Abstract
Background
Endometriosis is a common gynaecological condition affecting 6 to 11% of reproductive‐age women and may cause dyspareunia, dysmenorrhoea, and infertility. One treatment strategy is medical therapy with gonadotrophin‐releasing hormone analogues (GnRHas) to reduce pain due to endometriosis. One of the adverse effects of GnRHas is a decreased bone mineral density. In addition to assessing the effect on pain, quality of life, most troublesome symptom and patients' satisfaction, the current review also evaluated the effect on bone mineral density and risk of adverse effects in women with endometriosis who use GnRHas versus other treatment options.
Objectives
To assess the effectiveness and safety of GnRH analogues (GnRHas) in the treatment of painful symptoms associated with endometriosis and to determine the effects of GnRHas on bone mineral density of women with endometriosis.
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO and the trial registries in May 2022 together with reference checking and contact with study authors and experts in the field to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) which compared GnRHas with other hormonal treatment options, including analgesics, danazol, intra‐uterine progestogens, oral or injectable progestogens, gestrinone and also GnRHas compared with no treatment or placebo. Trials comparing GnRHas versus GnRHas in conjunction with add‐back therapy (hormonal or non‐hormonal) or calcium‐regulation agents were also included in this review.
Data collection and analysis
We used standard methodology as recommended by Cochrane. Primary outcomes are relief of overall pain and the objective measurement of bone mineral density. Secondary outcomes include adverse effects, quality of life, improvement in the most troublesome symptoms and patient satisfaction.
Due to high risk of bias associated with some of the studies, primary analyses of all review outcomes were restricted to studies at low risk of selection bias. Sensitivity analysis including all studies was then performed.
Main results
Seventy‐two studies involving 7355 patients were included. The evidence was very low to low quality: the main limitations of all studies were serious risk of bias due to poor reporting of study methods, and serious imprecision.
Trials comparing GnRHas versus no treatment
We did not identify any studies.
Trials comparing GnRHas versus placebo
There may be a decrease in overall pain, reported as pelvic pain scores (RR 2.14; 95% CI 1.41 to 3.24, 1 RCT, n = 87, low‐certainty evidence), dysmenorrhoea scores (RR 2.25; 95% CI 1.59 to 3.16, 1 RCT, n = 85, low‐certainty evidence), dyspareunia scores (RR 2.21; 95% CI 1.39 to 3.54, 1 RCT, n = 59, low‐certainty evidence), and pelvic tenderness scores (RR 2.28; 95% CI 1.48 to 3.50, 1 RCT, n = 85, low‐certainty evidence) after three months of treatment. We are uncertain of the effect for pelvic induration, based on the results found after three months of treatment (RR 1.07; 95% CI 0.64 to 1.79, 1 RCT, n = 81, low‐certainty evidence). Besides, treatment with GnRHas may be associated with a greater incidence of hot flushes at three months of treatment (RR 3.08; 95% CI 1.89 to 5.01, 1 RCT, n = 100, low‐certainty evidence).
Trials comparing GnRHas versus danazol
For overall pain, for women treated with either GnRHas or danazol, a subdivision was made between pelvic tenderness, partly resolved and completely resolved. We are uncertain about the effect on relief of overall pain, when a subdivision was made for overall pain (MD ‐0.30; 95% CI ‐1.66 to 1.06, 1 RCT, n = 41, very low‐certainty evidence), pelvic pain (MD 0.20; 95% CI ‐0.26 to 0.66, 1 RCT, n = 41, very low‐certainty evidence), dysmenorrhoea (MD 0.10; 95% CI ‐0.49 to 0.69, 1 RCT, n = 41, very low‐certainty evidence), dyspareunia (MD ‐0.20; 95% CI ‐0.77 to 0.37, 1 RCT, n = 41, very low‐certainty evidence), pelvic induration (MD ‐0.10; 95% CI ‐0.59 to 0.39, 1 RCT, n = 41, very low‐certainty evidence), and pelvic tenderness (MD ‐0.20; 95% CI ‐0.78 to 0.38, 1 RCT, n = 41, very low‐certainty evidence) after three months of treatment. For pelvic pain (MD 0.50; 95% CI 0.10 to 0.90, 1 RCT, n = 41, very low‐certainty evidence) and pelvic induration (MD 0.70; 95% CI 0.21 to 1.19, 1 RCT, n = 41, very low‐certainty evidence), the complaints may decrease slightly after treatment with GnRHas, compared to danazol, for six months of treatment.
Trials comparing GnRHas versus analgesics
We did not identify any studies.
Trials comparing GnRHas versus intra‐uterine progestogens
We did not identify any low risk of bias studies.
Trials comparing GnRHas versus GnRHas in conjunction with calcium‐regulating agents
There may be a slight decrease in bone mineral density (BMD) after 12 months treatment with GnRHas, compared to GnRHas in conjunction with calcium‐regulating agents for anterior‐posterior spine (MD ‐7.00; 95% CI ‐7.53 to ‐6.47, 1 RCT, n = 41, very low‐certainty evidence) and lateral spine (MD ‐12.40; 95% CI ‐13.31 to ‐11.49, 1 RCT, n = 41, very low‐certainty evidence).
Authors' conclusions
For relief of overall pain, there may be a slight decrease in favour of treatment with GnRHas compared to placebo or oral or injectable progestogens. We are uncertain about the effect when comparing GnRHas with danazol, intra‐uterine progestogens or gestrinone. For BMD, there may be a slight decrease when women are treated with GnRHas, compared to gestrinone. There was a bigger decrease of BMD in favour of GnRHas, compared to GnRHas in conjunction with calcium‐regulating agents. However, there may be a slight increase in adverse effects when women are treated with GnRHas, compared to placebo or gestrinone.
Due to a very low to low certainty of the evidence, a wide range of outcome measures and a wide range of outcome measurement instruments, the results should be interpreted with caution.
Keywords: Female; Humans; Calcium; Calcium, Dietary; Danazol; Danazol/therapeutic use; Drug-Related Side Effects and Adverse Reactions; Dysmenorrhea; Dyspareunia; Dyspareunia/drug therapy; Dyspareunia/etiology; Endometriosis; Endometriosis/complications; Endometriosis/drug therapy; Gestrinone; Gonadotropin-Releasing Hormone; Pelvic Pain; Pelvic Pain/drug therapy; Pelvic Pain/etiology; Progestins; Progestins/therapeutic use
Plain language summary
Gonadotrophin‐releasing hormone analogues for pain associated with endometriosis
What are the benefits and risks of gonadotrophin‐releasing hormone analogues (GnRHas) for pain associated with endometriosis?
Key messages
GnRHas offer more pain reduction compared to placebo or progestogens. However, the largest decrease in bone mineral density (BMD) was found when using GnRHas, compared to a different hormone called gestrinone and GnRHas together with calcium‐regulating agents (acting on bone).
Most adverse effects were hot flushes for patients treated with GnRHas or gestrinone (hormone) and weight gain when treated with danazol (hormone).
Further, well‐designed research is needed to provide better understanding of the benefits and risks of using GnRHas and other hormonal treatment options for pain associated with endometriosis.
What is endometriosis?
Endometriosis is a common condition, affecting women of childbearing age, and is usually due to the presence of endometrial‐like tissue in places other than the uterus. The most reported symptoms are pain and infertility.
What are GnRHas?
GnRHas are a group of drugs often used to treat endometriosis. GnRHas are a synthetic form of the hormone gonadorelin, released by the hypothalamus in the brain. They stimulate the pituitary gland in the brain to produce luteinising hormone (LH) and follicle‐stimulating hormone (FSH), both reproductive hormones. These reproductive hormones further stimulate the production of progesterone and oestrogen in the ovaries, the hormones that control your menstrual cycle.
However, continued use of GnRHas results in a suppression of ovarian function and therefore reduces oestrogen and progesterone levels. This will in turn result in a decrease of endometrial tissue and therefore reduce complaints of endometriosis.
Because GnRHas temporarily stop the production of reproductive hormones, they mimic symptoms of menopause, including a decrease in bone mineral density (the amount of calcium and other minerals present in your bones).
What did we want to find out?
In the current review, we looked at women with endometriosis, who were treated with GnRHas, and compared this treatment with other forms of hormonal treatment.
We wanted to find out if GnRHas were better than any other hormonal treatment to improve pain and to see their effect on bone mineral density.
Additionally, we wanted to find out if GnRHas were associated with an improved quality of life and also unveil any unwanted effects.
What did we do?
The review involved searching for studies that investigated the effect of GnRHas compared with placebo (dummy treatment) and other hormonal treatment in women with endometriosis. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 72 trials that involved 7355 women with endometriosis.
‐ A difference in overall pain, reported as pain reduction was seen in favour of GnRHas compared to placebo. We also saw that women treated with GnRHas had less pelvic pain reduction and an increase in endometriotic lesions after six months of treatment, compared to danazol.
‐ After six months of treatment, there was a greater decrease of pain for women treated with GnRHas compared to gestrinone.
‐ No difference was seen in pain scores between women treated with GnRHas compared to other hormonal treatment options.
‐ Most adverse effects were seen in women treated with GnRHas compared to placebo (hot flushes), with danazol (weight gain) and gestrinone (hot flushes).
‐ A greater decrease in bone mineral density was found in GnRHas compared to gestrinone and GnRHas in conjunction with calcium‐regulating agents.
‐ For the other comparisons examined in the current review, we are uncertain of the effect between the examined groups. It should be noted, however, that the evidence was often of (very) low quality in the analyses undertaken for the other comparisons.
What are the limitations of the evidence?
The included studies were of low quality mainly due to poor reporting of study methods and the inaccuracy with which the results were reported.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to May 2022.
Summary of findings
Summary of findings 1. GnRHas compared to no treatment for relief of overall pain associated with endometriosis and its related adverse effects.
| GnRHas compared to no treatment for relief of overall pain associated with endometriosis | ||||||
| Population: Women with endometriosis Settings: Gynaecology clinics Intervention: GnRHas Comparison: No treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | GnRHas | |||||
| No studies included for any outcomes | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. GnRHas compared to placebo for relief of overall pain associated with endometriosis and its related adverse effects.
| GnRHas compared to placebo for relief of overall pain associated with endometriosis | ||||||
| Population: Women with endometriosis Settings: Gynaecology clinic Intervention: GnRHas Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | GnRHas | |||||
| Relief of overall pain ‐ reported as pelvic pain ‐ 3 months | 372 per 1000 |
796 per 1000 (525 to 1205) |
RR 2.14 (1.41 to 3.24) |
87 (1 study) |
⊕⊕⊝⊝ low1 | |
| Relief of overall pain ‐ reported as dysmenorrhoea ‐ 3 months | 439 per 1000 |
988 per 1000 (698 to 1387) |
RR 2.25 (1.59 to 3.16) |
87 (1 study) | ⊕⊕⊝⊝ low1 | |
| Relief of overall pain ‐ reported as dyspareunia ‐ 3 months | 387 per 1000 |
855 per 1000 (538 to 1370) |
RR 2.21 (1.39 to 3.54) |
87 (1 study) | ⊕⊕⊝⊝ low1 | |
| Relief of overall pain ‐ reported as pelvic tenderness scores‐ 3 months | 357 per 1000 |
814 per 1000 (529 to 1250) |
RR 2.28 (1.48 to 3.50) |
87 (1 study) | ⊕⊕⊝⊝ low1 | |
| Relief of overall pain ‐ reported as pelvic induration scores ‐ 3 months | 405 per 1000 |
434 per 1000 (259 to 726) |
RR 1.07 (0.64 to 1.79) |
87 (1 study) | ⊕⊕⊝⊝ low1 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence based on a single trial
Summary of findings 3. GnRHas compared to analgesics for relief of overall pain associated with endometriosis and its related adverse effects.
| GnRHas compared to analgesics for relief of overall pain associated with endometriosis | ||||||
| Population: Women with pain due to endometriosis Settings: Gynaecological clinics Intervention: GnRHas Comparison: Analgesics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Analgesics | GnRHas | |||||
| No studies included for any outcomes | ||||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 4. GnRHas compared to danazol for relief of overall pain associated with endometriosis and its related adverse effects.
| GnRHas compared to danazol for relief of overall pain associated with endometriosis | ||||||
| Population: Women with pain due to endometriosis Settings: Gynaecological clinics Intervention: GnRHas Comparison: Danazol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Danazol | GnRHas | |||||
| Relief of overall pain ‐ reported as pelvic tenderness, partly resolved ‐ 6 months | 316 per 1000 | 363 per 1000 (155 to 862) | RR 1.15 (0.49 to 2.73) | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ reported as pelvic tenderness, complete resolved ‐ 6 months | 579 per 1000 | 637 per 1000 (388 to 1048) | RR 1.10 (0.67 to 1.81) | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ 6 months | The mean relief of overall pain in the control groups was ‐4.6 | The mean relief of overall pain in the intervention group was 0.4 higher (‐0.86 to 1.66) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ reported as pelvic pain ‐ 6 months | The mean relief of pelvic pain in the control groups was ‐0.5 | The mean relief of pelvic pain in the intervention group was 0.5 higher (0.10 to 0.90) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ reported as dysmenorrhoea ‐ 6 months | The mean relief of dysmenorrhoea in the control groups was ‐2.4 | The mean relief of dysmenorrhoea in the intervention group was 0.4 higher (‐0.12 to 0.92) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ reported as pelvic induration ‐ 6 months | The mean relief of pelvic induration in the control groups was ‐0.7 | The mean relief of pelvic induration in the intervention group was 0.7 higher (0.21 to 1.19) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Relief of overall pain ‐ reported as pelvic tenderness ‐ 6 months | The mean relief of pelvic tenderness in the control groups was ‐0.7 | The mean relief of pelvic tenderness in the intervention group was 0.2 lower (‐0.75 to 0.35) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence based on a single trial
2 Small number of events
Summary of findings 5. GnRHas compared to intra‐uterine progestogens device for relief of overall pain associated with endometriosis and its related adverse effects.
| GnRHas compared to intra‐uterine progestogens device for relief of overall pain associated with endometriosis | ||||||
| Population: Women with pain due to endometriosis Settings: Gynaecological clinics Intervention: GnRHas Comparison: Intra‐uterine progestogens device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intra‐uterine progestogens device | GnRHas | |||||
| No studies included with only low risk of bias | ||||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 6. Effect of GnRHas versus other hormonal treatment on bone mineral density.
| Effect of GnRHas versus other hormonal treatment on bone mineral density | ||||||
|
Population: Women with endometriosis, treated with GnRHas with effect on bone mineral density Settings: Gynaecological clinics Intervention: GnRHas Comparison: Other hormonal treatment | ||||||
| Outcomes | Illustrative comparative risks* 95% CI |
Relative effect (95% CI) |
No of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Other hormonal treatment | GnRHas | |||||
|
GnRHas vs gestrinone Percentage change values ‐ 6 months |
The mean percentage change in BMD in the control groups was 0.88 | The mean percentage change in BMD in the intervention group was 1.96 lower (3.62 to 0.30) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
GnRHas vs gestrinone Percentage change values ‐ 12 months |
The mean percentage change in BMD in the control groups was 2.06 | The mean percentage change in BMD in the intervention group was 5.10 lower (7.39 to 2.81) |
‐ | 41 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
GnRHas vs GnRHas in conjunction with calcium‐regulating agents Anterior‐posterior spine ‐ 12 months |
The mean change in BMD in the control groups was 2.1 | The mean change in BMD in the intervention group was 7.00 lower (7.53 to 6.47) |
‐ | 43 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
GnRHas vs GnRHas in conjunction with calcium‐regulating agents Lateral spine ‐ 12 months |
The mean change in BMD in the control groups was 7.5 | The mean relief of pelvic pain in the intervention group was 12.40lower (13.31 to 11.49) |
‐ | 43 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence based on a single trial
2 Small number of events
Background
Description of the condition
Endometriosis is a common gynaecological condition. Endometriosis is characterised as an inflammatory condition leading to fibrotic tissue formation, predominantly found in the pelvic peritoneum, ovaries and rectovaginal septum (Burney 2012; Vigano 2018). Endometriosis is associated with symptoms such as dysmenorrhoea, dyspareunia, abdominal pain and infertility (Vercellini 2014). It affects 6% to 11% of women of reproductive age, but the prevalence increases in women with infertility or pelvic pain (Darwish 2006; Eskenazi 1997; Mahmood 1991; Meuleman 2009; Wheeler 1989).
The precise pathogenesis of endometriosis remains unclear, however there are some hypotheses that have widely been accepted. Possible theorised mechanisms of the pathogenesis of endometriosis include induction, in situ development and retrograde menstruation or implantation (Levander 1955; Sampson 1940; Van der Linden 1997). The 'induction theory', introduced by Levander and Normann in 1955, is based on the assumption that specific substances released during the degeneration of the endometrium induce endometriosis from omnipotent blastema, present in connective tissues (Bontis 1997; Levander 1955). The ‘in‐situ development theory’ states that endometriosis develops from either the Wolffian duct or knob, or from Müllerian tissue (Batt 2007; Van der Linden 1997). The most common hypothesis is based on retrograde menstruation; this theory proposes that endometriosis arises by the dissemination of endometrial‐like tissue to ectopic sites where implantation, hypertrophy and invasion of pelvic structures occurs (Burney 2012; Robboy 2010; Sampson 1940; Viganò 2004). This ectopic growth provokes an inflammatory response, resulting in the symptoms mentioned above (Guidice 2010). Endometriosis is generally believed to be an oestrogen‐dependent disorder (Zondervan 2020). Local oestradiol stimulates the activation of pain fibres and promotes sprouting of nociceptors that contribute to persistent inflammatory pain, that often worsens over time (Guidice 2010).
To treat chronic pelvic pain associated with endometriosis, repeated courses of medical therapy or surgical therapy (or both) are often required. Hormonal treatment, such as gonadotrophin‐releasing hormone analogues (GnRHas), suppress ovarian function and alter the endometrium as well as the endometriosis tissue, which in turn often results in amenorrhoea and relief of endometriosis‐related pain symptoms (Stratton 2011). In the current review, only the GnRH agonists are included, not the GnRH antagonists.
Description of the intervention
The GnRHas are a family of compounds that differ from natural gonadotrophin‐releasing hormone (GnRH), a ten‐amino‐acid hormone (decapeptide), by modifications in the decapeptide at positions six and ten (Shaw 1991). They may be administered intranasally, or by subcutaneous or intramuscular injection. Buserelin, goserelin, leuprorelin, nafarelin and triptorelin are some of the most common GnRHas. Hypo‐oestrogenic side effects (relating to low levels of oestrogen), such as hot flushes, mood swings, sleep disturbances and bone mass loss are common when prescribing GnRHas. This is considered significant as it could increase the risk of women developing osteoporosis and place them at risk of osteoporotic fractures. To prevent bone loss and other hypo‐oestrogenic symptoms, it is therefore recommended to prescribe hormonal add‐back therapy concomitantly.
Other common treatments for endometriosis‐associated symptoms (including infertility and pain), are analgesics, danazol and progestogens (Brown 2012), including intra‐uterine systems, combined oral contraceptive pills (Brown 2018) and surgical therapies (Bafort 2020). A combination of surgery with hormonal treatment (pre‐surgical, post‐surgical or pre‐ and post‐surgical hormonal therapy), is also used as a treatment option for people with endometriosis. Only post‐surgical medical therapy provides a reduction in pain symptoms, reduced rate of recurrence and increased chance of pregnancy (Chen 2020)
The European Society of Human Reproduction and Embryology (ESHRE) recommends the use of GnRHas (nafarelin, leuprorelin, buserelin, goserelin or triptorelin) as one of the options for reducing endometriosis‐associated pain, however, evidence is limited regarding dosage or duration of treatment (Becker 2022). In addition, clinicians are recommended to prescribe hormonal add‐back therapy to coincide with the start of GnRH agonist therapy, to prevent bone loss and hypo‐oestrogenic symptoms during treatment. It is recommended to give careful consideration to the use of GnRHas in young women and adolescents, since these individuals may not have reached maximum bone density (Becker 2022).
How the intervention might work
Non‐analgesic medical treatment of endometriosis aims to suppress the ectopic endometrium deposits in premenopausal women by inducing atrophy within the hormonally dependent ectopic endometrium, making the endometrial‐like tissue inactive. The observation that endometriosis is rarely diagnosed in hypo‐oestrogenic postmenopausal women led to the concept of medical treatment of endometriosis by induction of a pseudo‐menopause.
Gonadotrophin‐releasing hormone analogues are a potent synthetic analogue of the hypothalamic hormone gonadorelin. This stimulates the pituitary gland to produce luteinising hormone (LH) and follicle‐stimulating hormone (FSH). However, with continued use, suppression occurs due to exhaustion and desensitivity of the gonadotrophic pituitary cells. This suppresses ovarian function and therefore reduces oestrogen and progesterone levels, introducing a hypogonadotropic hypogonadal state.
In endometriosis, this treatment leads to a reduction in the endometriosis implants and induces atrophy within them (Chen 2020). By reducing the endometriosis implants, GnRHas can provide a reduction in pain and other endometriosis‐related complaints.
Why it is important to do this review
Endometriosis occurs in approximately one in every 10 women within the reproductive general population (Macer 2012). It is a chronic disease with severe pain that impacts negatively on physical, mental and social well‐being (Klein 2014). In addition, the cost of endometriosis is high in both economic and psychosocial terms (Matthias 1996, Simoens 2012).
Treatment availability is dependent upon available resources but also upon the preferences of the individual woman and the gynaecologist. This particularly relates to their decisions concerning the conservation of fertility or requirements for contraception. One of the available treatment options is treatment with GnRHas. While GnRHas are not free of side effects, it is important to know how well they perform in comparison to other medical treatments and placebo.
This review will evaluate the effect of GnRHas specifically on the relief of pain and on bone mineral density in symptomatic women with endometriosis. This review is a combination of two previously published Cochrane Reviews on GnRHas for bone mineral density (Farmer 2003) and for pain associated with endometriosis (Brown 2010). It was decided to merge both reviews, in order to provide the best overview of the use of GnRHas in women with endometriosis.
Objectives
To assess the effectiveness and safety of GnRH analogues (GnRHas) in the treatment of painful symptoms associated with endometriosis and to determine the effects of GnRHas on bone mineral density of women with endometriosis.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing the use of GnRHas in the treatment of symptomatic endometriosis were eligible for inclusion. Cross‐over trials were included in the review providing that data from before‐and‐after cross‐over were available; only the first‐arm data were used for analysis. We excluded trials that used self‐reporting of endometriosis, as well as quasi‐randomised and non‐randomised studies (case control studies, cohort studies).
Types of participants
Premenopausal women with symptoms ascribed to endometriosis were eligible for inclusion. For a trial to be included, the clinical diagnosis of endometriosis must have been made by direct visualisation (laparoscopy or laparotomy) or from ultrasonographic imaging or magnetic resonance imaging (MRI). We excluded women with asymptomatic disease or infertility as the only presenting complaint.
Types of interventions
We included RCTs reporting the following comparisons for relief of pain associated with endometriosis and its related adverse effects.
GnRHas versus no treatment.
GnRHas versus placebo.
GnRHas versus analgesics.
GnRHas versus danazol.
GnRHas versus intra‐uterine progestogens.
GnRHas versus oral or injectable progestogens.
GnRHas versus gestrinone.
We also included RCTs comparing the following for relief of pain associated with endometriosis and its related adverse effects.
Different doses of GnRHas.
Different treatment duration of GnRHas.
Different routes of administration of GnRHas.
Different treatment regimens of GnRHas.
GnRHas versus GnRHas in conjunction with add‐back therapy (hormonal or non‐hormonal).
GnRHas versus GnRHas in conjunction with calcium‐regulating agents.
When we identified trials that assessed the effect of GnRHas on bone mineral density (BMD), we considered them for inclusion providing the treatment period exceeded six months. The reason for this decision is that shorter treatment periods do not seem to treat the disease effectively (Audebert 1998).
The following trials were excluded from this review.
Trials comparing GnRHas with surgical therapies, the combined oral contraceptive pill, progesterone receptor modulators or selective oestrogen receptor modulators (SERMs), as these are included in separate Cochrane Reviews (Bafort 2020; Brown 2018; Fu 2017; Van Hoesel 2021, respectively).
Trials comparing GnRHas with gonadotrophin antagonists, as this is a registered title of a Cochrane Review to be conducted by Cochrane Gynaecology and Fertility (Houda 2014).
Trials comparing GnRHas with alternative and complementary medicine such as Chinese herbs or acupuncture, as these are addressed by published Cochrane Reviews (Flower 2012; Zhu 2011, respectively).
Trials where GnRHas were administered in post‐surgical participants as adjuvant therapy.
Types of outcome measures
The choice of outcome measures is based on the core outcome set (COS) determined for endometriosis research (Duffy 2020). This COS was developed by conducting a systematic review and a widely‐supported Delphi study, in which it was determined which outcome measures in endometriosis research should definitely be assessed in endometriosis trials. The COS is expected to provide a more uniform way for conducting, performing and reporting endometriosis research.
Primary outcomes
Overall pain, defined by using both quantitative measures such as visual analogue scales or categorical outcomes, at the end of treatment and at three, six, nine, 12, 18 and 24 months' follow‐up, where possible
The objective measurement of bone mineral density (BMD), including dual‐energy photon absorptiometry (DPA), dual‐energy X‐ray absorptiometry (DEXA), single‐energy photon absorptiometry (SPA), single‐energy X‐ray absorptiometry (SXA) and quantitative computed tomography (QCT). Measurements taken at the lumbar spine and femoral head will be considered, whilst those at the distal forearm will be excluded because these measurements are of cortical bone which is less affected by GnRHa therapy (Whitehouse 1990; Ylikorkala 1990). Bone density measurements at the end of treatment and in the follow‐up period will be included. Measurements will be grouped according to the anatomical location of measurements and the timing of measurements.
For overall pain, we applied the core outcome set (COS). Overall pain is one of the outcome measures recommended with the COS. However, many studies still distinguish between different sub‐forms of pain, and do not use overall pain as an outcome measure. In the current review, it was decided to include both overall pain and other sub‐forms of pain, i.e. dysmenorrhoea, dyspareunia and pelvic pain. This is to provide as much useful information as possible for shared decision‐making with patients.
Secondary outcomes
Adverse effects (e.g. hot flushes, insomnia, reduced libido, vaginal dryness and headaches), both short‐term (during therapy) and long‐term (extending beyond the treatment period)
Quality of life and factors affecting quality of life (by quality of life scores)
Improvement in the most troublesome symptoms
Patients' satisfaction with treatment
Cost‐effectiveness and pregnancy rates are not outcomes of this review.
Search methods for identification of studies
The search strategy of Cochrane Gynaecology and Fertility was utilised to identify all publications that describe or might describe randomised trials of GnRHas in the treatment of symptomatic endometriosis.
Electronic searches
There were no language or date restrictions in the searches. The following electronic databases, trial registers and websites were searched:
Cochrane Gynaecology and Fertility Group's specialised register of controlled trials; searched from inception to 26 May 2022, ProCite platform (Appendix 1);
CENTRAL, via the Cochrane Register of Studies Online (CRSO); now containing output from two trials registries (clinicaltrials.gov https://clinicaltrials.gov/ and the International Clinical Trials Registry Platform (ICTRP) https://www.who.int/clinical‐trials‐registry‐platform) and CINAHL, searched 26 May 2022, Web platform (Appendix 2);
MEDLINE, searched from 1946 to 26 May 2022, Ovid platform (Appendix 3);
Embase, searched from 1980 to 26 May 2022, Ovid platform (Appendix 4);
PsycINFO, searched from 1806 to 26 May 2022, Ovid platform (Appendix 5).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). The Embase search was combined with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/what-we-do/methodology/search-filters/).
Other web‐based electronic sources of trials searched were:
ISI Web of Science, for conference abstracts;
LILACS database, as a source of trials from the Portuguese and Spanish regions;
Clinical Study Results, for clinical trial results of marketed pharmaceuticals;
OpenSIGLE database, for grey literature;
Google, for grey literature and for trials that were not yet indexed in the major databases.
Searching other resources
Any relevant journals and conference abstracts that were not covered in the Cochrane Gynaecology and Fertility specialised register were handsearched in liaison with the Group's Information Specialist, Marian Showell.
The reference lists of relevant articles retrieved by the search were handsearched, and we personally communicated with experts in the field to obtain any additional trials. In addition, we approached all distributors of GnRHas for details of unpublished trials of GnRHas known to, or undertaken by, them or their parent companies.
Data collection and analysis
Selection of studies
Two review authors (VV and MK) independently scanned the search results for relevant titles and abstracts and removed those that were clearly irrelevant. The full texts of all potentially eligible studies were retrieved. Two review authors (VV and MK) independently examined the full‐text articles for compliance with the inclusion criteria. Authors corresponded with study investigators to clarify study eligibility. Communication with study authors was documented in the appendices. Where required, disagreements regarding study eligibility were resolved by consensus or by the assessment of a third review author (JM).
Data extraction and management
Data extraction was conducted independently by two review authors (VV and MK). Data extraction forms were developed and pilot‐tested by the authors. Where studies had multiple publications, the main trial report was used as the reference and additional details supplemented from secondary papers. Authors corresponded with study investigators in order to resolve any data queries, as required. When disagreements arose between the two review authors, a third review author was contacted to resolve the dispute (JM).
Assessment of risk of bias in included studies
Assessment of the risk of bias in the included studies was undertaken by two of the review authors, using the Cochrane risk of bias tool (Higgins 2011). The assessment is based on allocation (random sequence generation and allocation concealment), blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias. Where uncertainty arose or there was a discrepancy between the two review authors (VV and MK), a third review author was contacted to make further assessment. When a study protocol was available, we assessed whether there are differences between the study protocol and published results.
If necessary, additional information was sought from the principal investigator of the original trial. All judgements were fully described and the conclusions presented in the risk of bias table.
Measures of treatment effect
For continuous data like pain and BMD (bone mineral density) scores, mean differences (MDs) with 95% confidence intervals (CIs) were reported using change‐from‐baseline scores. When similar outcomes were reported on different scales we intended to calculate standardised mean differences (SMDs). Ordinal data (e.g. quality of life scores) were treated as continuous data. A summary statistic for each outcome was calculated using a fixed‐effect model and a 95% CI. For dichotomous data, we calculated for each study a Mantel‐Haenszel risk ratio (RR) with corresponding 95% CI.
Unit of analysis issues
Data were presented according to each woman randomised. In cross‐over trials only the first‐arm data were used for analysis where data were available, and where data were not available, we intended to contact the primary author.
Dealing with missing data
The data were analysed on an intention‐to‐treat basis as far as possible, and attempts were made to obtain missing data from the original investigators. If studies reported sufficient detail to calculate MDs, but provided no information on an associated standard deviation (SD), the outcome was assumed to have an SD equal to the highest SD from other studies within the same analysis. For other outcomes, only the available data were analysed.
Assessment of heterogeneity
The review authors considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Statistical heterogeneity was assessed using the I2 statistic (Higgins 2019). An I2 measurement greater than 50% indicates substantial heterogeneity. Sensitivity analyses including all studies were conducted where it was taken into account if the quality of the studies contributed to the heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Data synthesis
When studies were sufficiently similar, the data from primary studies were combined using the fixed‐effect model. The primary analysis only included trials with a low risk of selection bias and no high risk of bias in other domains.
Subgroup analysis and investigation of heterogeneity
Data derived in all outcome measurements were divided into subgroups by dosage (low or high, as defined by study); duration of treatment (three, six, nine, 12, 18 and 24 months); route of administration (intranasal, intramuscular, subcuticular or depot injection); and drug regimens.
Sensitivity analysis
Sensitivity analyses were conducted for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether our conclusions differed in the following situations.
If all studies were included in the analysis (studies with unclear risk of selection bias and high risk of bias in other domains).
If studies with outlying results were excluded.
If alternative imputation strategies were adopted.
If a random‐effects model was adopted.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table using GRADEpro GDT software and Cochrane methods (Schünemann 2021). This table evaluated the overall quality of the body of evidence for the main review outcomes, namely overall pain, the objective measurement of BMD, and adverse effects, for the following main comparisons.
GnRHas versus no treatment.
GnRHas versus placebo.
GnRHas versus danazol.
Additional summary of findings tables were also prepared for the main review outcomes for other important comparisons (GnRHas versus analgesics; and GnRHas versus intra‐uterine progestogen devices). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias. Judgements about evidence quality (high, moderate, low or very low) were made by two review authors working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We extracted study data, formatted our comparisons in data tables and prepared a summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
This is a combination of two previous reviews (Brown 2010; Farmer 2003). The inclusion and exclusion criteria, as well as the participants, differ slightly from the two previous reviews. Brown 2010 only included women with a clinical diagnosis of endometriosis, so the diagnosis had to be made by direct visualisation (laparoscopy). In this review, we also include women with a clinical diagnosis of endometriosis from ultrasonographic imaging or magnetic resonance imaging (MRI). Farmer 2003 included premenopausal women suffering from endometriosis diagnosed visually by laparoscopy or laparotomy, or presumptively, from symptom history. Here, too, the inclusion criteria differ slightly from each other.
A total of seventy‐two studies were included. Two studies were still awaiting classification, however, as data were still not available at the current review, we also excluded these articles. A total of fifty‐six studies were excluded, and five are still awaiting classification. See study tables: "Characteristics of included studies", "Characteristics of excluded studies"; "Characteristics of studies awaiting classification". Figure 1 summarises the results of the search.
1.
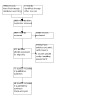
Study flow diagram
Included studies
Seventy‐two randomised controlled trials met our eligibility criteria and were included in this review (Abdou 2018; Adamson 1994; Agarwal 1997; AN Zoladex 1996; Audebert 1997; Bergqvist 1997; Bergqvist 1998; Bergqvist 2000; Burry 1989; Burry 1992; Chang 1996; Cheng 2005; Cirkel 1995; Crosignani 1996; Crosignani 2006; Dawood 1995; Dlugi 1990; Dmowski 1989a; Edmonds 1994; Fedele 1989; Ferreira 2010; Finkelstein 1998; Finkelstein 1999; Franke 2000; Fraser 1991; Freundl 1998; Fukushima 1993; Gnoth 1999; Gomes 2007; Harada 2009; Henzl 1988; Hornstein 1995; Hornstein 1998; Howell 1995; Hurst 2000; Irahara 2001; Jelley 1986; Kennedy 1990; Kiilholma 1995; Lemay 1988; Ling 1999; Mäkäräinen 1996; Miller 2000; Minaguchi 1986; Moghissi 1998; NEET 1992; Odukoya 1995; Orwoll 1994; Ozaki 2020; Palagiano 1994; Petta 2005; Rock 1993; Rolland 1990; Rotondi 2002; Roux 1995; Schlaff 2006; Shaw 1986; Shaw 1990; Sillem 1999; Skrzypulec 2004; Strowitzki 2012; Surrey 1992; Surrey 2002; Tahara 2000; Tang 2017; Tummon 1988; Tummon 1989; Vercellini 1994; Vercellini 1996; Wheeler 1992; Whitehouse 1990; Zupi 2005). See Characteristics of included studies for description.
All the studies were randomised controlled trials and came from a variety of different countries:
Brazil: Ferreira 2010; Gomes 2007; Petta 2005
Canada: Lemay 1988
Egypt: Abdou 2018
France: Audebert 1997; Roux 1995
Finland: Kiilholma 1995; Mäkäräinen 1996
Germany: Cirkel 1995; Freundl 1998; Gnoth 1999; Sillem 1999; Strowitzki 2012
International (multi‐centre): AN Zoladex 1996; Bergqvist 1997; Bergqvist 2000; Crosignani 2006; Fraser 1991; Henzl 1988; NEET 1992; Rolland 1990
Italy: Crosignani 1996; Fedele 1989; Palagiano 1994; Rotondi 2002; Vercellini 1994; Vercellini 1996; Zupi 2005
Japan: Fukushima 1993; Harada 2009; Irahara 2001; Minaguchi 1986; Ozaki 2020; Tahara 2000; Tang 2017
Netherlands: Franke 2000
Poland: Skrzypulec 2004
Sweden: Bergqvist 1998
Taiwan: Chang 1996; Cheng 2005
United Kingdom: Edmonds 1994; Howell 1995; Jelley 1986; Kennedy 1990; Odukoya 1995; Shaw 1986; Shaw 1990; Whitehouse 1990
United States of America: Adamson 1994; Agarwal 1997; Burry 1989; Burry 1992; Dawood 1995; Dlugi 1990; Dmowski 1989a; Finkelstein 1998; Finkelstein 1999; Hornstein 1995; Hornstein 1998; Hurst 2000; Ling 1999; Miller 2000; Moghissi 1998; Orwoll 1994; Rock 1993; Schlaff 2006; Surrey 1992; Surrey 2002; Tummon 1988; Tummon 1989; Wheeler 1992
The included studies comprised 7355 women having complaints of endometriosis. Where reported, ages ranged from 18 to 48 years. All women with endometriosis had a clinical diagnosis of endometriosis made by direct visualisation (laparoscopy or laparotomy) or from ultrasonographic imaging or magnetic resonance imaging (MRI).
The following interventions were tested in the included trials:
GnRHas versus placebo (five trials; Bergqvist 1998; Dlugi 1990; Ling 1999; Miller 2000; Skrzypulec 2004)
GnRHas versus danazol (twenty‐nine trials; Adamson 1994; AN Zoladex 1996; Audebert 1997; Burry 1989; Burry 1992; Chang 1996; Cheng 2005; Cirkel 1995; Dawood 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Fukushima 1993; Gnoth 1999; Henzl 1988; Jelley 1986; Kennedy 1990; NEET 1992; Odukoya 1995; Palagiano 1994; Rock 1993; Rolland 1990; Rotondi 2002; Shaw 1990; Tummon 1988; Tummon 1989; Vercellini 1994; Wheeler 1992; Whitehouse 1990)
GnRHas versus intra‐uterine progestogens (three trials; Ferreira 2010; Gomes 2007; Petta 2005)
GnRHas versus oral or injectable progestogens (seven trials; Abdou 2018; Crosignani 2006; Harada 2009; Ozaki 2020; Schlaff 2006; Strowitzki 2012; Zupi 2005)
GnRHas versus gestrinone (one trial; Vercellini 1996)
Trials comparing different doses of GnRHas (eight trials; Adamson 1994; Bergqvist 1997; Burry 1989; Henzl 1988; Minaguchi 1986; Shaw 1986; Tahara 2000; Tang 2017)
Trials comparing different treatment duration of GnRHas (two trials; Hornstein 1995; Orwoll 1994)
Trials comparing different route of administration of GnRHas (four trials; Agarwal 1997; Bergqvist 2000; Dmowski 1989a; Lemay 1988)
Trials comparing different GnRHas treatment regimens (one trial; Crosignani 1996)
Trials comparing GnRHas versus GnRHas in conjunction with add‐back therapy (hormonal or non‐hormonal) (seventeen trials; Bergqvist 1997; Edmonds 1994; Franke 2000; Freundl 1998; Gnoth 1999; Hornstein 1998; Howell 1995; Hurst 2000; Irahara 2001; Kiilholma 1995; Mäkäräinen 1996; Moghissi 1998; Sillem 1999; Surrey 1992; Surrey 2002; Vercellini 1994; Zupi 2005)
Trials comparing GnRHas versus GnRHas in conjunction with calcium‐regulating agents (three trials; Finkelstein 1998; Finkelstein 1999; Roux 1995)
Trials mentioning the effect of GnRHas on BMD (thirty trials; Crosignani 1996; Crosignani 2006; Dawood 1995; Dlugi 1990; Edmonds 1994; Finkelstein 1998; Finkelstein 1999; Franke 2000; Freundl 1998; Fukushima 1993; Gnoth 1999; Harada 2009; Hornstein 1998; Howell 1995; Irahara 2001; Moghissi 1998; Orwoll 1994; Rock 1993; Roux 1995; Schlaff 2006; Sillem 1999; Surrey 1992; Surrey 2002; Tahara 2000; Tang 2017; Tummon 1988; Vercellini 1996; Wheeler 1992; Whitehouse 1990; Zupi 2005)
No trials comparing GnRHas versus no treatment or trials comparing GnRH analogues versus analgesics were identified.
Six trials were included in two different comparisons, due to the fact that these trials included three different treatment groups, and therefore could be included in different comparisons (Adamson 1994; Bergqvist 1997; Burry 1989; Dmowski 1989a; Henzl 1988; Zupi 2005). Adamson 1994, Burry 1989, Dmowski 1989a, and Henzl 1988 compared varying dosage of GnRHas in addition to a comparison with danazol, and Bergqvist 1997 compared varying dosage of GnRHas in addition to a comparison with add‐back therapy (hormonal or non‐hormonal). Zupi 2005 compared GnRHas with GnRHas in conjunction with add‐back, and compared GnRHas with oral or injectable progestogens.
Excluded studies
Of the fifty‐six studies that were excluded, ten studies did not have the stated outcome measures (Acien 1989; Calvo 2000;Donnez 1989; el‐Roeiy 1988; Maouris 1991; Matalliotakis 2000; Tapanainen 1993; Valimaki 1989; Wright 1995; Yee 1986). Fifteen studies reported wrong comparisons, see 'Types of Interventions' for details (Agarwal 2015; Cooke 1989; Dmowski 1989; Donnez 2004; Fernandez 2004; Ferrero 2011; Imani 2009; Luciano 2004; Magini 1993; Miller 1990; Newton 1996; Shaw 2001; Surrey 1993; Taskin 1997; Toomey 2003; Warnock 1998). Four studies looked at the outcome in post‐surgical participants (Adiyono 2006; Matalliotakis 2004; Soysal 2004; Takaesu 2013); endometriosis was not the main condition discussed in eleven studies (Al‐Azemi 2009; Dodin 1991; Eldred 1992; Fraser 1996; Lindsay 1996; Matta 1988; Mukherjee 1996; Ripps 2003 Somekawa 1999; Sorensen 1997; Sowter 1997; Surrey 1995); and two studies had included a wrong population (Shaw 1992; Ylikorkala 1995). Nine studies were not randomised controlled trials (Bergqvist 1990; Choktanasiri 2001; Claesson 1989; Dawood 1990; Fedele 1993; Franssen 1992; Harada 2000 Pierce 2000; Vercellini 2009). Chan 1993 and Chen 2009 were both still awaiting assessment, as they were in the original review (Brown 2010) and therefore they were excluded.
The other five studies have been placed under 'awaiting classification'. These are abstracts of articles that are not available in full text and do not contain enough information to enable us to make a decision about inclusion or exclusion. Therefore, we decided to place these five studies under 'awaiting classification' (Aisaka 2000; Archer 2004; Gregoriou 1997; Long 2009; Vella 1995).
Risk of bias in included studies
Details on the quality of each individual study are described in the table Characteristics of included studies where the individual quality criteria were rated for each study. Authors have been contacted for more information when required.
Of the seventy‐two articles included, only three were at overall low risk of bias (Cheng 2005; Ling 1999; Vercellini 1996). Ten of these seventy‐two were indicated as having high risk of bias (AN Zoladex 1996; Burry 1989; Cirkel 1995; Dlugi 1990; Edmonds 1994; Fukushima 1993; Harada 2009; Howell 1995; Schlaff 2006; Surrey 2002). The other fifty‐nine were assigned as having overall unclear risk of bias.
For selection bias, six studies reported low risk of bias, without high risk of bias on the other domains, and were therefore included in the main analysis (Abdou 2018; Cheng 2005; Finkelstein 1998; Lemay 1988; Ling 1999; Odukoya 1995).
See Figure 2 for the risk of bias graph and Figure 3 for the risk of bias summary.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twenty‐two trials were at low risk of selection bias related to random sequence generation, as they used computer randomisation or random number tables (Abdou 2018; Cheng 2005; Cirkel 1995; Crosignani 1996; Ferreira 2010; Finkelstein 1998; Finkelstein 1999; Fraser 1991; Gomes 2007; Harada 2009; Lemay 1988; Ling 1999; Miller 2000; Odukoya 1995; Ozaki 2020; Petta 2005; Skrzypulec 2004; Surrey 1992; Surrey 2002; Tahara 2000; Vercellini 1994; Zupi 2005. The remaining trials were at unclear risk of selection bias as they did not describe the method of randomisation used.
Twelve trials were at low risk of selection bias related to allocation concealment (Abdou 2018; Adamson 1994; Chang 1996; Cheng 2005; Dlugi 1990; Finkelstein 1998; Harada 2009; Jelley 1986; Lemay 1988; Ling 1999; Odukoya 1995; Vercellini 1996). The remaining trials did not describe allocation concealment and were at unclear risk of this bias.
Blinding
Thirty‐six studies described blinding of patients and personnel, and they were judged to be at low risk of performance bias (Adamson 1994; Agarwal 1997; Bergqvist 1997; Bergqvist 1998; Burry 1989; Burry 1992; Cheng 2005; Dawood 1995; Dlugi 1990; Franke 2000; Fraser 1991; Freundl 1998; Gnoth 1999; Harada 2009; Henzl 1988; Hornstein 1995; Hornstein 1998; Hurst 2000; Kennedy 1990; Kiilholma 1995; Ling 1999; Mäkäräinen 1996; Miller 2000; Moghissi 1998; NEET 1992; Orwoll 1994; Rolland 1990; Roux 1995; Shaw 1990; Sillem 1999; Skrzypulec 2004; Surrey 1992; Surrey 2002; Vercellini 1996; Wheeler 1992; Whitehouse 1990). The remaining trials were assigned as having unclear risk of bias.
Forty‐two studies described blinding of outcome assessors, and they were judged to be at low risk of detection bias (Adamson 1994; Agarwal 1997; Bergqvist 1997; Bergqvist 1998; Burry 1989; Burry 1992; Chang 1996; Cheng 2005; Dawood 1995; Dlugi 1990; Franke 2000; Fraser 1991; Freundl 1998; Fukushima 1993; Gnoth 1999; Harada 2009; Henzl 1988; Hornstein 1995; Hornstein 1998; Hurst 2000; Kennedy 1990; Kiilholma 1995; Lemay 1988; Ling 1999; Mäkäräinen 1996; Miller 2000; Moghissi 1998; NEET 1992; Orwoll 1994; Petta 2005; Rolland 1990; Roux 1995; Schlaff 2006; Shaw 1990; Sillem 1999; Skrzypulec 2004; Surrey 1992; Surrey 2002; Vercellini 1996; Wheeler 1992; Whitehouse 1990; Zupi 2005). The remaining trials were assigned as having unclear risk of bias.
Incomplete outcome data
Eight trials were considered to be at high risk of attrition bias due to high loss to follow‐up (AN Zoladex 1996; Cirkel 1995; Dlugi 1990; Fukushima 1993; Harada 2009; Howell 1995; Schlaff 2006; Surrey 2002). Five trials were considered to be at unclear risk of bias due to insufficient data (Chang 1996; Orwoll 1994; Sillem 1999; Tang 2017; Tummon 1988) and the rest of the trials were assigned as having low risk of bias.
Selective reporting
None of the protocols from the original review were viewed but almost all the published reports of all included articles included all expected outcomes and were therefore judged to be at low risk of reporting bias. Only Burry 1989, Edmonds 1994 and Shaw 1986 did not report any results of changes in symptoms, while this had been asked and reported during follow‐up; they were assessed as being at high risk of reporting bias.
Other potential sources of bias
There was no evidence of other potential sources of bias identified.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
We identified six studies at low risk of selection bias and not at high risk of any other bias (Abdou 2018; Cheng 2005; Finkelstein 1998; Lemay 1988; Ling 1999; Odukoya 1995). The results of these studies were included in the original reviews. We also performed a sensitivity analysis, which included all studies. Results are stated below.
1. GnRHas versus no treatment for relief of overall pain associated with endometriosis and its related adverse effects
In a previous version of this review, there was only one study which compared GnRHas with no treatment (Fedele 1993) for the outcome of overall pain, reported as relief of painful symptoms (dysmenorrhoea). We have excluded this study from the current review because it was not possible to read this article in full text. No further studies comparing GnRHas versus no treatment were identified.
2. GnRHas versus placebo for relief of overall pain associated with endometriosis and its related adverse effects
Five studies were identified which compared GnRHas with placebo, all with a different outcome measure (Bergqvist 1998; Dlugi 1990; Ling 1999; Miller 2000; Skrzypulec 2004). Only Ling 1999 was at low risk of bias.
Four were included in sensitivity analysis, however, these results should be interpreted with caution. Skrzypulec 2004 did not provide usable data.
2.1 Relief of overall pain
Relief of overall pain (reported as decrease in pain scores) was reported by three studies (Bergqvist 1998; Ling 1999; Miller 2000), but only one study (Ling 1999) was included in the primary analysis. GnRHas may improve pelvic pain compared to placebo (RR 2.14; 95% CI 1.41 to 3.24, 1 RCT, n = 87), dysmenorrhoea (RR 2.25; 95% CI 1.59 to 3.16, 1 RCT, n = 87), dyspareunia (RR 2.21; 95% CI 1.39 to 3.54, 1 RCT, n = 59) and pelvic tenderness (RR 2.28; 95% CI 1.48 to 3.50,1 RCT, n = 85) after three months of treatment. We are uncertain of the effect of GnRHas compared to placebo for pelvic induration, based on the results after three months of treatment (RR 1.07; 95% CI 0.64 to 1.79, 1 RCT, n = 81). We graded all outcomes as having low‐certainty evidence; Analysis 1.1.
1.1. Analysis.

Comparison 1: GnRHas versus placebo, Outcome 1: Relief of overall pain ‐ dichotomous ‐ decrease of pain
The sensitivity analysis including the studies with unclear or high risk of bias suggested treatment with GnRHas compared to placebo (Analysis 2.1, Bergqvist 1998) at six months follow up may improve the relief of dyspareunia (RR 0.28; 95% CI 0.09 to 0.89, 1 RCT, n = 49) and pelvic tenderness (RR 0.22; 95% CI 0. 09 to 0.55, 1 RCT, n = 49). We are uncertain of the effect of GnRHas compared to placebo for dyschezia (RR 0.26 95%CI 0.03 to 2.17, 1 RCT, n = 49). One study (Analysis 2.1.; Miller 2000) found pain, using the Endometriosis Symptom Severity Score (ESSS), may improve following GnRHa therapy compared to placebo with an MD 2.90 (95% CI 2.80 to 3.00, 1 RCT, n = 120, Analysis 2.3), as measured one month after treatment.
2.1. Analysis.

Comparison 2: GnRHas versus placebo ‐ all studies included, Outcome 1: Relief of overall pain ‐ dichotomous
2.3. Analysis.

Comparison 2: GnRHas versus placebo ‐ all studies included, Outcome 3: Relief of overall pain ‐ continuous
2.2 Bone mineral density
No studies at overall low risk of bias were included in this analysis.
In the sensitivity analysis including unclear or high risk of bias studies, Dlugi 1990 was the only study that reported effects on BMD. "Due to the variety of different methodologies and anatomic sites used to assess changes in bone mineral density, sample sizes were small. In addition, very few placebo patients had data included in the analysis. Consequently, between treatment group data could not be adequately evaluated. Nevertheless, 15 patients treated with leuprolide acetate, who had spinal bone mineral density assessed by dual photon absorptiometry demonstrated a mean decrease of 3.6% (P = 0.001) from baseline to the end of treatment. Eight patients treated with leuprolide acetate had spinal bone mineral density measured by quantitative computed tomography. These patients had a mean decrease in their bone mineral density of 11.8% (P < 0.001)".
2.3 Adverse effects
Again, only Ling 1999 was included in the primary analysis. Treatment with GnRHas may be associated with greater incidence of hot flushes at three months of treatment (RR 3.08; 95% CI 1.89 to 5.01, 1 RCT, n = 100, low‐certainty evidence) (Analysis 1.2).
1.2. Analysis.

Comparison 1: GnRHas versus placebo, Outcome 2: Adverse effects ‐ dichotomous
We performed a sensitivity analysis including the studies with unclear or high risk of bias and found treatment with GnRHas may also be associated with greater incidence of vasodilatation (RR 2.69; 95% CI 1.51 to 4.81, 1 RCT, n = 63) and headache (RR 3.55; 95% CI 1.09 to 11.53, 1 RCT, n = 63) at six months of treatment (Dlugi 1990) and sleep disturbances at 12 months treatment (RR 2.31; 95% CI 1.33 to 4.02, 1 RCT, n = 49, Bergqvist 1998) compared to placebo. We are uncertain of the effect of GnRHas compared to placebo, for hot flushes at 12 months treatment (RR 1.62; 95% CI 0.87 to 3.02, 1 RCT, n = 49, Bergqvist 1998) (Analysis 2.4). Nevertheless, these results should be interpreted with caution in view of the high risk of bias in these studies.
2.4. Analysis.

Comparison 2: GnRHas versus placebo ‐ all studies included, Outcome 4: Adverse effects ‐ dichotomous
2.4 Quality of life
No studies with overall low risk of bias were included in this analysis.
One high risk of bias study reported on quality of life (Miller 2000). At one month of follow‐up, GnRHas treatment resulted in lower physical and mental health according to the Short Form (36) Health Survey (SF‐36) questionnaire (MD ‐0.46; 95% CI ‐0.48 ‐ 0.44, 1 RCT, n = 120, Analysis 2.5).
2.5. Analysis.

Comparison 2: GnRHas versus placebo ‐ all studies included, Outcome 5: Quality of life ‐ continuous
3. GnRHas versus analgesics for relief of overall pain associated with endometriosis and its related adverse effects
No studies comparing GnRHas and analgesics were identified.
4. GnRHas versus danazol for relief of overall pain associated with endometriosis and its related adverse effects
Twenty‐nine studies were identified which compared GnRHas with danazol (Adamson 1994; AN Zoladex 1996; Audebert 1997; Burry 1989; Burry 1992; Chang 1996; Cheng 2005; Cirkel 1995; Dawood 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Fukushima 1993; Gnoth 1999; Henzl 1988; Jelley 1986; Kennedy 1990; NEET 1992; Odukoya 1995; Palagiano 1994; Rock 1993; Rolland 1990; Rotondi 2002; Shaw 1990; Tummon 1988; Tummon 1989; Vercellini 1994; Wheeler 1992; Whitehouse 1990).
Of these twenty‐nine studies, only two were low risk of selection bias (Cheng 2005; Odukoya 1995). All twenty‐nine studies were included in sensitivity analysis, nevertheless, these results should be interpreted with caution.
4.1 Relief of overall pain
Relief of overall pain was reported by Cheng 2005 and Odukoya 1995.
Dichotomous data suggested very low‐certainty evidence of the effect of GnRHas versus danazol on relief of overall pain, reported as partly and completely resolved pelvic tenderness, after six months of treatment ((RR 1.15, 95% CI 0.49 to 2.73, 1 RCT, n = 41) and (RR 1.10, 95% CI 0.67 to 1.81, 1 RCT, n = 41)), respectively (Cheng 2005). One study reported that "there was no difference between the two drugs (aka leuprolide acetate versus danazol) in the relief of pain symptoms at the end of three months, but the mean score was lower at the end of three months with leuprolide acetate injections" (Odukoya 1995).
Continuous data suggested very low‐certainty evidence of the effect of GnRHas versus danazol on relief of overall pain (MD ‐0.13, 95% CI ‐0.74 to 0.49, 1 RCT, n = 41), pelvic pain (MD 0.26, 95% CI ‐0.35 to 0.88, 1 RCT, n = 41), dysmenorrhoea (MD 0.10, 95% CI ‐0.51 to 0.72, 1 RCT, n = 41), dyspareunia (MD ‐0.20, 95% CI ‐0.82 to 0.41, 1 RCT, n = 41), pelvic induration (MD ‐0.12, 95% CI ‐0.73 to 0.49, 1 RCT, n = 41 (Cheng 2005)) and pelvic tenderness (MD ‐0.21, 95% CI ‐0.82 to 0.41, 1 RCT, n = 41), all after three months of treatment (Cheng 2005).
Similarly, after six months of treatment, we found very low‐certainty evidence of the effect of GnRHas versus danazol on relief of overall pain (MD 0.40, 95% CI ‐0.86 to 1.66, 1 RCT, n = 41), pelvic pain (MD 0.50, 95% CI 0.10 to 0.90, 1 RCT, n = 41), dysmenorrhoea (MD 0.40, 95% CI ‐0.12 to 0.792, 1 RCT, n = 41), dyspareunia (MD ‐0.40, 95% CI ‐0.90 to 0.10, 1 RCT, n = 41) and pelvic tenderness (MD ‐0.20, 95% CI ‐0.75 to 0.35, 1 RCT, n = 41) (Cheng 2005).
We performed a sensitivity analysis including unclear or high risk of bias studies and found pain scores were reported by twenty‐two studies (Adamson 1994; Audebert 1997; Chang 1996; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Fukushima 1993; Henzl 1988; Jelley 1986; Kennedy 1990; NEET 1992; Odukoya 1995; Palagiano 1994; Rock 1993; Rolland 1990; Rotondi 2002; Tummon 1989; Vercellini 1994; Wheeler 1992; Whitehouse 1990), but only thirteen of them could be included in meta‐analyses (Adamson 1994; Audebert 1997; Chang 1996; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Jelley 1986; NEET 1992; Palagiano 1994; Tummon 1989; Wheeler 1992). Dichotomous data showed probably little or no difference in the effect of GnRHas versus danazol on overall pain relief after six months of treatment: reported as pelvic pain (RR 0.96, 95% CI 0.83 to 1.11, I2 = 0%, 6 RCTs, n = 625, (Adamson 1994; Cirkel 1995; Fedele 1989; NEET 1992; Palagiano 1994; Wheeler 1992)), dysmenorrhoea (RR 1.01, 95% CI 0.96 to 1.06, I2 = 0%, 6 RCTs, n = 644, (Adamson 1994; Cirkel 1995; Fedele 1989; NEET 1992; Palagiano 1994; Wheeler 1992)), dyspareunia (RR 1.10, 95% CI 0.90 to 1.34, I2 = 13%, 5 RCTs, n = 342, (Adamson 1994; Cirkel 1995; Fedele 1989; NEET 1992; Palagiano 1994)), pelvic induration (RR 0.78, 95% CI 0.32 to 1.89, I2 = 0%, 2 RCTs, n = 151, (Cirkel 1995; NEET 1992), pelvic tenderness partly resolved (RR 1.28, 95% CI 0.59 to 2.76, I2 = 0%, 2 RCTs, n = 96, (Cheng 2005; Cirkel 1995) and pelvic tenderness completely resolved (RR 0.97, 95% CI 0.84 to 1.12, I2 = 0%, 2 RCTs, n = 194 (Cheng 2005; Wheeler 1992)). Also, we are uncertain of the effect on pain reduction when pelvic induration and pelvic tenderness were combined (Kennedy 1990; Analysis 4.1).
4.1. Analysis.

Comparison 4: GnRHas versus danazol ‐ all studies included, Outcome 1: Relief of overall pain ‐ dichotomous
Continuous data suggested very low‐certainty evidence of the effect of GnRHas versus danazol on relief of overall pain (SMD 0.00, 95% CI ‐0.46 to 0.46, I2 = 81%, 3 RCTs, n = 85 (Cheng 2005; Dmowski 1989a; Tummon 1989)), pelvic pain (SMD 0.35, 95% CI ‐0.08 to 0.79, I2 = 65%, 2 RCTs, n = 90 (Cheng 2005; Fraser 1991)), dyspareunia (SMD 0.25, 95% CI ‐0.19 to 0.69, I2 = 90%, 2 RCTs, n = 90 (Cheng 2005; Fraser 1991)) and pelvic induration (SMD 0.40 95% CI ‐0.04 to 0.83, I2 = 73%, 2 RCTs, n = 90 (Cheng 2005; Fraser 1991)), all after six months of treatment. Pelvic tenderness after six months of treatment with GnRHas compared to danazol may be decreased in favour of GnRHas with SMD ‐0.59 (95% CI ‐1.03 to ‐0.15, I2 = 66%, 2 RCTs, n = 90 (Cheng 2005; Fraser 1991)) (Analysis 4.2). Two studies that could not be included in the meta‐analyses of continuous pain outcomes reported similar results. One study reported "The investigators' symptom severity scores also dropped after 6 months for both the nafarelin and danazol groups. There were no symptoms in 57% of the patients in the nafarelin group or 48% of patients in the danazol group" (Rolland 1990). This is comparable with Rotondi 2002, who stated that "Both treatments were associated with a significant reduction in mean total subjective scores but with no difference between the treatments", respectively. Nevertheless, as we included all types of risk of bias, these results should be interpreted with caution.
4.2. Analysis.

Comparison 4: GnRHas versus danazol ‐ all studies included, Outcome 2: Relief of overall pain ‐ continuous
No usable data could be extracted from Henzl 1988, Rock 1993 and Vercellini 1994.
4.2 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
When we included all studies in the analysis, six studies reported data on BMD (Dawood 1995; Fukushima 1993; Rock 1993; Tummon 1988; Wheeler 1992; Whitehouse 1990). Two studies (Dawood 1995; Wheeler 1992) reported the percentage change values of BMD at the lumbar spine after six months of treatment. Three studies (Fukushima 1993; Tummon 1988; Whitehouse 1990) reported absolute values of BMD at the lumbar spine after six months of treatment. We are uncertain of the effect of GnRHas or danazol between the two groups for absolute values of BMD (SMD 0.21 95% CI ‐0.31 to 0.73, I2 = 94%, 3 RCTs, n = 81) (Analysis 4.3).
4.3. Analysis.

Comparison 4: GnRHas versus danazol ‐ all studies included, Outcome 3: Bone mineral density of spinal bone mass ‐ continuous
Data reported by Rock 1993 could not be used in a meta‐analysis; it was reported that "the mean percent loss following 12 and 24 weeks of Zoladex therapy was 3.6% (n = 37; 95% CI ‐5.0 to ‐2.2) and 5.4% (n = 38; 95% CI ‐7.2 to ‐3.6), respectively. Danazol‐treated women showed a mean percent loss in bone mineral density at week 12 of 0.4% (n = 17; 95% CI ‐2.9 to +2.1) and a mean percent increase in 1.0% (n = 17; 95% CI ‐1.4 to +3.4) at week 24".
4.3 Adverse effects
Cheng 2005 reported adverse effects. We are uncertain about the effect of GnRHas compared to danazol on adverse effects reported by patients treated for six months. The adverse effects mentioned were vaginal dryness (RR 1.45, 95% CI 0.52 to 4.05, 1 RCT, n = 59), hot flushes (RR 15.50, 95% CI 0.93 to 259.61, 1 RCT, n = 59), gastrointestinal complaints (RR 0.15, 95% CI 0.01 to 2.74, 1 RCT, n = 59), weight gain (RR 0.26, 95% CI 0.08 to 0.82, 1 RCT, n = 59) acne (RR 0.08, 95% CI 0.00 to 1.35, 1 RCT, n = 59) and generalised spasm (RR 0.08, 95% CI 0.00 to 1.35, 1 RCT, n = 59), all very low‐certainty evidence.
We performed a sensitivity analysis including unclear or high risk of bias studies and found eighteen studies that reported on adverse effects (AN Zoladex 1996; Audebert 1997; Burry 1989; Burry 1992; Chang 1996; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Henzl 1988; Jelley 1986; Kennedy 1990; NEET 1992; Rock 1993; Rolland 1990; Rotondi 2002; Wheeler 1992). A total of forty different types of adverse effects were reported, of which a meta‐analysis could be performed in twenty‐three studies. Eight of the most commonly reported adverse effects were vaginal dryness, hot flushes, headaches, muscle cramps/myalgia, sleep disturbance/insomnia, altered libido, weight gain and acne. Adverse effects were more frequently reported in groups receiving GnRHas than those receiving danazol. The evidence suggested that, compared to danazol, GnRHas probably lead to more vaginal dryness (RR 1.82, 95% CI 1.53 to 2.18, I2 = 11%, 12 RCTs, n = 1340 (AN Zoladex 1996; Audebert 1997; Burry 1992; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Jelley 1986; NEET 1992; Palagiano 1994; Rock 1993; Rolland 1990)), more hot flushes (RR 1.50, 95% CI 1.42 to 1.60, I2 = 69%, 16 RCTs, n = 1998 (AN Zoladex 1996; Audebert 1997; Burry 1992; Chang 1996; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Henzl 1988; Jelley 1986; NEET 1992; Palagiano 1994; Rock 1993; Rolland 1990; Rotondi 2002; Wheeler 1992)), more headaches (RR 1.43, 95% CI 1.21 to 1.69, I2 = 0%, 12 RCTs, n = 1103 (AN Zoladex 1996; Audebert 1997; Burry 1992; Cirkel 1995; Dmowski 1989a; Fedele 1989; Fraser 1991; Palagiano 1994; Rock 1993; Rolland 1990; Rotondi 2002)), more sleep disturbance/insomnia (RR 2.04, 95% CI 1.61 to 2.59, I2 = 45%, 7 RCTs, n = 881, AN Zoladex 1996; Cirkel 1995; Dmowski 1989a; Jelley 1986; NEET 1992; Rolland 1990; Wheeler 1992)), and more altered libido (RR 1.58, 95% CI 1.30 to 1.92, I2 = 58%, 9 RCTs, n = 1286). On the other hand, compared to danazol, GnRHas probably lead to fewer muscle cramps/myalgia (RR 0.16, 95% CI 0.09 to 0.29, I2 = 0%, 8 RCTs, n = 884 (AN Zoladex 1996; Burry 1992; Cirkel 1995; Fedele 1989; Fraser 1991; Jelley 1986; NEET 1992; Rolland 1990)), less weight gain (RR 0.38 (95% CI 0.29 to 0.49, I2 = 65%, 9 RCTs, n = 1081 (Audebert 1997; Burry 1992; Cheng 2005; Fedele 1989; Jelley 1986; Palagiano 1994; Rock 1993; Rotondi 2002; Wheeler 1992)) and less acne (RR 0.59, 95% CI 0.47 to 0.73, I2 = 29%, 10 RCTs, n = 1040 (Audebert 1997; Burry 1992; Cheng 2005; Cirkel 1995; Dmowski 1989a; Fedele 1989; Jelley 1986; Rock 1993; Rolland 1990; Rotondi 2002)). All adverse effects reported in the previously mentioned eighteen articles, are reported in Analysis 4.4.
4.4. Analysis.

Comparison 4: GnRHas versus danazol ‐ all studies included, Outcome 4: Adverse effects ‐ dichotomous
The results, reported by Adamson 1994, Burry 1989 and Kennedy 1990, could not be extracted for current analyses.
4.4 Quality of life
No studies with overall low risk of bias were identified for this analysis.
For the sensitivity analysis, including all studies, only one study mentioned the effect of GnRHas compared to danazol on quality of life (Burry 1992), but results could not be extracted for current analyses. "The quality of life measurements in the U.S. study were obtained by a questionnaire composed of 22 simple questions that comprised the Psychological General Well‐Being Index plus a modification of Part II of the Nottingham Health Profile, which requires only a patient's visual qualification of psychological state rather than a numerical quantification. The results of these questionnaires failed to reveal statistically significant differences between the entire treatment group."
4.5 Improvement of most troublesome symptoms
No studies with overall low risk of bias were identified for this analysis.
In the sensitivity analysis, including studies with unclear or high risk of bias, six studies could be included that reported on "improvement of most troublesome symptom" (AN Zoladex 1996; Burry 1992; Henzl 1988; Kennedy 1990; Rolland 1990; Shaw 1990). A distinction was made between overall improvement and complete resolution, both after six months of treatment. We are uncertain of the effect between the two groups (RR 1.08, 95% CI 0.99 to 1.18, I2 = 39%, RCTs, n = 747 (AN Zoladex 1996; Burry 1992; Henzl 1988; Kennedy 1990; Rolland 1990; Shaw 1990) and RR 1.14, 95% CI 0.99 to 1.32, I2 = 43%, 5 RCTs, n = 534, (AN Zoladex 1996; Burry 1992; Kennedy 1990; Rolland 1990; Shaw 1990), respectively) (Analysis 4.5).
4.5. Analysis.

Comparison 4: GnRHas versus danazol ‐ all studies included, Outcome 5: Improvement of most troublesome symptoms ‐ dichotomous
5. GnRHas versus intra‐uterine progestogens for relief of overall pain associated with endometriosis and its related adverse effects
Three studies assessed outcome measures when GnRHas were compared to intra‐uterine progestogens (Ferreira 2010; Gomes 2007; Petta 2005).
5.1 Relief of overall pain
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including high risk of bias studies and found very low‐certainty evidence for an effect on relief of overall pain between groups for the effectiveness of pain relief, measured after six months of treatment with GnRHas or intra‐uterine progestogens (MD ‐0.76, 95% CI ‐1.62 to 0.10, I2 = 22%, 2 RCTs, n = 58 (Ferreira 2010; Gomes 2007)) (Analysis 5.1).
5.1. Analysis.

Comparison 5: GnRHas versus intra‐uterine progestagen device ‐ all studies included, Outcome 1: Relief of overall pain ‐ continuous
5.2 Quality of life
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including high risk of bias studies and found very low‐certainty evidence for an effect on quality of life scores, measured by the psychological well‐being questionnaire index (PGWBI) between both groups, after six months of treatment (MD ‐2.00, 95% CI ‐10.26 to 6.26, 1 RCT, n = 82, Petta 2005) (Analysis 5.2).
5.2. Analysis.

Comparison 5: GnRHas versus intra‐uterine progestagen device ‐ all studies included, Outcome 2: Quality of life ‐ continuous
6. GnRHas versus oral or injectable progestogens for relief of overall pain associated with endometriosis and its related adverse effects
Seven studies were identified which compared GnRHas with oral or injectable progestogens (Abdou 2018; Crosignani 2006; Harada 2009; Ozaki 2020; Schlaff 2006; Strowitzki 2012; Zupi 2005). Crosignani 2006 did not provide usable data on relief of overall pain and changes in BMD. Data about adverse effects were provided, and included in meta‐analysis. Abdou 2018 was considered at low risk of selection bias, and was included in the original review.
6.1 Relief of overall pain
Only one study reported relief of overall pain, after three months of treatment with either GnRHas or oral progestogens (Abdou 2018).
There may be an improvement in overall pain, reported as pelvic pain (MD ‐2.50, 95% CI ‐3.55 to ‐1.45, 1 RCT, n = 261, low certainty of evidence) and dyspareunia (MD ‐2.10, 95% CI ‐2.83 to ‐1.37, 1 RCT, n = 261, low certainty of evidence) after three months of treatment, in favour of oral progestogens. We are uncertain about the effect on back pain of both GnRHas and oral progestogens (MD 0.50, 95% CI ‐0.40 to 1.40, 1 RCT, n = 261) (Analysis 6.1).
6.1. Analysis.

Comparison 6: GnRHas versus oral or injectable progestogens, Outcome 1: Relief of overall pain ‐ continuous
We performed a sensitivity analysis including all studies. Two studies (Schlaff 2006; Strowitzki 2012) reported relief of overall pain in dichotomous data, comparing GnRHas with oral or injectable progestogens. Schlaff 2006 did not provide usable data on relief of overall pain, except for pelvic tenderness, but stated that, "treatment with DMPA‐SC 104 was statistically equivalent (P < 0.02) to treatment with leuprolide at month 6 for the reduction of four of the five signs and symptoms, namely dysmenorrhoea, dyspareunia, pelvic pain, and pelvic tenderness". Strowitzki 2012 reported results on different outcome measurements, namely pelvic pain, dysmenorrhoea, dyspareunia, pelvic induration and pelvic tenderness. For both, all reported outcomes were after six months of treatment. Therefore, a meta‐analysis could only be performed for pelvic tenderness; we are uncertain whether the two groups differ in effects on pelvic tenderness (RR 1.11, 95% CI 0.95 to 1.29, I2 = 0%, 2 RCTs, n = 419) (Analysis 7.1).
7.1. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 1: Relief of overall pain ‐ dichotomous
Continuous outcome data were reported by four studies (Abdou 2018; Harada 2009; Strowitzki 2012; Zupi 2005). However, these studies had different specific outcome measurements and treatment periods. Consequently, meta‐analysis could only be performed for dyspareunia after six months of treatment with GnRHas or oral or injectable progestogens. The results of Harada 2009 and Zupi 2005 were combined, and we are uncertain about the effect of GnRHas or oral or injectable progestogens between both groups (MD ‐0.10 95% CI ‐0.10 to 0.22, I2 = 0%, 2 RCTs, n = 180) (Analysis 7.2).
7.2. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 2: Relief of overall pain ‐ continuous
Crosignani 2006 only mentioned the results of overall pain in figures, but stated that "6 months of treatment with subcutaneous formulation of depot medroxyprogesterone acetate 104 mg/0.65 mL (DMPA‐SC 104) resulted in statistically equivalent (P < 0.02) reductions of all five signs and symptoms of endometriosis (dysmenorrhoea, dyspareunia, pelvic pain, pelvic tenderness and induration) compared with leuprolide treatment. Similarly, scores for all three prespecified scales of the SF‐36 (physical function, role physical and social functioning) significantly improved at month 6 relative to pre‐treatment in both treatment groups, (P ≤ 0.001)".
6.2 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including high risk of bias studies. Four studies reported the effect of GnRHas and oral versus injectable progestogens on bone mineral density (Crosignani 2006; Harada 2009; Schlaff 2006; Zupi 2005). Again, however, different outcome measures have been used, namely percentage change in BMD and absolute values after six and 12 months of treatment. This means that a meta‐analysis could not be undertaken. The difference in percentage change values after six months of treatment may decrease according to one study (MD ‐1.60, 95% CI ‐2.57 to ‐0.63, 1 RCT, n = 87, Harada 2009). One study found an uncertain difference (Zupi 2005) after six months (MD ‐0.04 95% CI ‐0.08 to 0.01, 1 RCT, n = 87), but BMD may decrease following GnRHas versus oral or injectable progestogens after 12 months of treatment (MD ‐0.05 95% CI ‐0.10 to ‐0.01, 1 RCT, n = 87) (Analysis 7.3).
7.3. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 3: Bone mineral density of spinal bone mass ‐ continuous
Crosignani 2006 could not be included in meta‐analysis, but "In the leuprolide group, significant (P < 0.001) reductions from pre‐treatment in both total hip and lumbar spine BMD (median percentage changes of –2.10 and –4.00, respectively) were observed at month 6. However, the DMPA‐SC 104 group showed significant reduction from pre‐treatment (P < 0.001) only in lumbar spine BMD (median percentage changes: total hip, –0.50; lumbar spine, –1.00). Compared with the leuprolide group, reductions in both total hip and lumbar spine BMD were significantly smaller (P < 0.001) in the DMPA‐SC 104 group".
"The leuprolide group experienced significant (P < 0.001) reductions from baseline in both total hip and lumbar spine BMD at month 6 (median percentage changes of ‐1.65 and ‐3.95, respectively). In comparison, the DMPA‐SC 104 group showed a significant reduction from baseline in lumbar spine BMD only (median percentage changes were as follows: total hip BMD, ‐0.30 [P = 0.063]; lumbar spine BMD, ‐1.10 [P < 0.001]). Compared with leuprolide, reductions in both total hip and lumbar spine BMD were significantly less (P < 0.001) for the DMPA‐SC 104 group" (Schlaff 2006).
6.3 Adverse effects
Only one study was included in the original analysis (Abdou 2018); it had with low‐certainty evidence.
There may be a decrease of vaginal bleeding seen in women treated with GnRHas, compared to oral progestogens (RR 0.33, 95% CI 0.23 to 0.48, 1 RCT, n = 242). Also, there may be less weight gain in women treated with GnRHas instead of oral progestogens (RR 0.31, 95% CI 0.10 to 0.92, 1 RCT, n = 242). We are uncertain about the effect of GnRHas compared to oral progestogens, for headache, after three months of treatment (RR 1.53, 95% CI 0.88 to 2.67, 1 RCT, n = 242) (Analysis 6.2).
6.2. Analysis.

Comparison 6: GnRHas versus oral or injectable progestogens, Outcome 2: Adverse effects ‐ dichotomous
We performed a sensitivity analysis including high risk of bias studies. All the seven studies who were identified, mentioned adverse effects when comparing GnRHas with oral or injectable progestogens (Abdou 2018; Crosignani 2006; Harada 2009; Ozaki 2020; Schlaff 2006; Strowitzki 2012; Zupi 2005). A meta‐analysis could be performed for headache, intermenstrual bleeding and hot flushes after six months of treatment.
For headache, an improvement was found between both groups in favour of GnRHas compared to oral or injectable progestogens (RR 1.46 95% CI 1.04 to 2.03, I2 = 0%, 3 RCTs, n = 110, (Crosignani 2006; Harada 2009; Schlaff 2006)). Hot flushes may improve in women treated with GnRHas, compared to oral or injectable progestogens (RR 2.11, 95% CI 1.70 to 2.62, I2 = 88%, 4 RCTs, n = 902, (Crosignani 2006; Harada 2009; Schlaff 2006; Zupi 2005)). In contrast, intermenstrual bleeding was decreased in women treated with GnRHas, compared to oral or injectable progestogens (RR 0.58 95% CI 0.50 to 0.67, I2 = 84%, 4 RCTs, n = 902, (Crosignani 2006; Harada 2009; Schlaff 2006; Zupi 2005)) (Analysis 7.4).
7.4. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 4: Adverse effects ‐ dichotomous
There may be an increase in the number of menopausal symptoms by the Kupperman Index (MD 6.80, 95% CI 2.37 to 11.23, 1 RCT, n = 70) and hot flushes (MD 1.10, 95% CI 0.71 to 1.49, 1 RCT, n = 70) in favour of GnRHas, and a decrease of breast pain (MD ‐0.20, 95% CI ‐0.39 to ‐0.01, 1 RCT, n = 70), and metrorrhagia (MD ‐0.90, 95% CI ‐1.31 to ‐0.49, 1 RCT, n = 70). We are uncertain about the effect of GnRH compared to oral or injectable progestogens, when results are reported after four months of treatment, when compared for depression (MD 0.20, 95% CI ‐0.18 to 0.58, 1 RCT, n = 70), oedema (MD 0.20, 95% CI ‐0.14 to 0.54, 1 RCT, n = 70), and headache (MD 0.10, 95% CI ‐0.32 to 0.52, 1 RCT, n = 70) (Analysis 7.5).
7.5. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 5: Adverse effects ‐ continuous
6.4 Quality of life
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including high risk of bias studies. Quality of life, measured by SF‐36 was reported by four studies (Harada 2009; Schlaff 2006; Strowitzki 2012; Zupi 2005). Harada 2009 reported results after six months of treatment and for all domains, but we are uncertain about the effect found between two groups. After 12 months of treatment, Zupi 2005 also reported no significant differences in the results of the SF‐36 (Analysis 7.6).
7.6. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 6: Quality of life ‐ continuous
Crosignani 2006 data were not usable for meta‐analysis, but stated that "mean scores for all four prespecified Endometriosis Health Profile‐30 (EHP‐30) scales (pain, emotional well‐being, self‐image and intercourse) as well as the two remaining scales (social support, control and powerlessness), significantly improved in both groups at month six compared with pretreatment. Similarly, scores for all three prespecified scales of the SF‐36 (physical function, role physical and social functioning) significantly improved at month six relative to pretreatment". In addition, "mean scores for all four prespecified EHP‐30 scales (pain, emotional well‐being, self‐image, and intercourse) and two additional EHP‐30 scales (social support as well as control and powerlessness) significantly improved in both groups at month six compared with the case of baseline (P < 0.05), and these improvements were maintained at the 12‐month post‐treatment follow‐up. Similarly, scores for all three prespecified scales of the SF‐36 (physical function, role‐physical, and social functioning) significantly improved at month six relative to baseline, with improvements maintained through 12 months of follow‐up, in both groups (P < 0.05)" (Schlaff 2006). Strowitzki 2012 stated that "at the end of treatment, QoL showed more pronounced absolute improvements in the dienogest (DNG) group than in the leuprolide acetate group, including both the physical health (DNG, 10.2 points; LA, 7.0 points) and the mental health (DNG, 3.3 points; LA, 1.9 points) summary scale scores. Compared with LA, DNG was also associated with greater relative improvements in specific SF‐36 scale categories. In particular, DNG produced greater improvements in the categories 'physical functioning' (DNG, 18.0%; LA, 6.8%), 'role‐physical' (DNG, 75.7%; LA, 33.6%), 'vitality' (DNG, 28.3%; LA, 12.3%), and 'social functioning' (DNG, 21.4%; LA, 8.7%), which relate to everyday physical activity, productivity at work, energy levels, and ability to interact in society, respectively".
6.5 Improvement of most troublesome symptom
No studies with overall low risk of bias were identified for this analysis.
For the sensitivity analysis, Strowitzki 2012 was the only study that reported dichotomous results on improvement of the most troublesome symptom. We are uncertain about the effect between the two groups after six months of treatment (RR 1.00, 95% CI 0.79 to 1.28, 1 RCT, n = 229) (Analysis 7.7).
7.7. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 7: Improvement of most troublesome symptoms ‐ dichotomous
Harada 2009 and Ozaki 2020 both reported values for overall symptoms. Harada 2009 reported no difference between two groups, after six months of treatment (MD ‐0.70, 95% CI ‐1.52 to 0.12, 1 RCT, n = 253). Ozaki 2020 reported results after four months of treatment, and used a numerical rating scale (NRS) and a verbal rating scale (VRS). For GnRHas compared to oral or injectable progestogens, there may be an improvement for NRS after four months of treatment (MD 2.60, 95% CI 0.37 to 4.83, 1 RCT, n = 70) and also for "improvement of most troublesome symptom" measured by VRS (MD 0.70, 95% CI 0.06 to 1.34, 1 RCT, n = 70) (Analysis 7.8).
7.8. Analysis.

Comparison 7: GnRHas versus oral or injectable progestogens ‐ all studies included, Outcome 8: Improvement of most troublesome symptoms ‐ continuous
7. GnRHas versus gestrinone for relief of overall pain associated with endometriosis and its related adverse effects
Only one study compared GnRHas with gestrinone (Vercellini 1996), with unclear and low risk for selection bias. This study was not included in the original review, but results below are from the sensitivity analyses.
7.1 Relief of overall pain
Vercellini 1996 reported relief of overall pain on a continuous scale, after three and six months of treatment with GnRHas or gestrinone. We are uncertain about the effect found for overall pain, reported as dysmenorrhoea, dyspareunia, and non‐menstrual pelvic pain, all measured on a NRS and VRS scale (Analysis 8.1).
8.1. Analysis.

Comparison 8: GnRHas versus gestrinone ‐ all studies included, Outcome 1: Relief of overall pain ‐ continuous
7.2 Bone mineral density
BMD was measured by Vercellini 1996, who reported the percentage of change after six and 12 months of treatment with either GnRHas or gestrinone. After both treatment periods, there may be a decrease found in favour of GnRHas ((MD ‐1.96 95% CI ‐3.62 to ‐0.30, 1 RCT, n = 41) and (MD ‐5.10 95% CI ‐7.39 to ‐2.81, 1 RCT, n = 41), respectively) (Analysis 8.2).
8.2. Analysis.

Comparison 8: GnRHas versus gestrinone ‐ all studies included, Outcome 2: Bone mineral density of spinal bone mass ‐ continuous
7.3 Adverse effects
After six months of treatment, there may be an improvement for hot flushes after treatment with GnRHas, compared to gestrinone (RR 2.29 95% CI 1.21 to 4.32, 1 RCT, n = 55). For the remainder of all the reported adverse effects, we are uncertain about the effect on BMD between the two groups (Analysis 8.3).
8.3. Analysis.

Comparison 8: GnRHas versus gestrinone ‐ all studies included, Outcome 3: Adverse effects ‐ dichotomous
8. Trials comparing different doses of GnRHas for relief of overall pain associated with endometriosis and its related adverse effects
A total of eight trials compared different doses of GnRHas (Adamson 1994; Bergqvist 1997; Burry 1989; Henzl 1988; Minaguchi 1986; Shaw 1986; Tahara 2000; Tang 2017). Tahara 2000 and Bergqvist 1997 both compared 200 μg nafarelin with 400 μg nafarelin for a treatment period of six months. Adamson 1994, Burry 1989 and Henzl 1988 all compared 400 μg nafarelin with 800 μg nafarelin for a treatment period of six months. Tang 2017 compared 1.88 mg leuprorelin with 3.75 mg leuprorelin for a treatment period of six months. As this comparison only included studies with unclear or high risk of bias, results stated below are results of the sensitivity analysis. Results from Minaguchi 1986 and Shaw 1986 could not be used for meta‐analysis.
8.1 Relief of overall pain
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies suited for meta‐analysis. We are uncertain about the effect found for overall pain, reported as pelvic pain after two months (MD 0.20, 95% CI ‐1.07 to 1.47, 1 RCT, n = 15), four months (MD ‐0.10, 95% CI ‐1.07 to 0.87, 1 RCT, n = 15) and six months (MD 0.30, 95% CI ‐0.61 to 1.21, 1 RCT, n = 15) of treatment with 200 μg nafarelin compared to 400 μg nafarelin (Tahara 2000) (Analysis 9.1).
9.1. Analysis.

Comparison 9: GnRHas versus GnRHas (varying dosage) ‐ all studies included, Outcome 1: Relief of overall pain ‐ 200 μg versus 400 μg nafarelin ‐ continuous
Besides, we are also uncertain about the effect found for overall pain, reported as pelvic pain (RR 1.24, 95% CI 0.71 to 2.16, 1 RCT, n = 77), dysmenorrhoea (RR 3.00, 95% CI 0.13 to 71.74, 1 RCT, n = 90), and dyspareunia (RR 1.05, 95% CI 0.49 to 2.26, 1 RCT, n = 57) after six months of treatment with 400 μg nafarelin compared to 800 μg nafarelin (Adamson 1994) (Analysis 9.2).
9.2. Analysis.

Comparison 9: GnRHas versus GnRHas (varying dosage) ‐ all studies included, Outcome 2: Relief of overall pain ‐ 400 μg versus 800 μg nafarelin ‐ dichotomous
Bergqvist 1997, Henzl 1988 and Tang 2017 did not provide sufficient data to include them in the meta‐analysis.
8.2 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies suited for meta‐analysis. In one study, it was reported that "bone mineral density of the lumbar spine at 24 weeks of treatment was significantly lower than before treatment in the control group, with a mean bone loss of 5.56%. The decrease in bone mineral content was less in the half‐dose group, with a mean bone loss of 1.38%. It has been reported that nafarelin at a dosage of 200 mg daily does not cause the significant reduction in bone density that is seen with a dosage of 400 mg daily. Our data show that loss of BMD was largely eliminated with half‐dose nafarelin during 6 months of GnRH agonist therapy" (Tahara 2000).
Tang 2017 stated that "the BMD was decreased in both groups at 20 weeks after treatment, and the degree of loss of BMD in the control group (= 3.75 mg leuprorelin) (5.6%) was higher than in the research group (= 1.88 mg leuprorelin)(1.2%; P < 0.05)".
8.3 Adverse effects
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies suited for meta‐analysis. We are uncertain about the effect found for vasomotor symptoms after two months, four months and six months of treatment with 200 μg nafarelin compared to 400 μg nafarelin (Tahara 2000). In addition, rhinitis, upper respiratory infections, and irregular bleeding were reported without any significant difference between either group (Bergqvist 1997) (Analysis 9.3).
9.3. Analysis.

Comparison 9: GnRHas versus GnRHas (varying dosage) ‐ all studies included, Outcome 3: Adverse effects ‐ 200 μg versus 400 μg nafarelin ‐ dichotomous
Tang 2017 compared 1.88 mg leuprorelin with 3.75 mg leuprorelin for a treatment period of two, three, four and six months. After two months, there was no difference in menopausal symptoms, measured by the Kupperman Index (MD 1.20, 95% CI ‐3.14 to 5.54, 1 RCT, n = 50). However, after three, four and six months, there may be a difference in favour of 3.75 mg leuprorelin compared to 1.88 mg leuprorelin ((MD 5.70, 95% CI 2.12 to 9.28, 1 RCT, n = 50) (MD 9.50, 95% CI 6.55 to 12.45, 1 RCT, n = 50) (MD 13.20, 95% CI 10.22 to 16.18, 1 RCT, n = 50), respectively) (Analysis 9.4).
9.4. Analysis.

Comparison 9: GnRHas versus GnRHas (varying dosage) ‐ all studies included, Outcome 4: Adverse effects ‐ 3.75 mg versus 1.88 mg leuprolide acetate ‐ continuous
Burry 1989 reported the results of weight gain during treatment, but it was not possible to extract data for meta‐analysis.
8.4 Improvement of most troublesome symptom
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Henzl 1988 measured overall improvement, and we are uncertain about the effect between 400 μg nafarelin with 80 0μg nafarelin for a treatment period of six months (RR 0.94, 95% CI 0.78 to 1.14, 1 RCT, n = 143) (Analysis 9.5).
9.5. Analysis.

Comparison 9: GnRHas versus GnRHas (varying dosage) ‐ all studies included, Outcome 5: Improvement of most troublesome symptoms ‐ 400 μg versus 800 μg nafarelin ‐ dichotomous
9. Trials comparing different treatment duration of GnRHas for relief of overall pain associated with endometriosis and its related adverse effects
Two trial mentioned the effect of different treatment duration of GnRHas (two trials; Hornstein 1995; Orwoll 1994). As this comparison only included studies with unclear or high risk of bias, results stated below are the results of the sensitivity analysis.
Hornstein 1995 and Orwoll 1994 both compared a total treatment period of three months with intranasal 200 μg nafarelin twice daily combined with three consecutive months of placebo use, with a total treatment period of six months with intranasal 200 μg nafarelin twice daily.
9.1 Relief of overall pain
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Hornstein 1995 measured relief of overall pain, with a comparison of a total treatment period of three months with a total treatment period of six months with intranasal 200 μg nafarelin twice daily. There may be an improvement for pelvic pain (MD 0.16, 95% CI 0.13 to 0.19, 1 RCT, n = 179) and pelvic tenderness (MD 0.05, 95% CI 0.03 to 0.07, 1 RCT, n = 179) after six months of treatment, in favour of six months treatment compared to three months of treatment (with intranasal 200 μg nafarelin twice daily and three months of placebo). For overall pain, reported as dysmenorrhoea (MD ‐0.09, 95% CI ‐0.11 to ‐0.07, 1 RCT, n = 179) and dyspareunia (MD ‐0.14, 95% CI ‐0.17 to ‐0.11, 1 RCT, n = 179), the opposite results were found. There may be a slight decrease after six months of treatment, in favour of three months treatment, compared to six months of treatment with intranasal 200 μg nafarelin twice daily. No significant difference was found for pelvic induration between the two groups (MD ‐0.03, 95% CI ‐0.06 to 0.00, 1 RCT, n = 179) (Analysis 10.1).
10.1. Analysis.

Comparison 10: GnRHas versus GnRHas (duration of treatment) ‐ all studies included, Outcome 1: Relief of overall pain ‐ 3 months vs 6 months ‐ continuous
9.2 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. For BMD, after a total treatment period of three months with intranasal 200 μg nafarelin twice daily combined with three consecutive months of placebo use, there might be a decrease in BMD, compared with a total treatment period of six months with intranasal 200 μg nafarelin twice daily. This applied to both spinal bone mass (MD 1.60, 95% CI 1.51 to 1.69, 1 RCT, n = 183) and proximal femoral bone (MD 1.90, 95% CI 1.72 to 2.08, 1 RCT, n = 183) (Orwoll 1994) (Analysis 10.2 and Analysis 10.3).
10.2. Analysis.

Comparison 10: GnRHas versus GnRHas (duration of treatment) ‐ all studies included, Outcome 2: Bone mineral density of spinal bone mass ‐ 3 months vs 6 months ‐ continuous
10.3. Analysis.

Comparison 10: GnRHas versus GnRHas (duration of treatment) ‐ all studies included, Outcome 3: Bone mineral density of proximal femoral bone ‐ 3 months vs 6 months ‐ continuous
10. Trials comparing different route of administration of GnRHas for relief of overall pain associated with endometriosis and its related adverse effects
Four trials comparing different route of administration of GnRHas were included in this comparison (Agarwal 1997; Bergqvist 2000; Dmowski 1989a; Lemay 1988).
Bergqvist 2000 compared subcutaneously administered goserelin depot with intranasal nafarelin 200 μg twice daily. Dmowski 1989a and Lemay 1988 both compared subcutaneously administered buserelin depot 200 μg once daily with intranasal buserelin 200 μg thrice daily.
The results of Dmowski 1989a could not be used, because no distinction was made in the article between subcutaneously administered buserelin depot 200 μg once daily and intranasal nafarelin 200 μg buserelin thrice daily.
10.1 Relief of overall pain
Lemay 1988 compared subcutaneously administered buserelin depot 200 μg once daily with intranasal 400 μg buserelin thrice daily. We are uncertain about the effect on pelvic pain (RR 1.00, 95% CI 0.53 to 1.87, 1 RCT, n = 5), dysmenorrhoea (RR 1.22, 95% CI 0.73 to 2.06, 1 RCT, n = 9), dyspareunia (RR 1.00, 95% CI 0.57 to 1.75, 1 RCT, n = 7), pelvic induration (RR 0.86, 95% CI 0.47 to 1.55, 1 RCT, n = 8) and pelvic tenderness (RR 1.50, 95% CI 0.69 to 3.27, 1 RCT, n = 10) between either group, after six months of treatment (Analysis 11.1). As this comparison included only one low‐risk study, a sensitivity analysis reported comparable results.
11.1. Analysis.

Comparison 11: GnRHas versus GnRHas (route of administration), Outcome 1: Relief of overall pain ‐ IN versus SC ‐ dichotomous
Agarwal 1997 compared intramuscularly administered leuprolide acetate depot 3.75 mg once monthly with intranasal nafarelin 200 μg twice daily. We are uncertain about the effect on overall pain, reported as pelvic pain (RR 0.96, 95% CI 0.73 to 1.26, 1 RCT, n = 192), dysmenorrhoea (RR 1.29, 95% CI 0.72 to 2.30, 1 RCT, n = 192), dyspareunia (RR 0.88, 95% CI 0.62 to 1.25, 1 RCT, n = 166), pelvic induration (RR 1.41, 95% CI 0.82 to 2.41, 1 RCT, n = 192) and pelvic tenderness (RR 1.23, 95% CI 0.88 to 1.73, 1 RCT, n = 192) between either group, after six months of treatment (Analysis 12.1; Analysis 12.2).
12.1. Analysis.

Comparison 12: GnRHas versus GnRHas (route of administration) ‐ all studies included, Outcome 1: Relief of overall pain ‐ IN versus SC ‐ dichotomous
12.2. Analysis.

Comparison 12: GnRHas versus GnRHas (route of administration) ‐ all studies included, Outcome 2: Relief of overall pain ‐ IN versus IM ‐ dichotomous
Bergqvist 2000 reported that "the total pain score, i.e. the three parameters dysmenorrhoea, dyspareunia and pelvic pain, was reduced in both groups, for goserelin by 45% and for nafarelin by 43% 3 months after the end of treatment".
10.2 Adverse effects
Lemay 1988 compared subcutaneously administered buserelin depot 200 μg once daily with intranasal 400 μg buserelin thrice daily. For hot flushes (RR 0.86, 95% CI 0.48 to 1.55, 1 RCT, n = 13), vaginal dryness (RR 0.86, 95% CI 0.17 to 4.37, 1 RCT, n = 13), decreased libido (RR 0.86, 95% CI 0.07 to 10.96, 1 RCT, n = 13) and headaches (RR 1.71, 95% CI 0.20 to 14.55, 1 RCT, n = 13), we are uncertain about the effects found between the two groups (Analysis 11.2). As this comparison included only one low‐risk study, a sensitivity analysis reported comparable results (Analysis 12.3).
11.2. Analysis.

Comparison 11: GnRHas versus GnRHas (route of administration), Outcome 2: Adverse effects ‐ IN vs SC ‐ dichotomous
12.3. Analysis.

Comparison 12: GnRHas versus GnRHas (route of administration) ‐ all studies included, Outcome 3: Adverse effects ‐ IN vs SC ‐ dichotomous
Bergqvist 2000 and Agarwal 1997 reported adverse effects after comparing intramuscular administered goserelin or leoprolide acetate depot with intranasal nafarelin 200 μg twice daily. For hot flushes (RR 0.95, 95% CI 0.88 to 1.02, I2 = 38%, 2 RCTs, n = 404), headaches (RR 0.83, 95% CI 0.63 to 1.10, 1 RCT, n = 213), sweating (RR 1.13, 95% CI 0.71 to 1.79, 1 RCT, n = 213), and vaginal dryness (RR 0.52, 95% CI 0.27 to 1.00, 1 RCT, n = 213), we are uncertain about the effect found between the two groups. This is in contrast with the results of vaginal bleeding, because there may be an improvement in favour of subcutaneously administered goserelin depot compared to intranasal nafarelin 200 μg twice daily (RR 19.19, 95% CI 1.12 to 328.28, 1 RCT, n = 213) (Analysis 12.4).
12.4. Analysis.

Comparison 12: GnRHas versus GnRHas (route of administration) ‐ all studies included, Outcome 4: Adverse effects ‐ IN versus IM depot ‐ dichotomous
10.3 Bone mineral density
Agarwal 1997 stated the percentage of decrease of BMD when intranasal nafarelin was compared to intramuscular leuprolide acetate. After six months of treatment, there may be a reduction in BMD in favour of nafarelin (MD ‐2.00, 95% CI ‐2.10 to ‐1.90, 1 RCT, n = 152).
11. Trials comparing different GnRHas treatment regimens for relief of overall pain associated with endometriosis and its related adverse effects
There was only one trial included, comparing different GnRHa treatment regimens (Crosignani 1996). Crosignani 1996 compared a monthly injection of leuprolide with a three‐monthly injection of leuprolide. As this comparison only included one study with an unclear risk of bias, the results stated below are the results of the sensitivity analysis.
11.1 Relief of overall pain
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Only Crosignani 1996 was included in the comparison 'Trials comparing different GnRHa treatment regimens for revealing painful symptoms associated with endometriosis and its related adverse effects'. We are uncertain about the effect found for overall pain, reported as pain symptom scores after three and six months of treatment. Results were subdivided into non‐menstrual pelvic pain (MD ‐0.20, 95% CI ‐0.52 to 0.12, 1 RCT, n = 30), dyspareunia (MD ‐0.10, 95% CI ‐0.51 to 0.31, 1 RCT, n = 30), pelvic induration (MD 0.10, 95% CI ‐0.40 to 0.60, 1 RCT, n = 30) and pelvic tenderness (MD ‐0.30, 95% CI ‐0.71 to 0.11, 1 RCT, n = 30), during a treatment period of three months. For a treatment period of six months, the results were similar for non‐menstrual pelvic pain (MD 0.10, 95% CI ‐0.15 to 0.35, 1 RCT, n = 30), dyspareunia (MD 0.00, 95% CI ‐0.50 to 0.50, 1 RCT, n = 30), and pelvic tenderness (MD 0.40, 95% CI ‐0.10 to 0.90, 1 RCT, n = 30). However, for pelvic induration after six months of treatment, there may be a difference between the two groups (MD 0.40, 95% CI 0.10 to 0.70, 1 RCT, n = 30) (Analysis 13.1).
13.1. Analysis.

Comparison 13: GnRHas versus GnRHas (different treatment regimens) ‐ all studies included, Outcome 1: Relief of overall pain ‐ monthly versus 3‐monthly depot leuprolide acetate ‐ continuous
11.2 Adverse effects
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Adverse effects were reported by Crosignani 1996; we are uncertain about the effect on hot flushes (RR 1.00, 95% CI 0.70 to 1.43, 1 RCT, n = 24), vaginal dryness (RR 0.67, 95% CI 0.13 to 3.44, 1 RCT, n = 30), abdominal pain (RR 3.00, 95% CI 0.13 to 68.26, 1 RCT, n = 30), arthralgia (RR 3.00, 95% CI 0.13 to 68.26, 1 RCT, n = 30) and depression (RR 3.00, 95% CI 0.13 to 68.26, 1 RCT, n = 30), after six months of treatment when a monthly injection of leuprolide was compared with a three‐monthly injection of leuprolide (Analysis 13.2).
13.2. Analysis.

Comparison 13: GnRHas versus GnRHas (different treatment regimens) ‐ all studies included, Outcome 2: Adverse effects ‐ monthly versus 3‐monthly depot leuprolide acetate ‐ dichotomous
11.3 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Crosignani 1996 reported the results of monthly versus three‐monthly doses of leuprolide acetate on BMD. The trial stated that there might be a slight variation of lumbar spine bone mineral density observed at the end of GnRHa treatment in both study groups (P < 0.01), the percentage decrease over basal being 5.2% and 4.9%, respectively. But the study did not provide sufficient data for comparison of the groups; authors were contacted for the previous version of the review, but have not replied to date.
12. Trials comparing GnRHas versus GnRHas in conjunction with add‐back therapy (hormonal or non‐hormonal) for relief of overall pain associated with endometriosis and its related adverse effects
Sixteen trials compared GnRHas versus GnRHas in conjunction with add‐back therapy (hormonal or non‐hormonal) (Bergqvist 1997; Edmonds 1994; Franke 2000; Freundl 1998; Gnoth 1999; Hornstein 1998; Howell 1995; Hurst 2000; Irahara 2001; Kiilholma 1995; Mäkäräinen 1996; Moghissi 1998; Sillem 1999; Surrey 1992; Surrey 2002; Vercellini 1994; Zupi 2005). As this comparison only included studies with unclear or high risk of bias, the results stated below are the results of the sensitivity analysis.
12.1 Relief of overall pain
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. A total of ten studies reported relief of overall pain as a primary or secondary outcome measure (Bergqvist 1997; Freundl 1998; Hornstein 1998; Howell 1995; Hurst 2000; Mäkäräinen 1996; Moghissi 1998; Surrey 2002; Vercellini 1994; Zupi 2005).
Relief of overall pain was presented both as a dichotomous and as a continuous outcome measure. Freundl 1998 was the only study that reported dichotomous outcomes; we are uncertain about the effect between GnRHas and GnRHas in conjunction with add‐back therapy (dysmenorrhoea, RR 0.63, 95% CI 0.58 to 159.04, 1 RCT, n = 28; dyspareunia, RR 6.07, 95% CI 0.86 to 43.04, 1 RCT, n = 28; and non‐menstrual pelvic pain, RR 1.30, 95% CI 0.26 to 6.62, 1 RCT, n = 28) (Analysis 14.1). Zupi 2005 reported continuous outcomes; we are uncertain about the effect found between groups for overall pain, reported as dysmenorrhoea after six months of treatment (not estimable), dyspareunia after six months of treatment (MD ‐0.20, 95% CI ‐0.40 to 0.80, 1 RCT, n = 90), and overall pain (MD ‐1.50, 95% CI ‐7.92 to 4.92, 1 RCT, n = 90); or dysmenorrhoea (not estimable) and dyspareunia (MD 0.20, 95% CI ‐0.03 to 0.43, 1 RCT, n = 90) both after 12 months of treatment. In contrast, values for pelvic pain may improve slightly in favour of GnRHas in conjunction with add‐back therapy. This applied to both six months of treatment (MD ‐0.20, 95% CI ‐0.39 to ‐0.01, 1 RCT, n = 90) and 12 months of treatment (MD ‐0.10, 95% CI ‐0.14 to ‐0.06, 1 RCT, n = 90) (Analysis 14.2). This corresponds partly to the data extracted from Howell 1995, which stated that "both groups showed similar improvement in pelvic symptoms (dysmenorrhoea, dyspareunia, and other pelvic pain) and pelvic signs (tenderness and induration) during the course of treatment with no significant difference between the two groups (P > 0.05)".
14.1. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 1: Relief of overall pain ‐ dichotomous
14.2. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 2: Relief of overall pain ‐ continuous
Hornstein 1998 did not include data in the meta‐analysis, but no significant difference was found between GnRHas alone (group A), compared with three different add‐back groups (group B received daily oral norethindrone acetate 5 mg with placebo for oestrogen; group C received norethindrone 5 mg and conjugated equine oestrogens 0.625 mg; group D received norethindrone 5 mg and conjugated equine oestrogens 1.25 mg), comparing dysmenorrhoea, non‐menstrual pelvic pain, and pelvic tenderness. "The mean decreases in group D were significantly less than those of group A at week 4, 8 (P < 0.05), 12 (P < 0.01), and 48 (P < 0.05)" (Hornstein 1998). All participants in the study of Kiilholma 1995 showed subjective improvement on goserelin acetate therapy. The authors reported that "the response was equally good in patients with or without HRT (P = not significant [NS]). The pelvic symptoms score decreased from 4.7 and 4.7 in goserelin acetate plus HRT and goserelin acetate plus placebo patients to 0.9 and 0.5 after 6 months, respectively".
Bergqvist 1997, Hurst 2000, Mäkäräinen 1996, Moghissi 1998 and Vercellini 1994 did not provide sufficient data to include their data in the meta‐analysis.
Surrey 2002 compared four different groups, all receiving GnRHas. Group A received daily oral placebos for oestrogen and progestin add‐back. Patients included in group B received daily oral norethindrone acetate 5 mg and placebo for oestrogen. Patients in group C received oral norethindrone acetate 5 mg and conjugated equine oestrogens 0.625 mg daily. Those in group D received oral norethindrone acetate 5 mg and conjugated equine oestrogens 1.25 mg daily. The authors reported that "the median changes in overall pelvic pain, dysmenorrhoea, and dyspareunia scores from pre‐therapy baseline throughout the follow‐up period for the four groups are displayed in Figures 1–3, respectively. Decreases in symptom scores from baseline remained statistically significant through month 12 of follow‐up for all groups with the exceptions of dysmenorrhoea for groups A and D and deep dyspareunia for group D, which all remained suppressed through month 8 of follow‐up. The only significant difference between groups regarding return to baseline scores occurred between groups A and D for dysmenorrhoea, where group D patients returned to baseline levels sooner".
12.2 Bone mineral density
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. The effect of add‐back therapy on the GnRHas‐induced loss of bone mineral density was reported by thirteen studies (Edmonds 1994, Franke 2000; Freundl 1998; Gnoth 1999; Hornstein 1998; Howell 1995; Irahara 2001; Moghissi 1998; Schlaff 2006; Sillem 1999; Surrey 1992; Surrey 2002; Zupi 2005).
A meta‐analysis was undertaken for continuous outcome measures, and subdivided into percentage change values and absolute values. The percentage change may improve when treated with GnRHas compared with GnRHas in conjunction with add‐back therapy (MD ‐3.88, 95% CI ‐4.27 to ‐3.49, I2 = 93%, 2 RCTs, n = 46, Freundl 1998; Surrey 1992). Gnoth 1999 also mentioned a significant loss of BMD in the lumbar spine for the GnRHa + placebo group compared to that for the GnRHa + add‐back group (6.5 vs. 2.0%, respectively, P = 0.001). For absolute values, after six months of treatment with either GnRHas or GnRHas in conjunction with add‐back therapy, there may be a difference in change in favour of GnRHas in conjunction with add‐back therapy (MD 0.02, 95% CI 0.02 to 0.02, I2 = 70%, 5 RCTs, n = 199, Gnoth 1999; Sillem 1999; Surrey 1992; Zupi 2005). Only Zupi 2005 reported results after 12 months of treatment, without any changes between either group (Analysis 14.3).
14.3. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 3: Bone mineral density of spinal bone mass ‐ continuous
Bone mineral density loss is reduced by 50% to 2.5% overall by the addition of add‐back therapy and there may be an improvement in the rate of return to normal of bone mineral density during post‐treatment follow‐up (Edmonds 1994). We are uncertain about the effect on BMD after six months of treatment with GnRHas, compared to GnRHas in conjunction with add‐back therapy (Franke 2000). Another study, Howell 1995, reported a "reduction of bone mineral density at the lumbar spine in group 1 (= GnRHas) of ‐4.1% compared with ‐2.3% in group 2 (= GnRHas + add‐back)". There was for both groups a reduction compared to baseline. A comparison between the two groups has not been made. Hornstein 1998 compared GnRHas alone (group A), and three different add‐back groups (group B: received daily oral norethindrone acetate 5 mg with placebo for oestrogen; group C: received norethindrone 5 mg and conjugated equine oestrogens 0.625 mg; group D: received norethindrone 5 mg and conjugated equine oestrogens 1.25 mg). "All three add‐back groups had significantly less bone loss than the agonist‐only group at 24‐ and 52‐week measurements (P ≤ 0.001)". This corresponds to the results found by Irahara 2001 and Sillem 1999. "The control group (= GnRHas alone) significantly (P < 0.01) decreased BMD of the lumber spine (mean percentage change: –6.3%) after six months of treatment; however, add‐back therapy prevented this BMD reduction (mean percentage change: –0.8%)" (Irahara 2001) and "both groups, a significant decrease in lumbar bone mineral density (LBMD) was observed after six months of treatment. When compared to baseline values, the mean relative bone loss was 4%, in both groups equally (P < 0.01, 0 months vs. 6 months). Absolute values decreased from 1.28 ± 0.18 g/cm2 (mean ± standard deviation) to 1.23 ± 0.16 g/cm2 in group A and from 1.19 ± 0.11 g/cm2 to 1.14 ± 0.1 g/cm2 in group B. No change in bone mineral density was observed at the femoral neck or Ward's triangle" (Sillem 1999). Moghissi 1998 reported that the mean percentage loss was significantly higher in the group without add‐back therapy than in either group with once daily doses of 0.3 mg of conjugated oestrogen and 5 mg of medroxyprogesterone acetate, or once daily doses of 0.625 mg of conjugated oestrogen and 5 mg of medroxyprogesterone acetate at week 12 (P < 0.001). Data could not be included in meta‐analyses.
12.3 Adverse effects
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Eleven studies reported adverse effects when comparing GnRHas with GnRHas in conjunction with add‐back therapy (Bergqvist 1997; Edmonds 1994; Franke 2000; Freundl 1998; Hornstein 1998; Howell 1995; Hurst 2000; Kiilholma 1995; Mäkäräinen 1996; Moghissi 1998; Zupi 2005). Only six of them could be included in meta‐analysis, namely for assessment of hot flushes, loss of libido, vaginal dryness, headache and vaginal bleeding, all after six months of treatment. For hot flushes and vaginal dryness, there may be an improvement in both groups, in favour of GnRHas (RR 1.59, 95% CI 1.32 to 1.93, I2 = 92%, 4 RCTs, n = 215 (Edmonds 1994; Freundl 1998; Howell 1995; Zupi 2005) and RR 1.40, 95% CI 1.11 to 1.76, I2 = 30%, 3 RCTs, n = 404 (Edmonds 1994; Howell 1995; Moghissi 1998), respectively). "Hot flushes were reported more frequently by the patients receiving goserelin acetate plus placebo than by those receiving HRT. The difference was significant (P < 0.01) after 4 weeks of trial medication and highly significant at 6 months (P < 0.0001)" (Kiilholma 1995). In addition, Hornstein 1998, who compared the GnRHas alone (group A) with three different add‐back groups (group B: received daily oral norethindrone acetate 5 mg with placebo for oestrogen; group C: received norethindrone 5 mg and conjugated equine oestrogens 0.625 mg; group D: received norethindrone 5 mg and conjugated equine oestrogens 1.25 mg) stated that "the percentage of patients experiencing hot flashes was dramatically less in the three add‐back groups than in group A", after 52 weeks of treatment. We are uncertain of the effect found between GnRHas and GnRHas in conjunction with add‐back therapy for loss of libido (RR 1.07, 95% CI 0.58 to 1.97, I2 = 89%, 2 RCTs, n = 96, Edmonds 1994; Howell 1995) and headache (RR 0.91, 95% CI 0.67 to 1.24, I2 = 0%, 3 RCTs, n = 126, Edmonds 1994; Freundl 1998; Howell 1995). Vaginal bleeding was seen more commonly in women treated with GnRH in conjunction with add‐back therapy (RR 0.57, 95% CI 0.35 to 0.93, I2 = 70%, 3 RCTs, n = 185, Bergqvist 1997; Howell 1995; Zupi 2005) (Analysis 14.4). This is comparable to the results of Mäkäräinen 1996, who stated: "significantly fewer patients in the MPA group (=GnRHas + add‐back) had hot flushes and sweating at 3 and 6 months than in the placebo group (=GnRHas alone). Other side effects recorded occurred with similar frequency in both groups". The results mentioned above, are partly in contrast to Hurst 2000, who reported "as expected, hot flush and headache mean scores during time period three are usually lower for the oestradiol group than for the placebo group, although these differences were not statistically significant". Besides these outcome measures, "in the GnRH agonist plus placebo group, the Kupperman index score decreased by 75% at 4 weeks, 129% at 12 weeks, and 113% at 24 weeks (P = 0.0004). The difference between groups at 24 weeks was statistically significant (P = 0.003)" (Franke 2000).
14.4. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 4: Adverse effects ‐ dichotomous
The results reported by Surrey 2002 could not be extracted for current analyses.
12.4 Quality of life
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Quality of life, measured by SF‐36, was reported by only one study (Zupi 2005). After 12 months of treatment, Zupi 2005 reported that there may be a slight difference in the results of the SF‐36, in the domains 'physical function' and 'vitality'. Both domains reported better quality of life in women treated with GnRHas in conjunction with add‐back therapy. For the other domains, we are uncertain of the effect after 12 months of treatment (Analysis 14.5).
14.5. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 5: Quality of life ‐ continuous
12.5 Improvement of most troublesome symptom
No studies with overall low risk of bias were identified for this analysis.
We performed a sensitivity analysis including all studies. Surrey 1992 reported results of improvement of overall pain, after four weeks of treatment with either GnRHas of GnRHas in conjunction with add‐back therapy. There may be a greater improvement in women treated with GnRHas, compared to GnRHas in conjunction with add‐back (Analysis 14.6).
14.6. Analysis.

Comparison 14: GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included, Outcome 6: Improvement of most troublesome symptoms ‐ continuous
This is in contrast to Edmonds 1994, which reported no difference in pain scores after six months of treatment with GnRHas or with GnRHas in conjunction with add‐back therapy.
13. Trials comparing GnRHas versus GnRHas in conjunction with calcium‐regulating agents for relief of overall pain associated with endometriosis and its related adverse effects
A total of three trials compared GnRHas versus GnRHas in conjunction with calcium‐regulating agents (Finkelstein 1998; Finkelstein 1999; Roux 1995).
Since Finkelstein 1998 reported the same results for BMD as Finkelstein 1999, only Finkelstein 1998 was included in the analysis on BMD. As this comparison only included one study with a low risk of bias, the results stated below are the results of the sensitivity analysis.
13.1 Bone mineral density
Finkelstein 1998 reported that there may be a slight decrease in BMD after 12 months treatment (MD ‐7.00 95% CI ‐7.53 to ‐6.47, 1 RCT, n = 43) with GnRHas, compared to GnRHas in conjunction with calcium‐regulating agents (Analysis 15.1; Analysis 16.1).
15.1. Analysis.

Comparison 15: GnRHas versus GnRHas in conjunction with calcium‐regulating agents, Outcome 1: Bone mineral density of spinal bone mass ‐ continuous
16.1. Analysis.

Comparison 16: GnRHas versus GnRHas in conjunction with calcium‐regulating agents ‐ all studies included, Outcome 1: Bone mineral density of spinal bone mass ‐ continuous
Roux 1995 compared triptorelin alone (group 0) with triptorelin and salmon calcitonin 100 IU daily (group 1) and triptorelin and salmon calcitonin 200 IU daily (group 2). After six months of treatment, the mean values (± SD) of BMD (g/cm2) of group 0 were 1.031 ± 0.091, compared to 1.009 ± 0.152 in group 1 and 0.987 ± 0.143 in group 2.
13.2 Adverse effects
Finkelstein 1998 reported results about adverse effects, but it was not possible to report these outcomes in a meta‐analysis. "Mild nausea (P < 0.001) and arthralgias (P = 0.05) were reported more common in the women who received human parathyroid hormone, although only 1 woman reported that these symptoms affected her routine activities".
Data of Roux 1995 could not be used in the meta‐analysis, but "[t]here was no difference in side effects in the three groups (GnRHas combined with placebo (group 0), salmon calcitonin 100 IU daily (group 1) and salmon calcitonin 200 IU daily (group 2)). Nasal symptoms were reported by 12 (31%) patients. Thirty patients (75%) experienced hot flushes, which were attributed to the menopausal status rather than the spray. However, 11 of 40 patients (27.5%) experienced arthralgia, predominantly of the hand joints, including 3 patients with a clinical diagnosis of carpal syndrome and 1 patient with spontaneous knee effusion".
13.3 Improvement of most troublesome symptom
We are uncertain about the effect mentioned by Finkelstein 1998 related to improvement of the most troublesome symptom, both overall improvement (RR 0.96, 95% CI 0.84 to 1.08, 1 RCT, n = 43) and complete resolution (RR 0.95, 95% CI 0.64 to 1.41, 1 RCT, n = 43), when GnRHas were compared to GnRHas in conjunction with calcium‐regulating agents for a treatment period of 12 months (Analysis 15.2; Analysis 16.2).
15.2. Analysis.

Comparison 15: GnRHas versus GnRHas in conjunction with calcium‐regulating agents, Outcome 2: Improvement of most troublesome symptoms ‐ dichotomous
16.2. Analysis.

Comparison 16: GnRHas versus GnRHas in conjunction with calcium‐regulating agents ‐ all studies included, Outcome 2: Improvement of most troublesome symptoms ‐ dichotomous
14. Trials assessing the effect of GnRHas on BMD
Thirty‐one trials were included, across the 13 comparisons (Agarwal 1997; Crosignani 1996; Crosignani 2006; Dawood 1995; Dlugi 1990; Edmonds 1994; Finkelstein 1998; Finkelstein 1999; Franke 2000; Freundl 1998; Fukushima 1993; Gnoth 1999; Harada 2009; Hornstein 1998; Howell 1995; Irahara 2001; Moghissi 1998; Orwoll 1994; Rock 1993; Roux 1995; Schlaff 2006; Sillem 1999; Surrey 1992; Surrey 2002; Tahara 2000; Tang 2017; Tummon 1988; Vercellini 1996; Wheeler 1992; Whitehouse 1990; Zupi 2005). The effects on BMD are reported separately in the relevant comparisons and are also visible in Analysis 4.3; Analysis 7.3; Analysis 8.2; Analysis 10.2; Analysis 10.3; Analysis 12.5; Analysis 14.3; Analysis 15.1; Analysis 16.1.
12.5. Analysis.

Comparison 12: GnRHas versus GnRHas (route of administration) ‐ all studies included, Outcome 5: Bone mineral density of spinal bone mass ‐ IN vs IM depot ‐ continuous
Discussion
Summary of main results
There were no studies found comparing GnRHas with either no treatment or analgesics.
Trials comparing GnRHas versus placebo for relief of overall pain associated with endometriosis and its related adverse effects
There may be a decrease in reported pelvic pain scores, dysmenorrhoea scores, dyspareunia scores, and pelvic tenderness scores after three months of treatment. We are uncertain of the effect of GnRHas compared to placebo for pelvic induration, based on the results found after three months of treatment.
Additionally, treatment with GnRHas may be associated slightly with a greater incidence of hot flushes at three months of treatment.
Trials comparing GnRHas versus danazol for relief of overall pain associated with endometriosis and its related adverse effects
For pelvic pain in women treated with either GnRHas or danazol, a subdivision was made between pelvic tenderness, partly resolved and completely resolved. These dichotomous data indicated the uncertainty of the effect of GnRHas or danazol for the effectiveness of pelvic tenderness after six months of treatment with either GnRHas or danazol.
We are uncertain about the effect of GnRHas compared to danazol for relief of overall pain, when a subdivision was made for overall pain, pelvic pain, dysmenorrhoea, dyspareunia, pelvic induration, and pelvic tenderness. For all results, this was after three months of treatment. After six months of treatment, we are still uncertain about the effect of GnRHas or danazol on overall pain, dysmenorrhoea, dyspareunia, and pelvic tenderness. For pelvic pain and pelvic induration, these complaints may decrease after treatment with GnRHas, compared to danazol, during six months of treatment.
Trials comparing GnRHas versus intra‐uterine progestogens for relief of overall pain associated with endometriosis and its related adverse effects
We are uncertain about the effect on VAS scores between groups after six months of treatment. Besides, we are also uncertain about the effect of GnRHas compared to intra‐uterine progestogens for relief of overall pain after six months of treatment.
For quality of life, we are uncertain about the effects, measured by the psychological well‐being questionnaire index (PGWBI) between groups, after six months of treatment.
Trials comparing GnRHas versus oral or injectable progestogens for relief of overall pain associated with endometriosis and its related adverse effects
There may be an improvement in pelvic pain and dyspareunia after three months of treatment, in favour of oral progestogens. We are uncertain about the effects on back pain of both GnRHas and oral progestogens after three months of treatment.
Additionally, there may be a decrease of vaginal bleeding in women treated with GnRHas, compared to oral progestogens. Also, there may be slightly less weight gain in women treated with GnRHas instead of oral progestogens. We are uncertain about the effect of GnRHas compared to oral progestogens, for headache, after three months of treatment.
Trials comparing GnRHas versus gestrinone for relief of overall pain associated with endometriosis and its related adverse effects
We are uncertain about the effects found for dysmenorrhoea, dyspareunia, and non‐menstrual pelvic pain, all measured on NRS and VRS scales.
For BMD, there may be a decrease found in favour of GnRHas after six and 12 months of treatment with either GnRHas or gestrinone.
After six months of treatment, there may be an improvement in hot flushes after treatment with GnRHas compared to gestrinone.
Trials comparing different route of administration of GnRHas for relief of overall pain associated with endometriosis and its related adverse effects
We are uncertain about the effect on pelvic pain, dysmenorrhoea, dyspareunia, pelvic induration and pelvic tenderness when comparing buserelin depot 200 μg once daily with intranasal buserelin 200 μg thrice daily, after six months of treatment.
When comparing subcutaneously administered buserelin depot 200 μg once daily with intranasal buserelin 200 μg thrice daily, for hot flushes, vaginal dryness, decreased libido and headaches, we are uncertain about the effects found between the two groups.
Trials comparing GnRHas versus GnRHas in conjunction with calcium‐regulating agents for relief of overall pain associated with endometriosis and its related adverse effects
There may be a slightly bigger decrease in BMD after 12 months treatment with GnRHas, compared to GnRHas in conjunction with calcium‐regulating agents.
We are uncertain about the effect related to improvement of the most troublesome symptom, both overall improvement and complete resolution, when GnRHas were compared to GnRHas in conjunction with calcium‐regulating agents for a treatment period of 12 months.
Overall completeness and applicability of evidence
Unfortunately, some data are still missing. Despite the attempts made to contact the authors, the missing data could not be included in the review. Nevertheless, it is believed that, due to the large number of patients included (7355 in total), the evidence is comprehensive and has covered a variety of treatment options and outcome measurements. In particular, the comparisons comparing GnRHas with danazol, including 23 studies, and GnRHas compared with GnRHas in conjunction with add‐back therapy, including 17 studies, cover a wide number of studies.
One issue of concern is the methods of reporting relief of overall pain in the trials. Some trials report overall pain whilst others provide details of specific endometriosis‐associated pain such as dysmenorrhoea, dyspareunia, pelvic pain, pelvic induration, and pelvic tenderness. In addition, these outcome measures were requested at many different follow‐up periods. This means that a meta‐analysis was impossible at times. When it was possible, the analysis contained only a limited amount of studies.
In addition, it should be noted that not all hormonal treatment options (e.g. gestrinone and danazol) reported in this review are still common treatment options for women with endometriosis. Since they are still treatment options that might be available, it has been decided to include these comparisons in the current review.
Quality of the evidence
This was a systematic review of 72 trials including 7355 women.
We prepared Summary of findings tables using GRADEpro and Cochrane methods. We judged the evidence for the comparisons included in the main analysis as low quality. This is primarily due to few studies with small sizes reporting each outcome. For the sensitivity analysis, due to poorly reported methods, the quality of evidence reviewed was either very low or low. Overall, the quality of the evidence was very mixed with only six of 72 trials reporting adequate details on all assessed categories, and so the evidence has therefore been declared as having an overall low risk of bias. A total of nine studies were at low risk for selection bias, without having high risk of bias in other domains, and were therefore included in the original analysis. Ten of 72 studies reported high risk of bias on one category, and most of the studies reported unclear risk of bias, due to missing data, mainly for 'selection bias'.
A strength of this review is that all the included studies involved premenopausal women with symptoms of endometriosis. The diagnosis of endometriosis was made by direct visualisation (laparoscopically or laparotomically diagnosed endometriosis) or from ultrasonographic imaging/MRI. In this way, an attempt was made to include all women with complaints, with a clear diagnosis of endometriosis. Women without complaints, for example, women with only infertility, were not included. This is especially important for the outcome measures 'relief of overall pain', 'quality of life', 'improvement of most troublesome symptom' and 'patient satisfaction'. Weaknesses of this review are that the included studies were small and many were at unclear of high risk of bias.
In addition to the above points, the form of reporting results by some included articles is debatable. Some included articles have described the results for continuous outcome measures (such as pain complaints) as a dichotomous outcome measure (improvement, yes/no). This can cause the results to be interpreted incorrectly and the effects to be underestimated or overestimated. We would therefore recommend that continuous outcomes should not be reported as dichotomous outcomes. This allows for a more careful way of reporting results.
Potential biases in the review process
The searches for this review included electronic searching by multiple sources and is felt to be comprehensive. At least two review authors independently extracted data and conducted the risk of bias assessment. The publications included spanned over 34 years (from 1988 to 2022) during which time the methods and data reporting practices have changed significantly. Especially for older publications, attempts to contact authors were often unsuccessful, resulting in poor scoring in risk of bias assessment due to inadequate information. Inaccessibility to useful information available with these authors, but not included in papers, might have also limited the meta‐analysis and especially the Summary of findings tables.
Agreements and disagreements with other studies or reviews
Other reviews concerning the use of GnRHas in treating endometriosis‐associated pain agree with GnRHas being effective in reducing overall pain (Jackson 2006; Kalaitzopoulos 2021; Rafique 2017). There are several proposals within reviews and guidelines on the specific duration of treatment available, varying between six and 12 months. This does not completely correlate with the findings of our review, as we as well have found that GnRHas could be effective in reducing pelvic tenderness. However, our evidence is still of very low certainty.
Most reviews describe danazol as an effective orally used treatment in reducing endometriosis‐associated pain. However, in the current ESHRE, WES and SOGC guidelines, danazol is no longer described as a recommended treatment option because of a high risk of quality of life‐reducing side effects. ACOG is the only guideline that still recommends danazol as a treatment option (Kalaitzopoulos 2021).
Authors' conclusions
Implications for practice.
Women who have complaints of endometriosis can be treated in different ways. The current review suggests that, for relief of overall pain, there may be a decrease in favour of treatment with GnRHas compared to placebo or oral or injectable progestogens. We are uncertain about the effects when comparing GnRHas with danazol, intra‐uterine progestogens or gestrinone. Not all aspects of pain relief are discussed in all the trials and generalisability about the relief of specific aspects of pain may be difficult.
This review showed that there may be a slight decrease in BMD when women were treated with GnRHas, compared to gestrinone. There was also a greater decrease of BMD when women were treated with GnRHas alone, compared to GnRHas in conjunction with calcium‐regulating agents. Thus, there may be an increase in adverse effects when women are treated with GnRHas, compared to placebo or gestrinone. This must be taken into account in the decision‐making process together with the patient, as endometriosis is a common disease, with many different treatment options that need to be individualised to the wishes and stage of life of the individual patient.
Implications for research.
Due to the limited number of low‐risk studies and the high heterogeneity in the sensitivity analyses, we recommend further research into the effects of GnRHas and other hormonal treatment options on our stated outcome measures. We recommend considering a modern large study with low risk of bias to validate the practice that has become common practice. In addition, it remains important to clearly describe the method in future articles, so that more articles can be labelled as low risk of bias in future reviews.
History
Protocol first published: Issue 7, 2021
Notes
This review represents a merging of two existing Cochrane Reviews (Brown 2010; Farmer 2003).
Acknowledgements
We thank everyone at Cochrane Gynaecology and Fertility, in particular Marian Showell, Information Specialist, who contributed to the search strategy. We would like to thank Julie Brown, Alice Pan, Roger Hart, Jessica E. Farmer, Andrew Prentice, Andrew Breeze, Gaity Ahmad, Andrew Watson, and Andy Pick for contributing to the previous versions of the review.
We would like to thank the following peer reviewers for their valuable comments:
Dr Andrew Watson, Consultant Obstetrician and Gynaecologist. Tameside Hospital, Greater Manchester, UK
Jack Wilkinson, Centre for Biostatistics, University of Manchester
Edgardo Somigliana, Università degli Studi di Milano and Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
Angela Beros, Cochrane Gynaecology and Fertility Group.
We thank Anne Lethaby for copy‐editing the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility (CGF) specialist register search strategy
ProCite platform
Searched from inception to 26 May 2022
Keywords CONTAINS "endometriosis" or "The Endometriosis Health Profile" or "dyspareunia" or "pelvic pain" or "pain‐dyspareunia" or "pain‐endometriosis" or "pain‐pelvic" or "dyschezia" or "bone density" or "bone mineral density" or "bone turnover" or "bone turnover estimates" or "bone turnover markers" or "bone metabolism" or "bone metabolism indicators" or Title CONTAINS "endometriosis" or "The Endometriosis Health Profile" or "dyspareunia" or "pelvic pain" or "pain‐dyspareunia" or "pain‐endometriosis" or "pain‐pelvic" or "dyschezia" or "bone density" or "bone mineral density" or "bone turnover" or "bone turnover estimates" or "bone turnover markers" or "bone metabolism" or "bone metabolism indicators"
AND
Keywords CONTAINS "Gonadorelin" or "GnRh" or "GnRHa" or "GnRH agonist" or "GnRH agonists" or "GnRHa‐gonadotropin" or "Gonadotrophin releasing agonist" or "Gonadotrophin releasing hormones" or "gonadotrophins" or "gonadotropin" or "gonadotropin releasing hormone agonist" or "goserelin acetate" or "Gosereline " or "Luteinising hormone releasing hormone" or "LHRH" or "LHRH agonists" or "LHRH antagonists" or "leuprorelin" or "leuprorelin acetate" or "leuprolin" or "leuprolide depot" or "Leuprolide" or "leuprolide acetate" or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Nafarelin" or "Nafarelin Study Group" or "triptorelin" or "Zoladex" or "Lupron" or "luprorelix" or "decapeptyl" or "decapeptyl" or "decapeptyl‐daily" or "decapeptyl‐depot" or "elagolix" or "Relugolix" or linzagolix or Title CONTAINS "GnRH agonist" or "GnRH agonists" or "Gonadorelin" or "GnRh" or "GnRHa"
(509 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched on 26 May 2022
#1 bone turnover:TI,AB,KY 3588
#2 bone loss:TI,AB,KY 5102
#3 bone adj2 densit*:TI,AB,KY 12767
#4 MESH DESCRIPTOR Bone Density EXPLODE ALL TREES 4790
#5 MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES 888
#6 Endometrio*:TI,AB,KY 3002
#7 dyspareunia:TI,AB,KY 1187
#8 (Dyschesia or Dyschezia):TI,AB,KY 52
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 20748
#10 MESH DESCRIPTOR Gonadotropin‐Releasing Hormone EXPLODE ALL TREES 2677
#11 gonadotropin‐releasing:TI,AB,KY 2463
#12 gonadotrophin‐releasing:TI,AB,KY 512
#13 (luteini?ing hormone‐releasing hormone*):TI,AB,KY 500
#14 (lhrh* or lhfshrh):TI,AB,KY 773
#15 gonadorelin*:TI,AB,KY 715
#16 (fsh releasing hormone*):TI,AB,KY 1
#17 (lh rh*):TI,AB,KY 206
#18 (buserelin or goserelin or leuprolide):TI,AB,KY 2450
#19 (triptorelin or nafarelin):TI,AB,KY 990
#20 (leuprorelin or naferelin):TI,AB,KY 475
#21 (GnRH* or Gn‐RH*):TI,AB,KY 4012
#22 (leuprorelin or naferelin):TI,AB,KY 475
#23 (suprecur or suprefact):TI,AB,KY 29
#24 (Zoladex or lupron):TI,AB,KY 402
#25 (prostap or enantone):TI,AB,KY 32
#26 (lucrin or trenantone*):TI,AB,KY 28
#27 (synarel or synarella):TI,AB,KY 10
#28 (decapeptyl or gonapeptyl):TI,AB,KY 157
#29 (Elagolix or Relugolix):TI,AB,KY 183
#30 (luliberin or cystorelin):TI,AB,KY 1
#31 (dirigestran or factrel or gonadoliberin):TI,AB,KY 7
#32 linzagolix or deslorelin 124
#33 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 7631
#34 #9 AND #33 1028
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 26 May 2022
1 exp Endometriosis/ (24263) 2 dyspareunia.tw. (4401) 3 (Dyschesia or Dyschezia).tw. (397) 4 Endometrio*.tw. (34198) 5 ((cycl* or menstru*) adj2 pain*).tw. (2194) 6 Bone Density/ (58624) 7 (bone adj2 densit*).tw. (59256) 8 bone loss.tw. (33045) 9 bone turnover.tw. (13572) 10 or/1‐9 (150269) 11 exp gonadotropin‐releasing hormone/ (33621) 12 (gonadotropin‐releasing or gonadotrophin‐releasing).tw. (18551) 13 (GnRH* or Gn‐RH*).tw. (24822) 14 luteini?ing hormone‐releasing hormone*.tw. (5915) 15 (lhrh* or lhfshrh).tw. (6524) 16 gonadorelin*.tw. (247) 17 fsh releasing hormone*.tw. (54) 18 lh rh*.tw. (3398) 19 (buserelin or goserelin or leuprolide).tw. (4245) 20 (triptorelin or nafarelin).tw. (1080) 21 (leuprorelin or naferelin).tw. (533) 22 (suprecur or suprefact).tw. (30) 23 (Zoladex or lupron).tw. (561) 24 (prostap or enantone).tw. (31) 25 (lucrin or trenantone$).tw. (18) 26 (synarel or synarella).tw. (13) 27 (decapeptyl or gonapeptyl).tw. (225) 28 (Elagolix or Relugolix).tw. (159) 29 (luliberin or cystorelin).tw. (190) 30 (dirigestran or factrel or gonadoliberin).tw. (169) 31 (linzagolix or deslorelin).tw. (325) 32 or/11‐31 (49323) 33 10 and 32 (2784) 34 randomized controlled trial.pt. (568945) 35 controlled clinical trial.pt. (94879) 36 randomized.ab. (561946) 37 randomised.ab. (111642) 38 placebo.tw. (234504) 39 clinical trials as topic.sh. (199923) 40 randomly.ab. (382777) 41 trial.ti. (262776) 42 (crossover or cross‐over or cross over).tw. (93495) 43 or/34‐42 (1523247) 44 exp animals/ not humans.sh. (5009925) 45 43 not 44 (1401679) 46 33 and 45 (636)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 26 May 2022
1 exp bone density/ (105245) 2 (bone adj2 densit*).tw. (85252) 3 bone loss.tw. (41805) 4 bone turnover.tw. (19885) 5 exp endometriosis/ (40870) 6 Endometrio*.tw. (49868) 7 dyspareunia.tw. (8221) 8 (Dyschesia or Dyschezia).tw. (795) 9 ((cycl* or menstru*) adj2 pain*).tw. (3256) 10 or/1‐9 (220324) 11 exp gonadorelin/ (35087) 12 ((gonadotropin‐releasing or gonadotrophin‐releasing) adj2 (analog* or agonist* or antagonist*)).tw. (6773) 13 ((GnRH* or Gn‐RH*) adj2 (analog* or agonist* or antagonist*)).tw. (15643) 14 (GnRH a or GnRHa).tw. (4062) 15 luteini?ing hormone‐releasing hormone*.tw. (5572) 16 (lhrh* or lhfshrh).tw. (7690) 17 gonadorelin*.tw. (395) 18 fsh releasing hormone*.tw. (17) 19 lh rh*.tw. (2759) 20 (buserelin or goserelin or leuprolide).tw. (6162) 21 (triptorelin or nafarelin).tw. (1686) 22 (leuprorelin or naferelin).tw. (822) 23 (suprecur or suprefact).tw. (1328) 24 (Zoladex or lupron).tw. (3828) 25 (prostap or enantone).tw. (418) 26 (lucrin or trenantone*).tw. (429) 27 (synarel or synarella).tw. (352) 28 (decapeptyl or gonapeptyl).tw. (2140) 29 (Elagolix or Relugolix).tw. (325) 30 (luliberin or cystorelin).tw. (187) 31 (dirigestran or factrel or gonadoliberin).tw. (291) 32 (linzagolix or deslorelin).tw. (396) 33 or/11‐32 (61600) 34 Clinical Trial/ (1024043) 35 Randomized Controlled Trial/ (704627) 36 controlled clinical trial/ (465536) 37 multicenter study/ (322883) 38 Phase 3 clinical trial/ (60413) 39 Phase 4 clinical trial/ (4746) 40 exp randomization/ (93845) 41 Single Blind Procedure/ (46061) 42 Double Blind Procedure/ (191894) 43 Crossover Procedure/ (70243) 44 Placebo/ (366605) 45 Randomi?ed controlled trial$.tw. (284966) 46 Rct.tw. (46655) 47 (random$ adj2 allocat$).tw. (49498) 48 Single blind$.tw. (28511) 49 Double blind$.tw. (222906) 50 ((treble or triple) adj blind$).tw. (1528) 51 placebo$.tw. (336711) 52 prospective study/ (764038) 53 or/34‐52 (2656999) 54 case study/ (85016) 55 case report.tw. (475125) 56 abstract report/ or letter/ (1191797) 57 Editorial.pt. (715507) 58 Letter.pt. (1192288) 59 Note.pt. (893698) 60 or/54‐59 (3416613) 61 53 not 60 (2524171) 62 10 and 33 and 61 (1388)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 26 May 2022
1 exp menstrual disorders/ (1348) 2 Endometrio*.tw. (362) 3 1 and 2 (23) 4 Endometrio*.tw. (362) 5 dyspareunia.tw. (623) 6 (Dyschesia or Dyschezia).tw. (9) 7 4 or 5 or 6 (955) 8 3 or 7 (955) 9 exp Gonadotropic Hormones/ (4368) 10 (gonadotropin‐releasing or gonadotrophin‐releasing).tw. (1118) 11 (GnRH* or Gn‐RH*).tw. (1121) 12 luteini?ing hormone‐releasing hormone*.tw. (239) 13 (lhrh* or lhfshrh).tw. (219) 14 gonadorelin*.tw. (5) 15 fsh releasing hormone*.tw. (1) 16 lh rh*.tw. (46) 17 (buserelin or goserelin or leuprolide).tw. (120) 18 (triptorelin or nafarelin).tw. (30) 19 (leuprorelin or naferelin).tw. (12) 20 (Zoladex or lupron).tw. (25) 21 (prostap or enantone).tw. (1) 22 (lucrin or trenantone*).tw. (1) 23 (decapeptyl or gonapeptyl).tw. (3) 24 (Elagolix or Relugolix).tw. (2) 25 (luliberin or cystorelin).tw. (7) 26 (dirigestran or factrel or gonadoliberin).tw. (2) 27 (linzagolix or deslorelin).tw. (9) 28 or/9‐27 (5185) 29 8 and 28 (17)
Data and analyses
Comparison 1. GnRHas versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Relief of overall pain ‐ dichotomous ‐ decrease of pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Decreases in pelvic pain scores ‐ 3 months | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.41, 3.24] |
| 1.1.2 Decreases in dysmenorrhoea scores ‐ 3 months | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.59, 3.16] |
| 1.1.3 Decreases in dyspareunia scores ‐ 3 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.39, 3.54] |
| 1.1.4 Decreases in pelvic tenderness scores ‐ 3 months | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.48, 3.50] |
| 1.1.5 Decreases in pelvic induration scores ‐ 3 months | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.79] |
| 1.2 Adverse effects ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Hot flushes/flashes ‐ 3 months | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.89, 5.01] |
Comparison 2. GnRHas versus placebo ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Relief of overall pain ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Dyspareunia ‐ 6 months | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.09, 0.89] |
| 2.1.2 Dyschezia/ bowel pressure ‐ 6 months | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.17] |
| 2.1.3 Pelvic tenderness ‐ 6 months | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.09, 0.55] |
| 2.2 Relief of overall pain ‐ dichotomous ‐ decrease of pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Decreases in pelvic pain scores ‐ 3 months | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.41, 3.24] |
| 2.2.2 Decreases in dysmenorrhoea scores ‐ 3 months | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.59, 3.16] |
| 2.2.3 Decreases in dyspareunia scores ‐ 3 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.39, 3.54] |
| 2.2.4 Decreases in pelvic tenderness scores ‐ 3 months | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.48, 3.50] |
| 2.2.5 Decreases in pelvic induration scores ‐ 3 months | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.79] |
| 2.3 Relief of overall pain ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Decrease of overall pain by Severity scale mean scores ‐ 1 month | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [2.80, 3.00] |
| 2.4 Adverse effects ‐ dichotomous | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Hot flushes/flashes ‐ 3 months | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.89, 5.01] |
| 2.4.2 Vasodilatation ‐ 6 months | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.51, 4.81] |
| 2.4.3 Headache‐ 6 months | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [1.09, 11.53] |
| 2.4.4 Hot flushes/flashes ‐ 12 months | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.87, 3.02] |
| 2.4.5 Sleep disturbances ‐ 12 months | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.33, 4.02] |
| 2.5 Quality of life ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Physical component ‐ 1 month | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.48, ‐0.44] |
| 2.5.2 Mental component ‐ 1 month | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.48, ‐0.44] |
2.2. Analysis.

Comparison 2: GnRHas versus placebo ‐ all studies included, Outcome 2: Relief of overall pain ‐ dichotomous ‐ decrease of pain
Comparison 3. GnRHas versus danazol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Relief of overall pain ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Pelvic tenderness, partly resolved ‐ 6 months | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.49, 2.73] |
| 3.1.2 Pelvic tenderness, complete resolved ‐ 6 months | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.67, 1.81] |
| 3.2 Relief of overall pain ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Relief of overall pain‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.66, 1.06] |
| 3.2.2 Pelvic pain ‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.26, 0.66] |
| 3.2.3 Dysmenorrhoea ‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.49, 0.69] |
| 3.2.4 Dyspareunia ‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.77, 0.37] |
| 3.2.5 Pelvic induration ‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.59, 0.39] |
| 3.2.6 Pelvic tenderness ‐ 3 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 3.2.7 Relief of overall pain ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.86, 1.66] |
| 3.2.8 Pelvic pain ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [0.10, 0.90] |
| 3.2.9 Dysmenorrhoea ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.12, 0.92] |
| 3.2.10 Dyspareunia ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.90, 0.10] |
| 3.2.11 Pelvic induration ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.21, 1.19] |
| 3.2.12 Pelvic tenderness ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.75, 0.35] |
| 3.3 Adverse effects ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Vaginal dryness/vaginitis ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.52, 4.05] |
| 3.3.2 Hot flushes/flashes ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.50 [0.93, 259.61] |
| 3.3.3 Gastrointestinal ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.74] |
| 3.3.4 Weight gain ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.82] |
| 3.3.5 Acne ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 3.3.6 Generalised spasm ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
3.1. Analysis.

Comparison 3: GnRHas versus danazol, Outcome 1: Relief of overall pain ‐ dichotomous
3.2. Analysis.

Comparison 3: GnRHas versus danazol, Outcome 2: Relief of overall pain ‐ continuous
3.3. Analysis.

Comparison 3: GnRHas versus danazol, Outcome 3: Adverse effects ‐ dichotomous
Comparison 4. GnRHas versus danazol ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Relief of overall pain ‐ dichotomous | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 Pelvic pain ‐ 6 months | 6 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 4.1.2 Dysmenorrhoea ‐ 6 months | 6 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 4.1.3 Dyspareunia ‐ 6 months | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.90, 1.34] |
| 4.1.4 Pelvic induration ‐ 6 months | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.89] |
| 4.1.5 Pelvic tenderness ‐ 6 months | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.39, 2.00] |
| 4.1.6 Pelvic tenderness, partly resolved ‐ 6 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.59, 2.76] |
| 4.1.7 Pelvic tenderness, complete resolved ‐ 6 months | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.12] |
| 4.1.8 Pelvic tenderness and induration combined, complete resolved ‐ 6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.38] |
| 4.1.9 Pelvic tenderness and induration combined, partly resolved ‐ 6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.35, 6.89] |
| 4.2 Relief of overall pain ‐ continuous | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Relief of overall pain‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.74, 0.49] |
| 4.2.2 Pelvic pain ‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.35, 0.88] |
| 4.2.3 Dysmenorrhoea ‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.72] |
| 4.2.4 Dyspareunia ‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.82, 0.41] |
| 4.2.5 Pelvic induration ‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.73, 0.49] |
| 4.2.6 Pelvic tenderness ‐ 3 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.82, 0.41] |
| 4.2.7 Relief of overall pain ‐ 6 months | 3 | 85 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.46, 0.46] |
| 4.2.8 Pelvic pain ‐ 6 months | 2 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.08, 0.79] |
| 4.2.9 Dysmenorrhoea ‐ 6 months | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.16, 1.08] |
| 4.2.10 Dyspareunia ‐ 6 months | 2 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.19, 0.69] |
| 4.2.11 Pelvic induration ‐ 6 months | 2 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.04, 0.83] |
| 4.2.12 Pelvic tenderness ‐ 6 months | 2 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.03, ‐0.15] |
| 4.3 Bone mineral density of spinal bone mass ‐ continuous | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Absolute values ‐ 6 months | 3 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.31, 0.73] |
| 4.4 Adverse effects ‐ dichotomous | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.4.1 Vaginal dryness/vaginitis ‐ 6 months | 12 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.53, 2.18] |
| 4.4.2 Hot flushes/flashes ‐ 6 months | 17 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.42, 1.60] |
| 4.4.3 Headaches ‐ 6 months | 11 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.21, 1.69] |
| 4.4.4 Infections and flu like symptoms ‐ 6 months | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.60 [1.31, 9.88] |
| 4.4.5 Muscle cramps/myalgia ‐ 6 months | 8 | 884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.09, 0.29] |
| 4.4.6 Sleep disturbance/insomnia ‐ 6 months | 7 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.61, 2.59] |
| 4.4.7 Skin rash ‐ 6 months | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.66] |
| 4.4.8 Gastrointestinal ‐ 6 months | 4 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.74] |
| 4.4.9 Weight gain ‐ 6 months | 9 | 1081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.29, 0.49] |
| 4.4.10 Acne ‐ 6 months | 10 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.73] |
| 4.4.11 Breast atrophy/changes ‐ 6 months | 5 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.85] |
| 4.4.12 Emotional lability/altered mood ‐ 6 months | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.12, 6.30] |
| 4.4.13 Oedema/fluid retention ‐ 6 months | 4 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.40] |
| 4.4.14 Asthenia ‐ 6 months | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.09, 0.64] |
| 4.4.15 Depression ‐ 6 months | 4 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.20, 0.70] |
| 4.4.16 Generalised spasm ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 4.4.17 Voice alteration ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.34] |
| 4.4.18 Hirsutism ‐ 6 months | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.09, 0.37] |
| 4.4.19 Seborrhoea ‐ 6 months | 4 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.19, 0.42] |
| 4.4.20 Alopecia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.75] |
| 4.4.21 Altered libido ‐ 6 months | 9 | 1286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.30, 1.92] |
| 4.4.22 Sweating ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.51] |
| 4.4.23 Breast tenderness ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.04, 4.33] |
| 4.4.24 Fatigue ‐ 6 months | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.40, 1.26] |
| 4.4.25 Arthralgia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.61 [1.08, 286.40] |
| 4.4.26 Hunger ‐ 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.54] |
| 4.4.27 Nervousness ‐ 6 months | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.80] |
| 4.4.28 Irritability ‐ 6 months | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [1.67, 13.45] |
| 4.4.29 Nausea ‐ 6 months | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.17] |
| 4.4.30 Breast pain ‐ 6 months | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [0.19, 66.61] |
| 4.4.31 Back distress ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.53] |
| 4.4.32 Paraesthesia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.18, 3.77] |
| 4.4.33 Agressiveness ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.34] |
| 4.4.34 Pain ‐ 6 months | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.11, 2.31] |
| 4.4.35 Oily hair and skin ‐ 6 months | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| 4.4.36 Bleeding ‐ 6 months | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.34] |
| 4.4.37 Malaise ‐ 6 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 4.4.38 Chest aches ‐ 6 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.21] |
| 4.4.39 Dizzy spells ‐ 6 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.78] |
| 4.4.40 PMS feelings ‐ 6 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.32, 5.06] |
| 4.5 Improvement of most troublesome symptoms ‐ dichotomous | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 Overall improvement ‐ 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| 4.5.2 Overall improvement ‐ 6 months | 6 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.99, 1.18] |
| 4.5.3 Complete resolution ‐ 6 months | 5 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.99, 1.32] |
Comparison 5. GnRHas versus intra‐uterine progestagen device ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Relief of overall pain ‐ continuous | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Relief of overall pain ‐ 6 months | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.62, 0.10] |
| 5.1.2 Decrease of VAS score ‐ 6 months | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.11, 0.11] |
| 5.2 Quality of life ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Psychological Well‐Being Questionnaire index (PGWBI) ‐ 6 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐10.26, 6.26] |
Comparison 6. GnRHas versus oral or injectable progestogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Relief of overall pain ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 Pelvic pain ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐3.55, ‐1.45] |
| 6.1.2 Dyspareunia ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.83, ‐1.37] |
| 6.1.3 Back pain ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.40, 1.40] |
| 6.2 Adverse effects ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 Vaginal bleeding ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.23, 0.48] |
| 6.2.2 Headache ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.88, 2.67] |
| 6.2.3 Weight gain ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.92] |
| 6.2.4 Vaginal dryness ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [1.66, 13.55] |
| 6.2.5 Hot flushes ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [1.87, 4.65] |
Comparison 7. GnRHas versus oral or injectable progestogens ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Relief of overall pain ‐ dichotomous | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Pelvic pain ‐ 6 months | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.15] |
| 7.1.2 Dysmenorrhoea ‐ 6 months | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.06] |
| 7.1.3 Dyspareunia ‐ 6 months | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.67, 1.47] |
| 7.1.4 Pelvic induration ‐ 6 months | 2 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 7.1.5 Pelvic tenderness ‐ 6 months | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.40] |
| 7.2 Relief of overall pain ‐ continuous | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 Pelvic pain ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐3.55, ‐1.45] |
| 7.2.2 Dyspareunia ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.83, ‐1.37] |
| 7.2.3 Back pain ‐ 3 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.40, 1.40] |
| 7.2.4 Overall pain ‐ 6 months | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 7.2.5 Pelvic pain ‐ 6 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.88, ‐0.32] |
| 7.2.6 Absolute reduction mean VAS ‐ 6 months | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐8.49, 5.49] |
| 7.2.7 Dysmenorrhoea ‐ 6 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 7.2.8 Dyspareunia ‐ 6 months | 2 | 172 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.22] |
| 7.2.9 Lumbago‐ mean change ‐ 6 months | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐8.20, 5.00] |
| 7.2.10 Lumbago ‐ 6 months | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.39, 0.19] |
| 7.2.11 Lower abdominal pain ‐ 6 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.45, 0.05] |
| 7.2.12 Dyschezia ‐ 6 months | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.14, 0.54] |
| 7.2.13 Pain on internal examination ‐ 6 months | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 7.2.14 Lower abdominal pain, mean change ‐ 6 months | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐5.19, 10.99] |
| 7.2.15 Pelvic induration ‐ 6 months | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.04, 0.56] |
| 7.2.16 Pelvic tenderness‐ 6 months | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 7.2.17 Pelvic pain ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.75, ‐0.45] |
| 7.2.18 Overall pain ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐2.13, 9.73] |
| 7.2.19 Dysmenorrhoea ‐ 12 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 7.2.20 Dyspareunia ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.33] |
| 7.3 Bone mineral density of spinal bone mass ‐ continuous | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 Percentage change values ‐ 6 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.57, ‐0.63] |
| 7.3.2 Absolute values ‐ 6 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.08, 0.01] |
| 7.3.3 Absolute values ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.10, ‐0.01] |
| 7.4 Adverse effects ‐ dichotomous | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.4.1 Vaginal bleeding ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.23, 0.48] |
| 7.4.2 Headache ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.88, 2.67] |
| 7.4.3 Weight gain ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.92] |
| 7.4.4 Vaginal dryness ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [1.66, 13.55] |
| 7.4.5 Hot flushes ‐ 3 months | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [1.87, 4.65] |
| 7.4.6 Acne ‐ 4 months | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.40, 3.57] |
| 7.4.7 Alopecia ‐ 4 months | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.49, 3.99] |
| 7.4.8 Decreased libido ‐ 4 months | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.10] |
| 7.4.9 Vaginal dryness ‐ 4 months | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.54, 7.37] |
| 7.4.10 Weight gain ‐ 4 months | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.68, 4.09] |
| 7.4.11 Nausea ‐ 6 months | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.30, 1.32] |
| 7.4.12 Headache ‐ 6 months | 3 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.04, 2.03] |
| 7.4.13 Breast pain ‐ 6 months | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.22, 1.98] |
| 7.4.14 Intermenstrual bleeding ‐ 6 months | 4 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.50, 0.67] |
| 7.4.15 Hot flushes/flashes ‐ 6 months | 4 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.70, 2.62] |
| 7.4.16 Emotional changes ‐ 6 months | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.21 [1.64, 16.61] |
| 7.4.17 Insomnia ‐ 6 months | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.59, 8.50] |
| 7.4.18 Decreased libido ‐ 6 months | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.59, 8.50] |
| 7.5 Adverse effects ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.5.1 Climacteric symptoms by Kupperman index ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 6.80 [2.37, 11.23] |
| 7.5.2 Hot flushes/flashes ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.71, 1.49] |
| 7.5.3 Depression ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 7.5.4 Oedema ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.14, 0.54] |
| 7.5.5 Headache ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.32, 0.52] |
| 7.5.6 Breast pain ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 7.5.7 Metrorrhagia ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.31, ‐0.49] |
| 7.6 Quality of life ‐ continuous | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.6.1 Bodily pain ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐10.81, 3.41] |
| 7.6.2 General health ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.58, 3.98] |
| 7.6.3 Physical function ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.66, 1.66] |
| 7.6.4 Role physical ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐12.01, 5.01] |
| 7.6.5 Role emotional ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐16.67, 1.07] |
| 7.6.6 Mental health ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐4.04, 4.24] |
| 7.6.7 Social function ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐9.98, 0.38] |
| 7.6.8 Vitality ‐ 6 months | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.41, 4.01] |
| 7.6.9 General health ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐1.97, 9.37] |
| 7.6.10 Physical function ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐3.72, 7.72] |
| 7.6.11 Role physical ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐4.15, 6.75] |
| 7.6.12 Role emotional‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐1.60, 10.00] |
| 7.6.13 Mental health‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐4.39, 5.99] |
| 7.6.14 Social function ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.93, 2.53] |
| 7.6.15 Vitality ‐ 12 months | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐4.94, 8.34] |
| 7.7 Improvement of most troublesome symptoms ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.7.1 Complete resolution ‐ 6 months | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.28] |
| 7.8 Improvement of most troublesome symptoms ‐ continuous | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.8.1 Overall symptoms by numerical rating scale ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [0.37, 4.83] |
| 7.8.2 Overall symptoms by verbal rating scale ‐ 4 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.06, 1.34] |
| 7.8.3 Overall symptoms ‐ 6 months | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.52, 0.12] |
Comparison 8. GnRHas versus gestrinone ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Relief of overall pain ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1.1 Dysmenorrhoea, visual analog scale ‐ 3 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 8.1.2 Dysmenorrhoea, verbal rating scale ‐ 3 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 8.1.3 Dyspareunia, visual analog scale ‐ 3 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 1.39 [0.04, 2.74] |
| 8.1.4 Dyspareunia, verbal rating scale ‐ 3 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.12, 0.68] |
| 8.1.5 Non‐menstrual pain, visual analog scale ‐ 3 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.59, 1.57] |
| 8.1.6 Non‐menstrual pain, verbal rating scale ‐ 3 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.36, 0.44] |
| 8.1.7 Dysmenorrhoea, visual analog scale ‐ 6 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.49, ‐0.15] |
| 8.1.8 Dysmenorrhoea, verbal rating scale ‐ 6 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.58, ‐0.12] |
| 8.1.9 Dyspareunia, visual analog scale ‐ 6 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.25, 2.09] |
| 8.1.10 Dyspareunia, verbal rating scale ‐ 6 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.04, 0.62] |
| 8.1.11 Non‐menstrual pain, visual analog scale ‐ 6 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.94, 1.76] |
| 8.1.12 Non‐menstrual pain, verbal rating scale ‐ 6 months | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.20, 0.50] |
| 8.2 Bone mineral density of spinal bone mass ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.2.1 Percentage change values ‐ 6 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.96 [‐3.62, ‐0.30] |
| 8.2.2 Percentage change values ‐ 12 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐7.39, ‐2.81] |
| 8.3 Adverse effects ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 Hot flushes/flashes ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.21, 4.32] |
| 8.3.2 Headache ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.51, 11.38] |
| 8.3.3 Asthenia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.02] |
| 8.3.4 Mood change ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 7.99] |
| 8.3.5 Dermatitis ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.55] |
| 8.3.6 Dizziness‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.01] |
| 8.3.7 Joint pain‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.01] |
| 8.3.8 Drowsiness ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.01] |
| 8.3.9 Swelling ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| 8.3.10 Nausea‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.01] |
| 8.3.11 Tachycardia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.01] |
| 8.3.12 Vaginal dryness ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.83 [0.24, 96.16] |
| 8.3.13 Insomnia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 8.3.14 Hypertrichosis ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 8.3.15 Seborrhea‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 8.3.16 Skin rash ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 8.3.17 Constipation ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 8.3.18 Itching ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.15] |
| 8.3.19 Vaginal discharge ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.15] |
| 8.3.20 Paresthesia ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.15] |
| 8.3.21 Cramps ‐ 6 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.15] |
Comparison 9. GnRHas versus GnRHas (varying dosage) ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Relief of overall pain ‐ 200 μg versus 400 μg nafarelin ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 Pelvic pain ‐ 2 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.07, 1.47] |
| 9.1.2 Pelvic pain ‐ 4 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.07, 0.87] |
| 9.1.3 Pelvic pain ‐ 6 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.61, 1.21] |
| 9.2 Relief of overall pain ‐ 400 μg versus 800 μg nafarelin ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 Pelvic pain ‐ 6 months | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.16] |
| 9.2.2 Dysmenorrhea ‐ 6 months | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 71.74] |
| 9.2.3 Dyspareunia ‐ 6 months | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.49, 2.26] |
| 9.3 Adverse effects ‐ 200 μg versus 400 μg nafarelin ‐ dichotomous | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.3.1 Vasomotor symptoms (hot flashes or dizziness) ‐ 2 months | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.12] |
| 9.3.2 Vasomotor symptoms (hot flashes or dizziness) ‐ 4 months | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.27] |
| 9.3.3 Vasomotor symptoms (hot flashes or dizziness) ‐ 6 months | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.01] |
| 9.3.4 Rhinitis ‐ 6 months | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.67] |
| 9.3.5 Upper respiratory infection ‐ 6 months | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.47] |
| 9.3.6 Irregular bleeding ‐ 6 months | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.63] |
| 9.4 Adverse effects ‐ 3.75 mg versus 1.88 mg leuprolide acetate ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 Menopausal symptoms by Kupperman Index ‐ 2 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐3.14, 5.54] |
| 9.4.2 Menopausal symptoms by Kupperman Index ‐ 3 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 5.70 [2.12, 9.28] |
| 9.4.3 Menopausal symptoms by Kupperman Index ‐ 4 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 9.50 [6.55, 12.45] |
| 9.4.4 Menopausal symptoms by Kupperman Index ‐ 5 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 13.20 [10.22, 16.18] |
| 9.5 Improvement of most troublesome symptoms ‐ 400 μg versus 800 μg nafarelin ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.5.1 Overall improvement ‐ 6 months | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
Comparison 10. GnRHas versus GnRHas (duration of treatment) ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Relief of overall pain ‐ 3 months vs 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1.1 Pelvic pain ‐ 6 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.13, 0.19] |
| 10.1.2 Dysmenorrhoea ‐ 6 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.11, ‐0.07] |
| 10.1.3 Dyspareunia ‐ 6 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.17, ‐0.11] |
| 10.1.4 Pelvic induration ‐ 6 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 10.1.5 Pelvic tenderness ‐ 6 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.03, 0.07] |
| 10.2 Bone mineral density of spinal bone mass ‐ 3 months vs 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.2.1 Percentage change values ‐ 6 months | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.51, 1.69] |
| 10.3 Bone mineral density of proximal femoral bone ‐ 3 months vs 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.3.1 Percentage change values ‐ 6 months | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [1.72, 2.08] |
Comparison 11. GnRHas versus GnRHas (route of administration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Relief of overall pain ‐ IN versus SC ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1.1 Pelvic pain ‐ 6 months | 1 | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.87] |
| 11.1.2 Dysmenorrhea ‐ 6 months | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.73, 2.06] |
| 11.1.3 Dyspareunia ‐ 6 months | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.57, 1.75] |
| 11.1.4 Pelvic induration ‐ 6 months | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.47, 1.55] |
| 11.1.5 Pelvic tenderness ‐ 6 months | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.69, 3.27] |
| 11.2 Adverse effects ‐ IN vs SC ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.2.1 Hot flushes/flashes ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.55] |
| 11.2.2 Vaginal dryness ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.17, 4.37] |
| 11.2.3 Decreased libido ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 11.2.4 Headaches ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.20, 14.55] |
Comparison 12. GnRHas versus GnRHas (route of administration) ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Relief of overall pain ‐ IN versus SC ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 Pelvic pain ‐ 6 months | 1 | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.87] |
| 12.1.2 Dysmenorrhoea ‐ 6 months | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.73, 2.06] |
| 12.1.3 Dyspareunia ‐ 6 months | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.57, 1.75] |
| 12.1.4 Pelvic induration ‐ 6 months | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.47, 1.55] |
| 12.1.5 Pelvic tenderness ‐ 6 months | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.69, 3.27] |
| 12.2 Relief of overall pain ‐ IN versus IM ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.2.1 Pelvic pain ‐ 6 months | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 12.2.2 Dysmenorrhoea ‐ 6 months | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.72, 2.30] |
| 12.2.3 Dyspareunia ‐ 6 months | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.25] |
| 12.2.4 Pelvic induration ‐ 6 months | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.41] |
| 12.2.5 Pelvic tenderness ‐ 6 months | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.88, 1.73] |
| 12.3 Adverse effects ‐ IN vs SC ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.3.1 Hot flushes/flashes ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.55] |
| 12.3.2 Vaginal dryness ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.17, 4.37] |
| 12.3.3 Decreased libido ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 12.3.4 Headaches ‐ 6 months | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.20, 14.55] |
| 12.4 Adverse effects ‐ IN versus IM depot ‐ dichotomous | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.4.1 Hot flushes/flashes ‐ 6 months | 2 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.02] |
| 12.4.2 Headache ‐ 6 months | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 12.4.3 Sweating ‐ 6 months | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.79] |
| 12.4.4 Vaginal dryness ‐ 6 months | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 1.00] |
| 12.4.5 Vaginal bleeding ‐ 6 months | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.19 [1.12, 328.28] |
| 12.5 Bone mineral density of spinal bone mass ‐ IN vs IM depot ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.5.1 Percentage decrease of BMD ‐ 6 months | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.10, ‐1.90] |
Comparison 13. GnRHas versus GnRHas (different treatment regimens) ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Relief of overall pain ‐ monthly versus 3‐monthly depot leuprolide acetate ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1.1 Non‐menstrual pelvic pain ‐ 3 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.52, 0.12] |
| 13.1.2 Dyspareunia ‐ 3 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 13.1.3 Pelvic induration ‐ 3 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.40, 0.60] |
| 13.1.4 Pelvic tenderness ‐ 3 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.71, 0.11] |
| 13.1.5 Non‐menstrual pelvic pain ‐ 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 13.1.6 Dyspareunia ‐ 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.50, 0.50] |
| 13.1.7 Pelvic induration ‐ 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.10, 0.70] |
| 13.1.8 Pelvic tenderness ‐ 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.10, 0.90] |
| 13.2 Adverse effects ‐ monthly versus 3‐monthly depot leuprolide acetate ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.2.1 Hot flushes/flashes ‐ 6 months | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.43] |
| 13.2.2 Vaginal dryness ‐ 6 months | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 13.2.3 Abdominal pain ‐ 6 months | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 68.26] |
| 13.2.4 Arthralgia ‐ 6 months | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 68.26] |
| 13.2.5 Depression ‐ 6 months | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 68.26] |
Comparison 14. GnRHas versus GnRHas in conjunction with add‐back therapy ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Relief of overall pain ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1.1 Dysmenorrhoea ‐ 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.62 [0.58, 159.04] |
| 14.1.2 Dyspareunia ‐ 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [0.86, 43.04] |
| 14.1.3 Non‐menstrual pelvic pain ‐ 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.26, 6.62] |
| 14.2 Relief of overall pain ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.2.1 Pelvic pain ‐ 6 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 14.2.2 Dysmenorrhoea ‐ 6 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2.3 Dyspareunia ‐ 6 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.40, 0.80] |
| 14.2.4 Pelvic pain ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.14, ‐0.06] |
| 14.2.5 Overall pain ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐7.92, 4.92] |
| 14.2.6 Dysmenorrhoea ‐ 12 months | 1 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2.7 Dyspareunia ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.03, 0.43] |
| 14.3 Bone mineral density of spinal bone mass ‐ continuous | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.3.1 Percentage change values ‐ 6 months | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐3.88 [‐4.27, ‐3.49] |
| 14.3.2 Absolute values ‐ 6 months | 5 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [0.02, 0.02] |
| 14.3.3 Absolute values ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 14.4 Adverse effects ‐ dichotomous | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.4.1 Hot flushes/flashes ‐ 3 months | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.61, 2.15] |
| 14.4.2 Hot flushes/flashes ‐ 6 months | 4 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.32, 1.93] |
| 14.4.3 Loss of libido ‐ 6 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.58, 1.97] |
| 14.4.4 Vaginal dryness ‐ 6 months | 3 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.11, 1.76] |
| 14.4.5 Headaches ‐ 6 months | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 14.4.6 Sweating ‐ 6 months | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.31, 0.94] |
| 14.4.7 Sleeplessness ‐ 6 months | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.18] |
| 14.4.8 Peripheral oedema ‐ 6 months | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.54, 9.95] |
| 14.4.9 Vaginal bleeding ‐ 6 months | 3 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.93] |
| 14.4.10 Rhinitis ‐ 6 months | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.94] |
| 14.4.11 Upper respiratory infection ‐ 6 months | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.51] |
| 14.4.12 Emotional changes ‐ 6 months | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [1.26, 7.78] |
| 14.5 Quality of life ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.5.1 General health ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐10.15, 0.75] |
| 14.5.2 Physical function ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐8.80 [‐14.81, ‐2.79] |
| 14.5.3 Role physical ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐3.13, 8.73] |
| 14.5.4 Role emotional ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐3.82, 8.42] |
| 14.5.5 Mental health ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐6.17, 5.57] |
| 14.5.6 Social function ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐8.80, 1.20] |
| 14.5.7 Vitality ‐ 12 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐15.18, ‐5.22] |
| 14.6 Improvement of most troublesome symptoms ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.6.1 Improvement overall pain ‐ 4 weeks | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [5.59, 18.41] |
Comparison 15. GnRHas versus GnRHas in conjunction with calcium‐regulating agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Bone mineral density of spinal bone mass ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1.1 Anterior‐posterior spine ‐ 12 months | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐7.53, ‐6.47] |
| 15.1.2 Lateral spine ‐ 12 months | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐12.40 [‐13.31, ‐11.49] |
| 15.2 Improvement of most troublesome symptoms ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.2.1 Overall improvement ‐ 12 months | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.08] |
| 15.2.2 Complete resolution ‐ 12 months | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.41] |
Comparison 16. GnRHas versus GnRHas in conjunction with calcium‐regulating agents ‐ all studies included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Bone mineral density of spinal bone mass ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1.1 Anterior‐posterior spine ‐ 12 months | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐7.53, ‐6.47] |
| 16.1.2 Lateral spine ‐ 12 months | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐12.40 [‐13.31, ‐11.49] |
| 16.2 Improvement of most troublesome symptoms ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.2.1 Overall improvement ‐ 12 months | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.08] |
| 16.2.2 Complete resolution ‐ 12 months | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdou 2018.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 261 women were randomised; 142 women were analysed. Mean age: Dienogest 29.52 +‐ 3.32 vs leuprolide 29.77 +‐ 3.09 Inclusion criteria:
Exclusion criteria:
Setting: Egypt Timing: May 2014‐December 2016 |
|
| Interventions | Dienogest 2 mg orally once daily for 12 weeks with the first tablet taken on the first day after onset of menstrual bleeding versus Leuproline acetate depot 3.75 mg IM every 4 weeks for 12 weeks with the first injection given during the first 3 days of menstrual bleeding |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: no funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were divided randomly by using random number table (computer) into two groups (A and B). |
| Allocation concealment (selection bias) | Low risk | Software Open Epi version 3.21 was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 284 patients met requirements of inclusion criteria, out of which 261 patients were willing to participate in the study and consented for participation. Patients who dropped out from follow‐up were excluded from the study statistics and results (9 patients from group A and 10 patients from group B). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected. |
Adamson 1994.
| Study characteristics | ||
| Methods | Trial design: Prospective randomised double‐blind controlled study | |
| Participants | Participants: 213 patients. 124 patients were randomised who reported pain symptoms. Mean age: not stated Inclusion:
Exclusion:
Setting: United States of America Timing: not stated |
|
| Interventions | Nafarelin acetate 400 mcg twice daily intranasaly + placebo per os or 6 months (n = 45) versus Nafarelin acetate 200 mcg twice daily intranasaly + placebo per os for 6 months (n = 45) versus Danazol 400 mg twice daily per os + placebo intranasaly for 6 months (n = 34) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: not stated Note previous version: Authors responded to methods query. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Computerised randomisation". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | "Centralised randomisation, sequentially numbered, sealed opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed with intention‐to‐treat for main outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Agarwal 1997.
| Study characteristics | ||
| Methods | Trial design: "Multicentre, randomised, double‐blind, double‐placebo study" | |
| Participants | Participants: 208 women were randomised; 192 were analysed. Mean age: Nafarelin = 29.8 ± 0.6 and LA = 31.7 ± 0.6 (SEM) Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | Nafarelin 200 mcg twice daily intranasally + placebo every 4 weeks IM for 6 months (n = 105) versus Leuprolide acetate depot 3.75 mg every 4 weeks IM + placebo twice daily intranasally for 6 months (n = 103) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation using permuted blocks of random numbers". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details for attrition: 24 women withdrew due to:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
AN Zoladex 1996.
| Study characteristics | ||
| Methods | Trial design: "Multicentre, open, randomised study" | |
| Participants | Participants: 71 women were randomised; 48 were analysed. Group A: patients with infertility associated with endometriosis and who desired pregnancy Group B: other patients (those with symptomatic endometriosis but not desiring pregnancy at time of the study) Mean age: Goserelin = 29.5 and danazol = 29.85 Group A: Mean age: Goserelin = 29.7 (24‐36) and danazol = 29.9 (21‐35) Inclusion criteria:
Exclusion criteria:
Setting: Australia and New Zealand (9 centres) Timing: Not stated |
|
| Interventions | Goserelin acetate 3.6 mg every 4 weeks SC for 24 weeks (n = 35) versus Danazol 200 mg three times a day PO for 24 weeks (n = 36) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes, analysis was performed on both an 'intention‐to‐treat' basis and also on a 'patient‐treated' basis. Sample size calculation: not stated Funding: Authors received a grant of ICI Pharmaceuticals Australia and received the supply of goserelin acetate. Note previous version: Authors contacted regarding methods and data; awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised in a 1 to 1 ratio". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Analysis was performed on both an 'intention‐to‐treat' basis and also on a 'patient‐treated' basis". Details given for attrition: 19 in danazol and 4 in goserelin group withdrew due to: adverse effects 9 (Dan), unwilling to continue 8 (Dan) and 4 (Gos), withdrawn by investigator 1 (Dan), other 1 (Dan) |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Audebert 1997.
| Study characteristics | ||
| Methods | Trial design: Open, multi‐centre, central randomised study | |
| Participants | Participants: 120 eligible women; 71 were randomised; 55 were analysed Mean age: 31 ± 5.9 years Inclusion criteria:
Exclusion criteria:
Setting: France Timing: January 1989 to February 1991 |
|
| Interventions | Leuprorelin 3.75 mg SC depot every 28 days for 24 weeks (n = 33) versus Danazol 600‐800 mg PO daily for 24 weeks (n = 22) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: not stated Note previous version: Could not use data unless mean and SD specified; author contacted. Author replied that study was sponsored by a pharmaceutical company who hold the raw data. He is attempting to locate a contact for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Central randomisation". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study. No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sufficient reporting of attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Bergqvist 1997.
| Study characteristics | ||
| Methods | Trial design: "Double‐blind randomised study" | |
| Participants | Participants: 49 eligible women; 49 were randomised and 47 were analysed Mean age: mean age not stated, median age 30 years (range 21‐46years) Inclusion criteria:
Exclusion criteria: not stated Setting: Europe Timing: not stated |
|
| Interventions | Nafarelin 200 mcg daily IN + placebo PO for 6 months (n = 12) versus Nafarelin 400 mcg daily IN + placebo PO for 6 months (n = 12) versus Nafarelin 200 mcg daily IN + norethisterone 1.2 mg daily PO for 6 months (n = 25) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Need raw data for symptom scores. Authors contacted regarding methods and data. No response to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation was carried out on a block basis". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants (2/25) from the nafareline + norethisterone group withdrew from the trial due to mood swings (1 participant) and pregnancy (1 participant). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Bergqvist 1998.
| Study characteristics | ||
| Methods | Trial design: "Prospective, randomised, placebo‐controlled, double‐blind, parallel study" | |
| Participants | Participants: 49 women eligible; 49 were randomised and 46 were analysed Age: mean of 31 years (19‐44years) Stage: most mild‐to‐moderate (IV n = 1) Inclusion criteria:
Exclusion criteria:
Setting: Sweden Timing: Not stated |
|
| Interventions | Triptorelin 3.75 mg IM depot every 4 weeks for 24 weeks (n = 24) versus Placebo IM every 4 weeks for 24 weeks (n = 25) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Needs raw score for pain. Authors contacted and awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and researchers were blinded through the use of identical kits for injections. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and researchers were blinded through the use of identical kits for injections. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants withdrew from the study. Two from the placebo group (1 prior to the first injection due to pregnancy, and one after 4 months due to lack of effect), and one from the triptorelin group due to hypoestrogenic side effects and depression. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Bergqvist 2000.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 252 women were randomised; 224 were analysed. Mean age: 18‐45 years (median 31 years) Inclusion criteria:
Exclusion criteria:
Setting: Scandinavia (28 centres = 7 centres in Sweden, 8 centres in Norway, 6 centres in Denmark and 7 centres in Finland) Timing: not stated |
|
| Interventions | Goserelin depot, 3.6 mg, administered subcutaneously into the anterior abdominal wall every 28 ±3 days (Zoladex; AstraZeneca) (n = 130) versus Nafarelin 200 μg nasally twice daily, giving a total daily dose of 400 μg (Synarel; Syntex) (n = 122) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated
Sample size calculation: not stated
Funding: The study was supported by AstraZeneca Pharmaceuticals, Alderley Park, Macclesfield, Cheshire SK10 4TF, UK. Authors contacted regarding methods and data. Awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was being performed within each centre. No further details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Altogether, 39 women withdrew from the study, four of whom commenced any therapy, two because they changed their minds and two because they became pregnant between the date of randomisation and the planned date of starting the trial medication. Twenty‐four patients withdrew during the active treatment period, adverse events being the most common reason. Sixteen women, nine in the goserelin group and seven in the nafarelin group, withdrew owing to adverse events, two women in the nafarelin group, withdrew because of local side effects of the treatment such as nasal irritation, one woman because of a worsening of symptoms and five for other reasons. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Burry 1989.
| Study characteristics | ||
| Methods | Trial design: Parallel, double‐blind, double‐dummy randomised trial | |
| Participants | Participants: 53 women were randomised; 51 were analysed. Mean age: 23‐38 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: Not stated |
|
| Interventions | Group I: danazol, 800 mg/day (400 mg twice daily) (n = 10) Group II: danazol, 600 mg/day (200 mg three times a day) (n = 8) Group III: intranasal nafarelin, 800 μg/day (400 μg twice a day) (n = 10) Group IV: intranasal nafarelin, 400 μg/day (200 μg two times a day) (n = 25) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: No Sample size calculation: not stated Funding: not stated Included in this review, but did not contribute any data Not possible to contact leading author; unfortunately had passed away. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly allocated'. No further details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study had a parallel, double‐blind, double‐dummy design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study had a parallel, double‐blind, double‐dummy design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group I: Danazol, 800 mg/day (400 mg twice a day): Nine of the 10 patients included in this group completed the study; one withdrew because of severe headaches. Group II: Danazol, 600 mg/day (200 mg three times a day). All eight patients completed the study. Group III: Nafarelin acetate, 800 μg/day (400 μg twice a day). Nine of 10 patients included in this group completed the study: one withdrew because of mood swings. Group IV: Nafarelin acetate, 400 μg/day (200 μg twice a day). All 25 patients included in this group completed the study. |
| Selective reporting (reporting bias) | High risk | Change in symptoms was asked for, but not reported in published article. |
| Other bias | Low risk | No other risk of bias detected |
Burry 1992.
| Study characteristics | ||
| Methods | Trial design: "Multi‐centre, double‐blind study" | |
| Participants | Participants: 169 women eligible; 169 were randomised and 147 analysed for efficacy. Mean age: not stated Inclusion criteria:
Exclusion criteria: not stated Setting: United States of America Timing: not stated |
|
| Interventions | Nafarelin 400 μg daily IN for 6 months (n = 111) versus Danazol 600 mg daily PO for 6 months (n = 58) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Need more info on randomisation and participants and raw data for quality of life. Authors contacted, awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation procedure assigned two of every three patients to receive nafarelin, 400 μg daily (n = 111), and one of three patients to danazol, 600 mg daily (n = 58). No further details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind". No further details were provided on the method of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was "double‐blind". No further details were provided on the method of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sufficient details for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Chang 1996.
| Study characteristics | ||
| Methods | Trial design: "Randomised comparative study" | |
| Participants | Participants: 45 women eligible; 45 were randomised and 33 were analysed. Mean age: 33 years (LA) Inclusion criteria:
Exclusion criteria: Setting: Taiwan Timing: not stated |
|
| Interventions | Leuprorelin acetate 3.75 mg SC depot every 28 days for 20 weeks (n = 30) versus Danazol 200 mg QID (800 mg/day) PO for 20 weeks (n = 15) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Need raw data for pain. Authors contacted, and additional methodological data provided, no raw data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was in the ratio two LA to one danazol with this study having its randomisation list". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered, identical containers of identical drugs". No further details were provided of method used to conceal allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details were provided on attrition. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Cheng 2005.
| Study characteristics | ||
| Methods | Trial design: "Randomised, parallel, comparative study" | |
| Participants | Participants: 59 women eligible; 59 were randomised and 41 were analysed for efficacy. Mean age: 34.8 ± 6.6 (nafarelin) and 32.4 ± 7.2 (danazol) Inclusion criteria:
Exclusion criteria:
Setting: Taiwan Timing: started in January 1998 and ended in October 2000 |
|
| Interventions | Nafarelin acetate 200 μg twice daily (400 μg/day) IN for 180 days (n = 29) versus Danazol 200 mg (600 mg/day) PO for 180 days (n = 30) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: This study was partly supported by a grant (VGH94‐ 195) from Taipei Veterans General Hospital, Taipei, Taiwan, R.O.C. Note previous version: Authors provided additional data on methods. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by a pharmacy. Authors provided additional data to previous authors, and therefore this study was assigned as having low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered, identical envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, outcome assessors and clinicians were blinded according to author. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, outcome assessors and clinicians were blinded according to author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All 59 patients were considered as the intent‐to‐treat population". Forty‐one of 59 patients (22/29 nafarelin and 19/30 danazol recipients) who completed 90 days’ treatment, and who underwent laparoscopic examinations before and after treatment, qualified for the efficacy evaluation. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Cirkel 1995.
| Study characteristics | ||
| Methods | Trial design: "randomised controlled comparative clinical study" | |
| Participants | Participants: 60 women eligible; 60 were randomised and 55 were analysed. Mean age: 30 ± 0.5 (triptorelin depot) and 30 ± 0.8 (danazol) Inclusion criteria:
Exclusion criteria:
Setting: Germany Timing: February 1989 and December 1990 |
|
| Interventions | Triptorelin 3.75 mg IM depot every 28 days for 24 weeks (n = 30) versus Danazol 200 mg three times a day (600 mg/day) PO for 24 weeks (n = 25) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted and awaiting response regarding methods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated to a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial as blinding of study personnel and participants would not have been possible due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five women assigned to danazol could not be included further: three patients refused to fulfill the protocol after randomisation and two others conceived spontaneously before starting medication. Twenty‐four weeks after the end of endocrine therapy, 20 women (12/30 in the triptorelin and 8/25 in the danazol group) had an additional follow‐up for evaluation of clinical symptomatology as well as laboratory parameters. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Crosignani 1996.
| Study characteristics | ||
| Methods | Trial design: Parallel‐arm, randomised controlled trial | |
| Participants | Participants: 30 women randomised (Group 1 ‐ three‐monthly leuprolide n = 15, Group 2 ‐ monthly leuprolide n = 15). 27 women analysed (Group 1 ‐ three‐monthly leuprolide n = 14, Group 2 ‐ monthly leuprolde n = 13) Mean age: Group 1: 28.6 ± 6.0, Group 2: 31.0 ± 5.2 Inclusion:
Exclusion:
Setting: University clinics in Milan, Genoa, Rome, Italy Timing: not stated |
|
| Interventions | Group 1: Leuprolide acetate 11.25 mg intramuscularly every 84 days for 6 months versus Group 2: Leuprolide acetate 3.75 mg every 28 days for 6 months |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Takeda Italia Farmaceutici S p.A., Roma, Italy, for supplying leuprolide depot injections, preparing the randomisation procedures, and giving financial support to the study Note previous version: Was previously excluded for pain not being an outcome but should be included as pain score is an outcome Contacted authors for more information on concealment, awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible subjects were randomised according to a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was an "open‐label trial". No further details of the blinding process were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One woman from group 1 (three monthly) withdrew from study as she did not want to undergo repeated venipunctures and laparoscopy. Two women in group 2 (monthly) stopped treatment due to a desire to conceive. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Crosignani 2006.
| Study characteristics | ||
| Methods | Trial design: randomised, phase III, evaluator‐blinded, comparator‐controlled clinical trial | |
| Participants | Participants: Of 300 randomised patients, 299 received at least one dose of study medication. Continuation rates were similar between the treatment groups, with 90.2% of patients receiving DMPA‐SC 104 and 93.2% of those receiving leuprolide completing the 6‐month treatment period. Of the patients that completed the 6‐month treatment period, 71.7 and 73.5% of patients in the DMPA‐SC 104 and leuprolide groups completed the 12‐month follow‐up period, respectively. Mean age: DMPA‐SC 31.8 ± 6.7 years, leuprolide 30.9 ± 6.1 years
Setting: Europe, Asia, Latin America and New Zealand Timing: July 1, 2001, through August 11, 2003 |
|
| Interventions | 6 months of active treatment with depot medroxyprogesterone acetate 104 mg/0.65 mL ( DMPA‐SC 104) versus 6 months of active treatment with leuprolide 3.75 mg monthly, or in The Netherlands, 11.25 mg once every 3 months |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: Yes Sample size calculation: not stated Funding: no funding Note previous version: Authors contacts as values were given as median percentage change. No response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This study randomised patients in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was an evaluator‐blinded study, in which the principal investigator and any designated sub‐investigators and study coordinators at each centre were blinded to the randomisation of each patient. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | For the purpose of maintaining the blinding, an independent person maintained the randomisation code, received the study syringes and administered the study medication. This individual was instructed not to reveal the randomisation code or to discuss the patient’s route of administration with clinical study site personnel. In addition, patients were instructed not to discuss the route of administration. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In DMPA‐SC 104 group, a total of 54/153 withdrawn during follow‐up period ‐ 12 adverse events ‐ 10 protocol violation ‐ 22 consent withdrawn ‐ 10 patient contact loss In leuprolide group, a total of 46/146 withdrawn during follow‐up period ‐ 9 adverse events ‐ 10 protocol violation ‐ 18 consent withdrawn ‐ 9 patient contact loss |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Dawood 1995.
| Study characteristics | ||
| Methods | Trial design: Prospective, randomised, double‐blind study | |
| Participants | Participants: 12 women were randomised and analysed. Mean age: The mean age was 29.4 ± 1.6 years for the LA group and 30.5 ± 2.9 years for the danazol group.
No surgical treatment was permitted at the time of pretreatment laparoscopy. Exclusion criteria:
Setting: United states of America Timing: not stated |
|
| Interventions | Group 1: 3.7 mg leuprolide acetate monthly injection + oral placebo every day or Group 2: 800 mg danazol orally + monthly placebo injection |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: supported by a grant from TAP Pharmaceuticals, Inc., Deerfield, Illinois The author has not been reached for no working email address was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomized clinical trial' by randomisation code. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Dlugi 1990.
| Study characteristics | ||
| Methods | Trial design: "Phase III, randomised, double‐blind, multi‐centre study" | |
| Participants | Participants: 63 women eligible; 63 were randomised and 52 were analysed. Mean age: 29.8 ± 1.0 leuprolide versus 30.2 ± 1.0 placebo Inclusion criteria:
Exclusion criteria: not stated Setting: United States of America Timing: Not stated |
|
| Interventions | Leuprolide acetate 3.75 mg IM depot every 4 weeks for 20 weeks (n = 32) versus Placebo (diluent) 2 mL IM every 4 weeks for 20 weeks (n = 31) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Supported by a grant from TAP Pharmaceuticals, North Chicago, Illinois Note previous version: Authors contacted for details on allocation concealment and SEMs. Letter returned to sender; author moved with no forwarding address. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | "Patients were assigned a 3 digit patient number in sequential order from those numbers allocated to each investigator. The patient number encoded the random assignment to a treatment group". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sufficient details for attrition: 7/32 withdrawn as subsequently determined they had failed to meet entry requirements; 4 excluded because they had received fewer than 3 injections of the study drug. There were partial exclusions for efficacy data due to non‐compliance with intended study procedures and dosing regimens for 15 patients (7 = leuprolide and 8 = placebo). 27/31 placebo (24 terminated because of worsened symptoms, 1 because of salpingitis, 1 became pregnant and 1 was non‐compliant) and 3 (2 because of intolerable pain and 1 because of an adverse event) leuprolide patients prematurely terminated study. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Dmowski 1989a.
| Study characteristics | ||
| Methods | Trial design: "Open‐label, randomised, prospective study" | |
| Participants | Participants: 36 women eligible, 36 were randomised and 29 were analysed Mean age: 30.8 ± 0.6 (SE) (range, 27 to 38 years) Inclusion criteria:
Exclusion criteria: not stated Setting: United States of America Timing: Not stated |
|
| Interventions | Buserelin 400 μg three times a day (1200 μg/day) IN for 6 months (n = 10) versus Buserelin 200 mcg daily SC for 6 months (n = 9) versus Danazol 200 mg four times a day (800 mg/day) PO for 6 months (n = 10) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: supported in part by a grant from Hoechst‐Roussel Pharmaceuticals, Inc. Note previous version: Authors contacted regarding allocation concealment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2:1 buserelin: danazol. No further details of method used to generate the randomisation sequence were provided. "Those who were randomised into Buserelin were given an option of SC injections or IN sprays of the drug". |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail for attrition: 3 in SC buserelin, 2 in IN buserelin and 2 in danazol group. 2 withdrew for family reasons, 3 were non‐compliant, 1 had severe emotional side effects on IN buserelin and 1 was allergic to danazol. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Edmonds 1994.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 50 women were randomised and analysed. Mean age: not stated Inclusion criteria:
Exclusion criteria:
Setting: United Kingdom Timing: not stated |
|
| Interventions | Group 1: goserelin 3.6 mg/month as SC depot (n = 25) versus Group 2: goserelin 3.6 mg/month as SC depot + 17‐oestrodiol 25 ug through the skin twice weekly and medroxyprogesterone acetate 5 mg/day PO (n = 25) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author could not be contacted for further information because of lack of contact information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised controlled trial'. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Change in pain‐related symptoms was asked for, but not reported in published article. |
| Other bias | Low risk | No other risk of bias detected |
Fedele 1989.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled study | |
| Participants | Participants: 62 women were randomised and analysed. Mean age: Buserelin = 29.8 ± 3.3 and danazol 31.3 ± 4.3 Inclusion criteria:
Exclusion criteria:
Setting: Italy Timing: not stated |
|
| Interventions | Buserelin 400 μg three times a day IN for 6 months (n = 30) versus Danazol 200 mg three times a day PO for 6 months (n = 32) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted for information on raw data for pain scores, and methods. No response to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were assigned randomly to one of two treatment groups. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Ferreira 2010.
| Study characteristics | ||
| Methods | Trial design: Randomised, prospective open‐labelled study | |
| Participants | Participants: 44 women with endometriosis (confirmed laparoscopically/histologically), consecutively selected at the pain and endoscopy outpatient clinic Mean age: 28.8 ± 4.9 years for LNG‐IUS and 41.4 ± 5.8 years for GnRHa Inclusion criteria:
Exclusion:
Setting: Brazil Timing: not stated |
|
| Interventions | Levonorgestrel intrauterine system (LNG‐IUS) (n = 22) versus GnRHas (n = 22) 3.75 mg leuprolide IM monthly treatment for 6 months |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: Yes Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised by computer program (GraphPad Software) at a 1:1 ratio)". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | GnRHa (1 pregnancy before drug administered and 3 moved and lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Finkelstein 1998.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 43 women were randomised and 39 analysed. Mean age: Group 1: 31 ± 7 years, group 2: 32 ± 7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | GnRH analog nafarelin acetate (Synarel, Syntex Laboratories, Inc, Palo Alto, Calif), 200 μg intranasally twice daily, for 12 months (group 1, n = 22) versus Nafarelin acetate plus human PTH‐(1‐34), 40 μg (500 U) subcutaneously daily, for 12 months (group 2, n = 21) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: This work was supported by National Institutes of Health grants R29 DK43341 and RR1066 and a National of Institutes of Health Clinical Associate Physician Award (Dr Finkelstein). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by a research biostatistician not otherwise involved in the study. |
| Allocation concealment (selection bias) | Low risk | The women were randomly assigned. Randomisation was performed using computer‐generated cards that were numbered sequentially and kept in opaque, sealed envelopes until entry into the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Because we and our local institutional review board felt that the use of placebo hPTH‐(1‐34) injections was unethical, no placebo was used. Thus, neither the patients nor the investigators were blinded with respect to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The person reading the bone density scans, however, was blinded with respect to treatment assignment, as were the technicians who performed the biochemical analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four women in group 2 (4/21) withdrew from the study after 3 months (n = 2) or 6 months (n = 2). Reasons for withdrawal included hot flashes and mild weight gain (n = 1), excessive distance from study site (n = 1), discomfort from injections (n = 1), and depression (n = 1). All data from these women are included and analysed with their originally assigned group. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Finkelstein 1999.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 38 women were randomised and analysed. Mean age: Group 1: 34 ± 7 years, group 2: 34 ± 7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | Nafarelin acetate (Synarel, Syntex Laboratories, Inc., Palo Alto, CA; 200 mg, intranasally, twice daily) (group 1; n = 28) versus Nafarelin acetate plus human PTH [40 mg (500 U), sc, daily] for 6–12 months (group 2; n = 23) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: This work was supported by NIH Grants R29‐DK‐43341 and RR1066 and a NIH Clinical Associate Physician Award (to J.S.F.). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The women were randomly assigned using computer generated cards. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Franke 2000.
| Study characteristics | ||
| Methods | Trial design: prospective, randomized, placebo‐controlled, double‐blind trial | |
| Participants | Participants: 41 women were randomised, 40 women were analysed. Mean age: Group 1: 29.9 ± 6.0 years, group 2: 31.2 ± 5.3 years Inclusion criteria:
Exclusion criteria: not stated Setting: The Netherlands Timing: April 1, 1997 until July 1, 1999 |
|
| Interventions | Group 1: goserelin acetate SC 3.6 mg every 4 weeks + oral placebo (n = 23) versus Group 2: goserelin acetate SC 3.6 mg every 4 weeks + 2 mg 17ß‐E² and 1 mg norethisterone acetate daily (n = 18) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Trial sponsored by AstraZeneca and Novo Nordisk, who also supplied the active drugs and placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered envelopes. No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in group 1 discontinued treatment due to severe climacteric symptoms. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Fraser 1991.
| Study characteristics | ||
| Methods | Trial design: "Double‐blind, double‐dummy, randomised, parallel study" | |
| Participants | Participants: 49 women with endometriosis stage 1‐3 were randomised and 45 were analysed. Mean age: not stated Inclusion criteria:
Exclusion criteria:
Setting: Australia/New Zealand Timing: not stated |
|
| Interventions | Nafarelin 200 mcg twice a day (400 μg/d) IN + placebo PO for 6 months (n = 33)
versus
Danazol 200 mg three times a day (600 mg/d) PO + placebo IN for 6 months (n = 16) Subdivisions were made: Stage 1‐2: Subgroup A (27 patients; 2 dropouts) who received intranasal nafarelin acetate 200 pg twice daily, or Subgroup B (13 patients; no dropouts) who received oral danazol 200 mg 3 times daily. Stage 3: Subgroup A (6 patients; 1 dropout) who received intranasal nafarelin acetate 200 pg twice daily for 6 months followed by conservative laparotomy, or Subgroup B (3 patients; no dropouts) who received oral danazol 200 mg three times daily followed by conservative laparotomy. |
|
| Outcomes | ‐ Dyspareunia, pelvic pain, pelvic tenderness, induration ‐ Change in rAFS score ‐ Adverse effects | |
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Syntex Pharmaceuticals International supplied nafarelin acetate, and a grant to cover the expenses of the trial. Note previous version: Authors contacted with regards to allocation concealment. Author replied that the data were difficult to find but would try. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo pill + placebo nasal spray so patient and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo pill + placebo nasal spray so assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/33 participants "dropped out" of intranasal nafarelin group; no "drop outs" from danazol group (0/16) |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Freundl 1998.
| Study characteristics | ||
| Methods | Trial design: randomised, double‐blind, comparative study | |
| Participants | Participants: 27 women were randomised and analysed. Mean age: group A: 35 ± 5 years, group P: 34.8 ± 5 years Inclusion criteria:
Exclusion criteria: not stated Setting: Germany Timing: not stated |
|
| Interventions | Group A: received a combination of leuprorelin acetate depot with 20 mg ethinyloestradiol plus 0.15 mg desogestrel (n = 14) versus Group P: received leuprorelin acetate depot plus placebo (n = 13) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Contact with the author could not be established for further information; he unfortunately passed away. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective double‐blind, randomised placebo‐controlled trial". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Fukushima 1993.
| Study characteristics | ||
| Methods | Trial design: single‐blind, single‐centre, randomised controlled trial | |
| Participants | Participants: 28 women were randomised, 19 women were analysed. Mean age: Buserelin 34.6 ± 5.1 years, danazol 32.6 ± 8.5 years Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: not stated |
|
| Interventions | Danazol (Bonzol©, Tokyo Tanabe Co., Ltd., Tokyo, Japan) capsules at a dose of 400 mg/day versus Buserelin (Suprecur© Hoechst Japan, Tokyo, Japan) nasal spray at a dose of 900 μg/day |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author could not be contacted due to lack of contact information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind (to assessor of bone mineral density) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/28 did not complete the study due to reasons unrelated to the treatment they had been administered. No further details were provided. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Gnoth 1999.
| Study characteristics | ||
| Methods | Trial design: Randomised, double‐blind, placebo‐controlled, prospective trial | |
| Participants | Participants: 27 women were randomised and analysed. Mean age: group 1; 34.8 ± 5 years, group 2; 35 ± 5 years Inclusion criteria:
Exclusion criteria: Reduced bone mineral density prior to study entry
Setting: Germany Timing: not stated |
|
| Interventions | Group 1: leuprolin acetate 3.75 mg IM + oral placebo each day versus Group 2: leuprolin acetate 3.75 mg IM + 20μg ethinyl oestrodiol and 0.15 mg desogestrel per day |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author contacted for more information regarding selection bias; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By centralised randomisation process. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pretreatment measurements of one women weres not done. 1 early pregnancy post‐treatment. The reply from the authors stated that there were no exclusions post‐randomisation or losses to follow‐up, but remarked that one woman withdrew directly after the first medical investigation and randomisation. She did not tell the reasons for her decision. There were no measurements taken from her. This participant was completely excluded from the evaluation and replaced. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Gomes 2007.
| Study characteristics | ||
| Methods | Trial design: randomised, controlled clinical study | |
| Participants | Participants: 22 women were randomised; 18 were analysed. Mean age: LNG‐IUS = 29.2 ± 5.5 years and Lupron = 32.6 ± 5.3 years Inclusion criteria:
Exclusion criteria:
Setting: Brazil Timing: February 2003 to May 2005 |
|
| Interventions | LNG‐IUS IU for 6 months (n = 11) versus Lupron depot 3.75 mg IM every 4 weeks for 6 months (n = 11) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: The levonorgestrel‐releasing intrauterine system (Mirena) and GnRH agonist ampules were provided free of charge by Schering, São Paulo, Brazil. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated system". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes". No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail given for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Harada 2009.
| Study characteristics | ||
| Methods | Trial design: Multicentre, randomised, double‐blind trial | |
| Participants | Participants: 271 women were randomised; 255 women were analysed. Mean age: Dienogest: 33.5 ± 6.9 years, buserelin 33.8 ± 6.2 years Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: June 2003 to February 2005 |
|
| Interventions | Dienogest 1 mg orally morning and evening (n = 134) versus Buserelin intranasally 300 micrograms morning, noon and evening (n = 134) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: yes Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised by the centre according to the permuted block method and computer‐generated numbers. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence list was kept centrally to maintain the blindness of the study until the key was disclosed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The BMD measurement procedures were performed under double‐blind conditions. Assessors were blinded and participants were blinded with double dummy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The BMD measurement procedures were performed under double‐blind conditions. Assessors were blinded and participants were blinded with double dummy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was used for the primary outcome. In dienogest group, withdrawn = 16/137 ‐ 3 consent withdrawn ‐ 6 adverse events ‐ 1 prohibited concomitant therapy ‐ 2 protocol criteria violation ‐ 4 other In buserelin acetate group, withdrawn = 20/134 ‐ 3 consent withdrawn ‐ 7 adverse events ‐ 2 prohibited concomitant therapy ‐ 3 protocol criteria violation ‐ 5 other BMD was available for only 41/137 patients who had received dienogest and 46/134 who had received buserelin acetate. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Henzl 1988.
| Study characteristics | ||
| Methods | Trial design: "parallel, randomised, double‐placebo design" | |
| Participants | Participants: 236 women were randomised; 213 analysed Age: not stated (60% were over 30 years old) Inclusion criteria:
Exclusion critieria: not stated Setting: USA, Canada, Sweden Timing: not stated |
|
| Interventions | Nafarelin IN 200 mcg twice daily + placebo PO for 6 months (n = 77) versus Nafarelin IN 400 mcg twice daily + placebo PO for 6 months (n = 79) versus Danazol PO 400 mg twice daily+ placebo IN for 6 months (n = 80) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted regarding randomisation and allocation concealment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail given for attrition: 23 were excluded from the analyses: 9 for reasons not related to the study drugs 7 in 800 mcg nafarelin and 4 in danazol due to hot flushes 2 in danazol due to rapid rise in serum enzymes 1 in danazol because of a lack of efficacy Analysed: 70/77: Nafarelin IN 200 mcg BD + placebo PO for 6 months 73/79: Nafarelin IN 400 mcg BD + placebo PO for 6 months 70/80: Danazol PO 400 mg BD + placebo IN for 6 months |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Hornstein 1995.
| Study characteristics | ||
| Methods | Trial design: "double‐blind, prospective, multi‐centre, randomised clinical trial" | |
| Participants | Participants: 179 women were randomised and analysed. Mean age: Group 1 = 31.0 +/‐ 6.1 years and group 2 = 31.3 +/‐ 5.7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | Group 1: Nafarelin 200 mcg IN for 3 months + placebo IN for 3 months after (n = 91) versus Group 2: Nafarelin 200 mcg IN for 6 months (n = 88) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: Supported in part by a grant from Syntex Laboratories, Inc., Palo Alto, California Note previous version: Authors contacted and replied |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | "randomisation was done by a pharmacy" . |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo nasal spray to blind participants; participants, investigators, outcome assessors and clinicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo nasal spray to blind participants; participants, investigators, outcome assessors and clinicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised were followed for a minimum of 12 months after treatment. Of the 179 subjects enrolled, 39 in group I and 43 in group II patients completed the 18‐month study without requiring further treatment other than periodic analgesics. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Hornstein 1998.
| Study characteristics | ||
| Methods | Trial design: Multi‐centre randomised, double‐blind, placebo‐controlled trial | |
| Participants | Participants: 201 women were randomised and analysed. Mean age: Group A: 28.4 ± 6.1, group B: 28.7 ± 6.2, group C: 29.0 ± 5.6, group D: 27.9 ± 5.7 Inclusion criteria:
Exclusion criteria: not stated Setting: United States of America Timing: not stated |
|
| Interventions | All patients received GnRH agonist (Lupron depot 3.75 mg every 4 weeks for 52 weeks) and calcium supplementation as elemental calcium 1000 mg daily. Group A: received daily oral placebos for progestin and oestrogen (n = 51) Group B: received daily oral norethindrone acetate 5 mg with placebo for oestrogen (n = 55) Group C: received norethindrone 5 mg and conjugated equine oestrogens 0.625 mg (n = 47) Group D: received norethindrone 5 mg and conjugated equine oestrogens 1.25 mg (n = 48) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: yes Funding: Grant received from TAP Pharmaceuticals Inc. Ms Castro is a paid employee of TAP Pharmaceuticals Inc; Dr Weissberg was a paid employee of TAP Pharmaceuticals Inc. at the time the study was undertaken. Drs Hornstein and Surrey have received honoraria for participating in a limited number of lectureships and symposias sponsored by TAP Pharmaceuticals Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned randomly to a treatment group by permuted blocks of four at each of 26 study sites. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Howell 1995.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 50 women were randomised; 48 completed 24 weeks of treatment and underwent second laparoxcopic assessment. Mean age: Group 1: 29 ± 6 years, group 2: 30 ± 5 years Inclusion criteria:
Exclusion criteria:
Setting: United Kingdom Timing: not stated |
|
| Interventions | Group 1: 3.6 mg SC depot injection of goserelin every 4 weeks versus Group 2: 3.6 mg SC injection every four weeks and transdermal oestrogen 25 ug daily and 5 mg medroxyprogesterone acetate daily for 20 weeks commencing with the second goserelin depot |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Study sponsored by Zeneca Pharmaceuticals Note previous version: 20 participants did not complete all the bone mineral density assessments ‐ 2 did not complete treatment and the other 18 did not complete follow‐up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Were randomised". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial. No further details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/48 participants did not complete all the bone mineral density assessments. 2/48 did not complete treatment and the other 18/48 did not complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Hurst 2000.
| Study characteristics | ||
| Methods | Trial design: placebo‐controlled, prospective, randomised, double‐blinded study | |
| Participants | Participants: 13 women were randomised and analysed. Mean age: Oestrogen: 28.3 ± 5.0 years, placebo 32.4 ± 5.2 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | All were treated with leuprolide acetate (TAP Pharmaceuticals, Deerfield, IL) 3.75 mg intramuscularly for six months. After three months of therapy, Oral oestradiol 1 mg daily (n = 6) versus Identical placebo (n = 7) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author contacted regarding information on selection bias; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned into treatment or placebo groups by the hospital’s investigational drug service". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Irahara 2001.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 21 women were randomised and analysed. Mean age: Group 1: 36.0 ±10.0 years, group 2: 35.5 ± 8.6 years Inclusion criteria:
Exclusion criteria: not stated Setting: Japan Timing: not stated |
|
| Interventions | Group 1: monthly injection of 3.75 mg leuprolide acetate depot (n = 10) versus Group 2: monthly injection of 3.75 mg leuprolide acetate + 0.625 mg conjugated equine oestrogen + 2.5 mg medroxyprogesterone acetate every other day from 2nd month of GnRHa treatment (n = 11) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author contacted for further information; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Jelley 1986.
| Study characteristics | ||
| Methods | Trial design: "Open, prospective, randomised, parallel study", multi‐centre | |
| Participants | Participants: 80 women were randomised; 68 were analysed. Median age: Buserelin = 28 and danazol = 30 Inclusion criteria:
Exclusion criteria:
Setting: UK Timing: not stated |
|
| Interventions | Buserelin 300 mcg three times a day intranasally for 7 months (n = 34) versus Danazol 600 mg once daily per OS for 7 months (n = 34) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Preliminary findings for the first 68 women treated only. Attempted to contact author regarding data. Author not contactable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The code was derived from random number tables". No further details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | "A sealed envelope was provided for each patient, and opened only after the patient's name had been entered on it". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open study, no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open study, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Kennedy 1990.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 82 women were randomised and analysed. Mean age: Nafarelin group was 31.5 ± 5.20 years (range = 21 to 41 years), danazol group 30.2 ± 4.89 years (range = 21 to 42 years). Inclusion criteria:
Exclusion criteria: not stated Setting: United Kingdom Timing: not stated |
|
| Interventions | Nafarelin 200 μg twice daily IN with placebo tablets (n = 55) versus Danazol 200 mg three times daily orally with placebo nasal spray (n = 27) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Supported by Syntex (U.K.) Ltd., Maidenhead, United Kingdom Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated, stratification on a 2:1 ratio, according to disease severity. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight patients did not complete treatment and their scores were omitted; their stages at first laparoscopy were I = 2, II = 4, III = 1, and IV = 1. Four patients withdrew from the nafarelin group because of depression, muscle aches, mastalgia and bloating (1); giddiness, nausea, and vague paraesthesia on the right side of the face and left hand (1); travel abroad (1); and a maculopapular rash (1). Two patients withdrew from the danazol group because of a viral illness (1) and a maculopapular rash (1). One patient from each group was withdrawn from the study because of poor compliance. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Kiilholma 1995.
| Study characteristics | ||
| Methods | Trial design: Prospective, randomised, double‐blind, placebo‐controlled, comparative study | |
| Participants | Participants: 88 women were randomised; 76 women were analysed. Mean age: Goserelin acetate plus HRT treatment mean 32 years (20‐48 years), goserelin acetate plus placebo treatment mean 34 years (21‐47 years) Inclusion criteria:
Exclusion criteria: not stated Setting: Finland Timing: not stated |
|
| Interventions | 3.6 mg SC goserelin acetate plus HRT treatment with combination of 2 mg 17β‐E2 and 1 mg norethisterone acetate (NET; Kliogest; Novo Nordisk AS, Bagsvaerd, Denmark) (n = 43) versus 3.6 mg SC goserelin acetate plus placebo (n = 45) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Supported by Zeneca Pharma, Helsinki, Finland. Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective, randomized, double‐blind, placebo‐controlled, comparative study". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76/88 completed study. 35/43 goserelin acetate with hormone replacement therapy and 41/45 goserelin acetate plus placebo group. Two patients discontinued the treatment because of side effects that were considered to be related to the treatment. One of them (goserelin acetate plus placebo) experienced severe depression and the other one had continuous bleeding (goserelin acetate plus HRT). Three women had pregnancy confirmed during the post‐treatment follow‐up period. Two patients underwent surgery for endometriosis, one woman started hormonal contraception, two women were treated with hormonal or IVF therapy for infertility, one patient needed hormonal therapy for a disease other than endometriosis, and one patient did not return. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Lemay 1988.
| Study characteristics | ||
| Methods | Trial design: Randomised study | |
| Participants | Participants: 13 women were randomised and analysed. Age: 24 to 37 years Inclusion criteria:
Exclusion criteria:
Setting: Canada Timing: Not stated |
|
| Interventions | Buserelin 400 μg three times a day IN (n = 7) versus Buserelin 200 μg once a day SC injection (n = 6) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Author contacted regarding methods and replied. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised allocation. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered, identical envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Ling 1999.
| Study characteristics | ||
| Methods | Trial design: double‐blind, randomised, parallel‐group, placebo‐controlled trial | |
| Participants | Participants: 100 women were randomised; 95 women were analysed. Mean age: Depot leuprolide group: 32.3 years, placebo group 29.4 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America (12 sites) Timing: June 1995 to January 1997 |
|
| Interventions | Depot leuprolide injection every 4 weeks, for 3 injections (n = 50) versus Placebo injections every 4 weeks, for 3 injections (n = 50) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: This study was supported by a grant from TAP Holdings, Inc., which distributes depot leuprolide. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedules were prepared in random blocks of two and four, with treatment group assignment in a 1:1 ratio. Each group was represented once within each block of two and twice within each block of four. The schedules were prepared by an administrative staff member using a FORTRAN program to generate uniform random numbers. |
| Allocation concealment (selection bias) | Low risk | Study medication was packaged according to the randomisation schedules and was sent to each site in sets of four, as needed. Patient numbers were sequential within each set. Patient number assignment started with the lowest available number for each site and proceeded in ascending order. The randomisation schedules were kept in one appropriate, secure place. The blind was not broken until enrolment and classification for evaluable patient analyses were complete. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In placebo group, 46/50 completed study.
In leuprolide depot group, 49/50 completed study.
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Miller 2000.
| Study characteristics | ||
| Methods | Trial design: "prospective, randomised, double‐blind, parallel, placebo‐controlled study" | |
| Participants | Participants: 120 women were randomised; 120 were analysed. Age: 18‐40 Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | Leuprolide acetate 3.75 mg single IM for 4 weeks (n = 60) versus Placebo for 4 weeks (n = 60) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: All participants recruited were analysed. Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted regarding methods and raw data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned to groups in the order in which they were enrolled according to a computer generated schedule prepared before the start of the study" |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind. A placebo injection was used to blind participants, but no other details of blinding of trial personnel or outcome assessors were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated double‐blind. A placebo injection was used to blind participants, but no other details of blinding of trial personnel or outcome assessors were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it wa clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Minaguchi 1986.
| Study characteristics | ||
| Methods | Trial design: Randomised, multi‐centre study | |
| Participants | Participants: 191 women were randomised and analysed. Mean age: not stated Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: not stated |
|
| Interventions | Buserelin 300 mcg once daily intranasally for 6 months (n = 69) versus Buserelin 300 mcg twice daily intranasally for 6 months (n = 59) versus Buserelin 300 mcg three times a day intranasally for 6 months (n = 63) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted regarding methods and data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | "Envelope". No further details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding; open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding; open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women who were randomised were analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Moghissi 1998.
| Study characteristics | ||
| Methods | Trial design: Prospective, placebo‐controlled study, open‐label for goserelin therapy and double‐blind for HRT | |
| Participants | Participants: 345 women were randomised; 306 women were analysed. Mean age: Group 1: 29.6 ± 6.6 years, Group 2 30.7 ± 6.0 years Group 3: 29.4 ± 5.7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America (42 participating centres) Timing: not stated |
|
| Interventions | Group 1: goserelin 3.6 mg every 28 days + oral placebo (n = 119) Group 2: goserelin 3.6 mg every 28 days + conjugated oestrogen 0.3 mg daily + medroxyprogesterone acetate 5 mg daily (n = 113) Group 3: goserelin 3.6 mg every 28 days + conjugated oestrogen 0.625 mg daily + medroxyprogesterone 5 mg daily (n = 113) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: yes Funding: Sponsored by Zeneca Pharmaceuticals, Wilmington, Delaware Authors could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 306/345 patients completed therapy and 39 patients (10 HRT0, 14 HRT1, and 15 HRT2) were withdrawn. Group 1 10/119 Group 2 14/113 Group 3 15/113 The reasons for withdrawal during therapy included dropout (n = 24), investigator decision (n = 4), side effects (n = 3), pregnancy (n = 1), and other reasons (n = 7). Three patients, one in each group, completed therapy but did not want to be observed during the follow‐up period. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Mäkäräinen 1996.
| Study characteristics | ||
| Methods | Trial design: Randomised, double blind, placebo‐controlled trial | |
| Participants | Participants: 38 women were randomised; 29 women were analysed after second‐look laparoscopy. Mean age: 30.6 years (range 22 to 38 years) Inclusion criteria:
Exclusion criteria: not stated Setting: Finland Timing: not stated |
|
| Interventions | Once‐a‐month SC injections of goserelin acetate 3.6 mg (Zoladex depot; Zeneca Pharmaceutics, Cheshire, United Kingdom) randomly combined with either Medroxyprogesterone acetate 100 mg daily (n = 19) versus Placebo, one tablet daily (n = 19) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Zeneca Pharma, Helsinki, Finland, for supplying the active drugs and placebo Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients interrupted the treatment because of side effects (three in the medroxyprogesterone acetate group and one in the placebo group). Increasing pelvic symptoms in one patient receiving medroxyprogesterone acetate required laparoscopy already 4 months after the end of treatment. Four women (two in the medroxyprogesterone acetate group and two in the placebo group) became pregnant within 6 months after the treatment. Thus, 29 women (13/19 in the medroxyprogesterone acetate and 16/19 in the placebo group) were included in the second‐look laparoscopy, which was performed 6 months after the end of medical treatment. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
NEET 1992.
| Study characteristics | ||
| Methods | Trial design: Multi‐centre, parallel, randomised, double‐blind, double‐dummy study | |
| Participants | Participants: 315 women were randomised, 307 were analysed for safety and 263 were analysed for efficacy. Mean age: not stated Inclusion criteria:
Exclusion criteria:
Setting: Multiple sites within Europe Timing: not stated |
|
| Interventions | Nafarelin 200 mcg twice daily intranasal + placebo per os for 6 months (n = 206) versus Danazol 200 mg three times a day per os + placebo intranasal for 6 months (n = 101) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: no Sample size calculation: not stated Funding: Supported in part by Syntex Research, Palo Alto, California, US Note previous version: 8 participants who were randomised never took the study medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised so that 2 were assigned to receive nafarelin for every 1 assigned to receive danazol". No further information was provided on method used to generate random sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Odukoya 1995.
| Study characteristics | ||
| Methods | Trial design: Randomised study | |
| Participants | Participants: 21 women were randomised and analysed. Mean age: 33 ± 5 years Inclusion criteria:
Exclusion criteria: not stated Setting: United Kingdom Timing: not stated |
|
| Interventions | Leuprolide acetate 3.75 SC monthly for 3 months (n = 10) versus Danazol 400 mg daily PO for 3 months (n = 11) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: Yes Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted regarding methods (blinding) and SD data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was "computer generated". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | "concealed in an envelope only opened at commencement of treatment". No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or trial personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Orwoll 1994.
| Study characteristics | ||
| Methods | Trial design: randomised, double‐blinded, placebo‐controlled trial | |
| Participants | Participants: 183 women were randomised and 137 analysed. Mean age: 31 years (range 17 to 46 years) Inclusion criteria:
Exclusion criteria:
Setting: United States of America (9 academic medical centres) Timing: not stated |
|
| Interventions | 200 μg of nafarelin intranasally twice per day for 6 months (n = 92) versus 200 μg of nafarelin intranasally twice per day for 3 months followed by 3 months of placebo (n = 91) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: Supported by Syntex Laboratories, Inc Note: Drs. Orwoll and Hornstein have been salaried consultants for Syntex Laboratories, Inc. Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomised". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised, double‐blinded, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomised, double‐blinded, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; appeared that all women included also were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Ozaki 2020.
| Study characteristics | ||
| Methods | Trial design: prospective randomised study | |
| Participants | Participants: 74 women were randomised; 70 women were analysed. Mean age: Group G: 34.2 ± 5.4 years (32.4–36.1) Group D: 35.4 ± 5.7 years (32.4–36.9) Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: January 2011 to August 2014 |
|
| Interventions | Group D received dienogest (Dinagest®, 1 mg; Mochida Pharmaceutical Co., Ltd., Tokyo, Japan) at 2 mg/day for 4 months preoperatively. Group G received a low‐dose sustained‐release goserelin acetate (ZOLADEX®, 1.8 mg; Kissei Pharmaceutical Co., Ltd., Nagano, Japan) at 1.8 mg every 4 weeks for a total of administrations preoperatively. |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: The authors declared that they had no confict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratifed randomisation was conducted in the participants. "1:1 ratio computerized random number function in Microsoft Excel" |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 74 randomised, 70 analysed Group G: 35/37 were analysed; group D: 35/37 were analysed. Excluded in group G due to mucinous cyst (n = 1) and borderline malignancy (n = 1) Excluded in group D due to mucinous cyst (n = 1) and dincontinued intervention (n = 1) |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Palagiano 1994.
| Study characteristics | ||
| Methods | Trial design: Randomised, open study | |
| Participants | Participants: 50 women were randomised; 47 were analysed. Age: 20‐40 years Inclusion criteria:
Exclusion criteria: not stated Setting: Italy Timing: not stated |
|
| Interventions | Leuprolide acetate 3.75 mg IM monthly for 6 months (n = 30) versus Danazol 600 mg once daily per os for 6 months (n = 20) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted regarding methods and replied |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". No further details were provided of method used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation done by a pharmacy. No further details were provided of method used to conceal treatment group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants were lost to follow‐up due to adverse effects (leuprolide acetate). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Petta 2005.
| Study characteristics | ||
| Methods | Trial design: multi‐centre randomised, controlled clinical trial | |
| Participants | Participants: 83 women were randomised; 71 were analysed. Mean age: LNG‐IUS = 29.4 ± 4.8 years and Lupron depot = 30.5 ± 6.4 years Inclusion criteria:
Exclusion criteria:
Setting: Brazil ( 3 centres) Timing: February 2002 to May 2004 |
|
| Interventions | LNG‐IUS (Mirena) 20 mcg/day 5 years IU for 6 months (n = 39) versus Lupron 3.75 mg every 28 days IM for 6 months (n = 43) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: Data analysis did not follow intention‐to‐treat principles but included only those women who had completed the VAS pain diary correctly throughout the entire study period of 6 months (n = 71). Sample size calculation: yes Funding:The levonorgestrel‐releasing intrauterine system (Mirena) and GnRH analogue ampoules were provided free of charge by Schering, Sao Paulo, Brazil. Note previous version: Authors contacted regarding data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by "computer generated system". Three centres participated in the study and randomisation was performed separately for each centre. |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes" were used to conceal allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Blinding of participants would not have been possible due to nature of the intervention". No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded according to author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "data analysis did not follow intention‐to‐treat principles" but details given for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Rock 1993.
| Study characteristics | ||
| Methods | Trial design: "multi‐centre, open, parallel study" | |
| Participants | Participants: 315 women were randomised and analysed. Only 58 did BMD measurements. Mean age: Goserelin = 30.4 years and danazol = 29.7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America (17 centres) Timing: not stated |
|
| Interventions | Goserelin 3.6 mg every 28 days SC for 24 weeks (n = 208) versus Danazol 400 mg twice a day PO for 24 weeks (n = 107) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Study sponsored by a grant from ICI Pharmaceuticals Group, a business unit of Zeneca Inc, Wilmington, Delaware Note previous version: Authors contacted regarding methods and data; awaiting response "14 participants, with stage IV endometriosis were included because their investigators believed that significant components of stage IV endometriosis could benefit from hormonal treatment". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1 goserelin: danazol. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomised subjects were included in the overall analysis of treatment outcome". Details given for attrition:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Rolland 1990.
| Study characteristics | ||
| Methods | Trial design: parallel, randomised, double‐blind, double‐placebo study | |
| Participants | Participants: 194 women were randomised; 170 were analysed. Mean age: between 18 and 45 years Inclusion criteria:
Exclusion criteria:
Setting: 13 medical centres in seven European countries Timing: not stated |
|
| Interventions | Nafarelin 200 μg twice daily IN + placebo PO twice daily for 6 months (n = 127) versus Danazol 200 mg twice daily PO + placebo IN twice daily for 6 months (n = 67) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Authors contacted regarding methods and data. Letter returned with author unknown at Department |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1 nafarelin: danazol. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐placebo, double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐placebo, double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details for attrition:
Nafarelin: 20/127 discontinued treatment. Danazol: 4/67 discontinued treatment. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Rotondi 2002.
| Study characteristics | ||
| Methods | Trial design: Randomised trial | |
| Participants | Participants: 81 women were randomised in a 2:1 ratio to leuprolide n = 54, or danazol n = 27. Mean age: mean age not provided; median age was 32 years (range 19‐41) Inclusion criteria:
Exclusion criteria: not stated Setting: Italy, fertility centre of the second University of Naples Timing: 1992 to 1999 |
|
| Interventions | Leuprolide acetate 3.75 mg subcutaneously every 28 days, for 6 months versus Danazol 200 mg orally three times a day for 6 months |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Excluded from previous version of this review as pain was not an outcome. Included in this review, but did not contribute any data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/54 women from leuprolide group and 5/27 from danazol group withdrew due to "adverse findings". |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Roux 1995.
| Study characteristics | ||
| Methods | Trial design: single‐site, double‐masked, randomised, placebo‐controlled, dose‐ranging trial | |
| Participants | Participants: 42 women were randomised; 40 women were analysed. Mean age: 34.0 ± 6.5 years (range 20‐44 years) Inclusion criteria:
Exclusion criteria:
Setting: France Timing: not stated |
|
| Interventions | All participants received triptorelin 3.75 mg IM every four weeks + norgestrel acetate 5 mg/day during the first 3 weeks following injection and then 1 g calcium carbonate daily for 27 weeks. In addition: Group 0: placebo intranasal spray, Group 1: salmon calcitonin 100 IU IN daily, Group 2: salmon calcitonin 200 IU IN daily. |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: yes Funding: not stated Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly devided". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants failed to complete the study ‐ one was lost to follow‐up and one was excluded due to orthopaedic material in the lumbar spine. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Schlaff 2006.
| Study characteristics | ||
| Methods | Trial design: multi‐centre, randomised, evaluator‐blinded, comparator‐controlled trial | |
| Participants | Participants: 274 women were randomised; 190 women were analysed. Mean age: medroxyprogesterone acetate group 29.2 ± 6.3 years, leuprolide group 32.1 ± 6.6 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America (43 sites) and Canada (7 sites) Timing: not stated |
|
| Interventions | Subcutaneous depot medroxyprogesterone acetate (DMPA) (104 mg/0.65 mL) every 3 months (n = 136) versus Leuprolide (11.25 mg) intramuscular every 3 months (n = 138) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: yes Funding: not stated Author contacted; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | "An independent person maintained the randomization code, received the study syringes, and administered the study medication". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In this evaluator‐blinded study, the principal investigator and any designated sub‐investigators and study co‐ordinators at each centre were blinded to the randomisation of each participant. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a dropout rate of 35.3% in the DMPA‐SC 104 group (48/136) and of 26.1% in the leuprolide group (36/138) during the 6‐month treatment period. The majority of these participants either actively withdrew from the study (DMPA‐SC 104 = 21, leuprolide = 9) or were lost to follow‐up (14 and 11, respectively). Nine patients in each group (6.6% and 6.5% in the DMPA‐SC 104 and leuprolide groups, respectively) discontinued as a result of adverse side effects. Of those women who completed the 6 months of active treatment, 51 (58.0%) of 88 in the DMPA‐SC 104 group and 58 (56.9%) of 102 in the leuprolide group left the study during the 12‐month follow‐up period. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Shaw 1986.
| Study characteristics | ||
| Methods | Trial design: Randomised study | |
| Participants | Participants: 20 women were randomised; 19 analysed Mean age: 30.4 +/‐ 3.8 Inclusion criteria:
Exclusion criteria: not stated Setting: UK Timing: not stated |
|
| Interventions | Buserelin 200 mcg three times a day intranasally for 6 months (n = 10) versus Buserelin 300 mcg three times a day intranasally for 6 months (n = 10) |
|
| Outcomes | Symptomatic changes rAFS score Adverse effects |
|
| Notes | Intention‐to‐treat analysis: No Sample size calculation: not stated Funding: Hoechst UK supplied the Buserelin used in this study. No further details provided Note previous version: Authors contacted but unable to provide further details as trial was almost 20 years old |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". No further details of method used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | No details provided of method used to conceal allocation to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "this paper reports an open study" with no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "this paper reports an open study" with no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail for attrition: 1 from buserelin 300 mcg TDS group withdrew after 3 months due to adverse effects. |
| Selective reporting (reporting bias) | High risk | No comparisons between groups for symptomatic changes |
| Other bias | Low risk | No other risk of bias reported |
Shaw 1990.
| Study characteristics | ||
| Methods | Trial design: Multi‐centre, placebo‐controlled, randomised trial | |
| Participants | 82 women were randomised; 74 were analysed. Mean age: not stated Inclusion criteria:
Exclusion criteria: not stated Setting: United Kingdom (2 sites) Timing: not stated |
|
| Interventions | Nafarelin 200 mcg BD IN + placebo PO for 6 months (n = 55) versus Danazol 200 mg three times a day PO + placebo IN for 6 months (n = 26) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Note previous version: Authors contacted but unable to provide further details as trial was almost 20 years old |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded and received placebo nasal spray or tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details for attrition given: 8 withdrew:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Sillem 1999.
| Study characteristics | ||
| Methods | Trial design: Randomised, double‐blind, placebo‐controlled, trial | |
| Participants | Participants: 23 women were randomised. Mean age: Group A: 29.7 ± 4.5 years, group B: 31.4 ± 3.8 years Inclusion criteria:
Exclusion criteria:
Setting: Germany Timing: not stated |
|
| Interventions | All patients underwent a six months course of goserelin (Zoladex®, Zeneca GmbH, Plankstadt, Germany) 3.6 mg SC every four weeks, the first injection being given on cycle day 3‐5 at the initial visit. Group A: additionally placebo (n = 12) versus Group B: additionally 5 mg medrogestone orally twice daily (Prothil®, Solvay GmbH, Hannover, Germany) (n = 11) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated Author contacted; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Skrzypulec 2004.
| Study characteristics | ||
| Methods | Trial design: Placebo, randomised, parallel study. | |
| Participants | 34 women were randomised and analysed . Mean age: GnRHa = 31.02 ± 2.5 and placebo = 32.13 ± 1.5 (SD) Inclusion criteria:
Exclusion criteria:
Setting: Poland Timing: September 2003 to April 2004 |
|
| Interventions | GnRHa 50 mg once daily per os for 12 weeks (n = 16) versus Placebo per os for 12 weeks (n = 18) |
|
| Outcomes | Relief of overall pain: reported as dysmenorrhoea, dyspareunia, pelvic pain | |
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was in the ratio two goserelin: one danazol with each centre having its randomisation list", "The randomised trial of Zoladex and Danazol was a multi‐centre trial with randomisation envelopes provided by the sponsors ICI to each of the centres as plain sealed envelopes and computerised randomisation lists for each centre" (author's reply). |
| Allocation concealment (selection bias) | Unclear risk | "plain, sealed envelopes". No other details provided of method used to conceal allocation to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, outcome assessors and clinicians were all blinded according to author. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, outcome assessors and clinicians were all blinded according to author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women who were randomised were analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detetcted |
Strowitzki 2012.
| Study characteristics | ||
| Methods | Trial design: The study was a multi‐centre, randomised, open‐label, parallel‐group, non‐inferiority comparison. | |
| Participants | Participants: 252 women were randomised; 229 women were analysed. Mean age: 18–45 years Inclusion criteria:
Exclusion criteria:
Setting: Germany Timing: not stated |
|
| Interventions | Dienogest (DNG) 2 mg/day orally (n = 124) versus Intramuscular leuprolide acetate 3.75 mg depot every 4 weeks (n = 128) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: No. "Efficacy analyses were based on the per‐protocol set (PPS), which included all randomized patients without major protocol deviations (a 'conservative' strategy appropriate for non‐inferiority studies)". Sample size calculation: yes Funding: Funding for the study was provided by Bayer HealthCare. J.M., C.G., T.F., and C.S. are full‐time employees of Bayer HealthCare. Editorial support funded by Bayer HealthCare was provided by PAREXEL. This editorial support consisted of the preparation of manuscript drafts based on detailed author guidance. Authors contacted; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized (1:1 ratio)". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | weo details of method used to conceal allocation are provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 109/124 (87.9%) women in the DNG group and 120/128 (93.8%) women in the LA group completed the study. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Surrey 1992.
| Study characteristics | ||
| Methods | Trial design: prospective randomised trial | |
| Participants | Participants: 20 women were randomised; 17 women were analysed. Mean age: group A: 32.9 ± 1.1 years, group B: 28.9 ± 1.7 years Inclusion criteria:
Exclusion criteria:
Setting: United States of America Timing: not stated |
|
| Interventions | Group 1: leuprolide acetate 3.75 mg IM every 28 days (n = 10) versus Group 2: leuprolide acetate 3.75 mg IM every 28 days and norethindrone po 5 mg for the first four weeks and then 10 mg daily for the remaining 20 weeks (n = 10) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Sponsored by TAP Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised allocation (author reply). No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/20 participants lost to follow‐up. One patient assigned to receive GnRHa and norethindrone failed to complete therapy for reasons which were felt to be unrelated to the medication. She was not replaced and data related to this patient were excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Surrey 2002.
| Study characteristics | ||
| Methods | Trial design: post‐treatment follow‐up analysis of a randomised, double‐masked, placebo‐controlled trial | |
| Participants | Participants: 201 women were randomised; 99 women were analysed. Mean age: Group A: 29.0 ± 1.0 years Group B: 29.0 ± 1.1 years Group C: 29.4 ± 1.0 years Group D: 28.9 ± 1.2 years Inclusion criteria: Regular menstrual cycle
Exclusion criteria:
Setting: United States of America (26 study sites) Timing: not stated |
|
| Interventions | Patients in all groups were to receive a depot preparation of the GnRH agonist leuprolide acetate 3.75 mg intramuscularly every 4 weeks for 52 weeks. Group A: daily oral placebos for oestrogen and progestin add‐back (n = 51) Group B: daily oral norethindrone acetate (Aygestin, Lederle, Wayne, NJ) 5 mg and placebo for oestrogen (n = 55) Group C: received oral norethindrone acetate 5 mg and conjugated equine oestrogens (Premarin, Wyeth‐Ayerst, Philadelphia, PA) 0.625 mg daily (n = 47) Group D: received oral norethindrone acetate 5 mg and conjugated equine oestrogens 1.25 mg daily (n = 48) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: yes Funding: This work was supported by a grant from TAP Pharmaceutical Products, Inc., Lake Forest, Illinois. Financial Disclosure: Drs. Surrey and Hornstein have received grant support and honoraria as members of the speaker’s bureau of TAP Pharmaceuticals. Dr. Surrey is also a member of the Medical Advisory Board of TAP Pharmaceuticals. Ms. Fredrick performed the statistical analysis and is an employee of Abbott Laboratories, a parent company of TAP Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned randomly to one of four treatment groups by permuted blocks of four at each of the treatment sites". |
| Allocation concealment (selection bias) | Unclear risk | No details were presented on the method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐masked, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐masked, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 123/201 completed 280 days of therapy. Because of dropouts, the actual group‐specific sample sizes in the follow‐up period were lower than the numbers originally enrolled based on the initial power analyses. Thus, a post hoc analysis was performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Tahara 2000.
| Study characteristics | ||
| Methods | Trial design: Prospective, randomised, longitudinal pilot study | |
| Participants | Participants: 15 women were randomised and analysed. Mean age: Control group: 35.2 ± 2.6 years Half‐dose group: 34.7 ± 5.7 years Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: not stated |
|
| Interventions | Control group: full‐dose intranasal nafarelin treatment (200 μg twice daily) for 24 weeks (n = 7) versus Half‐dose group: full dose intranasal nafarelin treatment (200 μg twice daily) for 4 weeks followed by half‐dose intranasal nafarelin treatment (200 μg daily) for 20 weeks (n = 8) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were assigned, using a random number table, to two groups. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Tang 2017.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled study | |
| Participants | Participants: 50 women were randomised and analysed. Mean age: Full‐dose group: 31.52 ± 6.38 years Half‐dose group: 30.36 ± 5.54 years Inclusion criteria:
Exclusion criteria:
Setting: Japan Timing: June 2014 to June 2016 |
|
| Interventions | Research group = Half‐dose group: “draw‐back” treatment with 3.75 mg GnRHa (leuprorelin) in the first two injections (one injection per 28 days), after which the third injection contained 1.88 mg GnRHa, and this dosage was continued up to and including the sixth injection after achieving the suppression criteria (E2 < 50 pg/mL, endometrial thickness 5 mm; in total four injections at 1.88 mg) (n = 25) Control group = full‐dose group: 3.75 mg GnRHa for six injections (n = 25) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: by Science and Technology Development Fund Project of Shenzhen, China (grant no. JCYJ20140415162338852), Science and Technology Planning Project of Guangdong Province, China (grant no. 2013B021800095), Scientific Research Project of Shenzhen Health Family Planning, China (grant no. 201401038) and Natural Science Foundation of Guangdong Province, China (grant no. 2015A030313889) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected. |
Tummon 1988.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 38 women were randomised and analysed. Mean age: 31.4 ± 0.6 years Inclusion criteria:
Exclusion criteria: not stated Setting: United States of America Timing: not stated |
|
| Interventions | GnRHa group: 8 women received leuprolide 1.6 mg daily intranasally, 8 women received buserelin 1.2 mg daily IN and 9 received buserelin 200 μg daily subcutaneously (n = 25) versus Danazol, 200 mg four times daily orally (n = 13) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Supported by TAP Pharmaceuticals, Abbott Laboratories, North Chicago, Illinois, and by Hoechst‐Roussel Pharmaceuticals, Somerville, New Jersey Author could not be contacted due to lack of information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No further details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Tummon 1989.
| Study characteristics | ||
| Methods | Trial design: Prospective, randomised study | |
| Participants | Participants: 15 women were randomised and analysed. Mean age: 32.1 ± 0.9 years (range 27 to 38 years) Inclusion criteria:
Exclusion criteria: not stated Setting: united States of America Timing: not stated |
|
| Interventions | Leuprolide daily SC injectons of 0.5 mg for 7 days, then changed to 400 mcg four times a day IN for 26 weeks (n = 10) versus Danazol 200 mg four times a day PO for 26 weeks (n = 5) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: Supported in part by a grant from Abbott Laboratories, Inc., Chicago, Illinois Note previous version: Authors contacted regarding methods and data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1 ratio leuprolide: danazol. No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Vercellini 1994.
| Study characteristics | ||
| Methods | Trial design: Open‐label, randomised study | |
| Participants | Participants: 42 women were randomised and analysed. Mean age: Group 1: 29 ± 6 years Group 2: 31 ± 6 years Inclusion criteria:
Exclusion criteria:
Setting: Italy Timing: not stated |
|
| Interventions | Group 1: Danazol only, oraly 50 mg/day for 9 months (n = 21) versus Group 2: Leuprolide 3.75 mg in a 28‐day IM depot for 3 months, followed by oral danazol 50 mg/day for 6 months + danazol (n = 21) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/42 withdrew from this study, one in each group at the fifth month of treatment (for persistent pain) and one in each group during follow‐up (they requested additional therapy). One women in the Danazol group was lost to follow‐up. Group 1: 18/21 completed follow‐up. Group 2: 19/21 completed follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Vercellini 1996.
| Study characteristics | ||
| Methods | Trial design: a multicentre, randomised, double‐blind study | |
| Participants | Participants: 55 women were randomised; 49 women were analysed (41 underwent complete serial bone mineral content assessment). Mean age: Gestrinone: 31.9 ± 5.4 years Leuprolide acetate: 28.6 ± 6.2 years Inclusion criteria:
Exclusion criteria:
Setting: Italy Timing: not stated |
|
| Interventions | Oral gestrinone 2.5 mg twice a week (Dimetrose; Poli Industria Chimica, Rozzano, Milano, Italy) (n = 27) versus Leuprolide acetate IM 3.75 mg depot injections every 4 weeks (Enantone; Takeda Farmaceutici, Roma, Italy) (n = 28) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated; only a post hoc analysis Funding: Supported in part by Poli Industria Chimica, Rozzano, Milano, Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing randomisation codes were to be opened only at trial completion or in case of severe side effects. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/55 women withdrew from the study during the treatment period, 4/27 in the gestrinone group (three refused further therapy, in one case because of her general practitioner's unfavourable opinion, in two cases because of psychological intolerance to treatment blinding, and one failed to keep clinic appointments) and 2/28 in the leuprolide acetate group (they stopped treatment for concomitant nongynaecologic diseases). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Wheeler 1992.
| Study characteristics | ||
| Methods | Trial design: "double‐blind, multi‐centre, randomised trial" | |
| Participants | Participants: 270 women were randomised and 253 were analysed. Age: Leuprolide = 30.8 years and danazol = 29.9 years Inclusion criteria:
Exclusion criteria
Setting: United States of America (17 centres) Timing: October 14, 1986 to December 21, 1988 |
|
| Interventions | Leuprolide 3.75 mg monthly IM + placebo once daily PO for 24 weeks (n = 134) versus Danazol 800 mg once daily PO + placebo monthly IM for 24 weeks (n = 136) | |
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: yes Funding: TAP‐Abbott Research and Development Judith Knittle is Senior Clinical Project Manager at TAP Pharmaceuticals Inc. In addition to her role in the preparation of this manuscript, she was involved in the design and implementation of the study. James Miller, MD, is currently Medical Director at TAP Pharmaceuticals Inc; during the conduct of this trial, he was in private clinical practice in Seattle and was one of the principal investigators of the study. Note previous version: Authors contacted regarding methods and data; awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled, double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details given for attrition: 17 patients were excluded due to:
|
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Whitehouse 1990.
| Study characteristics | ||
| Methods | Trial design: Randomised controlled trial | |
| Participants | Participants: 24 women were randomised; 22 women were analysed. Mean age: Nafarelin 33.7 ± 7 years, danazol 30.0 ± 3.3 years Inclusion criteria:
Exclusion criteria:
Setting: United Kingdom Timing: not stated |
|
| Interventions | Nafarelin 200 μg twice daily by nasal insufflation (n = 15) versus Danazol 200 mg three times daily orally (n = 9) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated". No further details of method used to generate the randomisation sequence were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants did not complete trial ‐ one became pregnant and one failed to return for final assessment. The results from these participants were excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
Zupi 2005.
| Study characteristics | ||
| Methods | Trial design: Randomised, controlled study | |
| Participants | Participants: 150 women were randomised and analysed. Mean age: Group A: 35.8 ± 5.1 Group B: 35.1 ± 4.8 Group C: 36.1 ± 5.3 Inclusion criteria:
Exclusion criteria: not stated Setting: Italy Timing: March 1, 2000 to February 28, 2003 |
|
| Interventions | Group A: GnRH‐a plus add‐back therapy = leuprolide acetate (Enantone Depot 11.25 mg; Takeda, Rome, Italy) every 3 months for 12 months plus transdermal E2 (25 μg Esclima; Takeda) and daily oral norethindrone (5 mg Primolut‐Nor; Schering, Berlin, Germany) (n = 46) Group B: GnRH‐a alone = leuprolide acetate (Enantone Depot 11.25 mg; Takeda) every 3 months for 12 months (n = 44) Group C: oestroprogestin alone = received oral ethinyl E2 (30 μg ) plus gestodene daily (0.75 mg Ginoden; Schering) for 12 consecutive months (n = 43) |
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: yes Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized by means of a computer‐generated randomization number sequence" |
| Allocation concealment (selection bias) | Unclear risk | No details of method used to conceal allocation were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The VAS and SF‐36 were administered to the patients by a nurse who was blind to the study before treatment, after 6 and 12 months of therapy, and 6 months after discontinuation of treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other risk of bias detected |
ADI: additive diameter of implants score AFS: American Fertility Society AIDS: acquired immune deficiency syndrome ASRM: American Society for Reproductive Medicine BD: twice daily BMD: bone mineral density BMI: Body Mass Index DMPA: Depot medroxyprogesterone acetate DNG: dienogest E2: oestradiol ESS: Endometriosis symptom severity FSH: Follicle‐Stimulating Hormone GnRHa: Gonadotropin Releasing Hormone Agonist HRT: Hormone Replacement Therapy Im: intramuscular IN: intranasally IVF: in vitro fertilization LA: leuprolid acetate LH: luteinizing hormone LNG‐IUS: Levonorgestrel‐Intrauterine System NSAID: Nonsteroidal anti‐inflammatory drugs OC: oral contraceptive OCP: oral contracteptive pill Pap: Papanicolaou PO: per os PRL: prolactin PTH: Parathyroid hormone QID: four times a day rAFS: revised American Society for Reproductive Medicine SC: subcutaneously SD: standard deviation SEM: standard error of mean SF‐36: Short Form Health Survey ‐ 36 T4: Thyroxine TDS: three times daily IU: intra‐uterine VAS: Visual Analogue Scale 17β‐E2: 17β‐estradiol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acien 1989 | Outcome in this article did not match the outcome measurements of this review. |
| Adiyono 2006 | Wrong participants: post‐surgical treatment |
| Agarwal 2015 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Al‐Azemi 2009 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Bergqvist 1990 | Wrong study design; not clearly stated as randomised trial |
| Calvo 2000 | Outcome in this article did not match the outcome measurements of this review. |
| Chan 1993 | Study still awaiting classification, so excluded in this review |
| Chen 2009 | Study still awaiting classification, so excluded in this review |
| Choktanasiri 2001 | Wrong study design, not clearly stated as randomised trial |
| Claesson 1989 | Wrong study design; not clearly stated as randomised trial |
| Cooke 1989 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Dawood 1990 | Wrong study design; not clearly stated as randomised trial |
| Dmowski 1989 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Dodin 1991 | Not only women with endometriosis included, but also without endometriosis |
| Donnez 1989 | The article did not meet the stated inclusion criteria. The outcome measures as viewed in the current review were not answered in this article. |
| Donnez 2004 | The article did not meet the stated inclusion criteria. Wrong comparison |
| el‐Roeiy 1988 | The article did not meet the stated inclusion criteria. The outcome measures as viewed in the current review were not answered in this article. |
| Eldred 1992 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Fedele 1993 | Wrong study design; not clearly stated as randomised trial |
| Fernandez 2004 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Ferrero 2011 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Franssen 1992 | Retrospective study, so wrong study design |
| Fraser 1996 | Ineligible condition: not about endometriosis but rather menorrhagia |
| Harada 2000 | Wrong study design; not clearly stated as randomised trial |
| Henzl 1990a | Wrong study design; not clearly stated as randomised trial |
| Imani 2009 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Lindsay 1996 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Luciano 2004 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Magini 1993 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Maouris 1991 | The outcome measures as viewed in the current review were not answered in this article. |
| Matalliotakis 2000 | The outcome measures as viewed in the current review were not answered in this article. |
| Matalliotakis 2004 | Wrong participants: post‐surgical treatment |
| Matta 1988 | Wrong patient population |
| Miller 1990 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Mukherjee 1996 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Newton 1996 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Pierce 2000 | Once patients were enrolled, allocation of treatment was carried out according to a patient‐centred, partially randomised design. |
| Ripps 2003 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Shaw 1992 | Study included also patients without complaints; no separate results stated in this article. As described in the Methods section, we decided to exclude this study. |
| Shaw 2001 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Somekawa 1999 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Sorensen 1997 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Sowter 1997 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Soysal 2004 | Post‐surgical treatment. So, wrong type of intervention |
| Surrey 1993 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Surrey 1995 | Not only women with endometriosis included, but also without endometriosis. So, wrong patient population |
| Takaesu 2013 | This study was investigating the effect of GnRHas on postoperative outcome measures. This type of intervention is excluded from the current review. |
| Tapanainen 1993 | Outcome in this article did not match the outcome measurements of this review. |
| Taskin 1997 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Toomey 2003 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Valimaki 1989 | The outcome measures as viewed in the current review were not answered in this article. |
| Vercellini 2009 | Wrong participants: post‐surgical treatment |
| Warnock 1998 | The article did not meet the stated inclusion criteria. Wrong comparison |
| Wright 1995 | The outcome measures as viewed in the current review were not answered in this article. |
| Yee 1986 | The outcome measures as viewed in the current review were not answered in this article. |
| Ylikorkala 1995 | Ineligible participants |
Characteristics of studies awaiting classification [ordered by study ID]
Aisaka 2000.
| Methods | Randomisation: randomised trial |
| Participants | Number of women: 53 Age: not stated Inclusion criteria: not stated Exclusion criteria: not stated Setting: Japan Timing: not stated |
| Interventions | Group 1: leuprolin + mestranol 0.05 mg and norethisterone 1 mg/tab 1 tab/day versus Group 2: leuprolin alone |
| Outcomes | Bone mineral density |
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated This abstract did not contain enough information to make a proper decision about in‐/exclusion. |
Archer 2004.
| Methods | Trial design: Randomised trial |
| Participants | Participants: Mean age: Inclusion criteria:
Exclusion criteria: Setting: United States and Canada Timing: not stated |
| Interventions | DMPA‐SC 104 mg every 3 months versus LA 11.25 mg given intramuscularly every 3 months |
| Outcomes |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: not stated Funding: not stated This abstract did not contain enough information to make a proper decision about in‐/exclusion. |
Gregoriou 1997.
| Methods | Randomisation: by sequential numerical allocation to a randomisation list before commencing trial |
| Participants | Number of women: 40 Age: Mean age of group 1: 28.3 years and mean age of group 2: 29.1 years Inclusion criteria:
Exclusion criteria:
Setting: Greece Timing: not stated |
| Interventions | Group 1: leuprolide acetate depot 3.75 mg IM every 4 weeks versus Group 2: leuprolide acetate depot 3.75 mg every 4 weeks + 1.25 mg daily oral conjugated equine oestrogens on days 1 to 25 and 5 mg oral medroxyprogesterone acetate on days 16‐25 |
| Outcomes |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated This abstract did not contain enough information to make a proper decision about in‐/exclusion. |
Long 2009.
| Methods | Trial design: randomised trial |
| Participants | Participants: 70 women with moderate or severe endometriosis Mean age: in GnRHa group was 31±7 years and in the add‐back group was 32±7 years in those who completed the study Inclusion criteria: not stated Exclusion criteria: not stated Setting: China Timing: not stated |
| Interventions | GnRHa Zoladex 3.6 mg every 28 days (x3) (n = 35) versus Zoladex 3.6 mg every 28 days (x3)+ add‐back‐ oestradiol valerate 0.5 mg + dydrogesterone 5 mg daily (n = 35) |
| Outcomes |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated This abstract did not contain enough information to make a proper decision about in‐/exclusion. |
Vella 1995.
| Methods | Trial design: randomised trial |
| Participants | Participants: 30 Meang age: not stated Inclusion criteria: not stated Exclusion criteria: not stated Setting: not stated Timing: not stated |
| Interventions | Group 1: goserelin versus Group 2: goserelin + premarin (conjugated oestrogens) 1.25 mg |
| Outcomes |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Funding: not stated This abstract did not contain enough information to make a proper decision about in‐/exclusion. |
DPMA: depot medroxyprogesterone acetate GnRHa: gonadotropin‐releasing hormone analogues IM: intramuscular LA: leuprolide acetate
Differences between protocol and review
In the current review, abstracts and articles whose full text was not available were excluded. We applied the core outcome set (COS). Overall pain is one of the outcome measures recommended with the COS. However, many studies still distinguish between different sub‐forms of pain, and do not use overall pain as an outcome measure. In the current review, it was decided to include both overall pain and other sub‐forms of pain, i.e. dysmenorrhoea, dyspareunia, and pelvic pain. This was to provide as much useful information as possible for shared decision‐making with patients. In addition, it was decided to perform the main analysis with only low risk of bias studies, defined as low risk of selection bias and no high risk of bias for any other domain. The sensitivity analysis, on the other hand, was performed with all studies, i.e. both the low risk and the high risk of bias studies. We included only the studies from the main analyses (low risk of bias) in the summary of findings tables.
Contributions of authors
Veerle Veth took the lead in developing the review protocol and search strategy; screening the articles; performing risk of bias assessment, data extraction, and data analysis; grading and interpretation; and writing and revising all versions of this review update.
Majorie van de Kar contributed to the background, search strategy, data extraction, risk of bias, analysis, results, and discussion of the review for this update.
James Duffy provided feedback on the methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
Madelon van Wely provided feedback on the methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
Velja Mijatovic provided feedback on the methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
Jacques Maas provided feedback on the methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
VV: none known
MvK: none known
JD: none known
JM: none known
MvW: co‐ed of Cochrane Gynaecology and Fertility Satellite
VM: none known
New
References
References to studies included in this review
Abdou 2018 {published data only}
- Abdou AM, Ammar IMM, Alenmr AAA, Abdelrhman AA. Dienogest versus leuprolide acetate for recurrent pelvic pain following laparoscopic treatment of endometriosis. Journal of Obstetrics and Gynecology of India Aug 2018;4:306-13. [PMID: DOI: 10.1007/s13224-018-1119-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adamson 1994 {published data only}
- Adamson GD, Kwei L, Edgren RA. Pain of endometriosis: effects of nafarelin and danazol therapy. International Journal of Fertility & Menopausal Studies 1994;39(4):215-7. [PubMed] [Google Scholar]
Agarwal 1997 {published data only}
- Agarwal SK, Hamrang C, Henzl MR, Judd HL. Nafarelin vs. leuprolide acetate depot for endometriosis. Changes in bone mineral density and vasomotor symptoms. Nafarelin Study Group. Journal of Reproductive Medicine 1997;42(7):413-23. [PubMed] [Google Scholar]
AN Zoladex 1996 {published data only}
- AN Zoladex. Goserelin depot versus danazol in the treatment of endometriosis the Australian/New Zealand experience. Australian & New Zealand Journal of Obstetrics & Gynaecology 1996;36(1):55-60. [DOI] [PubMed] [Google Scholar]
Audebert 1997 {published data only}
- Audebert A, Lucas C, Joubert-Collin M. Efficacy and safety of slow-release leuprorelin 3,75 mg compared to danazol treatment. [Efficacite et tolerance de la leuproreline 3,75 MG a liberation prolongeedans le traitement de l'endometriose en comparaison au danazol]. References en Gynecologie Obstetrique 1997;5(1):49-57. [Google Scholar]
Bergqvist 1997 {published data only}
- Bergqvist A, Jacobson J, Harris S. A double-blind randomized study of the treatment of endometriosis with nafarelin or nafarelin plus norethisterone. Gynecological Endocrinology 1997;11(3):187-94. [DOI: 10.3109/09513599709152533] [DOI] [PubMed] [Google Scholar]
Bergqvist 1998 {published data only}
- Bergqvist A, Bergh T, Hogstrom L, Mattsson S, Nordenskjold F, Rasmussen C. Effects of triptorelin versus placebo on the symptoms of endometriosis. Fertility & Sterility 1998;69(4):702-8. [DOI] [PubMed] [Google Scholar]
Bergqvist 2000 {published data only}
- Bergqvist A and SCANDET group. A comparative study of the acceptability and effect of goserelin and nafarelin on endometriosis. Gynecological Endocrinology Aug 2000;14:425-432. [DOI: ] [DOI] [PubMed] [Google Scholar]
Burry 1989 {published data only}
- Burry KA, Patton PE, Illingworth DR. Metabolic changes during medical treatment of endometriosis: nafarelin acetate versus danazol.. American Journal of Obstetrics & Gynecology 1989;160(6):1454-9; discussion 1459-61. [DOI] [PubMed] [Google Scholar]
Burry 1992 {published data only}
- Burry K. Nafarelin in the management of endometriosis: quality of life assessment. American Journal of Obstetrics & Gynecology 1992;166:735-9. [DOI] [PubMed] [Google Scholar]
- Burry KA, Buttram V, Moghissi KMF. Quality of life during and after treatment of endometriosis with Nafarelin or Danazol (abstract). Fertility & Sterility 1990;54(pp.s13):0-029. [Google Scholar]
Chang 1996 {published data only}
- Chang SP, Ng HT. A randomized comparative study of the effect of leuprorelin acetate depot and danazol in the treatment of endometriosis. Chung Hua i Hsueh Tsa Chih - Chinese Medical Journal 1996;57(6):431-7. [PubMed] [Google Scholar]
Cheng 2005 {published data only}
- Cheng MH, Yu BK, Chang SP, Wang PH. A randomized, parallel, comparative study of the efficacy and safety of nafarelin versus danazol in the treatment of endometriosis in Taiwan. Journal of the Chinese Medical Association 2005;68(7):307-14. [DOI] [PubMed] [Google Scholar]
Cirkel 1995 {published data only}
- Cirkel U, Ochs H, Schneider HP. A randomized, comparative trial of triptorelin depot (D-Trp6-LHRH) and danazol in the treatment of endometriosis. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1995;59(1):61-9. [DOI] [PubMed] [Google Scholar]
- Cirkel U, Ochs H, Schneider HPG. GnRH analogue depot (triptorelin) versus danazol in the treatment of endometriosis. Gynecological Endocrinology (3rd International Symposium) 1993;7(Supp 2):43. [Google Scholar]
- Ochs H, Cirkel U, Schneider HP. Correlation between extent of ovarian suppression and regression of endometriosis: decapeptyl vs danazol. Gynecological Endocrinology (3rd International Symposium) 1993;7(Supp 2):43. [Google Scholar]
Crosignani 1996 {published data only}
- Crosignani PG, De Cecco L, Gastaldi A, Venturini PL, Oldani S, Vegetti W, et al. Leuprolide in a 3-monthly versus a monthly depot formulation for the treatment of symptomatic endometriosis: a pilot study. Human Reproduction 1996;11(12):2732-5. [DOI: 10.1093/oxfordjournals.humrep.a019199] [DOI] [PubMed] [Google Scholar]
Crosignani 2006 {published data only}
- Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Human Reproduction Sep 2006;21:248-56. [DOI: 10.1093/humrep/dei290] [DOI] [PubMed] [Google Scholar]
Dawood 1995 {published data only}
- Dawood MY, Ramos J, Khan-Dawood F. Depot leuprolide acetate versus danazol for treatment of pelvic endometriosis: changes in vertebral bone mass and serum estradiol and calcitonin. Fertility & Sterility 1995;63:1177-1183. [DOI: 10.1016/s0015-0282(16)57593-1] [DOI] [PubMed] [Google Scholar]
Dlugi 1990 {published data only}
- Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Lupron Study Group. Fertility & Sterility 1990;54(3):419-27. [DOI: 10.1016/s0015-0282(16)53755-8] [DOI] [PubMed] [Google Scholar]
Dmowski 1989a {published data only}
- Dmowski WP, Radwanska E, Binor Z, Tummon I, Pepping P. Ovarian suppression induced with Buserelin or danazol in the management of endometriosis: a randomized, comparative study. Fertility & Sterility 1989;51(3):395-400. [DOI: 10.1016/s0015-0282(16)60543-5] [DOI] [PubMed] [Google Scholar]
Edmonds 1994 {published data only}
- Edmonds DK, Howell R. Can hormone replacement therapy be used during medical therapy of endometriosis? British Journal of Obstetrics and Gynaecology May 1994;101:24-26. [DOI: 10.1111/j.1471-0528.1994.tb13681.x] [DOI] [PubMed] [Google Scholar]
Fedele 1989 {published data only}
- Fedele L, Bianchi S, Arcaini L, Vercellini P, Candiani GB. Buserelin versus danazol in the treatment of endometriosis-associated infertility. American Journal of Obstetrics & Gynecology 1989;161(4):871-6. [DOI: 10.1016/0002-9378(89)90739-4] [DOI] [PubMed] [Google Scholar]
- Fedele L, Marchini M, Bianchi S, Baglioni A, Zanotti F. Vaginal patterns during danazol and buserelin acetate therapy for endometriosis: structural and ultrastructural study. Fertility & Sterility 1993;59(6):1191-5. [DOI] [PubMed] [Google Scholar]
Ferreira 2010 {published data only}
- Ferreira RA, Vieira C, Rosa-e-Silava J, Rosa-e-Silva A, Nogueira A, Ferriani R. Effects of the levonorgestrel-releasing intrauterine system on cardiovascular risk markers in patients with endometriosis: a comparative study with the GnRH analogue. Contraception 2010;81:117-122. [DOI: 10.1016/j.contraception.2009.08.003] [DOI] [PubMed] [Google Scholar]
Finkelstein 1998 {published data only}
- Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency–related bone loss with human parathyroid hormone–(1-34). Journal of the American Medical Association 1998;280:1067-1073. [DOI: 10.1001/jama.280.12.1067] [DOI] [PubMed] [Google Scholar]
Finkelstein 1999 {published data only}
- Finkelstein JS, Arnold AL . Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. Journal of Clinical Endocrinology & Metabolism 1999;84:1214-1219. [DOI: 10.1210/jcem.84.4.5643] [DOI] [PubMed] [Google Scholar]
Franke 2000 {published data only}
- Franke HR, Van der Weijer PHM, Pennings TMM, Van der Mooren MJ. Gonadotropin-releasing hormone agonist plus “add-back” hormone replacement therapy for treatment of endometriosis: a prospective, randomized, placebo-controlled, double-blind trial. Fertility & Sterility Sept 2000;74:534-539. [DOI: 10.1016/s0015-0282(00)00690-7] [DOI] [PubMed] [Google Scholar]
Fraser 1991 {published data only}
- Fraser IS, Shearman RP, Jansen RP, Sutherland PD. A comparative treatment trial of endometriosis using the gonadotrophin-releasing hormone agonist, nafarelin, and the synthetic steroid, danazol. Australian & New Zealand Journal of Obstetrics & Gynaecology 1991;31(2):158-63. [DOI: 10.1111/j.1479-828x.1991.tb01807.x] [DOI] [PubMed] [Google Scholar]
Freundl 1998 {published data only}
- Freundl G, Gödtke K, Gnotha C, Godehardt E, Kienle E. Steroidal ‘Add-Back’ Therapy in Patients Treated with GnRH Agonists. Gynecologic and Obstetric Investigation 1998;45:22-30. [DOI: 10.1159/000052848] [DOI] [PubMed] [Google Scholar]
Fukushima 1993 {published data only}
- Fukushima M, Shindo M, Sato K. Hormone treatment related bone mineral content changes in Japanese women with endometriosis. Asia-Oceania Journal of Obstetrics and Gynaecology 1993;19(3):299-307. [DOI: 10.1111/j.1447-0756.1993.tb00389.x] [DOI] [PubMed] [Google Scholar]
- Fukushima M . Changes in bone mineral content following hormone treatment for endometriosis. . International Journal of Gynecology & Obstetrics 1995;50:S17-S21. [DOI: 10.1016/0020-7292(95)02510-j] [DOI] [PubMed] [Google Scholar]
Gnoth 1999 {published data only}
- Gnoth C, Gödtke K, Freundl G, Godehardt E, Kienle E. Effects of add-back therapy on bone mineral density and pyridinium crosslinks in patients with endometriosis treated with gonadotropin-releasing hormone agonists. Gynecologic and Obstetric Investigation 1999;47:37-41. [DOI: 10.1159/000010059] [DOI] [PubMed] [Google Scholar]
Gomes 2007 {published data only}
- Gomes MK, Ferriani RA, Rosa e Silva JC, Japur de Sa Rosa e Silva AC, Vieira CS, Candido dos Reis FJ. The levonorgestrel-releasing intrauterine system and endometriosis staging. Fertility & Sterility 2007;87(5):1231-4. [DOI: 10.1016/j.fertnstert.2006.11.044] [DOI] [PubMed] [Google Scholar]
Harada 2009 {published data only}
- Harada T, Momoeda M, Taketani Y, Aso T, Fukunaga M, Hagino H, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—a randomized, double-blind, multicenter, controlled trial. Fertility & Sterility 2009;91(3):675-681. [DOI: 10.1016/j.fertnstert.2007.12.080] [DOI] [PubMed] [Google Scholar]
Henzl 1988 {published data only}
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobson J. Administration of nasal nafarelin as compared with oral danazol for endometriosis. A multicenter double-blind comparative clinical trial. New England Journal of Medicine 1988;318(8):485-9. [DOI] [PubMed] [Google Scholar]
- Henzl MR. Role of nafarelin in the management of endometriosis. Journal of Reproductive Medicine 1989;34(12 Suppl):1021-4. [PubMed]
- Jacobs L, Field C, Thie J, Coulam C. Treatment of endometriosis with the GnRH agonist naferelin acetate. International Journal of Fertility 1991;36:30-5. [PubMed] [Google Scholar]
- Moghissi KS, Corson SL, Buttram V, Henzl MR. Evaluation of a GnRH agonist (nafarelin) versus danazol for treatment of endometriosis. Contributions to Gynecology & Obstetrics 1987;16:266. [Google Scholar]
Hornstein 1995 {published data only}
- Hornstein M, Yuzpe A, Burry K, Heinrichs L, Orwoll E. A prospective randomised double-blind trial of 3 versus 6 months nafarelin therapy for symptoms of endometriosis. Fertility & Sterility 1992;58:S84. [PubMed] [Google Scholar]
- Hornstein MD, Yuzpe AA, Burry KA, Heinrichs LR, Buttram VL Jr, et al. Prospective randomized double-blind trial of 3 versus 6 months of nafarelin therapy for endometriosis associated pelvic pain. Fertility & Sterility 1995;63(5):955-62. [PMID: ] [PubMed] [Google Scholar]
Hornstein 1998 {published data only}
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA LUPRON add-back study group. Leuprolide acetate depot and hormonal add-back in endometriosis: A 12- month study. Obstetrics and Gynecology Jan 1998;1:16-24. [DOI: 10.1016/s0029-7844(97)00620-0] [DOI] [PubMed] [Google Scholar]
Howell 1995 {published data only}
- Howell R, Edmonds DK, Dowsett M, Crook D, Lees B, Stevenson JC. Gonadotropin-releasing hormone analogue (goserelin) plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertility & Sterility 1995;64(3):474-481. [DOI: 10.1016/s0015-0282(16)57779-6.] [DOI] [PubMed] [Google Scholar]
Hurst 2000 {published data only}
- Hurst BS, Gardner SC, Tucker KE, Awoniyi CA, Schlaff WD. Delayed oral estradiol combined with leuprolide increases endometriosis-related pain. Journal of the Society of Laparoendoscopic Surgeons 2000;4:97-101. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Irahara 2001 {published data only}
- Irahara M, Uemura H, Yasui T, Kinoshita H, Yamada M, Tezuka M, et al. Efficacy of every-other-day administration of conjugated equine estrogen and medroxyprogesterone acetate on gonadotropin-releasing hormone agonists treatment in women with endometriosis. Gynecologic and Obstetric Investigation 2001;52:217-222. [DOI: 10.1159/000052978] [DOI] [PubMed] [Google Scholar]
Jelley 1986 {published data only}
- Jelley RY, Magill PJ. The effect of LHRH agonist therapy in the treatment of endometriosis (English experience). Progress in Clinical & Biological Research 1986;225:227-38. [PubMed]
- Jelley RY. Multicentre open comparative study of buserelin and danazol in the treatment of endometriosis. British Journal of Clinical Practice 1986;48(Suppl):64-8.
Kennedy 1990 {published data only}
- Kennedy SH, Williams IA, Brodribb J, Barlow DH, Shaw RW. A comparison of nafarelin acetate and danazol in the treatment of endometriosis. Fertility & Sterility June 1990;53(6):998-1003. [DOI: 10.1016/s0015-0282(16)53574-2] [DOI] [PubMed] [Google Scholar]
Kiilholma 1995 {published data only}
- Kiilholma P, Tuimala R, Kivinen S, Korhonen M, Hagman E. Comparison of the gonadotropin-releasing hormone agonist goserelin acetate alone versus goserelin combined with estrogen-progestogen add-back therapy in the treatment of endometriosis. Fertility & Sterility 1995;64(5):903-908. [DOI: 10.1016/s0015-0282(16)57900-x] [DOI] [PubMed] [Google Scholar]
Lemay 1988 {published data only}
- Lemay A, Maheux R, Huot C, Blanchet J, Faure N. Efficacy of intranasal or subcutaneous luteinizing hormone-releasing hormone agonist inhibition of ovarian function in the treatment of endometriosis. American Journal of Obstetrics & Gynecology 1988;158(2):233-6. [DOI: 10.1016/0002-9378(88)90128-7] [DOI] [PubMed] [Google Scholar]
- Lemay A, Maheux R, Quesnel G, Bureau M, Faure N, Merat P. LH-RH agonist treatment of endometriosis. Contributions to Gynecology and Obstetrics 1987;16:247-53. [PubMed] [Google Scholar]
Ling 1999 {published data only}
- Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic pain study group. Obstetrics & Gynecology 1999;93(1):51-58. [DOI: 10.1016/s0029-7844(98)00341-x] [DOI] [PubMed] [Google Scholar]
Mäkäräinen 1996 {published data only}
- Makarainen L, Rönnberg L, Kauppila A. Medroxyprogesterone acetate supplementation diminishes the hypoestrogenic side effects of gonadotropin-releasing hormone agonist without changing its efficacy in endometriosis. Fertility & Sterility 1996;65(1):29-34. [DOI: 10.1016/s0015-0282(16)58023-6] [DOI] [PubMed] [Google Scholar]
Miller 2000 {published data only}
- Miller JD. Quantification of endometriosis-associated pain and quality of life during the stimulatory phase of gonadotropin-releasing hormone agonist therapy: a double-blind, randomized, placebo-controlled trial. American Journal of Obstetrics & Gynecology 2000;182(6):1483-1488. [DOI: 10.1067/mob.2000.106846] [DOI] [PubMed] [Google Scholar]
Minaguchi 1986 {published data only}
- Minaguchi H, Uemura T, Shirasu K. Clinical study on finding optimal dose of a potent LHRH agonist (buserelin) for the treatment of endometriosis--multicenter trial in Japan. Progress in Clinical & Biological Research 1986;225:211-25. [PMID: ] [PubMed] [Google Scholar]
Moghissi 1998 {published data only}
- Moghissi KS, Schlaff WD, Olive DL, Skinner MA, Yin H. Goserelin acetate (Zoladex) with or without hormone replacement. Therapy for the treatment of endometriosis. Fertility & Sterility June 1998;69(6):1056-1062. [DOI: 10.1016/s0015-0282(98)00086-7] [DOI] [PubMed] [Google Scholar]
NEET 1992 {published data only}
- Kennedy SH, Williams IA, Brodribb J, Barlow DH, Shaw RW. A comparison of nafarelin acetate and danazol in the treatment of endometriosis. Fertility & Sterility 1990;53(6):998-1003. [DOI] [PubMed] [Google Scholar]
- NEET. Nafarelin for endometriosis: a large-scale, danazol-controlled trial of efficacy and safety, with 1-year follow-up The Nafarelin European Endometriosis Trial Group (NEET) . Fertility & Sterility 1992;57(3):514-22. [PMID: ] [PubMed] [Google Scholar]
Odukoya 1995 {published data only}
- Odukoya OA, Bansal A, Wilson AP, Weetman AP, Cooke ID. Serum-soluble CD23 in patients with endometriosis and the effect of treatment with danazol and leuprolide acetate depot injection. Human Reproduction 1995;10(4):942-946. [DOI: 10.1093/oxfordjournals.humrep.a136067] [DOI] [PubMed] [Google Scholar]
Orwoll 1994 {published data only}
- Orwoll ES, Yuzpe AA, Burry KA, Heinrichs L, Buttram VC, Hornstein MD. Nafarelin therapy in endometriosis: long-term effects on bone mineral density. American Journal of Obstetrics and Gynecology Nov 1994;171(5):1221-1225. [DOI: 10.1016/0002-9378(94)90136-8] [DOI] [PubMed] [Google Scholar]
Ozaki 2020 {published data only}
- Ozaki R, Kumakiri J, Jinushi M, Ikuma S, Murakami K, Kawasaki Y, et al. Comparison of effect of preoperative dienogest and gonadotropin‑releasing hormone agonist administration on laparoscopic cystectomy for ovarian endometriomas. Archives of Gynecology and Obstetrics July 2020;302:969-976. [DOI: 10.1007/s00404-020-05691-3] [DOI] [PubMed] [Google Scholar]
Palagiano 1994 {published data only}
- Palagiano A, Capuano V. Medical treatment of endometriosis: comparative study of leuprolide acetate and danazol. Minerva Ginecologica 1994;46(4):173-7. [PMID: ] [PubMed] [Google Scholar]
Petta 2005 {published data only}
- Petta CA, Ferriani RA, Abrao MS, Hassan D, Rosa ESJC, Podgaec S, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Human Reproduction 2005;20(7):1993-8. [DOI: 10.1093/humrep/deh869] [DOI] [PubMed] [Google Scholar]
- Vieira CS, Ferreira RA, Rosa e Silva JC, Rosa e Silva ACJS, Gomes MK, Ferriani RA. Comparative study of the influence of the levonorgestrel intra-uterine system and the GnRH analogues on cardiovascular risk markers in patients with endometriosis. Fertility & Sterility 2007;88(Suppl 1):211. [Google Scholar]
- Sa Rosa e Silva AC, Rosa e Silva JC, Nogueira AA, Petta CA, Abrao MS, Ferriani RA. The levonorgestrel-releasing intrauterine device reduces CA-125 serum levels in patients with endometriosis. Fertility & Sterility 2006;86(3):742-4. [DOI] [PubMed] [Google Scholar]
Rock 1993 {published data only}
- Allen TW. Zoladex versus danazol in endometriosis therapy. Journal of the American Osteopathic Association 1993;93(10):1013. [Google Scholar]
- Rock JA, Truglia JA, Caplan RJ. Zoladex (goserelin acetate implant) in the treatment of endometriosis: a randomized comparison with danazol. Obstetrics & Gynecology 1993;82(2):198-205. [PMID: ] [PubMed] [Google Scholar]
- Rock JA. A multicenter comparison of GnRH agonist (Zoladex) and danazol in the treatment of endometriosis . Fertility & Sterility 1991;56(pp.s49). [Google Scholar]
Rolland 1990 {published data only}
- Rolland R, Heijden PF. Nafarelin versus danazol in the treatment of endometriosis. American Journal of Obstetrics & Gynecology 1990;162(2):586-8. [DOI: 10.1016/0002-9378(90)90437-c] [DOI] [PubMed] [Google Scholar]
Rotondi 2002 {published data only}
- Rotondi M, Labriola D, Ammaturo FP, Amato G, Carella C, Izzo A, et al. Depot leuprorelin acetate versus danazol in the treatment of infertile women with symptomatic endometriosis. European Journal of Gynaecological Oncology 2002;23(6):523-526. [PMID: ] [PubMed] [Google Scholar]
Roux 1995 {published data only}
- Roux C, Pelissier C, Listrat V, Kolta S, Simonetta C, Guignard M, et al. Bone loss during gonadotropin releasing hormone agonist treatment and use of nasal calcitonin. Osteoporosis International 1995;5:185-190. [DOI: 10.1007/BF02106098] [DOI] [PubMed] [Google Scholar]
Schlaff 2006 {published data only}
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertility & Sterility February 2006;85(2):314-325. [DOI: 10.1016/j.fertnstert.2005.07.1315.] [DOI] [PubMed] [Google Scholar]
Shaw 1986 {published data only}
- Shaw RW, Matta W. Reversible pituitary ovarian suppression induced by an LHRH agonist in the treatment of endometriosis - comparison of two dose regimens. Clinical Reproduction and Fertility 1986;4(5):329-36. [PMID: ] [PubMed] [Google Scholar]
Shaw 1990 {published data only}
- Shaw RW. Nafarelin in the treatment of pelvic pain caused by endometriosis. American Journal of Obstetrics & Gynecology 1990;162(2):574-6. [DOI: 10.1016/0002-9378(90)90433-8] [DOI] [PubMed] [Google Scholar]
Sillem 1999 {published data only}
- Sillem M, Parviz M, Woitge HW, Kiesel L, Ulrich U, Holst T, et al. Add-back medrogestone does not prevent bone loss in premenopausal women treated with goserelin. Experimental and Clinical Endocrinology & Diabetes 1999;107:379-385. [DOI: 10.1055/s-0029-1212129] [DOI] [PubMed] [Google Scholar]
Skrzypulec 2004 {published data only}
- Skrzypulec V, Walaszek A, Drosdzol A, Nowosielski K, Piela B, Rozmus-Warcholinska W. Influence of GnRH analogue on the intensification of endometriosis symptoms and infertility treatment. Wiadomosci Lekarskie 2004;57 Suppl 1:301-4. [PMID: ] [PubMed] [Google Scholar]
Strowitzki 2012 {published data only}
- Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Detailed analysis of a randomized, multicenter, comparative trial of dienogest versus leuprolide acetate in endometriosis. International Journal of Gynecology and Obstetrics 2012;117(3):228-233. [DOI: 10.1016/j.ijgo.2012.01.009] [DOI] [PubMed] [Google Scholar]
- Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Human Reproduction January 2010;25(3):633-641. [DOI: 10.1093/humrep/dep46] [DOI] [PubMed] [Google Scholar]
Surrey 1992 {published data only}
- Surrey ES, Judd HL. Reduction of vasomotor symptoms and bone mineral density loss with combined norethindrone and long-acting gonadotropin-releasing hormone agonist therapy of symptomatic endometriosis: A prospective randomized trial. Journal of Clinical Endocrinology and Metabolism 1992;75(2):558-563. [DOI: 10.1210/jcem.75.2.1386374] [DOI] [PubMed] [Google Scholar]
Surrey 2002 {published data only}
- Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstetrics and Gynecology 2002;99(5 Pt 1):709-719. [DOI: 10.1016/s0029-7844(02)01945-2] [DOI] [PubMed] [Google Scholar]
Tahara 2000 {published data only}
- Tahara M, Matsuoka T, Yokoi T, Tasaka K, Kurachi H, Murata Y. Treatment of endometriosis with a decreasing dosage of a gonadotropin-releasing hormone agonist (nafarelin): a pilot study with low-dose agonist therapy ("draw-back" therapy). Fertility & Sterility 2000;73(4):799-804. [DOI: 10.1016/s0015-0282(99)00636-6] [DOI] [PubMed] [Google Scholar]
Tang 2017 {published data only}
- Tang H, Wu R, Li X, Zhou Y, Liu Z, Wang C, Chen Y, et al. Curative effect of 1.88-mg and 3.75-mg gonadotrophin-releasing hormone agonist on stage III–IV endometriosis: Randomized controlled study. The Journal of Obstetrics and Gynaecology Research October 2017;43(10):1550-1554. [DOI: 10.1111/jog.13420] [DOI] [PubMed] [Google Scholar]
Tummon 1988 {published data only}
- Tummon IS, Ali A, Pepping ME, Radwanska E, Binor Z, Dmowski WP. Bone mineral density in women with endometriosis before and during ovarian suppression with gonadotropin-releasing hormone agonists or danazol. Fertility & Sterility May 1988;49(5):792-796. [PMID: ] [PubMed] [Google Scholar]
Tummon 1989 {published data only}
- Tummon IS, Pepping ME, Binor Z, Radwanska E, Dmowski WP. A randomized, prospective comparison of endocrine changes induced with intranasal leuprolide or danazol for treatment of endometriosis. Fertility & Sterility 1989;51(3):390-4. [DOI: 10.1016/s0015-0282(16)60542-3] [DOI] [PubMed] [Google Scholar]
Vercellini 1994 {published data only}
- Vercellini P, Trespidi L, Panazza S, Bramante T, Mauro F, Crosignani PG. Very low dose danazol for relief of endometriosis-associated pelvic pain: a pilot study. Fertility & Sterility 1994;62(6):1136-42. [PMID: ] [PubMed] [Google Scholar]
Vercellini 1996 {published data only}
- Vercellini P, Soma M, Moro GL. Gestrinone versus a gonadotropin-releasing hormone agonist for the treatment of pelvic pain associated with endometriosis: A multicenter, randomized, double-blind study. Fertility & Sterility 196;66(6):911-919. [DOI: 10.1016/s0015-0282(16)58682-8] [DOI] [PubMed] [Google Scholar]
Wheeler 1992 {published data only}
- Wheeler JM, Knittle JD, Miller JD. Depot leuprolide acetate versus danazol in the treatment of women with symptomatic endometriosis: a multicenter, double-blind randomized clinical trial. II. Assessment of safety. The Lupron Endometriosis Study Group. American Journal of Obstetrics & Gynecology 1993;169(1):26-33. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Knittle JD, Miller JD. Depot leuprolide versus danazol in treatment of women with symptomatic endometriosis. American Journal of Obstetrics & Gynecology 1992;167(5):1367-71. [DOI: 10.1016/0002-9378(93)90126-4.] [DOI] [PubMed] [Google Scholar]
Whitehouse 1990 {published data only}
- Whitehouse RW, Adams JE, Bancroft K, Vaughan-Williams CA, Elstein M. The effects of nafarelin and danazol on vertebral trabecular bone mass in patients with endometriosis. Clinical Endocrinology 1990;33(3):365-373. [DOI: 10.1016/s0015-0282(16)58682-8] [DOI] [PubMed] [Google Scholar]
Zupi 2005 {published data only}
- Zupi E, Sbracia M, Marconi D, Sorrenti G, Zullo F, Palomba S. Role of medical therapy in the treatment of endometriosis associated pelvic pain: a randomized controlled study. Journal of Minimally Invasive Gynecology 2005;12(5):S6. [DOI: 10.3390/jcm10051085] [DOI] [Google Scholar]
References to studies excluded from this review
Acien 1989 {published data only}
- Acien P, Shaw RW, Irvine L, Burford GRG. CA 125 levels in endometriosis patients before, during and after treatment with danazol or LHRH agonists. European Journal of Gynaecological Oncology 1989;32(1):241-6. [DOI] [PubMed] [Google Scholar]
Adiyono 2006 {published data only}
- Adiyono W, Adisusianto I. The impact of combination laparoscopic surgery and GNRH analog on quality of life endometriosis patients. In: XVIII FIGO World Congress of Gynecology and Obstetrics. Vol. 2. 5-10 November Kuala Lumpur, Malaysia, 2006:143.
Agarwal 2015 {published data only}
- Agarwal AK, Daniels A, Drosman SR, Udoff L, Foster WG, Pike MC, et al. Treatment of endometriosis with the GnRHa deslorelin and add-back estradiol and supplementary testosterone. BioMed Research International 2015;2015:1-9. [PMID: 10.1155/2015/934164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Azemi 2009 {published data only}
- Al-Azemi M, Jones G, Sirkeci F, Walters S, Houdmont M, Ledger W. Immediate and delayed add-back hormonal replacement therapy during ultra long GnRH agonist treatment of chronic cyclical pelvic pain. British Journal of Obstetrics and Gynaecology 2009;116:1646-1656. [DOI: 10.1111/j.1471-0528.2009.02319.x] [DOI] [PubMed] [Google Scholar]
Bergqvist 1990 {published data only}
- Bergquist C. Effects of nafarelin versus danazol on lipids and calcium metabolism. American Journal of Obstetrics and Gynecology 1990;162(2):589-591. [DOI: 10.1016/0002-9378(90)90438-d] [DOI] [PubMed] [Google Scholar]
Calvo 2000 {published data only}
- Calvo Lugo GE, Sauceda Gonzalez LF, Jimenez Perea ML, Diaz Arias FJ. Treatment of pelvic endometriosis with goserelin acetate or nafarelin acetate. Comparative study. Ginecologia y Obstetricia de Mexico 2000;68:7-14. [PubMed] [Google Scholar]
Chan 1993 {published data only}
- Chan CLK, Soon SB, Loh FH. Comparative study of gestrinone, danazol and decapeptyl CR in the treatment of endometriosis. 2nd International Scientific Meeting of the Royal College of Obstetricians 1993:82. [Google Scholar]
Chen 2009 {published data only}
- Chen Q-Y, Bian M-L, Qiao J, Zhang Z-Y, Lin J-F, Zuo Y-W. Randomized blind, parallel-controlled and multiple centre clinical trial on the effectiveness and safety of leuprolide acetate in the treatment of endometriosis. Chinese Journal of New Drugs - Zhongguo 2009;18(9):797-801. [PubMed] [Google Scholar]
Choktanasiri 2001 {published data only}
- Choktanasiri W, Rojanasakul A. Buserelin acetate implants in the treatment of pain in endometriosis. Journal of the Medical Association of Thailand 2001;84(5):656-60. [PubMed] [Google Scholar]
Claesson 1989 {published data only}
- Claesson B, Bergquist C. Clinical experience treating endometriosis with nafarelin. Journal of Reproductive Medicine 1989;34(12 Suppl):1025-1028. [PMID: ] [PubMed] [Google Scholar]
Cooke 1989 {published data only}
- Cooke ID, Thomas EJ. The medical treatment of mild endometriosis. Acta Obstetricia et Gynecologica Scandinavica - Supplement 1989;150:27-30. [PubMed] [Google Scholar]
Dawood 1990 {published data only}
- Dawood MY. A comparison of the efficacy and safety of buserelin vs danazol in the treatment of endometriosis. Current Concepts in Endometriosis 1990:253-67. [PubMed] [Google Scholar]
Dmowski 1989 {published data only}
- Dmowski WP. Comparitive study of buserelin versus danazol in the management of endometriosis. Gynecological Endocrinology 1989;3(Suppl 2):21-31. [Google Scholar]
Dodin 1991 {published data only}
- Dodin S, Lemay A, Maheux R, Dumont M, Turcot-Lemay L. Bone mass in endometriosis patients treated with GnRH agonist implant or danazol. Obstetrics and Gynecology 1991;77(3):410-415. [PMID: ] [PubMed] [Google Scholar]
Donnez 1989 {published data only}
- Donnez J, Nisolle-Pochet M, Clerckx-Braun F, Sandow J, Casanas-Roux F. Administration of nasal buserelin as compared with subcutaneous buserelin implant for endometriosis. Fertility & Sterility 1989;52(1):27-30. [DOI] [PubMed] [Google Scholar]
Donnez 2004 {published data only}
- Donnez J, Dewart PJ, Hedon B, Perino A, Schindler AE, Blumberg J, et al. Equivalence of the 3-month and 28-day formulations of triptorelin with regard to achievement and maintenance of medical castration in women with endometriosis. Fertility & Sterility 2004;81(2):297-304. [DOI] [PubMed] [Google Scholar]
Eldred 1992 {published data only}
- Eldred JM, Haynes PJ, Thomas EJ. A randomized double blind placebo controlled trial of the effects on bone metabolism of the combination of nafarelin acetate and norethisterone. Clinical Endocrinology 1992;37:354-359. [DOI: 10.1111/j.1365-2265.1992.tb02338.x] [DOI] [PubMed] [Google Scholar]
el‐Roeiy 1988 {published data only}
- el-Roeiy A, Dmowski WP, Gleicher N, Radwanska E, Harlow L, Binor Z, et al. Danazol but not gonadotropin-releasing hormone agonists suppresses autoantibodies in endometriosis. Fertility & Sterility 1988;50(6):864-71. [DOI] [PubMed] [Google Scholar]
Fedele 1993 {published data only}
- Fedele L, Bianchi S, Bocciolone L, Di Nola G, Franchi D. Buserelin acetate in the treatment of pelvic pain associated with minimal and mild endometriosis: a controlled study. Fertility & Sterility 1993;59(3):516-21. [DOI] [PubMed] [Google Scholar]
Fernandez 2004 {published data only}
- Fernandez H, Lucas C, Hédon B , Meyer JL, Mayenga JM and Roux C. One year comparison between two add-back therapies in patients treated with a GnRH agonist for symptomatic endometriosis: a randomized double-blind trial. Human Reproduction April 2004;19:1465-1471. [DOI: 10.1093/humrep/deh250] [DOI] [PubMed] [Google Scholar]
Ferrero 2011 {published data only}
- Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reproductive Biology and Endocrinology 2011;9:1-7. [DOI: 10.1186/1477-7827-9-88] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franssen 1992 {published data only}
- Franssen AM, Heijden PF, Thomas CM, Doesburg WH, Willemsen WN, Rolland R. On the origin and significance of serum CA-125 concentrations in 97 patients with endometriosis before, during, and after buserelin acetate, nafarelin, or danazol. Fertility & Sterility 1992;57(5):974-9. [DOI] [PubMed] [Google Scholar]
Fraser 1996 {published data only}
- Fraser IS, Healy DL, Torode H, Song JY, Mamers P, Wilde F. Depot goserelin and danazol pre-treatment before rollerball endometrial ablation for menorrhagia. Obstetrics & Gynecology 1996;87(4):544-50. [DOI] [PubMed] [Google Scholar]
Harada 2000 {published data only}
- Harada T. Empirical leuprolide treatment of women with suspected endometriosis was effective in reducing chronic pain. Evidence-based Obstetrics and Gynecology 2000;2:45. [Google Scholar]
Henzl 1990a {published data only}
- Henzl MR, Monroe SE. Nafarelin: a new medical therapy for endometriosis. Progress in Clinical & Biological Research 1990;323:343-55. [PubMed] [Google Scholar]
Imani 2009 {published data only}
- Imani R, Thai-Cuarto D, Jimenez R, Burke J, Kroll R, O'Brien C. Petal study: Safety, tolerability and effectiveness of elagolix, an oral GnRH antagonist for endometriosis. In: Fertility & Sterility. Vol. 92. 2009:S111-S112. [DOI: 10.1016/j.fertnstert.2009.07.1100] [DOI]
Lindsay 1996 {published data only}
- Lindsay PC, Shaw RW, Bennink HJC, Kicovic P. The effect of add-back treatment with tibolone (Livial) on patients treated with the gonadotropin-releasing hormone agonist triptorelin (decapeptyl). Fertility & Sterility 1996;65(2):342-348. [DOI: 10.1016/s0015-0282(16)58096-0] [DOI] [PubMed] [Google Scholar]
Luciano 2004 {published data only}
- Luciano AA. Leuprolide acetate in the management of endometriosis-associated pain: A multicenter, evaluator-blind, comparative clinical trial. Global Congress of Gynecologic Endoscopy 33rd Annual Meeting of the AAGL "Advancing Minimally Invasive Gynecology Worldwide" 2004;11(Suppl 3):s5. [Google Scholar]
Magini 1993 {published data only}
- Magini A, Pellegrini S, Tavella K, Forti G, Massi GB, Serio M. Estrogenic suppression by different administration schedules of goserelin depot for treatment of endometriosis. Journal of Endocrinological Investigation 1993;16(10):775-80. [DOI] [PubMed] [Google Scholar]
Maouris 1991 {published data only}
- Maouris P, Dowsett M, Nichols J, Rose G, Edmonds DK. Pseudomenopause treatment for endometriosis: The endocrine effects of danazol compared with the use of the LH-RH agonist goserelin. Journal of Obstetrics & Gynaecology 1991;11:123-127. [DOI: 10.1016/s0015-0282(16)58023-6] [DOI] [Google Scholar]
- Maouris P, Dowsett M, Rose G, D E. Comparison of the endocrine effects of danazol and the LHRH agonist goserelin (Zoladex) in the treament of endometriosis. Silver Jubilee British Congress of Obstetrics and Gynaecology 1989:61. [Google Scholar]
Matalliotakis 2000 {published data only}
- Matalliotakis IM, Neonaki MA, Koumantaki YG, Goumenou AG, Kyriakou DS, Koumantakis EE. A randomized comparison of danazol and leuprolide acetate suppression of serum-soluble CD23 levels in endometriosis. Obstetrics & Gynecology 2000;95(6 Pt 1):810-813. [DOI: 10.1016/s0029-7844(99)00635-3] [DOI] [PubMed] [Google Scholar]
Matalliotakis 2004 {published data only}
- Matalliotakis IM, Arici A, Goumenou AG, Katassos T, Karkavitsas N, Koumantakis EE. Comparison of the effects of leuprorelin acetate and danazol treatments on serum CA-125 levels in women with endometriosis. International Journal of Fertility & Womens Medicine 2004;49(2):75-8. [PubMed] [Google Scholar]
Matta 1988 {published data only}
- Matta W, Shaw R. A comparative study between buserelin and danazol in the treatment of endometriosis. The British Journal of Clinical Practice 1988;40(4):69-72. [Google Scholar]
Miller 1990 {published data only}
- Miller JD. Leuprolide acetate for the treatment of endometriosis. Progress in Clinical & Biological Research 1990;323:337-41. [PubMed] [Google Scholar]
Mukherjee 1996 {published data only}
- Mukherjee T, Barad D, Turk R, Reeman R. A randomized, placebo-controlled study on the effect of cyclic intermittent etidronate therapy on the bone mineral density changes associated with six months of gonadotropin-releasing hormone agonist treatment. American Journal of Obstetrics and Gynecology 1997;175(1):105-109. [DOI: 10.1016/S0002-9378(96)70258-2] [DOI] [PubMed] [Google Scholar]
Newton 1996 {published data only}
- Newton C, Slota D, Yuzpe AA, Tummon IS. Memory complaints associated with the use of gonadotropin-releasing hormone agonists: a preliminary study. Fertility & Sterility 1996;65(6):1253-5. [DOI] [PubMed] [Google Scholar]
Pierce 2000 {published data only}
- Pierce SJ, Gazvani RM, Farquharson RG. Long-term use of gonadotropin-releasing hormone analogs and hormone replacement therapy in the management of endometriosis: a randomized trial with a 6-year follow-up. Fertility & Sterility Nov 2000;74(5):964-968. [DOI: 10.1016/s0015-0282(00)01537-5] [DOI] [PubMed] [Google Scholar]
Ripps 2003 {published data only}
- Ripps BA, VanGilder K, Minhas B, Welford M, Mamish Z. Alendronate for the prevention of bone mineral loss during gonadotropin-releasing hormone agonist therapy. The Journal of Reproductive Medicine October 2003 ;48(10):761-766. [PMID: ] [PubMed] [Google Scholar]
Shaw 1992 {published data only}
- Shaw RW. A randomised comparative study of the effects of goserelin and danazol for the treatment of endometriosis. Gynecological Endocrinology 1990;4(70 Suppl 2):45. [Google Scholar]
- Shaw RW. An open randomized comparative study of the effect of goserelin depot and danazol in the treatment of endometriosis. Zoladex Endometriosis Study Team. Fertility & Sterility 1992;58(2):265-272. [DOI: 10.1016/s0015-0282(16)55205-4] [DOI] [PubMed] [Google Scholar]
- Shaw RW. Goserelin depot: an analog of LHRH for the treatment of endometriosis. Drugs Under Experimental & Clinical Research 1990;16(Suppl):69-75. [PubMed] [Google Scholar]
Shaw 2001 {published data only}
- Shaw R, Garry R, McMillan L, Sutton C, Wood S, Harrison R, et al. A prospective randomized open study comparing goserelin (Zoladex) plus surgery and surgery alone in the management of ovarian endometriomas. Gynaecological Endoscopy 2001;10(3):151-7. [Google Scholar]
Somekawa 1999 {published data only}
- Somekawa Y, Chigughi M, Harada M, Ishibashi T. Use of vitamin K2 (Menatetrenone) and 1,25- dihydroxyvitamin D3 in the prevention of bone loss induced by leuprolide. The Journal of Clinical Endocrinology & Metabolism Aug 1999;84(8):2700-2704. [DOI: 10.1210/jcem.84.8.5920] [DOI] [PubMed] [Google Scholar]
Sorensen 1997 {published data only}
- Sorensen SS, Colov NP, Vejerslev LO. Pre- and postoperative therapy with GnRH agonist for endometrial resection. A prospective, randomized study. Acta Obstetricia et Gynecologica Scandinavica 1997;76(4):340-4. [DOI] [PubMed] [Google Scholar]
Sowter 1997 {published data only}
- Sowter MC, Bidgood K, Richardson JA. A prospective randomized trial of the effect of preoperative endometrial inhibition on the long-term outcome of transcervical endometrial resection. Gynaecological Endoscopy 1997;6(1):33-7. [Google Scholar]
Soysal 2004 {published data only}
- Soysal S, Soysal ME, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Human Reproduction 2004;19(1):160-167. [DOI: 10.1093/humrep/deh035] [DOI] [PubMed] [Google Scholar]
Surrey 1993 {published data only}
- Surrey ES, Fournet N, Voigt B, Judd HL. Effects of sodium etidronate in combination with low-dose norethindrone in patients administered a long-acting GnRH agonist: a preliminary report. Obstetrics & Gynecology 1993;81(4):581-6. [PubMed] [Google Scholar]
Surrey 1995 {published data only}
- Surrey ES, Voigt B, Fournet N, Judd HL. Prolonged gonadotropin-releasing hormone agonist treatment of symptomatic endometriosis: the role of cyclic sodium etidronate and low-dose norethindrone "add-back" therapy. Fertility & Sterility 1995;63(4):747-55. [PubMed] [Google Scholar]
Takaesu 2013 {published data only}
- Takaesu Y, Nishi H, Kojima J, Sasaki T, Nagamitsu Y, Kato R, et al. Dienogest compared with gonadotropin-releasing hormone agonist after conservative surgery for endometriosis. The Journal of Obstetrics and Gynaecology Research Sept 2016;42(9):1152-1158. [DOI: 10.1111/jog.13023] [DOI] [PubMed] [Google Scholar]
Tapanainen 1993 {published data only}
- Tapanainen J, Hovatta O, Juntunen K, Martikainen H, Ratsula K, Tuppala M, et al. Subcutaneous goserelin versus intranasal buserelin for pituitary down-regulation in patients undergoing IVF: a randomized comparative study. Human Reproduction 1993;8(12):2052-5. [DOI] [PubMed] [Google Scholar]
Taskin 1997 {published data only}
- Taskin O, Yalcinoglu AI, Kucuk S, Uryan I, Buhur A, F B. Effectiveness of tibolone on hypoestrogenic symptoms induced by goserelin treatment in patients with endometriosis. Fertility & Sterility 1997;67(1):40-5. [DOI] [PubMed] [Google Scholar]
Toomey 2003 {published data only}
- Toomey C, Krauss B, Hammerschlag R, Burry K. Endometriosis: traditional medicine vs hormone therapy. National Centre for Complementary and Alternative Medicine 2003.
Valimaki 1989 {published data only}
- Valimaki M, Nilsson CG, Roine R, Ylikorkala O. Comparison between the effects of nafarelin and danazol on serum lipids and lipoproteins in patients with endometriosis. The Journal of Clinical Endocrinology and Metabolism 1989;69(6):1097-103. [DOI: 10.1210/jcem-69-6-1097] [DOI] [PubMed] [Google Scholar]
Vercellini 2009 {published data only}
- Vercellini P, Somigliana E, Vigano P, Abbiati A, Barbara G, Crosignani PG. Endometriosis: current therapies and new pharmacological developments. Drugs 2009;69(6):649-75. [DOI] [PubMed] [Google Scholar]
Warnock 1998 {published data only}
- Warnock JK, Bundren JC, Morris DW. Depressive symptoms associated with gonadotropin-releasing hormone agonists. Depression and Anxiety 1998;7(4):171-7. [DOI] [PubMed] [Google Scholar]
Wright 1995 {published data only}
- Wright S, Valdes CT, Dunn RC, Franklin RR. Short-term lupron or danazol therapy for pelvic endometriosis. Fertility & Sterility 1995;63(3):504-7. [PMID: ] [PubMed] [Google Scholar]
Yee 1986 {published data only}
- Yee B. A preliminary report on the comparative use of buserelin (Hoe 766) and danazol in the treatment of endometriosis: the university of Southern California experience. Progress in Clinical & Biological Research 1986;225:175-88. [PubMed] [Google Scholar]
Ylikorkala 1995 {published data only}
- Ylikorkala O, Tiitinen A, Hulkko S, Kivinen S, Nummi S. Decrease in symptoms, blood loss and uterine size with nafarelin acetate before abdominal hysterectomy: a placebo-controlled, double-blind study. Human Reproduction 1995;10(6):1470-4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Aisaka 2000 {published data only}
- Aisaka K, Nakagawa K, Uesato T, Miwa A, Koshino T, Ooka F, et al. Effectiveness of long term GN-RH agonist administration for treatment of endometriosis combined with estrogen-progestogen add back therapy. In: XVI FIGO World Congress of O & G 2000. 2000. [DOI: ]
Archer 2004 {published data only}
Gregoriou 1997 {published data only}
- Gregoriou O, Konidaris S, Vitoratos N, Papadias C, Papoulias I, Chryssicopoulos A. Gonadotropin-releasing hormone analoque (leuprolide) plus hormone replacement therapy for the treatment of endometriosis: a randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 1997;67:no pagination. [PMID: ] [PubMed] [Google Scholar]
Long 2009 {published data only}
- Long Q, Zhang S. A randomized clinical trial of GnRHa and add-back therapy in the treatment of endometriosis. In: International Journal of Gynecology and Obstetrics . Vol. 107. 2009:S247. [DOI: 10.1016/S0020-7292] [DOI]
Vella 1995 {published data only}
- Vella A, Brincat M, Galea R, Muscat Baron Y. Skin thickness and bone density: effect of add-back therapy in women on GnRh analogue. In: 27th British Congress of Obstetrics and Gynaecology. Vol. 155. 1995.
Additional references
Audebert 1998
- Audebert A, Descamps P, Marret H, Ory-Lavollee L, Bailleul F, Hamamah S. Pre or post-operative medical treatment with nafarelin in stage III-IV endometriosis: a French multicenter study. European Journal of Obstetrics, Gynecology and Reproductive Biology 1998;79(2):145-48. [DOI: 10.1016/s0301-2115(98)00028-1] [DOI] [PubMed] [Google Scholar]
Bafort 2020
- Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JMN. Laparoscopic surgery for endometriosis. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD011031. [DOI: 10.1002/14651858.CD011031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batt 2007
- Batt RE, Smith RA, Buck Louis GM, Martin DC, Chapron C, Koninckx PR, Yeh J. Müllerianosis. Histology & Histopathology 22-10-2007;10:1161-6. [DOI] [PubMed] [Google Scholar]
Becker 2022
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, Hanegem N, Vulliemoz N, Vermeulen N, ESHRE Endometriosis Guideline Group. ESHRE guideline: management of women with endometriosis.. Human Reproduction Open 2022;2:1-26. [DOI: 10.1093/hropen/hoac009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bontis 1997
- Bontis JN, Vavilis DT. Etiopathology of endometriosis. Annals of the New York Academy of Sciences 17-06-1997;816:305-9. [DOI] [PubMed] [Google Scholar]
Brown 2012
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD002122. [DOI: 10.1002/14651858.CD002122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2018
- Brown J, Crawford TJ, Datta S, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD001019. [DOI: 10.1002/14651858.CD001019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burney 2012
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertility and Sterility 2012;3:511-9. [DOI: 10.1016/j.fertnstert.2012.06.029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020
- Chen I, Veth VB, Choudhry AJ, Murji A, Zakhari A, Black AY, et al. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD003678. [DOI: 10.1002/14651858.CD003678.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darwish 2006
- Darwish A, Hassanin MS, Abou Sekkin IA. Epidemiology and risk factors associated with laparoscopicaly diagnosed typical and atypical endometriosis among Egyptian women. Middle East Fertility Society Journal 2006;11:196-201. [Google Scholar]
Duffy 2020
- Duffy JMN, Hirsch M, Vercoe M, Abbott J, Barker C, Collura B, et al. A core outcome set for future endometriosis research: an international consensus development study [A core outcome set for future endometriosis research: an international consensus development study]. British Journal of Obstetrics and Gynaecology 2020;127(8):967-74. [DOI: 10.1111/1471-0528.16157] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eskenazi 1997
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America 1997;24:235-58. [DOI: 10.1016/s0889-8545(05)70302-8 Abstract] [PMID: ] [DOI] [PubMed] [Google Scholar]
Flower 2012
- Flower A, Liu JP, Lewith G, Little P, Li Q. Chinese herbal medicine for endometriosis. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD006568. [DOI: 10.1002/14651858.CD006568.pub3] [DOI] [PubMed] [Google Scholar]
Fu 2017
- Fu J, Song H, Zhou M, Zhu H, Wang Y, Chen H, Huang W. Progesterone receptor modulators for endometriosis. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD009881. [DOI: 10.1002/14651858.CD009881.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guidice 2010
- Giudice LC. Clinical practice: endometriosis. New England Journal of Medicine 2010;25:2389-98. [DOI: 10.1056/NEJMcp1000274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Houda 2014
- Houda MR, Grant NH. Gonadotrophin antagonists for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD011446. [DOI: 10.1002/14651858.CD011446] [DOI] [Google Scholar]
Jackson 2006
- Jackson B, Telner DE. Managing the misplaced: approach to endometriosis. Canadian Family Physician 2006;11:1420-1424. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Kalaitzopoulos 2021
- Kalaitzopoulos DR, Samartzis N, Kolovos GN, Mareti E, Samartzis EP, Eberhard M, Dinas K, Daniilidis A. Treatment of endometriosis: a review with comparison of 8 guidelines. BMC Womens Health 2021;1:397. [DOI: 10.1186/s12905-021-01545-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2014
- Klein S, D'Hooghe T, Meuleman C, Dirksen C, Dunselman G, Simoens S. What is the societal burden of endometriosis-associated symptoms? a prospective Belgian study. Reproductive Biomedical Online 2013;28(1):116-224. [DOI: 10.1016/j.rbmo.2013.09.020] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Levander 1955
- Levander G, Normann P. The pathogenesis of endometriosis; an experimental study. Acta Obstetricia et Gynecologica Scandinavica 1955;34(4):366-98. [DOI: 10.3109/00016345509158287] [DOI] [PubMed] [Google Scholar]
Macer 2012
- Macer ML and Taylor HS. Endometriosis and Infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstetrics and Gynecology Clinics of North America 2013;39(4):535-549. [DOI: 10.1016/j.ogc.2012.10.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmood 1991
- Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Human Reproduction 1991;6:544-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matthias 1996
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC , Steege JF . Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstetrics and Gynecology 1996;87(3):321-7. [DOI: 10.1016/0029-7844(95)00458-0] [DOI] [PubMed] [Google Scholar]
Meuleman 2009
- Meuleman C, Vandenabeele B, Fieuws S, Spiessend C, Timmermans D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners.. Fertility and Sterility 2009;92(1):68-74. [DOI: 10.1016/j.fertnstert.2008.04.056] [DOI] [PubMed] [Google Scholar]
Rafique 2017
- Rafique S, Decherney AH. Medical Management of Endometriosis. Clinical Obstetrics and Gynecology 2017;3:485-496. [DOI: 10.1097/GRF.0000000000000292] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robboy 2010
- Robboy, S J, & Bean, S M. Pathogenesis of endometriosis. Reproductive Biomedicine Online 01-07-2010;21(1):4-5. [DOI] [PubMed] [Google Scholar]
Sampson 1940
- Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. American Journal of Obstetrics and Gynecology October 01, 1940;40(4):549-557. [DOI: 10.1016/S0002-9378(40)91238-8] [DOI] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook 2021.
Shaw 1991
- Shaw RW. GnRH analogues in the treatment of endometriosis -rationale and efficacy. London: Kluwer Academic Publishers, 1991. [Google Scholar]
Simoens 2012
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Human Reproduction 2021;27(5):1292-9. [DOI: 10.1093/humrep/des073] [DOI] [PubMed] [Google Scholar]
Stratton 2011
- Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human Reproduction Update 2011;17(3):327–46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Linden 1997
- Linden PJ. Theories on the pathogenesis of endometriosis. Human Reproduction 1996;3:53-65. [DOI: 10.1093/humrep/11.suppl_3.53.] [DOI] [PubMed] [Google Scholar]
Van Hoesel 2021
- Van Hoesel MH, Chen YL, Zheng A, Wan Q, Mourad SM. Selective oestrogen receptor modulators (SERMs) for endometriosis. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD011169. [DOI: 10.1002/14651858.CD011169.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vercellini 2014
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nature Reviews, Endocrinology 2014;5:261-75. [DOI: 10.1038/nrendo.2013.255] [DOI] [PubMed] [Google Scholar]
Vigano 2018
- Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Human Reproduction 2018;1(33(3)):347-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Viganò 2004
- Viganò P, Parazzini F, Somigliana E, Vercellini P, . Endometriosis: epidemiology and aetiological factors. Best Practice & Research Clinical Obstetrics & Gynaecology 2004;18(2):177-200. [DOI: 10.1016/j.bpobgyn.2004.01.007] [DOI] [PubMed] [Google Scholar]
Wheeler 1989
- Wheeler JM. Epidemiology of endometriosis-associated infertility. Journal of Reproductive Medicine 1989;34:41-6. [PMID: ] [PubMed] [Google Scholar]
Whitehouse 1990
- Whitehouse RW, Adams JE, Bancroft K, Vaughan-Williams CA, Elstein M. The effects of nafarelin and danazol on vertebral trabecular bone mass in patients with endometriosis.. Clinical Endocrinology 1990;3(33):365-73. [PMID: MEDLINE: 91070821] [DOI] [PubMed] [Google Scholar]
Ylikorkala 1990
- Ylikorkala O, Nilsson G, Hirvonen E, Viinikka L. Evidence of similar increases in bone turnover during nafarelin and danazol use in women with endometriosis. Gynaecological Endocrinology 1990;4(4):251-60. [PMID: MEDLINE: 91188927] [DOI] [PubMed] [Google Scholar]
Zhu 2011
- Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD007864. [DOI: 10.1002/14651858.CD007864.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zondervan 2020
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. New England Journal of Medicine 2020 Mar 26;382(13):1244-1256. [DOI: 10.1056/NEJMra1810764] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brown 2010
- Brown J, Pan A, Hart RJ. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD008475. [DOI: 10.1002/14651858.CD008475.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farmer 2003
- Farmer JE, Prentice A, Breeze A. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD001297. [DOI: 10.1002/14651858.CD001297] [DOI] [PMC free article] [PubMed] [Google Scholar]


