ABSTRACT
Viruses are masters at using cellular pathways to aid their replication. Cryo-electron tomography of poliovirus-infected cells revealed how it utilizes macroautophagy to its advantage. Assembly of these non-enveloped virions takes place directly on membranes and requires PIK3C3/VPS34 activity to be completed, whereas the canonical autophagy inducer ULK1 restricts virus assembly. The tomograms further revealed that enterovirus-induced autophagy is selective for RNA-loaded virions, which may help ensure maximum infectivity of the virus-laden vesicles released through secretory autophagy.
KEYWORDS: Autophagy, cryo-electron tomography, cryo-EM, enteroviruses, membrane trafficking, membranes, poliovirus, virology, virus replication
Poliovirus belongs to the enterovirus genus. This large group comprises hundreds of viruses that infect animals and humans causing everything from simple common colds to serious illnesses. Enterovirus particles are small (30-nm diameter), icosahedral and non-enveloped (meaning they do not contain a lipid membrane). The capsid encloses one copy of the positive-sense, single-stranded RNA that, when delivered to the cytoplasm, is instantly recognized as an mRNA by the host-cell ribosome.
As with all positive-sense RNA viruses, enteroviruses hijack cytoplasmic membranes to sustain their genome replication. The virus uses this strategy to gather all necessary viral and cellular components on a membrane scaffold, allowing efficient replication of viral RNA while shielding it from innate immunity detection. In early infection, the secretory pathway organelles are disassembled to harness their membranes. Later in infection – typically around 6 h after virus entry – macroautophagy (henceforth autophagy) is induced. This supplies additional membranes to support genome replication and is additionally known to be involved in release of viruses in secretory autophagy vesicles. In principle, autophagy could trap viruses in the cytoplasm and deliver them to the lysosome for degradation, therefore decreasing the number of replicative virions. However, enteroviruses have evolved several mechanisms to overcome the antiviral role of autophagy, while utilizing it for their benefit.
In the present study [1], we took advantage of technological developments in cryo-electron tomography (cryo-ET) combined with focused-ion-beam sample preparation methods to image the flash-frozen cytoplasm of poliovirus-infected cells in three dimensions at nanometer resolution. A major reason for using this methodology was the fact that decades of conventional EM of enterovirus infection had never unambiguously visualized the capsids inside infected cells. Cryo-ET, conversely, gives detailed views of the infected cytoplasm with abundant macromolecular details including newly produced empty capsids and RNA-loaded virions (Figure 1(a)). The in situ structural analysis undeniably confirmed the extensive host cell reorganization upon poliovirus infection, especially at the later 6 h time point shown in Figure 1(a), where an accumulation of autophagy-like membranes are seen accompanied by an increase of the MAP1LC3/LC3-II:LC3-I ratio. The tethering of capsids to the cytoplasmic faces of replication membranes by a still unknown protein complex correlates with their RNA loading. Surprisingly, even the assembly of these membrane-less capsids happens directly on membranes – both single and autophagy-like membranes (Figure 1(b)). The frequently observed assembly intermediate is structurally equivalent to half a capsid, and more than 90% of such intermediates are found docked to replication membranes.
Figure 1.
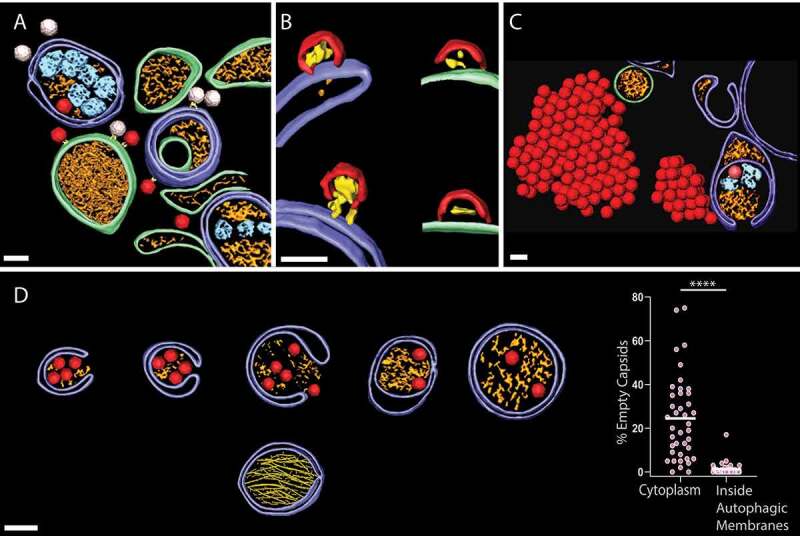
Cryo-electron tomography of the interplay between poliovirus replication and autophagy. (a) A cryo-electron tomogram acquired 6 h post infection provides a 3D view of the extensive reorganization of the cytoplasm by poliovirus. Dark red, RNA-loaded virions; white, empty capsids; purple, autophagy-like membranes; green, single-membrane structures; gold and cyan, vesicle contents. (b) Membrane-located capsid assembly intermediates (red). (c) Increased intracellular accumulation of new virions upon ULK1 inhibition. Color scheme as in A. (d) Upper row shows a series of phagophores and autophagosomes engulfing RNA-loaded virions. The lower image shows a separate type of autophagic membrane that contains bundles of protein filaments. Right: fraction of new capsids being empty (i.e., not containing the viral genome) in the cytoplasm and in the lumen of autophagic membranes. Each dot is one tomogram. (a-d) Scale bars: 50 nm.
Interestingly, we found that pharmacological inhibition of the class III phosphatidylinositol 3-kinase PIK3C3/VPS34 blocks poliovirus particle formation at the half-capsid intermediate stage. PIK3C3/VPS34 plays essential roles in endocytosis and autophagosome formation. To exclude an effect on virion endocytosis, the PIK3C3/VPS34 inhibitor Vps34-IN1 was added post-infection. Cryo-electron tomograms allow direct quantification of the concentration of structures in cells, and we could thus measure a near two-orders-of-magnitude decrease of intracellular complete virions upon PIK3C3/VPS34 inhibition, whereas the density of capsid intermediates docked on membranes remains unchanged. This effect is mirrored by a drastic decrease in released virions from PIK3C3/VPS34-inhibited cells. The effect of PIK3C3/VPS34 inhibition is not mediated by LC3 lipidation because PIK3C3/VPS34 inhibition still leads to a drastic decrease of virion release from LC3 triple knockout cells.
Given the robust autophagy induction by enteroviruses, it appeared puzzling that enteroviruses are reported to degrade the canonical autophagy inducer ULK1. To shed light on this, we infected cells treated with MRT68921, a small molecule inhibitor of ULK1 and its paralog ULK2. Cryo-electron tomography on these cells gave an unambiguous visual proof of the benefit to the virus of shutting down ULK1: the inhibited cells are chock-full of new virions, to the extent that they form extensive crystal-like arrays in the cytoplasm (Figure 1(c)). Virus release assays show a tenfold increase of released virions at a time point 2 h after that of the imaging, and the virions are still released in LC3-positive vesicles as shown by western blotting of released material and direct cryo-ET visualization of virus-containing vesicles just outside the plasma membrane. Consistent with findings of colleagues, this paints the picture of enteroviruses being dependent on several subsystems of autophagy, while still degrading as much of ULK1 as they can because it has the potential to limit virus replication.
Because secretory autophagy is known to be the origin of virus-containing extracellular vesicles, we next investigated whether this virus-induced form of autophagy has any kind of selectivity. The tomograms are indeed detailed enough that we can use them to catalog autophagic membranes based on their contents (Figure 1(a,d)). We calculated the percentage of capsids that are empty (i.e., lacking genome) in the cytoplasm and inside autophagic membranes. Strikingly, this revealed a strong selectivity in the virus-induced autophagy for RNA-loaded virions over empty capsids (Figure 1(d)). The mechanism of this selection is still mysterious, but its significance is clear: selecting only RNA-loaded, and hence infectious, particles for secretory autophagy will increase the infectivity of the released material. Last, while more than 50% of autophagic membranes contain virions, we observed several non-viral structures trapped in them. In particular, a distinct class of autophagic membranes contain protein filament bundles (Figure 1(d), lowermost autophagosome). We provided an initial structural characterization of these filaments and show that their formation is dependent on both LC3 and GABARAP proteins, representing additional proof of complexity of the virus-induced autophagy.
In summary, cryo-ET analysis of poliovirus-infected cells discovered several novel aspects of the crosstalk between poliovirus replication and autophagy. This analysis clearly raised as many new questions as it answered. The potential for higher-resolution subtomogram averages of the novel structures, for example, holds promise for further insights.
Acknowledgement
We would like to thank Daniel Klionsky for proofreading our manuscript.
Funding Statement
This work was supported by the European Commission [795892]; Human Frontier Science Program [CDA00047/2017-C]; Vetenskapsrådet [2021–01145]; Vetenskapsrådet [2018–05851].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Dahmane S, Kerviel A, Morado DR, et al. Membrane-assisted assembly and selective secretory autophagy of enteroviruses. Nat Commun. 2022;13(1):5986. [DOI] [PMC free article] [PubMed] [Google Scholar]


