Abstract
Follicle-stimulating hormone (FSH), a dimeric glycoprotein produced by pituitary gonadotrope cells, regulates spermatogenesis in males and ovarian follicle growth in females. Hypothalamic gonadotropin-releasing hormone (GnRH) stimulates FSHβ subunit gene (Fshb) transcription, though the underlying mechanisms are poorly understood. To address this gap in knowledge, we examined changes in pituitary gene expression in GnRH-deficient mice (hpg) treated with a regimen of exogenous GnRH that increases pituitary Fshb but not luteinizing hormone β (Lhb) messenger RNA levels. Activating transcription factor 3 (Atf3) was among the most upregulated genes. Activating transcription factor 3 (ATF3) can heterodimerize with members of the activator protein 1 family to regulate gene transcription. Co-expression of ATF3 with JunB stimulated murine Fshb, but not Lhb, promoter-reporter activity in homologous LβT2b cells. ATF3 also synergized with a constitutively active activin type I receptor to increase endogenous Fshb expression in these cells. Nevertheless, FSH production was intact in gonadotrope-specific Atf3 knockout [conditional knockout (cKO)] mice. Ovarian follicle development, ovulation, and litter sizes were equivalent between cKOs and controls. Testis weights and sperm counts did not differ between genotypes. Following gonadectomy, increases in LH secretion were enhanced in cKO animals. Though FSH levels did not differ between genotypes, post-gonadectomy increases in pituitary Fshb and gonadotropin α subunit expression were more pronounced in cKO than control mice. These data indicate that ATF3 can selectively stimulate Fshb expression in vitro but is not required for FSH production in vivo.
Keywords: transcription, GnRH, pituitary, knockout mouse, cell line
Reproduction is regulated by hormones in the hypothalamic-pituitary-gonadal axis. Gonadotropin-releasing hormone (GnRH) is secreted from the brain and stimulates the synthesis and secretion of the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), by gonadotrope cells of the anterior pituitary gland. The gonadotropins are dimeric glycoproteins composed of a common α-subunit (encoded by the Cga gene) noncovalently linked to hormone-specific β subunits (encoded by Fshb or Lhb). Gonadotropins travel via systemic circulation to the gonads, where they regulate steroidogenesis in both sexes, spermatogenesis in males, and follicle development and ovulation in females. Gonadotropin deficiency leads to impaired fertility in humans and mice (1-4). GnRH signals via the GnRH receptor (GnRHR), a G-protein coupled receptor on the surface of gonadotrope cells. GnRH is secreted from the median eminence in pulses, with low and high frequency pulses favoring FSH and LH synthesis, respectively (5, 6). Mechanisms of GnRH pulse frequency decoding by gonadotropes are incompletely resolved. However, repeated pulses of GnRH are required to stimulate LH production in particular (7). In the murine gonadotrope-like cell line, LβT2, pulsatile GnRH stimulates the expression of early growth response 1 (Egr1) (8). Early growth response 1 (EGR1) binds in concert with steroidogenic factor 1 and paired-like homeodomain transcription factor 1 to the proximal Lhb promoter, driving transcription (9). GnRH induces Egr1 expression via the extracellular regulated kinase 1/2 pathway (10). Mice lacking extracellular regulated kinase 1/2 (Mapk1/3) in gonadotropes or Egr1 globally are LH but not FSH deficient (8, 11-14). This mechanism of GnRH induction of Lhb transcription appears to be conserved across mammalian species, including humans (15).
In contrast, we lack comparable insight into the signaling molecules and transcription factors through which GnRH promotes Fshb transcription. Various models have been proposed based on in vitro studies in LβT2 and heterologous cells (16-21). However, none have been unequivocally substantiated in vivo. For example, the GnRHR classically signals via a Gαq/11-dependent pathway in vitro (22). It was proposed that at low GnRH pulse frequency, the GnRHR may couple to Gαs and stimulate Fshb transcription via a cAMP-PKA-CREB-dependent pathway (23, 24). This model was recently challenged, as FSH production is unaltered in gonadotrope-specific Gαs knockout mice. In contrast, mice lacking Gαq/11 in gonadotropes display both FSH and LH deficiency (25).
There is also compelling in vitro evidence that GnRH stimulates Fshb transcription via induction of members of the activator protein 1 (AP-1) family, including cFos, FosB, cJun, and JunB. Conserved and species-specific AP-1 binding sites have been identified in the proximal Fshb/FSHB promoters of sheep, humans, and mice (18, 19, 21). Two such elements are critical for GnRH regulation of ovine Fshb transcription in vitro but not in transgenic mice in vivo (21). FSH levels are unaffected in mice with gonadotrope-specific knockout of Jun, which encodes cJun, though a compensatory role for JunB could not be excluded (26). Global Junb knockout mice die during embryonic development (27), and gonadotrope-specific Junb knockouts have not been produced or analyzed to our knowledge. Fos, which encodes cFos, knockout mice are infertile but display defects throughout the hypothalamic-pituitary-gonadal axis that are not clearly linked to selective FSH deficiency (28). Fosb knockout mice are fertile though show defects in maternal behavior (29).
Given the apparent discrepancies between in vitro and in vivo results, we employed an alternative approach to identify GnRH-induced transcription factors that might stimulate Fshb expression in a manner analogous to EGR1 in the regulation of Lhb. We developed a protocol in GnRH-deficient (hpg) mice in which GnRH stimulates increases in pituitary Fshb but not Lhb expression. We then used bulk RNA-sequencing (RNA-seq) on whole pituitaries to screen for GnRH-induced transcription factors and focused on activating transcription factor 3 (ATF3). The data show that ATF3 selectively stimulates Fshb expression in vitro but that FSH synthesis is unimpaired in conditional Atf3 knockout mice in vivo.
Materials and Methods
Reagents
RQ1 RNase-Free DNase (M6101), random hexamer primers (C1181), MMLV-reverse transcriptase (M1701), and RNasin (N2511) were from Promega (Madison, WI, USA). Formalin solution (HT501128), GnRH (L7134), hyaluronidase (H3884), pancreatin (P3292), and collagenase (Type I-C0130), anti-Flag antibody (F7425, RRID:AB_439687), 3x-Flag peptide (F4799), and EZview Red ANTI-FLAG M2 Affinity Gel (F2426), and nitrocellulose (10 600 001) were from Millipore Sigma (St. Louis, MO, USA). Kisspeptin-54 (24 477) was from Cayman Chemicals (Cedarlane Labs, ON, Canada). TRIzol Reagent (15 596 026), Lipofectamine 3000 (L3000001), and o-phenylenediamine tablets were purchased from Thermo Fisher (Waltham, MA, USA). Blastaq 2X qPCR MasterMix-S (G891-1) was from Applied Biological Materials Inc. (Richmond, BC, Canada). Acrylamide:bis-acrylamide (37.5:1) (EC-890) was purchased from National Diagnostics (Atlanta, GA, USA). Western Lighting Plus ECL was purchased from PerkinElmer (Waltham, MA, USA). Oligonucleotides were from IDT (Coralville, IA, USA). Dulbecco's Modified Eagle Medium (DMEM; 319-005-CL), fetal bovine serum (098 150), and deoxynucleotide triphosphates were from Wisent (St-Bruno, QC, Canada). JunB antibody (610326, RRID:AB_397716) was purchased from BD Biosciences (Franklin Lakes, NJ, USA). cJun antibody (9165, RRID:AB_2130165) was from Cell Signaling Technologies (Danvers, MA, USA). The HRP-linked goat anti-rabbit secondary antibody (172-1034, RRID:AB_11125144) and HRP-linked goat anti-mouse secondary antibody (170-6516, RRID:AB_11125547) were from Bio-Rad (Hercules, CA, USA).
Animals
The mouse strains were described previously: hpg (30), floxed Atf3 (31), and GnRH receptor-IRES-Cre (GRIC) (32). Hpg animals possess a loss of function deletion in the Gnrh1 gene and are hypogonadal. Heterozygous hpg mice are fertile; therefore, they were crossed inter se to obtain hpg/hpg mice. To generate mice with a conditional deletion of Atf3 in gonadotropes, we crossed Atf3fx/+;GnrhrGRIC/+ females with Atf3fx/fx;Gnrhr+/+ males. This cross produces 4 different genotypes: Atf3fx/fx;Gnrhr+/+, Atf3fx/fx;GnrhrGRIC/+, Atf3fx/+;Gnrhr+/+, and Atf3fx/+;GnrhrGRIC/+. Atf3fx/fx;GnrhrGRIC/+ represent the conditional knockouts (cKO) and Atf3fx/fx;Gnrhr+/+ were used as controls. Primer pairs used for genotyping are in Table 1. All animal procedures were approved by the McGill University Downtown-A Facility Animal Care Committee under protocol #5204. Animals were housed on a 12L:12D cycle with lights on a 07h00 and were provided standard mouse chow ad libitum.
Table 1.
Primer list
| Genotyping | |
| Atf3 genotyping Fw | ACTGGGGCAAAGAAACATACC |
| Atf3 genotyping Rv | AAAAAGAATCGGGAAGACACT |
| Cre Fw | CCTGGAAAATGCTTCTGTCCG |
| Cre Rv | CAGGGTGTTATAAGCAATCCC |
| hpg Fw | TATGGCTTACAGTTCCAGGG |
| hpg Rv WT | AGGCTTGGAGAGCTGTAAGG |
| hpg Rv hpg | GTTTCAGTGCATCCTCTCAGG |
| qPCR hpg mice | |
| Actb Fw | GCTGCCTCAACACCTCAAC |
| Actb Rv | AGGTGACAGCATTGCTTCTC |
| B2m Fw | CAATGTGAGGCGGGTGGAA |
| B2m Rv | CGGCCTGTATGCTATCCAGAA |
| Fshb Fw | GTGCGGGCTACTGCTACACT |
| Fshb Rv | CAGGCAATCTTACGGTCTCG |
| Gapdh Fw | TGCCGTGAGTGGAGTCATAC |
| Gapdh Rv | ACAGCCGCATCTTCTTGTG |
| Lhb Fw | CTCCCGGTAGGTGCACACT |
| Lhb Rv | CCTTCACCACCAGCATCTGT |
| Rps11 Fw | GCACATTGAATCGCACAGTC |
| Rps11 Rv | CGTGACGAAGATGAAGATGC |
| Tuba1a Fw | CTGGAGCAGTTTGACGACAC |
| Tuba1a Rv | TGCCTTTGTGCACTGGTATG |
| qPCR cKO mice and LβT2b cells | |
| Atf3 Fw | TTACCGTCAACAACAGACCCCT |
| Atf3 Rv | CGCCTCCTTTTCCTCTCATCTT |
| Cga Fw | TCCCTCAAAAAGTCCAGAGC |
| Cga Rv | GAAGAGAATGAAGAATATGCAG |
| Egr1 Fw | GAGCGAACAACCCTATGAGC |
| Egr1 Rv | GAGTCGTTTGGCTGGGATAA |
| Fos Fw | GGAGCTGACAGATACACTCCAA |
| Fos Rv | GAGGCCACAGACATCTCCTC |
| Fshb Fw | GTGCGGGCTACTGCTACACT |
| Fshb Rv | CAGGCAATCTTACGGTCTCG |
| Gnrhr Fw | CACGGGTTTAGGAAAGCAAA |
| Gnrhr Rv | TTCGCTACCTCCTTTGTCGT |
| Junb Fw | AGGCAGCTACTTTTCGGGTC |
| Junb Rv | TTGCTGTTGGGGACGATCAA |
| Lhb Fw | ACTGTGCCGGCCTGTCAACG |
| Lhb Rv | AGCAGCCGGCAGTACTCGGA |
| Rpl19 Fw | CGGGAATCCAAGAAGATTGA |
| Rpl19 Rv | TTCAGCTTGTGGATGTGCTC |
Abbreviations: cKO, gonadotrope-specific Atf3 knockout; qPCR, quantitative polymerase chain reaction.
Hormone Injections in Mice
Hypogonadal mice (hpg/hpg) were injected once daily intraperitoneally (i.p.) with GnRH (1 µg) or saline for a total of 10 days. One hour after the last injection, animals were sacrificed, and pituitaries were snap frozen for bulk RNA-seq and quantitative polymerase chain reaction (qPCR) analyses.
To assess the efficacy of the gene knockout, 5-week-old control (Atf3fx/fx;Gnrhr+/+) or cKO (Atf3fx/fx;GnrhrGRIC/+) male and female mice were injected once i.p. with 1 µg GnRH. One hour after the injection, animals were sacrificed, and pituitaries were snap frozen for RNA extraction and qPCR analyses.
Juvenile female control and cKO mice (28-30 days) were injected with 1 nmol kisspeptin-54 as described in (33). Just prior to injection and for the next 4 hours, whole blood (4 µL) was obtained from the tail tip at 15-minute intervals. The blood was diluted 1:30 in phosphate-buffered saline (PBS) with a low-concentration detergent solution (0.05% Tween-20) and snap frozen for later measurement of LH by ELISA.
RNA Isolation for Bulk RNAseq, qPCR, and Complementary DNA Library Synthesis
Mouse pituitaries were homogenized in a Bead Ruptor Elite bead mill homogenizer (Omni International, Kennesaw, GA) cooled by liquid nitrogen and homogenized for 2 × 20 seconds at 4.2 m/s. Homogenates were processed for RNA extraction using the Agencourt RNAdvance Tissue Kit (Beckman Coulter, Indianapolis, IN) on a BioMek FXP Laboratory Automation Workstation (Beckman Coulter, Indianapolis, IN) according to the manufacturer's instructions. The purity and concentration of the RNA samples were evaluated on a NanoDrop One spectrophotometer (ThermoFisher Scientific) and quantified using Quant-iT RiboGreen RNA Assay Kit (ThermoFisher Scientific). The RNA integrity was assessed with an RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA). RNA integrity numbers for all samples were above 8.2 with a median RNA integrity number of 9.1. A total of 250 ng total RNA per sample was used as input material for the complementary DNA (cDNA) library preparation. Libraries were prepared using the Universal Plus mRNA-Seq Library Preparation Kit (NuGEN) according to the manufacturer's protocol. Ribosomal RNA and globin messenger RNA (mRNA) were removed from the library using the AnyDeplete kit (NuGEN). Library concentration was assessed fluorometrically using the Qubit dsDNA HS Kit (ThermoFisher Scientific). Libraries were validated for size and purity on the Fragment Analyzer using a Genomic DNA 50Kb Analysis Kit (DNF-467, Advanced Analytical Technologies).
Real-time Quantitative PCR Validation of Samples for RNA-Seq
Fifty ng of RNA prepared as described previously was reverse-transcribed with the Affinity Script reverse-transcriptase (Agilent). Next, samples were diluted 1:20 in molecular biology-grade H2O (Cellgro, Manassas, VA) and real-time qPCR (RT-qPCR) experiments were performed as previously described (34). Briefly, SYBR Green qPCR assays were performed (40 cycles) in an ABI Prism 7900HT thermal cycler (Applied Biosystems, Foster City, CA, USA) using 5 μL of cDNA template and 5 μL of master mix containing the specific primers for the targeted gene, Platinum Taq DNA polymerase, and the required qPCR buffer, following the manufacturer's recommendations. Three technical qPCR replicates were run for each biological replicate. Results were exported as cycle threshold (Ct) values, and Ct values of target genes were normalized to that of the average of 5 housekeeping genes (Rps11, Tuba1a, Gapdh, Actb, and B2m) in a subsequent analysis. Data were expressed as arbitrary units by using the formula E = 2500 × 1.93(housekeeping gene Ct value − gene of interest Ct value), where E is the expression level in arbitrary units. Primer sequences were previously described (35, 36).
RNA Preprocessing and Sequencing
Preliminary sequencing of cDNA libraries (average read depth of 90,000 reads) was performed using a MiSeq system (Illumina, Inc., San Diego, CA, USA) to confirm library quality. All the libraries were subsequently sequenced on Illumina HiSeq 2500 Rapid flow (Illumina Inc., San Diego, CA, USA) with paired-end 2 × 61-bp reads following standard Illumina protocols with an average depth of 30 million reads/sample.
A uniform pipeline was used to process all RNA-seq fastq files. STAR (v2.7.4) (37) was used to align the reads to mm10 genome build with Gencode v34 index. To quantify gene expression levels, kallisto (v0.46.0) (38) was used to pseudo-align RNA-seq reads to Gencode v34 transcripts (39). Throughout this study, Gencode v34 genome annotation was used as the reference gene annotations wherever applicable.
Differential Gene Expression Analysis
Due to the difference in sample sizes of males and females in this study, differential gene expression analysis was performed separately within each sex. Transcript expression quantification from kallisto were aggregated to gene level with the tximport (v1.14.2) (40) package, and differential gene expression analysis was carried out with DESeq2 (v1.26) (41), comparing samples of each sex in the GnRH-treated to vehicle-treated conditions.
Gonadectomy
Ovariectomies and castrations were performed in 6- to 8-week-old mice following McGill standard operating procedures #206 and #207. Sham controls were subjected to the same surgical procedures, but the gonads were not removed.
Natural Ovulation and Breeding Trials
Eight-week-old control and cKO females were paired with wild-type C57BL/6 males purchased from Charles River. Females were inspected daily at 07h00 for vaginal plugs. Females were then euthanized, and cumulus-oocyte complexes were harvested in PBS from ampullae of the oviducts on both sides. Cumulus cells were dissociated from the oocytes by incubating with 0.5 mg/mL hyaluronidase for 10 minutes at 37°C. The total number of oocytes retrieved from each female was counted under an inverted microscope.
For breeding trials, 8-week-old females were paired with wild-type C57BL/6 males for 6 months. Litter deliveries as well as number of pups per litter were recorded. Pups were removed from the breeders after 14 days to avoid interference with subsequent litters.
Tissue Collection
Six- to 10-week-old control and cKO mice (intact, sham operated, or gonadectomized) were sacrificed for organ and tissue collection. For intact female mice, vaginal smears were examined daily using 0.1% methylene blue to assess estrous cyclicity as previously described (42). Intact females were sacrificed on estrus morning (07h00). Male pituitaries, as well as pituitaries from animals subjected to surgeries, were collected at random times during the light phase of the light-dark cycle. Pituitaries were snap frozen in liquid N2 for later RNA extraction. Reproductive organs (testes, seminal vesicles, and one ovary) were weighed and then snap frozen in liquid N2. The remaining ovary was fixed overnight in 10% formalin. The next day, the ovary was washed twice with 1X PBS and then incubated in 70% ethanol. Fixed ovaries were sent for paraffin embedding, sectioning, and hematoxylin and eosin staining at the McGill Centre for Bone and Periodontal Research. All ovary sections were imaged using a Leica DM1000 microscope coupled to a Leica DFC310 FX digital color camera. Preovulatory follicles and corpora lutea in each image were identified by their histological features and counted in all images, avoiding double-counts. For sperm counts, after sacrifice, epididymal cauda from control and cKO animals were retrieved and placed in PBS. Epididymides were cut to allow sperm to swim out. Number of sperm from each epididymis was counted with a hemocytometer.
Blood Collection and Hormone Assays
Blood was collected by cardiac puncture and centrifuged at 800×g for 10 minutes to retrieve the serum. Samples were then diluted 1:10 or 1:20 in PBS with a low-concentration detergent solution for analyses of serum FSH and LH, respectively. FSH or LH levels were assessed using in-house ELISAs as previously described (43, 44). Detection ranges were 0.03125 to 0.5 ng/mL for FSH and 0.117 to 30 ng/mL for LH. For the FSH ELISA, we used the following capture [guinea pig anti-mouse-FSH antibody AFP-1760191, from National Institute of Diabetes and Digestive and Kidney Diseases-National Hormone & Pituitary Program (NIDDK-NHPP); RRID: AB_2665512] and detection [rabbit anti-rat-FSH antiserum FSH-S-11, AFP-C0972881, NIDDK-NHPP; RRID: AB_2687903] antibodies. For the LH ELISA, we used the following capture [anti-bovine LH beta subunit, 518B7; University of California; RRID: AB_2756886] and detection [rabbit LH antiserum, AFP240580Rb; NIDDK-NHPP; RRID: AB_2665533] antibodies.
Cell Culture and Plasmid Transfections
The expression plasmid for N-Flag-tagged ATF3 was purchased from Addgene (26 115) (45). The JunB expression vector was a gift from Dr. Alain Mauviel (Institut Curie, France) (46). The c-Fos expression plasmid was a gift from Dr. Dawn Devall (University of Massachusetts, MA, USA) (47). Rat ALK4-TD in pcDNA3.0 was a gift from Dr. Teresa Woodruff (Northwestern University, Evanston, IL, USA). Murine −1990/+1 Fshb-luc (48) and −232/+5 Lhb-luc were described previously (49). LβT2b cells were from Dr. Pamela Mellon [University of California, San Diego, CA, USA; refer to (50) for fingerprinting pattern for this subclone]. Cells were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C/5%CO2. For luciferase reporter assays, LβT2b cells were seeded at a density of 1.5 × 105 cells per well in 48-well plates. The following day, cells were transfected with promoter-reporter constructs and different expression plasmids as indicated in each figure legend using Lipofectamine 3000 following the manufacturer's instructions. The next day, cells were starved overnight in serum-free DMEM. The day after, cell lysates were prepared in passive lysis buffer and collected as previously described (51). Luciferase assays were performed on an Orion II microplate luminometer (Berthold Detection Systems, Oak Ridge, TN, USA). All conditions were performed in triplicate wells in at least 3 independent experiments. For gene expression analyses, cells were seeded at a density of 2 × 106 cells per well in 6-well plates. For Figure 1, the day after seeding, cells were starved overnight in serum-free DMEM. The next day, cells were treated as follows: For Figure 1D cells were treated with 10 nM GnRH and harvested with 1 mL TRIzol at 0, 15, 30, 60, 90, and 120 minutes after treatment started. For Figure 1E, cells were preincubated with 50 µM BAPTA-AM for 1 hour, then treated with 10 nM GnRH for 1 hour and harvested with 1 mL TRIzol. For Figure 2C, the day after seeding, cells were transfected with expression plasmids for ATF3, Junb, ALK4-TD, or empty vector (pcDNA3.0) using Lipofectamine 3000 following the manufacturer's instructions. On the next day, cells were starved overnight with serum-free DMEM. Cells were harvested with 1 mL TRIzol.
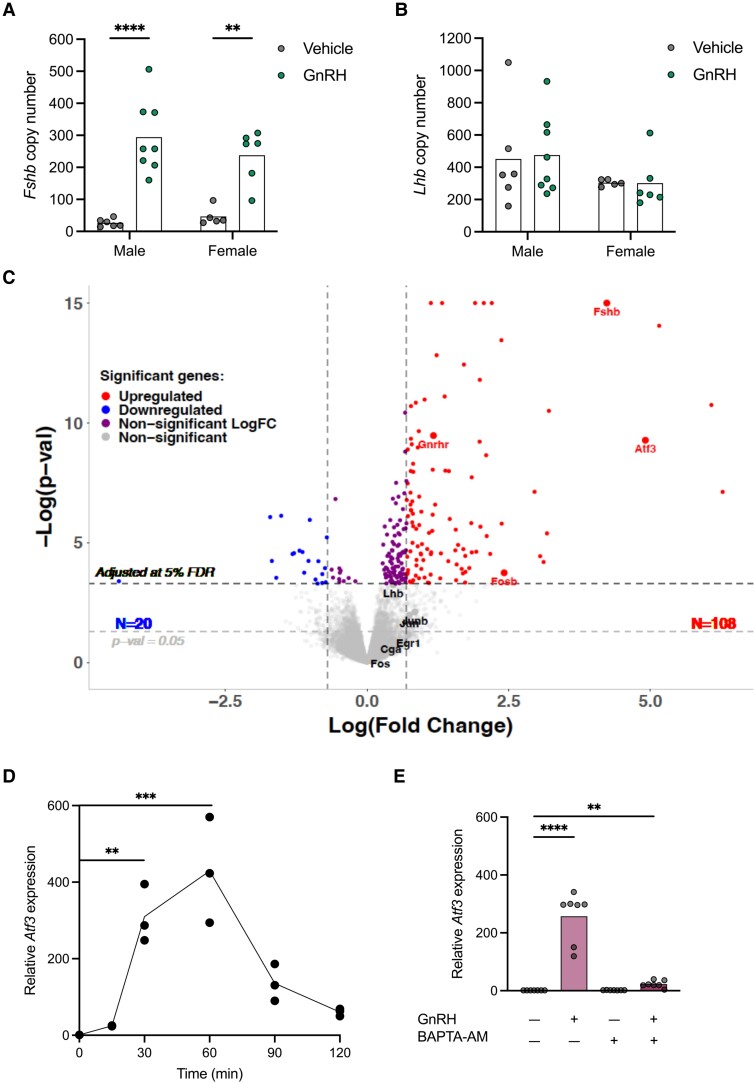
Figure 1.
GnRH induces Atf3 expression. (A-C) Hpg mice were treated once daily with vehicle or 1 µg GnRH i.p. for 10 consecutive days. GnRH-induced changes in pituitary (A) Fshb and (B) Lhb mRNA levels were assessed by qPCR from whole pituitaries. Shown are the relative copy number for each transcript for one representative experiment out of 2. Twenty-five animals were assessed, including 11 females (5 vehicle and 6 GnRH-treated) and 14 males (6 vehicle and 8 GnRH-treated). Data were analyzed by two-way ANOVA. (C) Pituitary RNA from male mice treated as in panels A and B was subjected to bulk RNA-seq. Volcano plot showing up (red) and down (blue) regulated genes. Select genes are labeled. (D) LβT2b cells were treated with 10 nM GnRH for the indicated lengths of time. RNA was collected at each time point and Atf3 expression was assessed by RT-qPCR. Each point represents an experimental replicate, n = 3. Groups were compared using one-way ANOVA followed by Tukey's multiple comparisons test. (E) LβT2b cells were pre-incubated with vehicle or 50 µM BAPTA-AM for 1 hour and then treated with 10 nM GnRH for 1 hour. Atf3 expression was assessed by RT-qPCR. Each point represents an experimental replicate. Bars represent the mean of the experimental replicates, n = 7. Groups were compared using the Kruskal-Wallis test followed by Dunn's correction for multiple comparisons. Asterisks indicate significant differences: ** P < 0.01; *** P < 0.001; **** P < 0.0001.
Abbreviations: ANOVA, analysis of variance; GnRH, gonadotropin-releasing hormone; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; RNA-seq, RNA sequencing; RT-qPCR, real time PCR.
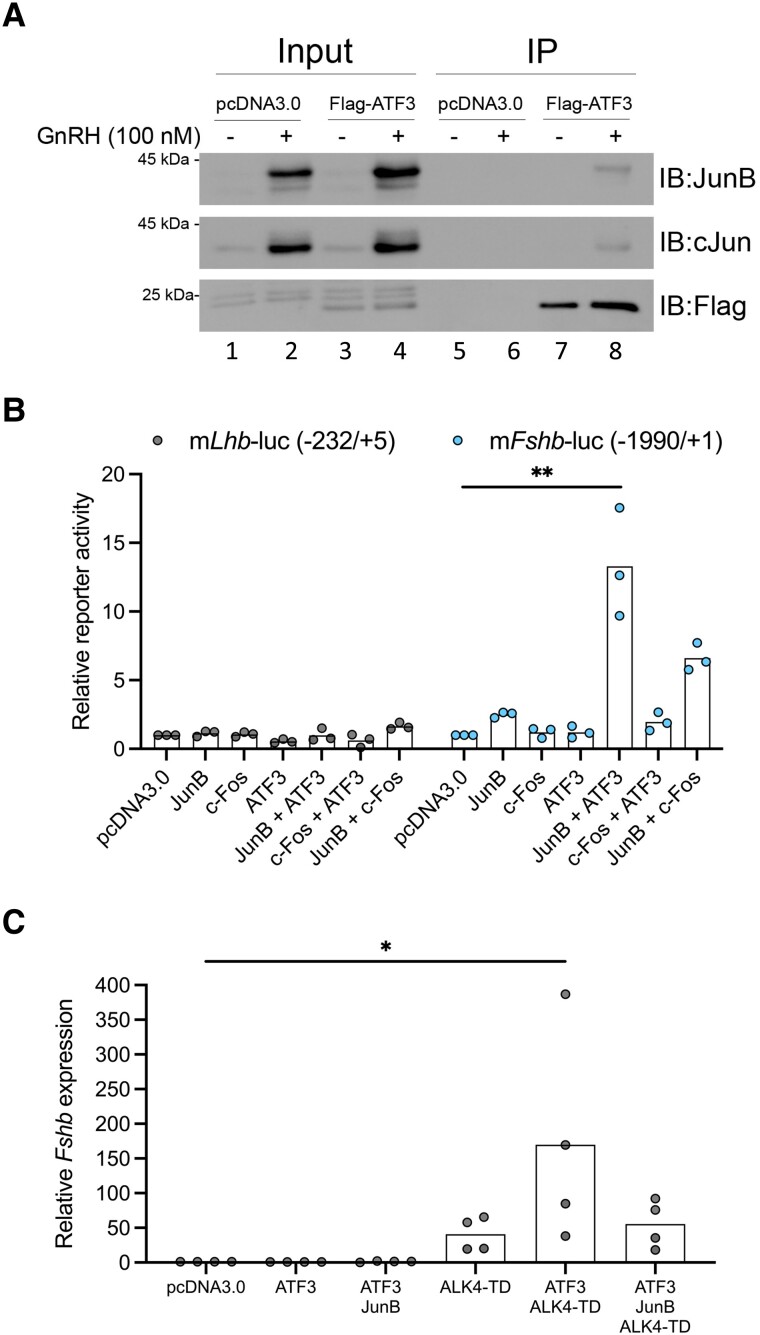
Figure 2.
ATF3 physically and functionally interacts with JunB. (A) LβT2b cells were transfected with 500 ng Flag-ATF3 or empty vector (pcDNA3.0). The next day, cells were treated with vehicle or 100 nM GnRH for 2 hours. Flag-ATF3 was immunoprecipitated with anti-Flag conjugated agarose beads. Input and eluates were subjected to sodium dodecyl sulfate-PAGE and immunoblotting with JunB, cJun, and Flag antibodies. Images shown are representative of 3 independent experiments. (B) LβT2b cells in 48-well plates were transfected with murine Lhb or Fshb promoter-reporters (225 ng/well) and with the indicated combinations of expression vectors for JunB (50 ng/well), c-Fos (50 ng/well), and/or ATF3 (50 ng/well). All conditions were balanced with pcDNA3.0. Cells were starved overnight, and reporter activity was measured the following day. Each point represents the average of technical triplicates in independent experiments, n = 3. (C) LβT2b cells in 6-well plates were transfected with the indicated expression vectors for ATF3, JunB, and ALK4-TD (500 ng/well each). The amount of transfected DNA was balanced with empty vector (pcDNA3.0). Cells were starved overnight, and RNA was extracted the following day. Fshb mRNA levels were measured by RT-qPCR using the pcDNA3.0 condition as control and ribosomal protein L19 (Rpl19) as the housekeeping gene. Each point represents the average of technical triplicates from independent experiments, n = 4. Bars represent the mean of the experimental replicates. Groups were compared using the Kruskal-Wallis test followed by Dunn's correction for multiple comparisons. Asterisks indicate significant differences: * P < 0.05, ** P < 0.01.
Abbreviations: GnRH, gonadotropin-releasing hormone; mRNA, messenger RNA: RT-qPCR, real time quantitative polymerase chain reaction.
RNA Extraction and RT-qPCR From Cultured Cells and Pituitaries of Knockout Mice
Frozen pituitaries were thawed and homogenized in TRIzol, and RNA was extracted following the manufacturer's protocol. Total RNA from pituitaries (200 ng) or LβT2b cells (500 ng) was reverse transcribed using Moloney murine leukemia virus reverse transcriptase and random hexamer primers. qPCR reactions were performed using Blastaq Master mix on a Corbett Rotorgene 6000 instrument (Corbett Life Science, Mortlake, NSW, Australia). For pituitary cDNA, expression of Rpl19, Fshb, Lhb, Cga, Gnrhr, Atf3, Jun, Junb, Fos, and Egr1 was analyzed in duplicate. For LβT2b cDNA, expression of Rpl19, Atf3, Fshb, and Junb was analyzed in triplicate. Gene expression was determined relative to that of the reference gene Rpl19 using the 2-ΔΔCt method. Primer sets are listed in Table 1.
Immunoprecipitation and Western Blotting
For immunoprecipitation experiments, 1.5 × 106 LβT2b cells were seeded in 6-well plates and transfected the next day with 500 ng of pcDNA3.0 or Flag-ATF3 expression plasmid using Lipofectamine 3000. The next day, cells were starved for 2 hours and then treated with vehicle (serum-free DMEM) or 100 nM GnRH for 2 hours. After treatment, cells were harvested in 300 µL of immunoprecipitation buffer [50 mM tris(hydroxymethyl)-aminomethane (Tris) pH 7.0, 150 mM NaCl, 1 mM ethylenediamine tetra-acetate, 0.1% Triton X-100], and incubated on ice for 15 minutes on an orbital mixer. Lysates were sonicated 3 times for 15 seconds at 15-second intervals at 3 W. Lysates were cleared by centrifugation at 12 000×g for 20 minutes at 4°C. An aliquot of 50 µL (input) was stored at −20°C, and the balance was incubated with pre-equilibrated Flag beads overnight at 4°C on a rotator. Next, beads were washed twice with 1X Tris-buffered saline (TBS) and incubated with 3xFlag peptide (125 ng/µL) for 2 hours at 4°C on a rotator to elute bound proteins. Western blotting was carried out as previously described (52, 53). Twenty-five µL of eluted proteins and inputs were mixed with 5X Laemmli buffer (250 mM Tris, 10% w/v sodium dodecyl sulfate, 0.2% w/v bromophenol blue, 50% v/v glycerol) and proteins were resolved as previously described in (53) using 10% sodium dodecyl sulphate–polyacrylamide gels (acrylamide:bis-acrylamide 37.5:1). Proteins were transferred to nitrocellulose membranes, which were blocked with 5% skimmed milk in Tris-buffered saline with 0.1% Tween® 20 detergent (0.05% Tween-20) for 1 hour at room temperature. Membranes were then incubated with antibodies against cJun (1:500), JunB (1:500), or Flag (1:2000) overnight at 4°C. The following day, membranes were washed 3 x 10 minutes with Tris-buffered saline with 0.1% Tween® 20 detergent and incubated with secondary antibodies against mouse (JunB) or rabbit (cJun, Flag) immunoglobin G conjugated to horseradish peroxidase. Bands were visualized using commercial electrochemiluminescence as per manufacturer instructions and imaged using an Amersham Imager 600 (GE Healthcare). Between each primary antibody incubation, membranes were stripped with 0.2 M NaOH for 40 minutes at room temperature and reblocked with 5% skimmed milk.
Statistical Analysis
Data from control and cKO mice (intact, sham, and gonadectomized groups), luciferase assays, and gene expression experiments were compared with Welch's t-tests (serum hormone data in intact animals), Mann-Whitney U, or Kruskal-Wallis tests (gene expression data), analyses of variance [ANOVA; serum hormone data in gonadectomized (GDX) vs intact animals], or a restricted maximum likelihood mixed effects model (LH release over time) as indicated in the figure legends, using GraphPad 9.0 (La Jolla, CA, USA). Post hoc pairwise comparisons were made with Tukey corrections for multiple comparisons (one-way ANOVA), Bonferroni corrections (two-way ANOVA), or Dunn's corrections (Kruskal-Wallis). Alpha was set at P = .05.
Results
Once Daily GnRH Potently Stimulates Fshb and Atf3 Expression in the Murine Pituitary
We treated hpg mice once daily with saline (vehicle) or 1 µg of GnRH i.p. for 10 consecutive days. One hour following the last injection, we observed marked increases in pituitary Fshb but not Lhb mRNA levels with this treatment paradigm in both males and females (Fig. 1A and 1B). We next performed bulk RNA-seq to assess GnRH-induced changes in pituitary gene expression. In accordance with the RT-qPCR results, Fshb was among the most upregulated genes in response to GnRH [Fig. 1C and Supplementary Fig. S1 (54).; Tables 2 and 3]. Gnrhr, but not Lhb or Cga, mRNA levels were also significantly induced by GnRH in males. Activating transcription factor 3 (Atf3; previously called LRG-21), an immediate early gene, was among the most upregulated genes in this analysis. In contrast, Fos, Jun, Junb, and Egr1 were not significantly induced, at least not in this analysis [Fig. 1C and Supplementary Fig. S1 (54)]. Fosb was induced in males only. A full list of differentially expressed genes can be found in Supplementary Tables S1 and S2 (54). As our goal was to identify GnRH-induced transcription factors that might stimulate Fshb transcription in an analogous manner to EGR1's regulation of Lhb, we focused our attention on Atf3.
Table 2.
GnRH-induced genes in male pituitaries
List of top (fold) significantly upregulated genes in pituitaries of male hpg mice treated once daily with 1 µg GnRH for 10 consecutive days as determined by bulk RNA-seq
| Gene | Log2 fold change | Adjusted P-value |
|---|---|---|
| Sprr1a | 6.272709 | 4.56E-05 |
| Npy | 6.07413 | 2.88E-08 |
| Rrad | 5.153462 | 3.05E-11 |
| Atf3 | 4.909689 | 5.79E-07 |
| Fshb | 4.23021 | 2.27E-25 |
| Homer1 | 3.209056 | 4.47E-08 |
| Cldn1 | 3.175397 | .001183 |
| Egr3 | 3.115625 | .010628 |
| Arc | 3.05516 | .006801 |
| Gm13889 | 2.955878 | 4.56E-05 |
| Fosb | 2.419963 | .024105 |
| Nr4a3 | 2.376182 | .000557 |
| Slc4a11 | 2.371501 | 1.07E-10 |
| Phex | 2.201548 | 3.22E-14 |
| Egr2 | 2.173954 | .005868 |
| Vgf | 2.11232 | .001454 |
| Kcna4 | 2.102733 | 1.92E-06 |
| Nefm | 2.060885 | 3.74E-27 |
| Fa2h | 2.001593 | .000698 |
| Serpinb1a | 1.99041 | 3.50E-09 |
| Lrrc2 | 1.928273 | .005312 |
Genes of particular interest are bolded.
Abbreviations: GnRH, gonadotropin-releasing hormone; RNA-seq, RNA sequencing.
Table 3.
GnRH-induced genes in female pituitaries
List of top (fold) significantly upregulated genes in pituitaries of female hpg mice treated once daily with 1 µg GnRH for 10 consecutive days as determined by bulk RNA-seq
| Gene | Log2 fold change | Adjusted P-value |
|---|---|---|
| Sprr1a | 10.04407 | 3.76E-08 |
| Atf3 | 5.347378 | 1.43E-08 |
| Npy | 4.644629 | .000506 |
| Bpifa1 | 4.467802 | .202106 |
| Rrad | 4.230058 | 1.37E-14 |
| Trim38 | 3.484956 | .00099 |
| Gm13889 | 3.251251 | 9.92E-07 |
| Foxf1 | 3.043353 | .000541 |
| Fshb | 2.98742 | 1.1E-06 |
| Grm4 | 2.750789 | .005369 |
| Homer1 | 2.663686 | .004503 |
| Slc4a11 | 2.543487 | 2.83E-05 |
| Arc | 2.016107 | .00155 |
| Nptx2 | 1.984718 | .020328 |
| Serpinb1a | 1.947116 | .001337 |
| Lrrc2 | 1.893953 | .061562 |
| Kcna4 | 1.89138 | 9.92E-07 |
| Timp1 | 1.803807 | 1.81E-09 |
| Nefl | 1.802612 | 9.85E-12 |
Genes of particular interest are bolded.
Abbreviations: GnRH, gonadotropin-releasing hormone; RNA-seq, RNA sequencing.
GnRH Induction of Atf3 Is Calcium-dependent in LβT2 Cells
Atf3 was induced by GnRH in LβT2 cells (by microarray) or by the GnRH analog, Buserelin, in pituitaries of ovariectomized/GnRH-immunized mice (36, 55). Here, we similarly observed rapid, robust, and transient induction of Atf3 by GnRH in LβT2b cells (Fig. 1D). Fshb expression was not upregulated in this experiment (data not shown). However, these cells need to be incubated for an additional 2 hours to reliably observe an increase in Fshb levels (53). We recently reported that FSH production is reduced in mice lacking Gαq/11 in their gonadotropes and in mice with a modified GnRHR that alters GnRH-induced Ca2+ signaling (25, 53). Therefore, we examined whether GnRH stimulation of Atf3 expression was similarly Ca2+ dependent. Preincubation of LβT2b cells with the intracellular Ca2+ chelator BAPTA-AM abolished Atf3 induction by GnRH (Fig. 1E). BAPTA-AM also blocks GnRH-stimulated Fshb in these cells (53).
ATF3 Synergizes With JunB to Stimulate Fshb Promoter-reporter Activity
ATF3 is a bZIP transcription factor with similarity to Fos proteins (56). Like Fos, it can heterodimerize with Jun family members (57, 58). We therefore examined potential physical and functional cooperativity between the ATF3 and Jun proteins. We first transfected LβT2b cells with empty expression vector (pcDNA3.0) or a vector encoding N-Flag-tagged ATF3. We then treated cells with vehicle or 100 nM GnRH for 2 hours to induce expression of endogenous Jun proteins. Both JunB and cJun were upregulated (lanes 2 and 4) and coimmunoprecipitated with Flag-ATF3 in GnRH-treated cells (lane 8, Fig. 2A). When coexpressed in LβT2b cells, ATF3 and JunB stimulated murine Fshb promoter-reporter activity, significantly more so than the cFos-ATF3 or cFos-JunB combinations (Fig. 2B). In contrast, ATF3-JunB did not stimulate murine Lhb promoter-reporter activity (Fig. 2B).
ATF3 Synergizes With ALK4-TD to Induce Fshb mRNA Expression
We next examined ATF3's capacity alone and in combination with JunB to stimulate endogenous Fshb expression in LβT2b cells. In contrast to the results with the Fshb promoter-reporter, overexpressed ATF3 and JunB did not increase Fshb mRNA levels (Fig. 2C). GnRH can synergistically stimulate Fshb expression with activins (25). We therefore asked whether the effects of ATF3 on the endogenous Fshb locus, which is tightly compacted in LβT2 cells (34), might depend on contemporaneous activin signaling. Therefore, we transfected LβT2b cells with a constitutively active form of the activin type I receptor, ALK4 (ALK4-TD). As previously reported, ALK4-TD stimulated Fshb expression (48) (Fig. 2C). Interestingly, this effect was enhanced by overexpressed ATF3 alone but not with JunB (Fig. 2C).
Atf3 Is Not Required for Gonadotropin Production In Vivo
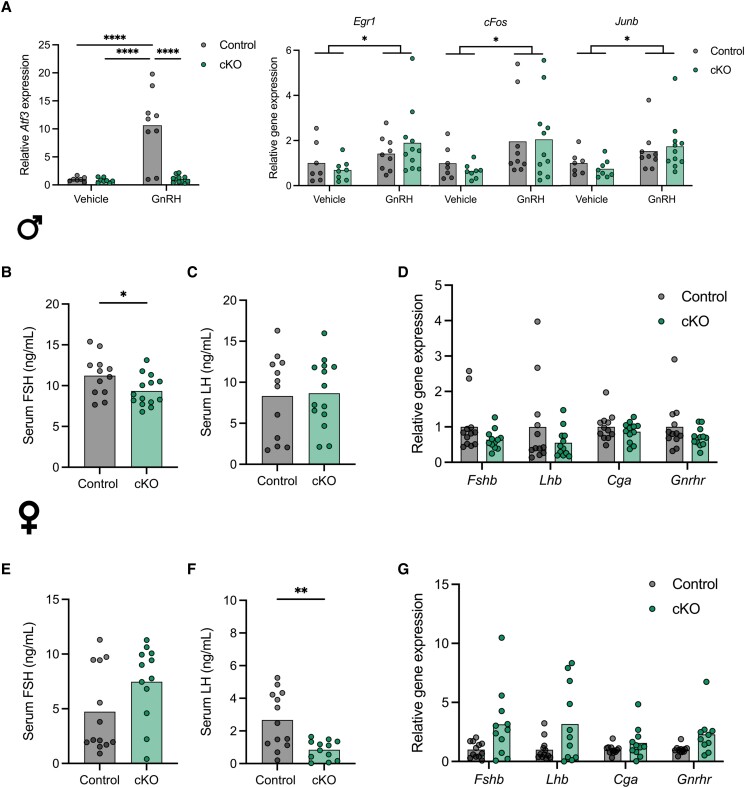
In light of these in vitro observations, we next examined ATF3's role in FSH production in vivo. We generated conditional (gonadotrope-specific) knockouts (cKO) by crossing Atf3fx/fx and Gnrhr-IRES-Cre (GRIC) mice. Atf3fx/fx (without Cre) littermates were used as controls. To demonstrate the efficacy of the gene knockout, we treated adult control and cKO mice with 1 µg GnRH. After 1 hour, pituitaries were collected and Atf3 mRNA levels assessed by RT-qPCR. GnRH stimulated a robust increase in Atf3 expression in controls that was completely blocked in cKOs (Fig. 3A, left panel). GnRH also induced modest but significant increases in Egr1, Fos, and Junb (Fig. 3A, right panels) but not Jun (not shown) mRNA levels after 1 hour, which did not differ between cKO and control mice.
Figure 3.
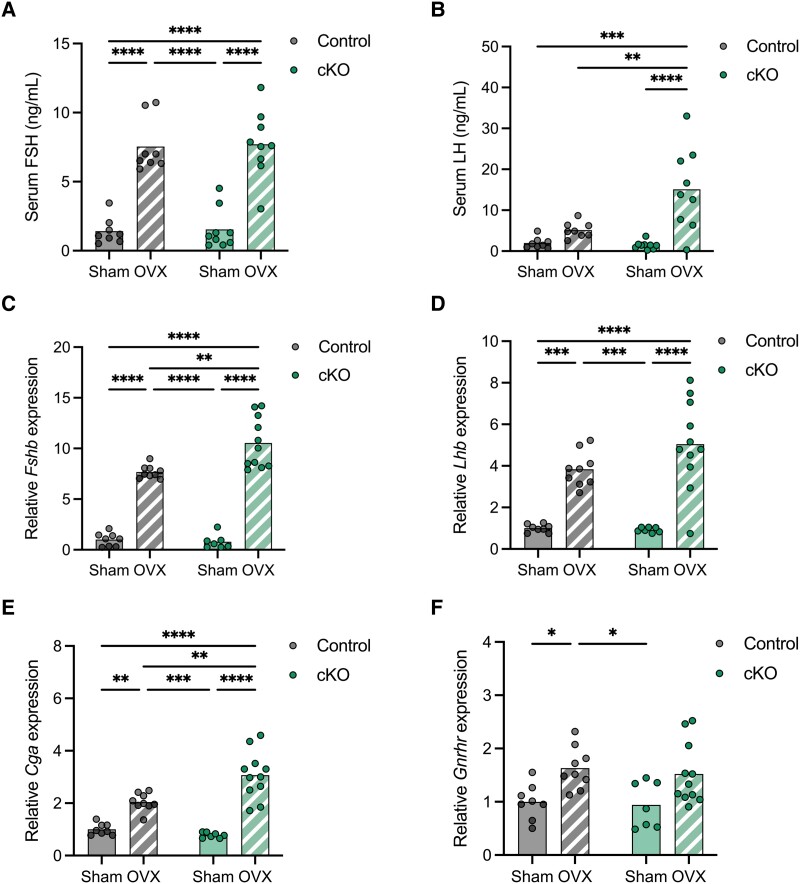
Gonadotrope-specific deletion of Atf3 does not impair gonadotropin production in male or female mice. (A) Adult gonadotrope-specific Atf3 cKO (green) or control mice (gray) of both sexes were treated once intraperitoneally with GnRH or vehicle. After 1 hour, pituitaries were harvested for analysis of Atf3, Egr1, Fos, and Junb mRNA levels by RT-qPCR. Data were normalized to the vehicle treated controls, and ribosomal protein L19 (Rpl19) was the housekeeping gene. Serum FSH (B) and LH (C) levels in 8- to 10-week-old control and cKO males were measured by ELISA; n = 12, Control; n = 14, cKO. Serum FSH (E) and LH (F) levels in 9- to 12-week-old control and cKO females on estrus morning were measured by ELISA. n = 13, Control; n = 12, cKO. Gene expression in pituitary glands from 8- to 10-week-old control and cKO males (D) or 9- to 12-week-old females (G). Fshb, Lhb, Cga, and Gnrhr mRNA levels were assessed by RT-qPCR. Results were normalized to control animals. Males: n = 12, Control; n = 12, cKO. Females: n = 12, Control; n = 11, cKO. In all panels bars represent group means. Each point represents an individual animal. In (A), groups were compared using two-way ANOVA followed by Bonferroni correction for multiple comparisons. In (B)-(G), all pairwise comparisons were done using t-tests. Asterisks indicate significant differences: * P < 0.05; ** P < 0.01; **** P < 0.0001.
Abbreviations: ANOVA, analysis of variance; cKO, conditional knockout; ELISA, enzyme-linked immunosorbent assay; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; mRNA, messenger RNA; RT-qPCR, real time quantitative polymerase chain reaction.
Having confirmed the efficacy of the knockout, we measured circulating gonadotropin levels and reproductive organ weights in adult control and cKO mice of both sexes. In a first cohort, serum FSH levels were modestly lower in cKO than in control males (Fig. 3B). Serum LH levels did not differ between genotypes (Fig. 3C). There were no genotype differences in pituitary expression of Fshb, Lhb, Cga, or Gnrhr in males (Fig. 3D). In females, serum FSH levels trended higher in cKOs than in controls on the morning of estrus, though this was not statistically significant (Fig. 3E). In contrast, cKO females exhibited lower LH levels than controls (Fig. 3F). At the pituitary level, Fshb, Lhb, Cga, and Gnrhr mRNA levels appeared higher in cKO than control females, but these trends were not statistically significant (Fig. 3G).
Reproductive Organs and Fertility Are Normal in Atf3 cKO Mice
In males, there were no genotype differences in seminal vesicle or testis weights or in epidydimal sperm counts (Fig. 4A-4C). Uterine weights were modestly but significantly greater in cKO than control females (Fig. 4D), but their ovarian weights were similar (Fig. 4E). The number of antral follicles and corpora lutea did not differ significantly between genotypes (Fig. 4F). cKO females appeared to ovulate fewer eggs than controls in natural cycles (on estrus morning), but this difference was not statistically significant (Fig. 4G). Moreover, there were no differences in litter sizes between cKO and control females (Fig. 4H) or in the number of litters per month (data not shown). We did not conduct breeding trials in males.
Figure 4.
Gonadotrope-specific Atf3 knockout mice are normogonadal and fertile. Seminal vesicle (A) and testes (B) weights in control and cKO males. Organ weights were normalized to body weight. n = 13, Control; n = 13, cKO. (C) Epididymal sperm counts in control and cKO animals. n = 9 Control; n = 9, cKO. Uterine (D) and ovarian (E) weights in control or cKO females. Organ weights were normalized to body weight. n = 13, Control; n = 12, cKO. (F) Number of antral (preovulatory) follicles and corpora lutea in one ovary from control and cKO females. n = 4, Control; n = 4, cKO. (G) Control and cKO females were mated at 9 weeks of age with wild-type C57BL/6 males and cumulus-oocyte complexes counted on the morning of vaginal plugging. n = 5, Control; n = 6, cKO. (H) Nine-week-old females from both genotypes were mated with wild-type C57BL/6 males for 6 months and the number of pups per litter was monitored. The average per female is shown. n = 6, Control; n = 5, cKO. Each point represents one animal. Bars represent group means. In all cases groups were compared using t-tests. An asterisk (*) indicates significant differences: P < 0.05.
Abbreviations: cKO, conditional knockout.
Post-gonadectomy Increases in LH Secretion Are Enhanced in Atf3 cKO Animals
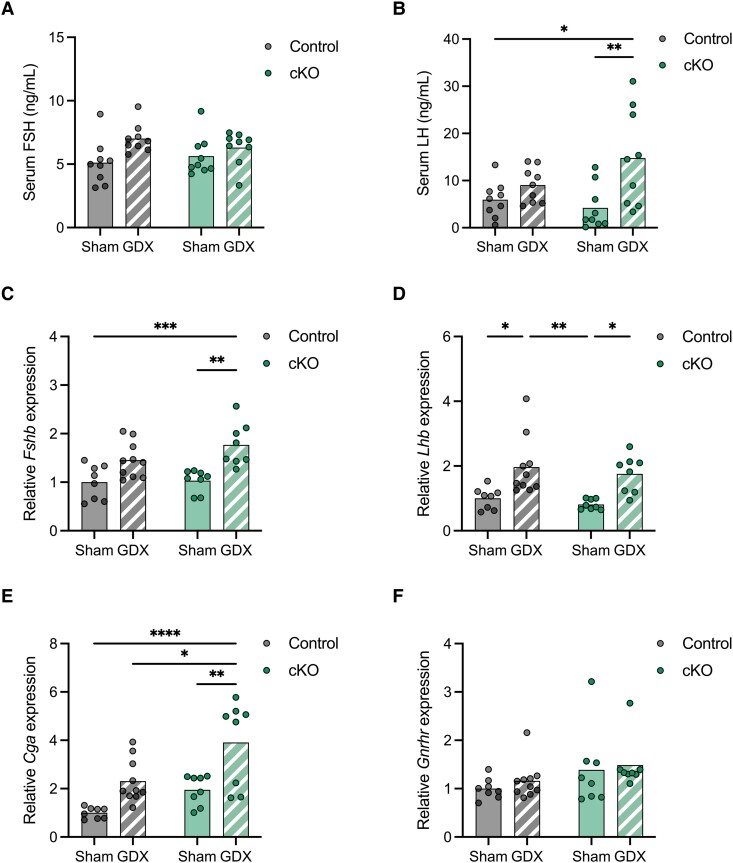
To further assess potential changes in gonadotropin regulation in Atf3 cKO mice, we gonadectomized animals (GDX) to increase endogenous GnRH secretion and subsequent signaling in gonadotropes. Depending on the strain, FSH either increases post-castration in male mice (59) or is unchanged (53). Here, there was a nonsignificant trend for serum FSH to be higher in GDX than sham-operated males, but there was no difference between control and cKO mice (Fig. 5A). Interestingly, pituitary Fshb and Cga mRNA levels were elevated in GDX males, but this was only significant in cKOs (Fig. 5C and 5E). Serum LH levels were higher in GDX than sham males, but this was statistically significant in cKO mice only (Fig. 5B). Pituitary Lhb mRNA levels were higher in GDX than in sham-operated males in both genotypes, which did not differ (Fig. 5D). Pituitary Gnrhr mRNA levels were not affected by gonadal status or genotype (Fig. 5F).
Figure 5.
LH but not FSH secretion is enhanced in castrated Atf3 cKO males. Ten- to 12-week-old control or cKO males were sham-operated (Sham, solid color) or GDX (hatched). Serum FSH (A) and LH (B) levels were measured by ELISA. n = 9, Sham-Control; n = 9, GDX-Control; n = 9, Sham-cKO; n = 9, GDX-cKO. Pituitary RNA was isolated and Fshb (C), Lhb (D), Cga (E), and Gnrhr (F) mRNA levels were assessed by RT-qPCR. n = 8, Sham-Control; n = 10, GDX-Control; n = 8, Sham-cKO; n = 8, GDX-cKO. Points and bars represent individual animals and group means, respectively. Groups were compared using two-way ANOVA followed by the Bonferroni correction for multiple comparisons. Asterisks indicate significant differences: *: P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Abbreviations: ANOVA, analysis of variance; cKO, conditional knockout; FSH, follicle-stimulating hormone; GDX, gonadectomized; LH, luteinizing hormone; mRNA, messenger RNA; RT-qPCR, real time quantitative polymerase chain reaction.
In females, serum FSH levels were higher in ovariectomized (OVX) relative to sham-operated mice. This was true in both controls and cKOs, which did not differ (Fig. 6A). However, OVX-associated increases in pituitary Fshb and Cga mRNA levels were more pronounced (significantly so) in cKOs relative to controls (Fig. 6C and 6E). As in males, serum LH levels were higher in OVX compared to sham-operated mice, but this was only statistically significant in cKO animals (Fig. 6B). Pituitary Lhb mRNA levels were greater in OVX than sham females of both genotypes, which did not differ (Fig. 6D). Gnrhr mRNA levels were higher in OVX females, but this was only statistically significant in the control genotype (Fig. 6F).
Figure 6.
LH but not FSH secretion is enhanced in ovariectomized Atf3 cKO females. Ten- to 12-week-old control or cKO females were sham-operated (Sham, solid color) or OVX (hatched). Serum FSH (A) and LH (B) levels were measured by ELISA. n = 8, Sham-Control; n = 8, OVX-Control; n = 9, Sham-cKO; n = 9, OVX-cKO. Pituitary RNA was isolated and Fshb (C), Lhb (D), Cga (E), and Gnrhr (F) mRNA levels were assessed by RT-qPCR. n = 8, Sham-Control; n = 9, OVX-Control; n = 7, Sham-cKO; n = 11, OVX-cKO. Points and bars represent individual animals and group means, respectively. Groups were compared using two-way ANOVAs and the Bonferroni correction for multiple comparisons was used. Asterisks indicate significant differences: * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Abbreviations: ANOVA, analysis of variance; cKO, conditional knockout; FSH, follicle-stimulating hormone; LH, luteinizing hormone; mRNA, messenger RNA; OVX, ovariectomized; RT-qPCR, real time quantitative polymerase chain reaction.
Kisspeptin Stimulates LH Release Equivalently in Control and cKO Females
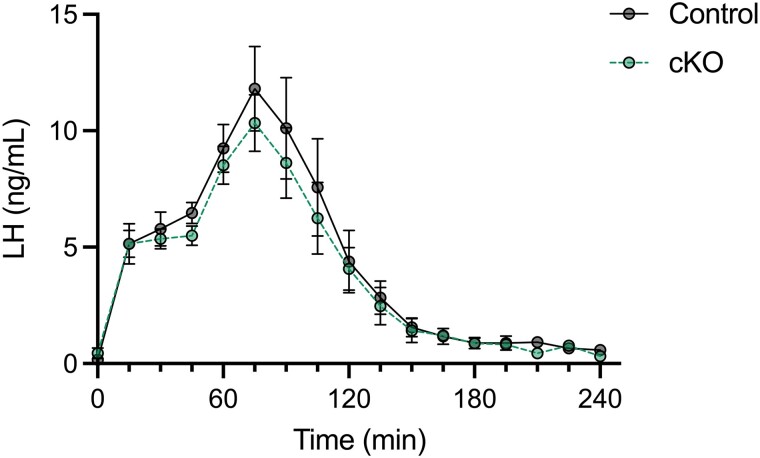
As LH secretion appeared to be enhanced post-gonadectomy in cKO mice, we examined the effects of the gene knockout on kisspeptin-stimulated LH release. We recently reported that exogenous kisspeptin-54 stimulates a pattern of LH release in juvenile female mice similar to that of the endogenous LH surge in adult females (43). Here, a single injection of kisspeptin-54 stimulated a robust increase in LH secretion in both control and cKO females, with no differences between them (Fig. 7).
Figure 7.
Kisspeptin-induced LH release is not altered in Atf3 cKO females. Secretion of LH in response to kisspeptin-54 in juvenile control (grey, continuous line) and cKO (green, dashed line) females. Twenty-eight- to 30-day old females from each genotype were injected with 1 nmol of Kisspeptin-54 at time 0. Then blood was collected at 15-minute intervals and assayed for LH by ELISA. Each point represents the group mean ± SEM. n = 5 per genotype. Groups were compared using a mixed effects model using restricted maximum likelihood estimation.
Abbreviations: cKO, conditional knockout; LH, luteinizing hormone.
Discussion
The mechanisms through which GnRH regulates Fshb expression have not been established. Here, we used an in vivo screening approach in an attempt to address this gap. We operated under the assumption that GnRH induces the production of a transcription factor that stimulates Fshb, akin to GnRH's regulation of Lhb transcription via EGR1 (9, 15, 60). Rather than examining the effects of pulsatile GnRH, we used once-daily GnRH, which stimulates FSH but not LH production in hpg mice (7). This enabled us to examine mechanisms that might mediate GnRH-dependent FSH synthesis in isolation. ATF3 emerged as an attractive candidate given its concordant upregulation with Fshb. Moreover, the ATF3 protein synergistically stimulated Fshb but not Lhb promoter-reporter activity with JunB in homologous LβT2b cells. Nevertheless, conditional deletion of Atf3 in gonadotropes did not impair FSH synthesis in mice in vivo. Indeed, if anything, ATF3 appeared to predominantly play a repressor role in gonadotropes, as post-castration and -ovariectomy increases in Fshb and Cga mRNA levels were modestly enhanced in Atf3 cKOs. In light of our data, ATF3 can be added to a growing list of factors implicated in Fshb regulation in vitro [cf (61)] that later proved to be dispensable, or at least nonessential, in vivo (26, 62-64).
Indeed, it was our knowledge of this history that motivated us to screen for GnRH-induced transcription factors in vivo rather than in vitro. Though Atf3 was previously shown to be robustly upregulated by a GnRHR agonist in LβT2 cells (11), we nevertheless pursued it here given that (1) it was among the most upregulated genes in gonadotropes in vivo in our RNA-seq analysis and (2) other transcription factors previously implicated in GnRH-regulated Fshb transcription in vitro, such as several Jun and Fos family members (63-65), were not significantly induced by the once-daily GnRH that upregulated both Fshb and Atf3 mRNA levels in hpg mice. Our initial analysis in Atf3 cKO mice was encouraging, as we observed a small but significant decrease in serum FSH in males. However, this was not reproduced in a second cohort of testis-intact (sham) mice or following castration, and pituitary Fshb mRNA levels were elevated in cKO females on estrus morning. Though we did not observe this genotype difference in a second cohort of ovary-intact (sham) females, they were randomly cycling. Therefore, there could be estrous cycle stage-dependent changes in FSH regulation by ATF3 that we did not detect, given the nature and extent of our analyses. That said, we did not observe effects on folliculogenesis, ovulation, or fertility in cKO females, suggesting that any changes in FSH production or secretion were likely to be modest.
We are confident that incomplete knockout (recombination) of the Atf3 locus does not explain the intact FSH production in cKO mice. Indeed, GnRH induction of Atf3 expression was completely blocked in cKO pituitaries. Moreover, these mice displayed statistically significant phenotypes, even if modest in nature. Of particular note, post-gonadectomy increases in LH secretion were enhanced in cKOs relative to controls in both sexes. The underlying mechanism is unclear, as Lhb mRNA levels were statistically equivalent between genotypes, and, at least in vitro, ATF3 did not directly regulate the murine Lhb promoter. As Cga mRNA levels were increased in cKO mice, it is possible that enhanced α rather than β subunit production explains the amplified LH response. It is not clear, however, why this would not have also led to enhanced FSH secretion in gonadectomized cKO mice. As there is often a close relationship between Fshb mRNA levels and FSH secretion (66), it is all the more surprising that serum FSH was not enhanced in these mice given that both FSH subunits were upregulated.
One plausible explanation for the maintenance of FSH production in Atf3 cKO mice is compensation, perhaps by one or more members of the CREB/AP-1 family, given ATF3's similarity to Fos proteins (67, 68). For example, FSH production is not significantly altered in gonadotrope-specific Jun knockout mice (26). However, Junb is upregulated in pituitaries of these animals, raising the possibility that JunB might compensate for the absence of cJun. GnRH induced modest but significant increases in Fos and Junb in pituitaries of both control and Atf3 cKO mice (Fig. 3A; in contrast to the hpg mice, Fig. 1C). Although their induction was not amplified in cKO mice, it is possible that these or other family members may compensate for the absence of Atf3. Still, we appreciate that AP-1 proteins possess similar but not redundant functions (69-71). We should note that ATF3 is structurally related to JDP2 (72), but we do not think that the latter compensates for the former in Atf3 cKO mice. JDP2, but not ATF3, attenuates GnRH induction of Fshb promoter activity in LβT2 cells, and Jdp2 knockouts have distinct pituitary and reproductive phenotypes from those described here (73). Indeed, in our estimation, the reduced FSH levels in Jdp2 knockouts are the best available evidence that AP-1 or related proteins regulate Fshb transcription in vivo.
Establishing whether there is functional redundancy between Fos, Jun, and ATF proteins in FSH regulation may require the conditional deletion of several family members in combination. Knocking out Atf3 and Junb together might be a good starting point. That said, here too, there may be compensation. We also acknowledge that this family of proteins may only play a minor role in GnRH induction of Fshb transcription in vivo as opposed to in vitro. If this is the case, it begs the question of what role, if any, ATF3 plays in GnRH action in gonadotropes given its robust induction.
An important caveat to consider is that we identified ATF3 in the context of once-daily GnRH administration. However, we investigated ATF3's functional role in vivo, where GnRH is released in multiple pulses per day. Therefore, it is possible that GnRH induction of Fshb expression might have been blocked or blunted had we performed the Atf3 knockout in hpg mice treated once per day with GnRH. Such a result, however, would have told us what can happen under the experimental conditions employed rather what does happen in real physiology. As a result, the in vivo screening method we employed may ultimately be limited in its predictive value. Nevertheless, there were several other differentially regulated genes in pituitaries of GnRH-treated hpg mice; many of which have unknown functions in gonadotropes. These represent new candidates for consideration in the continuing effort to determine mechanisms through which GnRH regulates FSH synthesis.
Acknowledgments
The authors thank Drs. Terry Hébert, Alain Mauviel, Pamela Mellon, and Teresa Woodruff for providing reagents.
Contributor Information
Carlos A I Alonso, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Caroline D David, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Chirine Toufaily, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Ying Wang, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Xiang Zhou, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Luisina Ongaro, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
German Nudelman, Department of Neurology, Center for Advanced Research on Diagnostic Assay, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Venugopalan D Nair, Department of Neurology, Center for Advanced Research on Diagnostic Assay, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Frederique Ruf-Zamojski, Department of Neurology, Center for Advanced Research on Diagnostic Assay, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Ulrich Boehm, Department of Experimental Pharmacology, Center for Molecular Signaling, Saarland University School of Medicine, Homburg 66421, Germany.
Stuart C Sealfon, Department of Neurology, Center for Advanced Research on Diagnostic Assay, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Daniel J Bernard, Department of Pharmacology and Therapeutics, McGill University, Montreal, QC H3G 1Y6, Canada.
Funding
Canadian Institutes of Health Research Project Grant PJT-169184 (D.J.B.); National Institutes of Health Grant DK46943 (S.C.S.).
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or GEO GSE220809.
References
- 1. Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15(2):205‐206. [DOI] [PubMed] [Google Scholar]
- 2. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201‐204. [DOI] [PubMed] [Google Scholar]
- 3. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101(49):17294‐17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss J, Axelrod L, Whitcomb RW, Harris PE, Crowley WF, Jameson JL. Hypogonadism caused by a single amino acid substitution in the beta subunit of luteinizing hormone. N Engl J Med. 1992;326(3):179‐183. [DOI] [PubMed] [Google Scholar]
- 5. Wildt L, Häusler A, Marshall G, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376‐385. [DOI] [PubMed] [Google Scholar]
- 6. Weiss J, Jameson JL, Burrin JM, Crowley WF Jr. Divergent responses of gonadotropin subunit messenger RNAs to continuous versus pulsatile gonadotropin-releasing hormone in vitro. Mol Endocrinol (Baltimore, Md). 1990;4(4):557‐564. [DOI] [PubMed] [Google Scholar]
- 7. Charlton HM, Halpin DM, Iddon C, et al. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology. 1983;113(2):535‐544. [DOI] [PubMed] [Google Scholar]
- 8. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol (Baltimore, Md). 2007;21(5):1175‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19(4):2567‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol(Baltimore, Md). 2002;16(2):221‐233. [DOI] [PubMed] [Google Scholar]
- 11. Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S. Identification of distinct gene expression profiles associated with treatment of LbetaT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene. 2003;308:67‐77. [DOI] [PubMed] [Google Scholar]
- 12. Lee SL, Sadovsky Y, Swirnoff AH, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (egr-1). Science (New York, NY). 1996;273(5279):1219‐1221. [DOI] [PubMed] [Google Scholar]
- 13. Brown JL, Xie J, Brieño-Enriquez MA, et al. Sex- and age-specific impact of ERK loss within the pituitary gonadotrope in mice. Endocrinology. 2018;159(3):1264‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bliss SP, Miller A, Navratil AM, et al. ERK Signaling in the pituitary is required for female but not male fertility. Mol Endocrinol (Baltimore, Md). 2009;23(7):1092‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15(2):77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim S, Luo M, Koh M, et al. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27(11):4105‐4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385(1-2):28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279(1):152‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149(11):5577‐5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller WL, Shafiee-Kermani F, Strahl BD, Huang HJ. The nature of FSH induction by GnRH. Trends Endocrinol Metabol. 2002;13(6):257‐263. [DOI] [PubMed] [Google Scholar]
- 21. Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL. Transcriptional regulation of the ovine follicle-stimulating hormone-beta gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology. 2001;142(6):2267‐2274. [DOI] [PubMed] [Google Scholar]
- 22. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30(1):10‐29. [DOI] [PubMed] [Google Scholar]
- 23. Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30(4):1028‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciccone NA, Lacza CT, Hou MY, et al. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol (Baltimore, Md). 2008;22(8):1908‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamatiades GA, Toufaily C, Kim HK, et al. Deletion of gαq/11 or gαs proteins in gonadotropes differentially affects gonadotropin production and secretion in mice. Endocrinology. 2022;163(2):bqab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonak CR, Lainez NM, Boehm U, Coss D. GnRH receptor expression and reproductive function depend on JUN in GnRH receptor–expressing cells. Endocrinology. 2018;159(3):1496‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schorpp-Kistner M, Wang ZQ, Angel P, Wagner EF. Junb is essential for mammalian placentation. EMBO J. 1999;18(4):934‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie C, Jonak CR, Kauffman AS, Coss D. Gonadotropin and kisspeptin gene expression, but not GnRH, are impaired in cFOS deficient mice. Mol Cell Endocrinol. 2015;411:223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86(2):297‐309. [DOI] [PubMed] [Google Scholar]
- 30. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269(5626):338‐340. [DOI] [PubMed] [Google Scholar]
- 31. Taketani K, Kawauchi J, Tanaka-Okamoto M, et al. Key role of ATF3 in p53-dependent DR5 induction upon DNA damage of human colon cancer cells. Oncogene. 2012;31(17):2210‐2221. [DOI] [PubMed] [Google Scholar]
- 32. Wen S, Schwarz JR, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701‐2711. [DOI] [PubMed] [Google Scholar]
- 33. Owen CM, Zhou X, Bernard DJ, Jaffe LA. Kisspeptin-54 injection induces a physiological luteinizing hormone surge and ovulation in mice. Biol Reprod. 2021;104(6):1181‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruf-Zamojski F, Fribourg M, Ge Y, et al. Regulatory architecture of the LβT2 gonadotrope cell underlying the response to gonadotropin-releasing hormone. Front Endocrinol (Lausanne). 2018;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stern E, Ruf-Zamojski F, Zalepa-King L, et al. Modeling and high-throughput experimental data uncover the mechanisms underlying fshb gene sensitivity to gonadotropin-releasing hormone pulse frequency. J Biol Chem. 2017;292(23):9815‐9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276(50):47195‐47201. [DOI] [PubMed] [Google Scholar]
- 37. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England). 2013;29(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525‐527. [DOI] [PubMed] [Google Scholar]
- 39. Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res. 2012;22(9):1760‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939‐4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ongaro L, Alonso CAI, Zhou X, et al. Development of a highly sensitive ELISA for measurement of FSH in serum, plasma, and whole blood in mice. Endocrinology. 2021;162(4):bqab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Q, Mora-Jensen H, Weniger MA, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106(7):2200‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mauviel A, Chung KY, Agarwal A, Tamai K, Uitto J. Cell-specific induction of distinct oncogenes of the jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J Biol Chem. 1996;271(18):10917‐10923. [DOI] [PubMed] [Google Scholar]
- 47. Harrison RJ, McNeil GP, Dobner PR. Synergistic activation of neurotensin/neuromedin N gene expression by c-jun and glucocorticoids: novel effects of fos family proteins. Mol Endocrinol (Baltimore, Md). 1995;9(8):981‐993. [DOI] [PubMed] [Google Scholar]
- 48. Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol Endocrinol (Baltimore, Md). 2004;18(3):606‐623. [DOI] [PubMed] [Google Scholar]
- 49. Fortin J, Bernard DJ. SMAD3 and EGR1 physically and functionally interact in promoter-specific fashion. Cell Signal. 2010;22(6):936‐943. [DOI] [PubMed] [Google Scholar]
- 50. Ruf-Zamojski F, Ge Y, Pincas H, et al. Cytogenetic, genomic, and functional characterization of pituitary gonadotrope cell lines. J Endocr Soc. 2019;3(5):902‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Libasci V, Bernard DJ. Activin A induction of FSHbeta subunit transcription requires SMAD4 in immortalized gonadotropes. J Mol Endocrinol. 2010;44(6):349‐362. [DOI] [PubMed] [Google Scholar]
- 52. Schang G, Toufaily C, Bernard DJ. HDAC Inhibitors impair fshb subunit expression in murine gonadotrope cells. J Mol Endocrinol. 2019;62(2):67‐78. [DOI] [PubMed] [Google Scholar]
- 53. Toufaily C, Fortin J, Alonso CA, et al. Addition of a carboxy-terminal tail to the normally tailless gonadotropin-releasing hormone receptor impairs fertility in female mice. eLife. 2021;10:e72937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alonso CAI, Toufaily C, Wang Y, et al. Supplementary data for: ATF3 Stimulates FSHβ expression in vitro but is dispensable for FSH production in murine gonadotropes in vivo. 2023. Figshare. https://figshare.com/articles/dataset/Alonso_et_al_supplementary_figure_and_tables/22217203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol (Baltimore, Md). 2005;19(10):2624‐2638. [DOI] [PubMed] [Google Scholar]
- 56. Katz HR, Arcese AA, Bloom O, Morgan JR. Activating transcription factor 3 (ATF3) is a highly conserved pro-regenerative transcription factor in the vertebrate nervous system. Front Cell Dev Biol. 2022;10:824036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodríguez-Martínez JA, Reinke AW, Bhimsaria D, Keating AE, Ansari AZ. Combinatorial bZIP dimers display complex DNA-binding specificity landscapes. eLife. 2017;6:e19272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hsu JC, Bravo R, Taub R. Interactions among LRF-1, JunB, c-jun, and c-fos define a regulatory program in the G1 phase of liver regeneration. Mol Cell Biol. 1992;12(10):4654‐4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schang G, Ongaro L, Brule E, et al. Transcription factor GATA2 may potentiate follicle-stimulating hormone production in mice via induction of the BMP antagonist gremlin in gonadotrope cells. J Biol Chem. 2022;298(7):102072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol (Baltimore, Md). 1999;13(5):752‐763. [DOI] [PubMed] [Google Scholar]
- 61. Kucka M, Bjelobaba I, Clokie SJ, Klein DC, Stojilkovic SS. Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol Endocrinol. 2013;27(11):1840‐1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL. Transcriptional activation of the ovine follicle-stimulating hormone beta-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology. 1998;139(11):4455‐4465. [DOI] [PubMed] [Google Scholar]
- 63. Bonfil D, Chuderland D, Kraus S, et al. Extracellular signal-regulated kinase, jun N-terminal kinase, p38, and c-src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145(5):2228‐2244. [DOI] [PubMed] [Google Scholar]
- 64. Thompson IR, Ciccone NA, Xu S, Zaytseva S, Carroll RS, Kaiser UB. GnRH pulse frequency-dependent stimulation of FSHβ transcription is mediated via activation of PKA and CREB. Mol Endocrinol (Baltimore, Md). 2013;27(4):606‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reddy GR, Xie C, Lindaman LL, Coss D. GnRH increases c-fos half-life contributing to higher FSHβ induction. Mol Endocrinol (Baltimore, Md). 2013;27(2):253‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93(8):2465‐2485. [DOI] [PubMed] [Google Scholar]
- 67. Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol. 2009;302(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kehat I, Hasin T, Aronheim A. The role of basic leucine zipper protein-mediated transcription in physiological and pathological myocardial hypertrophy. Ann N Y Acad Sci. 2006;1080:97‐109. [DOI] [PubMed] [Google Scholar]
- 69. Hilberg F, Aguzzi A, Howells N, Wagner EF. . c-Jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365(6442):179‐181. [DOI] [PubMed] [Google Scholar]
- 70. Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71(4):577‐586. [DOI] [PubMed] [Google Scholar]
- 71. Wang ZQ, Ovitt C, Grigoriadis AE, Möhle-Steinlein U, Rüther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360(6406):741‐745. [DOI] [PubMed] [Google Scholar]
- 72. Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol Cell Biol. 1997;17(6):3094‐3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jonak CR, Lainez NM, Roybal LL, Williamson AD. Coss D. c-JUN dimerization protein 2 (JDP2) is a transcriptional repressor of follicle-stimulating hormone β (FSHβ) and is required for preventing premature reproductive senescence in female mice. J Biol Chem. 2017;292(7):2646‐2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or GEO GSE220809.