Heart transplantation continues to be the final therapeutic option for patients with end-stage heart disease of varying etiologies, with more than 6,000 heart transplants performed annually worldwide. As survival of patients with heart disease has improved over time due to the availability of better medical, surgical, and device-based therapies, the heart transplant recipient population has changed. Similarly, candidate acceptance criteria for heart transplantation have expanded over the years. This focused report aims to document the changes that have taken place in the adult heart transplant recipient pool over the years and to identify important recipient characteristics and transplant processes that influence post-transplant outcomes. An analysis of changes in donor characteristics was published previously1.
This 38th annual adult heart transplant report is based on data submitted to the International Society for Heart and Lung Transplantation (ISHLT) International Thoracic Organ Transplant (TTX) Registry on 108,034 adult recipients of deceased donor heart transplants performed between January 1992 and June 2018. In response to the changing regulatory environment, the ISHLT Registry is currently updating the processes involved in data acquisition. Therefore, the patient cohort examined in this report is derived from the same database or datasets used in the 2019 annual report2. We refer the reader to the 2019 report for a detailed description of the cohort and for additional core analyses not directly related to this year’s focus theme.
Statistical methods
Data collection, conventions, and statistical methods
The 2021 ISHLT International TTX Registry report, as in past years, was developed using data submitted to the Registry as of November 2, 2018, by national and multinational transplant collectives as well as individual transplant centers. Since the Registry’s inception, 481 adult heart transplant centers have contributed data. This report presents an overview of characteristics of adult heart recipients of deceased donor heart-alone transplants and their associations with post-transplant outcomes, with a particular focus on how the recipient profile has changed over time. The results reported herein seek to provide as granular detail as possible with data retained in the ISHLT International TTX Registry for transplants through June 30, 2018. In addition to the data presented within the primary manuscript, extended analyses are provided in the online slide sets. The ISHLT website also contains slide sets for previous annual reports. This report references specific online e-slides when particular data are discussed but not shown due to space limitations. E-slide H(a) numbers refer to the online adult heart transplant slides (https://ishlt.org/research-data/registries/ttx-registry/ttx-registry-slides)
TaggedPThe ISHLT International TTX Registry website (https://ishlt.org/research-data/registries/ttx-registry#data-fields-look-up-tables-forms) provides detailed spreadsheets of the data elements collected in the Registry. The Registry required submission of core donor, recipient, and transplant procedure variables at the time of transplantation and at annual follow-up with low rates of missing data. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that depend on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
Unless otherwise specified, analyses of combined heart-lung transplants are not included in analyses of heart transplants. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., cardiac allograft vasculopathy), but it does capture specific time periods that can narrow the event time. For the report’s analyses, we use the mid-point between the annual follow-ups as a surrogate for the event date (i.e., the event occurred between the first and the second annual follow-up visits). On the follow-up where a death is reported, some under-reporting of secondary outcomes and other information is probable. Thus, to reduce the potential for underestimating event rates or other outcomes, some analyses are restricted to include only surviving recipients. For time-to-event analyses, we censored the follow-up of recipients who did not experience the event of interest at the last time the recipient was reported not to have had the event, which would either be the most recent annual follow-up or the time of retransplantation. We truncated time-to-event graphs (e.g., survival graphs) when the number of individuals at risk was <10. Previous Registry reports provide additional details regarding specific donor and recipient characteristics and outcomes.
Focus theme: recipient characteristics
Recipient characteristics
Many centers around the world have expanded adult heart transplant candidate acceptance criteria over time as clinical experience with heart transplantation has accrued, and post-transplant clinical outcomes have improved. This, combined with changes in population demographics, has led to significant changes in recipient characteristics, as shown in Table 1. Here, we divided adult heart transplants into three eras: 1992–2000, 2001–2009, and 2010–2018, with a similar number of transplants performed in each era.
Table 1.
Distribution of recipient characteristics by transplant era (transplants: January 1, 1992 − June 30, 2018)
| Jan 1992 – Dec 2000 (n = 37,616) | Jan 2001 – Dec 2009 (n = 33,588) | Jan 2010 – June 2018 (n = 36,830) | p-value | |
|---|---|---|---|---|
| Geographic Location: | ||||
| - Europe | 16,663 (44.3%) | 14,161 (42.2%) | 13,103 (35.6%) | <0.0001 |
| - North America | 19,119 (50.8%) | 17,186 (51.2%) | 20,265 (55.0%) | |
| - Other | 1,834 (4.9%) | 2,241 (6.7%) | 3,462 (9.4%) | |
| Age (years) | 54 (28–65) | 54 (25–66) | 55 (25–68) | <0.0001 |
| Male | 80.7% | 77.6% | 74.4% | <0.0001 |
| Weight (kg) | 75.7 (53.5–101.6) | 78.0 (54.0–106.1) | 80.0 (54.4–108.9) | <0.0001 |
| Height (cm) | 174.0 (157.0–188.0) | 174.0 (157.5–188.0) | 174.0 (157.5–188.0) | 0.0463 |
| BMI (kg/m2) | 25.0 (19.1–32.4) | 25.7 (19.3–33.7) | 26.5 (19.6–34.6) | <0.0001 |
| Blood type: | ||||
| - A | 45.2% | 43.3% | 42% | <0.0001 |
| - AB | 5.2% | 5.3% | 5.8% | |
| - B | 12.2% | 13.1% | 14.1% | |
| - O | 37.4% | 38.2% | 38.1% | |
| PRA ≥ 20% | 5.2% | 10.0% | 17.9% | <0.0001 |
| PRA ≥ 80% | 0.9% | 2.3% | 3.6% | <0.0001 |
| CMV antibody positive | 67.7% | 64.0% | 59.6% | <0.0001 |
| EBV antibody positive | 85.0%1 | 85.6% | 87.1% | 0.0001 |
| Hep B antibody positive | 3.8%2 | 5.2% | 4.9% | <0.0001 |
| Hep C antibody positive | 2.5%2 | 2.1% | 1.9% | 0.0006 |
| Diabetes | 16.7%2 | 22.3% | 27.0% | <0.0001 |
| History of malignancy | 3.8%2 | 5.8% | 8.7% | <0.0001 |
| History of smoking | - | 48.9%3 | 45.0% | <0.0001 |
| Pre-transplant dialysis | 3.0%2 | 4.1% | 4.8% | <0.0001 |
| Previous cardiac surgery | 37.8%2 | 43.6% | 50.1% | <0.0001 |
| BiLirubin (mg/dL) | 0.8 (0.3–2.5)2 | 0.8 (0.3–3.1) | 0.7 (0.3–2.3) | <0.0001 |
| Creatinine (mg/dl) | 1.2 (0.7–2.2)2 | 1.2 (0.7–2.2) | 1.2 (0.7–2.2) | <0.0001 |
| GFR (mL/min/1.73 m2) * | 72.0 (36.6–130.5)2 | 75.6 (36.9–143.6) | 77.8 (37.7–149.1) | <0.0001 |
| PCW mean (mmHg) | 20.0 (6.7–33.0)2 | 18.0 (6.0–32.0) | 17.0 (5.0–31.0) | <0.0001 |
| PA mean (mmHg) | 31.0 (14.0–50.0)2 | 28.0 (14.0–47.0) | 26.0 (13.0–45.0) | <0.0001 |
| PVR (Woods unit) | 2.2 (0.7–6.0)2 | 2.2 (0.6–5.6) | 2.1 (0.6–5.1) | <0.0001 |
| Inotrope use | 46.7%2 | 43.4% | 35.7% | <0.0001 |
| IABP use | 6.0% | 6.2% | 6.6% | 0.0672 |
| ECMO use | 0.2%4 | 0.7% | 1.1% | <0.0001 |
| Type of MCS use | ||||
| - None | - | 77.2% | 55.6% | <0.0001 |
| - VAD | - | 18.9% | 40.4% | |
| - TAH | - | 0.7% | 1.3% | |
| - BIVAD | - | 3.3% | 2.7% | |
| VentiLator use | 3.4% | 3.2% | 1.7% | <0.0001 |
| HospitaLized | 60.7% | 47.6% | 44.6% | <0.0001 |
BMI, body mass index; PRA, panel reactive antibody; CMV, cytomegalovirus; EBV, Epstein Barr virus; GFR, Glomerular Filtration Rate; PCW, pulmonary capillary wedge; PA, pulmonary artery pressure; PVR, pulmonary vascular resistance; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; MCS, mechanical circulatory support; VAD, ventricular assist device; TAH, total artificial heart.
Summary statistics excluded transplants with missing data
Continuous factors are expressed as median (5th − 95th percentiles)
Comparisons for categorical variables were made using the chi-square statistic
Comparisons for continuous variables were made using the Wilcoxon test
Based on October 1999 − December 2000 transplants
Based on April 1994 − December 2000 transplants
Based on July 2004 − December 2009 transplants
Based on April 1995 − December 2000 transplants
GFR was estimated using the Cockcroft-Gault formula
From the 1992–2000 era to the 2010–2018 era, the median recipient age increased slightly worldwide. This increase in recipient age was mainly driven by changes in North America, as shown in Figure 1A, where median recipient age increased from 53 to 57 years. Recipient age was relatively constant in Europe (~54 years) and Other regions (~51 years). Similar to the increase in recipient age over time, recipient body mass index (BMI) also increased over time, from a median of 25.0 kg/m2 in the 1992–2000 era to 26.5 kg/m2 in the 2010–2018 era, mainly due to an increase in median weight from 75.7 kg to 80.0 kg. As shown in Figure 1B, this increase was seen mainly in North America, and is consistent with current prevalence estimates for overweight and obesity worldwide3. Interestingly, recipient kidney function at the time of transplantation, as estimated by the glomerular filtration rate (GFR, Cockcroft-Gault formula), appeared to improve in all 3 regions over time, as shown in Figure 1C. There are several potential explanations for this observation, including the increasing use of simultaneous heart-kidney transplantation for candidates with severe kidney disease (therefore resulting in higher GFR in those receiving heart only transplants),4 improved kidney function in the setting of pre-transplant ventricular assist device support,5 fewer patients transplanted for ischemic cardiomyopathy (who often have risk factors for kidney disease such as hypertension and diabetes; eSlide H(a) 9), and increasing candidate weight (which is used to calculate eGFR). In support of the final explanation is the observation that median recipient creatinine was stable at 1.2 mg/dl over time.
Figure 1.
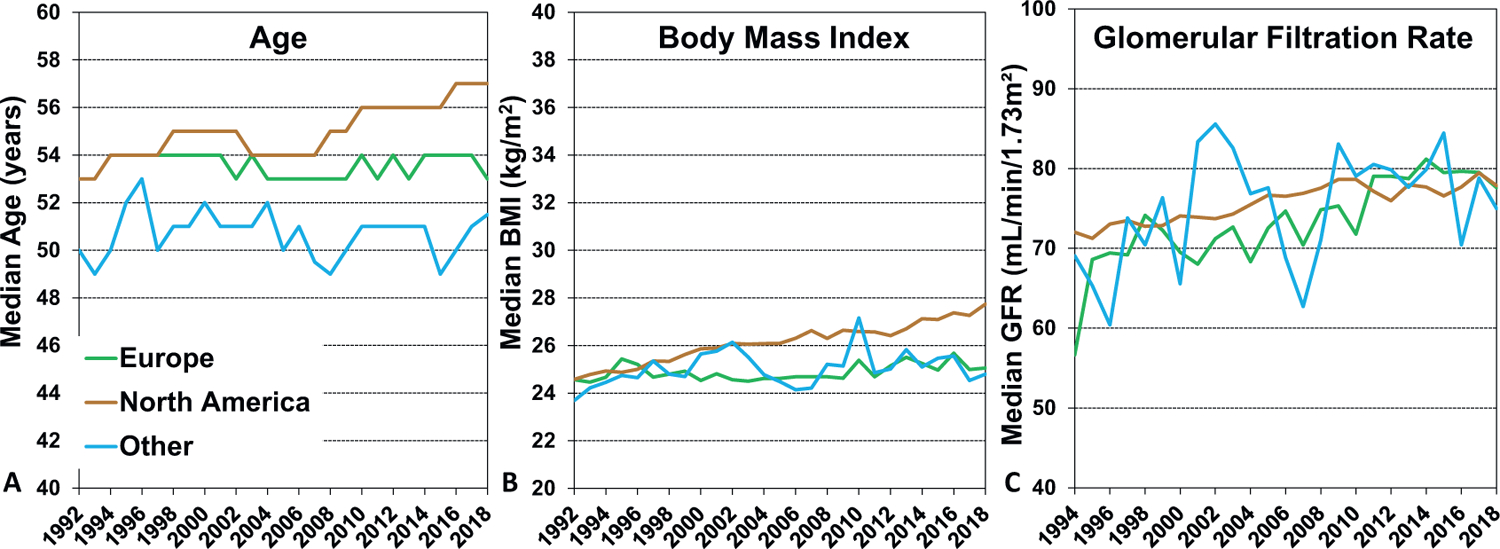
A: Median recipient age by year and geographic location (transplants: January 1992 - June 2018). B: Median recipient body mass index by year and geographic location (transplants: January 1992 − June 2018). C: Median recipient estimated glomerular filtration rate (Cockcroft-Gault formula) by year and geographic location (transplants: January 1994 − June 2018)
The sex distribution of heart transplant recipients has changed over time, with an increase in the proportion of female recipients from 19.3% in 1992–2000 to 25.6% in 2010–2018. It is unclear whether women are still under-represented as heart transplant candidates, or whether we have approached the true prevalence of women who meet the criteria for a heart transplant, as women are more likely to have heart failure with preserved ejection fraction and present with heart failure at an older age. The distribution of recipients by blood type has changed slightly over time, with a decrease in blood type A recipients from 45.2% to 42%, and corresponding increases in the other blood types (particularly type B) from 1992–2000 to the most recent era (Table 1 and eSlide H(a) 8).
There has been a substantial increase in the transplantation of allosensitized patients over time, likely due to the accrual of experience in managing these patients both before and after transplant, increased availability of therapies for pre-transplant desensitization and post-transplant rejection treatment,6 and improved methods for human leukocyte antigen (HLA) typing, antibody detection, and quantification.7 As such, the percentage of recipients with a pre-transplant panel of reactive antibodies (PRA) > 20% increased from 5.2% in 1992–2000 to 17.9% in the most recent era, and the percent of highly sensitized patients (PRA > 80%) increased from 0.9% to 3.6% over the same period (Table 1).
In parallel with the increase in transplantation of allosensitized patients, centers are also transplanting candidates with a greater burden of medical comorbidities. The prevalence of diabetes (both Type 1 and Type 2) increased from 16.7% in 1992–2000 to 22.3% in 2001–2009, and 27.0% in the most recent era (Table 1 and eSlide H(a) 13). Similarly, recipients with a previous history of malignancy increased from 3.8% to 8.7% over the same period (Table 1 and eSlide H(a) 12), along with an increase the proportion of recipients with a history of previous cardiac surgery from 37.8% to 50.1%. Finally, transplantation of candidates on dialysis increased from 3.0% in the first era to 4.8% in the most recent era (Table 1 and eSlide H(a) 12). This likely represents patients with acute kidney injury who are felt to have a high likelihood of renal recovery, as patients with end-stage renal disease are increasingly being referred for simultaneous heart-kidney transplantation.4
The use of temporary and durable mechanical circulatory support (MCS) has changed significantly over time, with technological advances and changes in allocation policies (Table 1). The use of intra-aortic balloon pumps (IABP) increased from 6.0% to 6.6% over time, while extracorporeal membrane oxygenation (ECMO) use increased from 0.2% to 1.1%. When comparing 2001–2009 to 2010–2018, use of ventricular assist devices (VADs) increased from 18.9% to 40.4%, total artificial hearts (TAH) increased from 0.7% to 1.3%, and use of biventricular assist devices declined from 3.3% to 2.7%. With the increased use of MCS, pre-transplant inotrope use fell from 46.7% to 35.7% over the three eras studied. Increased use of bridge-to-transplant LVADs, with less use of inotropic support, may partly account for the decline in patients hospitalized prior to transplant (60.7% to 44.6%). The cohorts analyzed in this report predate the United States revised donor heart allocation policy, implemented in October 2018,8 and therefore do not capture more recent data showing increased use of temporary mechanical support (IABP and ECMO)9 and a fall in bridge-to-transplant LVAD support in the United States.10
The distribution of recipient pre-transplant diagnosis (indication for transplant) has changed over time, with an increase in nonischemic cardiomyopathy, hypertrophic cardiomyopathy (particularly in Europe), restrictive cardiomyopathy, and congenital heart disease, and a decrease in transplantation for ischemic and valvular cardiomyopathy (eSlide H(a) 9).
Donor-recipient sex matching has garnered considerable recent interest.11–13 Over the years, there has been a slight increase in female-female transplants, and a corresponding decline in female-male transplants (eSlide H (a) 10), perhaps due to the inferior survival seen after sex-mismatched transplants (see multivariable survival analyses below). This could be explained by immunological differences resulting from sex-mismatched transplants,14 or to the increasing attention paid to sizematching the donor and recipient, recognizing that the inferior outcomes seen after sex-mismatched transplants may be due to heart size differences.1 Indeed, female donor-male recipient transplants are often undersized, when assessed by predicted heart mass, and this may partly account for subsequent increased mortality.15
Survival analyses
One-year survival
We next examined unadjusted associations between recipient risk factors and one-year post-transplant survival. One-year survival has improved in the most recent era (2012–2017, eSlide H(a) 15), compared to the two earlier eras. In unadjusted analyses stratified by region, one-year survival is in general higher in North America when compared to European and Other regions, and significant improvement in survival over time is also seen in North America (Figure 2). This finding may be due to greater use of hearts from donors with advanced age and other high-risk features in European countries, as described in more detail in the 2020 Registry Report1. There is also less emphasis on one-year survival as a transplant center performance and quality control metric in Europe than the United States, which may encourage the performance of higher-risk transplants, resulting in increased early mortality.16,17 Not surprisingly, when stratifying 1-year survival by recipient age (Figure 3), we see decreased survival in older recipients (age ≥ 60 years). In Europe, the youngest recipients (age 18–39 years) have significantly better 1-year survival than the other two recipient age categories (40–59 years and ≥60 years), while in North America and Other regions, survival was similar between 18–39 and 40–59-year-olds. Finally, for all recipient age categories, 1-year survival has improved over time (eSlide H(a) 18), particularly in the most recent era (2012–2017).
Figure 2.
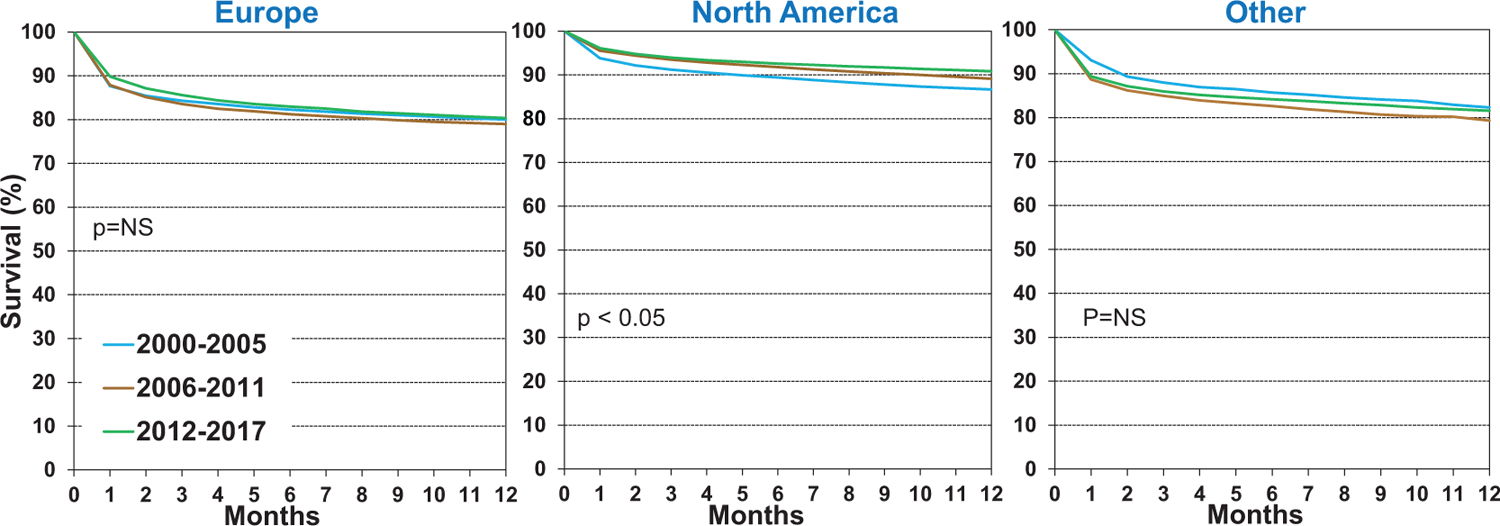
Kaplan-Meier survival within 12 months by location and era (transplants: January 2000-June 2017)
Figure 3.
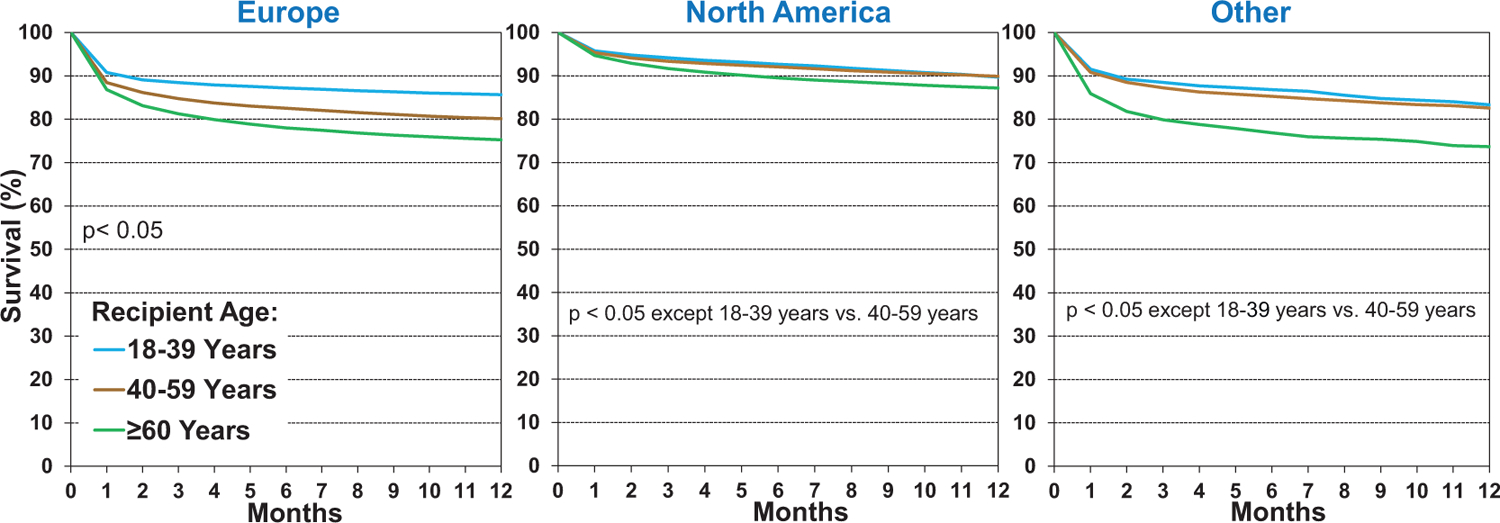
Kaplan-Meier survival within 12 months by location and recipient age (transplants: January 2000-June 2017)
As mentioned previously, transplantation of allosensitized patients has increased over time. Fortunately, short-term survival of allosensitized patients has improved over the past two decades (Figure 4), even in highly sensitized patients with PRA ≥ 80%, likely due to improved technologies for monitoring donor-specific antibody development, and enhanced prevention and treatment options for acute rejection. In fact, in the current era (2012–2017), 1-year survival of allosensitized patients is similar to that of non-sensitized patients.
Figure 4.
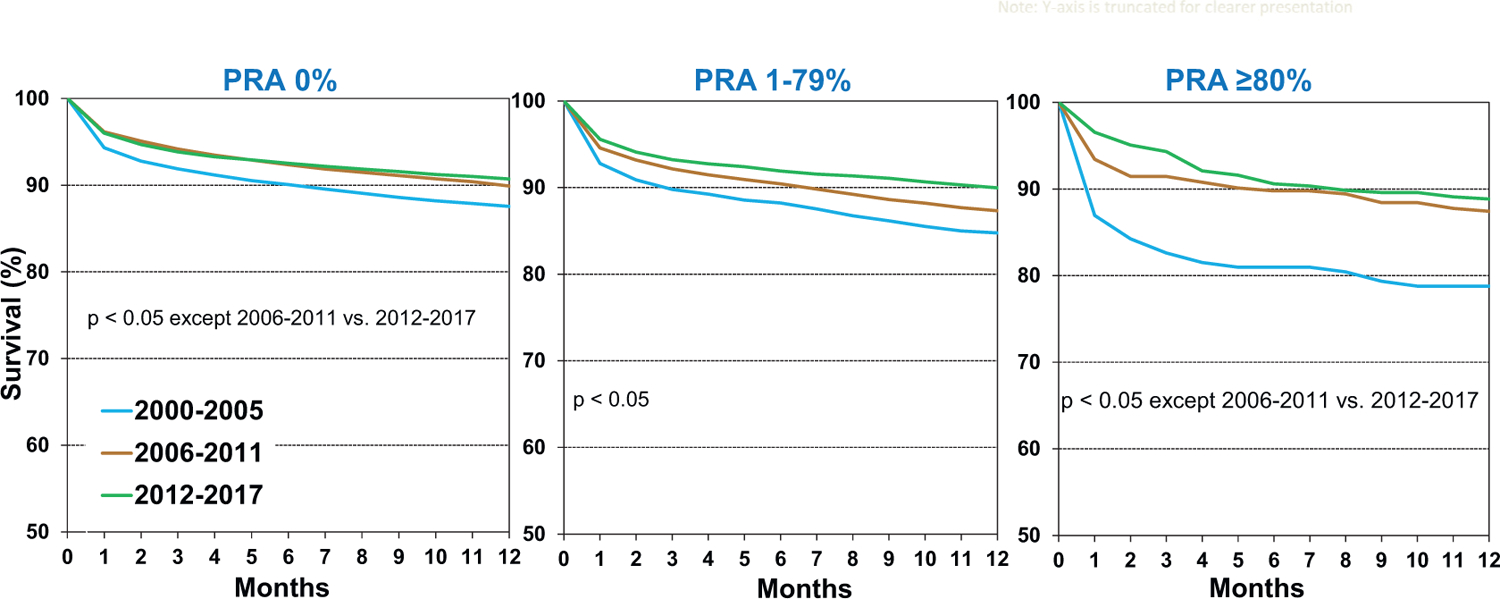
Kaplan-Meier survival within 12 months by recipient panel of reactive antibodies (PRA) and era (transplants: January 2000-June 2017)
In general, 1-year post-transplant survival has also improved in patients with varying severity of chronic kidney disease at the time of transplantation (eSlide H(a) 20); however, 1-year mortality continues to exceed 20% in patients with eGFR <30 ml/min/1.73m2 at transplant, which lends evidence in support of simultaneous heart-kidney transplantation in these candidates.
Figure 5 displays 1-year survival over time in recipients with and without pre-transplant ventricular assist device (VAD) support. While post-transplant survival was initially worse in patients with bridge-to-transplant VADs (2000–2005), it has since improved, and is now approaching that seen in patients without bridge-to-transplant VAD support. This likely reflects improved VAD patient selection and management prior to transplant, improvement in VAD technology, and improved surgical and peri-operative management of these recipients. One-year survival of recipients with pre-transplant total artificial hearts and biventricular assist devices remains lower than those supported with VADs, as these patients are generally sicker, with a greater burden of end-organ dysfunction at the time of transplant (eSlide H(a) 22).
Figure 5.
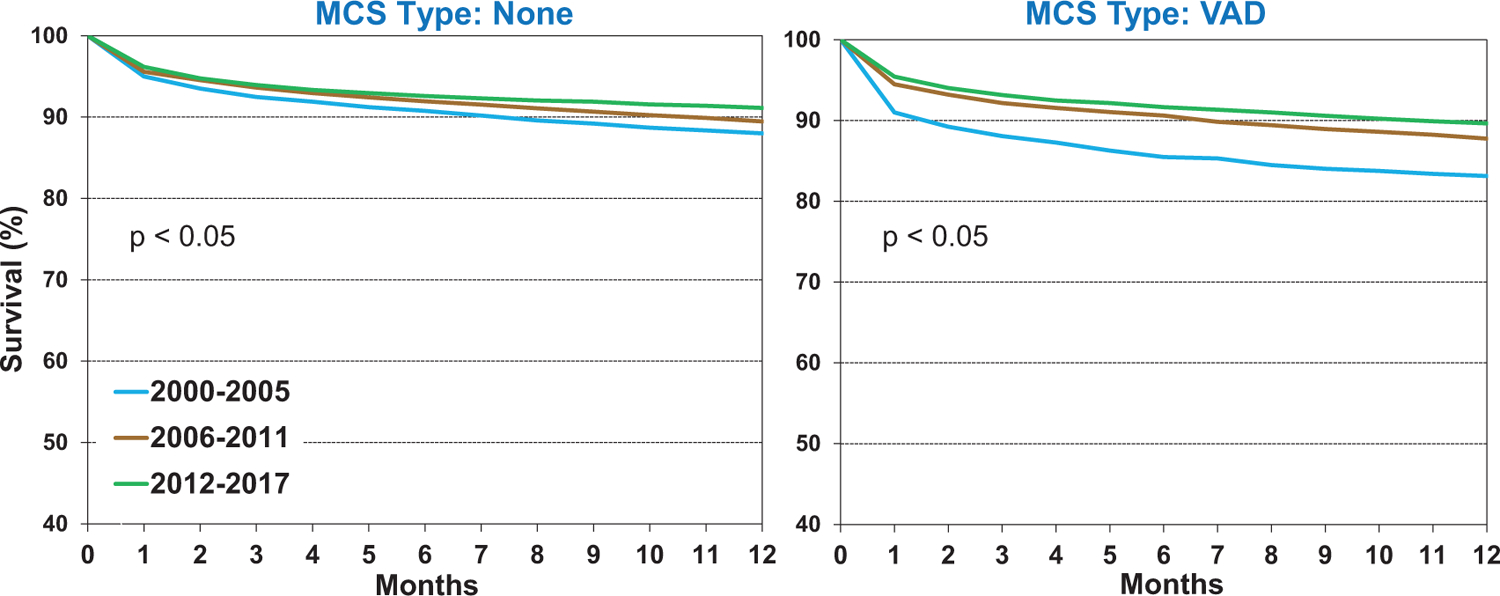
Kaplan-Meier survival within 12 months by recipient mechanical circulatory support (MCS) and era (transplants: January 2000-June 2017). VAD. ventricular assist device
Additional 1-year survival analyses show that recipient diabetes appears to have a small effect on 1-year survival (eSlide H(a) 23), in contrast to its association with 5-year survival, as discussed in the next section. As expected, patients on pre-transplant dialysis have high one-year mortality, which supports consideration of simultaneous or staged heart-kidney transplantation in these patients (eSlide H(a) 24). Finally, prior malignancy does not appear to influence short-term post-transplant survival (eSlide H(a) 24).
Five-year survival, conditional on survival to one year
To examine associations between recipient characteristics and longer-term post-transplant survival, we analyzed 5-year survival conditional on survival to one year (i.e., excluding recipients who died within the first-year posttransplant). As seen in eSlide H(a) 26, 5-year conditional survival has improved significantly in the most recent era (2008–2013), despite transplantation of more complex recipients with a greater burden of comorbidities, and increasing use of higher-risk donors. Examination of survival by location and era (eSlide H(a) 27) shows that 5-year conditional survival has improved over time in Europe, more so in North America, and not in Other regions. In opposition to 1-year survival, 5-year conditional survival is better in Europe (88.5%) compared to North America (86.9%), and Other regions (85.0%) in the most recent cohort, which may be due to lower risk recipients, better access to healthcare, and perhaps shorter ischemic times, compared to non-European countries. Another compelling finding of this Report concerned 5-year survival by location and recipient age, where major differences were observed between European and North American recipients (Figure 6). While young recipients (18–39 years) have the highest survival in Europe, they have the lowest survival in North America. There are several plausible explanations, such as access to medical care for young adults in Europe where universal healthcare is more prevalent, and perhaps worse adherence with medications and clinical follow-up in North America. Age-related disparities have been seen after lung transplantation as well, where 18–29-year olds with cystic fibrosis have worse survival than those 30 years or older.18 Differences in outcomes for chronic diseases have also been observed between young adults in the United States and Europe. For example, patients with cystic fibrosis in the United Kingdom maintain higher lung function over time, compared to their counterparts in the United States.19 These results highlight the need to focus on improving intermediate-term survival among young adult heart transplant recipients in North America. Finally, analysis of 5-year conditional survival by recipient age and era (eSlide H(a) 29) revealed no significant improvement in the youngest cohort (18–39 years) over time, whereas survival has improved in those who were at least 40 years old at the time of transplantation.
Figure 6.
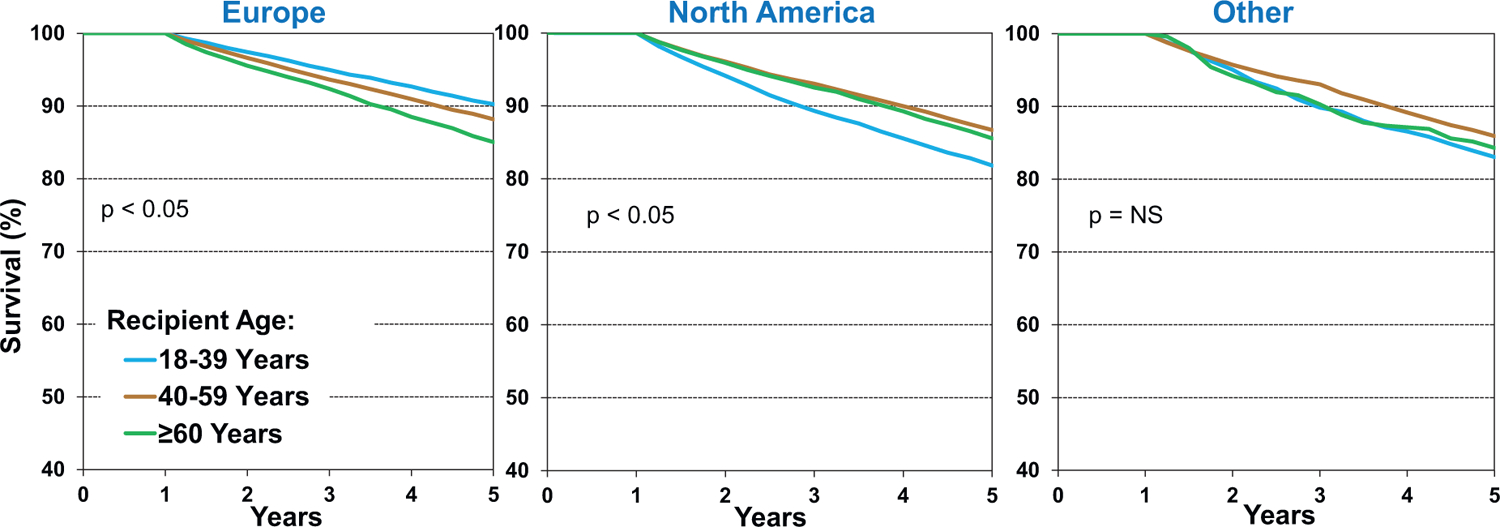
Kaplan-Meier survival within 5 years, conditional on survival to 1 year, by location and recipient age (transplants: January 1996-June 2013)
In contrast to 1-year survival (Figure 4 and eSlide H(a) 19), 5-year conditional survival has not improved over time in allosensitized recipients (Figure 7). It is possible that these patients continue to succumb to late antibody-mediated rejection, development of cardiac allograft vasculopathy (CAV), and other longer-term post-transplant complications. Similarly, 5-year conditional survival has not improved over time for patients with severe chronic kidney disease (eGFR <30 ml/min/1.73m2), again highlighting the need to consider these candidates for simultaneous or staged kidney transplantation (eSlide H(a) 31), and to develop protocols or therapies to prevent the decline in kidney function that is seen too often after transplantation.
Figure 7.
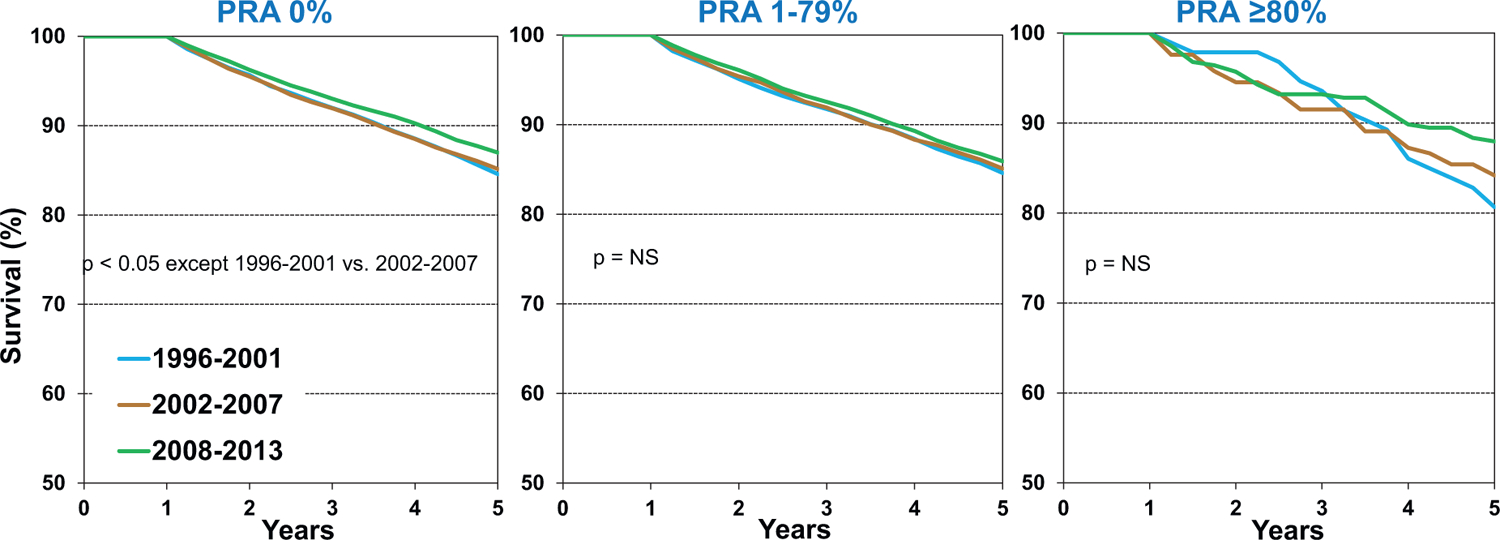
Kaplan-Meier survival within 5 years, conditional on survival to 1 year, by recipient panel of reactive antibodies (PRA) and era (transplants: January 1996-June 2013)
We also present 5-year conditional survival by era and presence of diabetes (eSlide H(a) 32) and history of malignancy (eSlide H(a) 33). Not surprisingly, 5-year survival of recipients with diabetes has generally improved over time, but remains inferior to that of patients without diabetes. In contrast, 5-year survival of patients with a history of malignancy is equivalent to those with no prior cancer diagnosis, which suggests that centers are appropriately selecting cancer survivors for transplantation.
Freedom from cardiac allograft vasculopathy, conditional on survival to discharge
CAV is a leading cause of long-term graft dysfunction and graft loss after heart transplantation. While CAV pathogenesis is complex, and involves both alloimmune and nonimmune processes, it is apparent that both donor and recipient risk factors predispose to CAV development. We examined associations between donor risk factors and CAV development in last year’s focus theme report1 and now examine associations between recipient risk factors and CAV. It should be noted that definitions of CAV may have varied between centers and collectives submitting data to the ISHLT TTX Registry—a standardized definition, such as the ISHLT CAV grading system, was not required.
Unfortunately, there has been little to no progress in freedom from CAV over the past two decades, overall (eSlide H(a) 35) and when stratified by recipient age (eSlide H(a) 36). At the turn of the century, statins20 and proliferation signal inhibitors21,22 were shown to prevent CAV development, and have been widely adopted in clinical practice. Since then, however, there have been no major developments in drug therapy for CAV. Early use of ramipril, an angiotensin-converting enzyme inhibitor, appeared to improve coronary artery microvascular function, but did not reduce coronary artery plaque volume in a single-center study.23 More recently, rituximab, a monoclonal B-cell depleting antibody, unexpectedly caused an increase in coronary artery plaque volume in a multicenter clinical trial.24 Thus, there remains a pressing need to identify effective therapies for CAV prevention and treatment.
Analysis stratified by recipient PRA and transplant era, as shown in Figure 8, was notable for an improvement in freedom from CAV from the first (1996–2001) to the second (2002–2007) era, but this was not sustained in the most recent era (2008–2013), for patients with PRA <80%. This may be a consequence of transplanting higher risk donor hearts and higher risk recipients. There appears to be an improvement in freedom from CAV in the most highly sensitized patients (PRA ≥80%) over time; however, these comparisons do not meet our a priori significance threshold, likely due to the small number of patients in these categories.
Figure 8.
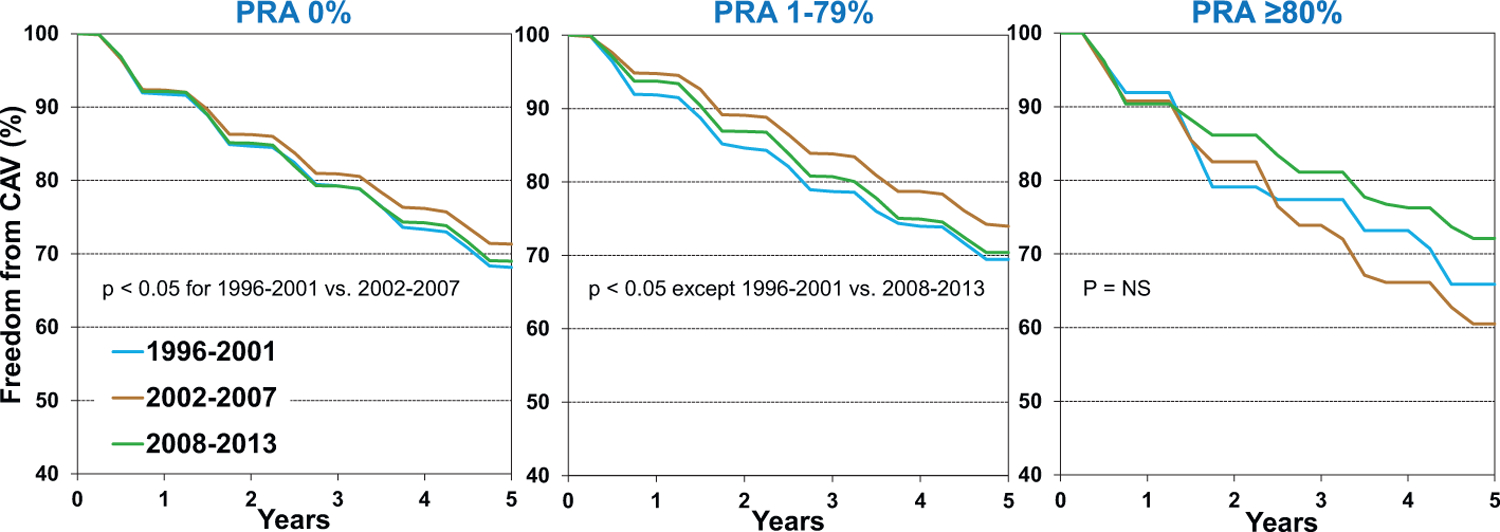
Freedom from cardiac allograft vasculopathy (CAV) conditional on survival to discharge by panel of reactive antibodies (PRA) and era (transplants: January 1996-June 2013)
Of interest is the observation that recipient diabetes does not appear to be associated with CAV development, as shown in Figure 9. However, it is important to note that this analysis is based on patients who had diabetes at the time of transplantation and does not account for those who developed post-transplant diabetes. Similarly, kidney function at the time of transplant does not appear to be a risk factor for subsequent CAV development. Thus, these analyses do not explore associations between the development of post-transplant diabetes (or kidney disease) and CAV development, and these complex relationships are best studied in dedicated analyses using multivariable models in order to account for potential confounders.
Figure 9.
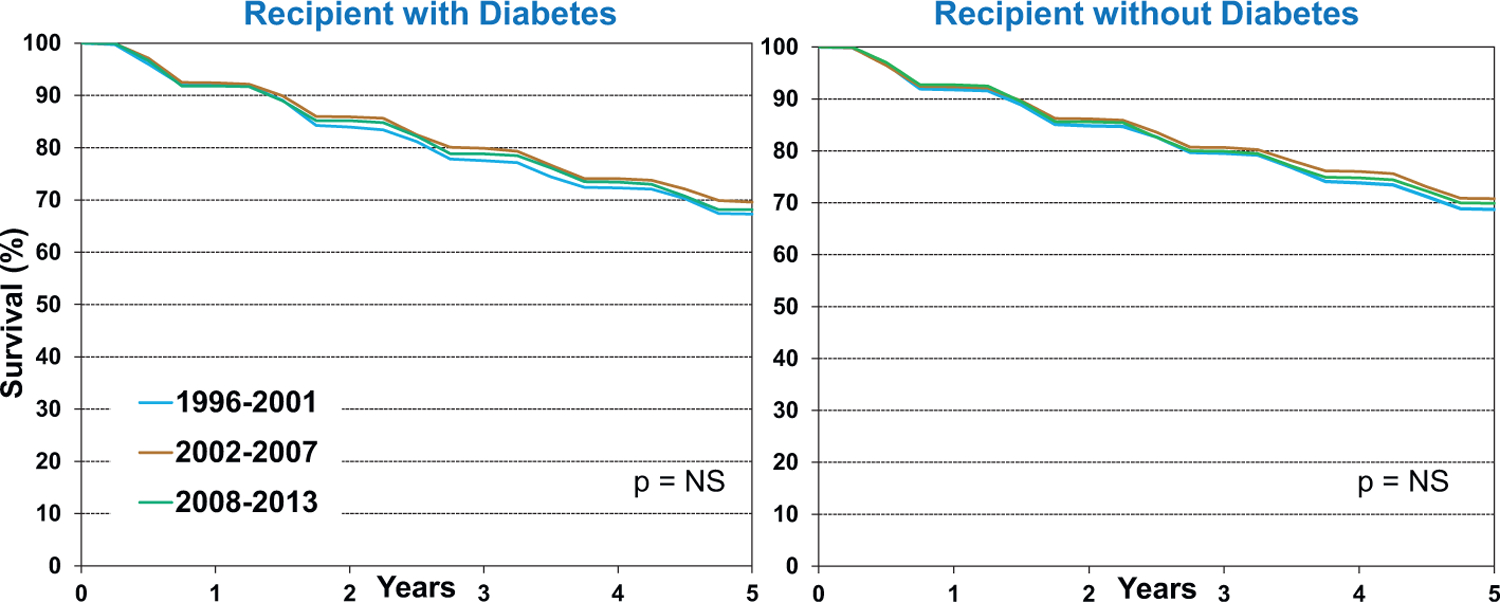
Freedom from cardiac allograft vasculopathy (CAV) conditional on survival to discharge by recipient diabetes and era (transplants: January 1996-June 2013)
Finally, analysis of freedom from CAV by recipient diagnosis (indication for transplant) reveals few significant differences (eSlide H(a) 40), likely due to the small number of patients in certain categories (such as restrictive cardiomyopathy, hypertrophic cardiomyopathy, and congenital heart disease) and the multiple comparisons performed.
Multivariable analyses
We next performed multivariable proportional hazards regression analyses to identify independent risk markers for and potential risk factors associated with subsequent mortality and morbidity. Covariates included in the multivariable models are listed in Supplemental Table 1. These analyses establish independent associations between risk factors and outcomes but cannot establish causality. It is also important to recognize that categorical and continuous risk factors for post-transplant morbidity and mortality will differ by time since transplant. Further, long-term data reflects patients transplanted in earlier eras, and thus the findings may not apply to current conditions. Specific associations observed among these data should, therefore, be interpreted with caution and are better explored in more detailed analyses of Registry data.
One-year survival
Categorical variables significantly associated with increased 1-year mortality after adult heart transplantation (for transplants performed between 1/2000 and 6/2017, Figure 10) include the indication for transplant—valvular and restrictive cardiomyopathy, congenital heart disease, ischemic cardiomyopathy, and retransplantation all confer a higher risk for death than nonischemic cardiomyopathy. Prior cardiac surgery and the use of MCS devices (VAD and ECMO) were also associated with increased 1-year mortality in multivariable analyses, as were indicators of critical illness, such as hospitalization at the time of transplant and mechanical ventilation. We again call attention to the fact that these analyses were performed on transplants occurring before allocation changes in the United State that resulted in greater use of ECMO and other temporary MCS devices.
Figure 10.
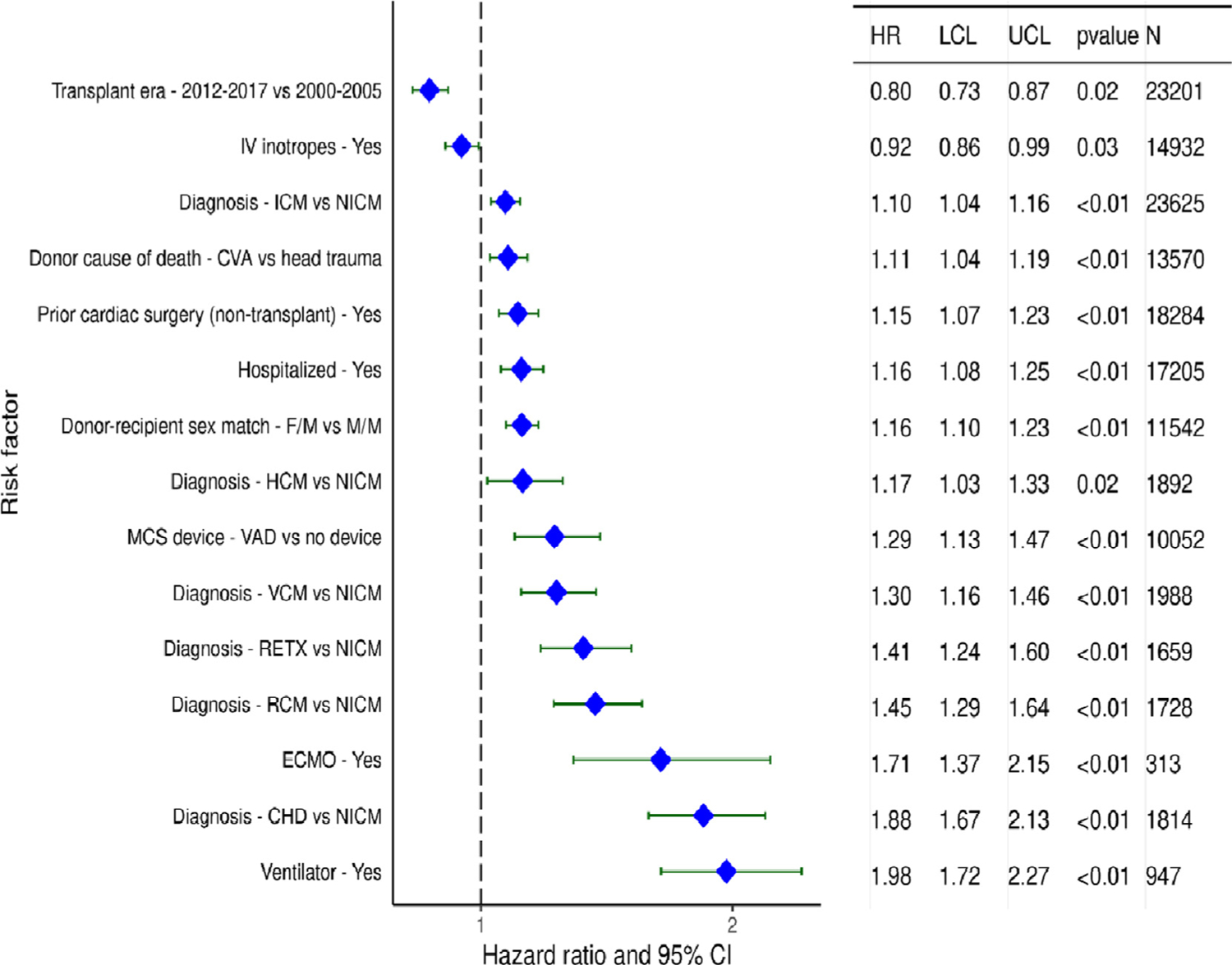
Statistically significant categorical risk factors for 1-year mortality with 95% confidence limits (transplants: January 2000-June 2017; N=67,223). IV: intravenous, ICM: ischemic cardiomyopathy, NICM: non-ischemic cardiomyopathy, CVA: cerebrovascular accident, F: female, M: male, HCM: hypertrophic cardiomyopathy, MCS: mechanical circulatory support, VAD: ventricular assist device, VCM: valvular cardiomyopathy, RETX: retransplant, RCM: restrictive cardiomyopathy, ECMO: extracorporeal membrane oxygenation, CHD: congenital heart disease
Transplant era is also significantly associated with 1-year mortality (p<0.0001). We next examined interactions between transplant era and risk factors of interest, to determine whether the risk of mortality for the identified risk factors is influenced by transplant era. No significant interaction was seen between transplant era and recipient diabetes (eSlide H(a) 43), or transplant era and MCS device type (eSlide H(a) 44) on the outcome of interest.
Continuous variables that were significantly associated with increased risk for 1-year mortality include older donor age, higher recipient BMI, longer ischemic time, worse recipient kidney and liver function, lower transplant center volume, and higher recipient pulmonary vascular resistance and PRA at the time of transplant (eSlides H(a) 45–58).
Both younger and older recipient age was associated with increased risk of 1-year mortality in multivariable analysis (Figure 11). Analysis of recipient kidney function at the time of transplant shows an increase in the hazard ratio (HR) for 1-year mortality with decreasing eGFR. There is a 50% increase in the HR for eGFR of 40 vs. 80 ml/min/1.73m2, and doubling of the HR for eGFR of <30 vs 80 ml/min/1.73m2 (Figure 12). These results may provide data for eventual development of evidence-based guidelines for selecting candidates for simultaneous heart-kidney transplantation.
Figure 11.
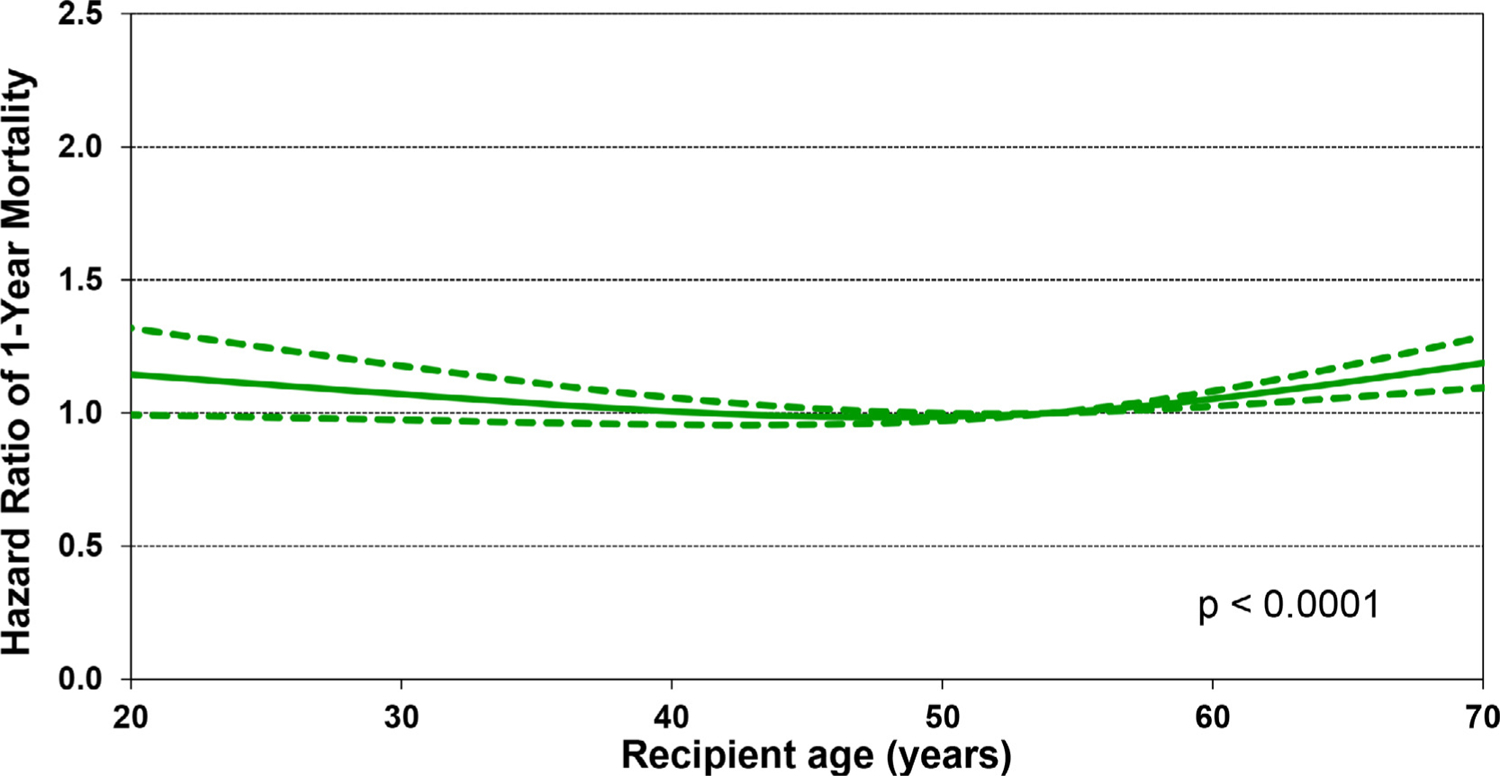
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient age (transplants: January 2000-June 2017; N = 67,223)
Figure 12.
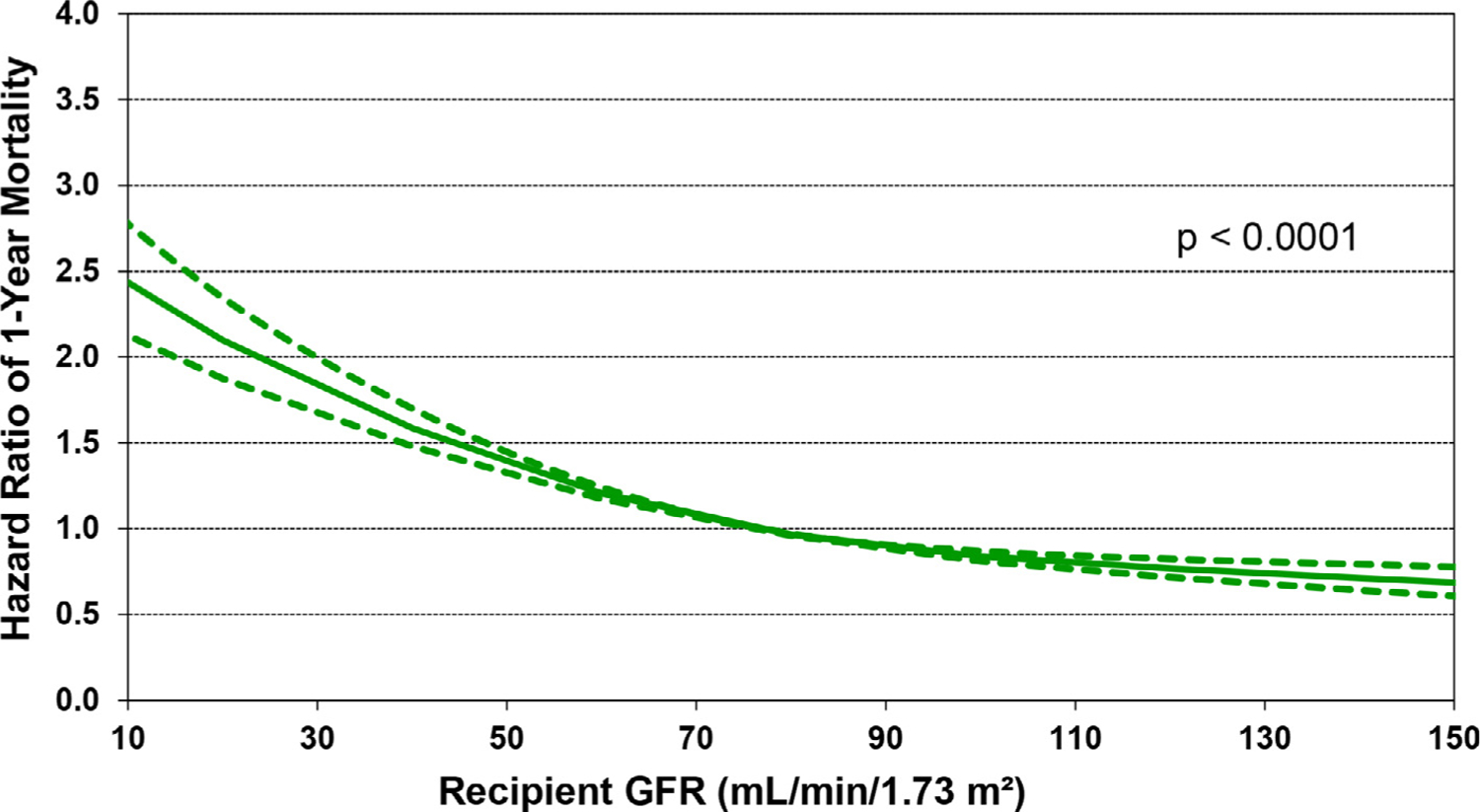
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient glomerular filtration rate (GFR) (transplants: January 2000-June 2017; N = 67,223). GFR was estimated using the Cockcroft-Gault formula
Of additional interest is eSlide H(a) 50, which shows that increasing recipient BMI is associated with increased 1-year mortality, in a linear fashion (Figure 13). Other recipient risk factors/markers for 1-year mortality are rather intuitive and include pulmonary hypertension (as measured by pulmonary vascular resistance and mean pulmonary artery pressure, (eSlides H(a) 51–52), hepatic dysfunction (as measured by total bilirubin, (Figure 14) and allosensitization (PRA, Figure 15). In addition, the relationship between donor age and recipient mortality (eSlide H(a) 56) was explored in more detail in the 2020 Registry Report,1 as was the association between ischemic time and mortality in the 2017 Registry Report.25 Transplant center volume, finally, is significantly associated with 1-year mortality, with the hazard increasing more steeply when fewer than 50–60 transplants were performed in the preceding 3 years (Figure 16). In addition, there were no significant interactions between transplant era and covariates of interest (i.e., recipient age, eGFR, and PRA) with respect to 1-year survival.
Figure 13.
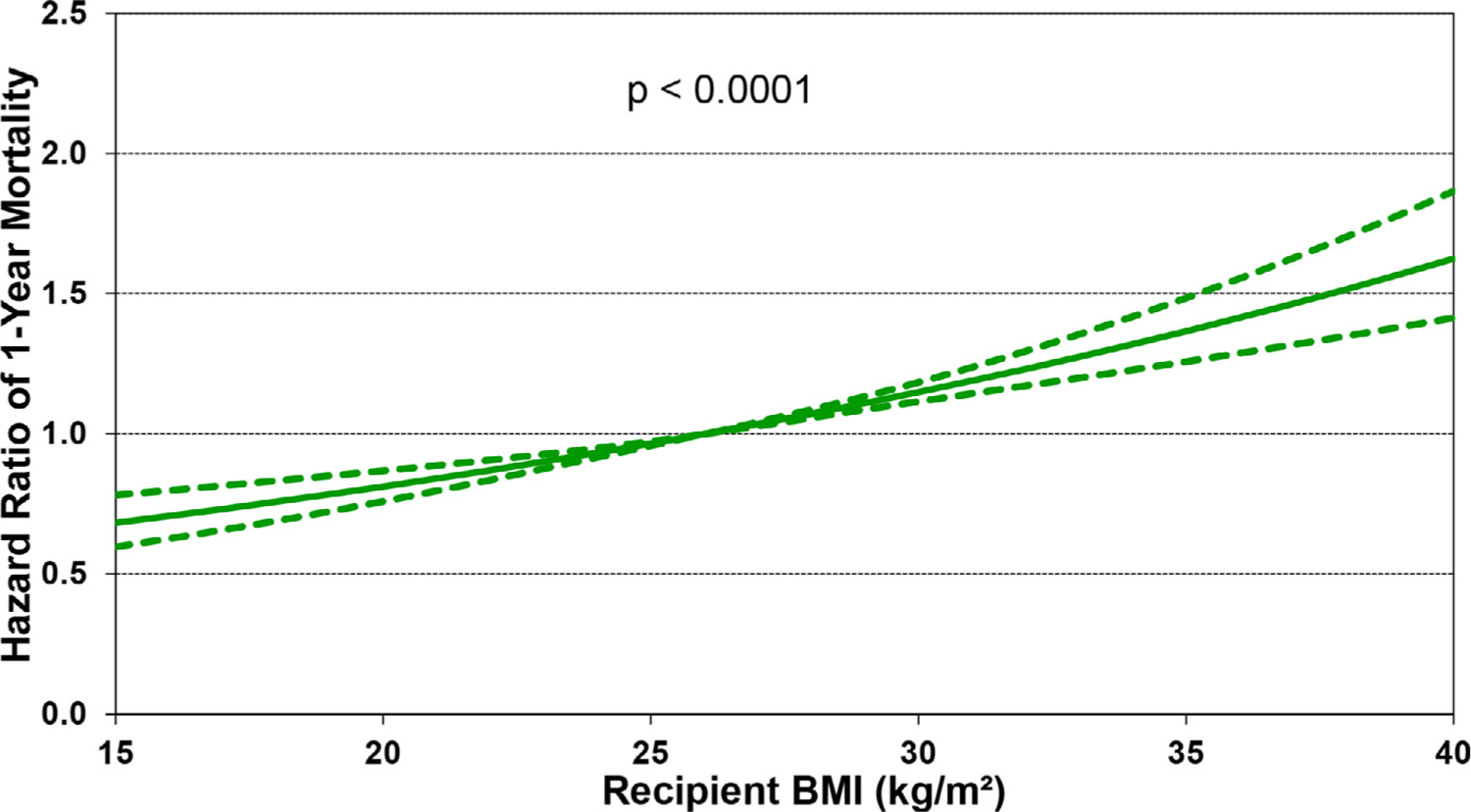
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient body mass index (BMI) (transplants: January 2000-June 2017; N = 67,223).
Figure 14.
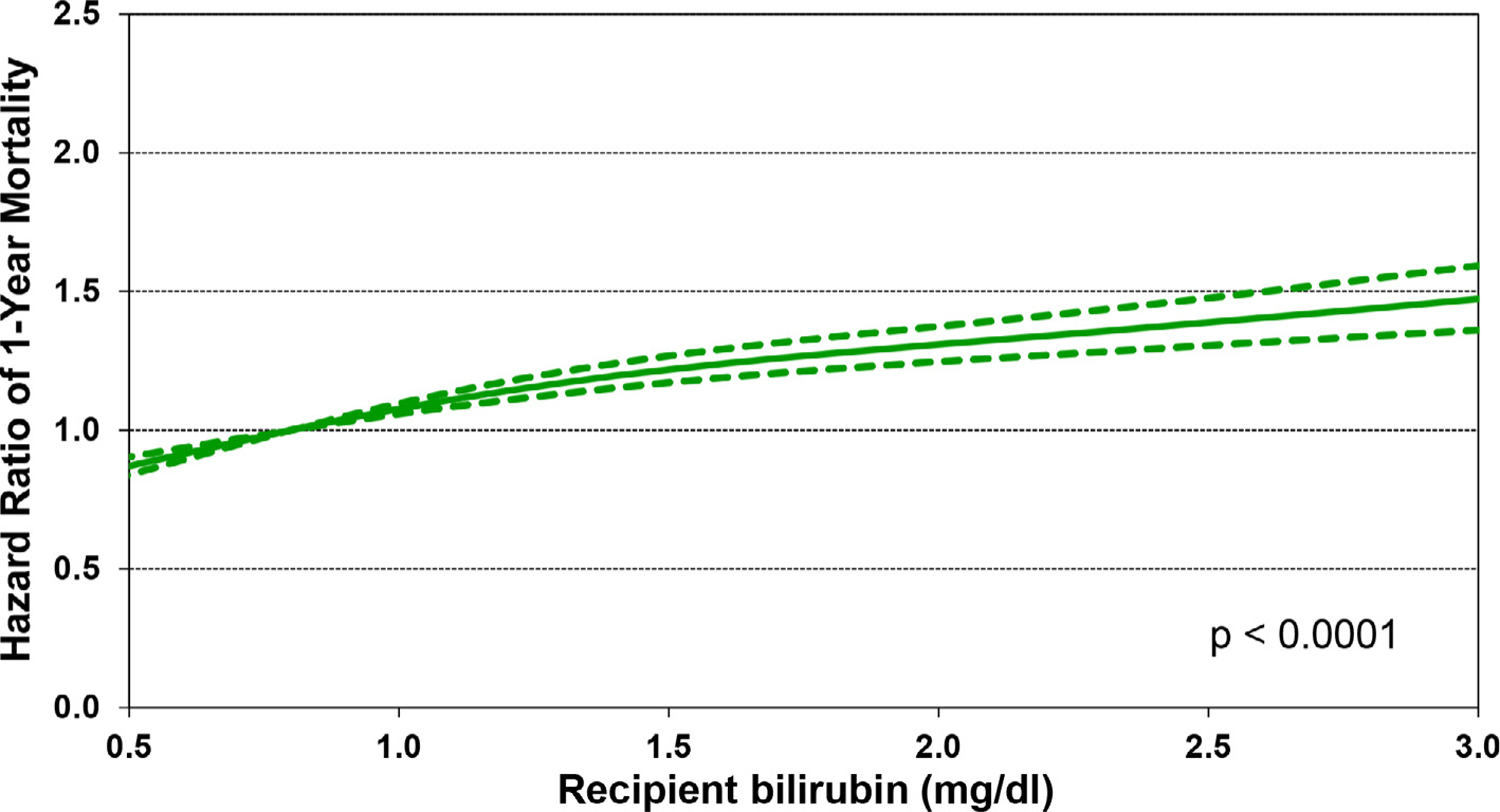
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient bilirubin (transplants: January 2000-June 2017; N = 67,223)
Figure 15.
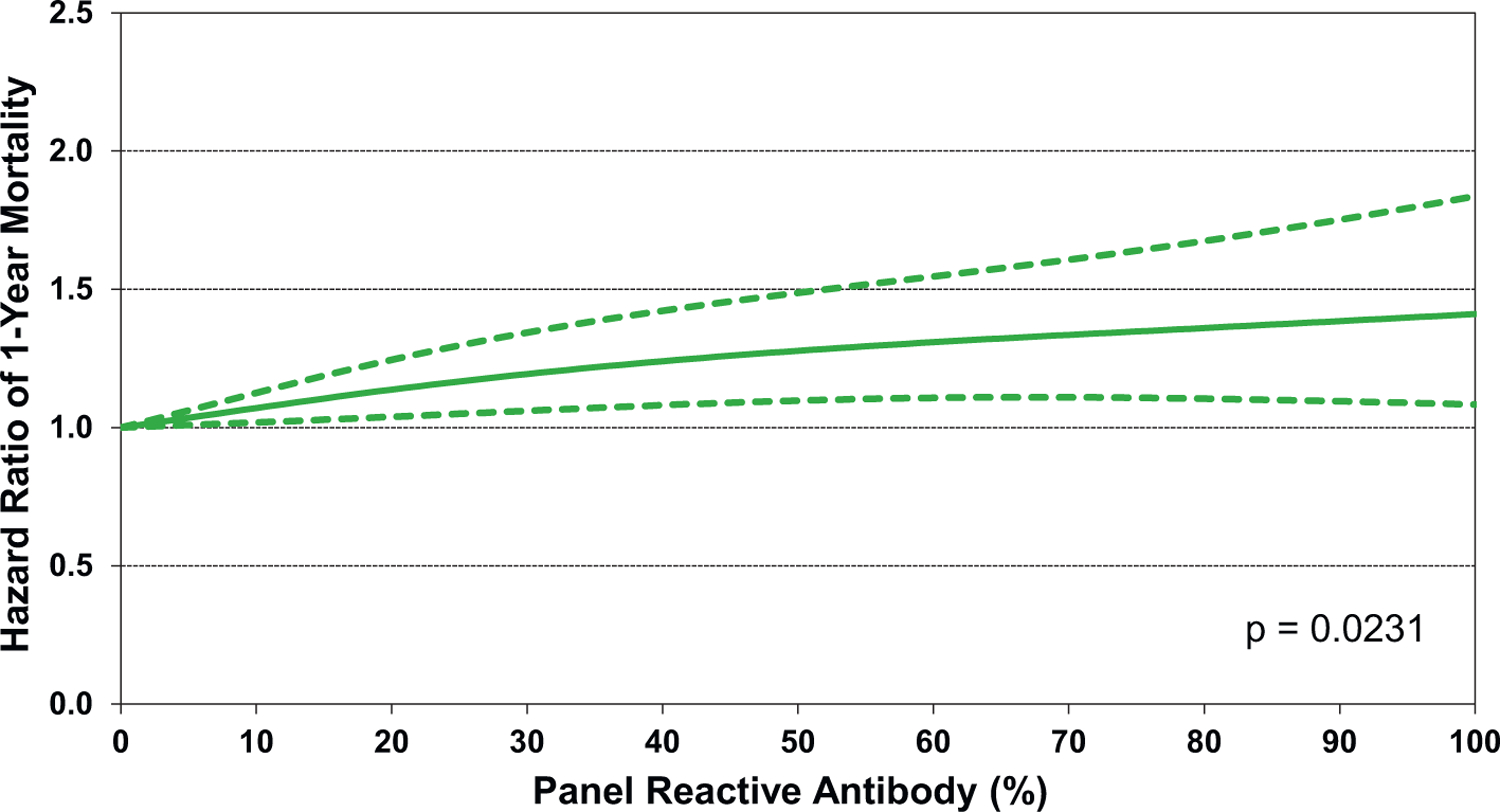
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient panel of reactive antibodies (PRA) (transplants: January 2000-June 2017; N = 67,223)
Figure 16.
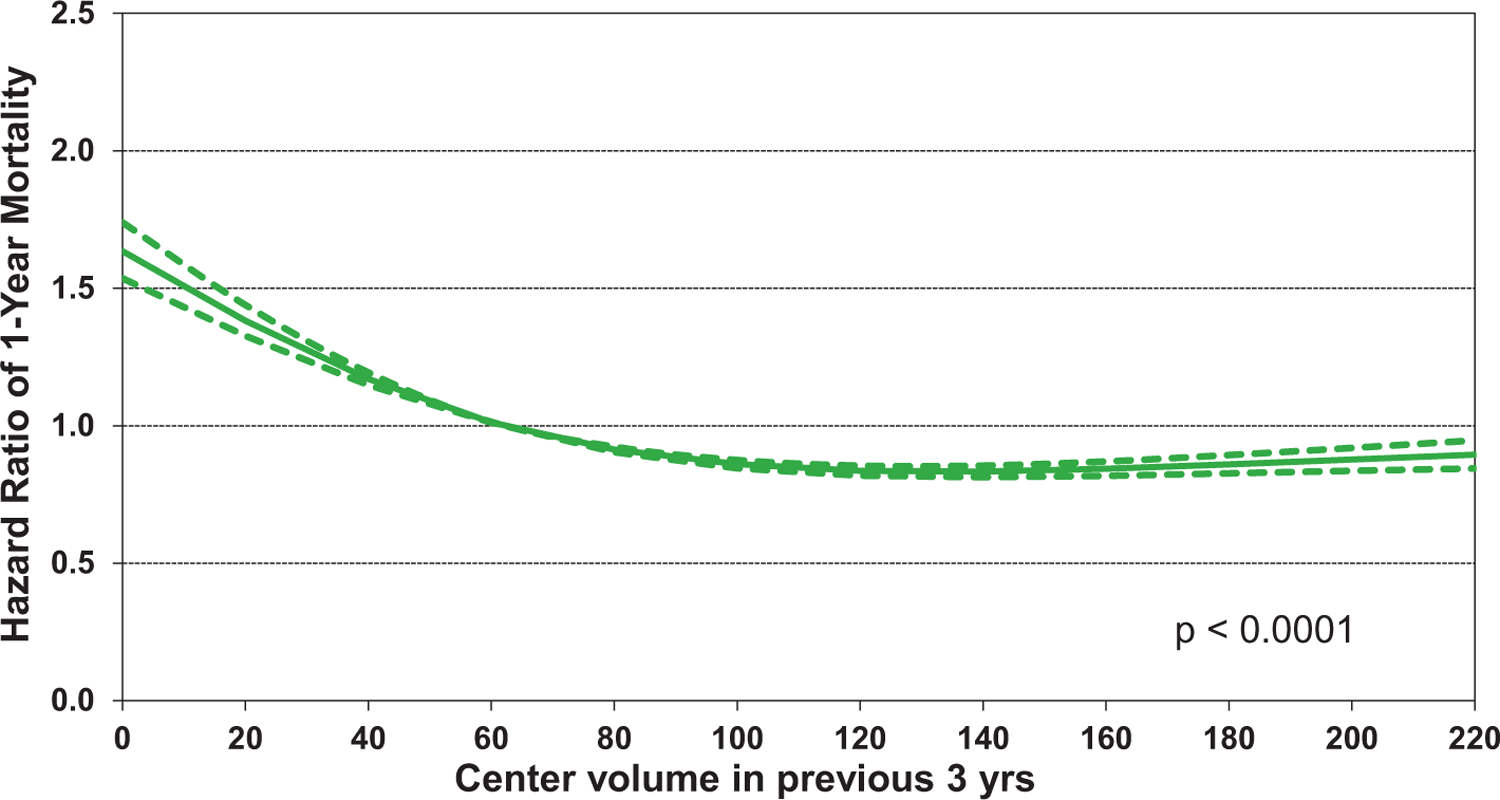
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by transplant center volume in previous three years (transplants: January 2000-June 2017; N = 67,223)
Five-year survival, conditional on survival to one year
Categorical variables associated with increased 5-year mortality, conditional on survival to one year, for transplants performed between 1/1996 and 6/2013, (Figure 17) again include specific indications for transplant and recipient hospitalization at transplant. Recall that hypertrophic cardiomyopathy and congenital heart disease were associated with increased one-year mortality (HR 1.17 and 1.88, respectively). When examining conditional 5-year survival, hypertrophic cardiomyopathy is associated with reduced mortality (HR 0.58), as is congenital heart disease (HR 0.77). Restrictive cardiomyopathy, ischemic cardiomyopathy, and retransplantation are still associated with increased mortality, with HR 1.14–1.35. It is possible that underlying comorbidities are influencing longer-term survival.
Figure 17.
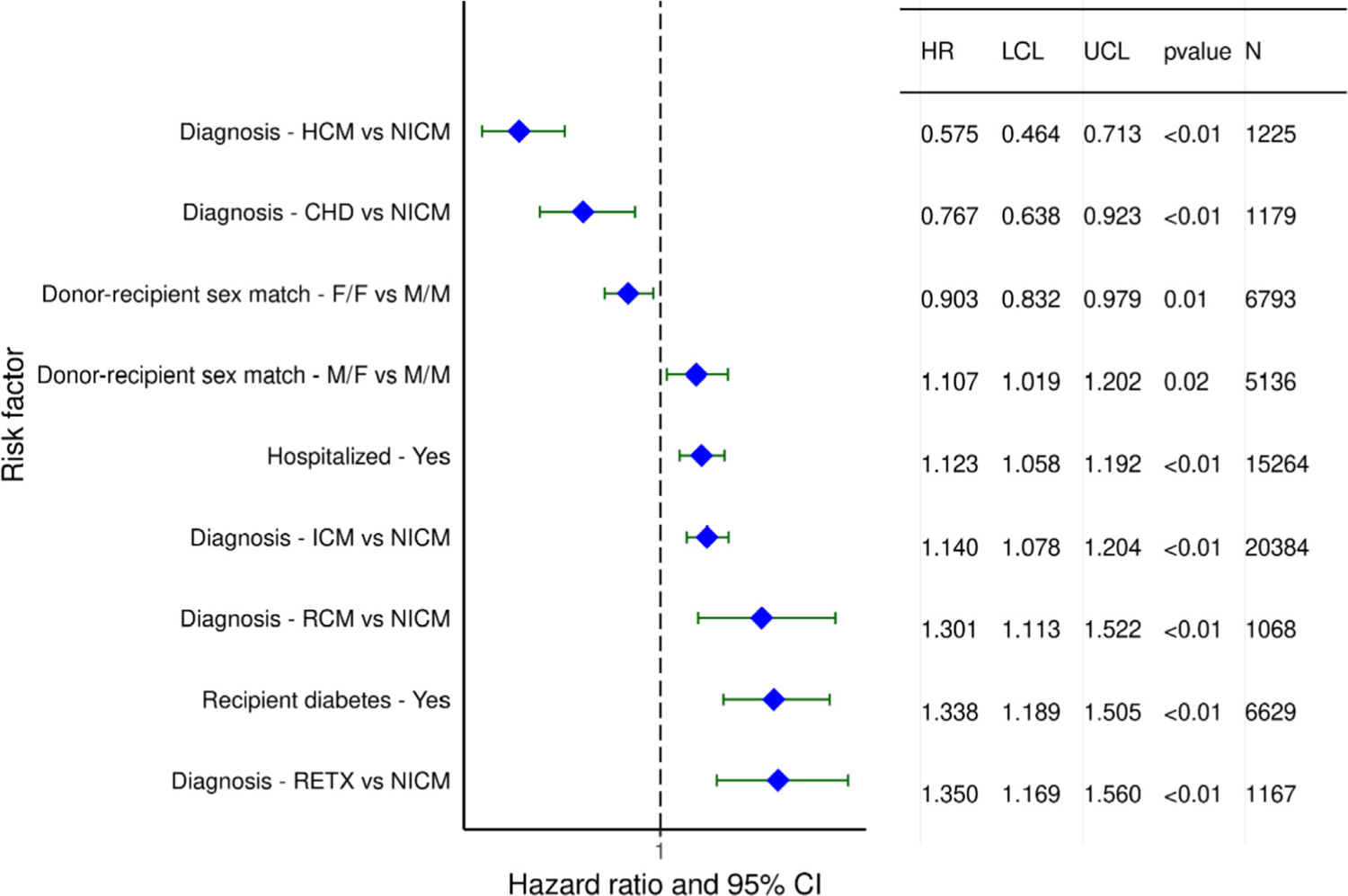
Statistically significant categorical risk factors for 5-year mortality, conditional on survival to 1 year, with 95% confidence limits (transplants: January 1996-June 2013; N=53,105). HCM: hypertrophic cardiomyopathy, NICM: nonischemic cardiomyopathy, CHD: congenital heart disease, F: female, M: male, ICM: ischemic cardiomyopathy, RCM: restrictive cardiomyopathy, RETX: retransplant
Donor-recipient sex mismatch continues to be significantly associated with 5-year conditional survival. We saw that female donor-male recipients had increased 1-year mortality compared to the male donor-male recipient combination (HR 1.16). We now see that the male donor-female recipient combination again has increased 5-year mortality compared to male donor-male recipients (HR 1.1). In contrast, the female donor-female recipient combination is associated with lower risk (HR 0.90) compared to male-male. While size-mismatch may partly account for these results, these sex-related differences in survival suggest that hormonal influences on the immunological response may also come into play.
Finally, recipient diabetes at transplant, which was not associated with 1-year mortality, is now associated with 5-year mortality. This suggests that the chronic sequelae of diabetes, including vascular disease and chronic kidney disease, may affect longer-term post-transplant outcomes. There was no significant interaction between transplant era and recipient diabetes (eSlide H(a) 61).
Continuous variables associated with 5-year mortality, conditional on survival to 1 year, are similar to those associated with 1-year survival, and include recipient age, BMI, pulmonary vascular resistance, and kidney function; donor age; and transplant center volume. Notably, other risk factors associated with 1-year survival, such as recipient PRA and ischemic time, are no longer significantly associated with 5-year survival. Associations between individual continuous risk factors and HRs for 5-year mortality are displayed in eSlides H(a) 63–71. Importantly, there are no significant interactions between the transplant era and the individual risk factors.
Cardiac allograft vasculopathy within 5 years, conditional on survival to discharge
Results of multivariable analyses for the development of CAV within 5 years, conditional on survival to discharge, are shown in eSlides H(a) 73–82. Significant categorical risk factors for CAV development include donor death due to cerebrovascular accident, and recipient diagnoses of ischemic cardiomyopathy and retransplantation (Figure 18). Of interest is the observation that female sex, of either the donor or recipient, is associated with reduced risk of CAV development. For example, the female donor-female recipient combination has a HR of 0.72 for CAV, compared to male donor-male recipient. The female donor-male recipient combination has a HR of 0.85, while the HR for male donor-female recipient is 0.90, all compared to male-male transplants. These results run counter to prior studies that show either a higher incidence of CAV development in female donors26 and female recipients,27 or no sex-based differences in CAV development,28 and deserve further investigation.
Figure 18.
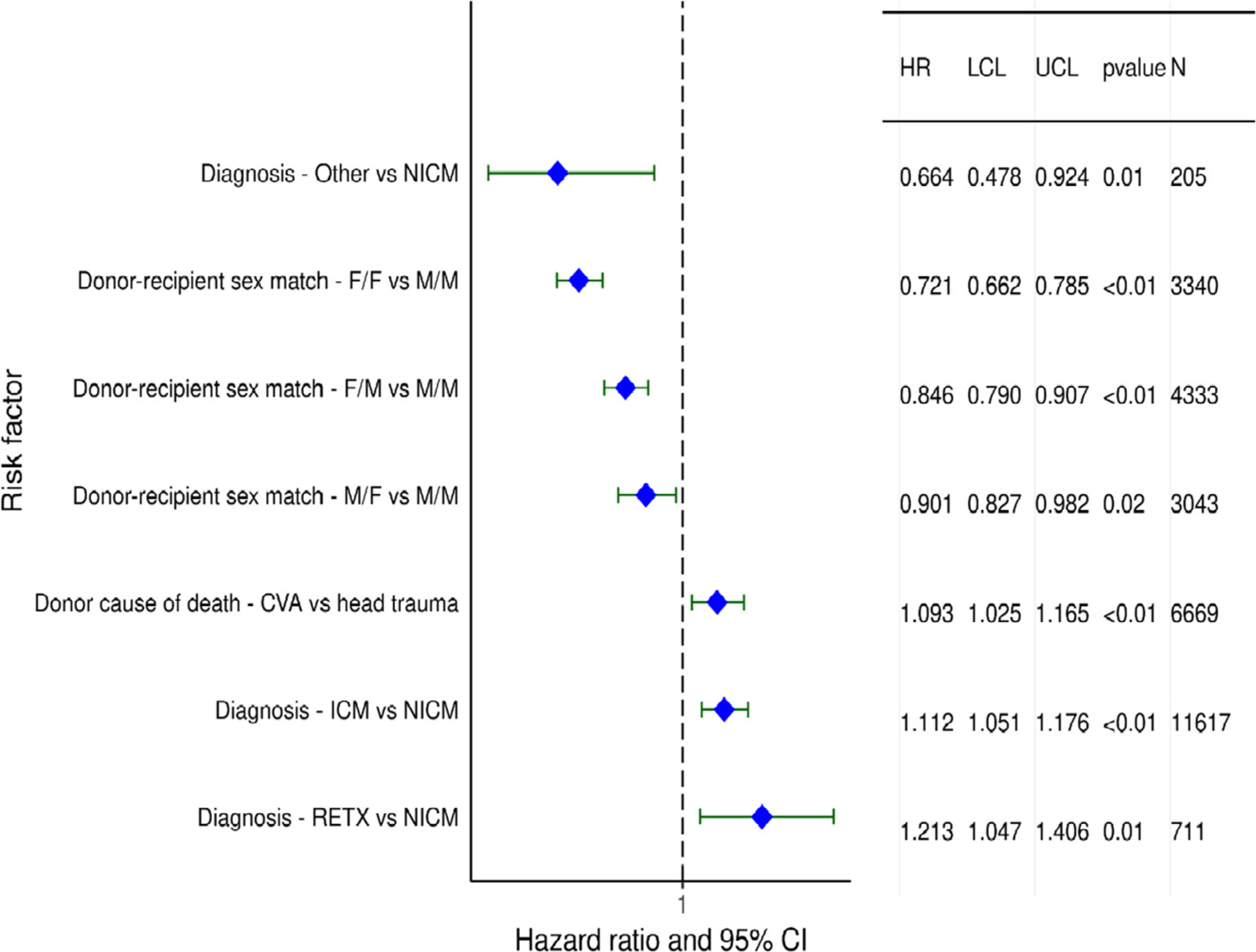
Statistically significant categorical risk factors for cardiac allograft vasculopathy (CAV) within 5 years, conditional on survival to discharge, with 95% confidence limits (transplants: January 1996-June 2013; N = 26,554) NICM: nonischemic cardiomyopathy, F: female, M: male, CVA: cerebrovascular accident, ICM: ischemic cardiomyopathy, RETX: retransplant
Statistically significant continuous risk factors for CAV development include donor age, recipient age, recipient BMI, and transplant center volume. Notably, recipient and donor age have the opposite effect on CAV development. As shown in Figure 19, younger recipient age is associated with increased risk of CAV, albeit the confidence intervals are quite wide for the lower age ranges. It is conceivable that younger recipients have a more robust immune response that predisposes them to CAV development. However, another explanation is that CAV is less likely to be diagnosed in older recipients who have more renal dysfunction and therefore are less likely to have screening coronary angiography. Not surprising is the observation that older donor hearts predispose the recipient to CAV (Figure 20), as has been shown by many prior studies.1,29−31 Finally, there appears to be a very modest association of center volume with CAV, with higher volume centers having a slightly higher HR of recipient CAV (HR 1.03–1.16) for centers performing more than 120 transplants over the previous 3 years, eSlide H(a) 82), perhaps due to transplantation of more medically complex recipients with a higher burden of comorbidities at the larger centers. Notably, there were no significant interactions between transplant era and recipient age, kidney function, and PRA at transplant.
Figure 19.
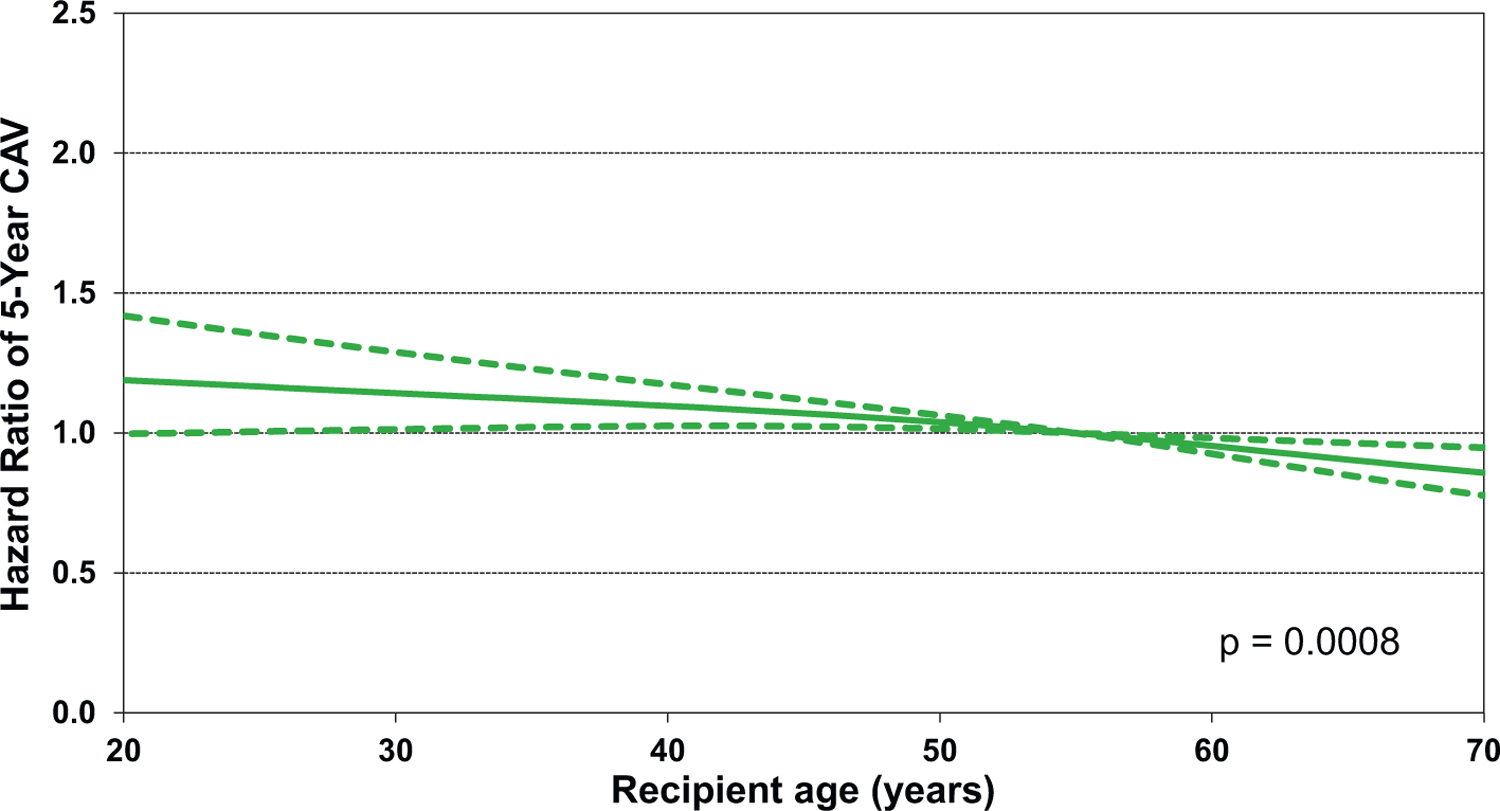
Multivariable hazard ratio plot for cardiac allograft vasculopathy (CAV) within 5 years with 95% confidence limits, by recipient age (transplants: January 1996-June 2013; N = 26,554)
Figure 20.
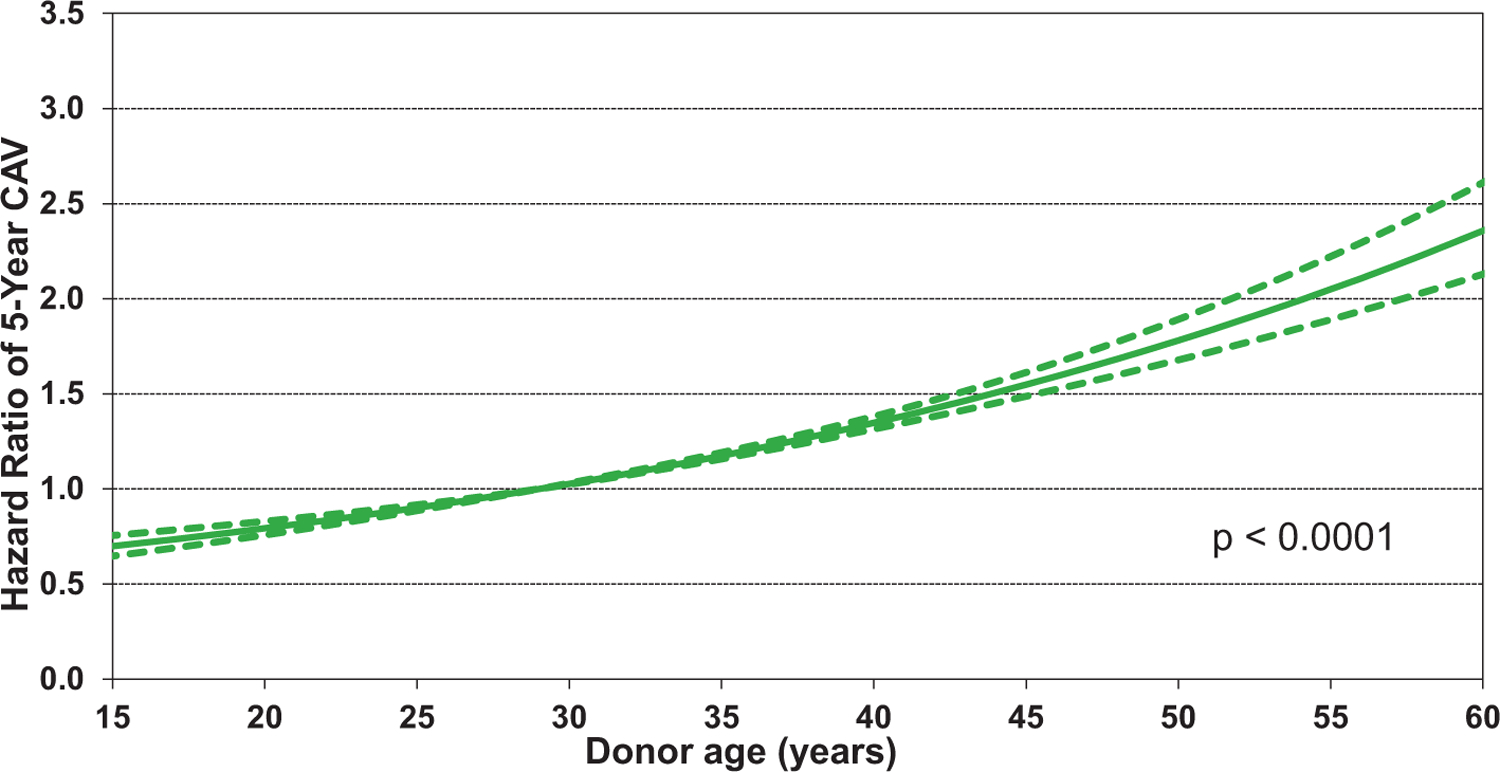
Multivariable hazard ratio plot for cardiac allograft vasculopathy (CAV) within 5 years with 95% confidence limits, by donor age (transplants: January 1996-June 2013; N = 26,554)
Conclusions
In this 2021 ISHLT Adult Heart Transplantation Report, we closely examined changes in the clinical characteristics of adult heart transplant recipients over time, and the impact of recipient characteristics on post-transplant outcomes. We observed many changes, including transplantation of older recipients over time, as well as transplantation of recipients with increasing medical complexity, including diabetes, obesity, prior cardiac surgery, and allosensitization. We also saw increased transplantation of recipients supported with temporary and durable MCS, particularly LVADs, bearing in mind that the analysis cohort ended in June 2018. Encouragingly, despite the increased medical complexity of transplant recipients, we saw consistent improvements in post-transplant survival, with regional differences. Short-term (1-year) survival is better in North America than in Europe, for example, while the opposite is true of longer-term (5-year) survival, likely reflecting underlying differences in the donor and recipient populations in different parts of the world. Finally, there has been little, if any, reduction in CAV development over time, which underscores the urgent need for effective therapies to prevent and treat this seemingly inexorable cause of graft failure after heart transplantation.
Supplementary Material
Acknowledgment
The authors wish to thank Ms. Lyna Cherikh, United Network of Organ Sharing Research Summer Intern, for her assistance with preparing the figures/table for the manuscript and for reviewing the manuscript.
Disclosure statement
Daniel C. Chambers received travel support from Astellas Pharma, Inc, and serves as a consultant and speaker for Roche Ltd; Kiran K. Khush serves as a consultant and speaker for CareDx, Inc; Josef Stehlik serves as a consultant for Medtronic, receives research support from Natera and received funding from ISHLT; Michael Perch receives research funding from Roche, travel support from Boeringer-Ingelheim, and is a speaker for Mallinckrodt, Glaxo Smith Kline, and Astra-Zeneca; Wida S. Cherikh, Aparna Sadavarte, and Alice Toll received funding from ISHLT; Don Hayes, Jr.; Michael O. Harhay; Aparna Sadavarte; Eileen Hsich; Luciano Potena; and Tajinder P. Singh do not have any relevant disclosures.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2021.07.015.
References
- 1.Khush KK, Potena L, Cherikh WS, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am 2010;39:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-fifth adult heart transplantation report2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1155–68. [DOI] [PubMed] [Google Scholar]
- 5.Yalcin YC, Muslem R, Veen KM, et al. Impact of continuous flow left ventricular assist device therapy on chronic kidney disease: a longitudinal multicenter study. J Card Fail 2020;26:333–41. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Choi J, Toyoda M, Vo A, Peng A, Jordan SC. Desensitization: overcoming the immunologic barriers to transplantation. J Immunol Res 2017;2017:6804678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sverchkova A, Anzar I, Stratford R, Clancy T. Improved HLA typing of Class I and Class II alleles from next-generation sequencing data. HLA 2019;94:504–13. [DOI] [PubMed] [Google Scholar]
- 8.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: Preliminary results of the US 2018 adult heart allocation revision. Am J Transplant 2020;20:2781–90. [DOI] [PubMed] [Google Scholar]
- 9.Varshney AS, Berg DD, Katz JN, et al. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the united network for organ sharing donor heart allocation system changes. JAMA Cardiol 2020;5:703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullan CW, Chouairi F, Sen S, et al. Changes in use of left ventricular assist devices as bridge to transplantation with new heart allocation policy. JACC Heart Fail 2021;9:420–9. [DOI] [PubMed] [Google Scholar]
- 11.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the international society for heart and lung transplantation registry. J Heart Lung Transplant 2012;31:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moayedi Y, Fan CPS, Cherikh WS, et al. Survival outcomes after heart transplantation: does recipient sex matter? Circ Heart Fail 2019;12:e006218. [DOI] [PubMed] [Google Scholar]
- 13.Reed RM, Netzer G, Hunsicker L, et al. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. JACC Heart Fail 2014;2:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau A, West L, Tullius SG. The impact of sex on alloimmunity. Trends Immunol 2018;39:407–18. [DOI] [PubMed] [Google Scholar]
- 15.Kransdorf EP, Kittleson MM, Benck LR, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant 2019;38:156–65. [DOI] [PubMed] [Google Scholar]
- 16.Chandraker A, Andreoni KA, Gaston RS, et al. Time for reform in transplant program-specific reporting: AST/ASTS transplant metrics taskforce. Am J Transplant 2019;19:1888–95. [DOI] [PubMed] [Google Scholar]
- 17.Nathan AS, Blebea C, Chatterjee P, et al. Mortality trends around the one-year survival mark after heart, liver, and lung transplantation in the United States. Clin Transplant 2020;34:e13852. [DOI] [PubMed] [Google Scholar]
- 18.Sethi J, Bugajski A, Patel KN, et al. Recipient age impacts long-term survival in adult subjects with cystic fibrosis after lung transplantation. Ann Am Thorac Soc 2021;18:44–50. [DOI] [PubMed] [Google Scholar]
- 19.Goss CH, MacNeill SJ, Quinton HB, et al. Children and young adults with CF in the USA have better lung function compared with the UK. Thorax 2015;70:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333:621–7. [DOI] [PubMed] [Google Scholar]
- 21.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med 2003;349:847–58. [DOI] [PubMed] [Google Scholar]
- 22.Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation 2004;110:2694–700. [DOI] [PubMed] [Google Scholar]
- 23.Fearon WF, Okada K, Kobashigawa JA, et al. Angiotensin-converting enzyme inhibition early after heart transplantation. J Am Coll Cardiol 2017;69:2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starling RC, Armstrong B, Bridges ND, et al. Accelerated allograft vasculopathy with rituximab after cardiac transplantation. J Am Coll Cardiol 2019;74:36–51. [DOI] [PubMed] [Google Scholar]
- 25.Lund LH, Khush KK, Cherikh WS, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1037–46. [DOI] [PubMed] [Google Scholar]
- 26.Erinc K, Yamani MH, Starling RC, et al. The influence of donor gender on allograft vasculopathy: evidence from intravascular ultrasound. Transplant Proc 2004;36:3129–31. [DOI] [PubMed] [Google Scholar]
- 27.Grupper A, Nestorovic EM, Daly RC, et al. Sex related differences in the risk of antibody-mediated rejection and subsequent allograft vasculopathy post-heart transplantation: a single-center experience. Transplant Direct 2016;2:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauptman PJ, Davis SF, Miller L, Yeung AC. The role of nonimmune risk factors in the development and progression of graft arteriosclerosis: preliminary insights from a multicenter intravascular ultrasound study. multicenter intravascular ultrasound transplant study group. J Heart Lung Transplant 1995;14:S238–42. [PubMed] [Google Scholar]
- 29.Escobar A, Ventura HO, Stapleton DD, et al. Cardiac allograft vasculopathy assessed by intravascular ultrasonography and nonimmunologic risk factors. Am J Cardiol 1994;74:1042–6. [DOI] [PubMed] [Google Scholar]
- 30.Nagji AS, Hranjec T, Swenson BR, et al. Donor age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann Thorac Surg 2010;90:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valantine H Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant 2004;23: S187–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


