Abstract
Nanostructure imaging mass spectrometry (NIMS) with fluorinated gold nanoparticles (f-AuNPs) is a nanoparticle assisted laser desorption/ionization approach that requires low laser energy and has demonstrated high sensitivity. Here we describe NIMS with f-AuNPs for the comprehensive analysis of metabolites in biological tissues. F-AuNPs assist in desorption/ionization by laser-induced release of the fluorocarbon chains with minimal background noise. Since the energy barrier required to release the fluorocarbons from the AuNPs is minimal, the energy of the laser is maintained in the low μJ/pulse range, thus limiting metabolite in-source fragmentation. Electron microscopy analysis of tissue samples after f-AuNP NIMS shows a distinct “raising” of the surface as compared to MALDI ablation, indicative of a gentle desorption mechanism aiding in the generation of intact molecular ions. Moreover, the use of perfluorohexane to distribute the f-AuNPs on the tissue creates a hydrophobic environment minimizing metabolite solubilization and spatial dislocation. The transfer of the energy from the incident laser to the analytes through the release of the fluorocarbon chains similarly enhances the desorption/ionization of metabolites of different chemical nature, resulting in heterogeneous metabolome coverage. We performed the approach in a comparative study of the colon of mice exposed to three different diets. F-AuNP NIMS allows the direct detection of carbohydrates, lipids, bile acids, sulfur metabolites, amino acids, nucleotide precursors as well as other small molecules of varied biological origins. Ultimately, the diversified molecular coverage obtained provides a broad picture of a tissue’s metabolic organization.
Keywords: nanostructure initiator mass spectrometry, perfluorinated gold nanoparticles, mass spectrometry imaging, metabolomics, gut microbiome, mice, fibre free diet
TOC

Mass spectrometry imaging (MSI) was designed to detect and spatially localize molecules on surfaces.1–4 Many MSI strategies have been proposed, of which currently the most employed is matrix-assisted laser desorption ionization (MALDI) coupled to time-of-flight mass spectrometry (TOF-MS).5,6 This approach leverages the co-crystallisation of the sample with a highly volatile UV-adsorbing matrix, usually sinapinic acid (SA), α-cyano-4-hydroxycinnamic acid (CHCA), or 2,5-dihydroxybenzoic acid (DHB), which transfers the energy from an incident laser beam to the analytes,7,8 thus aiding the soft ionization and desorption of analyte molecules.
MALDI-MS can provide high throughput analysis, however its application for comparative metabolomics MSI studies, for example in systems biology or personalized medicine, remains limited.9–11 This is primarily due to signal variability stemming from the uneven formation of the matrix-analyte crystals in so called “sweet spots”,12 and little ionization consistency for molecules of diverse chemical nature.13 Moreover, the generation of background ions from the matrix can interfere with the detection of low molecular weight molecules (m/z < 700 Da), including metabolites and drugs.13,14 Although a more even crystallization of the matrix on the surface of the sample can be achieved by sublimation,15,16 the noise deriving from the ionization of the matrix significantly suppresses the detection of small molecules, thus complicating the application of MALDI for comparative global metabolomics and pharmaceutical studies.
Over the last two decades, several efforts have been made to minimize MALDI background interference, leading to the further development of existing matrix-free secondary ion mass spectrometry (SIMS), and multiple new approaches including desorption electrospray ionization (DESI), laser ablation electrospray ionization (LAESI),17 and nanostructure imaging mass spectrometry (NIMS).5,18 SIMS desorbs and ionizes the analyte by focusing an ion beam on the surface of the sample, typically a collated primary high-energy atomic ion beam of Ga+, Cs+, O2+, or a ion cluster beam of C60+, Au3+, Ar2500+ or Bi3+.19–21 While the use of an atomic ion beam calls for a trade-off between spatial resolution and in source fragmentation events and generates complex spectra,5,20 the use of a cluster ion beam (cluster projectiles) significantly reduces this issue.22,23 For example, SIMS with argon gas clusters has been successfully applied for 2- and 3-dimensional in depth profiling of drugs and metabolites at single cell level with exquisitely high spatial resolution (up to 10–100 nm).24–26 However, it remains unknown whether the approach covers the detection of central carbon metabolites, which are of key relevance in metabolomics studies. Additionally, by using large clusters such as Ar2500+ the desorption can be very efficient however the ionization less so, as well as the use of high lateral resolution which may bias the detection toward the most abundant and easily ionizable molecules in the sample (e.g. lipids in biological tissues).
DESI is characterized by a very soft desorption/ionization (D/I)27 operated under ambient conditions where a sample can be directly analyzed without any prior preparation. A stream of charged solvent droplets are electrosprayed directly onto the sample surface, solvating and liberating the analytes as gaseous ions that are then focused into the mass spectrometer.28 DESI allows for real-time monitoring of biological and industrial processes.29,30 However, ambient conditions are not always desirable as they are often incompatible with the stability of the analyte towards, for example, oxidation, and metabolic biotransformations may occur after sampling. Furthermore, the use of droplets limits the spatial resolution and requires the optimization of the solvent depending on the specific type of sample and targeted molecules.31
NIMS is an alternative approach for surface assisted laser desorption ionization (SALDI) of small molecules and metabolites18 also applied to MSI.32–35 It originally employed etched porous silicon surfaces nanostructured with a thin layer of an initiator molecule (e.g., heptadodecafluoro-1,1,2,2-tetrahydrodecyl-tetramethyldisiloxane). In the resulting clathrate structures, the energy is transferred from the incident laser to the initiator, which is vaporized from the surface thus facilitating the D/I of the analytes. This approach can also be coupled with SIMS ion sources using, for example, Bi3+ or Ga+ ion beams (nano-NIMS),18 thus offering the possibility to reach lateral resolution on the nanometer scale. However, the classic NIMS protocol involves the use of hydrofluoric acid (HF), an extremely hazardous chemical that can be handled only under special safety protocols in certified laboratories. We have therefore recently developed an improved approach for NIMS based on functionalized gold nanoparticles (f-AuNPs).36
Different types of carbon, silica or metal nanostructured surfaces and nanoparticles have been previously employed for the enhancement of the D/I of small molecules and lipids, as these can offer extended dynamic ranges thanks to the ample surface area and efficient energy transfer properties combined with reduced background noise.13,37–44 Among these, AuNPs are particularly appealing for MSI since their biocompatibility and interesting physical properties.45 For instance, bare AuNPs have been applied for the D/I of carbohydrates46 and amino acids,47 aminothiols,48 fatty acids and nucleobases,46 or for the selective detection of glutathione.49 Despite these benefits, AuNPs require high laser energies for the D/I of metabolites, with associated laser-induced fragmentation as well as adsorption of metabolites at the NP surface.36,37,50 These limitations have prevented their widespread application for the simultaneous detection of a broad range of metabolites in space.
By contrast, the use of AuNPs functionalized with perfluorinated chains allows the use of lower laser energies due to a mostly kinetic D/I enhancement effect (release of the fluorocarbons chains) which prevents the direct contact of the analyte with the thermal energy at the AuNP surface.37 Moreover, the non-adherent nature of the perfluorinated layer on the surface of the particle impedes the selective adsorption of metabolites, making the approach amenable to the detection of a broad range of metabolites and small molecules.
Since the first implementation and application of f-AuNPs as a versatile approach for multimodal molecular imaging of biological specimens by MSI, X-ray and electron microscopy with breathable liquids,36 this technology has been further characterized becoming a powerful tool for metabolomics MSI directly from the surface of biological tissues, requiring minimal sample preparation while providing low background interference and minimal metabolite dislocation. This approach can be considered an advanced method of nanoparticle assisted laser desorption ionization (nano-PALDI), which leverages the NIMS concept by using nanostructured nanoparticles as initiators of the D/I process.
Here, we present NIMS with f-AuNPs for the targeted and untargeted analysis of metabolites from biological tissues. We further assess the applicability of the approach for comparative untargeted metabolomics MSI studies by exploring variations occurring in mouse colon under different diet regimens.
NIMS with f-AuNPs permits heterogeneous molecular coverage, allowing the direct detection of carbohydrates, lipids, bile acids, sulfur metabolites, amino acids, nucleotide precursors as well as other small molecules of biological origin with high sensitivity and minimal metabolite dislocation in space.
Results and Discussion
f-AuNPs for metabolomics NIMS.
AuNPs can be functionalized with a variety of ligands by performing gold-thiol reactions with straightforward synthetic protocols.51–53 For NIMS, we functionalized the surface of octanethiol AuNPs with 1H,1H,2H,2H-perfluorodecanethiol chains via dithiol coupling in a one-step reaction.36 This led to the formation of a highly ordered perfluorinated nanostructured monolayer on the surface of the AuNPs (Figure 1A), which allows the translation of the NIMS technology directly on the surface of the AuNPs without the use of the hazardous HF.
Figure 1.
(A) Schematic of the perfluorinated monolayer on the surface of the f-AuNP. When the laser hits the nanoparticle, the energy is transferred to the surface causing the thermal release of the fluorinated chains; (B) phase separation between the f-AuNPs dissolved in perfluorohexane and the surface of the sample.
The formation of the perfluorocarbon layer on the AuNP provides several advantages for the detection of metabolites and other small molecules in biological tissues. First, the resulting highly hydrophobic f-AuNPs can be solubilized in the biocompatible and water-repellent solvent perfluorohexane (PFH).54 The topical distribution of the f-AuNPs dissolved in PFH creates a non-adherent film, which remains distinctly separate from the hydrophilic surface of the sample (e.g., tissues, cell extracts) (Figure 1B). This aspect is of key relevance as it prevents the solubilization and spatial dislocation of the analytes (Figure S1), thus enabling NIMS of metabolites at a resolution only limited by the laser spot size. For example, by further optimization of the geometry of the laser source, as recently reported by Kompauer et al,2 the D/I may be operated at smaller laser diameters (higher lateral resolution) without reducing the incident energy. Moreover, PFH is a stable, non-reactive and highly volatile solvent,55 which spontaneously and rapidly vaporizes from the surface of the sample after uniform distribution of the f-AuNPs, without chemical modification of the analytes (Figure 1B).
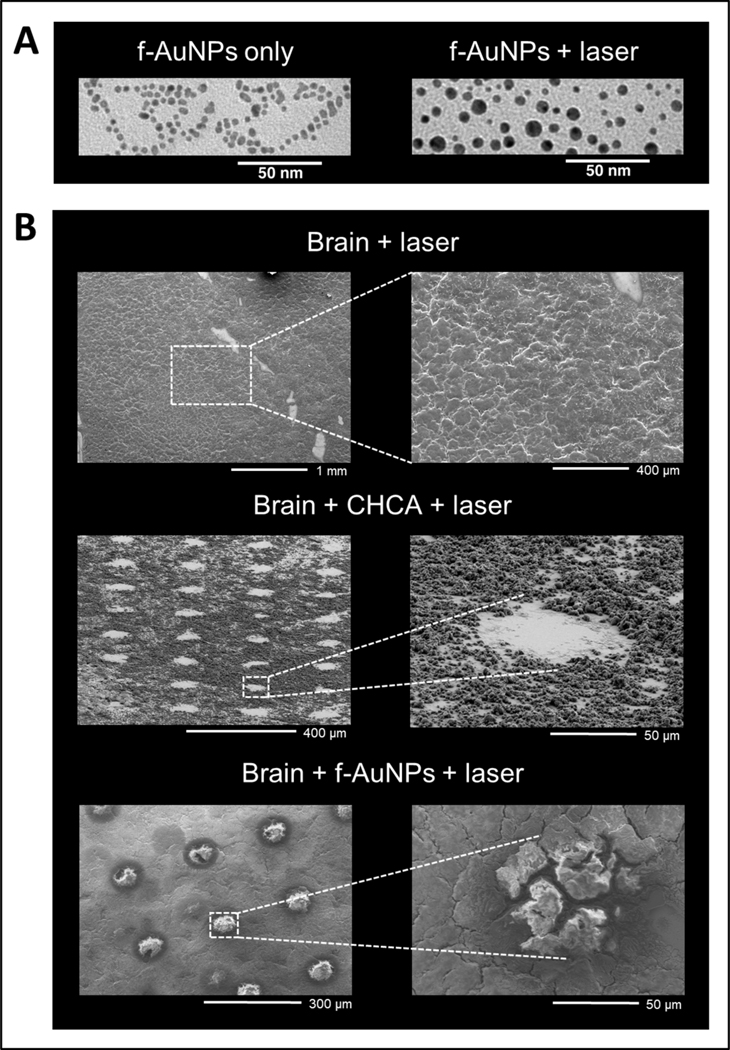
For analyte D/I, f-AuNPs function similarly to NIMS with porous silicon containing initiators by providing a highly structured surface for efficient energy transfer and facile analyte desorption. When the laser light hits the f-AuNPs, the energy absorbed by the gold nanoparticle is transferred to the surface where it cleaves the disulfide bonds (Figure 1A). The release of the fluorinated chains further promotes the desorption of the analytes from the surface of the sample.36 Interestingly, a portion of the laser energy is transformed into thermal energy, as shown by the apparent melting of the f-AuNPs to form larger particles in transmission electron microscopy (TEM) images of the f-AuNPs after laser irradiation (Figure 2A). Since the diameter of the f-AuNPs is on the order of nanometers, the morphology of the tissue is also preserved during the desorption process (Figure 2B).
Figure 2.
(A) TEM images of f-AuNPs before and after laser irradiation; (B) SEM images obtained after laser irradiation of: mouse brain tissue only, mouse brain tissue with α-cyano-hydroxycinnamic acid (CHCA) matrix, and mouse brain tissue with f-AuNPs.
Another benefit of using f-AuNPs for NIMS is linked to the low energy required for cleavage of the disulfide bonds,36 which enables the use of relatively low laser energy (μJ/pulse range). This limits in-source fragmentation events and thermal degradation of labile analytes.36 Scanning electron microscopy analysis (SEM) of brain tissue surfaces after MALDI and f-AuNP NIMS, shows a distinct “raising” of the tissue surfaces as compared to MALDI ablation (Figure 2B). This is associated with the low laser energy and the likely release of water vapor, supporting a more gentle D/I mechanism which aids in the generation of intact molecular ions. Therefore, f-AuNPs promote a softer form of LDI at lower laser energies when compared to bare AuNPs.36,37 Bare AuNPs primarily leverage a thermally-driven D/I mechanism prone to analyte in-source fragmentation and the generation of metal cluster ions. Moreover, the fluorinated surface of the f-AuNPs prevent chemical and physical interactions that may occur between the surface of the AuNP and the metabolites.37
Compared with other approaches, the absence of the analyte-matrix co-crystallization step, together with the uniform distribution of the f-AuNPs on the sample and the reduced laser energy required for the D/I, allow increased reproducibility and reduced matrix interference. Interestingly, since the f-AuNP solution in PFH possesses both hydrophobic and lipophobic behaviour, the D/I enhancement effect can be coupled to the use of other matrices for nanoscaping – the selective pre-concentration of the analytes in the sample – without interference. For example, anion exchange resin nanoparticles (e.g. DOWEX) can be used to selectively enhance the signal detected from cell extracts through a pre-treatment step (Figure S2).
Altogether these aspects make f-AuNPs a viable and versatile solution for MSI of metabolites in biological tissues, offering high throughput detection of small molecules (<700 Da) combined with the excellent sensitivity and minimal background noise of NIMS.18
f-AuNPs for metabolomics NIMS of the mouse colon.
Currently available methods for imaging metabolites provide limited coverage in the low molecular mass range (MW<700 Da) and are generally biased toward the detection of the most abundant, easily ionizable lipids.56 To address this, we explored NIMS with f-AuNPs for the detection of different classes of metabolites and assessed its applicability by performing the approach for the targeted and global exploration of the metabolic content of the mouse colon (Figure 3). Fresh frozen samples obtained from control mice fed with the standard diet were embedded in optimal cutting temperature compound (OCT) and cut to obtain 5 μm tissue sections. Each slice was then transferred onto the surface of a clean piranha etched p-doped silicon chip and covered with the f-AuNPs solution in PFH (3 mg/mL). The chip was kept in a desiccator at room temperature until direct analysis using a Q-TOF MS system equipped with a MALDI source. Analytes were detected by high-resolution measurements in the 25 – 700 m/z range in full scan positive acquisition mode. Laser energy was optimized to reduce in source fragmentation and to avoid the generation of gold clusters while promoting D/I of metabolites at 30 μm lateral resolution. The obtained m/z list was putatively annotated against the METLIN database57 using an in-house script (±5 ppm error, considering the [M+H]+ and [M-H2O+H]+ ions and the [M+Na]+ and [M+K]+ adducts). Metabolite identities were confirmed by the presence of the M+1 and M+2 isotopes and other related adducts with consistent spatial distribution, and by performing MS/MS experiments when possible. Phosphocholine ([M+H]+, 184.0733 m/z) was used for tissue alignment.
Figure 3.
Schematic of: (A) the in vivo study; (B) the f-AuNPs NIMS workflow for metabolite imaging in mouse colon samples.
Overall, our approach allowed for the global profiling and mapping of approximately 1000 features, and consistent annotation of approximately 80 metabolites distributed over six chemically diverse groups (Figure 4, Table S1). Due to the absence of chromatographic separation in MSI experiments, isobaric compounds were detected as sum of intensities. Although metabolite dislocation in the gut tissue can be excluded due to the use of the non-adherent f-AuNPs in PFH as D/I matrix, attention has to be paid in colon tissue slicing/handling since slight metabolite displacements may occur in the colon lumen due to the absence of an organized tissue structure.
Figure 4.
Number of detected features vs annotated metabolites obtained in the analysis of 5 μm mouse colon slices. Mice were fed with standard diet and metabolome coverage is reported for each chemical class.
As expected, our data show that lipids are the most abundant class among the detected metabolites, followed by the bile acids. More interestingly, the approach also enabled the detection of highly hydrophilic compounds as to provide coverage for the central carbon metabolism, including carbohydrates, amino acids, nucleotides and a variety of related precursors (Figure 4, Figure S3, Table S1). For example, glucose can be targeted as its stable [M+K]+ adduct, or hydrocholesterol as its [M+Na]+ adduct (Figure 5, Table S1). Matrix interference (e.g., gold clusters) in the low m/z range is very limited due to the low laser energy employed and does not compromise the detection of low molecular weight metabolites.36
Figure 5.
Mass spectrum obtained by f-AuNPs NIMS of a 5 μm mouse colon tissue slice. The insets show the distribution of targeted metabolites in the mice colon. Ion abundances were normalized according to the total signal in each pixel and phosphocholine was used for tissue alignment.
It is also worth noting that while attempting to understand metabolic dysregulations at a systems level, under certain conditions the activation or down regulation of a specific metabolic pathway can be inferred by measuring the abundance of few representative metabolites, used as proxy for the activity of the pathway.58–60 Combining this observation with the highly heterogeneous metabolome coverage provided by the use of NIMS with f-AuNPs, we believe that this approach enables the comprehensive interpretation of metabolism with spatial resolution at a system level.
In addition to metabolites of murine origin, several bacterial metabolites were also detected in the colon samples (Table S1). For example, butyrate and acetate were detected as [M+H]+ and [M+K]+ ions. The interplay between these microbial metabolites and the mucosa/epithelial cells of the gut has recently raised substantial interest, particularly with several observed correlations with human processes. For example, butyrate has been found to play a key role in the regulation of stem cell differentiation.61
In this context, our approach represents an advantageous tool for disentangling the contribution of gut metabolism in human health by spatially identifying the distribution of microbial metabolites. In comparison to previously applied approaches, NIMS with f-AuNPs provides high and, perhaps more importantly, heterogeneous coverage of multiple metabolite classes. This powerful tissue imaging technique can serve as a convenient platform for global and targeted exploration of the molecular makeup of the system with high sensitivity and lateral resolution.
Metabolic organization of the mouse colon and comparative analysis of diet-related rearrangements.
Low matrix interference and high reproducibility are essential factors for comparative analysis of MSI data. However, MSI experiments based on previously reported approaches (e.g., MALDI) usually provide poor reproducibility and high interference in the low molecular m/z range. Here we evaluated the suitability of NIMS with f-AuNPs for comparative global MSI studies by elucidating the metabolic variations occurring in the mouse colon under different dietary conditions. The study design involved nine mice fed with three different diets: a standard control diet (SD), a fiber deficient diet (FDD), and a polysaccharide and fiber deficient diet (PFD) (Figure 3A). Three biological replicates were collected for each condition and 5 μm tissue slices were analyzed according to the presented MSI protocol (Figure 3B). For comparative analysis, metabolite intensities were normalized to the total ion current in each pixel in the spectra and phosphocholine was used as reference for tissue alignment.
First, we defined the overall metabolic organization of the mouse colon under the SD diet regimen. Detected metabolites were divided according to relevant metabolic classes. Each class was determined by the sum of the normalized intensity values for representative molecules and their adducts (identified using an in-house R script to match with the METIN database within a 5 ppm error window). As shown in Figure 6A, the colon lumen contains high levels of lipid metabolites, which are of particularly high intensity in the center of the lumen, whereas bile acids appear uniformly distributed across the lumen as well as the colon mucosal and epithelial layers. Bile acids are endogenous molecules synthesized from cholesterol and further modified by gut bacteria.62 Our approach detected primary (e.g., cholic acid and muricholic acid as [M+H]+, [M+K]+ and [M+H-H2O]+, and chenodeoxycholic acid as [M+H]+ and [M+K]+), and conjugated bile acids (e.g., taurolithocholic acid 3-sulfate as [M+H]+). Primary bile acids are converted enzymatically to secondary bile acids via 7-dehydroxylation performed by the metabolic machinery of the gut microbiota.63 For instance, we detected isomers of lithocholic acid and isolithocholic acid as [M+H]+ and [M+K]+, and deoxycholic acid as [M+H]+ and [M+K]+ (Table S1). Bile acids have both lipophilic and hydrophilic properties and are secreted into the duodenum to facilitate the absorption of lipids and lipo-soluble vitamins. Due to this amphipathic nature, bile acids act as emulsifiers and can therefore distribute evenly between the gut epithelia/mucosa and the lumen, as demonstrated in Figure 6B.
Figure 6.
Overall metabolic organization of the mouse colon under standard diet: (A) lipids, (B) bile acids, (C) carbohydrates, (D) SCFA. Metabolite intensities were normalized according to the total ion signal in each pixel and reported as sum of intensities for each metabolic class. Phosphocholine was used for tissue alignment.
The overall distribution of metabolites involved in carbohydrate metabolism appears uniform in the colon lumen and epithelia, with slightly higher relative content in the lumen (Figure 6C). Interestingly, the host-mouse does not possess the enzymatic capability to digest plant fibers and cellulose. However, the mouse gut harbours more than 500 bacterial species, including obligate and facultative anaerobes, that possess a wide repertoire of fiber- and cellulose-degrading enzymes64 (e.g., Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia64–67). Consequently, the presence of carbohydrate metabolites in the lumen is likely mostly due to bacterial breakdown of dietary polysaccharides and fibers. Moreover, microbial communities in the colon are crucial to non-digestible carbohydrate/fiber fermentation, which results in the formation of short chain fatty acids (SCFA) (e.g., acetate, propionate, butyrate). SCFA can be assimilated and used by the host, or function locally as signalling molecules involved in intestinal and immune homeostasis.68,69 Consistent with this, we detected the presence of SCFA in the colon lumen (Figure 6D).
To explore the metabolic variations occurring in the mouse colon under different diets, we assessed the distribution of each metabolic class as obtained by the sum of the single normalized metabolite intensities detected for each class (Figure 7). The carbohydrate content in both the PFD and FDD conditions are significantly (p < 0.01) reduced (averaged sum of normalized intensities difference > 30%) compared to the SD. The lack of fiber in the FDD and PFD samples also results in a significant reduction of SCFA. These observations may highlight the occurrence of metabolic rearrangements in the mouse colon which similarly affect fiber and polysaccharide breakdown/utilization as well as the production of microbial fermentation end-products. The absence of fiber or polysaccharides in the mouse diet also induces a significant decrease in lipids, resulting in an even layer between the lumen and the mucosa (Figure 7). This difference is mostly due to a decrease in linoleic acid and/or related isobars, which appear to accumulate in the lumen center (Figure S4). Of note, the detected bile acids are only slightly affected by different diet regimens, which may indicate that the diet does not affect gut microbial regulation of bile acid production. We also revealed large presence of sulfur metabolites in the SD regimen, and their substantial decrease under FDD, and, to a lesser extent (p <0.01, averaged sum of normalized intensities difference < 30%), under the PFD (Figure 7). In this case, the difference is linked to a decrease in sulfate intensity (SD vs FDD) and/or distribution (SD vs FDD and SD vs PFD) (Figure 7 and Figure S5). Gut microbes play a major role in the metabolism of sulfur in the colon lumen70 as they can reduce sulfate while oxidizing organic compounds. Gut microbiota rearrangements involving sulfate-reducing bacteria, or increased degradation of the colonic mucus barrier resulting in enhanced sulfate release by mucinolytic bacteria71, may be at the basis of this observation.
Figure 7.
Metabolic rearrangement of the mouse colon under different diet regimen [standard diet (SD), fiber depleted diet (FDD) or polysaccharides and fiber depleted diet (PFD)]. Metabolite intensities were normalized according to the total ion signal in each pixel and reported as sum of intensities for each metabolic class. Phosphocholine was used for tissue alignment.
Much is still unknown about the role of the gut microbiota in the digestion of dietary polysaccharides and fibers72, although several recent studies point to its key functional contribution and interaction with host metabolism. In particular, some commensal bacteria have adapted to switch between multiple different substrates, while others exhibit more selective behaviour.73 For example, Ruminococcus flavefaciens and Fibrobacter succinogens can degrade polysaccharides and cellulose and members of the genus Bacteroides can break down a wide range of plant polysaccharides. Interpreted in this scenario, our data point to the occurrence of important microbiome remodeling in the mice colon driven by the absence of dietary fibers or polysaccharides. These rearrangements may ultimately reflect in the spatial organization of intestinal metabolism, as here unraveled on the micrometer scale by using NIMS with f-AuNPs for comprehensive metabolome profiling in space.
Conclusions
NIMS with f-AuNPs leverages nanostructured gold surfaces functionalized with perfluorinated alkanes to facilitate the detection of small molecules by LDI mass spectrometry. This approach assists in the ablation process and can catalyze the D/I of metabolites directly from the surface of the sample, with minimal noise from the nanoparticles. This results in an increase in D/I efficiency accompanied by a decrease in the energy required for desorption. The use of perfluorohexane to distribute the f-AuNPs also creates a non-adherent environment which remains homogenous and separate from the hydrophilic surface of the sample, permitting MSI metabolomics experiments at a lateral resolution only limited by the diameter of the laser. Comparing with previous NIMS protocols for MSI, NIMS with functionalized gold nanoparticles does not involve the use of HF or other highly hazardous chemicals, thus facilitating the translation of the approach to the laboratory routine.
When applied to the comparative exploration of the mouse colon, our approach allowed for the direct detection of more than 1000 features, of which approximately 80 metabolites were consistently annotated among biological replicates. F-AuNPs provided heterogeneous metabolome coverage, including chemically diverse small molecules of both animal and microbial origin. Moreover, the approach unraveled significant rearrangements in the metabolic organization of the mouse colon, stimulated by a fiber and/or polysaccharide depleted diet. In particular, the decrease in carbohydrate, SCFA, lipid and sulfate in the polysaccharide and/or fiber depleted diets may be linked to variations in the relative composition of the colon microbiota.
In summary, the versatility of f-AuNPs NIMS can provide a comprehensive view on the distribution of metabolites in biological samples. We believe that this approach can offer a means to gain a mechanistic understanding from functional comparative MSI metabolomic studies. Our current and future efforts are in the direction of interfacing f-AuNPs NIMS with LDI sources with optimized laser focusing geometry,2 and with ion beam cluster ion sources (e.g. Bi3+ cluster ion source) to explore the potential of this technology for the detection of metabolites localization at the single cell level.
Methods
Nanoparticles synthesis.
F-AuNPs were synthesized as previously described.36 Briefly, 1 mL of 2% (v/v) octanethiol functionalized gold nanoparticles (2–4 nm diameter, 20 mg/mL in toluene, Sigma, Milwaukee, WI) were washed with 20 mL neat ethanol, followed by 20 mL acetone, sonicated for 1 minute and centrifuged for 20 minutes at 3000 rpm between each wash. The washing steps were repeated three times. After removal of the supernatant, 1 mL hexane was added to the gold nanoparticles. The solution was tested for the presence of tetraoctyl ammonium ions (466 m/z), which can contaminate commercially available octanethiol functionalized gold nanoparticles. Next 200 μL of 1H,1H,2H,2H-perfluoredecanethiol was added under N2 flow and the resulting solution stirred for 72 hours with a magnetic stir bar in a capped 30 mL glass vial. Glass is necessary to prevent contamination of nanoparticles for high resolution mass spectrometric measurements. After the reaction was complete, the solvent was evaporated under N2 flow and the f-AuNPs were washed with hexane (three times), acetone (three times), and methanol/acetone/water (1:1:1 v/v/v) (two times). The particles were sonicated for 30 seconds and centrifugated at 3000 rpm at 4 °C between each wash. The supernatant was removed and the nanoparticles were dried at 90 °C in the oven and stored. A solution of f-AuNPs in PFH (3 mg/mL) was prepared and tested for contaminants before use. The f-AuNPs in PFH were stored in glass vials at room temperature until use.
Mass spectrometric conditions.
Metabolites were detected using a Waters Synapt G2-Si quadrupole time of flight (Q-TOF) mass spectrometer equipped with a MALDI source (Waters, Milford, MA). HDImaging v1.4 and MassLynx v4.2 software, were used for data acquisition. Tissue samples were imaged with a 30 μm step size using a solid state Nd:YAG laser operating at 355 nm (laser energy 175, sampling rate 3 s, and repetition rate of 2500 Hz). Untargeted measurements were performed in resolution MS mode, in the 25–700 m/z range in positive mode. Calibration was performed prior analysis using red phosphorus clusters as a reference standard, and during spectra acquisition using tryptophan (205.0105 m/z) as internal lock mass. The nanoscaping data (Figure S2), obtained from cell extracts, were acquired on a Bruker Microflex MALDI-TOF MS with the MSP Biotarget adapter (Bruker Daltonics, Billerica, MA) at 35% laser power.
Metabolite identification was based on the accurate mass of the molecule matched against the METLIN database, observed isotope pattern and presence of related adducts at consistent spatial distribution. MS/MS experiments were performed to confirm metabolite identities when possible.
In vivo study.
Mice colon samples were obtained in the frame of a study investigating the spatial architecture of the mice colon. The study obtained the approval by the relevant institutional ethics committee and was conducted in accordance with the protocols approved by the Austrian laws (BMWF-66.006/ 0032-WF/V/3b/2014). In brief, mice were housed 12h/12h day/night cycle in a controlled environment, providing free access to food and water. Nine wild-type C57BL/6 mice maintained on a standard diet (R/M-H Ssniff, Germany) were divided in three groups (n=3 per group) and fed for 1 week under a standard control diet (SD), a fiber-deficient diet (FDD), or a polysaccharide- and fiber-deficient diet (PFD) (Ssniff, Germany). Mice were sacrificed on day 7, and the colon was cryopreserved in optimal cutting material (OCT, Thermo Scientific, USA) and samples stored at −80°C until analysis. Mouse brain tissues for SEM analysis were obtained in compliance with the National Institutes of Health and institutional guidelines (TSRI, International accredited). CB17-PrkdcSCID mice were euthanized by overdose of isoflurane. Mouse brain tissues were cryopreserved in OCT and samples stored at −80°C until analysis.
Cell extracts.
Bacteroides fragilis cell extracts were kindly donated by the Forest Rohwer’s lab, at San Diego State University. Briefly, cells were grown in brain heart infusion (BHI) media until 0.8 OD600, centrifuged at 13,000 rpm and 4 °C, the supernatant was removed, cells were washed twice in minimal media and flash frozen in liquid nitrogen.
Sample preparation.
The frozen tissue samples were embedded in OCT and cryosectioned to obtain tissue slices (5 μm thickness) using a Leica 1900 cryostat (Buffalo Glove, IL). During this step attention should be posed to avoid any contact between the OCT material and the surface of the sample (i.e. via the cutting blade), since some OCT component may contaminate the surface of the sample and contribute to the MS background noise. The slices were thaw-mounted on a piranha etched p-type silicon chip and the f-AuNPs solution in PFH (3 mg/mL) was topically distributed on the surface of the sample. Standard solutions of analytes were also prepared, deposited on the piranha etched p-type silicon chip, evaporated and covered with the f-AuNPs solution in PFH. Each chip was kept under vacuum at room temperature until analysis. Bacteroides fragilis cells were resuspended in 2/2/1 acetonitrile/methanol/water and underwent x3 freeze/thaw cycles in liquid nitrogen/ice cold water bath. After centrifugation the supernatant was evaporated and resuspended in 50/50 acetonitrile/water. The DOWEX 200–400 mesh anionic resin particles (BioRad, Richmond, CA) were suspended in 50/50 acetonitrile/water (1 mg/mL). After mixing with the DOWEX resin, samples were spotted (1 μL) and dried on the plate for MS analysis.
Transmission electron microscopy.
F-AuNPs were deposited on 400 mesh copper grids and characterized by a Philips CM100 electron microscope (FEI, Hillsborough, OR) at 80 kV before and after irradiation (355 nm, laser energy 6.6 mJ, 2,000 shots). Images were collected using a Megaview III CCD camera (Olympus Soft Imaging Solutions, Munster, Germany).
Scanning electron microscopy.
SEM analysis were obtained with a Hitachi S4800 SEM at 5 kV. f-AuNPs were distributed on the surface of a clean piranha etched p-type silicon chip or one drop of the f-AuNPs solution in PFH (3 mg/mL) was topically distributed on the surface of a tissue slice. PFH was dried under vacuum at room temperature and the sample irradiated by a MALDI source with a laser with a 250 μm step size (included in the AB SCIEX TOF/TOF 5800 MS system).
Data analysis.
Data analysis was performed in HDImaging v1.4 software (Waters, Milford, MA), also used to preprocess and normalize metabolite intensities. Statistical analysis was performed on normalized ion intensities for each metabolic class between different diet conditions (two sided t-test, assuming unequal variance distribution). The significance threshold was set at p < 0.01. Metabolite abundances were considered different for statistically significant (p < 0.01) variations > 30% (averaged intensity), as semi-quantitative estimation of the variation. The obtained data sets were putatively annotated against the METLIN database based on a mass deviation of 5 ppm using an in-house R script. MS/MS data were processed in MassLynx v4.2 software. Data analysis was performed in the KNIME Analytics Platform (Konstanz, Germany).
Supplementary Material
Footnotes
Supporting Information Available. Supporting material (list of detected metabolites, supporting figures for metabolome coverage, metabolites dislocation and OCT distribution, mass spectra for the combined use of f-AuNPs with the DOWEX nanoparticles resin for metabolite pre-concentration, distribution of targeted metabolites in mice colon dysregulated under different diet regimens) is available free of charge via the Internet at http://pubs.acs.org.
Conflicts of interest. The authors declare no conflict of interest.
References
- (1).Buchberger AR; DeLaney K; Johnson J; Li L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem 2017, 90, 240–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kompauer M; Heiles S; Spengler B. Atmospheric Pressure MALDI Mass Spectrometry Imaging of Tissues and Cells at 1.4-Mum Lateral Resolution. Nat. Methods 2017, 14, 90–96. [DOI] [PubMed] [Google Scholar]
- (3).Ly A; Buck A; Balluff B; Sun N; Gorzolka K; Feuchtinger A; Janssen KP; Kuppen PJK; Van De Velde CJH; Weirich G; Erlmeier F; Langer R; Aubele M; Zitzelsberger H; McDonnell L; Aichler M; Walch A. High-Mass-Resolution MALDI Mass Spectrometry Imaging of Metabolites from Formalin-Fixed Paraffin-Embedded Tissue. Nat. Protoc 2016, 11, 1428–1443. [DOI] [PubMed] [Google Scholar]
- (4).Sturtevant D; Lee YJ; Chapman KD Matrix Assisted Laser Desorption/IonizationMass Spectrometry Imaging (MALDI-MSI) for Direct Visualization of Plant Metabolites in Situ. Current Opinion in Biotechnology 2016, 53–60. [DOI] [PubMed] [Google Scholar]
- (5).Vickerman JC Molecular Imaging and Depth Profiling by Mass Spectrometry—SIMS, MALDI or DESI? Analyst 2011, 136, 2199–2217. [DOI] [PubMed] [Google Scholar]
- (6).Aichler M; Walch A. MALDI Imaging Mass Spectrometry: Current Frontiers and Perspectives in Pathology Research and Practice. Lab. Investig 2015, 95, 422–431. [DOI] [PubMed] [Google Scholar]
- (7).Mirabelli MF; Zenobi R. Observing Proton Transfer Reactions Inside the MALDI Plume: Experimental and Theoretical Insight into MALDI Gas-Phase Reactions. J. Am. Soc. Mass Spectrom. 2017, 28, 1676–1686. [DOI] [PubMed] [Google Scholar]
- (8).Zenobi R; Knochenmuss R. Ion Formation in MALDI Mass Spectrometry. Mass Spectrom. Rev 1998, 17, 337–366. [Google Scholar]
- (9).Etalo D; de Vos RC; Joosten MHAJ; Hall R. Spatially-Resolved Plant Metabolomics: Some Potentials and Limitations of Laser-Ablation Electrospray Ionization (LAESI) Mass Spectrometry Metabolite Imaging. Plant Physiol. 2015, 169, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bontha SV; Maluf DG; Mueller TF; Mas VR Systems Biology in Kidney Transplantation: The Application of Multi-Omics to a Complex Model. American Journal of Transplantation 2017, 17, 11–21. [DOI] [PubMed] [Google Scholar]
- (11).Hood L; Heath JR; Phelps ME; Lin B. Systems Biology and New Technologies Enable Predictive and Preventative Medicine. Science 2004, 306, 640–643. [DOI] [PubMed] [Google Scholar]
- (12).Jackson SN; Woods AS The Development of Matrix-Assisted Laser Desorption Ionization (MALDI) Mass Spectrometry. In The Encyclopedia of Mass Spectrometry 2016; 124–131. [Google Scholar]
- (13).Shi R; Dai X; Li W; Lu F; Liu Y; Qu H; Li H; Chen Q; Tian H; Wu E; Wang Y; Zhou R; Lee S; Lifshitz Y; Kang Z; Liu J. Hydroxyl-Group-Dominated Graphite Dots Reshape Laser Desorption/Ionization Mass Spectrometry for Small Biomolecular Analysis and Imaging. ACS Nano 2017, 11, 9500–9513. [DOI] [PubMed] [Google Scholar]
- (14).Shroff R; Rulísek L; Doubsky J; Svatos A. Acid-Base-Driven Matrix-Assisted Mass Spectrometry for Targeted Metabolomics. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 10092–10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yang J; Caprioli RM Matrix Sublimation/Recrystallization for Imaging Proteins by Mass Spectrometry at High Spatial Resolution. Anal. Chem 2011, 83, 5728–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Holzlechner M; Reitschmidt S; Gruber S; Zeilinger S; Marchetti-Deschmann M. Visualizing Fungal Metabolites during Mycoparasitic Interaction by MALDI Mass Spectrometry Imaging. Proteomics 2016, 16, 1742–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nemes P; Vertes A. Laser Ablation Electrospray Ionization for Atmospheric Pressure, in Vivo, and Imaging Mass Spectrometry. Anal. Chem 2007, 79, 8098–8106. [DOI] [PubMed] [Google Scholar]
- (18).Northen TR; Yanes O; Northen MT; Marrinucci D; Uritboonthai W; Apon J; Golledge SL; Nordström A; Siuzdak G. Clathrate Nanostructures for Mass Spectrometry. Nature 2007, 449, 1033–1036. [DOI] [PubMed] [Google Scholar]
- (19).Winograd N. The Magic of Cluster SIMS. Anal. Chem 2005, 77, 142A–149A. [Google Scholar]
- (20).Winograd N. Imaging Mass Spectrometry on the Nanoscale with Cluster Ion Beams. Anal. Chem 2015, 87, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kraft ML; Weber PK; Longo ML; Hutcheon ID; Boxer SG Phase Separation of Lipid Membranes Analyzed with High-Resolution Secondary Ion Mass Spectrometry. Science 2006, 313, 1948–1951. [DOI] [PubMed] [Google Scholar]
- (22).Weibel DE; Lockyer N; Vickerman JC C60 Cluster Ion Bombardment of Organic Surfaces. Appl. Surf. Sci 2004, 231, 146–152. [Google Scholar]
- (23).Ostrowski SG; Szakal C; Kozole J; Roddy TP; Xu J; Ewing AG; Winograd N. Secondary Ion MS Imaging of Lipids in Picoliter Vials with a Buckminsterfullerene Ion Source. Anal. Chem 2005, 77, 6190–6196. [DOI] [PubMed] [Google Scholar]
- (24).Passarelli MK; Newman CF; Marshall PS; West A; Gilmore IS; Bunch J; Alexander MR; Dollery CT Single-Cell Analysis: Visualizing Pharmaceutical and Metabolite Uptake in Cells with Label-Free 3D Mass Spectrometry Imaging. Anal. Chem 2015, 87, 6696–6702. [DOI] [PubMed] [Google Scholar]
- (25).Passarelli MK; Ewing AG Single-Cell Imaging Mass Spectrometry. Current Opinion in Chem. Biol 2013, 17, 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Passarelli MK; Pirkl A; Moellers R; Grinfeld D; Kollmer F; Havelund R; Newman CF; Marshall PS; Arlinghaus H; Alexander MR; Dollery CT; Gilmore IS The 3D OrbiSIMS - Label-Free Metabolic Imaging with Subcellular Lateral Resolution and High Mass-Resolving Power. Nat. Methods 2017,14, 1175. [DOI] [PubMed] [Google Scholar]
- (27).Venter A; Sojka PE; Cooks RG Droplet Dynamics and Ionization Mechanisms in Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem 2006, 78, 8549–8555. [DOI] [PubMed] [Google Scholar]
- (28).Takáts Z; Wiseman JM; Gologan B; Cooks RG Mass Spectrometry Sampling under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [DOI] [PubMed] [Google Scholar]
- (29).Watrous J; Roach P; Heath B; Alexandrov T; Laskin J; Dorrestein PC Metabolic Profiling Directly from the Petri Dish Using Nanospray Desorption Electrospray Ionization Imaging Mass Spectrometry. Anal. Chem 2013, 85, 10385–10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ray A; Bristow T; Whitmore C; Mosely J. On-Line Reaction Monitoring by Mass Spectrometry, Modern Approaches for the Analysis of Chemical Reactions. Mass Spectrom. Rev 2017, Jun 19. [DOI] [PubMed] [Google Scholar]
- (31).Tillner J; Wu V; Jones EA; Pringle SD; Karancsi T; Dannhorn A; Veselkov K; McKenzie JS; Takats Z. Faster, More Reproducible DESI-MS for Biological Tissue Imaging. J. Am. Soc. Mass Spectrom. 2017, 28, 2090–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Greving MP; Patti GJ; Siuzdak G. Nanostructure-Initiator Mass Spectrometry Metabolite Analysis and Imaging. Anal. Chem 2011, 83, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Guinan TM; Gustafsson OJR; McPhee G; Kobus H; Voelcker NH Silver Coating for High-Mass-Accuracy Imaging Mass Spectrometry of Fingerprints on Nanostructured Silicon. Anal. Chem 2015, 87, 11195–11202. [DOI] [PubMed] [Google Scholar]
- (34).Francese S; Bradshaw R; Denison N. An Update on MALDI Mass Spectrometry Based Technology for the Analysis of Fingermarks – Stepping into Operational Deployment. Analyst 2017, 142, 2518–2546. [DOI] [PubMed] [Google Scholar]
- (35).Rocha B; Ruiz-Romero C; Blanco FJ Mass Spectrometry Imaging: A Novel Technology in Rheumatology. Nat. Rev. Rheumatol 2016, 13, 52. [DOI] [PubMed] [Google Scholar]
- (36).Kurczy ME; Zhu ZJ; Ivanisevic J; Schuyler AM; Lalwani K; Santidrian AF; David JM; Giddabasappa A; Roberts AJ; Olivos HJ; O’Brien PJ; Franco L; Fields MW; Paris LP; Friedlander M; Johnson CH; Epstein AA; Gendelman HE; Wood MR; Felding BH, et al. Comprehensive Bioimaging with Fluorinated Nanoparticles Using Breathable Liquids. Nat. Commun 2015, 6, 5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Picca RA; Calvano CD; Cioffi N; Palmisano F. Mechanisms of Nanophase-Induced Desorption in LDI-MS. A Short Review. Nanomaterials 2017, 7, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Taira S; Sugiura Y; Moritake S; Shimma S; Ichiyanagi Y; Setou M. Nanoparticle-Assisted Laser Desorption/Ionization Based Mass Imaging with Cellular Resolution. Anal. Chem 2008, 80, 4761–4766. [DOI] [PubMed] [Google Scholar]
- (39).Kawasaki H; Yonezawa T; Watanabe T; Arakawa R. Platinum Nanoflowers for Surface-Assisted Laser Desorption/Ionization Mass Spectrometry of Biomolecules. J. Phys. Chem. C 2007, 111, 16278–16283. [Google Scholar]
- (40).Pilolli R; Palmisano F; Cioffi N. Gold Nanomaterials as a New Tool for Bioanalytical Applications of Laser Desorption Ionization Mass Spectrometry. Anal. Bioanal. Chem 2012, 402, 601–623. [DOI] [PubMed] [Google Scholar]
- (41).Lee KH; Chiang CK; Lin ZH; Chang HT Determining Enediol Compounds in Tea Using Surface-Assisted Laser Desorption/Ionization Mass Spectrometry with Titanium Dioxide Nanoparticle Matrices. Rapid Commun. Mass Spectrom. 2007, 21, 2023–2030. [DOI] [PubMed] [Google Scholar]
- (42).Silina YE; Fink-Straube C; Hayen H; Volmer DA Analysis of Fatty Acids and Triacylglycerides by Pd Nanoparticle-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Methods 2015, 7, 3701–3707. [Google Scholar]
- (43).Dong X; Cheng J; Li J; Wang Y. Graphene as a Novel Matrix for the Analysis of Small Molecules by MALDI-TOF MS. Anal. Chem 2010, 82, 6208–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Go EP; Apon JV; Luo G; Saghatelian A; Daniels RH; Sahi V; Dubrow R; Cravatt BF; Vertes A; Siuzdak G. Desorption/Ionization on Silicon Nanowires. Anal. Chem 2005, 77, 1641–1646. [DOI] [PubMed] [Google Scholar]
- (45).Jain PK; Lee KS; El-Sayed IH; El-Sayed MA Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [DOI] [PubMed] [Google Scholar]
- (46).Tang HW; Wong MY; Lam W; Cheng YC; Che CM; Ng KM Molecular Histology Analysis by Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry Using Gold Nanoparticles as Matrix. Rapid Commun. Mass Spectrom. 2011, 25, 3690–3696. [DOI] [PubMed] [Google Scholar]
- (47).Su CL; Tseng WL Gold Nanoparticles as Assisted Matrix for Determining Neutral Small Carbohydrates through Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Anal. Chem 2007, 79, 1626–1633. [DOI] [PubMed] [Google Scholar]
- (48).Huang YF; Chang HT Nile Red-Adsorbed Gold Nanoparticle Matrixes for Determining Aminothiols through Surface-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Chem 2006., 78, 1485–1493. [DOI] [PubMed] [Google Scholar]
- (49).Wang J; Jie M; Li H; Lin L; He Z; Wang S; Lin JM Gold Nanoparticles Modified Porous Silicon Chip for SALDI-MS Determination of Glutathione in Cells. Talanta 2017, 168, 222–229. [DOI] [PubMed] [Google Scholar]
- (50).Zhu Z-J; Ghosh PS; Miranda OR; Vachet RW; Rotello VM Multiplexed Screening of Cellular Uptake of Gold Nanoparticles Using Laser Desorption/Ionization Mass Spectrometry. J. Am. Chem. Soc 2008, 130, 14139–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Nicolardi S; Van Der Burgt YEM; Codée JDC; Wuhrer M; Hokke CH; Chiodo F. Structural Characterization of Biofunctionalized Gold Nanoparticles by Ultrahigh-Resolution Mass Spectrometry. ACS Nano 2017, 11, 8257–8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zhou J; Ralston J; Sedev R; Beattie DA Functionalized Gold Nanoparticles: Synthesis, Structure and Colloid Stability. J. Colloid Interface Sci. 2009, 331, 251–262. [DOI] [PubMed] [Google Scholar]
- (53).Woehrle GH; Brown LO; Hutchison JE Thiol-Functionalized, 1.5-Nm Gold Nanoparticles through Ligand Exchange Reactions: Scope and Mechanism of Ligand Exchange. J. Am. Chem. Soc 2005, 127, 2172–2183. [DOI] [PubMed] [Google Scholar]
- (54).Biffinger JC; Kim HW; DiMagno SG The Polar Hydrophobicity of Fluorinated Compounds. ChemBioChem. 2004, 5, 622–627. [DOI] [PubMed] [Google Scholar]
- (55).Cooper DL; Allan NL; Powell RL Theoretical Studies of Fluorocarbons Part III. Primary, Secondary, Tertiary and Quaternary Centres. J. Fluor. Chem 1990, 49, 421–432. [Google Scholar]
- (56).Miura D; Fujimura Y; Wariishi H. In Situ Metabolomic Mass Spectrometry Imaging: Recent Advances and Difficulties. Journal of Proteomics. 2012, 75, 5052–5060. [DOI] [PubMed] [Google Scholar]
- (57).Smith CA; O’Maille G; Want EJ; Qin C; Trauger SA; Brandon TR; Custodio DE; Abagyan R; Siuzdak G. METLIN: A Metabolite Mass Spectral Database. Therapeutic Drug Monitoring 2005, 27, 747–751. [DOI] [PubMed] [Google Scholar]
- (58).DeBerardinis RJ; Mancuso A; Daikhin E; Nissim I; Yudkoff M; Wehrli S; Thompson CB Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci 2007, 104, 19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Allen EL; Ulanet DB; Pirman D; Mahoney CE; Coco J; Si Y; Chen Y; Huang L; Ren J; Choe S; Clasquin MF; Artin E; Fan ZP; Cianchetta G; Murtie J; Dorsch M; Jin S; Smolen GA Differential Aspartate Usage Identifies a Subset of Cancer Cells Particularly Dependent on OGDH. Cell Rep. 2016, 17, 876–890. [DOI] [PubMed] [Google Scholar]
- (60).Ke H; Lewis IA; Morrisey JM; McLean KJ; Ganesan SM; Painter HJ; Mather MW; Jacobs-Lorena M; Llinás M; Vaidya AB Genetic Investigation of Tricarboxylic Acid Metabolism during the Plasmodium Falciparum Life Cycle. Cell Rep. 2015, 11, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kaiko GE; Ryu SH; Koues OI; Collins PL; Solnica-Krezel L; Pearce EL; Oltz EM; Stappenbeck TS The Colonic Crypt Protects Stem Cells from Article The Colonic Crypt Protects Stem Cells From. Cell 2016, 165, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).De Aguiar Vallim TQ; Tarling EJ; Edwards PA Pleiotropic Roles of Bile Acids in Metabolism. Cell Metabolism. 2013, 17, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wahlström A; Sayin SI; Marschall HU; Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metabolism. 2016, 24, 41–50. [DOI] [PubMed] [Google Scholar]
- (64).Lupp C; Robertson ML; Wickham ME; Sekirov I; Champion OL; Gaynor EC; Finlay BB Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [DOI] [PubMed] [Google Scholar]
- (65).Murphy EF; Cotter PD; Healy S; Marques TM; O’Sullivan O; Fouhy F; Clarke SF; O’Toole PW; Quigley EM; Stanton C; Ross PR; O’Doherty RM; Shanahan F. Composition and Energy Harvesting Capacity of the Gut Microbiota: Relationship to Diet, Obesity and Time in Mouse Models. Gut 2010, 59, 1635–1642. [DOI] [PubMed] [Google Scholar]
- (66).Bercik P; Denou E; Collins J; Jackson W; Lu J; Jury J; Deng Y; Blennerhassett P; MacRi J; McCoy KD; Verdu EF; Collins SM The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609. [DOI] [PubMed] [Google Scholar]
- (67).Benson AK; Kelly SA; Legge R; Ma F; Low SJ; Kim J; Zhang M; Oh PL; Nehrenberg D; Hua K; Kachman SD; Moriyama EN; Walter J; Peterson DA; Pomp D. Individuality in Gut Microbiota Composition Is a Complex Polygenic Trait Shaped by Multiple Environmental and Host Genetic Factors. Proc. Natl. Acad. Sci 2010, 107, 18933–18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Morrison DJ; Preston T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Ghazalpour A; Cespedes I; Bennett BJ; Allayee H. Expanding Role of Gut Microbiota in Lipid Metabolism. Curr. Opin. Lipidol 2016, 27, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Carbonero F; Benefiel AC; Alizadeh-Ghamsari AH; Gaskins HR Microbial Pathways in Colonic Sulfur Metabolism and Links with Health and Disease. Front. Physiol 2012, 3, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Desai MS; Seekatz AM; Koropatkin NM; Kamada N; Hickey CA; Wolter M; Pudlo NA; Kitamoto S; Terrapon N; Muller A; Young VB; Henrissat B; Wilmes P. Stappenbeck TS; Núñez G; Martens EC. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Flint HJ; Bayer EA; Rincon MT; Lamed R; White BA Polysaccharide Utilization by Gut Bacteria: Potential for New Insights from Genomic Analysis. Nature Reviews Microbiology 2008, 6, 121–131. [DOI] [PubMed] [Google Scholar]
- (73).McWilliam Leitch EC; Walker AW; Duncan SH; Holtrop G; Flint HJ Selective Colonization of Insoluble Substrates by Human Faecal Bacteria. Environ. Microbiol 2006, 72, 667–679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.