Abstract
The emergence of human severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses significant challenges to global public health. Despite the extensive efforts of researchers worldwide, there remains considerable opportunities for improvement in timely diagnosis, specific treatment, and effective vaccines for SARS-CoV-2. This is due, in part, to the large number of asymptomatic carriers, rapid virus mutations, inconsistent confinement policies, untimely diagnosis and limited clear treatment plans. The emerging of nanozymes offers a promising approach for combating SARS-CoV-2 due to their stable physicochemical properties and high surface areas, which enable easier and multiple nano-bio interactions in vivo. Nanozymes inspire the development of sensitive and economic nanosensors for rapid detection, facilitate the development of specific medicines with minimal side effects for targeted therapy, trigger defensive mechanisms in the form of vaccines, and eliminate SARS-CoV-2 in the environment for prevention. In this review, we briefly present the limitations of existing countermeasures against coronavirus disease 2019 (COVID-19). We then reviewed the applications of nanozyme-based platforms in the fields of diagnostics, therapeutics and the prevention in COVID-19. Finally, we propose opportunities and challenges for the further development of nanozyme-based platforms for COVID-19. We expect that our review will provide valuable insights into the new emerging and re-emerging infectious pandemic from the perspective of nanozymes.
Graphical Abstract
Keywords: Nanozymes, COVID-19, SARS-CoV-2, Nanomedicine
Highlights
Nanozyme-based platforms have demonstrated improved sensitivity for SARS-CoV-2 antigen detection, reduced cost, and facilitated rapid diagnosis, making them a promising tool for COVID-19 diagnostics.
Nanozyme-based platforms offer potential benefits in targeted therapy for COVID-19 due to their ability to specifically counteract and eliminate SARS-CoV-2 with potentially fewer side effects.
Nanozyme-based platforms have been shown to act as immunostimulatory molecules, activating the defense response of the immune system against SARS-CoV-2, potentially providing a therapeutic avenue for COVID-19 treatment.
Introduction
As of May 22, 2023, over 765 million individuals have been affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in over six million confirmed deaths. SARS-CoV-2 is extremely infectious and can be transmitted between people within six feet [1]. At the present stage, most infections are mild or moderate and may have cough, fever, headache, nasal congestion and shortness of breath [2]. However, long-term SARS-CoV-2 infection will inevitably produce sequelae, chest radiographic abnormalities, impaired pulmonary diffusion capacity or depression symptoms [3]. Once an elderly patient who normally has underlying diseases is infected with SARS-CoV-2, the "cytokine storm" in the body after infection may lead to acute respiratory distress syndrome (ARDS), severe sepsis or septic shock, and even multiorgan dysfunction [4]. In response to the coronavirus disease 2019 (COVID-19) epidemic caused by SARS-CoV-2, there is still large amounts of works that need to be done. (1) Rapid diagnostic methods are still lacking [5]. Nucleic acid-based testing recommended by the World Health Organization (WHO) is time-consuming and complicated [6]. (2) Current treatments or medicines are not targeted []. Many of the approved drugs are aimed primarily at symptom relief [7]. (3) Effective and secure COVID-19 vaccines remain urgently needed [8]. At this stage, SARS-CoV-2 continues to mutate to obtain stronger infectivity [9]. In particular, Omicron variants can escape the protection of developed antibodies. In such a complex scenario, there is a greater urgency to develop rapid detection capability as well as to deploy targeted therapies and create more effective vaccines to prevent the progression of COVID-19 [10].
Enzymes, with significant catalytic activity, control the basic metabolic and life-sustaining activities of biological systems on the earth [11]. Most natural enzymes are spherical proteins or bioorganic molecules, which are easily affected by environmental factors such as temperature, pH or humidity [11]. Nanozymes are particular nanomaterials with inherent mimetic enzyme properties, combining the strengths of nanomaterials and natural enzymes [12]. Nanozymes exhibit higher stability, tunable catalytic activity, multienzyme activity, smart response and self-assembly capability [13]. To date, a great number of nanozymes have been developed to imitate the natural ones, such as peroxidase (POD), oxidase (OXD), catalase (CAT), and superoxide dismutase (SOD) [14]. In recent years, nanozymes have shown great application prospects in biomedicine, such as antimicrobial activity, biosensing, disease detection and cancer treatment [15].
In the past few decades, nanozymes have shown their great effectiveness in fighting against various viruses. Duan et al. utilized Fe3O4 magnetic nanoparticles (MNPs) as a nanozyme probe to detect Ebola virus [16]. The sensitivity of the nanozyme strip is 100 times higher than that of the standard strip method, and it is much faster and simpler. Qin et al. targeted the lipid envelope of influenza virus and destroyed the integrity of neighboring proteins by using iron oxide nanozymes (IONzymes), causing its inactivation [17]. Inspired by the wide antiviral applications of nanozymes, many scholars believe that nanozymes have great potential in treating SARS-CoV-2 infections [18]. (1) Nanozyme-based platforms can improve the sensitivity of antigen testing of SARS-CoV-2, reduce the cost and be conducive to rapid diagnosis. (2) Nanozyme-based platforms can assist in targeted COVID-19 treatment, specifically by resisting and clearing SARS-CoV-2 and producing fewer side effects. (3) Nanozyme-based platforms may act as immunostimulatory molecules to activate the defense responses of the immune response to SARS-CoV-2. However, few reviews have summarized the possible contribution of nanozymes in fighting against COVID-19 (Fig. 1).
Fig. 1.
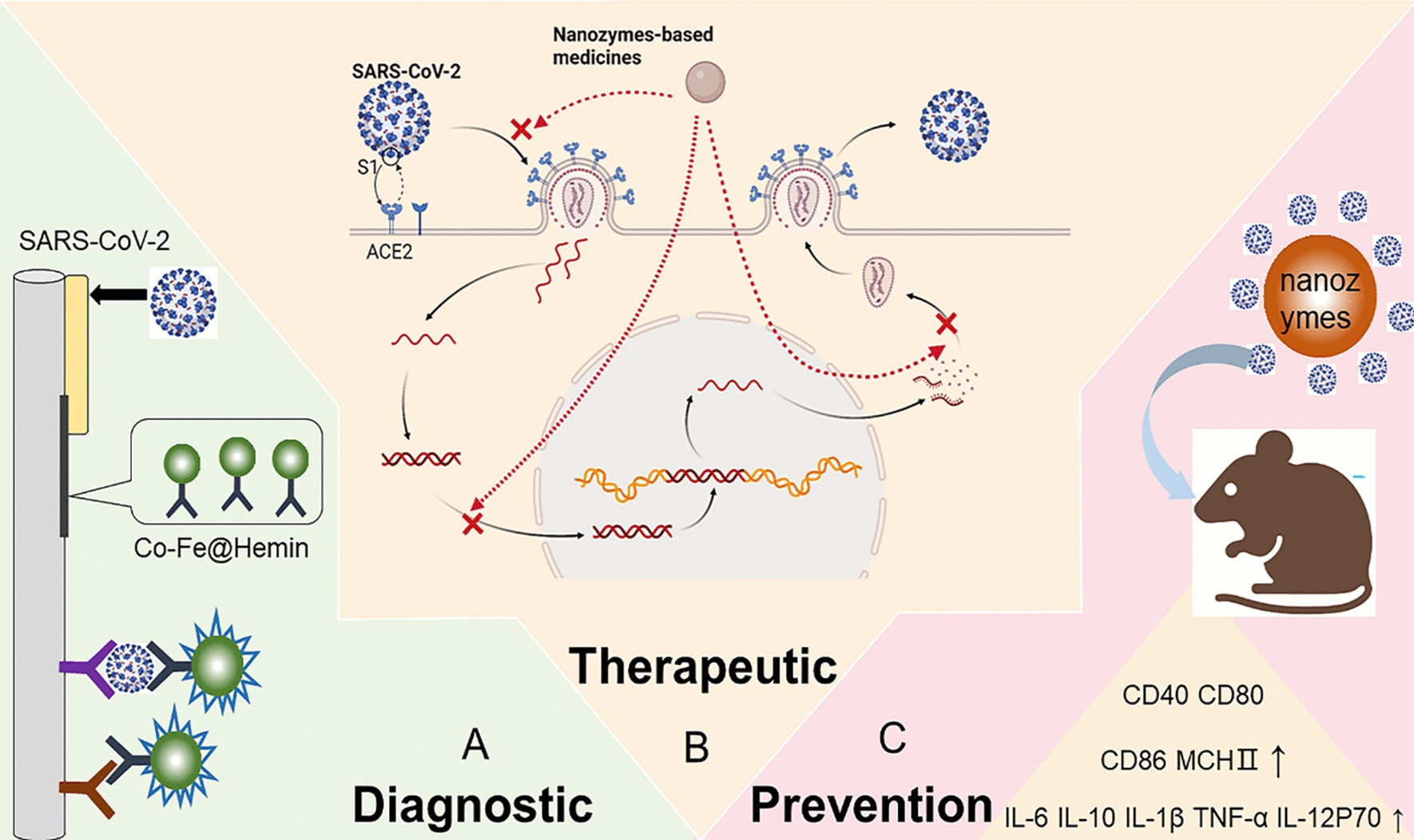
Summary of nanozyme-based platforms in combating COVID-19. A Nanozyme chemiluminescent paper assay for SARS-CoV-2 antigen in diagnosis. B Possible mechanism of nanozyme-based medicines preventing SARS-CoV-2 infection in therapeutic. C Nanozyme‐based mucosal vaccines are necessary to prevent COVID-19. Created with BioRender.com
In this review, we discussed the limitations of existing countermeasures in combating COVID-19. Moreover, we reviewed the applications of nanozyme-based platforms in the fields of diagnosis, treatment and prevention of COVID-19 and their safety considerations. Finally, we proposed opportunities and challenges for the further development of nanozyme-based platforms for COVID-19. We expect that our paper will put forward new opinions in facing the COVID-19 pandemic from the perspective of nanozymes (Table 1).
Table 1.
Application of nanozymes in COVID-19
| Nanozymes | Catalytic Activity | Application | References |
|---|---|---|---|
| Co–Fe@hemin | Peroxidase-like activity | Rapid and sensitive detection of SARS-CoV-2 antigen | [29] |
| Au@Pt | Peroxidase-like activity | Colorimetric detection of spike protein of SARS-CoV-2 | [47] |
| FeS2 | High peroxidase-like activity | Rapid and ultrasensitive nucleic acid detection of SARS-CoV-2 | [123] |
| MnO2 | Oxidase-like activity | Point-of-care testing of SARS-CoV-2 | [124] |
| Au@Pt | Peroxidase-like activity | Quantitative detection of SARS-CoV-2 nucleocapsid protein | [125] |
| Pt@Au | Peroxidase-like activity | Quantitative detection of SARS-CoV-2 | [36] |
| Pd-Ir | Peroxidase activity | Nucleocapsid protein from SARS-CoV-2 | [126] |
| Ag-TiO2 | Peroxidase-like activity | Inhibition of SARS-CoV-2 entry into cells | [127] |
| MCeC@MΦ | Superoxide dismutase and catalase-like activities | Treatment of cytokine storm caused by COVID-19 | [128] |
| CuNPs | Photo-activity | Confers self-sterilizing ability and reusability to masks | [116] |
| TiO2 | Photo-activity | Clears SARS-CoV-2 from the surface of an item | [129] |
Current diagnosis and nanozyme-based diagnosis of COVID-19
Current testing for SARS-CoV-2
When a new infectious pathogen appears, quick and precise diagnosis is considered to be the primary requirement for quickly tracking and isolating cases [19]. Early diagnoses with good sensitivities and high accuracies are very important when COVID-19 is spreading throughout an area, but practical detection options for the identification of SARS-CoV-2 are somewhat limited. There are three main ways to detect SARS-CoV-2 infections: nucleic acid detection, antibody detection, and antigen detection [20].
Principles of current detection
Among these techniques, the reverse transcription-polymerase chain reaction (RT‒PCR) method in nucleic acid detection is the gold standard for early diagnosis of COVID-19 [21]. Viral RNA is extracted from a sample of a person’s nose or throat after treatment with chemical solutions [22]. Reverse transcriptase and primers will reverse-transcribe the viral RNA to single-stranded complementary DNA (cDNA) [23]. Then, DNA polymerase extends the second strand, generating double-stranded DNA. DNA is amplified continuously in the RT‒PCR machine. Organic dyes are connected with the DNA strand and release fluorescence. When the fluorescence exceeds a certain level, the patient is considered to be contracted with SARS-CoV-2. It was reported that when diagnosing SARS-CoV-2, the sensitivity of PCR can reach 0.14 copy/μL [24], and the specificity can reach 96–100%. Antibody testing is another way to diagnose COVID-19 [25]. Antibodies are derived from the immune system as it combats SARS-CoV-2, suggesting that the body is fighting pathogens [26]. The detection of antibodies can be achieved by serologic testing by measuring the IgG and IgM levels in blood or plasma, which are produced by neutralizing virus antigens [27]. IgM is the first antibody to appear as a first immune response, suggesting a recent infection, and can be used as an aid in the diagnosis of early infection. IgG is produced later, as it lasts longer and is mostly used as an indicator of previous or subsequent infections [28]. Seroconversion kinetics vary considerably between antibody detection kits. In summary, the serum sample and sample diluent are added to the test strip, left flat at room temperature for about 10 min and the results are then evaluated according to the colour of the test and control lines [28]. The antigen test is based on the recognition of SARS-CoV-2 proteins and is used to identify the active replication virus in the early stages of infection. Antigen testing methods utilize techniques similar to serological methods, using enzyme-linked immunosorbent assays (ELISA) or chemiluminescent immunoassays [5]. As point of care devices, antigen testing methods can be conducted by minimally trained personnel in various primary and even community environments, with test results provided within 5–30 minutes and obtainable in a single clinical contact.
Limits of the current diagnosis scheme
Although nucleic acid detection is greatly encouraged by the WHO, it requires biosafety laboratories, professional instruments and skilled experts [18]. Moreover, it takes at least 1–2 hours to complete RNA extraction, reverse transcription, gene amplification and data analysis [29]. Hence, nucleic acid detection is not suitable for on-site and real-time screening [30]. At the same time, nucleic acid detection is very difficult for many poor neighborhoods or health caring systems, particularly in developing nations, due to the high cost.
Regarding antibody detection, the results may be false-positive due to cross-reactivity when the antibody is bound to an antigen that is distinct from the targeted antigen [31]. The reason for the cross reaction is the similarity between the molecules. Antibodies are only produced after at least 10 days of virus infection [32], so this general late response may lead to false-negatives [33]. Negative results of antibody tests may not confirm that the patient is not infected [34]. At the same time, serological testing as an invasive method is not convenient and not suitable for rapid detection at all. It appears that serologic tests are better suited for extensive screening of patients with infection but are not suitable for early diagnosis of COVID-19 [35].
Compared with nucleic acid testing or antibody testing, antigen detection can detect the virus itself, which is convenient for large-scale population screening [36]. However, most antigen tests can provide only qualitative results, not quantitative results [37]. ELISA based on antigen determination is very time-consuming and has complex washing and incubation steps [38].
It is necessary to control the incidence of COVID-19 by quick diagnosis of emerging patients [39]. There is still an urgency to have a simpler, faster, more cost-effective and more reliable approach for SARS-CoV-2 antigen identification [40].
Nanozyme-based detection of SARS-CoV-2
Nanozymes with high surface volume ratios enable them to have large reservoirs and abundant anchoring sites to load and deliver agents, so they can diagnose various virus infections quickly, sensitively and in real time [41]. It may be a good idea to apply nanozymes to the rapid diagnosis of COVID-19 in antigen diagnosis.
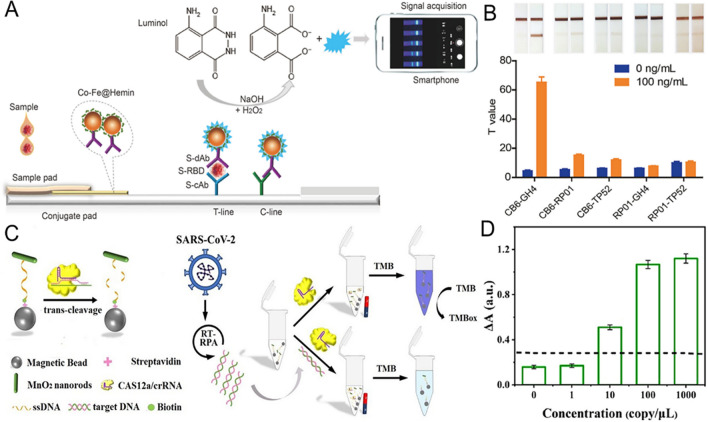
Nanozymes for chemiluminescence paper test
Traditional antigen detection is mainly based on colloidal gold immune assays or fluorometric immunochromatographic assays [42]. Liu et al. investigated a novel chemiluminescence paper using nanozymes for rapid and highly responsive determination of SARS-CoV-2 spike antigen [29] (Fig. 2A, B). Compared with traditional colloidal gold or fluorescent test paper and nucleic acid tests, this nanozyme chemiluminescence paper has the following advantages: (1) The key point of this test paper is a powerful Co-Fe@heme peroxidase nanoprobe, which promotes chemiluminescence and amplifies the immune reaction signal. Chemiluminescence signal amplification will improve the accuracy of the paper-based assay and reduce the incidence of false-negatives in earlier screening for SARS-CoV-2 disease. (2) The time taken for testing can be accomplished in less than 16 min, which is much faster than the nucleic acid test. (3) Importantly, they use Co–Fe@heme peroxidase nanozymes to replace natural horseradish peroxidase (HRP), which is the central material in conventional chemiluminescent immunodiagnostics [43]. Natural enzymes such as HRP are high-cost, unstable and complex to produce [44]. By comparison, Co–Fe@heme nanozymes are considerably more vigorous in hot and alkaline environments. Therefore, nanozyme chemiluminescent testing strips can be stably kept at environmental temperatures, which is conducive to their transportation and field application. (4) Nanozymes are relatively inexpensive and can be synthesized from easily available materials, while naturally occurring HRP needs complicated extraction and purification. As a result, the general cost of nanozyme-based chemiluminescent test paper is comparatively lower, which can greatly reduce the financial burden on national health care. This study is susceptible to errors from both reconstituted antigens and pseudoviruses, so it is important to further validate the accuracy of pharyngeal swab or nasal swab samples from infected persons. Parallel comparisons with other commercial kits for large-scale application are needed.
Fig. 2.
Nanozyme-based detection of SARS-CoV-2. A Schematic illustration of the Co–Fe@hemin nanozyme chemiluminescent paper test for SARS-CoV-2. B Screening of paired antibodies using a Co–Fe@hemin nanozyme colorimetric strip. A positive signature was rated by 100 ng/mL of S-RBD protein. C A protocol for detecting SARS-CoV-2 using the CRISPR‒Cas12a system based on the ssDNA-MnO2-MB reporter. D Detecting differing levels of SARS-CoV-2 E pseudovirus based on the RPA-conjugated MnO2 nanozyme-mediated CRISPR‒Cas12a system.
(Copyright 2020. Biosens Bioelectron, Copyright 2022, ACS Appl Mater Interfaces)
Nanozymes for colorimetric detection
Colorimetric assays have potential applications in detecting SARS-CoV-2. Bartolomeo et al. utilized gold nanoparticles (NPs) to cause a change in the extinction spectra of the relevant solutions within just a few minutes to obtain a rapid and credible determination of SARS-CoV-2 [45]. In addition, studies have shown that the addition of different metals to metal NPs to build polymetallic nanostructures contributes to an improvement in enzyme activities, so developing polymetallic nanozymes has become an efficient tactic [46]. Zhao et al. utilized the brilliant POD catalytic action of polyclonal antibody-linked Au@Pt NPs to enable efficient and sensitive colorimetric analysis of the spike-in (S1) protein of SARS-CoV-2 [47]. They prepared homogeneous and monodispersed gold nanoparticles as their seeds and then added platinum atoms into the gold nanoparticles, which led to considerable modification of the enzymatic catalytic pathways from the production of reactive oxygen species (ROS) to the process of rapid electron transfer (ET). With the benefits of being fast, simple to handle and highly flexible, colorimetric assays based on Au@Pt nanoparticles can assist in the quick diagnosis of COVID-19. Their peroxidase catalysis is markedly strengthened by the multiporous nanostructures and electronic-rich Pt envelope of the Au@Pt NPs. The results of this experiment will contribute to the understanding of the catalytic mechanisms of nanozymes and the development of colorimetric biological sensors for actual diagnostics on the basis of metal NPs.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) based testing systems have been used to detect nucleic acids [48–52], proteins [53–55], and small molecules [56–58]. Fluorescent assays are often used in conjunction with CRISPR‒Cas. Wu et al. built a nanozyme-mediated colorimetric detection method for SARS-CoV-2 using a CRISPR‒Cas12a-based system [59] (Fig. 2C, D). Manganese dioxide nanostrips were used as a reporting tool on the basis of oxidative enzyme-like activities. Active Cas12a mediates the reverse fragmentation of single-stranded DNA linkers between MnO2 nanorods and label magnetic beads in the presence of SARS-CoV-2, which reduces the oxygenation of tetramethylbenzidine, triggering color variation. The application of MnO2 nanozymes in place of naturally occurring enzymes allows the usage of H2O2 to be avoided, making it cost-effective and simple to operate. In addition, chromatographic analyses based on nanozymes and CRISPR‒Cas12a systems allow the measurement to be easily manipulated and are suitable for high volume microtiter-based analyses. The MnO2 nanozyme-guided CRISPR‒Cas12a system could be used as an outstanding tool for testing SARS-CoV-2.
Nanozyme-linked immunochromatographic sensor for quantitative detection
Liang et al. developed a nanozyme-linked immunochromatographic sensor (NLICS) for the quantification of SARS-CoV-2 nucleocapsid protein antigens in human blood samples [60]. Au@Pt NPs, the nucleocapsid protein antigens in serum samples, the primary specific monoclonal antibodies (mAb1) and the secondary mAb2 form a sandwich construction (Au@PtNPs-mAb1-nucleocapsid protein-mAb2). Finally, the base material solution undergoes a calorific reaction mediated by the intercalated structure. The light filtered through the reaction matrix was measured by a light meter, and the outcome was transferred simultaneously to a microphone via a Bluetooth connection for data processing and analyses. This will be important for the prompt identification of suspected cases of SARS-CoV-2 infection, particularly at the early phases of virus exposure. Compared with other antigen detection methods, this is a quantitative detection method, and the diagnosis of infected people will become more accurate. It provides a fresh idea for the highly sensitive and quantitative diagnosis of COVID-19.
Current therapeutics and nanozyme-based therapeutics against COVID-19
Current medicines and treatment schemes against COVID-19
Medicines
The following categories of medicines are being investigated or are in development for the control of COVID-19: antiviral medicines (such as remdesivir and favepiravir), anti-inflammatories (dexamethasone and statins), antibodies (such as convalescent plasma and hyperimmune immunoglobulins), targeted immunomodulatory therapies (such as tocilizumab, sarilumab, anakina and ruxolitinib), anticoagulants (such as heparin), and antifibrotic medicines (such as tyrosine kinase inhibitors) [61]. Pfizer declared in November 2021 that Paxlovid taken within the first few days of symptom onset resulted in an 89% reduction in hospitalisation rates in high-risk patients [62].
Treatment schemes
Virus inhibition is the most effective measure in the earlier stage of the infection. Hydroxychloroquine is hypothesized to suppress viral entrance and endocytosis in vitro and may produce favorable effects of immunomodulation in vivo [63]. Lopinavir, an antiretroviral protease inhibitor, has shown some antiviral activities against SARS-CoV-2 [64]. Other antiviral medicines that may be used in COVID-19 are also being explored, including famotidine, favipiravir, arbidol and camostat (TMPRSS2 inhibitor) [65]. In the later stage, alternative therapeutic strategies are necessary; for example, immunomodulators may help to protect against progression of the disorder, and anticoagulants may be helpful to avoid comorbidities. Glucocorticoids can alleviate inflammation-mediated lung injury, thereby reducing the risk of progressing to respiratory failure and death. Dexamethasone has a greater survival benefit for COVID-19 patients who are recruited after the first week of their illness or received respiratory support [66]. It has been reported that therapy with methylprednisolone is also connected with decreased mortality risk. Taking monoclonal antibodies that target key inflammation mediator interferons such as beta-1b is conducive to alleviating symptoms, shortening hospital stay and preventing organ damage with mild to modest COVID-19 [67]. More than 75% of inpatients with COVID-19 require supplemental oxygen therapy. Alternative approaches that are being explored include subcutaneous low original weight heparin, nursing home plasma-derived super immunoglobulin and monoclonal antibodies against SARS-CoV-2 [68, 69].
Limits of current therapeutics
In the context of the quick spread of SARS-CoV-2 and the growing demand for a rapid response, a globally recognized specific treatment is still lacking. The main goal of current medicines is to control excessive inflammatory and immune reactions in serious conditions [70]. Most of these medicines are administered systemically, resulting in multiple medicine interactions. At the same time, these medicines are accompanied by many adverse reactions [71]. For instance, adverse reactions are higher in patients treated with hydroxychloroquine, and the most common is diarrhea [72]. Lopinavir can also induce hepatic injury [73]. Remdesivir has a broad spectrum of antiviral activities, including against coronaviruses and filoviruses. However, the actual drug delivery concentration that reaches the lungs, and more precisely, the host infected cells, is insignificant. With regard to Paxlovid, it must be taken immediately after the onset of symptoms. More importantly, its availability to low-income settings is very poor, making for huge differences in treatment opportunities [62, 74]. Therefore, with the rapid growth of the COVID-19 incidence rate, we urgently need to find more accurate treatment methods or medicines. More targeted drugs can not only reduce unnecessary drug consumption but also reduce side effects.
Nanozyme-based treatment against COVID-19
As with most nanomaterials, nanozymes are also used as treatment options for diseases, such as serving as drug carriers or passively targeting diverse antiviral treatments [75]. Various forms of nanozymes possess considerable potential for antiviral applications [76]. A novel cold-adapted nanozyme based on a manganese-based nanosized metal-organic framework is developed to inactivate influenza virus H1N1 even at −20 °C. Multiwall and single-wall carbon nanotubes, carbon dots and carbon nanodiamonds both have the potential to directly suppress virus reproduction [77]. Due to the benefits of both natural enzymes and nanomaterials, nanozymes have become a good choice for the treatment of COVID-19.
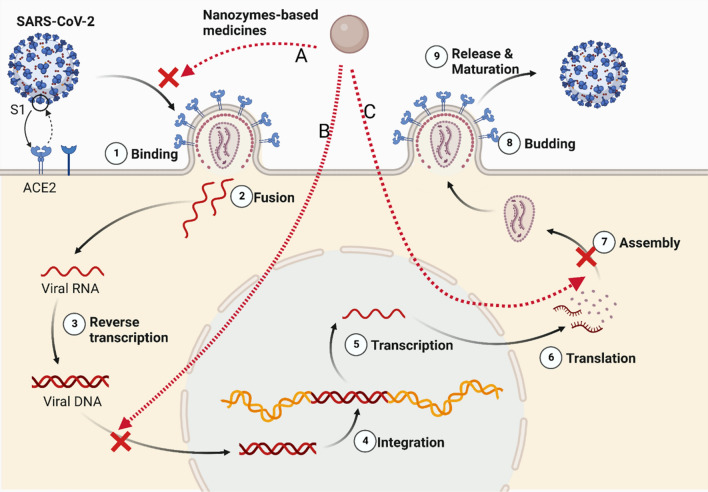
The structural proteins of SARS-CoV-2 are composed of nucleocapsid protein (N), membrane protein (M), envelope protein (E), and spike protein (S). [78]. When SARS-CoV-2 comes into contact with the host cell, the S glycoprotein binds to the membrane receptor angiotensin-converting enzyme 2 (ACE2) and enters the host cell through endocytic action [79]. The S protein is cleaved into S1 and S2 subunits during their biosynthesis in the infected cells. The S1 fraction is involved in the recognition and adhesion of the cell membrane, and the S2 fraction is used for membrane fusion and entry into host cells [80]. Therefore, the inhibitory effect of nanozymes on endocytosis will become a strategic goal to inhibit SARS-CoV-2 infection. After the fusion of the virus envelope and endoplasmic membrane, the virus genome is liberated to the cytoplasm and transformed into RNA-dependent RNA polymerase (RdRp) for gene replication. The inhibition of replication by nanozymes following virus entry into host cells is also considered to be an effective therapeutic target (Fig. 3).
Fig. 3.
Possible mechanism of nanozyme-based medicines preventing SARS-CoV-2 infection. A Inhibiting the endocytosis of SARS-CoV-2 by nanozymes. B Blocking SARS-CoV-2 replication by nanozymes. C Blocking SARS-CoV-2 assembly and proliferation by nanozymes. Created with BioRender.com
Due to reasons related to existing at the nanoscale, most nanozymes may have high affinity for virus attachment. Strong nano-bio interactions can recognize virus structures in situ. Nanozymes can interact with viral structures and directly inactivate or destroy them without harming host cells, which will minimize their side effects and degradation [81, 82]. Compared to traditional drugs, nanozyme agents typically have better biological distribution and increase accumulation in the target position, thus showing higher treatment effectiveness and fewer secondary effects [83].
Ag-TiO2 single-atom nanozymes for inhibiting SARS-CoV-2 entry
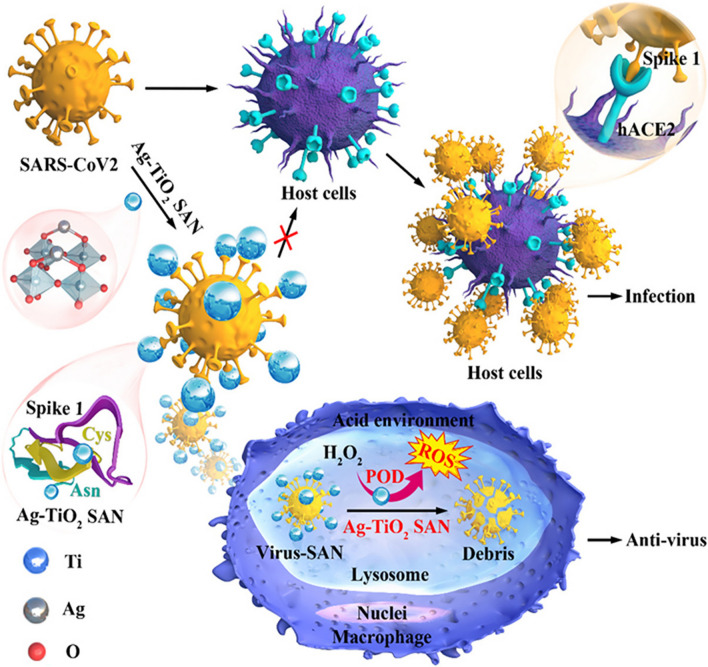
Wang et al. successfully designed Ag-TiO2 single-atom nanozymes (SANs) with significant inhibition of SARS-CoV-2 entry activities (Fig. 4). The SAN Ag atom is tightly bound to both cysteine and asparagine, which are the two most enriched amino acids on the receptor binding domain (RBD) of the spike 1 protein of SARS-CoV-2, thereby inhibiting the interaction between the S1 RBD and its receptor ACE2 [84]. Then, the SAN/virus compound will be engulfed by macrophages and aggregated with lysosomes. Ag-TiO2 SAN initiates ROS production via POD-like activity to efficiently kill viruses. It can efficiently promote virus phagocytosis by macrophages in vivo and exhibit enhanced uptake with up to 99.65% for SARS-CoV-2 pseudovirus in vitro. Ag-TiO2 SAN can specifically resist and clear SARS-CoV-2 through its strong adsorptive property and POD-like activity, making up for the shortcomings of many existing broad-spectrum medicines. Ag-TiO2 SAN enables gram-level synthesis of a new SAN to combat and scavenge SARS-CoV-2, but it is used only in pseudoviruses of viral elimination models. Further verification of accuracy for real clinical samples is also needed.
Fig. 4.
Schematic diagram of Ag-TiO2 SAN against SARS-CoV-2. The SAN Ag atom is tightly bound to both cysteine and asparagine, inhibiting the interaction between the S1 RBD and its receptor ACE2.
(Copyright 2021, Nano Today)
V2O5 nanozymes for inhibiting virus replication
Viral infection and replication are closely associated with oxidative stress. Singh et al. used vanadium pentoxide (V2O5) nanozymes to imitate natural glutathione peroxidase activity in function, catalyzing ROS neutralization in virus-infected cells to mitigate ROS and uniformly block viral reactivation and replication without adverse effects on cell physiology [85]. Meanwhile, increased levels of reactive oxygen or nitrogen species content are a distinctive characteristic of inflamed tissue. Nanozymes ROS scavenging ability can help the infected body relieve inflammation. The system is mainly applied to HIV‐infected individuals, and its use in SARS-CoV-2‐infected individuals is just around the corner. Researchers successfully revealed the potential usefulness of V+2O5 nanosheets against SARS-CoV-2 and suggested nanozymes as future platforms to develop interventions against COVID-19.
Nanozymes for the cytokine storm in COVID-19
During infection, excessive immune responses may cause overwhelming immune system impairment in the human body [86–88]. High levels of inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) and chemokines (C–C motif chemokine ligand (CCL)-2, CCL-3, and CCL-5) did not give good results in patients with poor outcomes [89–91]. Cytokine storms are the major contributor to ARDS in patients during COVID-19 infection and are an essential factor leading to COVID-19 aggravation or even death [4]. The decoy nanozymes, MCeC@MΦ, consist of mesoporous silica nanoparticle cores loaded with CeO2 nanocatalyst and Ce6 photosensitizer and biomimetic shells of macrophage membrane. The intense expression of cell membrane receptors in MCeC@MΦ allows the binding of decoy nanozymes to endotoxins or proinflammatory cytokines, which neutralizes endotoxin, sequesters proinflammatory cytokines and provides protection against overactivation of host immune cells [92]. At the same time, its superoxide dismutase, catalase-like activity and hydroxyl radical antioxidant capacity can be used to catalyze and remove multiple ROS in the body. MCeC@MΦ can significantly reduce the systemic hyperinflammatory response and rapid salvage of organ damage within 1 day in multidrug-resistant Escherichia coli bacteremia mice. This might open the possibilities of treating cytokine storms and immune-mediated inflammatory diseases caused by COVID-19.
Current prevention and nanozyme-based prevention of COVID-19
Epidemiological data show that SARS-CoV-2 is mainly transmitted through airway splashing during intimate face-to-face exposure, such as talking, coughing or sneezing [93]. Other propagation modes of COVID-19 include aerosol propagation and contact surface propagation [94]. Compared to SARS-CoV-1, SARS-CoV-2 is extremely well stabilized in aerosols and on interfaces, remaining virally infectious for several hours [95].
Current prevention measures for COVID-19
Daily defensive measures
Numerical modeling research and empirical data suggest that increasing the strength of public health care intervention can reduce the transmission rate of COVID-19 around the world [96–98]. Public health interventions include wearing masks and restricting crowd aggregation in public places, enforcing social distancing and implementing centralized isolation after infection [97]. If a positive infection is found, the community should immediately expand the monitoring scale, implement contact tracing and isolate the infection to block the transmission chain [99]. However, the public health measures adopted by different countries may vary because of the influence of demographic, political, regional and other influencing factors [100]. Some countries may face challenges in effectively managing the COVID-19 pandemic due to the lack of medical resources. Moreover, only daily protection is not enough to control the transmission rate. The most important part of daily protective measures is wearing masks. Personal protective equipment, especially mask, is a key component of the strategies to prevent SARS-CoV-2 [101]. However, discarded disposable masks may become a source of rapid transmission of the virus. Meanwhile, the disposal and decontamination of masks have brought great environmental impacts and economic burdens [102].
Herd immunity and vaccines
With the COVID-19 pandemic, many countries have put forward the concept of “herd immunity” [103]. Herd immunity is achieved when a certain threshold of immunity at the population level is reached [104]. When the threshold of immunity is high enough and this threshold is met (whether through natural infection or vaccination), it can protect most people in a specific geographical region within a given time period and can break the chain of transmission of specific contagious diseases, in theory [105]. Specifically, herd immunity can be realized when the effective reproduction number R drops below 1 without any intervention, which means that an infected person in a group generates less than one secondary case on average[106].
Effective vaccination is the best way to accelerate herd immunity and eliminate epidemics without causing more deaths [107]. Recently, different types of vaccines have been authorized for worldwide clinical use to combat the COVID-19 pandemic [108]. COVID-19 is transmitted through the respiratory tract, and the mucosal immune response plays a part in the primary line of protection in the battle against pathogens, so mucosal vaccines have attracted the attention of many scholars in recent years [109]. Mucosal vaccines trigger robust mucosal and systemic immunity reactions at the major sites of viral exposure [110]. However, mucosal vaccine preparations need to ensure the immune availability and immune stimulation ability of vaccine antigens on the mucosal surface. Nasal barriers often obstruct the transmission of antigens to submucosal antigen presenting cells (APCs)‐dendritic cells (DCs) and restrict the subsequently mature level of DCs to activate protective adaptive immunity [111, 112]. An efficient antigen delivery system should be developed to protect the antigen of mucosal vaccines from degradation and clearance.
Nanozyme-based prevention measures of COVID-19
CS‐IONzyme for mucosal vaccines
Nanozymes provide an ideal antigen delivery platform for vaccines, acting as adjacent platforms and mimicking virus structures, and they allow multivalent antigen presentation and antigen stability after administration [113]. Nanozymes can serve as adjuvants to enhance the immune response and initiate the defense system against SARS-CoV-2 to increase vaccine efficacy [114]. Nasal-based vaccines are a promising mucosal vaccine strategy for virus protection. Qin et al. developed a chitosan (CS)-functionalized iron oxide nanozyme (IONzyme)-based influenza whole inactivated virus (WIV) nasal vaccine [115]. CS‐IONzyme first increased the adherence level of H1N1 WIV to nasal mucosa and then vigorously motivated nasal epithelial cells to excrete the chemokine CCL20 while recruiting more dendritic cells to the submucosal area to form transepithelial dendrites (TED) for H1N1 WIV ingestion. Finally, dendritic cells complete antigen presentation and initiate the specific immune response. The adhesion of antigen to nasal mucosa was increased by 30 times, and the IgA-mucosal adaptive immunity was increased 8.9-fold. The CS-IONzyme-based nasal vaccine provided 100% protection against influenza with enhanced POD-like activity. Host infection with SARS-CoV-2 also occurs first through mucosal infection, so mucosal vaccines based on nanozymes are a new idea for COVID-19 vaccines. The study offers a prospective antiviral option for the design of IONzyme-based nasal vaccines to combat SARS-CoV-2.
Cu-nanozymes for photoactive antiviral face masks
Kumar et al. sprayed a mixture of copper and shellac nanoparticles onto a nonwoven medical surgical mask, conferring self-sterilizing and reusability ability to the masks [116]. CuNPs enhanced the hydrophobicity of the mask and repelled water droplets, resulting in the mask exhibiting excellent photoactivity for antimicrobial action. Relying on the strong photocatalytic properties of copper nanozymes, these masks have fast and efficient bactericidal effects on pathogens. Under the sun, through photocatalytic and photothermal effects, masks can produce high levels of free radicals that disrupt the virus membrane, allowing the masks to be self-cleaning and reusable. These masks can prevent the spread of virus particles, provide important protection against the COVID-19 epidemic and contribute considerably to the abatement of financial expenses and environmental impacts (Fig. 5).
Fig. 5.
Cu-nanozymes for photoactive antiviral face masks. The manufacturing principle of the self-cleaning mask was as follows: the nebulizer was conceived to intermix CuNPs, shellac and pressurized air at the intersection. After solar irradiation, through photothermal effect and photocatalytic effect processes, the mask inactivates the virus and becomes self-cleaning. Created with BioRender.com
Nanozyme-based surface coating against surface contact propagation
SARS-CoV-2 particles can remain on surfaces for a long time, which provide another route of transmission: surface contact [117]. Nanozymes can be sprayed onto various surfaces to improve the antiviral effectiveness of protective layers [118]. Some studies have explored the use of nanozymes as a means of decontaminating surfaces and reducing the spread of the virus. For example, photoactivated titanium dioxide successfully inactivated a wide spectrum of pathogens, including SARS-CoV-2, under a light irradiance level (0.4 mW/cm2 at a wavelength of 375 nm). Mechanistic research has shown that light-induced hydroxyl radicals target the viral genome, resulting in viral disintegration, instead of attacking the virion global structure and content [119]. Similarly, silver, gold, and copper nanozymes all have excellent abilities to damage viruses. Coronaviruses have been previously reported to remain viable on the object surface for up to 7 days, but SARS-CoV-2 viruses can only survive on copper surfaces for no more than 4 h [120]. Due to their increased contact with microbes by virtue of their small sizes, nanozymes are immensely effective for antiviral applications. The development of virus-resistant surfacing materials would be a powerful tool to protect against the transmission of COVID-19.
Challenges of nanozymes in combating COVID-19
Despite nanozymes having numerous benefits for combating SARS-CoV-2, including high physical and chemical stability, excellent durability and simplicity, many challenges still remain.
One of the main challenges is the lack of knowledge about the underlying mechanisms of nanozymes drugs action with COVID-19. Additional studies are required to fully capture the biological and biochemical interactions between nanozymes and SARS-CoV-2 as well as the potential side effects and toxicity of these biocatalysts. Once nanozymes enter the human body, they can interact with biological molecules (mainly proteins), giving nanozymes new biological characteristics, which may be more significant than the original properties of nanozymes [121].
Another challenge is the lack of standardization in the design and production of nanozymes, which can lead to varying levels of efficacy and specificity. For instance, Au nanozymes are poisonous at 1.4 nm but not at 15 nm. It is crucial to closely explore the characteristic parameters of nanodrugs, particularly the relations among dimension, surface chemistry, dose and pharmacokinetics. Their physicochemical characteristics, such as dimension, geometry, electrical charge or surface chemistry, can be modified if necessary. Modulating the chemical composition and controlling the morphology to change the size, surface modification and assembly of nanozymes provide a reference for researchers in making nanozymes with maximum security for practical biomedical applications.
SARS-CoV-2 is constantly mutating [122], and nanozyme-based viral assays should still be validated using more complex clinical samples to obtain a more comprehensive and accurate diagnosis. Thus, simply using recombinant antigens or pseudoviruses is completely inadequate. The strategy of producing nanozyme diagnostic strips at scale is mandatory to increase their availability worldwide. This cost advantage can significantly reduce the financial burden on the nation's health care resources and, crucially, can benefit areas of poor socioeconomic status. Moreover, a parallel comparison of this strip assay with other commercially available SARS-CoV-2 kits is still required to make the nanozyme-mediated detection strategy commercially available.
To hasten the clinical adoption of nanozyme-based vaccines, a comprehensive assessment of their benefits and risks is essential. Safety is one of the most important concerns. Controlled dosage and surface modification can help to reduce toxicity and increase specificity for SARS-CoV-2, but their relevant influence on the following dynamics and catalytic activity of nanozymes must be evaluated. The immunogenicity and cellular transmigration of nanozymes must be researched. Regulation of reaction conditions at larger scales is challenging when producing nanozyme-based vaccines for SARS-CoV-2, so further expansion synthesis must be validated. Otherwise, issues such as batch variation will inevitably hinder the vaccines’ industrialization process.
Conclusion
In the context of the SARS-CoV-2 global health outbreak, we have concluded the possible applications of nanozymes in fighting against SARS-CoV-2. (1) Regarding diagnosis, nanozyme-based detection provides a highly sensitive, rapid, inexpensive and even quantitative detection method compared to the current gold standard—the nucleic acid test. (2) In terms of treatment, they can specifically resist and clear SARS-CoV-2 by inhibiting its endocytosis or replication in the process of infecting host cells, which make up for the shortcomings of broad-spectrum medicines. Moreover, direct destruction of the viral structure can minimize its side effects and degradation. (3) Regarding prevention, nanozyme-based vaccines have better potential for appropriate exposure to the immune system and can protect vaccine antigens from degradation and removal. Nanozyme-based masks and surface coating will greatly reduce SARS-CoV-2 in the environment, reducing economic and environmental costs.
Although considerable progress has been made in recent years, there is still space for progress in the study of nanozymes in the fight against COVID-19. Here, we propose several key questions and directions of studies that can be researched in the future to increase the potential of clinical transformation of nanozymes. (1) For detection, to commercialize the nanozyme-mediated detection strategy, it is still necessary to evaluate nanozyme-based tests and other commercial kits. (2) For nanozyme-based medicines, we should evaluate their safety, pharmacokinetics, clinical toxicity, immunogenicity, and cellular fate. We should also consider the metabolism and subsequent catalytic activity of nanozymes in vivo. (3) For nanozyme-based vaccines, mass production and availability to the entire range of countries are the target directions. In conclusion, nanozyme-based tools are expected to play a frontline role in addressing COVID-19. Considering the continuous evolution of SARS-CoV-2, nanozymes must also "evolve" in morphology, composition and surface function to counteract.
Acknowledgements
Not applicable.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- POD
Peroxidase
- OXD
Oxidase
- CAT
Catalase
- SOD
Superoxide dismutase
- ARDS
Acute respiratory distress syndrome
- WHO
World health organization
- MNPs
Magnetic nanoparticles
- IONzymes
Iron oxide nanozymes
- PCR
Polymerase chain reaction
- RT‒PCR
Reverse transcription-polymerase chain reaction
- cDNA
Complementary DNA
- ELISA
Enzyme linked immunosorbent assay
- HRP
Horseradish peroxidase
- NPs
Nanoparticles
- ROS
Reactive oxygen species
- ET
Electron transfer
- NLICS
Nanozyme-linked immunochromatographic sensor
- CRISPR
Clustered regularly interspaced short palindromic repeats
- Cas
CRISPR-associated
- ACE2
Angiotensin converting enzyme 2
- RdRp
RNA-dependent RNA polymerase
- SANs
Single-atom nanozymes
- RBD
Receptor binding domain
- V2O5
Vanadium pentoxide
- IL
Interleukin
- TNF
Tumor necrosis factor
- CCL
C–C motif chemokine ligand
- APCs
Antigen presenting cells
- DCs
Dendritic cells
- CS
Chitosan
- WIV
Whole inactivated virus
- TED
Transepithelial dendrites
Author contributions
XW and KLF selected the topic and guided the review, JW and QPX summarized studies, JW wrote the manuscript, HYS, XHC, XXZ, XYZ, YJH, YZ, HFL and NL searched the references and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82071155, 82271023), the Project of Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials [Grant number RC2021-02 and RC202301], the Shanxi Applied Basic Research Program Outstanding Youth Cultivation Project Fund [Grant numbers 202203021223006].
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jia Wang and Qingpeng Xie contributed equally to this work.
Contributor Information
Jia Wang, Email: wj34588@163.com.
Qingpeng Xie, Email: peggy1119345469@163.com.
Haoyue Song, Email: shykycg@163.com.
Xiaohang Chen, Email: kqyx__cxh@163.com.
Xiaoxuan Zhang, Email: xiaoxuankq@163.com.
Xiangyu Zhao, Email: zxy199803122021@163.com.
Yujia Hao, Email: hh18003522704@126.com.
Yuan Zhang, Email: zhangyuan5458@163.com.
Huifei Li, Email: felicia_lihuifei@163.com.
Na Li, Email: sabrinal0906@163.com.
Kelong Fan, Email: fankelong@ibp.ac.cn.
Xing Wang, Email: kqwx100@163.com.
References
- 1.Freeman CM, Rank MA, Bolster LaSalle CM, Grys TE, Lewis JC. Effectiveness of Physical Distancing: Staying 6 Feet Over to Put Respiratory Viruses 6 Feet Under. Mayo Clin Proc. 2021;96:148–151. doi: 10.1016/j.mayocp.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Peeling RW, Heymann DL, Teo YY, Garcia PJ. Diagnostics for COVID-19: moving from pandemic response to control. Lancet. 2022;399:757–768. doi: 10.1016/S0140-6736(21)02346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Liao X, Liu Z, Ma Z, Dong J, Zheng G, Zi M, Wang F, He Q, Li G, et al. Healthy outcomes of patients with COVID-19 two years after the infection: a prospective cohort study. Emerg Microbes Infect. 2022;11:2680–2688. doi: 10.1080/22221751.2022.2133639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev. 2021; 34. [DOI] [PMC free article] [PubMed]
- 6.Perivolaropoulos C, Vlacha V. A reduction of the number of assays and turnaround time by optimizing polymerase chain reaction (PCR) pooled testing for SARS-CoV-2. J Med Virol. 2021;93:4508–4515. doi: 10.1002/jmv.26972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drożdżal S, Rosik J, Lechowicz K, Machaj F, Szostak B, Przybyciński J, Lorzadeh S, Kotfis K, Ghavami S, Łos MJ. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat. 2021;59:100794. doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi T. Mutations in SARS-CoV-2 are on the increase against the acquired immunity. PLoS ONE. 2022;17:e0271305. doi: 10.1371/journal.pone.0271305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Z, Guo S, Zhou Y, Li M, Wang M, Ying B. Applications of laboratory findings in the prevention, diagnosis, treatment, and monitoring of COVID-19. Signal Transduct Target Ther. 2021;6:316. doi: 10.1038/s41392-021-00731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashrafi AM, Bytesnikova Z, Barek J, Richtera L, Adam V. A critical comparison of natural enzymes and nanozymes in biosensing and bioassays. Biosens Bioelectron. 2021;192:113494. doi: 10.1016/j.bios.2021.113494. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh N, Salimi A. Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering. J Nanobiotechnol. 2021;2023:26. doi: 10.1186/s12951-021-00771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang M, Yan X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc Chem Res. 2019;52(8):2190. doi: 10.1021/acs.accounts.9b00140. [DOI] [PubMed] [Google Scholar]
- 14.Ren XY, Chen DX, Wang Y, Li HF, Zhang YB, Chen HY, Li X, Huo MF. Nanozymes-recent development and biomedical applications. J Nanobiotechnol. 2022; 20. [DOI] [PMC free article] [PubMed]
- 15.Wang D, Jana D, Zhao Y. Metal-organic framework derived nanozymes in biomedicine. Acc Chem Res. 2020;53:1389–1400. doi: 10.1021/acs.accounts.0c00268. [DOI] [PubMed] [Google Scholar]
- 16.Duan D, Fan K, Zhang D, Tan S, Liang M, Liu Y, Zhang J, Zhang P, Liu W, Qiu X, et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron. 2015;74:134–141. doi: 10.1016/j.bios.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Qin T, Ma R, Yin Y, Miao X, Chen S, Fan K, Xi J, Liu Q, Gu Y, Yin Y, et al. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics. 2019;9:6920–6935. doi: 10.7150/thno.35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumawat M, Umapathi A, Lichtfouse E, Daima HK. Nanozymes to fight the COVID-19 and future pandemics. Environ Chem Lett. 2021;19:3951–3957. doi: 10.1007/s10311-021-01252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oeschger TM, McCloskey DS, Buchmann RM, Choubal AM, Boza JM, Mehta S, Erickson D. Early warning diagnostics for emerging infectious diseases in developing into late-stage pandemics. Acc Chem Res. 2021;54:3656–3666. doi: 10.1021/acs.accounts.1c00383. [DOI] [PubMed] [Google Scholar]
- 20.Yuce M, Filiztekin E, Ozkaya KG. COVID-19 diagnosis—a review of current methods. Biosens Bioelectron. 2021;172:112752. doi: 10.1016/j.bios.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derakhshan MA, Amani A, Faridi-Majidi R. State-of-the-art of nanodiagnostics and nanotherapeutics against SARS-CoV-2. ACS Appl Mater Interfaces. 2021;13:14816–14843. doi: 10.1021/acsami.0c22381. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2021;19:171–183. doi: 10.1038/s41579-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabir MS, Clements MO, Kimmitt PT. RT-Bst: an integrated approach for reverse transcription and enrichment of cDNA from viral RNA. Br J Biomed Sci. 2015;72:1–6. doi: 10.1080/09674845.2015.11666788. [DOI] [PubMed] [Google Scholar]
- 24.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brunink S, Schneider J, Schmidt ML, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25. [DOI] [PMC free article] [PubMed]
- 25.Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sensors. 2021;6:593–612. doi: 10.1021/acssensors.0c02621. [DOI] [PubMed] [Google Scholar]
- 26.Tong P-B-V, Lin L-Y, Tran TH. Coronaviruses pandemics: can neutralizing antibodies help? Life Sci. 2020;255:117836. doi: 10.1016/j.lfs.2020.117836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacofsky D, Jacofsky EM, Jacofsky M. Understanding antibody testing for COVID-19. J Arthroplasty. 2020;35:S74–s81. doi: 10.1016/j.arth.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong G, Yi Y, Tuantuan L, Xiaowu W, Xiuyong L, Ang L, Mingfeng H. Evaluation of the auxiliary diagnostic value of antibody assays for the detection of novel coronavirus (SARS-CoV-2) J Med Virol. 2020;92:1975–1979. doi: 10.1002/jmv.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu D, Ju C, Han C, Shi R, Chen X, Duan D, Yan J, Yan X. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens Bioelectron. 2020;173:112817. doi: 10.1016/j.bios.2020.112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Xue M, Zhang J, Chen Q, Chen J, Wang Z, Zhou W, Chen P, Xia N, Ge S. A one-step dipstick assay for the on-site detection of nucleic acid. Clin Biochem. 2013;46:1852–1856. doi: 10.1016/j.clinbiochem.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertens P, De Vos N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, Van den Wijngaert S, Monteil V, Melin P, Stoffels K, et al. Development and potential usefulness of the COVID-19 Ag respi-strip diagnostic assay in a pandemic context. Front Med. 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand R, Thelaus L, Fernström N, Sunnerhagen T, Lindroth Y, Linder A, Rasmussen M. Rapid diagnostic testing for SARS-CoV-2: validation and comparison of three point-of-care antibody tests. J Med Virol. 2021;93:4592–4596. doi: 10.1002/jmv.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kent CJVmd. Different paths to the same destination: screening for Covid-19. 2020.
- 36.Liu B, Wu Z, Liang C, Lu J, Li J, Zhang L, Li T, Zhao W, Fu Y, Hou S, et al. Development of a smartphone-based nanozyme-linked immunosorbent assay for quantitative detection of SARS-CoV-2 nucleocapsid phosphoprotein in blood. Front Microbiol. 2021;12:692831. doi: 10.3389/fmicb.2021.692831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo G, Lee G, Kim MJ, Baek S-H, Choi M, Ku KB, Lee C-S, Jun S, Park D, Kim HG, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 38.Oishee MJ, Ali T, Jahan N, Khandker SS, Haq MA, Khondoker MU, Sil BK, Lugova H, Krishnapillai A, Abubakar AR, et al. COVID-19 pandemic: review of contemporary and forthcoming detection tools. Infect Drug Resist. 2021;14:1049–1082. doi: 10.2147/IDR.S289629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas H, Xu C, Yu Y, Gao W. Emerging telemedicine tools for remote COVID-19 diagnosis, monitoring, and management. ACS Nano. 2020;14:16180–16193. doi: 10.1021/acsnano.0c08494. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal DK, Nandwana V, Henrich SE, Josyula V, Thaxton CS, Qi C, Simons LM, Hultquist JF, Ozer EA, Shekhawat GS, Dravid VP. Highly sensitive and ultra-rapid antigen-based detection of SARS-CoV-2 using nanomechanical sensor platform. Biosens Bioelectron. 2022;195:113647. doi: 10.1016/j.bios.2021.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali J, Elahi SN, Ali A, Waseem H, Abid R, Mohamed MM. Unveiling the potential role of nanozymes in combating the COVID-19 outbreak. Nanomaterials (Basel) 2021;11:1328. doi: 10.3390/nano11051328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Lai J, Wu K, Huang X, Guo S, Zhang L, Liu J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta. 2018;180:260–270. doi: 10.1016/j.talanta.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 44.Deng J, Yang M, Wu J, Zhang W, Jiang X. A self-contained chemiluminescent lateral flow assay for point-of-care testing. Anal Chem. 2018;90:9132–9137. doi: 10.1021/acs.analchem.8b01543. [DOI] [PubMed] [Google Scholar]
- 45.Della Ventura B, Cennamo M, Minopoli A, Campanile R, Censi SB, Terracciano D, Portella G, Velotta R. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. Acs Sensors. 2020;5:3043–3048. doi: 10.1021/acssensors.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Z, Xu M, Lu M, Chen G, Tang D. Urchin-like (gold core)@(platinum shell) nanohybrids: a highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens Bioelectron. 2015;70:194–201. doi: 10.1016/j.bios.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Fu Z, Zeng WL, Cai SF, Li HL, Ding JW, Wang C, Chen YF, Han N, Yang R. Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J Colloid Interface Sci. 2021;604:113–121. doi: 10.1016/j.jcis.2021.06.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding X, Yin K, Li Z, Lalla RV, Ballesteros E, Sfeir MM, Liu C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat Commun. 2020;11:4711. doi: 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sridhara S, Goswami HN, Whyms C, Dennis JH, Li H. Virus detection via programmable Type III-A CRISPR-Cas systems. Nat Commun. 2021;12:5653. doi: 10.1038/s41467-021-25977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steens JA, Zhu Y, Taylor DW, Bravo JPK, Prinsen SHP, Schoen CD, Keijser BJF, Ossendrijver M, Hofstra LM, Brouns SJJ, et al. SCOPE enables type III CRISPR-Cas diagnostics using flexible targeting and stringent CARF ribonuclease activation. Nat Commun. 2021;12:5033. doi: 10.1038/s41467-021-25337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Shang X, Huang X. Next-generation pathogen diagnosis with CRISPR/Cas-based detection methods. Emerg Microb Infect. 2020;9:1682–1691. doi: 10.1080/22221751.2020.1793689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mimitou EP, Cheng A, Montalbano A, Hao S, Stoeckius M, Legut M, Roush T, Herrera A, Papalexi E, Ouyang Z, et al. Multiplexed detection of proteins, transcriptomes, clonotypes and CRISPR perturbations in single cells. Nat Methods. 2019;16:409–412. doi: 10.1038/s41592-019-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwinn MK, Machleidt T, Zimmerman K, Eggers CT, Dixon AS, Hurst R, Hall MP, Encell LP, Binkowski BF, Wood KV. CRISPR-mediated tagging of endogenous proteins with a luminescent peptide. ACS Chem Biol. 2018;13:467–474. doi: 10.1021/acschembio.7b00549. [DOI] [PubMed] [Google Scholar]
- 55.Chen Q, Tian T, Xiong E, Wang P, Zhou X. CRISPR/Cas13a signal amplification linked immunosorbent assay for femtomolar protein detection. Anal Chem. 2020;92:573–577. doi: 10.1021/acs.analchem.9b04403. [DOI] [PubMed] [Google Scholar]
- 56.Liang M, Li Z, Wang W, Liu J, Liu L, Zhu G, Karthik L, Wang M, Wang K-F, Wang Z, et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat Commun. 2019;10:3672. doi: 10.1038/s41467-019-11648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu C, Wang C, Li F, Zheng X, Xing X, Zhang C. Aptamer assisted CRISPR-Cas12a strategy for small molecule diagnostics. Biosens Bioelectron. 2021;183:113196. doi: 10.1016/j.bios.2021.113196. [DOI] [PubMed] [Google Scholar]
- 58.Xiong Y, Zhang J, Yang Z, Mou Q, Ma Y, Xiong Y, Lu Y. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J Am Chem Soc. 2020;142:207–213. doi: 10.1021/jacs.9b09211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu LN, Wang XJ, Wu XC, Xu SQ, Liu M, Cao XZ, Tang TS, Huang XX, Huang H. MnO2 nanozyme-mediated CRISPR-Cas12a system for the detection of SARS-CoV-2. Acs Appl Mater Interfaces. 9. [DOI] [PubMed]
- 60.Liang CL, Liu BC, Li JF, Lu JH, Zhang EH, Deng QT, Zhang L, Chen R, Fu YS, Li CY, Li TT. A nanoenzyme linked immunochromatographic sensor for rapid and quantitative detection of SARS-CoV-2 nucleocapsid protein in human blood. Sens Actuators B-Chem. 2021;349:9. doi: 10.1016/j.snb.2021.130718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 62.Usher AD. The global COVID-19 treatment divide. Lancet. 2022;399:779–782. doi: 10.1016/S0140-6736(22)00372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colson P, Rolain J-M, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vastag B. Old drugs for a new bug: influenza, HIV drugs enlisted to fight SARS. JAMA. 2003;290:1695–1696. doi: 10.1001/jama.290.13.1695. [DOI] [PubMed] [Google Scholar]
- 65.Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Ng Y-Y, Lo J, Chan J, Tam AR, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (London, England) 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch B-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tammam SN, El Safy S, Ramadan S, Arjune S, Krakor E, Mathur S. Repurpose but also (nano)-reformulate! The potential role of nanomedicine in the battle against SARS-CoV2. J Control Release. 2021;337:258–284. doi: 10.1016/j.jconrel.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zekarias A, Watson S, Vidlin SH, Grundmark B. Sex differences in reported adverse drug reactions to COVID-19 drugs in a global database of individual case safety reports. Drug Saf. 2020;43:1309–1314. doi: 10.1007/s40264-020-01000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Law MF, Ho R, Law KWT, Cheung CKM. Gastrointestinal and hepatic side effects of potential treatment for COVID-19 and vaccination in patients with chronic liver diseases. World J Hepatol. 2021;13:1850–1874. doi: 10.4254/wjh.v13.i12.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burki T. The future of paxlovid for COVID-19. Lancet Respir Med. 2022;10:e68. doi: 10.1016/S2213-2600(22)00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019;48:3683–3704. doi: 10.1039/C8CS00718G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyer S, Doktycz MJ. Nanozymes for antiviral therapy. Nanomedicine (Lond) 2012;7:1654–1655. [PubMed] [Google Scholar]
- 77.Kamat S, Kumari M, Jayabaskaran C. Nano-engineered tools in the diagnosis, therapeutics, prevention, and mitigation of SARS-CoV-2. J Control Release. 2021;338:813–836. doi: 10.1016/j.jconrel.2021.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen K-Y, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medhi R, Srinoi P, Ngo N, Tran HV, Lee TR. Nanoparticle-based strategies to combat COVID-19. Acs Applied Nano Materials. 2020;3:8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 82.Cagno V, Andreozzi P, D'Alicarnasso M, Jacob Silva P, Mueller M, Galloux M, Le Goffic R, Jones ST, Vallino M, Hodek J, et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 83.Zhang XD, Chen XK, Zhao YL. Nanozymes: versatile platforms for cancer diagnosis and therapy. Nano-Micro Letters. 2022;14:27. doi: 10.1007/s40820-021-00773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang DJ, Zhang B, Ding H, Liu D, Xiang JQ, Gao XJJ, Chen XH, Li ZJ, Yang L, Duan HX, et al. TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today. 2021;40:11. doi: 10.1016/j.nantod.2021.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh S, Ghosh S, Pal VK, Munshi M, Shekar P, Murthy DTN, Mugesh G, Singh A. Antioxidant nanozyme counteracts HIV-1 by modulating intracellular redox potential. EMBO Mol Med. 2021;13:19. doi: 10.15252/emmm.202013314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davidson S, Maini MK, Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res. 2015;35:252–264. doi: 10.1089/jir.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, Peiris JS. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, Chan JFW, Woo PCY, Yuen KY. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 92.Du XC, Zhang MZ, Zhou HT, Wang WJ, Zhang CM, Zhang L, Qu YY, Li WF, Liu XD, Zhao MW, et al. Decoy nanozymes enable multitarget blockade of proinflammatory cascades for the treatment of multi-drug-resistant bacterial sepsis. Research. 2022;2022:15. doi: 10.34133/2022/9767643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhen Q, Zhang A, Huang Q, Li J, Du Y, Zhang Q. Overview of the role of spatial factors in indoor SARS-CoV-2 transmission: a space-based framework for assessing the multi-route infection risk. Int J Environ Res Public Health. 2022;19:11007. doi: 10.3390/ijerph191711007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Portarapillo M, Di Benedetto A. Methodology for risk assessment of COVID-19 pandemic propagation. J Loss Prev Process Ind. 2021;72:104584. doi: 10.1016/j.jlp.2021.104584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valenzuela-Fernandez A, Cabrera-Rodriguez R, Ciuffreda L, Perez-Yanes S, Estevez-Herrera J, Gonzalez-Montelongo R, Alcoba-Florez J, Trujillo-Gonzalez R, de Artola DGM, Gil-Campesino H, et al. Nanomaterials to combat SARS-CoV-2: strategies to prevent, diagnose and treat COVID-19. Front Bioeng Biotechnol. 2022;10:42. doi: 10.3389/fbioe.2022.1052436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jüni P, Rothenbühler M, Bobos P, Thorpe KE, da Costa BR, Fisman DN, Slutsky AS, Gesink D. Impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ. 2020;192:E566–e573. doi: 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, Huang J, He N, Yu H, Lin X, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao Y, Tang B, Wu J, Cheke RA, Tang S. Linking key intervention timing to rapid decline of the COVID-19 effective reproductive number to quantify lessons from mainland China. Int J Infect Dis. 2020;97:296–298. doi: 10.1016/j.ijid.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Ren R, Kattan MW, Jehi L, Cheng Z, Fang K. Public health interventions' effect on hospital use in patients with COVID-19: comparative study. JMIR Public Health Surveill. 2020;6:e25174. doi: 10.2196/25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sacco PL, De Domenico M. Public health challenges and opportunities after COVID-19. Bull World Health Organ. 2021;99:529–535. doi: 10.2471/BLT.20.267757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwon S, Joshi AD, Lo CH, Drew DA, Nguyen LH, Guo CG, Ma W, Mehta RS, Shebl FM, Warner ET, et al. Association of social distancing and face mask use with risk of COVID-19. Nat Commun. 2021;12:3737. doi: 10.1038/s41467-021-24115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tuñón-Molina A, Takayama K, Redwan EM, Uversky VN, Andrés J, Serrano-Aroca Á. Protective face masks: current status and future trends. ACS Appl Mater Interfaces. 2021;13:56725–56751. doi: 10.1021/acsami.1c12227. [DOI] [PubMed] [Google Scholar]
- 103.Kwok KO, McNeil EB, Tsoi MTF, Wei VWI, Wong SYS, Tang JWT. Will achieving herd immunity be a road to success to end the COVID-19 pandemic? J Infect. 2021;83:381–412. doi: 10.1016/j.jinf.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kadkhoda K. Herd immunity to COVID-19 alluring and elusive. Am J Clin Pathol. 2021;155:471–472. doi: 10.1093/ajcp/aqaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fontanet A, Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao Y, Wu Y, Feng Y, Xu W, Xiong F, Zhang X. SARS-CoV-2 vaccine research and immunization strategies for improved control of the COVID-19 pandemic. Front Med. 2022;16:185–195. doi: 10.1007/s11684-021-0913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laine C, Cotton D, Moyer DV. COVID-19 vaccine: promoting vaccine acceptance. Ann Intern Med. 2021;174:252–253. doi: 10.7326/M20-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cho CS, Hwang SK, Gu MJ, Kim CG, Kim SK, Ju DB, Yun CH, Kim HJ. Mucosal vaccine delivery using mucoadhesive polymer particulate systems. Tissue Eng Regen Med. 2021;18:693–712. doi: 10.1007/s13770-021-00373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavelle EC, Ward RW. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol. 2022;22:236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 112.Qin T, Yin Y, Yu Q, Huang L, Wang X, Lin J, Yang Q. CpG oligodeoxynucleotides facilitate delivery of whole inactivated H9N2 influenza virus via transepithelial dendrites of dendritic cells in nasal mucosa. J Virol. 2015;89:5904–5918. doi: 10.1128/JVI.00296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jindal A, Sarkar S, Alam A. Nanomaterials-mediated immunomodulation for cancer therapeutics. Front Chem. 2021;9:17. doi: 10.3389/fchem.2021.629635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonam SR, Kotla NG, Bohara RA, Rochev Y, Webster TJ, Bayry J. Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today. 2021;36:19. doi: 10.1016/j.nantod.2020.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin T, Ma S, Miao XY, Tang Y, Huangfu DD, Wang JY, Jiang J, Xu N, Yin YC, Chen SJ, et al. Mucosal vaccination for influenza protection enhanced by catalytic immune-adjuvant. Adv Sci. 2020;7:15. doi: 10.1002/advs.202000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar S, Karmacharya M, Joshi SR, Gulenko O, Park J, Kim GH, Cho YK. Photoactive antiviral face mask with self-sterilization and reusability. Nano Lett. 2021;21:337–343. doi: 10.1021/acs.nanolett.0c03725. [DOI] [PubMed] [Google Scholar]
- 117.Marzoli F, Bortolami A, Pezzuto A, Mazzetto E, Piro R, Terregino C, Bonfante F, Belluco S. A systematic review of human coronaviruses survival on environmental surfaces. Sci Total Environ. 2021;778:146191. doi: 10.1016/j.scitotenv.2021.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin N, Verma D, Saini N, Arbi R, Munir M, Jovic M, Turak A. Antiviral nanoparticles for sanitizing surfaces: a roadmap to self-sterilizing against COVID-19. Nano Today. 2021;40:101267. doi: 10.1016/j.nantod.2021.101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tong YM, Shi GS, Hu GW, Hu XY, Han L, Xie XF, Xu YF, Zhang R, Sun J, Zhong J. Photo-catalyzed TiO2 inactivates pathogenic viruses by attacking viral genome. Chem Eng J. 2021;414:10. doi: 10.1016/j.cej.2021.128788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shirvanimoghaddam K, Akbari MK, Yadav R, Al-Tamimi AK, Naebe M. Fight against COVID-19: the case of antiviral surfaces. APL Mater. 2021;9:14. doi: 10.1063/5.0043009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Campos EVR, Pereira AES, de Oliveira JL, Carvalho LB, Guilger-Casagrande M, de Lima R, Fraceto LF. How can nanotechnology help to combat COVID-19? opportunities and urgent need. J Nanobiotechnol. 2020;18:23. doi: 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng X, Zou S, Li D, He J, Fang L, Wang H, Yan X, Duan D, Gao L. Nanozyme-strip for rapid and ultrasensitive nucleic acid detection of SARS-CoV-2. Biosens Bioelectron. 2022;217:114739. doi: 10.1016/j.bios.2022.114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu L, Wang X, Wu X, Xu S, Liu M, Cao X, Tang T, Huang X, Huang H. MnO(2) nanozyme-mediated CRISPR-Cas12a system for the detection of SARS-CoV-2. ACS Appl Mater Interfaces. 2022;14:50534–50542. doi: 10.1021/acsami.2c14497. [DOI] [PubMed] [Google Scholar]
- 125.Liang C, Liu B, Li J, Lu J, Zhang E, Deng Q, Zhang L, Chen R, Fu Y, Li C, Li T. A nanoenzyme linked immunochromatographic sensor for rapid and quantitative detection of SARS-CoV-2 nucleocapsid protein in human blood. Sens Actuators B Chem. 2021;349:130718. doi: 10.1016/j.snb.2021.130718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J, Li Y. One-pot high-yield synthesis of Pd nanocubes for Pd-Ir nanocube-based immunoassay of nucleocapsid protein from SARS-CoV-2. Anal Bioanal Chem. 2021;413:4635–4644. doi: 10.1007/s00216-021-03265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang D, Zhang B, Ding H, Liu D, Xiang J, Gao XJ, Chen X, Li Z, Yang L, Duan H, et al. TiO(2) supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today. 2021;40:101243. doi: 10.1016/j.nantod.2021.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du X, Zhang M, Zhou H, Wang W, Zhang C, Zhang L, Qu Y, Li W, Liu X, Zhao M, et al. Decoy nanozymes enable multitarget blockade of proinflammatory cascades for the treatment of multi-drug-resistant bacterial sepsis. Research (Washington, DC) 2022;2022:9767643. doi: 10.34133/2022/9767643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong Y, Shi G, Hu G, Hu X, Han L, Xie X, Xu Y, Zhang R, Sun J, Zhong J. Photo-catalyzed TiO(2) inactivates pathogenic viruses by attacking viral genome. Chem Eng J. 2021;414:128788. doi: 10.1016/j.cej.2021.128788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.