This cohort study measures the associated long-term effects of COVID-19 that are distinct from the risks associated with hospitalization for acute illnesses in general.
Key Points
Question
Is the risk of newly developing selected medical and mental health conditions greater within 1 year following hospitalization for severe COVID-19 compared with influenza or sepsis?
Findings
This population-based cohort study of 26 499 people hospitalized for COVID-19 compared with 17 516 historic controls with influenza, 282 473 historic controls with sepsis, and 52 878 people concurrently hospitalized with sepsis found that COVID-19 was associated with elevated 1-year risk of venous thromboembolism but not 12 other prespecified conditions.
Meaning
Apart from an elevated risk of venous thromboembolism, the burden of postacute conditions among those who survive hospitalization for COVID-19 may be comparable with other acute infections.
Abstract
Importance
People who survive hospitalization for COVID-19 are at risk for developing new cardiovascular, neurological, mental health, and inflammatory autoimmune conditions. It is unclear how posthospitalization risks for COVID-19 compare with those for other serious infectious illnesses.
Objective
To compare risks of incident cardiovascular, neurological, and mental health conditions and rheumatoid arthritis in 1 year following COVID-19 hospitalization against 3 comparator groups: prepandemic hospitalization for influenza and hospitalization for sepsis before and during the COVID-19 pandemic.
Design, Setting, and Participants
This population-based cohort study included all adults hospitalized for COVID-19 between April 1, 2020, and October 31, 2021, historical comparator groups of people hospitalized for influenza or sepsis, and a contemporary comparator group of people hospitalized for sepsis in Ontario, Canada.
Exposure
Hospitalization for COVID-19, influenza, or sepsis.
Main Outcome and Measures
New occurrence of 13 prespecified conditions, including cardiovascular, neurological, and mental health conditions and rheumatoid arthritis, within 1 year of hospitalization.
Results
Of 379 366 included adults (median [IQR] age, 75 [63-85] years; 54% female), there were 26 499 people who survived hospitalization for COVID-19, 299 989 historical controls (17 516 for influenza and 282 473 for sepsis), and 52 878 contemporary controls hospitalized for sepsis. Hospitalization for COVID-19 was associated with an increased 1-year risk of venous thromboembolic disease compared with influenza (adjusted hazard ratio, 1.77; 95% CI, 1.36-2.31) but with no increased risks of developing selected ischemic and nonischemic cerebrovascular and cardiovascular disorders, neurological disorders, rheumatoid arthritis, or mental health conditions compared with influenza or sepsis cohorts.
Conclusions and Relevance
In this cohort study, apart from an elevated risk of venous thromboembolism within 1 year, the burden of postacute medical and mental health conditions among those who survived hospitalization for COVID-19 was comparable with other acute infectious illnesses. This suggests that many of the postacute consequences of COVID-19 may be related to the severity of infectious illness necessitating hospitalization rather than being direct consequences of infection with SARS-CoV-2.
Introduction
Post–COVID-19 condition (PCC; also known as “long COVID”) is variably defined as the persistence of symptoms or sequelae occurring 4 to 12 weeks after probable or confirmed SARS-CoV-2 infection. Symptoms may include the development of new, returning, or ongoing health problems as postacute sequelae of infection.1,2,3,4 These negative health outcomes are associated with gross impairments in people’s ability to work and care for themselves, poor quality of life, and high health care use and costs.5,6,7,8,9,10,11,12,13,14,15,16,17,18
It has been difficult to discern whether adverse events caused by SARS-CoV-2 infection are more common than those caused by other severe, acute illnesses. People who survive hospitalization for influenza, sepsis, or other critical illnesses are known to have increased risks of cardiovascular, neurological, and other health conditions.19,20,21,22,23,24,25 Multiple prior studies reported associations of SARS-CoV-2 infection with increased short- and long-term risks of cardiovascular, neurological, mental health, and inflammatory autoimmune conditions; however, there are concerns that these associations may reflect bias in the chosen comparison group or residual confounding by illness severity or care provided, rather than unique, direct outcomes of SARS-CoV-2 infection.26,27,28,29,30,31,32,33 For example, many prior studies did not distinguish the effects of hospitalization for acute illness from the direct effects of SARS-CoV-2 infection, while exploratory analyses within these studies demonstrated that the risks of postacute complications of COVID-19 were largely driven by the subgroups of hospitalized individuals and those admitted to the intensive care unit (ICU).27,28,30,33 Many of these prior studies also did not include individuals who were vaccinated against SARS-CoV-2 and/or were conducted in cohorts of US veterans comprising mainly older white males, thus the affected populations had elevated baseline risk for severe illness, which is believed to be associated with increased risk of long-term health outcomes following infection.34 The findings from these studies may not reflect the direct effects of SARS-CoV-2 infection on the development of PCC or may overestimate the magnitude of these risks.
The direct effects of SARS-CoV-2 infection on the risk of developing new medical and mental health conditions remain undistinguished from the effects of hospitalization for acute illness. The objective of this population-level cohort study was to measure the associated long-term outcomes of COVID-19 that are distinct from the risks associated with hospitalization for acute illnesses in general. We conceptualized long-term outcomes to be the incident development of cardiovascular, neurological, and mental health conditions and rheumatoid arthritis within 1 year of hospitalization and used hospitalization for influenza and sepsis as comparators representative of other similar acute illnesses.
Methods
Study Design, Setting, and Data Sources
We conducted a population-based cohort study in Ontario, Canada, using linked clinical and health administrative databases. These databases are well validated and widely used to conduct population-level studies in Ontario (eTable 1 in Supplement 1). The administrative data sets used in this study were linked using unique encoded identifiers at the patient level and analyzed at ICES (formerly the Institute of Clinical and Evaluative Sciences). Ontario is Canada’s most populous province with more than 13 million adults and more than 1.4 million confirmed cases of SARS-CoV-2 infection. Residents of Ontario have public insurance for hospital care and physicians’ services, and those 65 years and older are provided prescription drug insurance.
ICES is a prescribed entity under Ontario’s Personal Health Information Protection Act (PHIPA). Section 45 of PHIPA authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, allocation of resources to, or planning for all or part of the health system. Projects that use data collected by ICES under section 45 of PHIPA, and use no other data, are exempt from research ethnics board review. The use of the data in this project is authorized under section 45 and approved by ICES’s Privacy and Legal Office. This study is reported in accordance with guidelines for the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD).35
Study Participants
The COVID-19 study cohort included all Ontario adults (aged ≥18 years) who were hospitalized with a principal diagnosis of COVID-19 between April 1, 2020, and October 31, 2021, and followed up for up to 1 year after hospitalization, censoring for death or loss to follow-up. The historical comparator cohorts consisted of people hospitalized for influenza or sepsis between January 1, 2014, and March 25, 2019, representing a group of people with severe infectious illnesses. Because care patterns and hospitalization thresholds may have changed during the pandemic, we also included a contemporary comparator cohort of people hospitalized for non–COVID-19 sepsis between April 1, 2020, and October 31, 2021. There were insufficient influenza cases during the pandemic to form a comparator. The index study date was the date of discharge from hospital.
We excluded people who died during their index hospitalization, were hospitalized for influenza or sepsis in the 1 year prior to index study date, had missing age or sex, were younger than 18 years or older than 105 years at index date, were nonresidents of Ontario, or were not eligible for the Ontario Health Insurance Plan for a period of 3 months or more in the year prior to the index date (Figure 1). To prevent people belonging to more than 1 of the 4 mutually exclusive comparator groups, we assigned individuals to each cohort in a stepwise fashion based on the first occurrence of hospitalization for a specific cause, and once assigned they were removed from the remaining pool of eligible people.
Figure 1. Creation of the Study Cohorts.
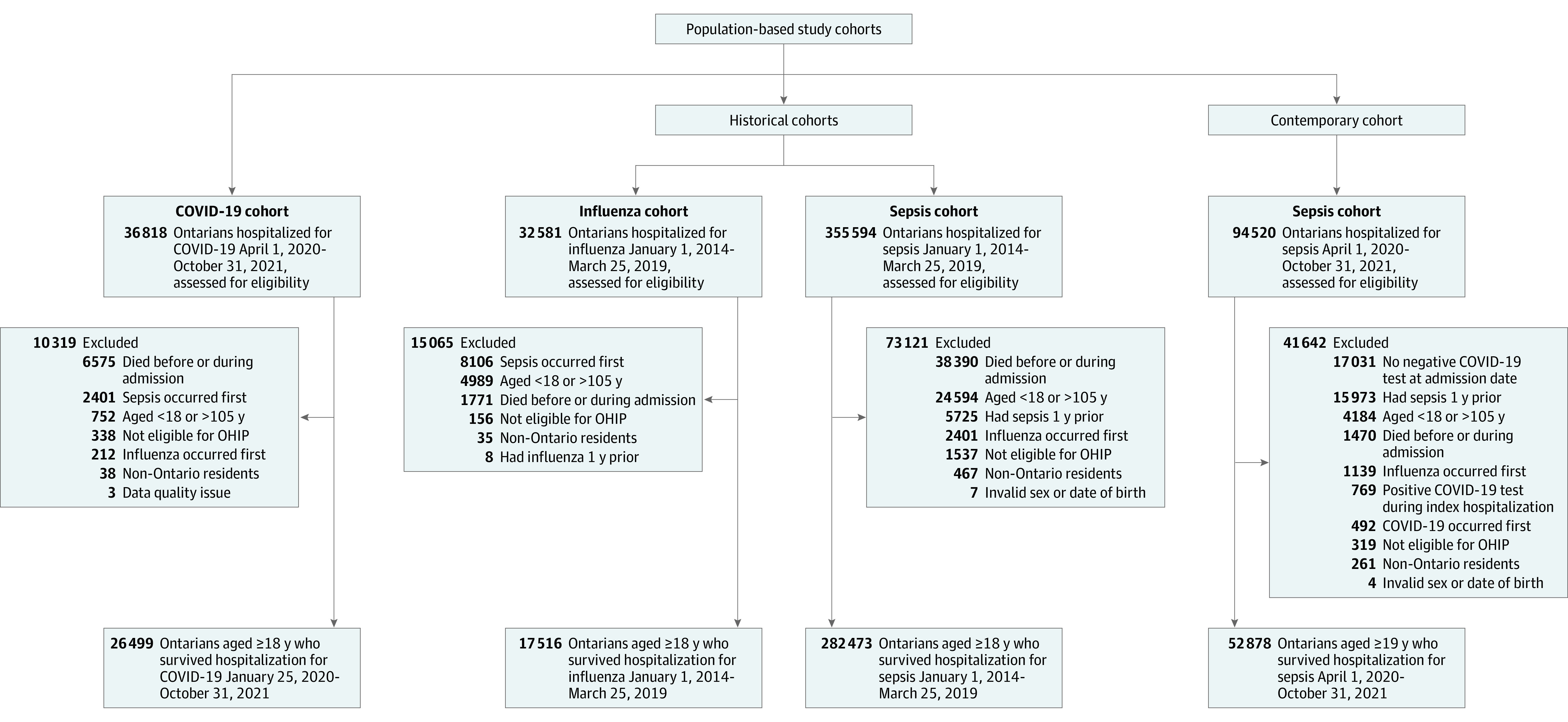
OHIP indicates the Ontario Health Insurance Plan.
Exposure Definitions
The primary exposure of hospitalization for COVID-19 was identified based on an established approach at ICES using laboratory-confirmed SARS-CoV-2 infection via polymerase chain reaction testing that was performed within 14 days prior to admission or 3 days following admission to hospital during the study period.36 This approach has been shown to have similar accuracy in identifying people hospitalized with a main diagnosis of COVID-19, which has a 98% sensitivity and 99% specificity.36 Hospitalization for influenza was determined using a previously validated algorithm that is 83% sensitive and 98% specific, and hospitalization for sepsis was identified using a previously validated algorithm based on the Sepsis-2 definition that is 72% sensitive and 85% specific.37,38
Outcomes Definitions
The primary outcomes were a prespecified set of individual cardiovascular, neurological, and mental health conditions and rheumatoid arthritis. People could experience more than 1 outcome, although the risk of developing each was analyzed separately. We specifically assessed the incidence of new onset of these conditions among people without a prior history of them (see eTable 2 in Supplement 1 for details on validated case ascertainment algorithms). These included cardiovascular conditions and procedures (including acute myocardial infarction, stroke, heart failure, hypertension, percutaneous coronary intervention, and coronary artery bypass grafting), venous thromboembolic disease (deep vein thrombosis and pulmonary embolism), neurological disorders (seizure, Parkinson disease, and dementia), rheumatoid arthritis, and mental health conditions (depression, anxiety, and substance use disorders). These conditions were chosen because they were known adverse outcomes following COVID-19, influenza, sepsis, or general critical illness based on a recent comprehensive review of the literature and the clinical and scientific experience of our team.6 Death following discharge from hospital was measured as a secondary outcome.
Covariates
Covariates included in the analytical models were prespecified and chosen as potential confounders based on the clinical and research expertise of our team. Baseline demographic and clinical variables at index date included age; sex; socioeconomic status; residence in a nursing home; rural residence; surname-based ethnicity39; comorbidities40; diagnosis of pre-existing physical, sensory, and intellectual disability41; Hospital Frailty Risk Score42; hospitalization for pneumonia within prior 5 years; ICU admission; use of mechanical ventilation during index hospitalization; index hospital length of stay; and COVID-19 vaccination status. The Hospital Frailty Risk Score (range, 0-50) is a validated measure of a patient’s function and comorbidity that reflects global illness severity and identifies a group of patients who are at greater risk of adverse outcomes, including hospital admission, length of stay, and 30-day mortality.42,43 We calculated the Hospital Frailty Risk Score using hospitalization records with a 5-year look back, including the person’s index hospitalization for COVID-19, influenza, and sepsis. The Hospital Frailty Risk Score is composed of a specific set of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes that are used to calculate it. Therefore, people who were not hospitalized before their index hospitalization and/or did not have any of the conditions contained within the Hospital Frailty Risk Score could have a score of zero. We categorized Hospital Frailty Risk Scores into 3 groups based on the distribution of scores within the cohort: (1) 0.1 to 4.9, (2) 5.0 to 8.9, and (3) at least 9, using a score of 0 as the referent group. Full vaccination status was defined as 2 doses, and partial status was defined as having 1 dose of an approved vaccine, which corresponded to the established provincial criteria during the study period that was before the availability of third and fourth booster doses. Comorbidities were identified using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes from the Ontario Health Insurance Plan database and the Discharge Abstract Database, using validated case ascertainment algorithms where available.44
Statistical Analysis
We used propensity score–based overlap weighting to achieve balance on all measured baseline characteristics between the exposure groups and to achieve optimal precision of the estimated associated effects of COVID-19 on the development of medical and mental health sequelae.45 A propensity score (PS), defined as the probability of being hospitalized for COVID-19 vs either influenza or sepsis, was estimated using multivariable random-effects logistic regression and included the covariates described previously.46,47 Hospital random effects were included in the PS to better balance patients discharged from the same hospital because they were treated similarly in terms of discharge processes.46 Propensity overlap weighting assigns weights to all people in the study cohort who were proportional to the probability of that person belonging to the opposite exposure group.45 The result is such that people at the extremes of the PS do not dominate results and worsen precision, as occurs with inverse probability of treatment weighting. We included multiple factors in the propensity weights that are risk factors for severe COVID-19. We additionally included the index hospital in which a person was admitted to account for potential hospital-specific differences in care and outcomes.48 All outcomes are reported using the weighted results, unless otherwise specified.
Each patient was weighted according to the overlap weight, defined as their probability of being assigned to the opposite exposure group based on the PS model.45 Overlap weights give larger weights to patients with a high probability of receiving either exposure who have the greatest overlap in observed risk factors and downweight patients in the extremes of the distribution. Overlap weights achieve near perfect balance for all covariates included in the PS and produce the smallest standard errors among all balancing weight approaches.45
We used Cox proportional hazard regression models, weighted by overlap weights, to estimate adjusted hazard ratios (aHRs) between patients hospitalized with COVID-19 compared with hospitalization for influenza or sepsis and included a robust variance estimator to account for weighting.49,50 We used separate cause-specific competing risk models to analyze each outcome, treating mortality as a competing risk.51 Each model included only study participants without a prior diagnosis of the specific outcome being investigated.
We explored the potential influence of time following hospital discharge by investigating associations with outcomes at fewer than 30 days and 30 days or more, which are clinically relevant time periods for PCC,1,2,3,4 and we stratified analyses according to ICU admission during a person’s index admission to hospital.
Statistical tests were 2-sided and performed at the 5% level of significance. All analyses were performed using SAS, version 9.4 (SAS Institute).
Results
Baseline Characteristics
After exclusions, the final pool of eligible participants included 26 499 people hospitalized for COVID-19, 2 historical comparator cohorts of 17 516 people who survived hospitalization for influenza and 282 473 people who survived hospitalization for sepsis, and a contemporary comparator cohort of 52 878 people who survived hospitalization for sepsis (Figure 1). The median (IQR) age varied across each group within the unweighted study cohort, and those admitted for COVID-19 were generally younger (61 [48-74] years) (eTable 3 in Supplement 1). There was a lower proportion of female patients hospitalized for COVID-19 compared with the other groups in the unweighted cohort (n = 12 301 [46.4%]). In total, 18% (n = 6575) of the COVID-19 group, 5% (n = 1771) of the historical influenza group, 11% (n = 38 390) of the historical sepsis group, and 1.6% (n = 1470) of the contemporary sepsis group died during their index hospitalization. Additionally, 7% (n = 1846) of the COVID-19 group were fully or partially vaccinated against SARS-CoV-2. The baseline characteristics of the weighted study cohorts are summarized in Table 1 and eTable 4 in Supplement 1. The characteristics of the study sample before weighting are summarized in eTable 3 in Supplement 1.
Table 1. Baseline Characteristics of Weighted Study Cohorts of Adults Who Survived Hospitalization for COVID-19 and Contemporary Sepsis.
| Characteristic | No. (%) | SDa | |
|---|---|---|---|
| COVID-19 (n = 26 499) | Contemporary sepsis (n = 52 878) | ||
| Age, median (IQR), y | 70 (57-81) | 70 (57-81) | 0.0 |
| Sex | |||
| Female | 4589.2 (48.0) | 4589.2 (48.0) | 0.0 |
| Male | 4979.9 (52.0) | 4979.9 (52.0) | 0.0 |
| Rural residence | 557.8 (5.8) | 557.8 (5.8) | 0.0 |
| Neighborhood income quintile | |||
| 1 | 2788.5 (29.1) | 2788.5 (29.1) | 0.0 |
| 2 | 2069.3 (21.6) | 2069.3 (21.6) | 0.0 |
| 3 | 1835.9 (19.2) | 1835.9 (19.2) | 0.0 |
| 4 | 1515.7 (15.8) | 1515.7 (15.8) | 0.0 |
| 5 | 1307.8 (13.7) | 1307.8 (13.7) | 0.0 |
| Missing | 52.0 (0.5) | 52.0 (0.5) | 0.0 |
| Surname-based ethnic group | |||
| Chinese | 322.3 (3.4) | 376.5 (3.9) | 0.0 |
| General population | 8758.8 (91.5) | 8839.9 (92.4) | 0.0 |
| South Asian | 484.8 (5.1) | 346.93 (3.6) | 0.1 |
| Missing | ≤5 (0.0) | 5.8 (0.1) | 0.0 |
| SARS-CoV-2 vaccination statusb | |||
| Full | 512.3 (5.4) | 2197.3 (23.0) | 0.5 |
| Partial | 1333.4 (13.9) | 1040.6 (10.9) | 0.1 |
| Unvaccinated | 7723.4 (80.7) | 6331.2 (66.2) | 0.3 |
| Chronic conditions | |||
| Asthma | 1899.5 (19.8) | 1899.5 (19.8) | 0.0 |
| Arrhythmia | 965.3 (10.1) | 965.3 (10.1) | 0.0 |
| Cancer | 1812.6 (18.9) | 1812.6 (18.9) | 0.0 |
| Chronic kidney disease | 2811.4 (29.4) | 2811.4 (29.4) | 0.0 |
| Cirrhosis (decompensated) | 260.9 (2.7) | 260.9 (2.7) | 0.0 |
| Coronary artery disease | 1374.2 (14.4) | 1374.2 (14.4) | 0.0 |
| Chronic obstructive pulmonary disease | 1217.6 (12.7) | 1217.6 (12.7) | 0.0 |
| Dementia | 1380.5 (14.4) | 1380.5 (14.4) | 0.0 |
| Depression/anxiety | 3013.2 (31.5) | 3013.2 (31.5) | 0.0 |
| Diabetes | 3895.5 (40.7) | 3895.5 (40.7) | 0.0 |
| Heart failure | 1726.2 (18.0) | 1726.2 (18.0) | 0.0 |
| Hypertension | 6409.1 (67.0) | 6409.1 (67.0) | 0.0 |
| Osteoarthritis | 3072.0 (32.1) | 3072.0 (32.1) | 0.0 |
| Parkinson disease | 220.0 (2.3) | 220.0 (2.3) | 0.0 |
| Seizure disorder | 366.9 (3.8) | 366.9 (3.8) | 0.0 |
| Stroke | 628.2 (6.6) | 628.2 (6.6) | 0.0 |
| Substance use disorder | 446.9 (4.7) | 446.9 (4.7) | 0.0 |
| Venous thromboembolism | 272.4 (2.8) | 272.4 (2.8) | 0.0 |
| Pre-existing disability | |||
| Developmental | 41.1 (0.4) | 36.2 (0.4) | 0.0 |
| Physical | 2420.7 (25.3) | 2468.6 (25.8) | 0.0 |
| Sensory | 1103.2 (11.5) | 1151.1 (12.0) | 0.0 |
| Hospital Frailty Risk Score | |||
| 0 | 311.7 (3.3) | 311.7 (3.3) | 0.0 |
| 0.1-4.9 | 4047.8 (42.3) | 4047.8 (42.3) | 0.0 |
| 5.0-8.9 | 2021.3 (21.1) | 2021.3 (21.1) | 0.0 |
| ≥9.0 | 3095.9 (32.4) | 3095.9 (32.4) | 0.0 |
| Missing | 92.4 (1.0) | 92.4 (1.0) | 0.0 |
| Resided in nursing home | 524.6 (5.5) | 524.6 (5.5) | 0.0 |
| Prior pneumonia, mean (SD) | 0.6 (0.3) | 0.6 (0.3) | 0.0 |
| Delirium during index hospitalization | 1424.5 (14.9) | 1424.5 (14.9) | 0.0 |
| ICU admission during index hospitalization | 1865.2 (19.5) | 1865.2 (19.5) | 0.0 |
| Mechanical ventilation during index hospitalization | 817.3 (8.5) | 817.3 (8.5) | 0.0 |
| Index hospitalization length of stay, median (IQR), d | 10 (5-21) | 7 (4-13) | 0.2 |
Abbreviation: ICU, intensive care unit.
An SD less than 0.10 indicates acceptable balance.
Full vaccination status was defined as 2 doses, and partial status was defined as having 1 dose of an approved vaccine.
Development of Incident Health Conditions and Mortality
Venous thromboembolic disease was more common after hospitalization for COVID-19 compared with hospitalization for influenza (aHR, 1.77; 95% CI, 1.36-2.31). The risks of all other outcomes were similar or lower after hospitalization for COVID-19 compared with hospitalization for sepsis, before or during the pandemic, or influenza (Figure 2 and Table 2). Within 1 year of discharge from hospital, mortality ranged between 6% and 23% across the study cohort (Table 2).
Figure 2. Primary Analysis of Outcomes.
Forest plot of the adjusted risk of incident cardiovascular, neurologic, and mental health conditions and rheumatoid arthritis within 1 year among adults who survived hospitalization for COVID-19 compared with those who survived hospitalization for influenza and sepsis in Ontario, Canada, between January 1, 2014, and October 31, 2021. PCI/CABG indicates percutaneous coronary intervention/coronary artery bypass grafting.
Table 2. Incident Health Conditions and Mortalitya.
| Outcome | Crude rate per 100 person-years (95% CI) | Hazard ratio (95% CI)b |
|---|---|---|
| COVID-19 vs prepandemic influenza (historical comparator) | ||
| Death within 1 y of discharge from index hospitalization, No. (%) | 1556 of 26 499 (5.9)/2161 of 17 516 (12.3) | NA |
| Acute myocardial infarction | 0.08 (0.05-0.13)/0.16 (0.10-0.23) | 1.00 (0.48-2.06) |
| Cardiovascular disease | 3.15 (2.90-3.43)/6.25 (5.82-6.70) | 0.78 (0.68-0.89) |
| Dementia | 1.58 (1.40-1.77)/3.09 (2.81-3.41) | 0.95 (0.79-1.14) |
| Depression/anxiety | 6.86 (6.43-7.32)/7.00 (6.51-7.52) | 0.94 (0.84-1.06) |
| Heart failure | 2.13 (1.92-2.35)/5.08 (4.69-5.50) | 0.62 (0.53-0.73) |
| Hypertension | 4.80 (4.36-5.30)/5.57 (4.91-6.32) | 0.85 (0.70-1.04) |
| Parkinson disease | 0.09 (0.06-0.14)/0.34 (0.26-0.45) | 0.39 (0.20-0.77) |
| PCI/CABG | 0.49 (0.40-0.60)/0.85 (0.72-1.01) | 0.66 (0.48-0.91) |
| Rheumatoid arthritis | 0.18 (0.13-0.25)/0.19 (0.13-0.27) | 1.00 (0.55-1.81) |
| Seizure | 0.19 (0.14-0.26)/0.34 (0.26-0.44) | 0.55 (0.33-0.91) |
| Stroke | 2.30 (2.09-2.53)/3.15 (2.88-3.44) | 0.86 (0.73-1.01) |
| Substance use disorder | 0.31 (0.24-0.40)/0.29 (0.22-0.39) | 0.82 (0.51-1.30) |
| Venous thromboembolism | 1.28 (1.13-1.45)/0.74 (0.62-0.89) | 1.77 (1.36-2.31) |
| COVID-19 vs sepsis (historical comparator) | ||
| Death within 1 y of discharge from index hospitalization, No. (%) | 1556 of 26 499 (5.9)/67 459 of 282 473 (23.9) | NA |
| Acute myocardial infarction | 0.08 (0.05-0.13)/0.18 (0.16-0.20) | 0.67 (0.37-1.24) |
| Cardiovascular disease | 3.15 (2.90-3.43)/7.29 (7.17-7.42) | 0.70 (0.64-0.78) |
| Dementia | 1.58 (1.40-1.77)/4.76 (4.66-4.86) | 0.84 (0.74-0.96) |
| Depression/anxiety | 6.86 (6.43-7.32)/7.29 (7.16-7.42) | 0.84 (0.78-0.92) |
| Heart failure | 2.13 (1.92-2.35)/6.87 (6.75-7.00) | 0.50 (0.45-0.57) |
| Hypertension | 4.80 (4.36-5.30)/6.53 (6.33-6.74) | 0.73 (0.64-0.84) |
| Parkinson disease | 0.09 (0.06-0.14)/0.28 (0.26-0.30) | 0.56 (0.34-0.92) |
| PCI/CABG | 0.49 (0.40-0.60)/0.79 (0.75-0.83) | 0.57 (0.44-0.73) |
| Rheumatoid arthritis | 0.18 (0.13-0.25)/0.20 (0.19-0.22) | 0.92 (0.59-1.42) |
| Seizure | 0.19 (0.14-0.26)/0.46 (0.43-0.48) | 0.55 (0.38-0.78) |
| Stroke | 2.30 (2.09-2.53)/4.00 (3.92-4.09) | 0.90 (0.80-1.00) |
| Substance use disorder | 0.31 (0.24-0.40)/0.54 (0.51-0.57) | 0.52 (0.39-0.71) |
| Venous thromboembolism | 1.28 (1.13-1.45)/1.21 (1.17-1.26) | 1.14 (0.97-1.35) |
| COVID-19 vs sepsis (contemporary comparator) | ||
| Death within 1 y of discharge from index hospitalization, No. (%) | 1556 of 26 499 (5.9)/11 938 of 52 878 (22.6) | NA |
| Acute myocardial infarction | 0.08 (0.05-0.13)/0.13 (0.10-0.17) | 0.71 (0.33-1.51) |
| Cardiovascular disease | 3.15 (2.90-3.43)/7.89 (7.55-8.25) | 0.66 (0.58-0.74) |
| Dementia | 1.58 (1.40-1.77)/5.07 (4.81-5.34) | 0.96 (0.82-1.11) |
| Depression/anxiety | 6.86 (6.43-7.32)/6.74 (6.41-7.09) | 1.09 (0.97-1.21) |
| Heart failure | 2.13 (1.92-2.35)/6.90 (6.58-7.23) | 0.58 (0.50-0.66) |
| Hypertension | 4.80 (4.36-5.30)/7.24 (6.70-7.82) | 0.73 (0.62-0.87) |
| Parkinson disease | 0.09 (0.06-0.14)/0.27 (0.22-0.33) | 0.84 (0.48-1.50) |
| PCI/CABG | 0.49 (0.40-0.60)/0.65 (0.57-0.75) | 0.82 (0.59-1.13) |
| Rheumatoid arthritis | 0.18 (0.13-0.25)/0.24 (0.19-0.30) | 0.77 (0.45-1.31) |
| Seizure | 0.19 (0.14-0.26)/0.44 (0.37-0.52) | 0.72 (0.47-1.10) |
| Stroke | 2.30 (2.09-2.53)/4.38 (4.15-4.62) | 1.00 (0.87-1.14) |
| Substance use disorder | 0.31 (0.24-0.40)/0.95 (0.85-1.06) | 0.39 (0.28-0.55) |
| Venous thromboembolism | 1.28 (1.13-1.45)/1.55 (1.42-1.70) | 0.96 (0.78-1.18) |
Abbreviations: NA, not applicable; PCI/CABG, percutaneous coronary intervention/coronary artery bypass grafting.
Models were adjusted for deciles of age; sex; neighborhood income quintile; rural residence; residence in a nursing home; all prevalent comorbidities; the presence of pre-existing sensory, developmental, or physical disabilities; a diagnosis of pneumonia in the prior 5 years; Hospital Frailty Risk Score groups; the presence of delirium during index hospitalization; admission to the intensive care unit; and use of mechanical ventilation during index hospitalization. Crude mortality, crude rate, and hazard ratio of incident cardiovascular, neurologic, and mental health conditions and rheumatoid arthritis within 1 year among adults who survived hospitalization for COVID-19 were compared with those among adults who survived hospitalization for influenza and sepsis in Ontario, Canada, between January 1, 2014, and October 31, 2021.
A hazard ratio less than 1 indicates a lower risk of the outcome for people hospitalized for COVID-19, and a hazard ratio greater than 1 indicates a higher risk of the outcome.
Association of Timing From Hospital Discharge With Outcomes
Within 30 days of discharge, COVID-19 was associated with a higher risk of 3 outcomes than the other exposure groups. First, hospitalization for COVID-19 was associated with a higher risk of venous thromboembolic disease compared with hospitalization for influenza (aHR, 3.04; 95% CI, 1.87-4.95), sepsis prepandemic (aHR, 1.74; 95% CI, 1.35-2.23), and sepsis during the pandemic (aHR, 1.43; 95% CI, 1.04-1.97). Second, hospitalization for COVID-19 was associated with elevated risk of stroke within the first 30 days of discharge compared with influenza (aHR, 1.40; 95% CI; 1.02-1.93), sepsis prepandemic (aHR, 1.19; 95% CI, 1.00-1.42), and sepsis during the pandemic (aHR, 1.23; 95% CI, 1.00-1.51) (Figure 3A). Third, hospitalization for COVID-19 was associated with elevated risk of depression and anxiety within the first 30 days of discharge compared with influenza (aHR, 1.49; 95% CI, 1.15-1.93) and sepsis before (aHR, 1.40; 95% CI, 1.20-1.64) and during the pandemic (aHR, 1.33; 95% CI, 1.09-1.62) (Figure 3A and eTable 5 in Supplement 1). However, after 30 or more days postdischarge, hospitalization for COVID-19 was not associated with risk of venous thromboembolism, stroke, depression and anxiety, or any other prespecified outcomes compared with the influenza or sepsis cohorts (Figure 3B and eTable 6 in Supplement 1).
Figure 3. Secondary Analysis of Outcomes After Hospital Discharge.

Forest plot of the adjusted risk of incident cardiovascular, neurologic, and mental health conditions and rheumatoid arthritis among adults who survived hospitalization for COVID-19 compared with those who survived hospitalization for influenza and sepsis in Ontario, Canada, between January 1, 2014, and October 31, 2021. PCI/CABG indicates percutaneous coronary intervention/coronary artery bypass grafting.
Association of ICU Admission With Outcomes
Among people admitted to the ICU during their index hospitalization, COVID-19 was not associated with a risk of developing any of the 13 prespecified outcomes within 1 year of hospital discharge compared with the influenza or sepsis cohorts (eTable 7 in Supplement 1).
Discussion
This population-based cohort study of all adults in Ontario who survived hospitalization for COVID-19 found that patients with COVID-19 did not have a greater risk of developing new cardiovascular, neurologic, or mental health conditions or rheumatoid arthritis compared with Ontario adults hospitalized for influenza or sepsis. COVID-19 was associated with an increased risk of venous thromboembolism, stroke, and depression or anxiety within the first 30 days following hospital discharge, but these risks appeared to dissipate beyond 30 days. To place the magnitude of these findings in context, we compared the crude annual incidence of acute myocardial infarction, stroke, and dementia in this study of hospitalized adults with the general population of adults in Canada. The crude annual incidence of acute myocardial infarction following hospitalization for COVID-19 was lower (80 vs 212 per 100 000), and the annual crude incidence of stroke (2300 vs 303 per 100 000) and dementia (1580 vs 1317 per 100 000) was higher than those of adults in the general population.52 It is important to note that a higher proportion of people hospitalized for COVID-19 died during hospitalization compared with influenza or sepsis, which influences the risk profile of survivors and may explain why the risks of many outcomes were lower among survivors of COVID-19 hospitalization compared with influenza or sepsis. Overall, the comparable rates of serious medical and mental health conditions following hospitalization for COVID-19, influenza, or sepsis suggest that many of these conditions may be related to the severity of illness from any infection that necessitated hospitalization,53,54,55 rather than being direct consequences of infection with SARS-CoV-2.
This study advances the understanding of the long-term sequelae of COVID-19 by disentangling the outcomes of hospitalization for acute and critical illness from pathological features specific to COVID-19. These observations do not diminish the effects of PCC on populations and health systems around the world, given the large number of people hospitalized for COVID-19 who have experienced severe mortality and substantial long-term morbidity. At a health system and policy level, it is important to recognize the long-term consequences of severe infectious illnesses, including COVID-19, influenza, and sepsis, and invest in longitudinal care to support people beyond their immediate discharge from hospital.
The present findings contrast with prior research, which reported that COVID-19 was associated with substantially increased risks of multiple long-term health outcomes.26,27,28,29,30,33 These associations may have been overestimated due to the choice of comparator groups in some studies. The risks reported among many of these previous studies appeared to be largely driven by comparing subgroups of people hospitalized for COVID-19 with groups that included both hospitalized and nonhospitalized individuals. Some studies compared hospitalization for COVID-19 with people hospitalized for any cause, which would include a broad range of diagnoses (including elective surgical procedures) and varying severity of illness, limiting comparability. Selecting comparator groups with similarly severe illnesses is particularly important because patients with COVID-19 may receive more careful follow-up and thus appear to have greater risk of incident conditions due to greater surveillance (ie, ascertainment bias). Many prior studies also did not report outcomes according to timing following hospital discharge.
Strengths and Limitations
This large population-based study included all adults in Ontario hospitalized for COVID-19, influenza, or sepsis and used well-validated case ascertainment methods, multiple comparator groups, and a rigorous approach to address confounding to understand and contextualize a range of postacute medical and mental health conditions associated with hospitalization for COVID-19. The present findings were consistent across these various analyses and further illuminate temporal associations between COVID-19 and venous thromboembolism, stroke, and depression or anxiety. We studied people who survived hospitalization for COVID-19, which limits the generalizability of the findings to this specific population. Furthermore, the study cohort was largely composed of older adults, with expected differences observed in median age between those with COVID-19 and those with sepsis and influenza. These demographic differences may reflect the predisposition of older adults to develop severe sepsis and influenza compared with those with COVID-19, which we accounted for in the propensity overlap weights. These findings may also not generalize to outpatient settings, which we did not include because testing for SARS-CoV-2 and influenza occurs less systematically. Near universal testing for SARS-CoV-2 in hospitals during the study period reduces the risk of selective detection of SARS-CoV-2 infection and strengthens our confidence in the findings pertaining to hospitalization. Second, the risk of artificially creating a “healthier” COVID-19 cohort through exclusion of people previously hospitalized for sepsis and influenza is likely minimal given the low numbers for each. Third, we lacked symptom and biochemical testing data to reflect the underlying severity of illness during index admission. We stratified analyses by ICU admission at any point during index hospitalization to help differentiate the associated risks among those who were critically ill. It is possible that barriers to accessing care for COVID-19 could be associated with greater illness severity at the time of hospitalization as well as with worse postdischarge outcomes, which we would expect to bias the results toward detectable differences in outcomes between the study groups. Fourth, the study period may not generalize to current population immunity and viral characteristics, as it was conducted prior to evolution of recent viral strains, widespread SARS-CoV-2 reinfections, or administration of more than 2 doses of COVID-19 vaccines.56 Still, we included 15% of people who were partially or fully vaccinated against COVID-19, which might further help explain the difference between these findings and prior studies among unvaccinated cohorts. Fifth, we did not measure other important person-centered outcomes such as fatigue, return to work, and quality of life. Sixth, as in any observational study, there is a risk of bias due to confounding by unmeasured factors such as race or other important measures of disease severity such as the use of extracorporeal membrane oxygenation. Some of these unmeasured factors may be associated with the observed differences in mortality during index admission.
Conclusions
This cohort study demonstrated that severe COVID-19 leading to hospitalization was associated with an elevated risk of venous thromboembolism within 1 year compared with influenza and sepsis but was otherwise not associated with a greater risk of other prespecified medical or mental health conditions. Many of the postacute consequences of COVID-19 may be related to the severity of infectious illness necessitating hospitalization rather than being direct consequences of infection with SARS-CoV-2.
eTable 1. Description of datasets
eTable 2. Description of validated case ascertainment algorithms used to measure primary outcomes including incident cardiovascular, neurological, and mental health conditions
eTable 3. Baseline characteristics of adults who survived hospitalization for COVID-19, compared to those who survived hospitalization for influenza and sepsis before weighting in Ontario between January 1, 2014, and October 31, 2021
eTable 4. Baseline characteristics of adults who survived hospitalization for COVID-19, compared to those who survived hospitalization for influenza and historical sepsis after weighting in Ontario between January 1, 2014, and October 31, 2021
eTable 5. Secondary analysis according to timing (<30 days) of outcomes following discharge from index hospitalization
eTable 6. Secondary analysis according to timing (≥30 days) of outcomes following discharge from index hospitalization
eTable 7. Secondary analysis among those admitted to ICU during index hospitalization
eReferences
Data Sharing Statement
References
- 1.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102-e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Post COVID-19 condition (long COVID). Public Health Agency of Canada . August 18, 2021. Accessed May 10, 2023. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html
- 3.COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP) . March 11, 2022. Accessed May 10, 2023. https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742
- 4.Long COVID or post-COVID conditions. Centers for Disease Control and Prevention . July 11, 2022. Updated December 16, 2022. Accessed May 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 5.Bach K. New data shows long Covid is keeping as many as 4 million people out of work. Brookings . August 24, 2022. Accessed May 10, 2023. https://www.brookings.edu/research/new-data-shows-long-covid-is-keeping-as-many-as-4-million-people-out-of-work/
- 6.Quinn KL, Katz GM, Bobos P, et al. Understanding the post COVID-19 condition (long COVID) in adults and the expected burden for Ontario. Ontario COVID-19 Science Advisory Table . September 7, 2022. Accessed May 10, 2023. https://covid19-sciencetable.ca/sciencebrief/understanding-the-post-covid-19-condition-long-covid-in-adults-and-the-expected-burden-for-ontario/
- 7.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863-876. doi: 10.1016/S2213-2600(22)00126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNaughton CD, Austin PC, Sivaswamy A, et al. Post-acute health care burden after SARS-CoV-2 infection: a retrospective cohort study. CMAJ. 2022;194(40):E1368-E1376. doi: 10.1503/cmaj.220728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker HR, Gulea C, Koteci A, et al. GP consultation rates for sequelae after acute covid-19 in patients managed in the community or hospital in the UK: population based study. BMJ. 2021;375:e065834. doi: 10.1136/bmj-2021-065834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skyrud KD, Hernæs KH, Telle KE, Magnusson K. Impacts of mild COVID-19 on elevated use of primary and specialist health care services: a nationwide register study from Norway. PLoS One. 2021;16(10):e0257926. doi: 10.1371/journal.pone.0257926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 13.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372(693):n693. doi: 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhaskaran K, Rentsch CT, Hickman G, et al. Overall and cause-specific hospitalisation and death after COVID-19 hospitalisation in England: a cohort study using linked primary care, secondary care, and death registration data in the OpenSAFELY platform. PLoS Med. 2022;19(1):e1003871. doi: 10.1371/journal.pmed.1003871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings G, Monaghan A, Xue F, Mockler D, Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10(24):5913. doi: 10.3390/jcm10245913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizarro-Pennarolli C, Sánchez-Rojas C, Torres-Castro R, et al. Assessment of activities of daily living in patients post COVID-19: a systematic review. PeerJ. 2021;9:e11026. doi: 10.7717/peerj.11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Navarro-Pardo E, Rodríguez-Jiménez J, Pellicer-Valero OJ. Post-COVID functional limitations on daily living activities are associated with symptoms experienced at the acute phase of SARS-CoV-2 infection and internal care unit admission: a multicenter study. J Infect. 2022;84(2):248-288. doi: 10.1016/j.jinf.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuschke D, Houston D. The impact of Long COVID on the UK workforce. Appl Econ Lett. Published online July 6, 2022. doi: 10.1080/13504851.2022.2098239 [DOI] [Google Scholar]
- 19.Kosyakovsky LB, Angriman F, Katz E, et al. Association between sepsis survivorship and long-term cardiovascular outcomes in adults: a systematic review and meta-analysis. Intensive Care Med. 2021;47(9):931-942. doi: 10.1007/s00134-021-06479-y [DOI] [PubMed] [Google Scholar]
- 20.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345-353. doi: 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 21.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312-1320. doi: 10.1001/archinte.167.12.1312 [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677. doi: 10.1136/bmj.m4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma AA, Hora T, Jung HY, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ. 2021;193(12):E410-E418. doi: 10.1503/cmaj.202795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28(5):911-923. doi: 10.1038/s41591-022-01810-6 [DOI] [PubMed] [Google Scholar]
- 25.Herridge MS, Azoulay É. The COVID-19 continuum of illness. Lancet Respir Med. 2022;10(7):630-631. doi: 10.1016/S2213-2600(22)00219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu TM, Seet CYH, Koh JS, et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open. 2021;4(4):e217498. doi: 10.1001/jamanetworkopen.2021.7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583-590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi: 10.1016/S2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377:e069590. doi: 10.1136/bmj-2021-069590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ. 2022;376:e068993. doi: 10.1136/bmj-2021-068993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815-827. doi: 10.1016/S2215-0366(22)00260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clift AK, Ranger TA, Patone M, et al. Neuropsychiatric ramifications of severe COVID-19 and other severe acute respiratory infections. JAMA Psychiatry. 2022;79(7):690-698. doi: 10.1001/jamapsychiatry.2022.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406-2415. doi: 10.1038/s41591-022-02001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung H, Austin PC, Brown KA, et al. Effectiveness of COVID-19 vaccines over time prior to Omicron emergence in Ontario, Canada: test-negative design study. Open Forum Infect Dis. 2022;9(9):ofac449. doi: 10.1093/ofid/ofac449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton MA, Calzavara A, Emerson SD, et al. Validating International Classification of Disease 10th Revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS One. 2021;16(1):e0244746. doi: 10.1371/journal.pone.0244746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolley RJ, Quan H, Jetté N, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open. 2015;5(12):e009487. doi: 10.1136/bmjopen-2015-009487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol. 2010;10(1):42. doi: 10.1186/1471-2288-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muggah E, Graves E, Bennett C, Manuel DG. The impact of multiple chronic diseases on ambulatory care use; a population based study in Ontario, Canada. BMC Health Serv Res. 2012;12(1):452. doi: 10.1186/1472-6963-12-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin E, Balogh R, Cobigo V, Ouellette-Kuntz H, Wilton AS, Lunsky Y. Using administrative health data to identify individuals with intellectual and developmental disabilities: a comparison of algorithms. J Intellect Disabil Res. 2013;57(5):462-477. doi: 10.1111/jir.12002 [DOI] [PubMed] [Google Scholar]
- 42.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf. 2019;28(4):284-288. doi: 10.1136/bmjqs-2018-008661 [DOI] [PubMed] [Google Scholar]
- 44.Mondor L, Maxwell CJ, Hogan DB, et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort. PLoS Med. 2017;14(3):e1002249. doi: 10.1371/journal.pmed.1002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417-2418. doi: 10.1001/jama.2020.7819 [DOI] [PubMed] [Google Scholar]
- 46.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188(1):250-257. [DOI] [PubMed] [Google Scholar]
- 47.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837-2849. doi: 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saxena FE, Bierman AS, Glazier RH, et al. Association of early physician follow-up with readmission among patients hospitalized for acute myocardial infarction, congestive heart failure, or chronic obstructive pulmonary disease. JAMA Netw Open. 2022;5(7):e2222056. doi: 10.1001/jamanetworkopen.2022.22056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390-400. doi: 10.1080/01621459.2016.1260466 29930437 [DOI] [Google Scholar]
- 50.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074-1078. doi: 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 51.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canadian Chronic Disease Surveillance System, 2019-2020 crude incidence rates. Public Health Agency of Canada . Accessed May 10, 2023. https://health-infobase.canada.ca/ccdss/data-tool/
- 53.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisk LE, Nichol G, Elmore JG. Toward unbiased evaluation of postacute sequelae of SARS-CoV-2 infection: challenges and solutions for the long haul ahead. Ann Intern Med. 2022;175(5):740-743. doi: 10.7326/M21-4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179(1):38-45. doi: 10.1001/jamainternmed.2018.5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437-446. doi: 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Description of datasets
eTable 2. Description of validated case ascertainment algorithms used to measure primary outcomes including incident cardiovascular, neurological, and mental health conditions
eTable 3. Baseline characteristics of adults who survived hospitalization for COVID-19, compared to those who survived hospitalization for influenza and sepsis before weighting in Ontario between January 1, 2014, and October 31, 2021
eTable 4. Baseline characteristics of adults who survived hospitalization for COVID-19, compared to those who survived hospitalization for influenza and historical sepsis after weighting in Ontario between January 1, 2014, and October 31, 2021
eTable 5. Secondary analysis according to timing (<30 days) of outcomes following discharge from index hospitalization
eTable 6. Secondary analysis according to timing (≥30 days) of outcomes following discharge from index hospitalization
eTable 7. Secondary analysis among those admitted to ICU during index hospitalization
eReferences
Data Sharing Statement



