Abstract
Background
Robotic nipple-sparing mastectomy (RNSM) has emerged as a new treatment option for breast cancer and risk-reducing mastectomy (RRM) for women who have a high risk of pathogenic variants. Even though several studies have reported that RNSM is a feasible procedure, some argue that it should only be performed by specialized surgeons, and data on oncologic outcomes and patient-reported outcomes (PROs) are limited. Recently, the United States Food and Drug Administration and several surgeons warned that robotic breast surgery should be performed only by specialized surgeons and recommended that the benefits, risks, and alternatives of all available treatment options be discussed with patients so they can make informed treatment decisions. The Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) has been established to evaluate, standardize, and teach this state-of-the-art procedure. We have designed a multicenter prospective cohort study entitled Mastectomy with Reconstruction Including Robot Endoscopic Surgery (MARRES) to report surgical, PRO, and oncologic outcomes.
Methods
MARRES is a multi-institution cohort study prospectively collecting data from patients undergoing mastectomy and reconstruction. The patient inclusion criteria are adult women older than 19 with breast cancer or a high risk of breast cancer (patients with BRCA1/2, TP53, PALB2 mutations, etc.), who have scheduled therapeutic or RRM and want immediate reconstruction. Surgical outcomes, including pre- and postoperative photos, oncologic outcomes, cost-effectiveness, and PRO, are collected. The primary endpoints are postoperative complication rates within 30 postoperative days and the Clavien-Dindo grade of postoperative complications within 180 postoperative days. The secondary endpoints are 5-year postoperative recurrence-free survival and cancer incidence rate (for those who underwent RRM), patient satisfaction with reconstruction expectations preoperative (baseline) and results within 6 to 12 postoperative months, surgeon satisfaction with postoperative results in 6 postoperative months, and cost-effectiveness of the definitive surgery. Patient recruitment will be completed in April 2025, and the target number of enrolled patients is 2000.
Discussion
This study will provide evidence about the surgical outcomes, oncologic outcomes, and patient satisfaction with RNSM and endoscopic nipple-sparing mastectomy (NSM), compared with conventional NSM.
Trial registration
ClinicalTrials.gov Identifier NCT04585074. Registered April 8, 2020.
Keywords: Breast neoplasms, Conventional nipple-sparing mastectomy, Endoscopic nipple-sparing mastectomy, Germline BRCA1/2 mutation, Robotic-assisted nipple-sparing mastectomy, Minimally invasive procedure, Immediate breast reconstruction
Background
Since Toth and Lappert first described a skin-sparing mastectomy (SSM) procedure, similar oncologic outcomes and better patient satisfaction and quality of life (QOL) have been reported for it compared with conventional mastectomy (CM) [1–3]. Immediate breast reconstruction (IBR) is facilitated by preserving the skin envelope at the time of mastectomy, and the success of SSM has paved the way for nipple-sparing mastectomy (NSM). Because NSM has shown better patient satisfaction and cosmetic results than SSM, with comparable oncologic outcomes, it has become popular [4, 5]. NSM with IBR has become widespread as indications have expanded. Increased BRCA1/2 genetic testing and public awareness have led to a rise in risk-reducing mastectomy (RRM), and there is increased interest in QOL after mastectomy [6, 7].
In NSM, various kinds of skin incisions can be selected [8–10]. To prevent visible scarring and minimize the incision size, endoscopic NSM (ENSM) was developed more than 20 years ago [11–13]. However, ENSM has several limitations: (1) complex devices are needed, (2) the approaches are difficult for endoscopic devices, especially in the medial part of the breast, and (3) it is labor-intensive for surgeons and assistants, especially for large and ptotic breasts. Therefore, ENSM has been performed only by specialized surgeons in East Asia.
Robots are used for various surgeries, including those for malignant diseases. Since the first reports of robotic nipple-sparing mastectomy (RNSM) by Toesca et al. and Park et al. in Korea, RNSM has been performed in selected patients and BRCA1/2 carriers [14, 15]. Even though several studies have shown that RNSM is a feasible procedure, it is performed by only a few specialized surgeons [16]. Furthermore, few data have been reported about its oncologic outcomes.
Recently, the United States Food and Drug Administration warned that robotic breast surgery should be performed only by specialized surgeons and recommended that the benefits, risks, and alternatives of all available treatment options be discussed with patients so they could make informed treatment decisions. Previous articles have also recommended that randomized controlled trials be conducted to assess the surgical safety, patient reported outcomes (PROs), and oncologic outcomes of RNSM and thus evaluate its clinical role in treating BRCA1/2 carriers and patients with breast cancer [17, 18].
The Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) has been established to evaluate, standardize, and teach this state-of-the-art procedure. Previously, the KoREa-BSG retrospectively analyzed the initial experiences of surgeons and showed that RNSM is technically feasible and reliable, with a short learning curve [19]. Therefore, to obtain a high level of evidence, we designed a multicenter prospective exploratory cohort study entitled Mastectomy with Reconstruction Including Robotic Endoscopic Surgery (MARRES) to collect data on surgical outcomes, PROs, and oncologic outcomes (Protocol serial numbers: KoREa-BSG 03 and KBCSG-23).
Evidence for immediate reconstruction
Guidelines and reviews for conventional NSM (CNSM) with IBR
The first NSM was described by Freeman in 1962 but only for RRM [20]. Subcutaneous mastectomy for patients with primary breast cancer was first reported by Hinton et al. in 1984 and showed rates of local recurrence and survival comparable to those with CM [21]. The use of NSM for women with breast cancer has been justified by demonstrating its oncologic and surgical safety. Previous studies confirmed acceptable locoregional recurrence, disease-free survival, and overall survival (OS) rates following NSM [4, 22–27]. The surgical outcomes of NSM, including rates of nipple necrosis and overall postoperative complications, were acceptable in previous reports [4, 22, 23]. The procedure’s advantages in terms of better aesthetic outcomes, improved patient satisfaction, and psychosexual benefits have also been investigated [28–30].
The National Comprehensive Cancer Network (NCCN) guideline comments that SSM showed similar oncologic outcome compared to CM [31]. However, SSM should be performed by an experienced breast surgeon. Similarly, NSM is comparable oncologic outcome to SSM, if cases are appropriately selected. NSM can be performed for any tumor size independent of axillary status, especially for early breast cancer, and for ductal carcinoma in situ and RRM, but having cT4b and cT4c breast cancers with skin involvement or skin edema, and clinical signs of nipple involvement and any R1 resection at the nipple margin should be a contraindication.
Guidelines and reviews for ENSM with IBR
Due to aesthetic concerns about the breast, breast surgery has been developed to satisfy both oncologic safety and cosmetic needs [13]. Endoscopic procedures have been used for various breast surgeries, including augmentation, mastectomy, excision, biopsy, capsulectomy, and reconstruction [32].
Endoscopic-assisted breast surgery (EABS) was introduced in the late 1990s to optimize the aesthetic effects by using a minimal incision in an inconspicuous location for benign breast disease or breast cancer [33]. Previous studies reported excellent patient satisfaction on patient-reported questionnaires after ENSM [34–36]. Additionally, endoscopy could offer better visualization with light handle retractions through a small incision and allow similar oncologic outcomes compared to CNSM [13, 37]. Indications and contraindications for EABS are similar to CNSM. Although ENSM is considered a safe and feasible procedure that leaves a relatively inconspicuous incision in patients with breast cancer [38], endoscopic techniques for breast surgery have not been widely performed because of the technical challenges with using rigid instruments [39].
Guidelines and reviews for RNSM with IBR
Since the first robotic surgical system was introduced in 2000, robotic surgical systems have spread widely into various surgical fields because of the comfort and ergonomic movement they allow, with magnified and high-resolution vision [40]. Robotic breast surgery has also been developed and investigated by several pioneers to establish clinical evidence for this new technique (Tables 1 and 2) [19, 41–47].
Table 1.
General information on previous studies about RNSM
| Study | Year | Study interval | Aim | Design | No. of patients (procedures) | Indication (n, %) |
|---|---|---|---|---|---|---|
| Ryu et al. [19] | 2020 | Nov 2016–Jan 2020 | To report the early experience of RNSM with IBR in the KoREa-BSG | Retrospective case series, multicenter | 73 (82) | Therapeutic (75, 91.5%)Risk-reducing (7, 8.5%) |
| Lee et al. [41] | 2021 | Nov 2016–Jan 2019 | To directly compare surgical outcomes between CNSM and RNSM | Retrospective case–control comparison study, single center |
CNSM 270 RNSM 41 |
Therapeutic (34, 83%) Risk-reducing (7, 17.1%) |
| Toesca et al. [42] | 2019 | June 2014–Jan 2019 | To present and discuss perioperative surgical outcomes and early oncologic follow-up data on consecutive patients undergoing RNSM | Prospective case series, single center | 73 (94) |
Invasive breast cancer (39, 41.5%) DCIS (21, 223%) Risk-reducing (34, 36.2%) |
| Sanson et al. [43] | 2018 | Nov 2015–Jan 2020 | To report the feasibility of RNSM with a large series of 138 procedures | Prospective, single center | 79 (138) |
Prophylactic (75%) Therapeutic (25%) |
| Lai et al. [44] | 2020 | July 2011–Sep 2019 | To critically compare RNSM vs. CNSM procedures from different aspects (clinical outcomes, patient-reported aesthetic results, and medical costs) in the management of patients with breast cancer | Retrospective case–control comparison study, single center |
CNSM 62 RNSM 54 |
Therapeutic |
| Houvenaeghel et al. [45] | 2021 | Nov 2016–Mar 2020 | To compare RNSM and CNSM in terms of the breast complication rate (main objective) and hospital stay, duration of surgery, cost evaluation, and patient satisfaction (secondary objectives) | Prospective case–control comparison study, single center |
CNSM 142 RNSM 87 |
Primary (70, 80.5%) Local recurrence (10, 11.5%) Prophylactic (7, 8.0%) |
| Loh et al. [46] | 2020 | April 2018–Jan 2020 | To report the use of RNSM in patients with breast cancer and analyze the learning curve of one surgeon in a single medical center | Retrospective single center | 78 (85) | Therapeutic |
| Kuo et al. [47] | 2019 | N/A | To report the combination of robotic mastectomy and immediate microsurgical free-flap reconstruction and exploit its oncologic and aesthetic advantages | Case series, single center | 3 | Therapeutic |
CNSM conventional nipple-sparing mastectomy, IBR immediate breast reconstruction, KoREa-BSG Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group, RNSM robotic nipple-sparing mastectomy
Table 2.
Summary of ongoing studies of RNSM
| Identifier | Sample size | Randomization | Indication | Intervention | Postoperative outcomes | Oncologic outcomes | PRO | Country of origin |
|---|---|---|---|---|---|---|---|---|
| NCT04585074 (current study) | 2000 | No |
Women with breast cancer High-risk women |
RNSM + ENSM CNSM |
Yes | Yes | Yes | South Korea |
| NCT04108117 | 300 | No |
Women with breast cancer High-risk women |
RNSM CNSM |
Yes | Yes | No | South Korea |
| NCT03440398 | 82 | Yes |
Women with breast cancer High-risk women |
RNSM CNSM |
Yes | Yes | Yes | Italy |
| NCT03892980 | 145 | No | High-risk women | RNSM | Yes | No | No | United States |
| NCT04537312 | 20 | No |
Women with breast cancer High-risk women |
RNSM | Yes | No | Yes | United States |
| NCT04457167 | 480 | No |
Women with breast cancer High-risk women |
RNSM CNSM |
Yes | No | No | France |
| NCT04151368 | 30 | No |
Women with breast cancer High-risk women |
RNSM | Yes | Yes | Yes | Canada |
| NCT04037852 | 180 | No | Women with breast cancer |
RNSM CNSM or ENSM |
Yes | Yes | Yes | Taiwan |
CNSM conventional nipple-sparing mastectomy, ENSM endoscopic nipple-sparing mastectomy, PRO Patient-reported outcome, RNSM robot-assisted nipple-sparing mastectomy
No intraoperative or postoperative mortality caused by RNSM has been reported and only one case of open conversion experienced in the literature [16]. The rates of postoperative complications have primarily been acceptable [16, 41, 42, 48]. In terms of oncologic safety, a few reports have indicated a favorable incidence of margin positivity and no locoregional recurrence within short-term follow-up [42, 47, 48]. The innovative robotic technique in breast surgery has been presented as a safe and feasible surgical procedure that is not inferior to the conventional methods in terms of early oncological outcomes [42].
As RNSM attracted attention and became the focus of more research, standardized RNSM guidelines were developed by a representative panel of 10 international experts in 2019 [49]. Previous studies have suggested that an indication and contraindication for RNSM is similar to CNSM [48–50].
Methods/Design
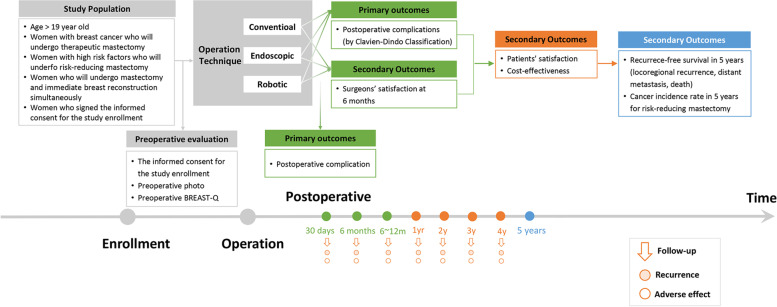
The MARRES study is a multi-institution cohort study being conducted by KoREa-BSG. It is prospectively collecting clinical data from patients undergoing mastectomy and reconstruction in academic hospitals in Korea (Fig. 1). The version 1.71 protocol for this study was approved on June 27, 2022. The principal investigator (PI) will periodically review and evaluate study conduct and progress according to the study protocol; data collection status for participant safety; and the quality, consistency, security, and accessibility of the accumulated data at regular intervals during the study. The protocol and study progress will be audited annually according to the policy of each Institutional Review Board. The PI and sub-PIs meet to communicate every one or two months. Only the PI and sub-PIs will have access to the final trial dataset, but the dataset could be shared with the permission of the PI upon reasonable request. We will publish the results of the dataset in a medical journal.
Fig. 1.
Flowchart of the prospective cohort study
The inclusion criteria for the patients are as follows: adult women older than 19 with breast cancer or a high risk of breast cancer (patients with BRCA1/2, TP53, PALB2 mutations, etc.) who are scheduled for a therapeutic or risk reducing mastectomy and want immediate reconstruction. The PI or a physician delegated by the PI provides information about the study and an adequate opportunity to consider all options to all potential trial participants and obtains signed informed consent forms before enrollment. Patients who plan breast-conserving surgery or who are not candidates for IBR will be excluded. The target number of enrolled patients is 2000.
Patients' clinicopathological factors, including height and weight, will be collected, along with data on surgical results, including photographic and oncological results and cost-effectiveness and patient satisfaction data. The basic characteristics, photographs, and satisfaction of patients will be collected preoperatively using the Breast-Q survey. Patient data will be collected within 6 months of surgery, including clinicopathological factors, surgery results (drainage amount, removal date, and complications), postoperative recovery evaluations, complications and adverse reactions, and cost. Between 6 months and 1 year after surgery, the results of surgery, recurrence, a satisfaction survey, intraoperative console video, and postoperative photographs will be collected. Every 12 months thereafter, data on adjuvant therapy (chemotherapy, radiation therapy, targeted therapy, and endocrine therapy), surgical results, recurrence, adverse events, and other unintended effects of the interventions will be investigated and collected until the end of the study period.
Personal information that can identify the subjects will be kept confidential even after the publication of the study. After enrollment, each participant’s name will be encrypted and replaced with a participant number. The datasets collected from each institution will be accumulated and stored in a designated computer on a central server. Access to that database is restricted to authorized researchers.
An interim analysis will be done after the recruitment of subjects is completed, no later than the 3rd year after the start of data collection. The PI will access those interim results and report them to the all sub-PIs. At that time, the PI and sub-PIs will determine whether the trial should be terminated.
Follow-up observation and data collection of the subjects will continue for 4–8 years after enrollment, and then the final analysis will be conducted. All patients will be guided to complete follow-up evaluations indicating recurrences and survival for at least 5 years.
Timeline
Actual Study Start Date: April 8, 2020
Estimated Primary Completion Date: April 7, 2025
Estimated Study Completion Date: April 7, 2030
Study population
Intervention details.
Procedure: Robotic nipple-sparing mastectomy
Patients undergoing RNSM and IBR are enrolled in this arm. RNSM should be performed using a robotic surgical system (da Vinci S, Si, X, Xi, or SP). Axillary or lateral incisions are used for this procedure.
Other names: Robot-assisted nipple-sparing mastectomy, robot mastectomy, robotic mastectomy, hybrid robotic nipple-sparing mastectomy, robot-assisted nipple-areolar complex, and skin-sparing mastectomy.
Procedure: Endoscopic nipple-sparing mastectomy
Patients undergoing ENSM and IBR are enrolled in this arm. ENSM should be performed using endoscopic tools for mastectomies [51].
Other names: Endoscopy-assisted nipple-sparing mastectomy, endoscopic-assisted nipple-sparing mastectomy, endoscopic nipple-sparing mastectomy, endoscopic subcutaneous mastectomy, video-assisted nipple-sparing mastectomy, endoscopic video-assisted breast surgery, videoendoscopic nipple-sparing mastectomy.
Procedure: Conventional mastectomy (including nipple-sparing mastectomy, skin-sparing mastectomy)
Patients undergoing a CM and immediate reconstruction are enrolled in this arm. CM should not be performed using a robotic or endoscopic surgical system. Any incisions can be used for this procedure. CM also includes NSM and SSM.
Other names: Total mastectomy, mastectomy, nipple-sparing mastectomy, skin-sparing mastectomy.
Endpoints
Primary endpoint
Postoperative complication rates within 30 postoperative days: Postoperative complication rates are calculated as the total number of postoperative complication cases per total operation cases.
Clavien-Dindo grade of postoperative complications in 180 postoperative days: The Clavien-Dindo grade of postoperative complications is evaluated. Only grade III or higher postoperative complications are used for this analysis.
Secondary endpoints
Recurrence-free survival (RFS) in 5 postoperative years: RFS events include locoregional recurrence, distant recurrence, and death by any cause. Contralateral breast cancer and a second primary malignancy are considered to be censored data.
Cancer incidence rate in 5 postoperative years: Cancer incidence rate for those who underwent a prophylactic mastectomy.
Patient satisfaction with reconstruction results preoperative (baseline) and 6 to 12 postoperative months: Reconstruction module with pre- and postoperative scales for satisfaction with the abdomen, as assessed by BREAST-Q version 2.0. (This scale should be completed only by patients who have had reconstruction using a TRAM flap or DIEP flap. Otherwise, it is skipped.) Satisfaction with the back, as assessed by BREAST-Q version 2.0. (This scale should only be completed by patients who have had reconstruction using an LD flap. Otherwise, it is skipped.) Satisfaction with implants, as assessed by BREAST-Q version 2.0. (This scale should only be completed by patients who have had reconstruction using implants. Otherwise, it is skipped.) In all scales, higher scores reflect better outcomes.
Surgeon's satisfaction with surgery within 6 postoperative months: Assessed using scoring criteria for cosmetic assessment [52], response options (overall symmetry, postoperative scar, NAC symmetry, etc.), and range (0–10). Higher scores reflect better outcomes.
Evaluation of the cost-effectiveness of the definitive surgery according to the surgical method: Assessed by conducting a patient’s survey 6 months to 1 year after the last surgery. This evaluation uses the EuroQol five-dimension scale, Korean version questionnaire. In all scales, higher scores reflect better outcomes.
Inclusion & exclusion criteria
Inclusion Criteria:
Female patients older than 19
Patients with breast cancer or a high risk of breast cancer (BRCA1/2, TP53, PALB2 mutations, etc.)
Patients scheduled for a therapeutic or risk reducing mastectomy (including conventional, skin-sparing, and areolar-conserving mastectomies)
Patients who want immediate reconstruction
Patients who provide written consent to participate in the study
Exclusion Criteria:
Patients scheduled for a breast-conserving surgery
Patients who do not want immediate reconstruction during mastectomy
Patients who undergo a different procedure on the other side breast simultaneously
Surgeon Inclusion Criteria:
Surgeons who are members of KoREa-BSG
Surgeons who participated in an education program for RNSM as an operator more than once
Stopping criteria
Patients are free to withdraw from the study at any point without limitation. All data collected before the withdrawal will be included. After a withdrawal, no additional data will be collected from that patient. If the PI determines that it is inappropriate to continue the clinical trial, some part or the entire study could be stopped.
Sample size determination
We determined the sample size for this study according to the real clinical experience of the PI, without statistical calculation, to explore the safety and effectiveness of the robotic surgical system and derive clinical outcomes from the procedures. The main institution performed 4038 cases of breast surgery and 746 cases of mastectomy and IBR (18.5%) between 2016 and 2018, including 86 RRMs (86/746, 11.5%). The iBRA study reported the uni- or bilateral RRMs accounted for 34.8% of mastectomies [53]. The mean value of the RRM rate between the main institution and the iBRA study was 23.15%. We estimated an approximate sample size of 11.5% for the main institution and 23.15% for the other institutions.Planned enrollment is 2000 total patients across the conventional, endoscopic, and robotic groups.
Pre-specified subgroup analysis
Because of the long duration and expensive cost of a randomized trial, we will extract random patients from our prospective cohort and conduct subgroup analyses as a randomized registry trial. In a previous study, the rates of grade III complications in the reference group and the robotic group were 34.8% and 17.2%, respectively. For the comparison of complication rates among different surgical methods, the required sample size was calculated as 112 (1:1 random match) according to the method used in a randomized registry trial previously introduced to detect a non-inferiority margin difference, which achieved 80% power between the group proportions of 0.0500. The robotic group proportion is assumed to be 39.8% under the null hypothesis of inferiority. The power was computed for the case in which the actual robotic group proportion is 17.1%. The test statistic used is the one-sided Z test (unpooled). The significance level of the test was targeted at 0.0250. The actual significance level achieved by this design is 0.0265.
Statistical analyses
We have categorized three study sets: Conventional vs. endoscopic, conventional vs. robotic, and conventional vs. endoscopic and robotic. Additionally, subgroup analyses of a randomized registry trial will be conducted to compare the surgical and oncologic outcomes between the conventional and minimally invasive (endoscopic and robotic) groups. Primary and some secondary outcomes (postoperative complications, the satisfaction of patients and surgeons, and the cost-effectiveness evaluation) and other categorical variables will be examined by the chi-square test or Fisher's exact test. Continuous variables will be examined by t-testing or ANOVA and Mann–Whitney testing or Kruskal–Wallis testing, if needed. The other secondary outcomes (RFS, OS, and cancer incidence rates in 5 years) will be examined using Kaplan–Meier plots and log-rank testing. No imputation will be performed for subjects who have missing data due to dropping out of the study. Data relating to major protocol non-adherence due to patients dropping out of the study will be excluded from the analysis population.
Discussion
This study will provide evidence about the surgical outcomes, oncologic outcomes, and patient satisfaction with RNSM and ENSM, compared with CM.
This prospective cohort study has some advantages over previous retrospective studies conducted in a single center or with a single surgeon. The sample size of the study is larger than previously published studies of RNSM. Additionally, our protocol will collect large-scale PRO data about cosmesis after RNSM. The participation of many surgeons from multiple institutions will allow us to observe the effects of different surgeons or institutions on the clinical outcomes of the surgical procedures. In Korea, an increasing number of breast surgeons has been performing RNSM because of active educational programs and systematic research activities. Therefore, the involvement of many skilled surgeons will produce accurate clinical outcomes for the procedures. However, because this is not a randomized controlled trial, selection bias might still occur in determining the surgical modality for each subject. To overcome that limitation and secure the surgical and oncologic outcomes from a new, innovative surgical procedure, we are planning a randomized controlled trial for the near future.
Through this study, we wish to confirm that minimally invasive breast surgery can be considered as a standard treatment for women with breast cancer and as a preventive treatment for women with a high risk of pathogenic variants.
Acknowledgements
The abstract of this study was accepted as a poster presentation at the 2021 San Antonio Breast Cancer Symposium, 7–10 December 2021.
Abbreviations
- CM
Conventional mastectomy
- CNSM
Conventional nipple-sparing mastectomy
- DIEP
Deep inferior epigastric perforators
- DTI
Direct-to-implant
- EABS
Endoscopic-assisted breast surgery
- ENSM
Endoscopic nipple-sparing mastectomy
- IBR
Immediate breast reconstruction
- KoREa-BSG
Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group
- LD
Latissimus dorsi
- MARRES
Mastectomy with Reconstruction Including Robot Endoscopic Surgery
- NAC
Nipple-areolar complex
- NSM
Nipple-sparing mastectomy
- OS
Overall survival
- OPBC
Oncoplastic Breast Consortium
- PI
Principal investigator
- PRO
Patient-reported outcomes
- QOL
Quality of life
- RFS
Recurrence-free survival
- RNSM
Robotic nipple-sparing mastectomy
- RRM
Risk-reducing mastectomy
- SSM
Skin-sparing mastectomy
- TRAM
Transverse abdominis rectus muscle
Authors’ contributions
HSP conceived of the study, and HSP, JMR, and JL contributed to its design and development. HSP, JMR, and JL wrote and revised the manuscript. KoREa-BSG supports the administration of the study. All authors read and approved the final manuscript.
Funding
This study is partially supported by Medtronic (PHO0215021). This funding source had no role in the design of this study and did not have any role in its execution, analyses, interpretation of the data, or decision to submit results.
Medtronic,PHO0215021,Jai Min Ryu
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The protocol for this trial was registered with clinicaltrials.gov (NCT04585074) on April 8, 2020. The investigational plan for the trial was reviewed and approved by the Institutional Review Boards of all participating institutions (Severance Hospital IRB No. 4–2020-0165). All participants will provide written informed consent to study participation before enrollment.
Consent for publication
Not applicable.
Competing interests
Hyung Seok Park received honoraria from AstraZeneca, Takeda, Ethicon, Medtronic, and Intuitive Surgical. Jai Min Ryu received a research grant from Medtronic for this study. No other relationships or activities have occurred that could appear to have influenced the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jai Min Ryu and Jeea Lee contributed equally to this work.
References
- 1.Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048–1053. doi: 10.1097/00006534-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Cocquyt VF, Blondeel PN, Depypere HT, Van De Sijpe KA, Daems KK, Monstrey SJ, Van Belle SJ. Better cosmetic results and comparable quality of life after skin-sparing mastectomy and immediate autologous breast reconstruction compared to breast conservative treatment. Br J Plast Surg. 2003;56(5):462–470. doi: 10.1016/S0007-1226(03)00198-X. [DOI] [PubMed] [Google Scholar]
- 3.Reefy S, Patani N, Anderson A, Burgoyne G, Osman H, Mokbel K. Oncological outcome and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction: a prospective observational study. BMC Cancer. 2010;10:171. doi: 10.1186/1471-2407-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mota BS, Riera R, Ricci MD, Barrett J, de Castria TB, Atallah AN, Bevilacqua JL: Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev. 2016;11(11):CD008932. [DOI] [PMC free article] [PubMed]
- 5.Agha RA, Al Omran Y, Wellstead G, Sagoo H, Barai I, Rajmohan S, Borrelli MR, Vella-Baldacchino M, Orgill DP, Rusby JE. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open. 2019;3(2):135–145. doi: 10.1002/bjs5.50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsharif E, Ryu JM, Choi HJ, Nam SJ, Kim SW, Yu J, Chae BJ, Lee SK, Lee JE: Oncologic Outcomes of Nipple-Sparing Mastectomy with Immediate Breast Reconstruction in Patients with Tumor-Nipple Distance Less than 2.0 cm. J Breast Cancer. 2019;22(4):613–623. [DOI] [PMC free article] [PubMed]
- 7.Weber WP, Haug M, Kurzeder C, Bjelic-Radisic V, Koller R, Reitsamer R, Fitzal F, Biazus J, Brenelli F, Urban C, et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res Treat. 2018;172(3):523–537. doi: 10.1007/s10549-018-4937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corso G, De Lorenzi F, Vicini E, Pagani G, Veronesi P, Sargenti M, Magnoni F, Naninato P, Maisonneuve P, Sangalli C et al: Nipple-sparing mastectomy with different approaches: surgical incisions, complications, and cosmetic results. Preliminary results of 100 consecutive patients at a single center. J Plast Reconstr Aesthet Surg. 2018;71(12):1751–1760. [DOI] [PubMed]
- 9.Lanthaler M, Spinelli R, Tasch C, Sieb M, Harfmann M, Nitto A, Pierer G, Bauer T. Influence of Incision Site on Postoperative Outcome in Skin-/Nipple-Sparing Mastectomy: Is There a Difference between Radial and Inframammary Incision? Breast Care (Basel) 2020;15(3):265–271. doi: 10.1159/000502408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Yoon C, Bae SJ, Cha C, Kim D, Lee J, Ahn SG, Roh TS, Kim YS, Jeong J. Comparison of complications according to incision types in nipple-sparing mastectomy and immediate reconstruction. Breast (Edinburgh, Scotland) 2020;53:85–91. doi: 10.1016/j.breast.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, Kurihara T, Tozaki M. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Indian J Surg Oncol. 2010;1(3):232–239. doi: 10.1007/s13193-011-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlander LD, Sundin J, Bakshandeh N. Endoscopy mastectomy and breast reconstruction: endoscopic breast surgery. Aesthetic Plast Surg. 1995;19(1):27–29. doi: 10.1007/BF00209307. [DOI] [PubMed] [Google Scholar]
- 13.Mok CW, Lai HW. Endoscopic-assisted surgery in the management of breast cancer: 20 years review of trend, techniques and outcomes. Breast (Edinburgh, Scotland) 2019;46:144–156. doi: 10.1016/j.breast.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Toesca A, Peradze N, Manconi A, Nevola Teixeira LF: Reply to the letter to the editor "Robotic-assisted Nipple Sparing Mastectomy: A feasibility study on cadaveric models" by Sarfati B. et al. J Plast Reconstr Aesthet Surg. 2017;70(4):558–560. [DOI] [PubMed]
- 15.Park HS, Kim JH, Lee DW, Song SY, Park S, Kim SI, Ryu DH, Cho YU. Gasless Robot-Assisted Nipple-Sparing Mastectomy: A Case Report. J Breast Cancer. 2018;21(3):334–338. doi: 10.4048/jbc.2018.21.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angarita FA, Castelo M, Englesakis M, McCready DR, Cil TD. Robot-assisted nipple-sparing mastectomy: systematic review. Br J Surg. 2020;107(12):1580–1594. doi: 10.1002/bjs.11837. [DOI] [PubMed] [Google Scholar]
- 17.Margenthaler JA. Robotic Mastectomy-Program Malfunction? JAMA Surg. 2020;155(6):461–462. doi: 10.1001/jamasurg.2019.6361. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RS, Sanders T, Park A, Buras R, Liang W, Harris C, Mylander C, Rosman M, Holton L, Singh D, et al. Prospective Study Comparing Surgeons' Pain and Fatigue Associated with Nipple-Sparing versus Skin-Sparing Mastectomy. Ann Surg Oncol. 2017;24(10):3024–3031. doi: 10.1245/s10434-017-5929-9. [DOI] [PubMed] [Google Scholar]
- 19.Ryu JM, Kim JY, Choi HJ, Ko B, Kim J, Cho J, et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience of the Korea robot-endoscopy minimal access breast surgery study group (KoREa-BSG). Ann Surg. 2022;275(5):985–91. [DOI] [PubMed]
- 20.Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962;30:676–682. doi: 10.1097/00006534-196212000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hinton CP, Doyle PJ, Blamey RW, Davies CJ, Holliday HW, Elston CW. Subcutaneous mastectomy for primary operable breast cancer. Br J Surg. 1984;71(6):469–472. doi: 10.1002/bjs.1800710623. [DOI] [PubMed] [Google Scholar]
- 22.Headon HL, Kasem A, Mokbel K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch Plast Surg. 2016;43(4):328–338. doi: 10.5999/aps.2016.43.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallon P, Feron JG, Couturaud B, Fitoussi A, Lemasurier P, Guihard T, Cothier-Savay I, Reyal F. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg. 2013;131(5):969–984. doi: 10.1097/PRS.0b013e3182865a3c. [DOI] [PubMed] [Google Scholar]
- 24.Stanec Z, Žic R, Budi S, Stanec S, Milanović R, Vlajčić Z, Roje Z, Rudman F, Martić K, Held R, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73(5):485–491. doi: 10.1097/SAP.0b013e31827a30e6. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZY, Kim HJ, Lee JW, Chung IY, Kim JS, Lee SB, Son BH, Eom JS, Kim SB, Gong GY, et al. Breast Cancer Recurrence in the Nipple-Areola Complex After Nipple-Sparing Mastectomy With Immediate Breast Reconstruction for Invasive Breast Cancer. JAMA Surg. 2019;154(11):1030–1037. doi: 10.1001/jamasurg.2019.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249(3):461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 27.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34(2):143–148. doi: 10.1016/j.ejso.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68(5):446–450. doi: 10.1097/SAP.0b013e3182394bba. [DOI] [PubMed] [Google Scholar]
- 29.Sherman KA, Woon S, French J, Elder E. Body image and psychological distress in nipple-sparing mastectomy: the roles of self-compassion and appearance investment. Psychooncology. 2017;26(3):337–345. doi: 10.1002/pon.4138. [DOI] [PubMed] [Google Scholar]
- 30.Sacchini V, Pinotti JA, Barros AC, Luini A, Pluchinotta A, Pinotti M, Boratto MG, Ricci MD, Ruiz CA, Nisida AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714. doi: 10.1016/j.jamcollsurg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 6 Dec 2022.
- 32.Eaves FF, 3rd, Bostwick J, 3rd, Nahai F, Murray DR, Styblo TM, Carlson GW: Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plastic Surg. 1995;22(4):683–695. [PubMed]
- 33.Yamashita K, Shimizu K: Endoscopic video-assisted breast surgery: procedures and short-term results. J Nippon Med Sch. 2006;73(4):193–202. [DOI] [PubMed]
- 34.Ho WS, Ying SY, Chan AC. Endoscopic-assisted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer. Surg Endosc. 2002;16(2):302–306. doi: 10.1007/s004640000203. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope-assisted subcutaneous mastectomy and reconstruction for breast cancer. Surgery. 2002;131(1 Suppl):S324–329. doi: 10.1067/msy.2002.120120. [DOI] [PubMed] [Google Scholar]
- 36.Lai HW, Wu HS, Chuang KL, Chen DR, Chang TW, Kuo SJ, Chen ST, Kuo YL. Endoscopy-Assisted Total Mastectomy Followed by Immediate Pedicled Transverse Rectus Abdominis Musculocutaneous (TRAM) Flap Reconstruction: Preliminary Results of 48 Patients. Surg Innov. 2015;22(4):382–389. doi: 10.1177/1553350614546003. [DOI] [PubMed] [Google Scholar]
- 37.Kuo YL, Chang CH, Chang TY, Chien HF, Liao LM, Hung CS, Lin SL, Chen ST, Chen DR, Lai HW. Endoscopy-Assisted Total Mastectomy with and without Immediate Reconstruction: An Extended Follow-Up Multicenter Study. Plastic Reconstruct Surg. 2021;147(2):267–278. doi: 10.1097/PRS.0000000000007587. [DOI] [PubMed] [Google Scholar]
- 38.Lee HY, Chang YW, Yu DY, Lee TY, Kim DW, Kim WY, Jung SP, Woo SU, Lee JB, Son GS. Comparison of Single Incision Endoscopic Nipple-Sparing Mastectomy and Conventional Nipple-Sparing Mastectomy for Breast Cancer Based on Initial Experience. J Breast Cancer. 2021;24(2):196–205. doi: 10.4048/jbc.2021.24.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathat G, Herlin C, Bonnel C, Captier G, Duraes M. Endoscopic Nipple-Sparing Mastectomy with Immediate Prepectoral Implant-Based Reconstruction: A Case Report. Am J Case Rep. 2019;20:1812–1816. doi: 10.12659/AJCR.919669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leal Ghezzi T, Campos Corleta O. 30 Years of Robotic Surgery. World J Surg. 2016;40(10):2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Park HS, Lee H, Lee DW, Song SY, Lew DH, Kim JY, Park S, Kim SI. Post-Operative Complications and Nipple Necrosis Rates Between Conventional and Robotic Nipple-Sparing Mastectomy. Front Oncol. 2020;10:594388. doi: 10.3389/fonc.2020.594388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toesca A, Invento A, Massari G, Girardi A, Peradze N, Lissidini G, Sangalli C, Maisonneuve P, Manconi A, Gottardi A, et al. Update on the Feasibility and Progress on Robotic Breast Surgery. Ann Surg Oncol. 2019;26(10):3046–3051. doi: 10.1245/s10434-019-07590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanson C, Roulot A, Honart JF, Rimareix F, Leymarie N, Sarfati B: Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A prospective Study of 138 Procedures. Chirurgia (Bucharest, Romania : 1990). 2021; 116(2):135–142. [DOI] [PubMed]
- 44.Lai HW, Chen ST, Mok CW, Lin YJ, Wu HK, Lin SL, Chen DR, Kuo SJ. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer- A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg. 2020;73(8):1514–1525. doi: 10.1016/j.bjps.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Houvenaeghel G, Barrou J, Jauffret C, Rua S, Sabiani L, Van Troy A, Buttarelli M, Blache G, Lambaudie E, Cohen M, et al. Robotic Versus Conventional Nipple-Sparing Mastectomy With Immediate Breast Reconstruction. Front Oncol. 2021;11:637049. doi: 10.3389/fonc.2021.637049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loh ZJ, Wu TY, Cheng FT. Evaluation of the Learning Curve in Robotic Nipple-sparing Mastectomy for Breast Cancer. Clin Breast Cancer. 2021;21(3):e279–e284. doi: 10.1016/j.clbc.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Kuo WL, Huang JJ, Huang YT, Chueh LF, Lee JT, Tsai HP, Chen SC. Robot-assisted Mastectomy Followed by Immediate Autologous Microsurgical Free Flap Reconstruction: Techniques and Feasibility in Three Different Breast Cancer Surgical Scenarios. Clin Breast Cancer. 2020;20(1):e1–e8. doi: 10.1016/j.clbc.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Park HS, Lee J, Lee DW, Song SY, Lew DH, Kim SI, Cho YU. Robot-assisted Nipple-sparing Mastectomy with Immediate Breast Reconstruction: An Initial Experience. Sci Rep. 2019;9(1):15669. doi: 10.1038/s41598-019-51744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai HW, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC, Cheng FT, Kuo WL, Peradze N, Song SY, et al. Consensus Statement on Robotic Mastectomy-Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg. 2020;271(6):1005–1012. doi: 10.1097/SLA.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 50.Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, Bonanni B, Curigliano G, Rietjens M, Viale G, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: Feasibility and safety study. Breast (Edinburgh, Scotland) 2017;31:51–56. doi: 10.1016/j.breast.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin H. Current Trends in and Indications for Endoscopy-Assisted Breast Surgery for Breast Cancer. Adv Exp Med Biol. 2021;1187:567–590. doi: 10.1007/978-981-32-9620-6_30. [DOI] [PubMed] [Google Scholar]
- 52.Ueda S, Tamaki Y, Yano K, Okishiro N, Yanagisawa T, Imasato M, Shimazu K, Kim SJ, Miyoshi Y, Tanji Y, et al. Cosmetic outcome and patient satisfaction after skin-sparing mastectomy for breast cancer with immediate reconstruction of the breast. Surgery. 2008;143(3):414–425. doi: 10.1016/j.surg.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Potter S, Conroy EJ, Cutress RI, Williamson PR, Whisker L, Thrush S, Skillman J, Barnes NLP, Mylvaganam S, Teasdale E, et al. Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (iBRA): a multicentre, prospective cohort study. Lancet Oncol. 2019;20(2):254–266. doi: 10.1016/S1470-2045(18)30781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.