treated with alteplase. JAMA. 2022;327(8):760-771. doi: 10.1001/jama.2022.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seiffge DJ, Wilson D, Wu TY. Administering thrombolysis for acute ischemic stroke in patients taking direct oral anticoagulants: to treat or how to treat. JAMA Neurol. 2021;78(5):515-516. doi: 10.1001/jamaneurol.2021.0287 [DOI] [PubMed] [Google Scholar]
- 39.Seiffge DJ, Meinel T, Purrucker JC, et al. Recanalisation therapies for acute ischaemic stroke in patients on direct oral anticoagulants. J Neurol Neurosurg Psychiatry. 2021;92(5):534-541. doi: 10.1136/jnnp-2020-325456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meinel TR, Wilson D, Gensicke H, et al. ; DOAC-IVT Writing Group for the International DOAC-IVT, TRISP, and CRCS-K-NIH Collaboration . Intravenous thrombolysis in patients with ischemic stroke and recent ingestion of direct oral anticoagulants. JAMA Neurol. 2023;80(3):233-243. doi: 10.1001/jamaneurol.2022.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broderick JP, Palesch YY, Demchuk AM, et al. ; Interventional Management of Stroke III Investigators . Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893-903. doi: 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidwell CS, Jahan R, Gornbein J, et al. ; MR RESCUE Investigators . A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914-923. doi: 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciccone A, Valvassori L, Nichelatti M, et al. ; SYNTHESIS Expansion Investigators . Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904-913. doi: 10.1056/NEJMoa1213701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saver JL, Jahan R, Levy EI, et al. ; SWIFT Trialists . Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241-1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 45.Nogueira RG, Lutsep HL, Gupta R, et al. ; TREVO 2 Trialists . Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231-1240. doi: 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winningham MJ, Haussen DC, Nogueira RG, et al. Periprocedural heparin use in acute ischemic stroke endovascular therapy: the TREVO 2 trial. J Neurointerv Surg. 2018;10(7):611-614. doi: 10.1136/neurintsurg-2017-013441 [DOI] [PubMed] [Google Scholar]
- 47.Nahab F, Walker GA, Dion JE, Smith WS. Safety of periprocedural heparin in acute ischemic stroke endovascular therapy: the Multi MERCI trial. J Stroke Cerebrovasc Dis. 2012;21(8):790-793. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist
eTable 1. Weighted Means/Proportions and Standardized Differences for the Association of EVT With Study Endpoints by Taking a VKA vs Not Taking a VKA
eTable 2. Weighted Means/Proportions and Standardized Differences for the Association of EVT With Study Endpoints by Abnormal INR Values for Patients Taking a VKA vs Not Taking a VKA
eTable 3. Hospital-Level Characteristics of the Study Population
eTable 4. Baseline Characteristics of Those Patients in the Study Registry Taking a VKA and Potentially Eligible for EVT Stratified by Those Who Were and Were Not Treated With EVT
eTable 5. Radiographic Endpoints According to Treatment Status
eTable 6. Baseline Characteristics of the Study Population Taking a VKA and With Documented INR According to Presenting INR
eTable 7. Endpoints According to VKA Treatment Status and Admission INR
eTable 8. Endpoints According to VKA Treatment Status in Patients Treated With IV tPA
eTable 9. Endpoints According to VKA Treatment Status in Patients Not Treated With IV tPA
eTable 10. Endpoints According to VKA Treatment Status and Admission INR in Patients Treated With IV tPA
eTable 11. Endpoints According to VKA Treatment Status and Admission INR in Patients Not Treated With IV tPA
Data Sharing Statement