Abstract
Background
Currently, the main pharmaceutical intervention for COVID-19 is vaccination. While antidepressant (AD) drugs have shown some efficacy in treatment of symptomatic COVID-19, their preventative potential remains largely unexplored. Analysis of association between prescription of ADs and COVID-19 incidence in the population would be beneficial for assessing the utility of ADs in COVID-19 prevention.
Methods
Retrospective study of association between AD prescription and COVID-19 diagnosis was performed in a cohort of community-dwelling adult mental health outpatients during the 1st wave of COVID-19 pandemic in the UK. Clinical record interactive search (CRIS) was performed for mentions of ADs within 3 months preceding admission to inpatient care of the South London and Maudsley (SLaM) NHS Foundation Trust. Incidence of positive COVID-19 tests upon admission and during inpatient treatment was the primary outcome measure.
Results
AD mention was associated with approximately 40% lower incidence of positive COVID-19 test results when adjusted for socioeconomic parameters and physical health. This association was also observed for prescription of ADs of the selective serotonin reuptake inhibitor (SSRI) class.
Conclusions
This preliminary study suggests that ADs, and SSRIs in particular, may be of benefit for preventing COVID-19 infection spread in the community. The key limitations of the study are its retrospective nature and the focus on a mental health patient cohort. A more definitive assessment of AD and SSRI preventative potential warrants prospective studies in the wider demographic.
Keywords: COVID-19, SSRI, Antidepressants, Drug repurposing, Respiratory infection, SARS-CoV-2
Background
More than 3 years since the declaration of the global pandemic, COVID-19 remains a major public health concern across the world. In the beginning of the pandemic, the main strategy for limiting COVID-19 spread in the population necessarily relied on non-pharmaceutical interventions of variable effectiveness, including individual measures such as personal protective equipment and social distancing, as well as society-wide restrictions such as lockdowns [1]. Later on, rapid development of vaccines provided a much-needed pharmaceutical approach for curbing COVID-19. Although mass vaccination has resulted in widespread immunity against SARS-CoV-2, some of the key concerns remain, including efficacy against newly emerging variants [2], level of protection in immunocompromised individuals [3], and the logistics of mass vaccination, particularly in lower-income economies [4]. Taken together, these considerations highlight a significant unmet need for development of alternative strategies for mitigating COVID-19.
One potentially promising approach involves repurposing of previously characterised drugs [5–8]. Notwithstanding the early high-profile failures of hydroxychloroquine [9] and ivermectin [10], more recently, it has been shown that antidepressant drugs (AD) may be associated with improved outcomes in COVID-19 patients [11–13]; furthermore, one AD (fluvoxamine) has shown efficacy in preventing severe COVID-19 in clinical trials [14–17], and another (fluoxetine) was associated with a slight decrease in mortality in a large cohort of COVID-19 patients [18]. The efficacy of fluvoxamine in symptomatic COVID-19 patients, however, remains controversial [19, 20], and the general utility of ADs for COVID-19 prevention has not been assessed.
Studies in cell-based models indicate that ADs may target cell biological mechanisms implicated in early stages of SARS-CoV-2 infection, hinting at the potential prophylactic effect of ADs [21–24]. To investigate the potential link between ADs and protection against COVID-19 infection, we present analysis of association between positive COVID-19 test result incidence and prior AD exposure in a cohort of community-dwelling mental health outpatients.
Methods
Study design, data source, and population
We conducted an observational, retrospective, matched cohort study of individuals admitted to the 4 inpatient care units (Bethlem Royal Hospital, Lambeth Hospital, Lewisham Hospital, and Maudsley Hospital) affiliated with the South London and Maudsley (SLaM) NHS Foundation Trust during the 1st wave of the COVID-19 pandemic of 2020. SLaM provides near-monopoly comprehensive mental health services to a geographic catchment of 1.3 million residents in four boroughs of south London. SLaM has used electronic health records across all its services since 2006 and its Clinical Record Interactive Search (CRIS) platform was set up in 2008 to provide researcher access to de-identified data from these records within a robust governance infrastructure. CRIS has been subsequently developed through a range of data linkages and natural language processing (NLP) algorithms [25], and the platform has provided data for over 250 peer-reviewed publications to date. Using CRIS, we extracted data on admissions of patients aged 18 years or older to SLaM inpatient facilities between 1 April and 31 December 2020. PCR or antigen tests for COVID-19 were routinely performed at admission and during the inpatient stay over that period. The criterion for inclusion in this study was the conclusive positive or negative COVID-19 test result(s) during inpatient stay in a SLaM inpatient unit. Characteristics of the study cohort are presented in Table 1.
Table 1.
Characteristics of the study cohort
| Characteristics | COVID-19 Negative, n= 5,462 (%) | COVID-19 Positive, n= 202 (%) | Test statistics |
|---|---|---|---|
| Gender* | 0.031£, 1, 0.89 | ||
| Female | 2,506 (46.1) | 92 (45.5) | |
| Male | 2,935 (53.9) | 110 (54.5) | |
| Missing data (% of total patients) | 21(0.4) | 0 (0.0) | |
| Ethnicity* | 0.887£, 1, 0.35 | ||
| Non-white | 3,052 (58.9) | 119 (62.3) | |
| White | 2,127 (41.1) | 72 (37.7) | |
| Missing data (% of total patients) | 283 (5.2) | 11 (5.4) | |
| Age group (no missing data)* | 19.601£, 6,<0.001 | ||
| 18-29 | 1,595 (29.2) | 39 (19.3) | |
| 30- 39 | 1,342 (24.6) | 59 (29.2) | |
| 40- 49 | 973 (17.8) | 25 (12.4) | |
| 50- 59 | 844 (15.5) | 41 (20.3) | |
| 60- 69 | 424 (7.8) | 18 (8.9) | |
| 70- 79 | 215 (3.9) | 13 (6.4) | |
| 80 & over | 69 (1.3) | 7 (3.5) | |
| Primary mental health diagnosis (ICD-10) at index date* | |||
| F01-F09: Mental disorders due to known physiological conditions | 120 (2.2) | 14 (6.9) | 4.340$, <0.001 |
| F10-F19: Mental and behavioural disorders due to psychoactive substance use | 240 (4.4) | 6 (3.0) | 0.975$, 0.33 |
| F20 -F29: Schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders | 2,538 (51.1) | 104 (56.5) | 1.404$, 0.16 |
| F30-F39: Mood [affective] disorders | 1,071 (21.6) | 28 (15.2) | 2.029$, 0.04 |
| F40-F48: Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders | 241 (4.9) | 5 (2.7) | 1.326$, 0.18 |
| F50-F59: Behavioural syndromes associated with physiological disturbances and physical factors | 99 (2.0) | 3 (1.6) | 0.344, 0.73 |
| F60-F69: Disorders of adult personality and behaviour | 474 (9.5) | 19 (10.3) | 0.361$, 0.719 |
| F70-F79: Intellectual disabilities | 16 (0.3) | 0 (0.0) | 0.771$, 0.44 |
| F80-F89: Pervasive and specific developmental disorders | 44 (0.9) | 0 (0.0) | 1.281$, 0.21 |
| F90-F98: Behavioural and emotional disorders with onset usually occurring in childhood and adolescence | 12 (0.2) | 0 (0.0) | 0.667$, 0.50 |
| Z00.4 - General psychiatric examination, not elsewhere classified | 109 (2.2) | 5 (2.7) | 0.477$, 0.63 |
| Missing primary diagnosis(% of total patients) | 498 (9.1) | 18 (8.9) | |
| HoNOS subscale scores >1* | |||
| Agitation problems | 2206 (44.2) | 117 (61.3) | 4.650$, <0.001 |
| Self-injury problems | 1006 (20.2) | 24 (12.6) | 3.150$, <0.001 |
| Drinking and substance misuse problems | 1710 (34.3) | 60 (31.4) | 0.815$, 0.42 |
| Cognitive problems | 1100 (22.0) | 68 (35.6) | 4.400$, <0.001 |
| Physical illness problems | 1218 (24.4) | 65 (34.0) | 3.025$, <0.001 |
| Hallucination problems | 2736 (54.8) | 131 (68.6) | 3.756$, <0.001 |
| Depressed problems | 2063 (41.3) | 65 (34.0) | 2.013$, 0.05 |
| Relationship problems | 1805 (36.2) | 67 (35.1) | 0.031$, 0.76 |
| Daily living problems | 1430 (28.7) | 74 (38.7) | 3.016$, <0.001 |
| Living condition problems | 1327 (26.6) | 57 (29.8) | 0.997$, 0.32 |
| Occupational problems | 1602 (32.1) | 73 (38.2) | 1.776$, 0.08 |
| Missing data (% of total patients) | 471 (8.6) | 11 (5.4) | |
| Type of medication start date mentioned 90 days before index admission date | |||
| Atypical (not mirtazapine) | 39 (0.7) | 2 (1.0) | 0.452$, 0.65 |
| MAOI | 8 (0.1) | 0 (0.0) | 0.544$, 0.59 |
| Mirtazapine | 518 (9.5) | 13 (6.4) | 1.456$, 0.15 |
| SNRI | 225 (4.1) | 3 (1.5) | 1.870$, 0.06 |
| SSRI | 1,020 (18.7) | 21 (10.4) | 2.983$, <0.001 |
| TCA | 113 (2.1) | 4 (2.0) | 0.087$, 0.93 |
| Any antidepressant | 1,513 (27.7) | 34 (16.8) | 3.404$, <0.001 |
*Percentages calculated excluding missing data
$To compare two groups difference in proportion test was used; Z value, p-value reported
£To compare difference in frequencies chi squared test was used; Chi squared test value, degree of freedom and p-value reported
Exposure and outcome
For exposure, we used a natural language processing (NLP) algorithm to identify the medications mentioned in the patient’s record in a 6-month window before or after referral, which provides a validated proxy measure for drug receipt [26]. The list of medications can be found in Table 2. We established use of the following medication classes: atypical (not mirtazapine), monoamine oxidase inhibitors (MAOI), mirtazapine, serotonin and norepinephrine reuptake inhibitors (SNRI), selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants (TCA), and any antidepressants mentioned above within 31, 62, or 90 days preceding the index hospital admission.
Table 2.
Drugs assessed in this study
| Drug Class | Drug name |
|---|---|
| Selective serotonin reuptake inhibitor (SSRI) |
citalopram dapoxetine escitalopram fluoxetine fluvoxamine paroxetine sertraline |
| Serotonin and norepinephrine reuptake inhibitor (SNRI) |
venlafaxine duloxetine reboxetine |
| Atypical |
mirtzaphine vortioxetine bupropion trazodone vilazodone |
| Tricyclic antidepressant (TCA) |
amitriptyline clomipramine imipramine lofepramine nortriptyline trimipramine |
| Monoamine oxidase inhibitor (MAOI) |
tranylcypromine phenelzine isocarboxazid moclobemide |
The primary outcome measure was the result of the laboratory test for COVID-19 (antigen or PCR). Any incidence of a positive COVID-19 test result during the inpatient stay was categorised as ‘positive’. For categorisation as ‘negative,’ all COVID test results during inpatient stay were required to be negative.
Covariates and predictors
We ascertained age at the time of admission, gender, and ethnicity (dichotomised to white and non-white) as recorded at the time of hospital admission. We identified the diagnosis given closest to hospital admission in structured fields. According to the International Statistical Classification of Diseases and Related Health Problems (WHO ICD-10) [27] criteria, we established the following diagnosis groups:
F01-F09: Mental disorders due to known physiological conditions;
F10-F19: Mental and behavioural disorders due to psychoactive substance use;
F20-F29: Schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders;
F30-F39: Mood [affective] disorders;
F40-F48: Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders;
F50-F59: Behavioural syndromes associated with physiological disturbances and physical factors;
F60-F69: Disorders of adult personality and behaviour;
F70-F79: Intellectual disabilities;
F80-F89: Pervasive and specific developmental disorders;
F90-F98: Behavioural and emotional disorders with onset usually occurring in childhood and adolescence.
Mental and physical health problems as well as functional difficulties were scored using the Health of the Nation Outcome Scales (HoNOS) [28]. This is a routinely used measure in British mental health services and the most recent scores were extracted at the time of the index admission. Each subscale is rated on a scale ranging from 0 (no problem) to 4 (severe or very severe problem); to simplify interpretation, we dichotomised the scores to ‘minor or no problems’ (scores 0 or 1) and ‘mild to severe problems’ (scores 2 to 4).
Statistical techniques
Initially, χ2 tests and Z-score statistics were used to analyse COVID-19 test results for each covariate. Logistic regression models were then assembled to quantify odds ratios (ORs) for the associations between antidepressant medication receipt and incidence of COVID-19 positive test result, applying the above sub-categorisation of antidepressants and timing as secondary analyses. 95% confidence intervals (CI) and P-values for ORs were calculated, and P-values < 0.05 were considered statistically significant. Initially unadjusted logistic regression analyses were carried out, followed by adjustments for sociodemographic factors (gender, age, ethnicity) and then further adjustments for those significant HoNoS subscales and primary mental health diagnosis. Finally, primary analyses (only patients receiving any antidepressant medication) were stratified by primary mental health diagnosis measured using ICD-10 diagnosis at the time of index hospital admission. All statistical analyses were conducted with STATA version15 [29].
Results
We leveraged data from the CRIS platform, which provides research access to deidentified electronic clinical records for SLaM18. During the study period (1 April–31 December 2020), 5664 cases of mental health inpatient care admission at SLaM facilities had been tested for COVID-19, with 202 (3.56%) testing positive. Characteristics of the study cohort are presented in Table 1. By ICD-10 code, the most prevalent primary diagnoses were in the F2 (schizophreniform) category, and the second most common in the F3 (mood disorders) category.
We then queried CRIS for mentions of ADs in the clinical records of patients within the time period of 90 days preceding the date of admission. The list of drugs included in the query is presented in Table 2. 27.7% percent of COVID-19-negative cases had at least one AD mention within 90 days preceding admission, compared to 16.8% of COVID-19-positive cases. Accordingly, the occurrence of a positive COVID-19 test result in patients with an AD mention was significantly lower than in those without (2.2 vs 4.1%, p = 0.000663, χ2 test). Most prescribed ADs belonged to the SSRI class, in line with their prevalence in treatment of major depressive disorder [30]: two thirds (67%) of the cases with AD mention within 90 days (Table 1), and the occurrence of a COVID-19-positive test result in patients with a recent SSRI record was significantly less than in those without (2.0 vs 3.9%, p = 0.002853, χ2 test). Associations with other AD classes were not statistically significant.
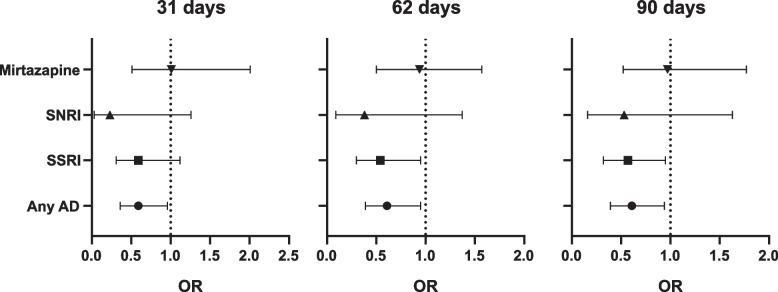
To further investigate the relationship between AD/SSRI and COVID-19 test results, we performed multiple logistic regression analysis (Table 3). We found significant adjusted associations for AD receipt within 31, 60, and 90 days before admission and incidence of positive COVID-19 test results. Similar associations were found for SSRI receipt within 62 and 90 days prior to admission in adjusted models (Fig. 1). Following stratification by diagnosis (Table 4), associations were similar in direction across all groups apart from substance use disorders and were strongest in organic disorders (F0), mood disorders (F3), and anxiety disorders (F4).
Table 3.
Strength of the association between any antidepressants medication receiving status and COVID-19 positive test result at when medication was mentioned 31 days, 62 days and 90 days for the first time before index hospital admission [Odds ratios (95% CI), P value]. Statistically significant association in bold
|
Type of medicati on |
Adjustments | 31 days | 62 days | 90 days |
|---|---|---|---|---|
|
Any drug received |
Unadjusted |
0.52 (0.34, 0.81), <0.001 |
0.51 (0.35, 0.76), <0.001 |
0.53 (0.36, 0.77), <0.001 |
|
Adjusted for sociodemographic factors (gender, age, ethnicity) |
0.55 (0.35, 0.86), 0.01 |
0.52 (0.35, 0.79), <0.001 |
0.54 (0.37, 0.80), <0.001 |
|
|
aFurther adjusted for HoNoS symptoms and primary mental health diagnosis) |
0.59 (0.36, 0.96), 0.03 |
0.61 (0.39, 0.95), 0.03 |
0.61 (0.39, 0.94), 0.02 |
|
| SSRIs | Unadjusted |
0.52 (0.30, 0.90), 0.02 |
0.50 (0.31, 0.8), <0.001 |
0.51 (0.32, 0.80), <0.001 |
|
Adjusted for sociodemographic factors (gender, age, ethnicity) |
0.59 (0.34, 1.03), 0.06 |
0.53 (0.32, 0.87), 0.01 |
0.55 (0.34, 0.88), 0.01 |
|
|
aFurther adjusted for HoNoS symptoms and primary mental health diagnosis) |
0.59 (0.31, 1.12), 0.10 |
0.54 (0.30, 0.95), 0.03 |
0.57 (0.32, 0.95), 0.03 |
|
| SNRIs | Unadjusted |
0.16 (0.02, 1.14), 0.07 |
0.25 (0.06, 1.02), 0.05 |
0.35 (0.11, 1.11), 0.07 |
|
Adjusted for sociodemographic factors (gender, age, ethnicity) |
0.16 (0.02, 1.12), 0.07 |
0.25 (0.06, 1.02), 0.05 |
0.35 (0.11, 1.11), 0.08 |
|
|
aFurther adjusted for HoNoS symptoms and primary mental health diagnosis) |
0.23 (0.03, 1.66), 0.15 |
0.38 (0.09, 1.37), 0.10 |
0.53 (0.16, 1.63), 0.24 |
|
| Mirtazapine | Unadjusted |
0.73 (0.38, 1.39), 0.34 |
0.65 (0.36, 1.18), 0.16 |
0.66 (0.37, 1.16), 0.15 |
|
Adjusted for sociodemographic factors (gender, age, ethnicity) |
0.71 (0.37, 1.38), 0.32 |
0.64 (0.35, 1.16), 0.14 |
0.65 (0.36, 1.15), 0.14 |
|
|
aFurther adjusted for HoNoS symptoms and primary mental health diagnosis) |
1.01 (0.51, 2.01), 0.97 |
0.94 (0.50, 1.57), 0.64 |
0.97 (0.52, 1.77), 0.91 |
[Odds ratios (95% CI), P value]. Statistically significant association in bold
aAdjusted for all significant HoNoS subscale and primary mental health diagnosis variables in the Table 1 (F01-F09: Mental disorders due to known physiological conditions, F30-F39: Mood [affective] disorders, Agitation problems, Self-injury problems, Cognitive problems, Physical illness problems, Hallucination problems, Depressed problems, Daily living Problems
Fig. 1.
Adjusted odds ratios (OR) at 31, 62, and 90 days (related to Table 3). Error bars correspond to 95% CI
Table 4.
Association between any antidepressant medication receiving status and COVID-19 positive test result stratified by primary mental health condition at the time of index hospital admission* [Odds ratios (95% CI), P value]. Statistically significant association in bold
| Primary diagnosis of the index mental health condition | 31 days | 62 days | 90 days |
|---|---|---|---|
| F01-F09: Mental disorders due to known physiological conditions | 0.27 (0.03, 2.87), 0.28 | 0.17 (0.01, 2.09), 0.17 | 0.16 (0.01, 1.97), 0.15 |
| F10-F19: Mental and behavioural disorders due to psychoactive substance use | 20.33 (1.59, 26.20), 0.02 | 7.82 (1.01, 22.27), 0.05 | 15.02 (1.09, 28.05), 0.04 |
| F20-F29: Schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders | 0.54 (0.21, 1.35), 0.19 | 0.75 (0.37, 1.53), 0.43 | 0.69 (0.34, 1.40), 0.31 |
| F30-F39: Mood [affective] disorders | 0.25 (0.05, 0.94), 0.04 | 0.28 (0.08, 0.98), 0.04 | 0.37 (0.13, 0.97), 0.04 |
| F40-F48: Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders | 0.18 (0.06, 1.59), 0.17 | 0.16 (0.04, 1.72), 0.15 | 0.19 (0.02, 2.02), 0.17 |
| F50-F59: Behavioural syndromes associated with physiological disturbances and physical factors | 0.72 (0.20, 16.08), 0.49 | 0.78 (0.25, 17, 09), 0.84 | 0.84 (0.23, 23.09), 0.94 |
| F60-F69: Disorders of adult personality and behaviour | 1.86 (0.48, 7.30), 0.37 | 1.86 (0.44, 7.84), 0.39 | 1.21 (0.29, 4.96), 0.78 |
*Adjusted for age, gender, ethnicity and all significant HoNoS subscale and primary mental health diagnosis variables in the table 1 (F01-F09: Mental disorders due to known physiological conditions, F30-F39: Mood [affective] disorders, Agitation problems, Self-injury problems, Cognitive problems, Physical illness problems, Hallucination problems, Depressed problems, Daily living problems
Discussion
The results of this study indicate that prior receipt of ADs and specifically SSRIs in community-dwelling mental health outpatients was associated with a decreased likelihood of COVID-19 incidence, suggesting that these drugs may have a protective effect against COVID-19 in this population. A key strength of this study is the large number of participants. Furthermore, the focus on the 1st wave of COVID-19 avoids the confounding effects of the ‘herd immunity’ in the population due to mass vaccination and/or previous exposure to COVID-19 in the following time period [31]. Importantly, exposure to both ADs and COVID-19 occurred prior to hospitalisation, reflecting the ‘real-life’ situational value of the study, which is further enhanced by the diverse socio-economical composition of the study cohort (Table 1). Our findings are consistent with decreased COVID-19 incidence in middle-aged and older adults with self-reported history of psychotropic drug use [32]. Interestingly, decreased COVID-19 incidence has also been reported for mental health inpatients using anti-psychotic medications [33], further underscoring the link between psychotropic drugs and protection against COVID-19.
SSRI treatment is associated with a dropout rate of 28% over the standard 6-month treatment course [34]. Given the study design, it can be expected that a proportion of cases did not adhere to the prescription regimen, suggesting that the observed effect may be an underestimation. Nevertheless, the effect size reported here is considerably larger than 8% reduction in overall mortality associated with record of SSRI in a large cohort of COVID-19 cases [18], being more similar to the 36% reduction by fluvoxamine in risk of hospitalisation for outpatients with COVID-19 [17] and to the 44% reduction in risk of intubation or death for hospitalised patients with COVID-19 [11]. In the context of the above studies, our findings hint that ADs/SSRIs may be at least as effective in preventing COVID-19 as in treating it, providing impetus for further investigation of their clinical utility in the general population.
The mechanisms underlying the putative protective effects of SSRIs in COVID-19 remain unclear. Some of the proposed cell biology mechanisms include blockade of viral replication [21, 23], modulation of endocytic trafficking [6, 22], phospholipidosis [35], and anti-inflammatory action through inhibition of cytokine release [36, 37]. In turn, candidate molecular targets for SSRI action include acid sphingomyelinase [12, 24], sigma receptor [38], and even the lipid bilayer of the cell membrane itself [39]. Further mechanistic insight into the role of SSRIs outside the central nervous system will require detailed investigation of physiology and cell biology of SSRIs in appropriate experimental systems, with particular consideration given to pharmacokinetics of therapeutically relevant SSRI concentrations.
Study limitations
The study has a number of important limitations, largely due to its retrospective nature and focus on a cohort of mental health patients. It cannot demonstrate a causal relationship between medications and COVID-19 test results; indeed, the protective effect is consistent with either decreased infection rate or increased recovery rate from COVID-19.
The time period of the study was limited to the first wave of the COVID-19 pandemic, which presented novel adverse effects on mental health [40]; also, during this period, COVID-19 was associated with the original strain of SARS-CoV-2 rather than its subsequently documented variants. Owing to the numbers of participants and prevalence of multiple drug prescriptions, it was not possible to investigate the association between COVID-19 and individual drugs; compared to SSRIs, the number of participants receiving other drug classes was low (Table 1), and it was also not possible to assess older-generation drugs, including fluvoxamine. The study did not account for dosage regimen; also, it was not possible to determine medication adherence from the available data.
Finally, although the association of interest appeared to be present across a range of diagnostic groups, the possibility cannot be ruled out that antidepressant use may have been a marker of personal or behavioural factors conferring protection, e.g. compliance with societal restrictions and/or personal protection measures in place at the time. To address the above limitations and to further corroborate the findings of this study, randomised prospective clinical studies for selected ADs in the general population will be of essence.
Conclusions
Our results suggest that ADs, and SSRIs in particular, may provide a degree of protection against COVID-19, specifically SARS-CoV-2 infection. This evidence lends some support to further investigation of drug repurposing as an complementary strategy to vaccination in appropriate contexts, especially considering the key advantages of ADs viz. well-characterised safety profile, low price, and ready availability. Identification of affordable and safe drugs that reduce the risk of COVID-19 is likely to be relevant for the global pandemic response, particularly in cases and situations where mass vaccination may be problematic.
In the longer term, there may be merit in investigating the utility of ADs/SSRIs for treatment of other respiratory infections using similar cell biological mechanisms to COVID-19, e.g. influenza [41]. For now, one can hope that the results from this study will contribute to the public health policy debate on COVID-19 management, help re-establish drug repurposing in the context of COVID-19 treatment and highlight the potential for wider clinical benefits of psychotropic drugs.
Acknowledgements
The authors are grateful to the CRIS team members at the Maudsley Biomedical Research Center for their invaluable help with data extraction, and to E. Barker for help with proofreading of the manuscript.
Abbreviations
- AD
Antidepressant
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CRIS
Clinical Record Interactive Search
- HoNOS
Health of the Nation Outcome Scale
- ICD-10
International Statistical Classification of Diseases and Related Health Problems (ICD), 10th revision
- MAOI
Monoamine oxidase inhibitor
- NHS
National Health Service
- NIHR
National Institute for Health and Care Research
- NLP
Natural language processing
- OR
Odds ratio
- PCR
Polymerase chain reaction
- SNRI
Serotonin and norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- SLaM
South London and Maudsley
- TCA
Tricyclic antidepressant
- WHO
World Health Organization
Authors’ contributions
Study concept: OOG, CM, DA. Study design: OOG, CM, GP. Project administration: OOG, CM, RS, DA, GP. Data extraction and verification: CM, RS, GP. Data analysis: OOG, GP. Manuscript writing and revisions: OOG, CM, RS, DA, GP. All authors read and approved the final manuscript.
Funding
OOG is funded by the Lewy Body Society and the National Natural Science Fund of China. RS, CM, DA, and GP are part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. RS is additionally part-funded by the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust, and the DATAMIND HDR UK Mental Health Data Hub (MRC grant MR/W014386). Funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Availability of data and materials
All data generated or analysed during this study are included in this published article (Tables 1, 2, 3, and 4).
Declarations
Ethics approval and consent to participate
The project was approved by the Research Ethics Committee of the Institute of Psychiatry, Psychology and Neuroscience, King’s College London (reference number 21–059 KHP).
Consent for publication
Not required.
Competing interests
RS declares research support received within the last 36 months from Janssen, GSK, and Takeda.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haug N, Geyrhofer L, Londei A, Dervic E, Desvars-Larrive A, Loreto V, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4(12):1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker EPK, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10(3):e326–e328. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Dashboard for Vaccine Equity [Internet]. UNDP Data Futures Platform. [Cited 5 Apr 2023]. Available from: https://data.undp.org/vaccine-equity/.
- 5.Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir Med. 2021;9(7):e63. doi: 10.1016/S2213-2600(21)00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glebov OO. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020;287(17):3664–3671. doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Wang F, Tang J, Nussinov R, Cheng F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit Health. 2020;0(0). 10.1016/S2589-7500. [DOI] [PMC free article] [PubMed]
- 9.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivermectin for preventing and treating COVID-19. [Cited 28 Jul 2022]. Available from: https://www.cochrane.org/CD015017/INFECTN_ivermectin-preventing-and-treating-covid-19. [DOI] [PMC free article] [PubMed]
- 11.Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Jannot AS, Neuraz A, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;4:1–14. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 12.Hoertel N, Sánchez-Rico M, Kornhuber J, Gulbins E, Reiersen AM, Lenze EJ, et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. J Clin Med. 2022;11(19):5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz BA, Hoerte N, Lenze EJ, FJalali, Reiersen AM. Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2. Transl Psychiatry. 2022;12(1):1–9. doi: 10.1038/s41398-022-02109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenze E. Fluvoxamine for early treatment of COVID-19: a fully-remote, randomized placebo controlled trial. clinicaltrials.gov; 2021 May [Cited 7 Jun 2021]. Report No.: NCT04668950. Available from: https://clinicaltrials.gov/ct2/show/NCT04668950.
- 15.Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021 [cited 2021 Jun 9];8(2). 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed]
- 17.Reis G, Moreira-Silva EA dos S, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2021 [cited 2021 Nov 8];0(0). Available from: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00448-4/fulltext. [DOI] [PMC free article] [PubMed]
- 18.Oskotsky T, Marić I, Tang A, Oskotsky B, Wong RJ, Aghaeepour N, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11):e2133090. doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19. N Engl J Med. 2022;387(7):599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy MW, Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Felker GM, et al. Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2023;329(4):296–305. doi: 10.1001/jama.2022.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fred SM, Kuivanen S, Ugurlu H, Casarotto PC, Levanov L, Saksela K, et al. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front Pharmacol. 2022 [cited 2023 Feb 16];12. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2021.755600. [DOI] [PMC free article] [PubMed]
- 22.Glebov OO. Low-dose fluvoxamine modulates endocytic trafficking of SARS-CoV-2 spike protein: a potential mechanism for anti-COVID-19 protection by antidepressants. Front Pharmacol. 2021;12:3466. doi: 10.3389/fphar.2021.787261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimniak M, Kirschner L, Hilpert H, Geiger N, Danov O, Oberwinkler H, et al. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci Rep. 2021;11(1):5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpinteiro A, Edwards MJ, Hoffmann M, Kochs G, Gripp B, Weigang S, et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep Med. 2020;1(8):100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera G, Broadbent M, Callard F, Chang CK, Downs J, Dutta R, et al. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open. 2016;6(3):e008721. doi: 10.1136/bmjopen-2015-008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis KAS, Broadbent M, Bishara D, Mueller C, Stewart R. Can medication mentions in CRIS be used for researching medication use in older people with dementia? Comparing the natural language processing app for medicines to GP prescribing. medRxiv; 2023 [cited 2023 Feb 21]. p. 2023.01.27.23285104. Available from: https://www.medrxiv.org/content/10.1101/2023.01.27.23285104v1.
- 27.ICD-10 Version:2019. [cited 2022 Aug 15]. Available from: https://icd.who.int/browse10/2019/en#/.
- 28.HoNOS. Healthy London Partnership. [cited 2022 Aug 11]. Available from: https://www.healthylondon.org/resource/outcome-measures/honos/.
- 29.Stata | FAQ: Citing Stata software, documentation, and FAQs. [cited 2022 Aug 15]. Available from: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/.
- 30.Tran BX, Ha GH, Vu GT, Nguyen LH, Latkin CA, Nathan K, et al. Indices of change, expectations, and popularity of biological treatments for major depressive disorder between 1988 and 2017: a scientometric analysis. Int J Environ Res Public Health. 2019;16(13):2255. doi: 10.3390/ijerph16132255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. [cited 2022 Aug 12]. Available from: https://www.who.int/news-room/questions-and-answers/item/herd-immunity-lockdowns-and-covid-19.
- 32.Ma Y, Li S, Yang H, Zhang Y, Li H, Xu F, et al. Effect of psychotropics on the risk of COVID-19 in middle-aged and older adults. Eur Neuropsychopharmacol . 2023;66:67–77. doi: 10.1016/j.euroneuro.2022.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemani K, Williams SZ, Olfson M, Leckman-Westin E, Finnerty M, Kammer J, et al. Association between the use of psychotropic medications and the risk of COVID-19 infection among long-term inpatients with serious mental illness in a New York state–wide psychiatric hospital system. JAMA Netw Open. 2022;5(5):e2210743. doi: 10.1001/jamanetworkopen.2022.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado M, Iskedjian M, Ruiz I, Einarson TR. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Curr Med Res Opin. 2006;22(9):1825–1837. doi: 10.1185/030079906X132415. [DOI] [PubMed] [Google Scholar]
- 35.Tummino TA, Rezelj VV, Fischer B, Fischer A, O’Meara MJ, Monel B, et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science. 2021;373(6554):541–547. doi: 10.1126/science.abi4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021 [cited 2023 Feb 16];12. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed]
- 37.Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One. 2017;12(10):e0186700. doi: 10.1371/journal.pone.0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci. 2021;5:1–10. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor R, Peyear TA, Koeppe RE, Andersen OS. Antidepressants are modifiers of lipid bilayer properties. J Gen Physiol. 2019;151(3):342–356. doi: 10.1085/jgp.201812263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creese B, Khan Z, Henley W, O’Dwyer S, Corbett A, Silva MVD, et al. Loneliness, physical activity, and mental health during COVID-19: a longitudinal analysis of depression and anxiety in adults over the age of 50 between 2015 and 2020. Int Psychogeriatr. 2021;33(5):505–514. doi: 10.1017/S1041610220004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020 [cited 2022 Aug 12];55(4). Available from: https://erj.ersjournals.com/content/55/4/2000607. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article (Tables 1, 2, 3, and 4).