Abstract
The past decade has brought significant advances in our understanding of the molecular mechanisms of thyroid carcinogenesis. Among thyroid carcinomas, the most successful class of targeted therapeutics appears to be selective kinase inhibitors. Actionable kinase fusions arise in around 10–15% of cases of thyroid cancer, a significant subset. A cohort of molecular testing platforms, both commercial and laboratory-derived, has been introduced into clinical practice to identify patients with targetable tumors, requiring pathologists to develop an integrative approach that utilizes traditional diagnostic cytopathology and histopathology, immunohistochemistry, and cutting-edge molecular assays for optimal diagnostic, prognostic, and therapeutic efficiency. Furthermore, there has been increasing scrutiny of the clinical behavior of kinase fusion–driven thyroid carcinoma (KFTC), still regarded as papillary thyroid carcinomas, and in characterizing molecular predictors of kinase inhibitor resistance with an aim to establish standardized, evidence-based treatment regimens. This review presents an overview of the current literature on the clinicopathologic and molecular features of KFTC as well as the latest investigational progress and encountered challenges for this unique subset of thyroid neoplasias.
Keywords: Kinase fusion, NTRK, RET, Inhibitor, Resistance, Thyroid cancer
Introduction
The thyroid gland is a frequent site for human cancer, particularly in women, and differentiated thyroid carcinomas, most commonly papillary thyroid carcinomas (PTCs) followed by follicular thyroid carcinomas (FTCs), account for nearly all cases. Although PTC and FTC are usually cured by surgery alone, lesions that are large display “high-risk” histology and have residual disease, and locoregional or distant metastasis may require additional treatment, the most traditional adjuvant therapeutic being radioactive iodine (RAI). Furthermore, de novo and acquired RAI resistance may develop as a harbinger of therapeutic failure and poor outcomes, with merely 10% of these patients reaching 10-year survival [1]. High-grade thyroid carcinomas, such as poorly differentiated thyroid carcinomas, differentiated high-grade thyroid carcinomas, and anaplastic thyroid carcinomas, are generally RAI-refractory with unfavorable 5-year disease-specific survival (50–60% and nearly none, respectively [2, 3]). Fortunately, recent pharmaceutical developments have brought a growing armamentarium of molecularly targeted therapy, mainly selective kinase inhibitors (Table 1) [4–13], which has revolutionized the clinical landscape of RAI-refractory thyroid cancer.
Table 1.
FDA-approved targeted therapy for thyroid cancer
| Agent | Target(s) | Thyroid indication(s) | Trial registration (reference) | Design | Histology (case no.) | ORR | Median PFS |
|---|---|---|---|---|---|---|---|
| Lenvatinib | VEGFR1/2/3, FGFR1/2/3/4, PDGFRα, RET, KIT | RAI-refractory thyroid cancer | SELECT (4) | Phase III | PTC (200), FTC (75), OTC (70), PDTC (47) | 64.8% | 18.3 months (lenvatinib) vs. 3.6 months (placebo) |
| Sorafenib | VEGFR1/2/3, RET, RAF, PDGFRβ, KIT | RAI-refractory thyroid cancer | DECISION (5) | Phase III | PTC (237), FTC (106), PDTC (40), WDC (3) | 12.2% | 10.8 months (sorafenib) vs. 5.8 months (placebo) |
| Cabozantinib | VEGFR1/2/3, RET, MET, FLT3, KIT | DTC failing prior anti-VEGF therapy | COSMIC-311 (6) | Phase III | PTC (102), FTC (90) | 15% | Not reached (cabozantinib) vs. 1.9 months (placebo) |
| MTC | EXAM (7) | Phase III | MTC (330) | 28% | 11.2 months (cabozantinib) vs. 4.0 months (placebo) | ||
| Vandetanib | VEGFR1/2/3, RET, EGFR | MTC | ZETA (8) | Phase III | MTC (331) | 45% | Not reached (vandetanib) vs. 19.3 months (placebo) |
| Dabrafenib, trametinib | BRAF (dabrafenib), MEK (trametinib) | BRAF V600E-mutant solid tumors** | ROAR/ BRF117019 (9) | Phase II | ATC (36) | 56% | 6.7 months |
| Selpercatinib | RET | RET fusion+ solid tumors** | LIBRETTO-001 (10) | Phase I/II | PTC (13), PDTC (3), ATC (2), OTC (1) | 79% | 20.1 months |
| RET-mutant MTC | LIBRETTO-001 (10) | Phase I/II | MTC (55 with and 88 without prior MKI treatment) | 69%, 73% | Not reached (previously treated with MKI); 23.6 months (MKI-naïve) | ||
| Pralsetinib | RET | RET fusion+ thyroid cancer | ARROW (11) | Phase I/II | RET fusion-positive thyroid cancer (11) | 89% | Not reached |
| RET-mutant MTC | ARROW (11) | Phase I/II | 55 with and 21 without prior MKI treatment | 60%, 71% | Not reached | ||
| Larotrectinib | NTRK1/2/3 | NTRK fusion+ solid tumors** | NAVIGATE, SCOUT, LOXO-TRK-14001 (12) | Phase I/II | PTC (20), ATC (7), FTC (2) | 71% | 2.2 months for ATC; not reached for other histologic types |
| Entrectinib | NTRK1/2/3, ROS1, ALK | NTRK fusion+ solid tumors** | ALKA-372–001, STARTRK-1, STARTRK-2 (13) | Phase I/II | NTRK fusion-positive thyroid cancer (5) | 20% | - |
ORR objective response rate, PFS progression-free survival, RAI radioactive iodine, DTC diferentiated thyroid carcinoma, PTC papillary thyroid carcinoma, FTC follicular thyroid carcinoma, PDTC poorly diferentiated thyroid carcinoma, WDC well-diferentiated carcinoma, OTC oncocytic thyroid carcinoma, ATC anaplastic thyroid carcinoma, MTC medullary thyroid carcinoma, MKI multi-kinase inhibitors vandetanib and cabozantinib
Histology-agnostic approval
The molecular pathogenesis of most thyroid carcinomas involves derangements of the mitogen-activated protein kinase (MAPK) and PI3K/Akt/mTOR pathways (Fig. 1). Both pathways can be activated by receptor tyrosine kinases (RTKs) on the cell surface, such as RET, NTRK, ALK, MET, and ROS1, through RAS activation (Fig. 1). BRAF encodes an intracytoplasmic serine-threonine kinase mediator of the MAPK pathway, in which an activating point mutation, p.V600E, accounts for over 50% of adult PTC occurrences [14]. In BRAF p.V600E-negative PTC, rearrangements of various kinase genes, mainly RET (around 28% and 14% in pediatric and adult PTC, respectively), NTRK (15% and 8%), ALK (4–6% and 3%), BRAF (1–19% and 3%), MET, and ROS1 (rare), serve as important tumorigenic drivers (Table 2) [15–38]. Kinase rearrangements have been identified in 9–20% of poorly differentiated thyroid carcinomas (PDTC) [15, 30, 39–41] and 1–6% of anaplastic thyroid carcinomas (ATC) [15, 33, 39, 42]. Follicular-derived carcinomas that lack BRAF p.V600E mutation and kinase rearrangements, such as the majority of FTC, follicular-patterned PTC, PDTC, and ATC, may arise from N/H/KRAS mutations, non-p.V600E BRAF mutations, the PAX8::PPARG fusion, and various alterations of the PI3K/Akt/mTOR pathway [43]. Furthermore, additional fusions have been noted in salivary type carcinomas (CRTC1::MAML) [44] and NUT carcinomas (NSD3::NUTM1) [45, 46]. Changes in the SWI/SNF complexes, histone-modifying enzymes, and mismatch repair proteins are thought to underlie the de novo development or secondary transformation towards high-grade neoplasms (PDTC, DHGTC, and ATC) [43]. Medullary thyroid carcinomas (MTC), derived from parafollicular cells, are mainly driven by RET and RAS mutations but have been found to carry kinase fusions in exceptionally rare cases [47–50].
Fig. 1.
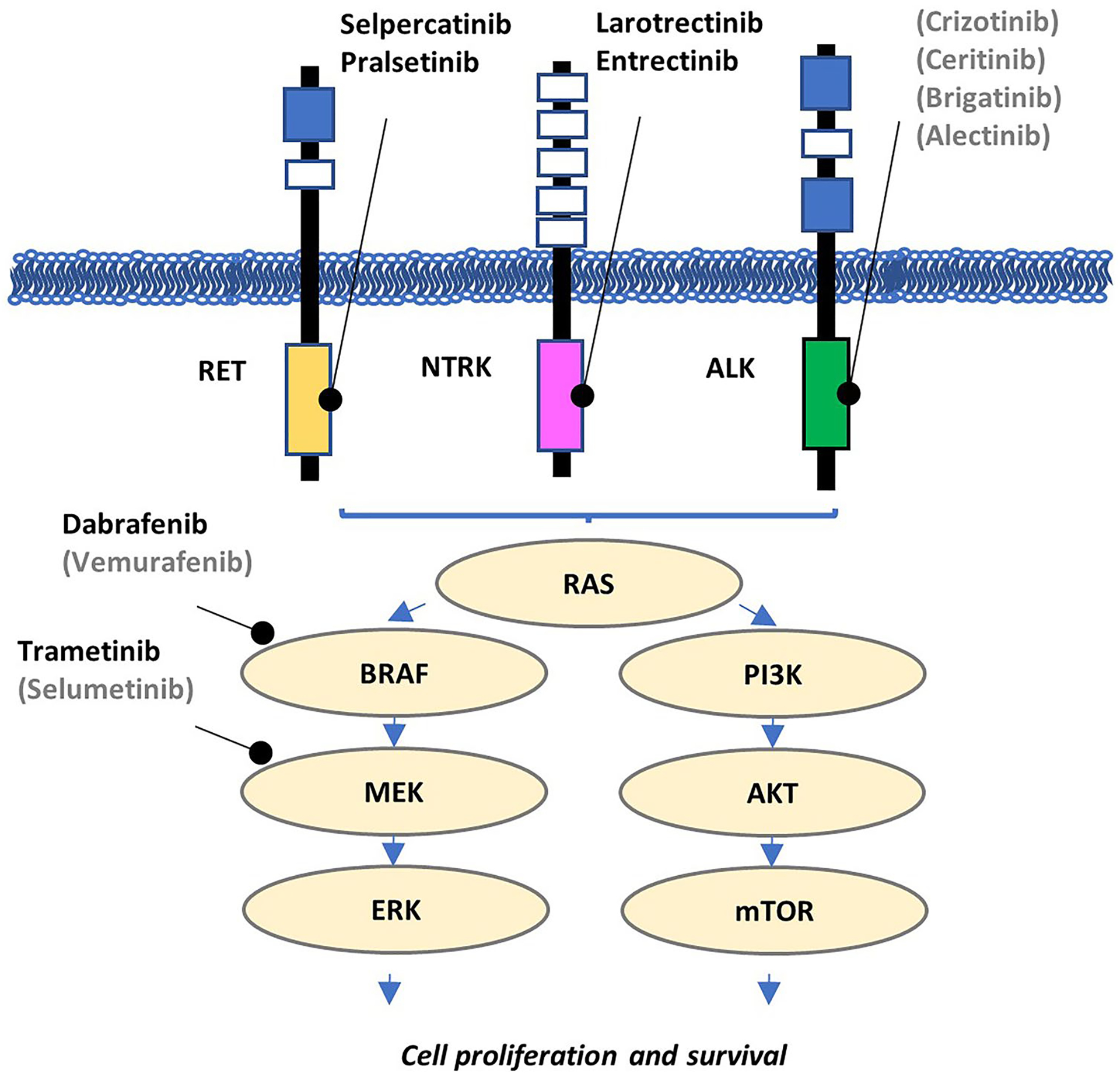
Oncogenic signaling pathways in thyroid carcinogenesis. The mitogen-activated protein kinase (MAPK) and PI3K pathways play a central role in thyroid oncogenesis and harbor the most common targetable molecular drivers. Targeted inhibitors listed in gray and in parentheses have yet to receive FDA approval
Table 2.
| Study | Testing approach | Method | Cohort Size | No. with kinase fusions | % fusion-positive | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK | BRAF | MET | NTRK1 | NTRK2 | NTRK3 | RET | ROS1 | |||||
| Adult papillary thyroid carcinoma | ||||||||||||
| Musholt et al. | Consecutive | RT-PCR | 119 | - | - | - | 15 | - | - | 17 | - | 27% |
| Brzeziańska et al. | Consecutive | RT-PCR | 33 | - | - | - | 4 | - | - | 7 | - | 33% |
| Chou et al. | Consecutive | IHC, FISH | 498 | 14 | - | - | - | - | - | - | - | 3% |
| Park et al. | Consecutive | IHC, FISH, NanoString | 341 | 4 | - | - | - | - | - | - | - | 1% |
| Lu et al. | Consecutive | NGS | 138 | 0 | 1 | - | 1 | - | 2 | 7 | 0 | 8% |
| Lee et al. | Consecutive | IHC, FISH, RT-PCR | 769 | 0 | - | - | 3 | - | - | 16 | - | 2% |
| Bastos et al. | Consecutive | RT-PCR | 116 | 4 | 0 | - | - | - | 6 | - | - | 9% |
| Liang et al. | Consecutive | NGS | 355 | 1 | 1 | 0 | 3 | 0 | 9 | 30 | 0 | 12% |
| Panebianco et al. | Selective | NGS | na | 27 | - | - | - | - | - | - | - | - |
| Chu et al. | Selective | NGS | 212 | 2 | 6 | 2 | 8 | 0 | 10 | 28 | 1 | 27% |
| Lee et al. | Consecutive | IHC, FISH, NGS | 525 | - | - | - | 2 | 0 | 10 | - | - | 2% |
| Nozaki et al. | Consecutive | FISH | 307 | 1 | - | - | 2 | - | 1 | - | 0 | 1% |
| Kong et al. | Consecutive | IHC, FISH | 315 | - | - | - | 3 | 0 | 16 | - | - | 6% |
| Pediatric papillary thyroid carcinoma | ||||||||||||
| Fenton et al. | Consecutive | RT-PCR | 33 | - | - | - | - | - | - | 15 | - | 45% |
| Prasad et al. | Consecutive | NGS | 27 | 0 | 0 | 0 | 1 | - | 6 | 6 | - | 48% |
| Cordioli et al. | Consecutive | RT-PCR | 35 | - | 4 | - | - | - | 3 | 13 | - | 57% |
| Sisdelli et al. | Consecutive | RT-PCR, FISH | 80 | - | 15 | - | - | - | - | - | - | 19% |
| Alzahrani et al. | Consecutive | NGS | 48 | 1 | 0 | 0 | 1 | 0 | 5 | 14 | 0 | 44% |
| Pekova et al. | Consecutive | NGS | 93 | 6 | 2 | 1 | 3 | 0 | 14 | 26 | 0 | 56% |
| Lee et al. | Consecutive | NGS, IHC, FISH | 106 | 6 | 0 | 0 | 2 | 0 | 2 | 20 | 0 | 28% |
| Macerola et al. | Consecutive | NanoString | 163 | 6 | 0 | - | 5 | - | 18 | 17 | - | 28% |
| Rogounovitch et al. | Consecutive | RT-PCR | 34 | - | 0 | - | - | - | 6 | 12 | - | 53% |
| Franco et al. | Consecutive | NGS | 131 | 0 | 1 | 2 | 4 | 0 | 5 | 34 | 0 | 35% |
| Ricarte-Filho et al. | Selective | NGS | 144 | - | - | - | 7 | 0 | 13 | - | - | 14% |
| Radiation-associated papillary thyroid carcinoma | ||||||||||||
| Bounacer et al. | Consecutive | RT-PCR | 15 | - | - | - | 2 | - | - | - | - | 13% |
| Rabes et al. | Consecutive | RT-PCR | 191 | - | - | - | 2 | - | - | 38 | - | 21% |
| Dinets et al. | Consecutive | RT-PCR | 70 | - | - | - | - | - | - | 24 | - | 34% |
| Efanov et al. | Consecutive | NGS | 65 | 5 | 7 | 0 | 2 | 0 | 7 | 22 | 0 | 66% |
| Poorly differentiated thyroid carcinoma | ||||||||||||
| Landa et al. | Selective | NGS | 84 | 3 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 10% |
| Panebianco et al. | Selective | NGS | na | 5 | - | - | - | - | - | - | - | - |
| Duan et al. | Consecutive | NGS | 41 | 1 | 0 | - | 1 | - | - | 6 | - | 20% |
| Chu et al. | Selective | NGS | 23 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 9% |
| Pekova et al. | Consecutive | NGS | 10 | - | - | - | 1 | 0 | 1 | - | - | 20% |
| Anaplastic thyroid carcinoma | ||||||||||||
| Duan et al. | Consecutive | NGS | 25 | 0 | 0 | - | 1 | - | - | 0 | - | 4% |
| Chu et al. | Selective | NGS | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 5% |
| Nozaki et al. | Consecutive | FISH | 16 | 0 | - | - | 1 | - | 0 | - | 0 | 6% |
| Xu et al. | Consecutive | NGS | 360 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 1% |
Kinase rearrangements produce valuable therapeutic targets by causing the fusion of the 3′ adenosine triphosphate (ATP)-binding kinase domain to a 5′ partner sequence that causes deregulated kinase activation through ligand-independent dimerization or loss of autoinhibition. Most partner genes involved in RTK rearrangement contain dimerization domains, such as the coiled-coil domains in NCOA4, TPR, PPL, CCDC6, TPM3, the WD domain in EML4, the PB1 domains in TFG and SQSTM1, the PNT domain in ETV6, and the RNA recognition motif in RBPMS, which allows the fusion oncoprotein to undergo dimerization and transactivation without the kinase’s physiologic ligand (Fig. 2A). Unlike RTK fusions, BRAF fusions may (e.g., AKAP9::BRAF) or may not (e.g., AGK::BRAF) contain partner-derived dimerization domains. The key oncogenic mechanism in BRAF fusions is believed to be the loss of 5′ autoinhibitory domains when replaced by a partner sequence (Fig. 2B). Regardless of the activation mechanisms, it is the retained active kinase domain that renders fusion oncoproteins vulnerable to inhibition by small molecules that obstruct ATP binding through ATP-competitive and non-competitive approaches [51]. These kinasetargeting agents have significantly improved the clinical outlook for patients with RAI-refractory thyroid cancers (Table 1) [4–13]. Timely and cost-effective detection of actionable kinase rearrangements has thus become a life-saving task entrusted to resourced pathology laboratories.
Fig. 2.
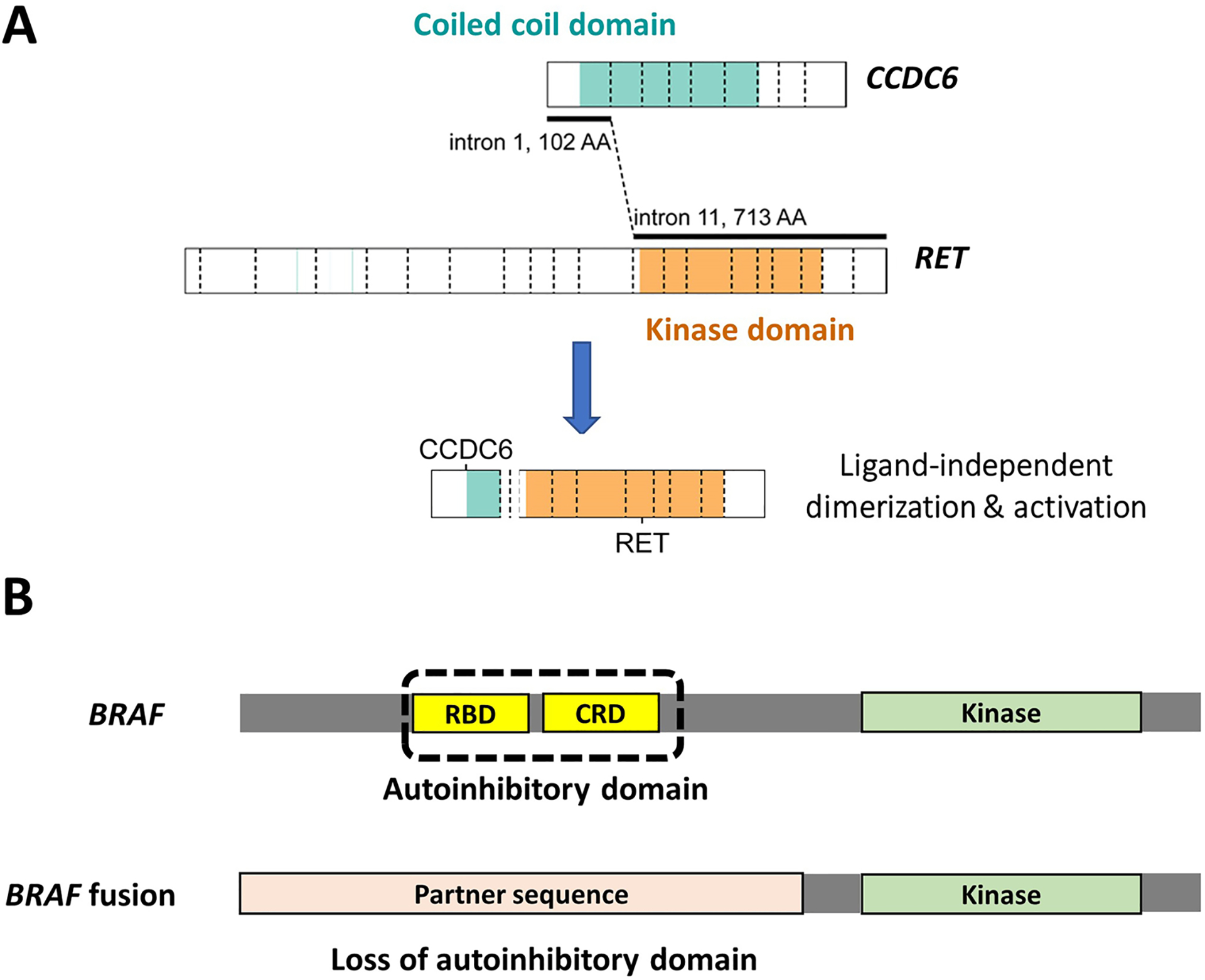
Oncogenic mechanisms of kinase fusions. Most tyrosine kinase fusions contain partner-derived dimerization domains that enable ligand-independent activation (A). BRAF fusions, however, may or may not have partner-derived dimerization domains and thought to be activated by loss of autoinhibition (B)
Since then, the clinical management of kinase fusion–related thyroid cancer (KFTC) has met with several challenges. As diagnostic techniques have evolved from the use of fluorescence in situ hybridization (FISH) and reverse transcriptionpolymerase chain reaction (RT-PCR) toward a growing dependence on next-generation sequencing (NGS) analysis, this improved molecular resolution is still relatively costly [52]. This has triggered recent research interest in the histologic and immunohistochemical correlates of KFTC that may inform strategic testing algorithms [15, 28, 38]. Furthermore, the literature has been notably heterogeneous on the prevalence and clinical behavior of KFTC (Tables 2 and 3), hampering the development of an evidence-based treatment standard. The decision to test tumors only in the face of advanced thyroid cancer (pre-operatively for unresectable tumors or post-operatively for incompletely resected tumors or those with distant disease) or with complete excision in order to bank genomic data from the outset remains controversial in regard to resource allocation, but arguable considering the pre-operative standard of care has become molecular testing for diagnostically indeterminate thyroid nodules, the majority of which are benign [53]. Lastly, secondary resistance-mediating mutations have emerged in KFTC patients treated with kinase inhibitors and are critical to incorporate into future therapeutic planning. This review presents recent advances and ongoing challenges in exploiting the molecular actionability of KFTC.
Table 3.
Clinical features of kinase fusion-related papillary thyroid carcinomas in published series
| Study | Queried kinases | %T1-T2 | %T3-T4 | %N1 | %M1 |
|---|---|---|---|---|---|
| Adult | |||||
| Chu et al. | ALK, BRAF, MET, NTRK1/2/3, RET, ROS1 | 37 | 61 | 79 | 6 |
| Chou et al. | ALK | 45 | 55 | 27 | 0 |
| Kong et al. | NTRK1/2/3 | 53 | 47 | 83 | 11 |
| Nozaki et al. | ALK, NTRK1/3, ROS1 | 75 | 25 | 75 | 0 |
| Panebianco et al. | ALK | 89 | 11 | 30 | 0 |
| Lee et al. | NTRK1/2/3 | 92 | 8 | 42 | 8 |
| Park et al. | ALK | 100 | 0 | 50 | 0 |
| Pediatric | |||||
| Cordioli et al. | BRAF, NTRK3, RET | 35 | 65 | 88 | 35 |
| Pekova et al. | ALK, BRAF, MET, NTRK1/2/3, RET, ROS1 | 44 | 56 | 81 | 17 |
| Franco et al. | ALK, BRAF, MET, NTRK1/2/3, RET, ROS1 | 47 | 49 | 93 | 40 |
| Ricarte-Filho et al. | NTRK1/2/3 | 55 | 45 | 80 | 45 |
| Prasad et al. | ALK, BRAF, MET, NTRK1/3, RET | 77 | 23 | 69 | 0 |
| Rogounovitch et al. | BRAF, NTRK3, RET | 78 | 22 | 83 | 6 |
| Alzahrani et al. | ALK, BRAF, MET, NTRK1/2/3, RET, ROS1 | 75 | 15 | 85 | 15 |
Histologic Features
The past decades have seen a growing number of histologicmolecular correlation research in search of histologic predictors of therapeutic targetability in thyroid cancer. RET is the most commonly rearranged kinase gene and has been associated with diffuse sclerosing papillary thyroid carcinoma (DSPTC). DSPTC is characterized by prominent lymphatic invasion (intra and extrathyroidal), stromal sclerosis, lymphocytic inflammation (commonly Hashimoto thyroiditis), squamous metaplasia, and numerous psammomatous calcifications, often clusters of smaller-sized psammomatous calcifications. DSPTC is not specific to RET-rearranged tumors but can also occur on occasion with BRAF V600E mutation in 24% of cases [54] and with ALK, BRAF, and NTRK fusions [15, 31, 34, 36]. RET rearrangements in PTC have also been linked the solid PTC [55], which has also been reported with BRAF, NTRK, and ROS1 fusions [15, 34]. Tall cell PTC, although primarily associated with the BRAF V600E mutation, may rarely be seen with ALK, BRAF, NTRK, and RET fusions [15, 29, 31, 56]. In addition to the reported associations with unusual subtypes of PTC, fusion-driven PTC commonly present with classical, follicular, and mixed papillary-follicular architectures [15, 34, 57], which, just like fusion-driven PDTC, ATC, and MTC, overlap morphologically with fusion-negative counterparts. Fortunately, recent KFTC studies have observed several distinct features, including multinodularity [15, 38, 57], lymphovascular spread [15, 38, 41], and intratumoral fibrosis [15, 38], which are present in the majority of KFTC (Fig. 3) and, when concurrent with negativity for BRAF p.V600E in follicular-derived carcinomas by immunostaining or molecular assays, encourages consideration of fusion testing if clinically indicated [15]. Of late, artificial intelligence has shown great promise in identifying kinase fusions on morphologic grounds in lung cancers [58] and may apply to thyroid tumors in the near future, although, for now, a simple BRAF test and pathologist architectural review of a hematoxylin and eosin-stained tumor slide are great predictors.
Fig. 3.
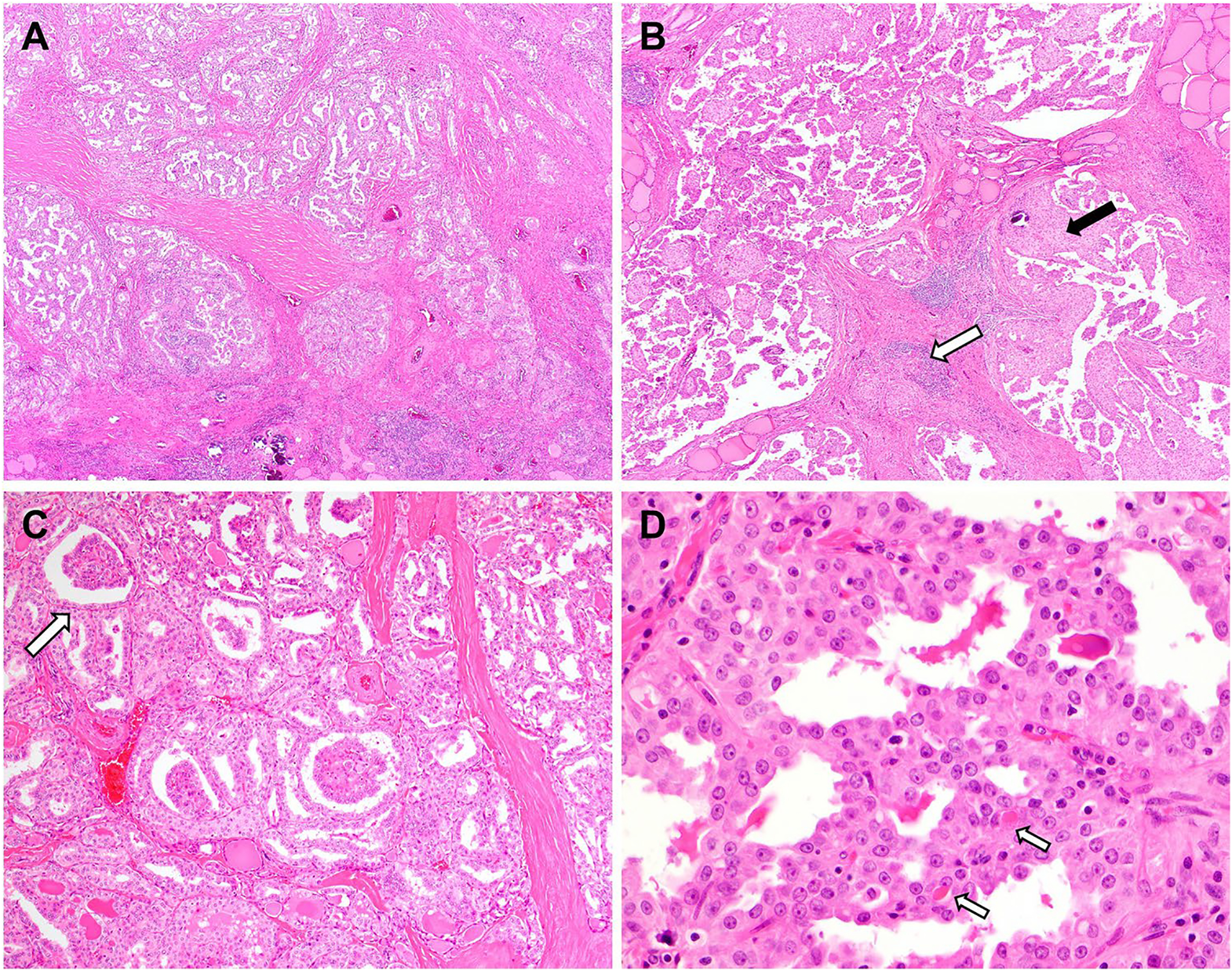
Histologic features of KFTC include multinodularity (A), lymphovascular spread (not shown) and prominent intratumoral fibrosis (A) that have been noted in several series. RET fusions are well-known to be associated with the diffuse sclerosing PTC (B) characterized by chronic lymphocytic inflammation (white arrow) and squamous metaplasia (black arrow) in addition to stromal fibrosis/sclerosis. NTRK rearranged tumors may show intriguing glomeruloid architectural formations (C, arrow). Primary secretory carcinomas are histologically reminiscent of its salivary counterpart with microcystic architecture (D) and eosinophilic secretions (arrows). The nuclei are vesicular with conspicuous nucleoli
Primary thyroid secretory carcinoma (SC) is a newly established entity in the 5th edition of the World Health Organization classification for endocrine tumors. Similar to its salivary gland counterpart, thyroid SC is characterized by the presence of ETV6::NTRK3 fusion, which has been consistently identified in the 13 cases reported thus far [15, 59, 60]. SC tend to present at advanced clinical stage with large size and cervical lymph node involvement at the initial diagnosis [60]. Histologically, microcystic to papillary growth, densely fibrotic stroma, and eosinophilic secretion are typically noted (Fig. 3). The tumor cells have moderate cytoplasm and vesicular nuclei with conspicuous nucleoli and frequent nuclear grooves. Although papillary growth and nuclear grooves are reminiscent of PTC, SC show a distinct immunophenotype with negativity for thyroglobulin (thus insensitive to RAI therapy) and TTF-1 while being positive for S100, GATA3, and mammaglobin.
Molecular Diagnosis
When evaluating the KFTC literature, it is important to understand the capabilities and limitations of various molecular platforms so that a fusion-positive-versus-fusion-negative comparison would not be biased by the putative “fusion-negative” group potentially containing fusions that are outside the scope of the employed methodology. Immunohistochemistry (IHC) and FISH offer fast turnaround time but lack molecular resolution. By molecular approaches, kinase gene rearrangements can be detected at the breakpoint (i.e., demonstrating a hybrid sequence formed by the partner genes) or through 3′-to-5′ expression imbalance (EI). 3′-to-5′ EI occurs when an overexpressed fusion product contains only the 3′ region of the queried gene, such as where the kinase domain is located in most RTK and BRAF. Fusion breakpoint and EI can each be characterized by direct RNA hybridization–based transcript enumeration (e.g., NanoString nCounter®), reverse transcription quantitative RTqPCR, digital PCR, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), or NGS [61] (Table 2). The most commonly applied clinical platforms are reviewed here (Fig. 4).
Fig. 4.
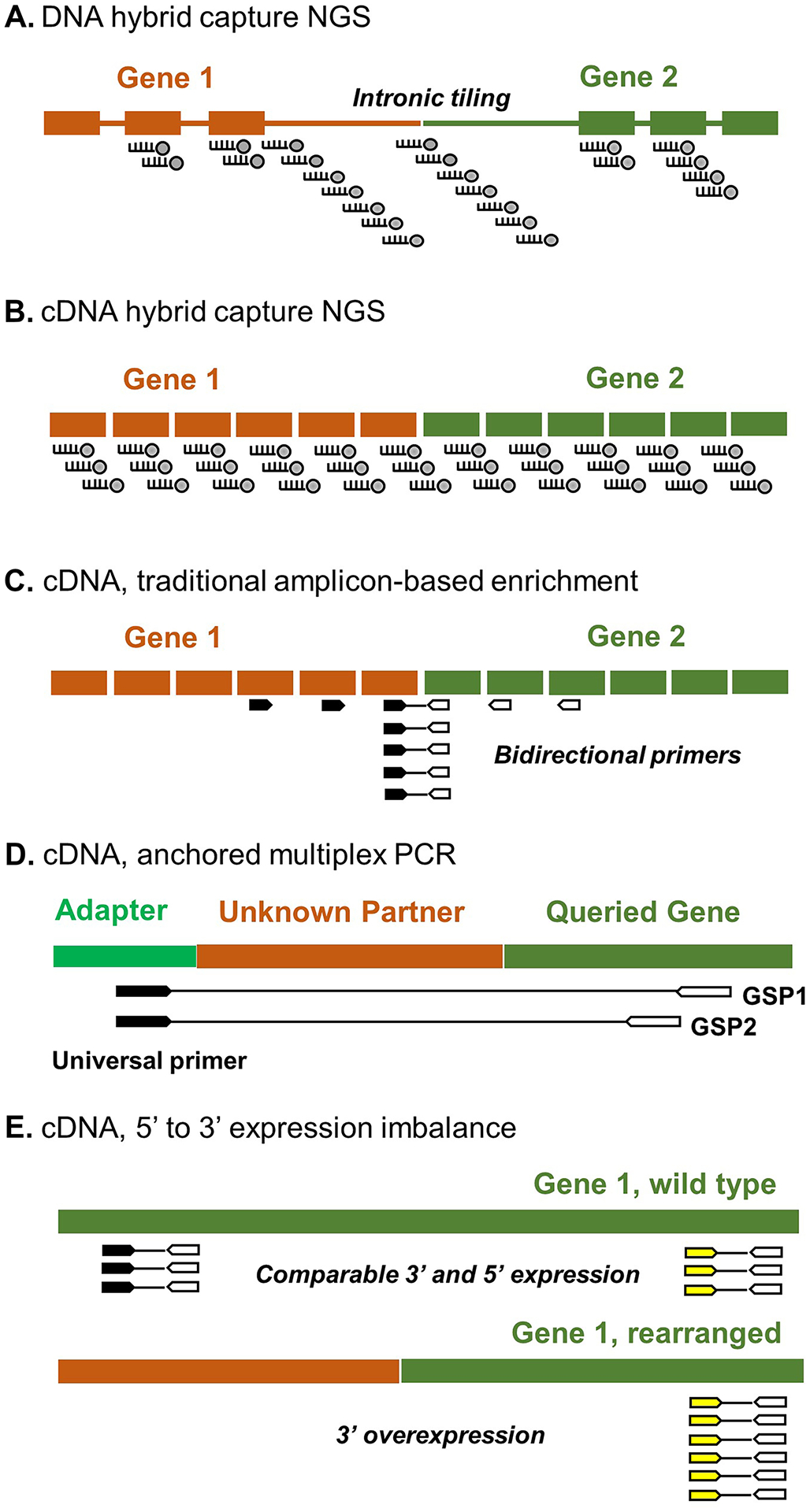
Common molecular platforms for clinical fusion testing. See text for details
Immunohistochemistry
Commercial IHC antibodies are currently available for querying rearrangements of NTRK (pan-TRK) [62, 63], RET [64], ALK [31, 32], and ROS1 [33]. With its unique edge in allowing fast turnaround time and in situ visualization of protein expression within cellular context, kinase-based IHC has gained wide clinical applications. However, the performance of kinase-based IHC appears to be heterogeneous and partner gene-dependent. Pan-TRK IHC has a reported sensitivity and specificity of 81.1% and 99.9%, respectively [63]. The sensitivity is high in NTRK1 (96%) and reasonably good in NTRK3 (79%) fusion tumors; NTRK2 fusions are rare but appear to be consistently labeled (100%) [63, 65]. RETbased IHC showed a sensitivity of 100% for KIF5B::RET, 88.9% for CCDC6::RET, and 50% for NCOA4::RET, with a specificity of around 82% [64]. ALK IHC has the most reliable performance, with nearly 100% sensitivity and specificity for ALK fusions [32]. In contrast, ROS1 IHC may often be compromised by nonspecific staining [33]. The use of multiplex fusion target immunohistochemistry as a screening tool has not been employed, but given the variable sensitivities of individual IHC markers, it seems to be of limited utility relative to NGS testing.
However, it is noteworthy that BRAF p.V600E-specific IHC is a valuable tool in identifying KFTC as the BRAF p.V600E mutation is mutually exclusive with kinase fusions in the pre-treatment setting. The commonly employed VE1 clone has a sensitivity of 89–100% and a specificity of 62–100% [66]. The VE1 antibody, however, does not label BRAF fusions and non-p.V600E mutations.
Fluorescence In Situ Hybridization
FISH has been the conventional gold standard for detecting gene rearrangements. The break-apart probe design waives the need for partner gene identity and breakpoint localization. However, FISH has limited multiplexing capability and may not be able to discern biologically nonproductive fusions such as those that are out-of-frame or lack therapeutically relevant structures such as the kinase domain in kinase fusions, leading to false positivity and treatment failure [67]. On the other hand, sources of false negativity include fusions that derive from short-segment inversions, such as the NCOA4::RET fusion, which often fail to produce visually apparent split signals [64].
Hybrid Capture NGS
Hybrid capture NGS employs probes that hybridize with the genomic region of interest and are biotinylated to allow subsequent capture by streptavidin-labeled beads. Hybrid sequences (split reads) at fusion breakpoint can be captured by kinase gene-targeting probes without requiring partner gene identity, thus allowing detection of novel fusions. Gene fusions often occur at intronic locations, where repetitive sequences may create considerable sequencing and bioinformatic difficulties. At the DNA level, breakpoint characterization requires intronic probe tiling (Fig. 4A), which can be costly, particularly when large introns are encountered. In contrast, when hybrid capture is performed in complementary DNA (cDNA) derived from tumor RNA, the introns are spliced out, and the probe design can focus on exonic regions and circumvent the cost and bioinformatic challenges of intronic sequencing (Fig. 4B). Furthermore, analysis at the RNA level reflects the transcriptional activity and splicing outcome of gene fusions. In samples with low tumor content, the overexpression of fusion sequences can be beneficial for increasing assay sensitivity. The main limitation of cDNA sequencing is its dependence on tumor RNA quality. Meticulous examination of quality metrics is essential for ensuring result validity.
RT-PCR and Bidirectional Amplicon-Based NGS
Fusion breakpoints can also be queried using bi-directional primer sets that target the rearranged genes (Fig. 4C). As primer design requires a focused interest in certain fusion partners and hotspot exons, non-targeted fusions may not be detectable. As listed in Table 2, earlier KFTC studies often employed multiplex RT-PCR that amplified the most frequent types of RET and NTRK rearrangements [21, 24]. The newest multiplex RT-PCR fusion assays have introduced microfluidic devices that automatically perform nucleic extraction and fusion detection with minimal personnel dependence, allowing ultra-rapid clinical testing [61]. Recently, the advent of amplicon-based NGS has significantly boosted the multiplexing capability by allowing hundreds of amplicons to be concurrently sequenced. Compared to hybrid capture NGS, amplicon-based NGS is advantageous for simple workflow, fast turnaround time, and relative tolerance for low-tumor samples, but, similar to RTPCR, is dependent on the starting probe design in terms of uncommon/novel fusion coverage.
Anchored Multiplex PCR-Based cDNA NGS
The anchored multiplex PCR (AMP) technology is a power platform invented by the Massachusetts General Hospital in Boston, MA, and commercially supplied by ArcherDx, Inc., that enables gene fusion detection in an amplicon-based and yet partner-agnostic manner [68]. This is achieved by deploying gene-specific primers (GSP) and adapter-complementary primers (Fig. 4D) that amplify fusion transcripts without preceding knowledge of partner gene identity. Multiple studies have utilized large AMP NGS panels with comprehensive coverage of oncogenic fusions in thyroid cancer [15, 16, 18], allowing more accurate assessment of KFTC prevalence. AMP assay specificity has been further improved by a twostep amplification protocol using two nested GSP pools for a given region [68].
3′ to 5′ Expression Imbalance
In KFTC, oncogenic fusion products are highly expressed and contain the 3′ kinase domain-encoding sequence but not the 5′ region of the kinase gene. As a result, when quantified separately, KFTC carry more transcripts of 3′ sequence than of 5′ sequence, leading to EI (i.e. 3′ region overexpression, Figure 4E). EI can be demonstrated using various methods such as direct RNA hybridization-based transcript enumeration (e.g., NanoString nCounter® [36]), quantitative RT-PCR [61], digital PCR, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), and NGS such as the Oncomine Focus Assay [69]. Although EI allows for fusion detection without knowing partner identity, the observed sensitivity may be suboptimal across different platforms [64]. The magnitude of EI is affected by endogenous tissue expression of the queried gene, tumor purity, and RNA quality [64]. A cutoff value for making positive fusion calls can be challenging to determine, as exemplified by the low sensitivity of ROS1 EI (29%) in the pulmonary setting due to high background expression [61]. To complement this limitation, most assays that employ EI have co-operating fusion-specific detection mechanisms that cover the prevalent fusion types to achieve an overall acceptable sensitivity [36, 61]. Another downside of EI is inability to identify fusion partner and breakpoint location.
Clinical Features
The reported prevalence of kinase fusions in thyroid tumors have been variable among the published series (Table 2) [15–38], depending on patient demographics, risk factors, tumor histology, and the analytic methods employed. KFTC has a well-established association with pediatric and radiation-associated thyroid cancers, traditionally classified as PTC, particularly those arising in post-Chernobyl radiation exposure victims. Histologically, PTC is the tumor type that shows the strongest association with kinase fusions, although whether or not many of these tumors truly have features of PTC, or are unique on their own, is a phenomenon continuing to evolve. It is noteworthy that studies which used IHC, FISH, and RT-PCR for fusion detection often focused on one to three kinase genes and may not be able to detect uncommon kinase fusions such as those of BRAF, MET, and ROS1. Coverage of partner genes may also be limited when using methods with low multiplexing capability. Since RET is the most frequently rearranged kinase in the thyroid, studies that did not cover RET tended to report lower KFTC prevalence. When drawing clinicopathologic comparison of KFTC against fusion-negative tumors, it is important to understand the scope and limitations of the source studies to ensure comparability of patient groups. Even when using NGS with comprehensive analysis of actionable genes, it is crucial to review panel details (e.g., tiled introns, DNA versus RNA sequencing, traditional amplicon versus AMP etc.) to properly interpret sequencing results.
For kinase-driven PTC, while most researchers have reported a predilection for early lymph node involvement, the observed distribution of primary tumor stage and the frequency of distant metastasis have each spanned a wide range in the literature (Table 3). To understand the cause of such variability, in addition to examining the analytic scope of the studies as discussed above, cohort identification approach is also important to consider. While most researchers have evaluated consecutive cohorts that included all available PTC patients from their chosen time period, some studies relied on data-mining of historical fusion testing results [15] and may have preferentially included patients who had more aggressive disease that triggered clinical testing, thus creating selection bias. Overall, the current literature on KFTC behavior is relatively scarce and notably heterogeneous. There is an ongoing need for additional clinical data, particularly those based on comprehensive molecular profiling, for more objective assessment of KFTC behavior.
Treatment and Acquired Resistance
The 2022 National Comprehensive Cancer Network (NCCN) guidelines recommend pursuing actionable molecular marker testing including RET, NTRK, and ALK rearrangements for advanced thyroid carcinoma to facilitate personalized utilization of kinase inhibitor therapy [70] (Table 1). The kinase domain of most RTK is composed of N-terminal and C-terminal lobes connected by a hinge region that is known as the ATP-binding site (Fig. 5A) [71]. The ATP-binding site is highly conserved among RTK, formed by the phosphate-binding loop, the catalytic loop, and the activation loop that contain the Asp-PheGly (DFG) and the Ala-Pro-Glu (APE) motifs (Fig. 5A). In the active state, the DFG motif assumes a “DFG-in” conformation that allows the aspartate to interact with the magnesium cofactor. In the inactive state, the DFG motif rotates the aspartate residue outward into a “DFG-out” conformation. On the back side behind the adenine ring binding site is a hydrophobic cleft. The access to this cleft is controlled by gatekeeper residues on the hinge. For TKI that bind to the kinase by passing through this gate, such as most multi-kinase inhibitors (MKIs), gatekeeper mutations may lead to steric hindrance and therapeutic resistance (Fig. 5A) [72]. Resistance may also result from other acquired mutations that conformationally alter the kinase domain such as at the solvent front (Fig. 5A) or through activation of bypassing signaling pathways (Fig. 5B). Recent advances in TKI therapy in thyroid cancer are reviewed here.
Fig. 5.
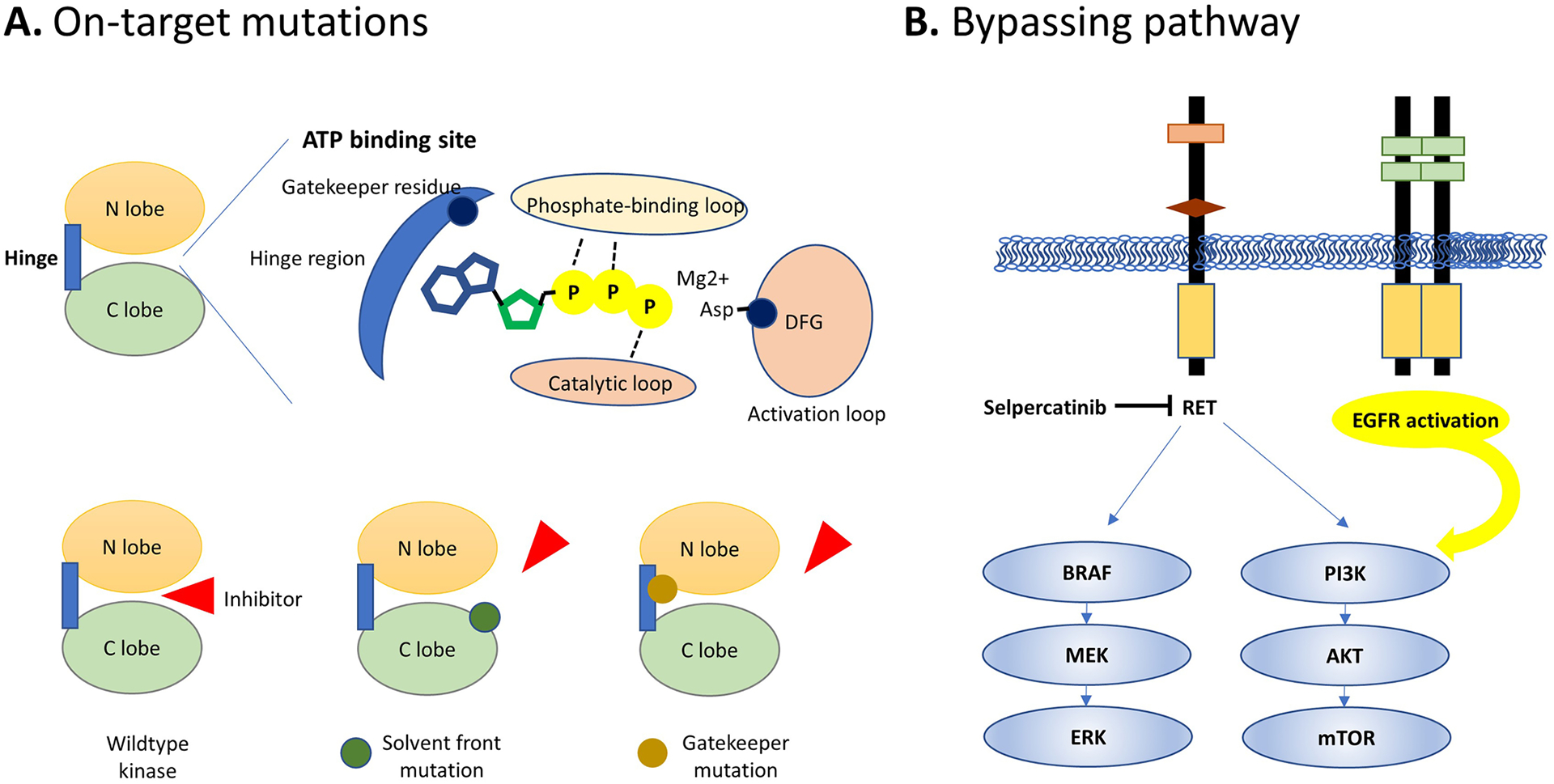
Mechanisms of acquired resistance to kinase inhibitor therapy. On-target resistance is mediated by acquired mutations at various locations in the kinase domain (A). Alternative pathway activation, such as another receptor tyrosine kinase that drives downstream MAPK and PI3K signaling, bypasses the inhibition of RET in this example
Multi-Kinase Inhibitors (MKIs)
Sorafenib and lenvatinib are currently the first-line TKI therapy for clinically significant RAI-refractory differentiated thyroid carcinoma. Their anti-tumoral effects mainly originate through the inhibition of endothelial growth factor receptors (VEGFR) signaling and suppressing angiogenesis. In the SELECT trial that included 392 randomized subjects, the median progression-free survival (PFS) was significantly better in the lenvatinib group compared to placebo (18.3 months versus 3.6 months) [4]. In the DECISION trial for sorafenib, the median PFS was improved to 10.8 months compared to 5.8 months in the placebo group [5]. Many MKI also demonstrate inhibitory activity against RET. However, their molecular non-selectivity leads to frequent toxicities and inferior pharmacokinetics which motivated subsequent development of selective RET inhibitors. One key difference between MKI and selective RET inhibitor is that MKI bind to the RET kinase domain by passing through the aforementioned structural gate and are therefore subjected to gatekeeper mutationmediated resistance, such as p.V804L/M [73]. Unlike MKI, selective RET inhibitors access the back cleft by wrapping around the gate wall without passing through it [72], thus remaining active against V804 mutants.
Selective RET Inhibitors
Selpercatinib and pralsetinib are selective RET inhibitors with improved efficacy and safety compared to MKI. In September 2022, Selpercatinib received United State Food and Drug Administration (FDA) accelerated approval for histology-agnostic treatment of advanced or metastatic solid tumors driven by RET rearrangements based on the LIBRETTO-001 trial (Table 1) [10]. The trial evaluated a total of 19 non-medullary thyroid carcinoma patients, achieving an objective response rate (ORR) of 79%, including 1 complete response and 14 partial responses, with the remaining patients experiencing stable disease [10]. The median PFS was 20.1 months [10]. Around 30% of the subjects required dose reductions and 2% terminated treatment due to side effects including abnormal liver function and hypersensitivity [10]. Pralsetinib received FDA approval in 2020 for thyroid cancer based on the ARROW trial (Table 1) that evaluated 11 RET fusion-positive thyroid cancer patients with an ORR of 89% [11]. Acquired resistance to selpercatinib has been reported through both on-target and bypassing mechanisms. We recently observed a ERC1::RET fusion ATC that developed EGFR amplification causing selpercatinib resistance [15]. In two lung carcinomas harboring KIF5B::RET and CCDC6::RET fusions, an MTC driven by RET p.M918T and p.V804 M/L mutations and 39 selpercatinib-resistant cell lines, two research groups found p.G810 C/S/R at the solvent front, p.Y806 C/N in the hinge region, and p.V738A at the β2 strand to confer resistance to both selpercatinib and pralsetinib [72, 74]. Second-generation agent TPX-0046 is in development to tackle solvent front p.G810 mutations but may be subjected to other structural hindrance based on in silico predictions [75].
NTRK Inhibitors
Larotrectinib and entrectinib are FDA-approved TRK inhibitors for the histology-agnostic treatment of adult and pediatric solid tumors driven by NTRK rearrangements. NTRK1/2/3 rearrangements are uncommon drivers of thyroid, lung, breast, pancreatic, and colonic carcinomas, sarcomas, melanomas, and gliomas, while being the defining genetic feature for several rare neoplasms such as secretory carcinomas and congenital mesoblastic nephromas. Combining three phase 1/2 trials that included 159 patients with a wide spectrum of tumor types, larotrectinib achieved an ORR of 79% with complete response seen in 16% [76]. Entrectinib, an inhibitor of not only TRK but also ALK and ROS1, showed an ORR of 57% in three phase 1/2 trials [13]. Both larotrectinib and entrectinib were well tolerated with <5% toxicity-related treatment termination. Dose reduction was documented in 8% (larotrectinib) and 30% (entrectinib) due to anemia, abnormal renal, hepatic or pancreatic function, and fatigue [13, 76]. Acquired resistance-mediating mutations have been identified at gatekeeper residues (NTRK1 p.F589L and NTRK3 p.F617L), the solvent front (NTRK1 p.G595R and NTRK3 p.G623R), and in the activation loop (NTRK1 p.G667C/S, NTRK3 p.G696A) in tumors of various organs [77–79]. Next-generation NTRK inhibitors selitrectinib and repotrectinib are being developed with demonstrated efficacy against solvent front and activation loop mutants [80].
ALK Inhibitors
ALK fusions are rare in thyroid cancer, with small numbers of reports in PTC, PDTC, ATC, and MTC (Table 2) [48, 81, 82]. Crizotinib is a first-generation ALK, ROS1, and MET inhibitor that received FDA approval in 2011 for ALK-rearranged lung cancer. Since the discovery of ALK-driven malignancies in virtually every organ, crizotinib has shown systemic therapeutic success including in the thyroid. However, most patients developed resistance in 1 to 2 years due to acquired kinase domain mutations and activation of bypassing signaling caused by mutations or amplifications of EGFR, KRAS, and MET [83, 84]. In scattered case reports, therapeutic response could sometimes be restored by switching to brigatinib and alectinib with some cases demonstrating lasting response (Table 4) [15, 48, 81, 85, 86].
Table 4.
Reported cases of thyroid carcinomas treated with ALK inhibitor forgings (mm)
| Study | Histology | ALK Fusion | ALK Inhibitor | Best Response, Duration |
|---|---|---|---|---|
| Demeure et al. [85] | PTC | EML4::ALK | Crizotinib | SD, over 6 months to the end of study |
| de Salins et al. [86] | OC | ALK fusion with unknown partner | Crizotinib | CR, 6 months |
| Chu et al. [15] | PDTC | STRN::ALK | Crizotinib | SD, 11 months |
| Leroy et al. [81] | ATC | STRN::ALK | Crizotinib, switched to ceritinib and then brigatinib for resistance | CR, 36 months (crizotinib); PR, 16 months (ceritinib); PR, 8 months (brigatinib) |
| Hillier et al. [48] | MTC | CCDC6::ALK | Crizotinib, switched to alectinib for tolerance | PR, over 280 days to the end of the study |
PTC papillary thyroid carcinoma, OC oncocytic carcinoma, PDTC poorly differentiated thyroid carcinoma, ATC anaplastic thyroid carcinoma, MTC medullary thyroid carcinoma, SD stable disease, CR complete response, PR partial response
Summary
Despite the significant recent advances in targeted therapy for thyroid cancer, several unsolved clinical needs remain. Although ALK, MET, and ROS1 fusions are well documented in thyroid tumors with commercially available inhibitors, these agents currently do not have FDA-approved thyroid indications due to scarcity of data. Furthermore, although most KFTC studies reported high frequencies of early lymph node spread of KFTC, other aspects of clinical behavior, including long-term survival, remain poorly understood due to inconsistent study designs and findings among the published clinical series and/or a lack of solid evidence due to case rarity and test selection bias. Fortunately, as comprehensive genomic profiling becomes increasingly accessible and affordable in both the pre-treatment and post-treatment settings, one can expect more high-quality evidence to arrive in the near future and to provide novel diagnostic, prognostic, and treatment resistance predictors that support effective tumor behavior modeling and management planning for optimal patient outcomes.
Funding
Dr. Sadow’s salary is supported, in part, by the National Cancer Institute of the National Institutes of Health, Bethesda, USA (1P01CA240239-04).
Footnotes
Ethical Approval The study was performed with Massachusetts General Hospital and Massachusetts General Brigham Internal Review Board approval to Dr. Sadow as Principal Investigator (2011P000013).
Competing Interests The authors declare no competing interests.
Availability of Data and Materials
This is a review article of published data accessible ad hoc by request.
References
- 1.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. The Journal of clinical endocrinology and metabolism. 2006;91:2892–9. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 2014;99:1245–52. [DOI] [PubMed] [Google Scholar]
- 3.Wong KS, Dong F, Telatar M, Lorch JH, Alexander EK, Marqusee E, et al. Papillary Thyroid Carcinoma with High-Grade Features Versus Poorly Differentiated Thyroid Carcinoma: An Analysis of Clinicopathologic and Molecular Features and Outcome. Thyroid : official journal of the American Thyroid Association. 2021;31:933–40. [DOI] [PubMed] [Google Scholar]
- 4.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. The New England journal of medicine. 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (London, England). 2014;384:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2021;22:1126–38. [DOI] [PubMed] [Google Scholar]
- 7.Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in Progressive Medullary Thyroid Cancer. Journal of Clinical Oncology. 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jr SAW, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in Patients With Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. Journal of Clinical Oncology. 2012;30:134–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Annals of oncology : official journal of the European Society for Medical Oncology. 2022;33:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. The New England journal of medicine. 2020;383:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. The lancet Diabetes & endocrinology. 2021;9:491–501. [DOI] [PubMed] [Google Scholar]
- 12.Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. European journal of endocrinology. 2022;186:631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. The Lancet Oncology. 2020;21:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DN, Sadow PM. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Human pathology. 2018;82:32–8. [DOI] [PubMed] [Google Scholar]
- 15.Chu YH, Wirth LJ, Farahani AA, Nosé V, Faquin WC, Dias-Santagata D, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2020;33:2458–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, et al. RET, NTRK, ALK, BRAF, and MET Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid : official journal of the American Thyroid Association. 2020;30:1771–80. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Cai W, Feng D, Teng H, Mao F, Jiang Y, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. The Journal of pathology. 2018;244:215–26. [DOI] [PubMed] [Google Scholar]
- 18.Franco AT, Ricarte-Filho JC, Isaza A, Jones Z, Jain N, Mostoufi-Moab S, et al. Fusion Oncogenes Are Associated With Increased Metastatic Capacity and Persistent Disease in Pediatric Thyroid Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022;40:1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YA, Lee H, Im SW, Song YS, Oh DY, Kang HJ, et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. The Journal of clinical investigation. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzahrani AS, Alswailem M, Alswailem AA, Al-Hindi H, Goljan E, Alsudairy N, et al. Genetic Alterations in Pediatric Thyroid Cancer Using a Comprehensive Childhood Cancer Gene Panel. The Journal of clinical endocrinology and metabolism. 2020;105. [DOI] [PubMed] [Google Scholar]
- 21.Bounacer A, Schlumberger M, Wicker R, Du-Villard JA, Caillou B, Sarasin A, et al. Search for NTRK1 proto-oncogene rearrangements in human thyroid tumours originated after therapeutic radiation. British journal of cancer. 2000;82:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinets A, Hulchiy M, Sofiadis A, Ghaderi M, Höög A, Larsson C, et al. Clinical, genetic, and immunohistochemical characterization of 70 Ukrainian adult cases with post-Chornobyl papillary thyroid carcinoma. European journal of endocrinology. 2012;166:1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabes HM, Demidchik EP, Sidorow JD, Lengfelder E, Beimfohr C, Hoelzel D, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:1093–103. [PubMed] [Google Scholar]
- 24.Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. The Journal of clinical endocrinology and metabolism. 2000;85:1170–5. [DOI] [PubMed] [Google Scholar]
- 25.Ricarte-Filho JC, Halada S, O’Neill A, Casado-Medrano V, Laetsch TW, Franco AT, et al. The clinical aspect of NTRK-fusions in pediatric papillary thyroid cancer. Cancer genetics. 2022;262–263:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musholt TJ, Musholt PB, Khaladj N, Schulz D, Scheumann GF, Klempnauer J. Prognostic significance of RET and NTRK1 rearrangements in sporadic papillary thyroid carcinoma. Surgery. 2000;128:984–93. [DOI] [PubMed] [Google Scholar]
- 27.Brzeziańska E, Karbownik M, Migdalska-Sek M, PastuszakLewandoska D, Włoch J, Lewiński A. Molecular analysis of the RET and NTRK1 gene rearrangements in papillary thyroid carcinoma in the Polish population. Mutation research. 2006;599:26–35. [DOI] [PubMed] [Google Scholar]
- 28.Lee YC, Hsu CY, Lai CR, Hang JF. NTRK-rearranged papillary thyroid carcinoma demonstrates frequent subtle nuclear features and indeterminate cytologic diagnoses. Cancer cytopathology. 2022;130:136–43. [DOI] [PubMed] [Google Scholar]
- 29.Kong Y, Bu R, Parvathareddy SK, Siraj AK, Siraj N, Al-Sobhi SS, et al. NTRK fusion analysis reveals enrichment in Middle Eastern BRAF wild-type PTC. European journal of endocrinology. 2021;184:503–11. [DOI] [PubMed] [Google Scholar]
- 30.Panebianco F, Nikitski AV, Nikiforova MN, Kaya C, Yip L, Condello V, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocrine-related cancer. 2019;26:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou A, Fraser S, Toon CW, Clarkson A, Sioson L, Farzin M, et al. A detailed clinicopathologic study of ALK-translocated papillary thyroid carcinoma. The American journal of surgical pathology. 2015;39:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park G, Kim TH, Lee HO, Lim JA, Won JK, Min HS, et al. Standard immunohistochemistry efficiently screens for anaplastic lymphoma kinase rearrangements in differentiated thyroid cancer. Endocrine-related cancer. 2015;22:55–63. [DOI] [PubMed] [Google Scholar]
- 33.Nozaki Y, Yamamoto H, Iwasaki T, Sato M, Jiromaru R, Hongo T, et al. Clinicopathological features and immunohistochemical utility of NTRK-, ALK-, and ROS1-rearranged papillary thyroid carcinomas and anaplastic thyroid carcinomas. Human pathology. 2020;106:82–92. [DOI] [PubMed] [Google Scholar]
- 34.Sisdelli L, Cordioli M, Vaisman F, Moraes L, Colozza-Gama GA, Alves PAG Jr., et al. AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatric blood & cancer. 2019;66:e27707. [DOI] [PubMed] [Google Scholar]
- 35.Efanov AA, Brenner AV, Bogdanova TI, Kelly LM, Liu P, Little MP, et al. Investigation of the Relationship Between Radiation Dose and Gene Mutations and Fusions in Post-Chernobyl Thyroid Cancer. Journal of the National Cancer Institute. 2018;110:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macerola E, Proietti A, Poma AM, Ugolini C, Torregrossa L, Vignali P, et al. Molecular Alterations in Relation to Histopathological Characteristics in a Large Series of Pediatric Papillary Thyroid Carcinoma from a Single Institution. Cancers. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastos AU, de Jesus AC, Cerutti JM. ETV6-NTRK3 and STRNALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. European journal of endocrinology. 2018;178:83–91. [DOI] [PubMed] [Google Scholar]
- 38.Prasad ML, Vyas M, Horne MJ, Virk RK, Morotti R, Liu Z, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–107. [DOI] [PubMed] [Google Scholar]
- 39.Duan H, Li Y, Hu P, Gao J, Ying J, Xu W, et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology. 2019;75:890–9. [DOI] [PubMed] [Google Scholar]
- 40.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation. 2016;126:1052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, et al. NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis. Cancers. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid : official journal of the American Thyroid Association. 2020;30:1505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal S, Bychkov A, Jung C-K. Emerging Biomarkers in Thyroid Practice and Research. Cancers. 2022;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol 2022. Mar;33(1):27–63. [DOI] [PubMed] [Google Scholar]
- 45.Allison DB, Rueckert J, Cornea V, Lee CY, Dueber J, Bocklage T. Thyroid Carcinoma with NSD3::NUTM1 Fusion: a Case with Thyrocyte Differentiation and Colloid Production. Endocr Pathol. 2022. Jun;33(2):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barletta JA, Gilday SD, Afkhami M, Bell D, Bocklage T, Boisselier P, et al. NUTM1-rearranged Carcinoma of the Thyroid: A Distinct Subset of NUT Carcinoma Characterized by Frequent NSD3-NUTM1 Fusions. Am J Surg Pathol. 2022. Aug 29. 10.1097/PAS.0000000000001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubbs EG, Ng PK, Bui J, Busaidy NL, Chen K, Lee JE, et al. RET fusion as a novel driver of medullary thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 2015;100:788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillier K, Hughes A, Shamberger RC, Shusterman S, PerezAtayde AR, Wassner AJ, et al. A Novel ALK Fusion in Pediatric Medullary Thyroid Carcinoma. Thyroid : official journal of the American Thyroid Association. 2019;29:1704–7. [DOI] [PubMed] [Google Scholar]
- 49.Ji JH, Oh YL, Hong M, Yun JW, Lee HW, Kim D, et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS genetics. 2015;11:e1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasaian K, Wiseman SM, Walker BA, Schein JE, Hirst M, Moore RA, et al. Putative BRAF activating fusion in a medullary thyroid cancer. Cold Spring Harbor molecular case studies. 2016;2:a000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dar AC, Shokat KM. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annual review of biochemistry. 2011;80:769–95. [DOI] [PubMed] [Google Scholar]
- 52.Sciacchitano S, Lavra L, Ulivieri A, Magi F, De Francesco GP, Bellotti C, et al. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget. 2017;8:49421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajab M, Payne RJ, Forest VI, Pusztaszeri M. Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada. Cancers. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology. 2016;69:45–53. [DOI] [PubMed] [Google Scholar]
- 55.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocrine pathology. 2002;13:3–16. [DOI] [PubMed] [Google Scholar]
- 56.Basolo F, Giannini R, Monaco C, Melillo RM, Carlomagno F, Pancrazi M, et al. Potent mitogenicity of the RET/PTC3 oncogene correlates with its prevalence in tall-cell variant of papillary thyroid carcinoma. The American journal of pathology. 2002;160:247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seethala RR, Chiosea SI, Liu CZ, Nikiforova M, Nikiforov YE. Clinical and Morphologic Features of ETV6-NTRK3 Translocated Papillary Thyroid Carcinoma in an Adult Population Without Radiation Exposure. The American journal of surgical pathology. 2017;41:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayer C, Ofek E, Fridrich DE, Molchanov Y, Yacobi R, Gazy I, Hayun I, Zalach J, Paz-Yaacov N, Barshack I. Direct identification of ALK and ROS1 fusions in non-small cell lung cancer from hematoxylin and eosin-stained slides using deep learning algorithms. Mod Pathol. 2022. Sep 3. 10.1038/s41379-022-01141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saliba M, Mohanty AS, Ho AL, Drilon A, Dogan S. Secretory Carcinoma of the Thyroid in a 49-Year-Old Man Treated with Larotrectinib: Protracted Clinical Course of Disease Despite the High-Grade Histologic Features. Head and neck pathology. 2022;16:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai MA, Mehrad M, Ely KA, Bishop JA, Netterville J, Aulino JM, et al. Secretory Carcinoma of the Thyroid Gland: Report of a Highly Aggressive Case Clinically Mimicking Undifferentiated Carcinoma and Review of the Literature. Head and neck pathology. 2019;13:562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu YH, Barbee J, Yang SR, Chang JC, Liang P, Mullaney K, et al. Clinical Utility and Performance of an Ultrarapid Multiplex RNA-Based Assay for Detection of ALK, ROS1, RET, and NTRK1/2/3 Rearrangements and MET Exon 14 Skipping Alterations. J Mol Diagn. 2022. Jun;24(6):642–654. 10.1016/j.jmoldx.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solomon JP, Hechtman JF. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer research. 2019;79:3163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon JP, Linkov I, Rosado A, Mullaney K, Rosen EY, Frosina D, et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2020;33:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang SR, Aypar U, Rosen EY, Mata DA, Benayed R, Mullaney K, et al. A Performance Comparison of Commonly Used Assays to Detect RET Fusions. Clinical cancer research : an official journal of the American Association for Cancer Research. 2021;27:1316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YC, Chen JY, Huang CJ, Chen HS, Yang AH, Hang JF. Detection of NTRK1/3 Rearrangements in Papillary Thyroid Carcinoma Using Immunohistochemistry, Fluorescent In Situ Hybridization, and Next-Generation Sequencing. Endocrine pathology. 2020;31:348–58. [DOI] [PubMed] [Google Scholar]
- 66.Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: A review. Seminars in diagnostic pathology. 2015;32:400–8. [DOI] [PubMed] [Google Scholar]
- 67.Rosenbaum JN, Bloom R, Forys JT, Hiken J, Armstrong JR, Branson J, et al. Genomic heterogeneity of ALK fusion breakpoints in non-smallcell lung cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31:791–808. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nature medicine. 2014;20:1479–84. [DOI] [PubMed] [Google Scholar]
- 69.Heydt C, Wölwer CB, Velazquez Camacho O, Wagener-Ryczek S, Pappesch R, Siemanowski J, et al. Detection of gene fusions using targeted next-generation sequencing: a comparative evaluation. BMC medical genomics. 2021;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Comprehensive Cancer Network. Thyroid Cancer (Version 2.2022). [cited October 14, 2022]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- 71.Román-Gil MS, Pozas J, Rosero-Rodríguez D, Chamorro-Pérez J, Ruiz-Granados Á, Caracuel IR, et al. Resistance to RET targeted therapy in Thyroid Cancer: Molecular basis and overcoming strategies. Cancer treatment reviews. 2022;105:102372. [DOI] [PubMed] [Google Scholar]
- 72.Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Annals of oncology : official journal of the European Society for Medical Oncology. 2021;32:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer discovery. 2020;10:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2020;15:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Repetto M, Crimini E, Ascione L, Boscolo Bielo L, Belli C, Curigliano G. The return of RET GateKeeper mutations? an in-silico exploratory analysis of potential resistance mechanisms to novel RET macrocyclic inhibitor TPX-0046. Investigational new drugs. 2022;40:1133–6. [DOI] [PubMed] [Google Scholar]
- 76.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. The Lancet Oncology. 2020;21:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. The New England journal of medicine. 2018;378:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russo M, Misale S, Wei G, Siravegna G, Crisafulli G, Lazzari L, et al. Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer. Cancer discovery. 2016;6:36–44. [DOI] [PubMed] [Google Scholar]
- 80.Murray BW, Rogers E, Zhai D, Deng W, Chen X, Sprengeler PA, et al. Molecular Characteristics of Repotrectinib That Enable Potent Inhibition of TRK Fusion Proteins and Resistant Mutations. Molecular cancer therapeutics. 2021;20:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leroy L, Bonhomme B, Le Moulec S, Soubeyran I, Italiano A, Godbert Y. Remarkable Response to Ceritinib and Brigatinib in an Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma Previously Treated with Crizotinib. Thyroid : official journal of the American Thyroid Association. 2020;30:343–4. [DOI] [PubMed] [Google Scholar]
- 82.Godbert Y, Henriques de Figueiredo B, Bonichon F, Chibon F, Hostein I, Pérot G, et al. Remarkable Response to Crizotinib in Woman With Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:e84–7. [DOI] [PubMed] [Google Scholar]
- 83.Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25:6662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toyokawa G, Seto T. Updated Evidence on the Mechanisms of Resistance to ALK Inhibitors and Strategies to Overcome Such Resistance: Clinical and Preclinical Data. Oncology research and treatment. 2015;38:291–8. [DOI] [PubMed] [Google Scholar]
- 85.Demeure MJ, Aziz M, Rosenberg R, Gurley SD, Bussey KJ, Carpten JD. Whole-genome sequencing of an aggressive BRAF wild-type papillary thyroid cancer identified EML4-ALK translocation as a therapeutic target. World J Surg. 2014;38(6):1296–1305. [DOI] [PubMed] [Google Scholar]
- 86.de Salins V, Loganadane G, Joly C, et al. Complete response in anaplastic lymphoma kinase-rearranged oncocytic thyroid cancer: A case report and review of literature. World J Clin Oncol. 2020;11(7):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article of published data accessible ad hoc by request.


