Abstract
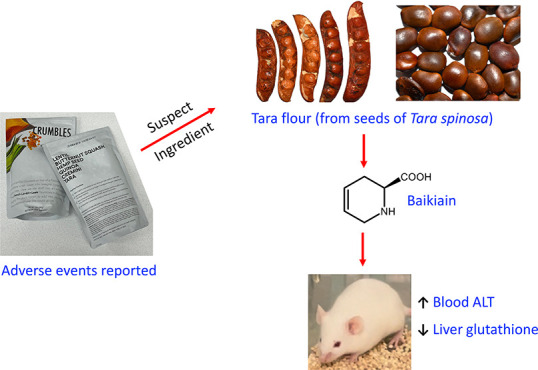
The French Lentil & Leek Crumbles frozen food product was recently recalled due to reports of gastrointestinal issues. So far, 393 adverse illness complaints and 133 hospitalizations have been reported from consumption of this food, and the tara (Tara spinosa) protein flour ingredient is hypothesized to be responsible. A multipronged approach resulted in identification of (S)-(−)-baikiain in tara as a compound of interest due to its abundance, possible metabolic fate, and close resemblance to irreversible inhibitors of L-pipecolate oxidase. Oral administration of baikiain in ND4 mice showed a statistically significant increase in blood ALT levels and a reduction in liver GSH.
On June 17, 2022, Daily Harvest (New York, NY) issued a voluntary recall of about 28,000 units of their newly launched French Lentil & Leek Crumbles frozen food product (1 serving size, 113 g) due to gastrointestinal (GI) issues. Case counts received by the FDA total 393 adverse illness events and 133 hospitalizations related to the consumption of this product within 39 US states due to complaints of GI, liver, gallbladder, and(or) bile duct problems.1
Initial testing of the frozen food product resulted in negative results for common food toxicants—microbial pathogens, mycotoxins, major allergens, heavy metals, pesticides, hepatitis A, and norovirus.2 Out of the 27 components in the Crumbles, Daily Harvest suspected tara flour was the potential problem since the ingredient was unique to this product (it had never been used in any other product sold by Daily Harvest). Compellingly, similar adverse health events were reported for consumption of the Revive Superfoods (Oakville, ON, Canada) Mango and Pineapple smoothies which also contain tara protein as an ingredient. Tara flour is a new plant-based protein ingredient manufactured from the seeds of the South American tree Tara spinosa (Feuillé ex Molina) Britton & Rose (synonym: Caesalpinia spinosa (Molina) Kuntze) which is one of the three accepted species in the genus Tara (Leguminosae).3Tara spinosa is primarily cultivated in Peru (responsible for >80% of the world supply) as a rich source of tannins based on a galloyl quinic acid structure. Tara pods (without seeds) represent approximately 65% (by mass) of the fruit and are rich in hydrolyzable tannins (between 40–60% by mass),4 which are used mainly for the industrial production of tannins, while the seeds are used as a source of gum.5 The objective of this research was to undertake a multipronged pharmacognosy approach to assess the quality and safety of the tara flour ingredient within the Daily Harvest’s Crumbles product.
Initial analytical/chemical studies focused on confirming that the Crumbles product was free from common food toxicants. Tara flour raw material was determined to be free from intentional, accidental, and economic adulteration or spiking with synthetics. Other toxic compounds were absent, including amatoxins, phallotoxins, aflatoxins, microcystins, and pyrrolizidine alkaloids. ICP-MS analysis of lead, chromium, cadmium, and arsenic showed that the product was within acceptable, safe limits. A high-quality DNA extracted from the tara protein flour was instrumental in ensuring the authenticity of raw materials used in Daily Harvest’s Crumbles product. Amplification of the ITS, psbA-trnH, trnL-trnF, and matK genomic regions and BLAST analysis against the NCBI databases resulted in 98.6–100% of sequence similarity to the sequence of Taraspinosa (Table S1).
Along with tara protein flour used in the Crumbles product, a botanically verified voucher sample was used to establish the comparative analytical fingerprints using LC-QToF-MS (Figure S1). For example, analysis of hydrolyzable mono, di, tri, and tetra O-galloylquinic acids (tannins) helped establish that tara flour originated from the cotyledons without significant contamination from the husk. Histochemical analysis of a ferric chloride-stained seed cross-section confirmed that the total phenols were mostly localized in the outer pericarp, inner testa, and tegmen regions (Figure S2). Instead, cotyledons were enriched with several small molecule (nonprotein) amino acids, fatty acids, and sugars (Figures S3 and S4). Mass, NMR, and optical rotation data confirmed the identity of two functionalized amino acids, L-3-hydroxymethyltyrosine (3-HMT) and L-3-hydroxymethylphenylalanine (3-HMP)6 and (S)-(−)-baikiain7 (Figures S5, S6, and S7), in the extract of tara cotyledons reference material and Daily Harvest tara flour, as shown in Figure 1.
Figure 1.
Three major nonprotein amino acids (from left to right: baikiain, 3-HMT, and 3-HMP) were identified in tara protein flour.
All three nonprotein amino acids were present at high levels in the tara flour: (S)-(−)-baikiain (3% w/w on dry weight basis), L-3-hydroxymethyltyrosine (1.5%) and L-3-hydroxymethylphenylalanine (0.6%). Although previously isolated from Caesalpinia spinosa(6) and from the toxic mushroom Russula subnigricans,7 no published toxicology studies have been reported on the pure compound baikiain. However, under physiological conditions, it is plausible that baikiain could metabolize into reactive intermediates 4,5-epoxypipecolic acid (via CYP-mediated oxidation) or 4- or 5-hydroxypipecolic acids (via hydration) that induce glutathione depletion and inactivation of detoxifying enzymes. Indeed, baikiain8 and its oxidized metabolite, 4,5-epoxypipecolic acid,9 are reported as strong, time-dependent, irreversible inhibitors of L-pipecolate oxidase, an enzyme that mediates protection against oxidative stress and “repair” of abnormal metabolites.10
Hemagglutination, mitogenic, and prooxidant activities were evaluated since compounds exhibiting these properties can cause toxic responses and induce nonspecific immune stimulation resulting in symptoms like some of the adverse events reported by consumers of the Daily Harvest Crumbles product. For example, consuming foods containing high levels of lectins (beans, grains, seeds, and nuts) may result in acute gastrointestinal distress, nutritional deficiencies, immune allergic reactions, and food poisoning.11−13
Crude extracts of tara protein flour (water, methanol, and dichloromethane) and the nonprotein amino acids isolated from tara flour showed no hemagglutination effect (Figure 2). The presence of the mitogen PHA was evaluated using an in vitro bioassay that selectively detects TLR4 activators. PHAs are water-soluble toxins that activate the TLR4 signaling pathway, but their activity is not reduced by treatment with the LPS-inhibitor polymyxin B.14 A crude water extract from tara flour (1 g of raw material extracted with 10 mL of endotoxin-free water at room temperature for 1 h) exhibited TLR4 stimulatory activity. However, the detected activity was inhibited by treatment with polymyxin B and was, therefore, likely due to LPS in the sample. This data and the hemagglutination results indicate that PHA and other toxic lectins are not present at detectable levels within the tara samples and compounds tested.
Figure 2.

Hemagglutination effect of tara flour extract and pure compounds in human erythrocytes. The highest concentration was 10 mg/mL for extracts, 2.5 mg/mL for pure compounds, and 0.5 mg/mL for PHA lectin control with 2× serial dilutions from left to right.
Two assay systems were employed to screen samples for prooxidant activities since oxidative stress could result in toxic effects such as depletion of cellular ATP. The first bioassay, using human hepatic cells to measure the production of intracellular reactive oxygen species (ROS), showed that neither water extract nor pure compounds produced any significant induction of ROS (Table S2). The second bioassay used red blood cells treated with carmustine to induce disruption in glutathione homeostasis (an in vitro system mimicking glucose-6-phosphate dehydrogenase-deficient cells).
No detectable depletion of glutathione was observed for any compounds (tested at 100 and 500 μg/mL) or crude water, methanol, or dichloromethane extracts of tara flour (tested at concentrations between 0.25 to 5 mg/mL). Results from both assays indicate that tara does not contain substances that cause oxidative damage.
Overall, the in vitro data indicates that tara flour does not exhibit hemagglutination, mitogenic, or prooxidant toxic attributes. In addition, it is unlikely that tara flour components exhibit hepatotoxicity since the extracts (tested up to 100 μg/mL) and the nonprotein amino acid compounds (tested up to 10 μg/mL) exhibited no cytotoxic effects on HepG2 cells. However, future research is warranted to determine whether there are metabolites/breakdown products of tara-derived compounds formed in vivo that could exhibit any of the in vitro activities tested.
The absence of toxic effects for tara extracts and isolated compounds observed using the in vitro bioassays may be due to the limitation of these systems to reflect the in vivo condition (e.g., complexity of organ systems, reactive intermediates, formation of toxic metabolites, etc.). Therefore, an initial investigation was performed using a mouse model to evaluate the potential in vivo toxicity of oral administration of baikiain, the most abundant nonprotein amino acids in tara flour. Markers of hepatic health were evaluated since liver problems were one of the adverse events reported by consumers of the Daily Harvest Crumbles product. For several reasons, a high dose (1 g/kg body weight) was selected for investigation. First, the Crumbles product was as food (1 serving size, 113 g), and therefore, it was likely consumed at levels substantially higher than a supplement or drug. Second, the toxicity may be due to a minor metabolite of baikiain formed under in vivo conditions (similar to acetaminophen). Ingestion of acetaminophen (at the recommended dose) is considered safe, but an overdose or underlying anomalies in detoxifying mechanisms can result in liver and kidney toxicity as well as glutathione depletion due to increased levels of the active metabolite N-acetyl-p-benzoquinone imine. High oral dosage levels (e.g., 1 g/kg) are used in rodent models investigating acetaminophen overdose toxicity.
A statistically significant increase in blood ALT levels (2-, 4- and 6-h postadministration) and depletion of liver total glutathione was observed in baikiain-treated mice compared to the control group (Table 1). Additional markers of liver and kidney toxicity (BUN, CRE, and AMY) were also significantly higher at the 4- and 6-h time points.
Table 1. Effect of Oral Administration of Baikiain (1g/kg) in ND4 Male Mice on Parameters of Acute Toxicitya.
| 0 h |
2 h-Post treatment |
4 h-Post treatment |
6 h-Post treatment |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Baikiain | Control | Baikiain | Control | Baikiain | Control | Baikiain | |
| ALT (U/L) | 34(1.8) | 31(0.90) | 48(2.7) | 81(10.0)* | 59(3.1) | 190(71)* | 66(3.9) | 230(81)* |
| BUN (mg/dL) | 22(1.0) | 21(1.3) | 24(1.1) | 27(2.0) | 32(1.2) | 42(2.7)* | 33(1.6) | 61(3.7)† |
| CRE (mg/dL) | 0.22(0.013) | 0.24(0.024) | 0.24(0.016) | 0.34(0.053) | 0.25(0.027) | 0.58(0.11)* | 0.2(0.00) | 0.63(0.075)* |
| ALB (g/dL) | 4.2(0.072) | 4.0(0.089) | 3.8(0.094) | 3.6(0.094) | 3.7(0.083) | 3.6(0.14) | 3.5(0.071) | 3.3(0.12) |
| ALP (U/L) | 136(9.9) | 129(6.2) | 128(9.6) | 110(4.9) | 123(7.1) | 122(6.2) | 115(5.5) | 118(5.1) |
| TBIL (mg/dL) | 0.30(0.0) | 0.30(0.0) | 0.30(0.0) | 0.29(0.011) | 0.30(0.0) | 0.27(0.017) | 0.28(0.013) | 0.26(0.016) |
| AMY (U/L) | 1100(45) | 1200(53) | 1200(27) | 1300(78) | 1300(36) | 1600(95)* | 1198(42.69) | 1532(131.8)¶ |
| GSH (μg/mg) in liver tissue | 675(49) | 207(61)† | ||||||
Values are means ± SEM, n = 10 mice per group. Two-tailed t test comparisons (¶p < 0.05, *p < 0.01, †p < 0.001).
In summary, the results of these initial studies support a working hypothesis that the adverse events reported by individuals consuming the Daily Harvest Crumbles product originate from the tara flour ingredient and are due, at least in part, to high levels of nonprotein amino acids (e.g., baikiain). It is further hypothesized that in vivo metabolism of metabolically unstable baikiain results in a toxic metabolite(s) that depletes glutathione and/or is an irreversible enzyme inhibitor (for L-pipecolate oxidase), resulting in adverse events which are dependent on the dose consumed and potentially exacerbated for individuals that have specific genetic predispositions.
Acknowledgments
Authors would like to thank Dr. Kumar Katragunta, Ms. Katherine Martin and Dr. Mohammad K. Ashfaq for their assistance in the research studies.
Glossary
ABBREVIATIONS
- ALB
albumin
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AMY
amylase
- BUN
blood urea nitrogen
- CRE
creatinine
- GSH
glutathione
- LPS
lipopolysaccharide
- PHA
phytohemagglutinin
- TBIL
total bilirubin
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.3c00100.
Materials and methods, sequence data, LC-MS profile, histochemical images, and NMR data (PDF)
Author Contributions
The manuscript was written through contributions of all authors and all authors have approved the final version. CRediT: Amar G. Chittiboyina conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, supervision, validation, visualization, writing-original draft, writing-review & editing; Zulfiqar Ali investigation, validation, writing-original draft; Bharathi Avula formal analysis, investigation, validation; Shabana I. Khan data curation, investigation, methodology, validation, visualization, writing-original draft; Tahir M. Mir investigation, validation; Jin Zhang investigation, validation; Fadime Aydoğan investigation, validation; Fazila Zulfiqar investigation, validation; Natascha Techen investigation, methodology, validation; Iffat Parveen investigation, validation; Pankaj Pandey investigation, software, validation; Sebastian J. Adams investigation, validation; Yan-Hong Wang investigation, validation; Jianping Zhao investigation; Gailen D. Marshall formal analysis, investigation, methodology, supervision, validation; Nirmal D. Pugh investigation, methodology, project administration, writing-original draft, writing-review & editing; Ikhlas A. Khan conceptualization, funding acquisition, investigation, project administration, resources, supervision, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Investigation of Adverse Event Reports: French Lentil & Leek Crumbles (June 2022). 2022. https://www.fda.gov/food/outbreaks-foodborne-illness/investigation-adverse-event-reports-french-lentil-leek-crumbles-june-2022 (accessed March 22, 2023).
- Updates on our voluntary recall of French Lentil + Leek Crumbles. 2022. https://www.daily-harvest.com/content/french-lentil-leek-crumbles-advisory# (accessed March 22, 2023).
- Acevedo-Rodríguez P.; Strong M. T.. Catalogue of seed plants of the West Indies. Smithsonian contributions to botany; Smithsonian Institution Scholarly Press: Washington DC, 2012. [Google Scholar]
- Aguilar-Galvez A.; Noratto G.; Chambi F.; Debaste F.; Campos D. Potential of tara (Caesalpinia spinosa) gallotannins and hydrolysates as natural antibacterial compounds. Food Chem. 2014, 156, 301–304. 10.1016/j.foodchem.2014.01.110. [DOI] [PubMed] [Google Scholar]
- Desai S.; Prajapati V.; Chandarana C.. Chemistry, biological activities, and uses of tara gum. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy H. N., Ed.; Springer, 2022; pp 265–289. [Google Scholar]
- Watson R.; Fowden L. Amino acids of Caesalpinia tinctoria and some allied species. Phytochemistry 1973, 12, 617–622. 10.1016/S0031-9422(00)84454-4. [DOI] [Google Scholar]
- Kusano G.; Ogawa H.; Takahashi A.; Nozoe S.; Yokoyama K. A new amino acid,(2S, 3R)-(−)-3-hydroxybaikiain from Russula subnigricans Hongo. Chem. Pharm. Bull. 1987, 35, 3482–3486. 10.1248/cpb.35.3482. [DOI] [Google Scholar]
- Zabriskie T. M. Mechanism-based inhibition of L-pipecolate oxidase by 4, 5-dehydro-L-pipecolic acid. J. Med. Chem. 1996, 39, 3046–3048. 10.1021/jm960331f. [DOI] [PubMed] [Google Scholar]
- Ho B.; Zabriskie T. M. Epoxide derivatives of pipecolic acid and proline are inhibitors of pipecolate oxidase. Bioorg. Med. Chem. Lett. 1998, 8, 739–744. 10.1016/S0960-894X(98)00106-1. [DOI] [PubMed] [Google Scholar]
- Natarajan S. K.; Muthukrishnan E.; Khalimonchuk O.; Mott J. L.; Becker D. F. Evidence for pipecolate oxidase in mediating protection against hydrogen peroxide stress. J. Cell. Biochem. 2017, 118, 1678–1688. 10.1002/jcb.25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamcová A.; Laursen K. H.; Ballin N. Z. Lectin activity in commonly consumed plant-based foods: calling for method harmonization and risk assessment. Foods 2021, 10, 2796. 10.3390/foods10112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Simpson B. K.; Sun H.; Ngadi M. O.; Ma Y.; Huang T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. 10.1080/10408398.2015.1096234. [DOI] [PubMed] [Google Scholar]
- Zubčević N.; Fočak M.; Suljević D. Highly specific hemagglutination activity of plant lectins in specific species: Case of Fabaceae and Solanaceae. Bulg. J. Agric. Sci. 2018, 24, 391–397. [Google Scholar]
- Unitt J.; Hornigold D. Plant lectins are novel Toll-like receptor agonists. Biochem. Pharmacol. 2011, 81, 1324–1328. 10.1016/j.bcp.2011.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



