Abstract
The classical view of the renin-angiotensin system (RAS) is that of the circulating hormone pathway involved in salt and water homeostasis and blood pressure regulation. It is also involved in the pathogenesis of cardiac and renal disorders. This led to the creation of drugs blocking the actions of this classical pathway, which improved cardiac and renal outcomes. Our understanding of the RAS has significantly expanded with the discovery of new peptides involved in this complex pathway. Over the last two decades, a counter-regulatory or protective pathway has been discovered that opposes the effects of the classical pathway. Components of RAS are also implicated in the pathogenesis of obesity and its metabolic diseases. The continued discovery of newer molecules also provides novel therapeutic targets to improve disease outcomes. This article aims to provide an overview of an updated understanding of the RAS, its role in physiological and pathological processes, and potential novel therapeutic options from RAS for managing cardiorenal disorders, obesity, and related metabolic disorders.
Keywords: cardio-protection, cardiovascular, hypertension, obesity, renin-angiotensin-aldosterone system
Introduction and background
The renin-angiotensin system (RAS) is a complex hormonal pathway that is a critical regulator of blood volume, electrolyte balance, and systemic vascular resistance. The classical understanding of RAS is that it comprises three significant components: renin, angiotensin II, and aldosterone [1, 2]. The discovery of new peptides in the last few decades has increased our understanding and the complexity of the RAS [3]. These new peptides form a counter-regulatory pathway of the RAS, which oppose the actions of the classic arm [4]. More recent studies continue to enhance our understanding of the newer peptides in the RAS and cross-talk between the two main pathways and their receptors.
The RAS is ubiquitous with the involvement of multiple organ systems, especially the kidneys, lungs, systemic vasculature, adrenal cortex, and brain [3]. The traditional view of circulating RAS is that of an endocrine system. Discoveries over the last few decades have led to a change in this view. Recent findings show that RAS components are produced in various tissues around the body, with paracrine and autocrine effects in these tissues [5, 6]. Local or tissue RAS (tRAS) is present in the heart, kidneys, adrenals, blood vessels, brain, adipose tissue, ovaries, testes, and skin [7, 8].
Both circulating and tRAS have a significant role in the physiological process of salt and water homeostasis, blood pressure regulation, and multiple cellular processes in different organ systems. They also have a significant role in pathophysiological conditions of hypertension, heart failure, other cardiovascular diseases, renal diseases, obesity, and metabolic disorders [5, 9]. In addition to describing the classical pathway of RAS, a novel and more detailed understanding of the counter-regulatory pathway of RAS will be discussed in this article.
Review
Classical pathway
Prorenin
Prorenin is an inactive precursor of renin, consisting of prosegment with 43 amino acids at the N-terminal compared to renin [10]. Many tissues in the body constitutively release prorenin. So far, no acute processes are known to induce the release of prorenin. Prorenin is produced in multiple tissues, including juxtaglomerular cells in the kidney, adrenal gland, placenta, uterus, retina, testes, and submandibular glands (also a part of local RAS) [11, 12]. The concentration of prorenin in the blood is thought to be ten times higher than that of renin under normal circumstances [13]. This concentration increases to 40-200 times that of renin in patients with diabetes complicated by retinopathy, nephropathy, and pregnancy [14-16]. The function of blood prorenin is not yet known [15].
Renin
Within the afferent arterioles of the kidney, specialized cells called juxtaglomerular (JG) cells contain prorenin. While prorenin is secreted constitutively in its inactive form, activation of JG cells causes the cleavage of prorenin to renin. This activation of prorenin to renin can be proteolytic or non-proteolytic [17]. The proteolytic activation of prorenin occurs in the kidney by enzymes like proconvertase 1 and cathepsin B [18, 19]. Mature renin is then stored in the granules of the JG cells and released into circulation by specific stimuli.
There are four primary stimuli for the release of renin [20-22]: 1) Changes in renal perfusion perceived by the pressure transducer mechanism in afferent arterioles (sense stretch from the mechanoreceptors of the arteriolar wall); 2) Delivery of NaCl (sodium chloride) to the distal convoluted tubule that is sensed by the chemoreceptors in the macular densa cell (which forms the JG apparatus with the JG cells in the afferent arteriole); 3) Increased beta-sympathetic flow acting through the beta-1 adrenergic receptors, particularly in the upright posture; 4) Negative feedback from humoral factors like angiotensin I, potassium (renin release is increased by hypokalemia and decreased by hyperkalemia), and ANP (atrial natriuretic peptide). Therefore, conditions leading to decreased renal perfusion and reduced tubular sodium content lead to renin enzyme release into the bloodstream. The half-life of plasma renin activity is 10-15 minutes [23]. Renin is the rate-limiting enzyme in RAS [24].
(Pro)renin receptor ((P)RR)
(P)RR is an ancient molecule present in nematodes, fish, and other mammals [25]. Its structure is highly conserved, with high structural homology between species [25]. It is ubiquitously expressed as a 350 amino acid, single transmembrane protein (also a part of local RAS) [26]. It was first cloned in 2002 [27]. (P)RR has multiple ligands that induce intracellular signaling pathways [28]. Both renin and prorenin bind to (P)RR, with prorenin having a greater affinity to bind to this receptor than renin [27, 29, 30]. When renin binds to (P)RR, it undergoes a conformational change, allowing the substrate (angiotensinogen) to attach. The attachment of renin to the (P)RR also increases its catalytic efficiency by fourfold to convert angiotensinogen to angiotensin I (Ang I) [27]. Besides its role in the RAS, this receptor has been demonstrated to play a role in many physiological functions (via different ligands), including the cell cycle through cell proliferation and differentiation, acid-base balance, autophagy, energy metabolism, T-cell homeostasis, embryonic development, blood pressure regulation, water balance, and maintenance of podocyte function [25, 26, 28, 31-33].
Studies have demonstrated prorenin and renin to affect disease pathogenesis independent of Ang II significantly and are receptor-mediated. In the presence of an Ang II type 1 receptor blocker (losartan), these molecules activate various mitogen-activated protein (MAP) kinases (p38 and P42/44). Activating the p38 MAPK/HSP27 pathway resulted in alterations in the actin filaments in cardiomyocytes, which could explain their role in hypertrophy and cardiomyopathy [34]. In the presence of a direct renin inhibitor (remikerin), Ang II type 1 receptor blocker (losartan), and angiotensin-converting enzyme blocker (enalapril), activation of (P)RR by renin in mesangial cells was shown to induce the p42/44 MAPK pathway [35]. This led to increased production of the fibrogenic cytokine transforming growth factor (TGF)-beta, which is also known to be directly induced by Ang II via the angiotensin type 1 receptor [36-38]. Renin also led to increased levels of plasminogen activator inhibitor-type 1 (PAI-1), collagen 1 messenger RNA (mRNA) and protein, and fibronectin (FN), which are known to induce fibrosis and apoptosis. This Ang II independent effect has been seen even within the central nervous system, with activation of the (P)RR by prorenin and renin leading to increase sympathetic outflow with the possible pathogenesis of resulting hypertension [39, 40].
Angiotensinogen
This molecule is a 485 amino acid alpha 2-globulin primarily synthesized and constitutively secreted by the liver. However, angiotensinogen mRNA has been found in many other tissues (as a part of local RAS), including the heart, brain, kidney, adrenal gland, placenta, ovary, vascular and adipose tissues [41, 42]. Renin cleaves this large molecule's N-terminal and leads to the formation of angiotensin I.
Angiotensin I (Ang I)
This is considered a biologically inert decapeptide, Ang (1-10) [43].
Angiotensin-Converting Enzyme 1 (ACE1)
This enzyme is an exopeptidase expressed on plasma membranes of vascular endothelial cells, mainly in pulmonary circulation [44]. It cleaves the two amino acids from the dipeptide Ang I (1-10) carboxy-terminal to make the octapeptide Angiotensin II or Ang II (1-8). This enzyme is also expressed in intestinal and urogenital tracts, various tissues within the heart, adipose tissue, and neuronal cells in the brain [44-47].
Angiotensin II (Ang II)
Ang II is the primary mediator of the physiological effects of RAS, including volume regulation, blood pressure, and aldosterone secretion [48]. However, the half-life of Ang II in circulation is very short (<60 seconds) [49]. This raises the possibility of Ang II being produced close to the site of action, possibly functioning as part of local RAS in different tissues [9].
The physiological effects of Ang II on extracellular volume and blood pressure regulation are mediated in six ways: 1) Inducing vasoconstriction by contraction of the vascular smooth muscle [50]; 2) Activation of Na-H exchanger on the proximal tubular cells, causing an increase sodium reabsorption [51, 52]; 3) Aldosterone secretion from the adrenal cortex in the zona glomerulosa [50, 53]. This is mediated through the transcription of CYP11B2 (which encodes aldosterone synthase) [54]; 4) Increasing central sympathetic outflow and stimulatory action on the sympathetic ganglia [55]; 5) Increasing release of catecholamines from the adrenal medulla and direct ganglion stimulation [56, 57]; and 6) Increasing release of vasopressin from the hypothalamus-posterior pituitary [58].
Ang II is implicated in many pathophysiological states and is known to induce oxidative stress, vascular smooth muscle contraction, endothelial dysfunction, fibrosis, and hypertrophic, anti-apoptotic, and pro-mitogenic effects [59-61]. Ang II has been implicated in the pathogenesis of hypertension, atherosclerotic disease, heart failure, obesity-mediated hypertension, and kidney disease through these effects [5, 62-65]. In obese individuals, high angiotensin II levels lead to increased body fat accumulation and the development of insulin resistance [66]. Weight loss was associated with reduced circulating Ang II levels [66].
Ang II's physiological and pathophysiological effects are mediated by two types of Ang receptors: type 1 and type 2 [67]. These receptors have different and often opposing physiological responses attributed to the cell signaling pathways: the AT1-R receptor stimulates protein phosphorylation, and the AT2-R receptor stimulates dephosphorylation [68].
Angiotensin II Type 1 Receptor (AT1-R)
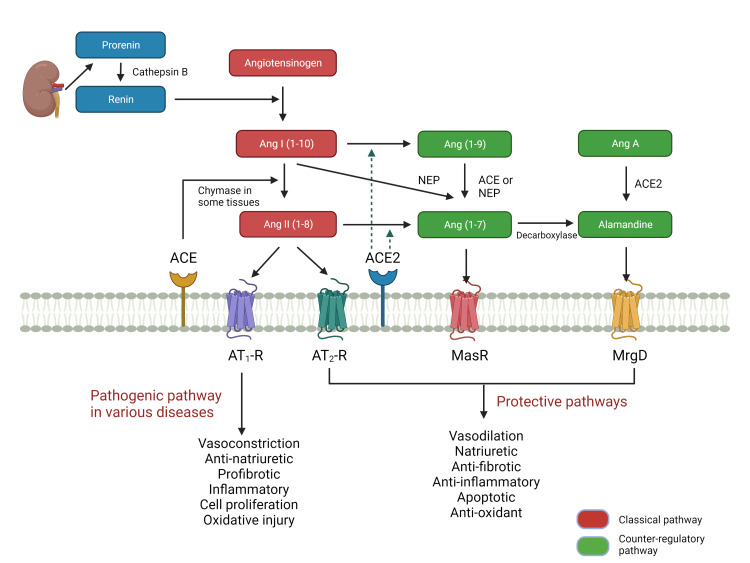
It is a G-protein coupled receptor [69]. It is widely distributed in many cell types, including the heart, vasculature, kidney, adrenal glands, pituitary, and central nervous system [70-73]. Ang II mediates its physiological effects of vasoconstriction and sodium and water reabsorption through the AT1-R [74]. In pathogenic states, the activation of the AT1-R leads to inflammation, fibrosis, oxidative stress, tissue remodeling, and increased blood pressure [75]. Activation of this receptor leads to activation of ADAM17, a disintegrin and metalloproteinase 17), which leads to multiple intracellular cascade effects. ADAM17 increases the transcription of EGFR (epidermal growth factor receptor), which leads to the activation of other downstream kinases, leading to increased fibrosis and vascular remodeling [74, 76]. ADAM17 produces TNF (tumor necrosis factor) alpha in the kidney, leading to an elevation in blood pressure [77]. ADAM17 cleaves and inactivates ACE2, thereby counteracting the beneficial effects of the counter-regulatory pathway, which is central to balancing the pathogenic aspects of the classical RAS pathway (Figure 1) [78]. The dysregulation of this receptor is central to the pathophysiology of cardiac and renal diseases [74, 79, 80]. This receptor is selectively blocked with angiotensin-receptor-blocking drugs or “-sartans.”
Figure 1. Classical and counter-regulatory pathways in the renin-angiotensin system.
Ang: Angiotensin; ACE: Angiotensin-converting enzyme; NEP: Neutral endopeptidase; AT-R: Angiotensin receptor; MrgD: Mas-related G-protein coupled receptor, member D; MasR: Mas receptor. Created using BioRender.com
Angiotensin II Type 2 Receptor (AT2-R)
It is a G-protein coupled receptor [69]. This receptor has a lower degree of expression compared to AT1-R. It exhibits only about 34% homology to AT1-R [81]. It is widely expressed in fetal tissues, and this expression decreases significantly in adult life [68, 82]. In adults, it is expressed in the heart, kidney, adrenal glands, and brain [83-85]. Despite its low expression, it is essential in mediating protective and opposing effects to Ang II via the AT1-R. These actions inhibit inflammation, fibrosis, and central sympathetic outflow and cause vasodilation and neuroregeneration [86, 87]. Stimulation of the AT2-R by Ang II leads to vasodilation and natriuresis, opposite to the vasoconstriction and anti-natriuresis caused by Ang II via the AT1-R [68, 88, 89].
Aldosterone
This steroidogenic hormone is synthesized in the zona glomerulosa of the adrenal cortex. The synthesis and secretion of this hormone are mainly regulated by angiotensin II, ACTH (adrenocorticotropic hormone), and extracellular potassium concentration [90, 91]. The effects of aldosterone are mediated through nuclear cytosolic receptors [92]. Mineralocorticoid receptors (MR) are found in the kidney and colon epithelial cells (for sodium transport) and the non-epithelial tissues in the heart and brain [93]. The half-life of aldosterone in plasma is less than 20 minutes [94].
Epithelial effects (classic effects): Aldosterone mediates its effects on electrolyte and water homeostasis by binding to the MR receptors on principal epithelial cells in the renal cortical collecting duct. Sodium is reabsorbed via the ENaC (epithelial sodium channel) on the apical membranes of principal cells in the collecting tubules. Aldosterone inactivates Nedd 4-2 (neural-precursor-cell-expressed, developmentally downregulated gene 4-2), which degrades ENaC [95, 96]. Aldosterone increases transcription of Sgk1 (serum and glucocorticoid inducible kinase 1), leading to the inactivation of Nedd 4-2 by phosphorylation - the number of active ENaC increase, leading to increased reabsorption of sodium [94, 97]. Aldosterone activates Na-K ATPase at the apical cells' basolateral membrane [98]. This leads to sodium transport in the extracellular space and increases potassium uptake in the apical cells.
Non-epithelial effects (non-classic effects): The effects of aldosterone on the cardiovascular and central nervous systems are complex and multifaceted. 1) Cardiovascular system: Aldosterone can increase vascular tone, potentially by increasing the production and sensitivity of catecholamines and decreasing responsiveness to the vasodilatory effects of acetylcholine [99, 100]. It also leads to the upregulation of Ang II receptors [99]. At physiologic levels, aldosterone induces the production of nitric oxide synthase [101]. Aldosterone can impair vascular function at pathogenic levels by reducing nitric oxide availability [101-103]. Aldosterone promotes the growth and remodeling of cardiac cells, which can lead to the development of cardiac inflammation, hypertrophy, and fibrosis [104, 105]; 2) Central nervous system: The effects of MR on the brain are context-dependent. We will limit the discussion to salt and water homeostasis. MRs are highly expressed in regions that regulate stress responses, including the hippocampus, amygdala, cerebellum, and prefrontal cortex [106, 107]. These regulate thirst, salt appetite, and blood pressure [108, 109].
Counter-regulatory (protective) pathway
Angiotensin-Converting Enzyme 2 (ACE2)
The discovery of this enzyme was first reported in 2000 [110]. This enzyme is a monocarboxypeptidase with the closest structural homology to ACE1 [111]. The catalytic domain of the ACE2 enzyme is 42% similar to ACE1 [110]. Two substrates of ACE2 in the RAS are Ang I (1-10) and Ang II (1-8). The catalytic efficiency of ACE2 is 300 times higher for Ang II than Ang I [111]. ACE2 is also widely expressed in the lungs, cardiovascular system, kidneys, adipose tissue, and brain [112-115]. The membrane-bound ACE2 levels are regulated by a metalloproteinase ADAM17, which cleaves it and creates a soluble ACE2 [116, 117]. Ang II (via AT1-R activation) is known to upregulate ADAM17 activity and decrease the activity of membrane-bound ACE2, potentially enhancing pathogenic disease processes [74, 118].
Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), which was responsible for the 2002-2004 SARS epidemic, was found to use ACE2 as its functional receptor. Previous studies have shown a significant amount of surface expression of ACE2 on lung alveolar epithelial cells and enterocytes of the small intestine [114]. These findings of tissue distribution helped us understand the disease pathogenesis. During the COVID-19 (coronavirus disease 2019) pandemic that started in 2019, the SARS-CoV-2 virus was also seen to use the ACE2 protein as its functional receptor [119, 120].
Angiotensin (1-9)
ACE2 converts Ang I (1-10) to Ang (1-9) by the hydrolysis of the C-terminal leucine [110]. This protein has been found to affect the cardiovascular system through its interaction with the AT-R [121-123]. It has anti-hypertrophic effects [121, 122, 124, 125]. Furthermore, it reduces hypertension-induced cardiovascular and renal inflammation [124, 126]. In addition, it causes blood pressure reduction through different mechanisms like vasodilation and natriuresis in experimental models [124, 126].
Angiotensin (1-7)
This peptide is the most important active product of ACE2. It has been determined to be a critical regulator of ACE2-mediated counter-regulation or protective pathway of RAS [3]. It is formed by the hydrolysis of C-terminal phenylalanine from Ang II (1-8) [127, 128]. Ang (1-7) can also be produced by NEP (neutral endopeptidase) activity on Ang I (1-10) and by propyl carboxypeptidase on Ang II (1-8) [129]. The half-life for circulating Ang (1-7) is approximately 10 seconds due to rapid metabolism by peptidases such as ACE 1 and dipeptidyl peptidase 3 [130-132]. Ang (1-7) has multiple biological activities opposing Ang II (1-8) actions [133-135]. It induces anti-inflammatory, vasodilatory, antiangiogenic, antihypertensive, and antifibrotic effects by binding to its G-protein-coupled receptor, MasR [136, 137]. Ang-(1-7) also improves lipid and glucose homeostasis [138]. In addition, Ang (1-7) prevented the development of left ventricular systolic or diastolic dysfunction in male Sprague-Dawley rats after induction of myocardial infarction [139].
A proto-oncogene MAS1 encodes MasR [140]. MasR is expressed in the brain, testes, heart, kidney, and blood vessels [4]. The Mas-related G-protein coupled receptor, member D (MrgD), is the second receptor for Ang (1-7) [141]. Ang (1-7) increases intracellular cAMP (cyclic adenosine monophosphate) levels, phosphokinase A (PKA) activity, and cAMP response element-binding phosphorylation via its action on MasR and MrgD receptors [141]. In addition, Ang (1-7) also activates the phosphatidylinositol-3-kinase-Akt pathway leading to nitric oxide synthase activation in the heart [142, 143].
Ang (1-7) can bind to the AT1-R at very high concentrations and potentially antagonize the effects of Ang II on this receptor [144]. Another study showed blockade of phenylephrine-mediated vasoconstriction by the action of Ang (1-7) on AT1-R (in the presence of MasR and AT2-R antagonists); this effect was lost in the AT1-R knockout mice [145]. It had been postulated that some of the Ang (1-7) effects might be mediated via the AT2-R [136, 146]. This was challenged by a later study, which showed the lack of increase in intracellular cAMP mediated by Ang (1-7) using an AT2-R blocker [141]. Hence, the effect of Ang (1-7) on AT2-R remains unclear. This further proves that the interactions between different components of the RAS via different receptors are very complex.
Alatensins
This term was introduced by Santos et al. for peptides created by the decarboxylation of the aspartate (Asp) residues of the angiotensin molecules to alanine (Ala) [3]. The first described member in this group (in 2008) was an octapeptide Ang A, created by the decarboxylation of the aspartate on Ang II (1-8) residue to alanine [147]. The second member of this group (first described in 2013) is a heptapeptide, Alamandine (Ala1-Ang-(1-7)) [148].
Angiotensin A (Ang A)
The affinity of Ang A to the AT1-R is similar to that of Ang II, but it exhibits a higher affinity to the AT2-R. Ang A has a lower intrinsic activity at the AT1-R, translating to weaker vasoconstrictive and hypertensive effects [147]. Under normal conditions, the concentration of Ang A (Ala1-Ang-(1-8)) is about 20% that of Ang II (1-8) [149]. The ratio of Ang A/Ang II is significantly increased in end-stage renal disease [147]. Due to its more potent effects at the AT2-R, it may oppose the deleterious effects of Ang II [147]. The downstream impact of MasR remains to be elucidated.
Alamandine (Ala)
Two mechanisms generate this heptapeptide: 1) Action of ACE2 on Ang A and 2) Decarboxylation of aspartate residue to alanine on Ang (1-7)[148, 150]. Alamandine acts through MrgD [148]. MasR is also the functional receptor for Ala [151]. Ala leads to a reduction in blood pressure through vasodilation, and a reduction in oxidative stress and inflammation [148, 152]. Ala attenuated fibrosis and cardiac dysfunction due to chronic hypertension in preclinical models [153]. In rats, Ala reduced hypertension and renal damage from a high salt diet by inhibiting protein kinase C. Protein kinase C causes an increase in reactive oxygen species [154]. In Sprague-Dawley rats, infusion of Ala before induced global cardiac ischemia leads to attenuation of reperfusion injury by improving post-ischemia LV pressure, and coronary flow, decreasing apoptotic protein expression, and increasing anti-oxidative protein expression. Ala is protected against renal reperfusion injury and renal dysfunction induced by a high salt diet in rats [154, 155]. These effects were through the action of Ala on the MrgD receptor [156]. In addition to reducing cardiac and renal injury, Ala, via its receptor MrgD, also reduces pulmonary fibrosis by reducing oxidative damage and increasing autophagy [157]. Alamandine could have unique effects, different from those of Ang (1-7), via its action on the MrgD receptor [158].
MrgD receptor
MrgD is widely expressed in the central nervous system, heart, kidney, sensory neurons of dorsal root and trigeminal ganglia, gastrointestinal tract, respiratory tract, and skin [159-162]. However, the downstream effects of MrgD remain to be elucidated.
Local or tissue RAS (tRAS)
The tissue renin-angiotensin system (tRAS) refers to the local production and action of the renin-angiotensin system (RAS) components in tissues outside the circulating RAS. These tissue systems produce critical components of RAS's classical and counter-regulatory pathways. Angiotensin II is produced by ACE1 and chymase (ACE-independent) action in various tissues [163]. The activity of cathepsin D on angiotensinogen in some tissues creates angiotensin I and the action of cathepsin G on angiotensinogen directly makes angiotensin II [164]. Dysregulation of the tRAS has been implicated in the pathogenesis of several diseases, including hypertension, heart failure, diabetic nephropathy, and metabolic disorders, including obesity [5, 165, 166].
Heart
The role of tRAS in the heart is to regulate cellular processes in various tissues in response to different stimuli, including ischemia. Various components of RAS are produced within multiple tissues, including renin, angiotensin II, ACE1, ACE2, and Ang (1-7). The local production of angiotensin II is also thought to be regulated by enzymes such as chymase, which is abundant in cardiac mast cells, in addition to the activity of ACE1 [167, 168]. The older hearts in animal models produce Ang II (1-8) primarily through an ACE1-independent pathway via the action of chymase [163]. The heart expresses both main types of angiotensin receptors: AT1-R and AT2-R, effects of Ang II (1-8) through these receptors can lead to cardiac remodeling [169]. Components of the counter-regulatory RAS pathway also produce and affect various physiological and pathogenic processes [7, 170]. The circulating and tissue RAS are thought to exert combined effects on multiple organ systems.
In human cardiomyocytes, ACE2 is expressed in atria and ventricles, smooth muscle cells, fibroblasts, and endothelial cells [171]. In addition to the action of ACE2, Ang (1-7) can be produced in the heart from Ang I (1-10) by propyl endopeptidase and neural endopeptidase (NEP) [129]. Ang (1-7) exerts various cardio-protective effects locally in the cardiac tissues. These include anti-hypertensive, anti-proliferative, anti-fibrotic, anti-arrhythmogenic, and anti-inflammatory effects [172]. MasR expression in the heart to various physiological and pathophysiological stimuli was demonstrated in DOCA (deoxycorticosterone acetate)-salt rats [173]. MrgD receptor is expressed in cardiomyocytes in rats. Genetic deletion of MrgD in rats led to the development of cardiomyopathy, highlighting the importance of this receptor and potentially of its ligand, alamandine, in the regulation of normal cardiac function [174].
Adipose Tissue
In the adipose tissue tRAS, all components of RAS are expressed. Under physiological conditions, these components affect adipose tissue development and differentiation through autocrine/paracrine effects [175, 176]. Adipose tRAS has been involved in developing obesity and its metabolic complications. Overactivation of the RAS in the white adipose tissue has been associated with the development of obesity, insulin resistance, glucose intolerance, and hypertension [177, 178]. In humans, expression of angiotensinogen mRNA is upregulated in obese individuals, which might contribute to the increased levels of local and circulating angiotensin II levels [179]. Angiotensinogen mRNA expression is higher in visceral adipose tissue than the subcutaneous adipose tissue in obese humans [180-182]. Nutritional status and BMI positively correlated with the expression of angiotensinogen mRNA [180]. A high-fat diet upregulated its expression while fasting, caloric restriction, and weight loss decreased its expression [183-185]. Angiotensinogen deficiency in adipocytes of mice attenuated obesity-related hypertension [186]. In rats, angiotensinogen and Ang II (1-8) influence the development and differentiation of white adipose tissue by releasing prostacyclin from adipocytes through autocrine/paracrine effects [175, 187, 188].
Administration of recombinant human ACE2 (rhACE2) significantly attenuated weight gain in high-fat diet (HFD)-fed mice, irrespective of food intake. This was due to increased energy expenditure (increased expression of uncoupling protein-1) through differentiation of brown adipose tissue (BAT), increase in BAT mass, and browning of subcutaneous white adipose tissue (sWAT). Administration of rhACE2 also improved insulin sensitivity, measured by homeostasis model assessment [189]. Ang (1-7) infusion led to similar results in HFD-fed mice [190]. In a different study, Ang (1-7) administration in HFD-fed mice led to decreased visceral adipose tissue expansion and suppressed lipogenesis via the action of Ang (1-7) on the MasR [191]. The role of alamandine on adipose tissue remains to be elucidated. A recent study demonstrated increased white adipose tissue (WAT) and reduction in BAT in MrgD knockout mice [192]. Nitric oxide (NO) and 5’-AMP-activated protein kinase (AMPK) regulated glucose metabolism through increased expression and expression of GLUT4 in the skeletal muscles [193]. Alamandine leads to the activation of the NO/AMPK pathway in mice via the MrgD receptor and might have a role in glucose metabolism [194]. This needs to be determined by future studies.
Brain
In the brain tRAS, angiotensin II is produced locally from angiotensinogen, independently of the systemic RAS. Renin has been found in neurons and astrocytes. The local production of angiotensin II is thought to be regulated by brain-specific enzymes such as chymase and cathepsin G. The brain tRAS expresses both AT1-R and AT2-R, which mediate the effects of locally produced angiotensin II [195]. The components for the counter-regulatory RAS are also present in the brain, including ACE2, Ang (1-7), MasR, and MgrD [195]. This tRAS is critical in regulating various physiological processes within the central nervous system (CNS), including blood pressure control, water balance, neuroendocrine regulation, and stress responses. Dysregulation of the brain tissue RAS has been implicated in the development of various neurological disorders, including hypertension, stroke, Alzheimer's disease, and Parkinson's disease [196].
Discussion
With the discovery of new components of the RAS, we have been able to improve our understanding of the role of RAS in various physiological and pathophysiological processes. RAS has important functions and pathogenic roles in various cardiac, renal, metabolic, and neurological functions and disorders. Traditionally, the focus was on the blockade of components of the classical pathway of RAS. These RAS blockers improved disease outcomes and contributed to an improved understanding of the RAS itself.
Blockade of the Classical Pathway of RAS or ACE/Ang II/AT1-R Axis Blockade
Direct renin inhibitor: This has not improved renal or cardiovascular outcomes in patients with type 2 diabetes [197, 198]. The use of this agent remains uncommon in clinical practice due to the lack of benefit noted from clinical trials.
Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB): These are used as first-line agents for the management of hypertension. These agents have improved cardiovascular (CV) outcomes, including reduced hospitalizations for heart failure and CV mortality [199-203]. These agents improve kidney outcomes like reduced microalbuminuria and slow the progression of chronic kidney disease and diabetic nephropathy [204-209]. These agents improved glycemic control in patients with type 2 diabetes by enhancing glucose uptake by skeletal muscle via the glucose transporter 4 (GLUT 4), thereby improving insulin sensitivity [210-212]. Interestingly, randomized controlled trials have shown a lower incidence of type 2 diabetes mellitus in individuals treated with ACEi and ARBs versus placebo [213-215]. These agents also exert positive effects on cardiac and renal outcomes through enhanced activity of the protective arm of the RAS [216, 217]. A randomized controlled trial to study the effects of olmesartan versus amlodipine in 80 patients with hypertension and type 2 diabetes showed a significant increase in ACE2 and Ang (1-7) levels in the olmesartan arm [218]. Inhibition of ACE1 in animal models leads to reduced food intake and body weight, and improved glucose tolerance and insulin sensitivity[219-221]. Large randomized controlled trials will be needed to prove these effects of ACEi on food intake, body weight, and glucose metabolism in humans.
Mineralocorticoid receptor antagonists (MRA): Spironolactone and eplerenone (steroidal MRAs) reduce hospitalizations and mortality in patients with heart failure with reduced ejection fraction [222, 223]. Finerenone (non-steroidal MRA) reduces heart failure-related hospitalizations and improves kidney outcomes in patients with diabetic kidney disease [224, 225];
Aldosterone synthase blocker: Baxdrostat, a selective aldosterone synthase inhibitor, has shown promising results in patients with resistant hypertension in a recent phase 2 clinical trial with dose-dependent reductions in blood pressure [226].
Current Therapeutic Endeavors Focus on Enhancing the Effects of the Counter-Regulatory (protective) Pathway of RAS
ACE2-Ang(1-7)-MasR axis and Ala-MrgD axis. Chymase inhibitors are another potential therapeutic option as this enzyme leads to ACE1-independent production of Ang II (1-10) in various tRAS. These agents might hold promise to improve cardiac and renal outcomes. They might also have potential in the treatment of obesity and metabolic disorders.
Current Therapeutic Options for the Treatment of Obesity Focus on Appetite Suppression
Enhancing energy expenditure by enhancing BAT mass and activity through the effects of the ACE2/Ang (1-7)/MasR axis might be a therapeutic strategy in the future. Pharmacological agents for this pathway include ACE2 activators, Ang (1-7) analogs, MasR agonists, alamandine analogs, and, MrgD receptor agonists. The use of these agents can further improve our understanding of RAS.
Conclusions
Discoveries of new peptides have greatly expanded our understanding of the renin-angiotensin system. Recent findings have also improved our knowledge about the pathogenesis process behind various cardiovascular, renal, and metabolic diseases. This has galvanized interest in finding newer therapeutic targets in RAS to improve cardiovascular and renal outcomes and reduce the burden of obesity and metabolic disorders. Currently, available drugs block the classical pathway of RAS. Extensive research is being done to create analogs and agonists of the counter-regulatory pathway of RAS. With the creation of such agents, there is potential for multi-target drugs that block the classical pathway and activate the counter-regulatory or protective pathway.
The authors have declared that no competing interests exist.
References
- 1.Classical and counter-regulatory renin-angiotensin system: potential key roles in COVID-19 pathophysiology. Almutlaq M, Alamro AA, Alroqi F, Barhoumi T. CJC Open. 2021;3:1060–1074. doi: 10.1016/j.cjco.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renin-angiotensin system and cardiovascular functions. Wu CH, Mohammadmoradi S, Chen JZ, Sawada H, Daugherty A, Lu HS. Arterioscler Thromb Vasc Biol. 2018;38:0. doi: 10.1161/ATVBAHA.118.311282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The renin-angiotensin system: going beyond the classical paradigms. Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. Am J Physiol Heart Circ Physiol. 2019;316:0. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7) Santos RAS, Sampaio WO, Alzamora AC, et al. Physiol Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.M. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Engeli S, Negrel R, Sharma AM. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 6.Physiology of local renin-angiotensin systems. Paul M, Poyan Mehr A, Kreutz R. Physiol Rev 86, 747-803. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 7.An update on the tissue renin angiotensin system and its role in physiology and pathology. Nehme A, Zouein FA, Zayeri ZD, Zibara K. J Cardiovasc Dev Dis. 2019;6:14. doi: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Update on tissue renin-angiotensin systems. Bader M, Ganten D. J Mol Med (Berl) 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 9.The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Atlas SA. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prorenin and other large molecular weight forms of renin. Sealey JE, Atlas SA, Laragh JH. Endocr Rev. 1980;1:365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- 11.Cellular biology of the renin-angiotensin systems. Re RN. Arch Intern Med. 1984;144:2037–2041. [PubMed] [Google Scholar]
- 12.Circulating versus tissue renin-angiotensin system: on the origin of (pro)renin. Krop M, Danser AH. Curr Hypertens Rep. 2008;10:112–118. doi: 10.1007/s11906-008-0022-1. [DOI] [PubMed] [Google Scholar]
- 13.Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. J Hypertens. 1998;16:853–862. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- 14.High plasma prorenin in diabetes mellitus and its correlation with some complications. Franken AA, Derkx FH, Man in't Veld AJ, et al. J Clin Endocrinol Metab. 1990;71:1008–1015. doi: 10.1210/jcem-71-4-1008. [DOI] [PubMed] [Google Scholar]
- 15.Prorenin as a reproductive hormone. New form of the renin system. Sealey JE, Glorioso N, Itskovitz J, Laragh JH. Am J Med. 1986;81:1041–1046. doi: 10.1016/0002-9343(86)90402-x. [DOI] [PubMed] [Google Scholar]
- 16.Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. Danser AH, van den Dorpel MA, Deinum J, et al. J Clin Endocrinol Metab. 1989;68:160–167. doi: 10.1210/jcem-68-1-160. [DOI] [PubMed] [Google Scholar]
- 17.Renin, prorenin and the putative (pro)renin receptor. Danser AH, Deinum J. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 18.Proteolytic processing of human prorenin in renal and non-renal tissues. Reudelhuber TL, Ramla D, Chiu L, Mercure C, Seidah NG. Kidney Int. 1994;46:1522–1524. doi: 10.1038/ki.1994.435. [DOI] [PubMed] [Google Scholar]
- 19.Cathepsin B is a prorenin processing enzyme. Neves FA, Duncan KG, Baxter JD. Hypertension. 1996;27:514–517. doi: 10.1161/01.hyp.27.3.514. [DOI] [PubMed] [Google Scholar]
- 20.Renin release. Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Physiology (Bethesda) 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 21.Control of renin synthesis and secretion. Kurtz A. Am J Hypertens. 2012;25:839–847. doi: 10.1038/ajh.2011.246. [DOI] [PubMed] [Google Scholar]
- 22.Renin release: sites, mechanisms, and control. Kurtz A. Annu Rev Physiol. 2011;73:377–399. doi: 10.1146/annurev-physiol-012110-142238. [DOI] [PubMed] [Google Scholar]
- 23.Half-life of plasma renin activity in normal subjects and in malignant hypertension. Skrabal F. Klin Wochenschr. 1974;52:1173–1174. doi: 10.1007/BF01466736. [DOI] [PubMed] [Google Scholar]
- 24.The preparation, purification, and amino acid sequence of a polypeptide renin substrate. Skeggs LT, Jr. Jr., Kahn JR, Lentz K, Shumway NP. J Exp Med. 1957;106:439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The biology of the (pro)renin receptor. Nguyen G, Muller DN. J Am Soc Nephrol. 2010;21:18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 26.The prorenin and (pro)renin receptor: new players in the brain renin-angiotensin system? Li W, Peng H, Seth DM, Feng Y. Int J Hypertens. 2012;2012:290635. doi: 10.1155/2012/290635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The (pro)renin receptor in health and disease. Ichihara A, Yatabe MS. Nat Rev Nephrol. 2019;15:693–712. doi: 10.1038/s41581-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 29.Biochemical properties of renin and prorenin binding to the (pro)renin receptor. Nabi AH, Suzuki F. Hypertens Res. 2010;33:91–97. doi: 10.1038/hr.2009.201. [DOI] [PubMed] [Google Scholar]
- 30.Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. Batenburg WW, Krop M, Garrelds IM, et al. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 31.(Pro)renin receptor in the kidney: function and significance. Arthur G, Osborn JL, Yiannikouris FB. Am J Physiol Regul Integr Comp Physiol. 2021;320:0. doi: 10.1152/ajpregu.00259.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Functions of the (pro)renin receptor (Atp6ap2) at molecular and system levels: pathological implications in hypertension, renal and brain development, inflammation, and fibrosis. Hoffmann N, Peters J. Pharmacol Res. 2021;173:105922. doi: 10.1016/j.phrs.2021.105922. [DOI] [PubMed] [Google Scholar]
- 33.Roles of the (pro)renin receptor in the kidney. Oshima Y, Morimoto S, Ichihara A. World J Nephrol. 2014;3:302–307. doi: 10.5527/wjn.v3.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Saris JJ, t Hoen PA, Garrelds IM, et al. Hypertension . 2006;48:564–571. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 35.Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Huang Y, Wongamorntham S, Kasting J, et al. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 36.Transforming growth factor beta in tissue fibrosis. Border WA, Noble NA. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 37.Angiotensin II stimulates canonical TGF-beta signaling pathway through angiotensin type 1 receptor to induce granulation tissue contraction. Ehanire T, Ren L, Bond J, et al. J Mol Med (Berl) 2015;93:289–302. doi: 10.1007/s00109-014-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Border WA, Noble NA. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 39.ANG II-independent prorenin/(pro)renin receptor signaling pathways in the central nervous system. Feng Y. Am J Physiol Heart Circ Physiol. 2015;309:0–733. doi: 10.1152/ajpheart.00526.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. Merrill DC, Thompson MW, Carney CL, et al. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angiotensinogen: molecular biology, biochemistry and physiology. Morgan L, Broughton Pipkin F, Kalsheker N. Int J Biochem Cell Biol. 1996;28:1211–1222. doi: 10.1016/s1357-2725(96)00086-6. [DOI] [PubMed] [Google Scholar]
- 42.Atlas of tissue renin-angiotensin-aldosterone system in human: a transcriptomic meta-analysis. Nehme A, Cerutti C, Dhaouadi N, et al. Sci Rep. 2015;5:10035. doi: 10.1038/srep10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renin-angiotensin-aldosterone system and immunomodulation. A state-of-the-art review. Laghlam D, Jozwiak M, Nguyen LS. Cells. 2021;10:1767. doi: 10.3390/cells10071767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angiotensin-converting enzyme and its clinical significance--a review. Studdy PR, Lapworth R, Bird R. J Clin Pathol. 1983;36:938–947. doi: 10.1136/jcp.36.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Falkenhahn M, Franke F, Bohle RM, et al. Hypertension. 1995;25:219–226. doi: 10.1161/01.hyp.25.2.219. [DOI] [PubMed] [Google Scholar]
- 46.The expression and localisation of the angiotensin-converting enzyme mRNA in human adipose tissue. Jonsson JR, Game PA, Head RJ, Frewin DB. Blood Press. 1994;3:72–75. doi: 10.3109/08037059409101524. [DOI] [PubMed] [Google Scholar]
- 47.The angiotensin converting enzyme (ACE) Coates D. Int J Biochem Cell Biol. 2003;35:769–773. doi: 10.1016/s1357-2725(02)00309-6. [DOI] [PubMed] [Google Scholar]
- 48.The angiotensin II type 1 receptor and receptor-associated proteins. Guo DF, Sun YL, Hamet P, Inagami T. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 49.Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Hypertension. 1997;30:42–49. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 50.The renal renin-angiotensin system. Harrison-Bernard LM. Adv Physiol Educ. 2009;33:270–274. doi: 10.1152/advan.00049.2009. [DOI] [PubMed] [Google Scholar]
- 51.Angiotensin II stimulation of Na-H antiporter activity is cAMP independent in OKP cells. Cano A, Miller RT, Alpern RJ, Preisig PA. Am J Physiol. 1994;266:0–1608. doi: 10.1152/ajpcell.1994.266.6.C1603. [DOI] [PubMed] [Google Scholar]
- 52.Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Cogan MG. Hypertension. 1990;15:451–458. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 53.Locally generated angiotensin II in the adrenal gland regulates basal, corticotropin-, and potassium-stimulated aldosterone secretion. Gupta P, Franco-Saenz R, Mulrow PJ. Hypertension. 1995;25:443–448. doi: 10.1161/01.hyp.25.3.443. [DOI] [PubMed] [Google Scholar]
- 54.Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. Nogueira EF, Xing Y, Morris CA, Rainey WE. J Mol Endocrinol. 2009;42:319–330. doi: 10.1677/JME-08-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Reid IA. Am J Physiol. 1992;262:0–778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 56.Angiotensin II induces catecholamine release by direct ganglionic excitation. Dendorfer A, Thornagel A, Raasch W, Grisk O, Tempel K, Dominiak P. Hypertension. 2002;40:348–354. doi: 10.1161/01.hyp.0000028001.65341.aa. [DOI] [PubMed] [Google Scholar]
- 57.Angiotensin II and the adrenal. Giacchetti G, Opocher G, Sarzani R, Rappelli A, Mantero F. Clin Exp Pharmacol Physiol Suppl. 1996;3:119–124. [PubMed] [Google Scholar]
- 58.Angiotensin II-induced vasopressin release is mediated through alpha-1 adrenoceptors and angiotensin II AT1 receptors in the supraoptic nucleus. Qadri F, Culman J, Veltmar A, Maas K, Rascher W, Unger T. https://jpet.aspetjournals.org/content/267/2/567. J Pharmacol Exp Ther. 1993;267:567–574. [PubMed] [Google Scholar]
- 59.Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Mehta PK, Griendling KK. Am J Physiol Cell Physiol. 2007;292:0–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 60.Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Rajagopalan S, Kurz S, Munzel T, et al. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theodore Cooper lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Dzau VJ. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 62.Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 63.The angiotensin II AT2 receptor is an AT1 receptor antagonist. AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 64.Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. Ferrario CM. J Renin Angiotensin Aldosterone Syst. 2006;7:3–14. doi: 10.3317/jraas.2006.003. [DOI] [PubMed] [Google Scholar]
- 65.Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2) Xu Z, Li W, Han J, et al. Sci Rep. 2017;7:44911. doi: 10.1038/srep44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Saiki A, Ohira M, Endo K, et al. Metabolism. 2009;58:708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 67.International union of basic and clinical pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected] Karnik SS, Unal H, Kemp JR, et al. Pharmacol Rev. 2015;67:754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Carey RM, Wang ZQ, Siragy HM. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 69.Structural basis for selectivity and diversity in angiotensin II receptors. Zhang H, Han GW, Batyuk A, et al. Nature. 2017;544:327–332. doi: 10.1038/nature22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Biochem Biophys Res Commun. 1992;183:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- 71.Brain angiotensin type-1 and type-2 receptors: cellular locations under normal and hypertensive conditions. Sumners C, Alleyne A, Rodriguez V, et al. Hypertens Res. 2020;43:281–295. doi: 10.1038/s41440-019-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II-angiotensin type 1 receptor system. Iwanaga Y, Kihara Y, Takenaka H, Kita T. J Mol Cell Cardiol. 2006;41:798–806. doi: 10.1016/j.yjmcc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Localization and function of angiotensin AT1 receptors. Allen AM, Zhuo J, Mendelsohn FA. Am J Hypertens. 2000;13:31–38. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 74.Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Eguchi S, Kawai T, Scalia R, Rizzo V. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angiotensin AT1/AT2 receptors: regulation, signalling and function. Kaschina E, Unger T. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 76.G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. Mifune M, Ohtsu H, Suzuki H, et al. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 77.Tumor necrosis factor-alpha produced in the kidney contributes to angiotensin II-dependent hypertension. Zhang J, Patel MB, Griffiths R, et al. Hypertension. 2014;64:1275–1281. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clinical relevance and role of neuronal AT(1) receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Xu J, Sriramula S, Xia H, et al. Circ Res. 2017;121:43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Naito T, Ma LJ, Yang H, et al. Am J Physiol Renal Physiol. 2010;298:0–691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Role of angiotensin II AT1 receptor activation in cardiovascular diseases. Billet S, Aguilar F, Baudry C, Clauser E. Kidney Int. 2008;74:1379–1384. doi: 10.1038/ki.2008.358. [DOI] [PubMed] [Google Scholar]
- 81.Expression, genomic organization, and transcription of the mouse angiotensin II type 2 receptor gene. Ichiki T, Inagami T. Circ Res. 1995;76:693–700. doi: 10.1161/01.res.76.5.693. [DOI] [PubMed] [Google Scholar]
- 82.Molecular characterization and chromosome localization of a human angiotensin II AT2 receptor gene highly expressed in fetal tissues. Lazard D, Briend-Sutren MM, Villageois P, Mattei MG, Strosberg AD, Nahmias C. https://europepmc.org/article/med/7719706. Recept Channels. 1994;2:271–280. [PubMed] [Google Scholar]
- 83.Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 84.Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Tsutsumi K, Saavedra JM. Am J Physiol. 1991;261:0–216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 85.Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM. Hypertension. 1998;32:78–83. doi: 10.1161/01.hyp.32.1.78. [DOI] [PubMed] [Google Scholar]
- 86.AT(2) receptor and tissue injury: therapeutic implications. Namsolleck P, Recarti C, Foulquier S, Steckelings UM, Unger T. Curr Hypertens Rep. 2014;16:416. doi: 10.1007/s11906-013-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Carey RM, Siragy HM. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 88.Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Siragy HM, Inagami T, Ichiki T, Carey RM. Proc Natl Acad Sci U S A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Hypertension. 2008;52:666–671. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- 90.Aldosterone biosynthesis, regulation, and classical mechanism of action. Williams GH. Heart Fail Rev. 2005;10:7–13. doi: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- 91.Regulation of aldosterone secretion. Quinn SJ, Williams GH. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- 92.Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Arriza JL, Weinberger C, Cerelli G, et al. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 93.Mineralocorticoid receptors: distribution and activation. Funder JW. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 94.Steroid hormones: relevance and measurement in the clinical laboratory. Holst JP, Soldin OP, Guo T, Soldin SJ. Clin Lab Med. 2004;24:105–118. doi: 10.1016/j.cll.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Kamynina E, Staub O. Am J Physiol Renal Physiol. 2002;283:0–387. doi: 10.1152/ajprenal.00143.2002. [DOI] [PubMed] [Google Scholar]
- 96.Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. Snyder PM, Olson DR, Thomas BC. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 97.SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. McCormick JA, Bhalla V, Pao AC, Pearce D. Physiology (Bethesda) 2005;20:134–139. doi: 10.1152/physiol.00053.2004. [DOI] [PubMed] [Google Scholar]
- 98.Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. Summa V, Mordasini D, Roger F, et al. J Biol Chem. 2001;276:47087–47093. doi: 10.1074/jbc.M107165200. [DOI] [PubMed] [Google Scholar]
- 99.The new biology of aldosterone. Connell JM, Davies E. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 100.Enhancement of aldosterone-induced catecholamine production by bone morphogenetic protein-4 through activating Rho and SAPK/JNK pathway in adrenomedullar cells. Goto J, Otsuka F, Yamashita M, et al. Am J Physiol Endocrinol Metab. 2009;296:0–916. doi: 10.1152/ajpendo.90840.2008. [DOI] [PubMed] [Google Scholar]
- 101.Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R, Feldman RD. Circulation. 2003;108:2400–2406. doi: 10.1161/01.CIR.0000093188.53554.44. [DOI] [PubMed] [Google Scholar]
- 102.Aldosterone inhibits inducible nitric oxide synthase in neonatal rat cardiomyocytes. Chun TY, Bloem LJ, Pratt JH. Endocrinology. 2003;144:1712–1717. doi: 10.1210/en.2002-220956. [DOI] [PubMed] [Google Scholar]
- 103.Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Proc Natl Acad Sci U S A. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Brown NJ. Nat Rev Nephrol. 2013;9:459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Weber KT, Brilla CG. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 106.Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. J Mol Endocrinol. 2000;24:321–328. doi: 10.1677/jme.0.0240321. [DOI] [PubMed] [Google Scholar]
- 107.J. Mechanisms of ligand specificity of the mineralocorticoid receptor. Fuller PJ, Yao Y, Yang J, Young MJ. J Endocrinol. 2012;213:15–24. doi: 10.1530/JOE-11-0372. [DOI] [PubMed] [Google Scholar]
- 108.Aldosterone in the brain. Geerling JC, Loewy AD. Am J Physiol Renal Physiol. 2009;297:0–576. doi: 10.1152/ajprenal.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Hypertension. 2012;60:1023–1030. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Donoghue M, Hsieh F, Baronas E, et al. Circ Res. 2000;87:0–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 111.Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. Vickers C, Hales P, Kaushik V, et al. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 112.Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Am J Physiol Regul Integr Comp Physiol. 2007;292:0–381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Gembardt F, Sterner-Kock A, Imboden H, et al. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Paizis G, Tikellis C, Cooper ME, et al. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) Lambert DW, Yarski M, Warner FJ, et al. J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Selective and specific regulation of ectodomain shedding of angiotensin-converting enzyme 2 by tumor necrosis factor alpha-converting enzyme. Iwata M, Silva Enciso JE, Greenberg BH. Am J Physiol Cell Physiol. 2009;297:1318–1329. doi: 10.1152/ajpcell.00036.2009. [DOI] [PubMed] [Google Scholar]
- 118.Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM- 17: a positive feedback mechanism in the RAS. Patel VB, Clarke N, Wang Z, et al. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 119.Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Gheblawi M, Wang K, Viveiros A, et al. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Ni W, Yang X, Yang D, et al. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adenoviral delivery of angiotensin-(1-7) or angiotensin-(1-9) inhibits cardiomyocyte hypertrophy via the mas or angiotensin type 2 receptor. Flores-Munoz M, Godinho BM, Almalik A, Nicklin SA. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0045564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Flores-Munoz M, Work LM, Douglas K, et al. Hypertension. 2012;59:300–307. doi: 10.1161/HYPERTENSIONAHA.111.177485. [DOI] [PubMed] [Google Scholar]
- 123.Gene therapy with angiotensin-(1-9) preserves left ventricular systolic function after myocardial infarction. Fattah C, Nather K, McCarroll CS, et al. J Am Coll Cardiol. 2016;68:2652–2666. doi: 10.1016/j.jacc.2016.09.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Angiotensin-(1-9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. Ocaranza MP, Moya J, Barrientos V, et al. J Hypertens. 2014;32:771–783. doi: 10.1097/HJH.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 125.Angiotensin-(1-9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Sotomayor-Flores C, Rivera-Mejias P, Vasquez-Trincado C, et al. Cell Death Differ. 2020;27:2586–2604. doi: 10.1038/s41418-020-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Angiotensin-(1-9) reduces cardiovascular and renal inflammation in experimental renin-independent hypertension. Gonzalez L, Novoa U, Moya J, et al. Biochem Pharmacol. 2018;156:357–370. doi: 10.1016/j.bcp.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 127.Recombinant human ACE2 and the angiotensin 1-7 axis as potential new therapies for heart failure. Patel VB, Lezutekong JN, Chen X, Oudit GY. Can J Cardiol. 2017;33:943–946. doi: 10.1016/j.cjca.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Patel VB, Zhong JC, Grant MB, Oudit GY. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. Santos RA, Ferreira AJ, Verano-Braga T, Bader M. J Endocrinol. 2013;216:0. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 130.Angiotensin-(1-7) and angiotensin-( 1-9): function in cardiac and vascular remodelling. McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA. Clin Sci (Lond) 2014;126:815–827. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 131.AT1 receptor signaling pathways in the cardiovascular system. Kawai T, Forrester SJ, O'Brien S, Baggett A, Rizzo V, Eguchi S. Pharmacol Res. 2017;125:4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vascular actions of angiotensin 1-7 in the human microcirculation: novel role for telomerase. Durand MJ, Zinkevich NS, Riedel M, et al. Arterioscler Thromb Vasc Biol. 2016;36:1254–1262. doi: 10.1161/ATVBAHA.116.307518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Mercure C, Yogi A, Callera GE, et al. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 134.ACE2, angiotensin-(1-7), and Mas: the other side of the coin. Bader M. Pflugers Arch. 2013;465:79–85. doi: 10.1007/s00424-012-1120-0. [DOI] [PubMed] [Google Scholar]
- 135.Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Oudit GY, Penninger JM. Curr Heart Fail Rep. 2011;8:176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 136.Angiotensin-(1-7) and vascular function: the clinical context. Touyz RM, Montezano AC. Hypertension. 2018;71:68–69. doi: 10.1161/HYPERTENSIONAHA.117.10406. [DOI] [PubMed] [Google Scholar]
- 137.Angiotensin (1-7) induces MAS receptor internalization. Gironacci MM, Adamo HP, Corradi G, Santos RA, Ortiz P, Carretero OA. Hypertension. 2011;58:176–181. doi: 10.1161/HYPERTENSIONAHA.111.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Therapeutic targeting of the angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas cascade in the renin-angiotensin system: a patent review. Ferreira AJ, Bader M, Santos RA. Expert Opin Ther Pat. 2012;22:567–574. doi: 10.1517/13543776.2012.682572. [DOI] [PubMed] [Google Scholar]
- 139.Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Loot AE, Roks AJ, Henning RH, et al. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 140.The meaning of Mas. Bader M, Alenina N, Young D, Santos RAS, Touyz RM. Hypertension. 2018;72:1072–1075. doi: 10.1161/HYPERTENSIONAHA.118.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.G-protein-coupled receptor MrgD is a receptor for angiotensin-(1-7) involving adenylyl cyclase, cAMP, and phosphokinase A. Tetzner A, Gebolys K, Meinert C, et al. Hypertension. 2016;68:185–194. doi: 10.1161/HYPERTENSIONAHA.116.07572. [DOI] [PubMed] [Google Scholar]
- 142.Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Dias-Peixoto MF, Santos RA, Gomes ER, et al. Hypertension. 2008;52:542–548. doi: 10.1161/HYPERTENSIONAHA.108.114280. [DOI] [PubMed] [Google Scholar]
- 143.Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 144.Angiotensin-(1-7) binding at angiotensin II receptors in the rat brain. Rowe BP, Saylor DL, Speth RC, Absher DR. Regul Pept. 1995;56:139–146. doi: 10.1016/0167-0115(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 145.Cardioprotective angiotensin-(1-7) peptide acts as a natural-biased ligand at the angiotensin II type 1 receptor. Galandrin S, Denis C, Boularan C, et al. Hypertension. 2016;68:1365–1374. doi: 10.1161/HYPERTENSIONAHA.116.08118. [DOI] [PubMed] [Google Scholar]
- 146.Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Walters PE, Gaspari TA, Widdop RE. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 147.Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Jankowski V, Vanholder R, van der Giet M, et al. Arterioscler Thromb Vasc Biol. 2007;27:297–302. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- 148.Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Lautner RQ, Villela DC, Fraga-Silva RA, et al. Circ Res. 2013;112:1104–1111. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 149.Angiotensin A/Alamandine/MrgD axis: another clue to understanding cardiovascular pathophysiology. Hrenak J, Paulis L, Simko F. Int J Mol Sci. 2016;17:1098. doi: 10.3390/ijms17071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alamandine: a new member of the angiotensin family. Villela DC, Passos-Silva DG, Santos RA. Curr Opin Nephrol Hypertens. 2014;23:130–134. doi: 10.1097/01.mnh.0000441052.44406.92. [DOI] [PubMed] [Google Scholar]
- 151.Decarboxylation of Ang-(1-7) to Ala(1)-Ang-(1-7) leads to significant changes in pharmacodynamics. Tetzner A, Naughton M, Gebolys K, et al. Eur J Pharmacol. 2018;833:116–123. doi: 10.1016/j.ejphar.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 152.Activation of the Mas receptors by AVE0991 and MrgD receptor using alamandine to limit the deleterious effects of Ang II-induced hypertension. Tanriverdi LH, Ozhan O, Ulu A, et al. Fundam Clin Pharmacol. 2023;37:60–74. doi: 10.1111/fcp.12829. [DOI] [PubMed] [Google Scholar]
- 153.Alamandine attenuates long‑term hypertension‑induced cardiac fibrosis independent of blood pressure. Wang L, Liu C, Chen X, Li P. Mol Med Rep. 2019;19:4553–4560. doi: 10.3892/mmr.2019.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alamandine alleviates hypertension and renal damage via oxidative-stress attenuation in Dahl rats. Gong J, Luo M, Yong Y, Zhong S, Li P. Cell Death Discov. 2022;8:22. doi: 10.1038/s41420-022-00822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alamandine protects against renal ischaemia-reperfusion injury in rats via inhibiting oxidative stress. Zhu J, Qiu JG, Xu WT, Ma HX, Jiang K. J Pharm Pharmacol. 2021;73:1491–1502. doi: 10.1093/jpp/rgab091. [DOI] [PubMed] [Google Scholar]
- 156.Alamandine protects the heart against reperfusion injury via the MrgD receptor. Park BM, Phuong HTA, Yu L, Kim SH. Circ J. 2018;82:2584–2593. doi: 10.1253/circj.CJ-17-1381. [DOI] [PubMed] [Google Scholar]
- 157.Alamandine via MrgD receptor attenuates pulmonary fibrosis via NOX4 and autophagy pathway. Liu Q, Zheng B, Zhang Y, Huang W, Hong Q, Meng Y. Can J Physiol Pharmacol. 2021;99:885–893. doi: 10.1139/cjpp-2020-0662. [DOI] [PubMed] [Google Scholar]
- 158.Alamandine but not angiotensin-(1-7) produces cardiovascular effects at the rostral insular cortex. Marins FR, Oliveira AC, Qadri F, et al. Am J Physiol Regul Integr Comp Physiol. 2021;321:0. doi: 10.1152/ajpregu.00308.2020. [DOI] [PubMed] [Google Scholar]
- 159.Alamandine and its receptor MrgD pair up to join the protective arm of the renin-angiotensin system. Schleifenbaum J. Front Med (Lausanne) 2019;6:107. doi: 10.3389/fmed.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 161.Neuro-immune interactions and the role of Mas-related G protein-coupled receptors in the gastrointestinal tract. Van Remoortel S, Lambeets L, Timmermans JP. Anat Rec (Hoboken) 2022;306:1131–1139. doi: 10.1002/ar.25008. [DOI] [PubMed] [Google Scholar]
- 162.MRGD, a MAS-related G-protein coupled receptor, promotes tumorigenisis and is highly expressed in lung cancer. Nishimura S, Uno M, Kaneta Y, et al. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0038618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Froogh G, Pinto JT, Le Y, et al. Am J Physiol Heart Circ Physiol . 2017;312:0. doi: 10.1152/ajpheart.00534.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsson LM. J Clin Endocrinol Metab. 1998;83:3925–3929. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- 165.Local adipose tissue renin-angiotensin system. Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Curr Hypertens Rep. 2008;10:93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Yvan-Charvet L, Quignard-Boulange A. Kidney Int. 2011;79:162–168. doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- 167.Mast cell chymase and tryptase as targets for cardiovascular and metabolic diseases. He A, Shi GP. Curr Pharm Des. 2013;19:1114–1125. doi: 10.2174/1381612811319060012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.ACE-versus chymase-dependent angiotensin II generation in human coronary arteries: a matter of efficiency? Tom B, Garrelds IM, Scalbert E, et al. Arterioscler Thromb Vasc Biol. 2003;23:251–256. doi: 10.1161/01.atv.0000051875.41849.25. [DOI] [PubMed] [Google Scholar]
- 169.Local angiotensin II aggravates cardiac remodeling in hypertension. Xu J, Carretero OA, Liao TD, et al. Am J Physiol Heart Circ Physiol. 2010;299:0–1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Dostal DE, Baker KM. Circ Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 171.The atlas of ACE2 expression in fetal and adult human hearts reveals the potential mechanism of heart-injured patients infected with SARS-CoV-2. Shao X, Zhang X, Zhang R, et al. Am J Physiol Cell Physiol. 2021;322:0. doi: 10.1152/ajpcell.00169.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Angiotensin-(1-7) Santos RA. Hypertension. 2014;63:1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 173.The cardiac expression of Mas receptor is responsive to different physiological and pathological stimuli. Dias-Peixoto MF, Ferreira AJ, Almeida PW, et al. Peptides. 2012;35:196–201. doi: 10.1016/j.peptides.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 174.Genetic deletion of the alamandine receptor MRGD leads to dilated cardiomyopathy in mice. Oliveira AC, Melo MB, Motta-Santos D, et al. Am J Physiol Heart Circ Physiol. 2018;316:0. doi: 10.1152/ajpheart.00075.2018. [DOI] [PubMed] [Google Scholar]
- 175.Angiotensinogen, angiotensin II and adipose tissue development. Ailhaud G, Fukamizu A, Massiera F, Negrel R, Saint-Marc P, Teboul M. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:0–35. doi: 10.1038/sj.ijo.0801501. [DOI] [PubMed] [Google Scholar]
- 176.Angiotensinogen, adipocyte differentiation and fat mass enlargement. Ailhaud G, Teboul M, Massiera F. Curr Opin Clin Nutr Metab Care. 2002;5:385–389. doi: 10.1097/00075197-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 177.Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Kalupahana NS, Massiera F, Quignard-Boulange A, et al. Obesity (Silver Spring) 2012;20:48–56. doi: 10.1038/oby.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Schutten MT, Houben AJ, de Leeuw PW, Stehouwer CD. Physiology (Bethesda) 2017;32:197–209. doi: 10.1152/physiol.00037.2016. [DOI] [PubMed] [Google Scholar]
- 179.Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Yasue S, Masuzaki H, Okada S, et al. Am J Hypertens. 2010;23:425–431. doi: 10.1038/ajh.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gene expression of angiotensinogen in adipose tissue of obese patients. Giacchetti G, Faloia E, Sardu C, et al. Int J Obes Relat Metab Disord. 2000;2:142–143. doi: 10.1038/sj.ijo.0801305. [DOI] [PubMed] [Google Scholar]
- 181.Increased adipose angiotensinogen gene expression in human obesity. Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Obes Res. 2000;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 182.The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. van Harmelen V, Elizalde M, Ariapart P, et al. Int J Obes Relat Metab Disord. 2000;24:673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- 183.Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Frederich RC, Jr. Jr., Kahn BB, Peach MJ, Flier JS. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- 184.Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Am J Physiol Regul Integr Comp Physiol. 2004;287:0–949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 185.Weight loss and the renin-angiotensin-aldosterone system. Engeli S, Bohnke J, Gorzelniak K, et al. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 186.Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Yiannikouris F, Gupte M, Putnam K, et al. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Angiotensin II as a trophic factor of white adipose tissue: stimulation of adipose cell formation. Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Endocrinology. 2001;142:487–492. doi: 10.1210/endo.142.1.7883. [DOI] [PubMed] [Google Scholar]
- 188.Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Darimont C, Vassaux G, Ailhaud G, Negrel R. Endocrinology. 1994;135:2030–2036. doi: 10.1210/endo.135.5.7956925. [DOI] [PubMed] [Google Scholar]
- 189.ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. Kawabe Y, Mori J, Morimoto H, et al. Am J Physiol Endocrinol Metab. 2019;317:1140. doi: 10.1152/ajpendo.00311.2019. [DOI] [PubMed] [Google Scholar]
- 190.Angiotensin 1-7 stimulates brown adipose tissue and reduces diet-induced obesity. Morimoto H, Mori J, Nakajima H, et al. Am J Physiol Endocrinol Metab. 2018;314:0. doi: 10.1152/ajpendo.00192.2017. [DOI] [PubMed] [Google Scholar]
- 191.Angiotensin(1-7) attenuates visceral adipose tissue expansion and lipogenesis by suppression of endoplasmic reticulum stress via Mas receptor. Ma C, Shi T, Song L, Liu J, Yuan M. Nutr Metab (Lond) 2022;19:82. doi: 10.1186/s12986-022-00716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brown adipose tissue transcriptome unveils an important role of the beta-alanine/alamandine receptor, MrgD, in metabolism. Cerri GC, Santos SHS, Bader M, Santos RAS. J Nutr Biochem. 2023;114:109268. doi: 10.1016/j.jnutbio.2023.109268. [DOI] [PubMed] [Google Scholar]
- 193.Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Lira VA, Soltow QA, Long JH, Betters JL, Sellman JE, Criswell DS. Am J Physiol Endocrinol Metab. 2007;293:1062–1068. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- 194.Alamandine acts via MrgD to induce AMPK/NO activation against ANG II hypertrophy in cardiomyocytes. Jesus ICG, Scalzo S, Alves F, et al. Am J Physiol Cell Physiol. 2018;314:0. doi: 10.1152/ajpcell.00153.2017. [DOI] [PubMed] [Google Scholar]
- 195.Within the brain: the renin angiotensin system. Jackson L, Eldahshan W, Fagan SC, Ergul A. Int J Mol Sci. 2018;19:876. doi: 10.3390/ijms19030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Role of brain renin angiotensin system in neurodegeneration: an update. Abiodun OA, Ola MS. Saudi J Biol Sci. 2020;27:905–912. doi: 10.1016/j.sjbs.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cardiorenal end points in a trial of aliskiren for type 2 diabetes. Parving HH, Brenner BM, McMurray JJ, et al. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 198.Renal outcomes with aliskiren in patients with type 2 diabetes: a prespecified secondary analysis of the ALTITUDE randomised controlled trial. Heerspink HJ, Persson F, Brenner BM, et al. Lancet Diabetes Endocrinol. 2016;4:309–317. doi: 10.1016/S2213-8587(15)00469-6. [DOI] [PubMed] [Google Scholar]
- 199.A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) study group. Kober L, Torp-Pedersen C, Carlsen JE, et al. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 200.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. Pfeffer MA, Braunwald E, Moye LA, et al. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 201.A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. Cohn JN, Tognoni G, Valsartan Heart Failure Trial I. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 202.Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Pitt B, Segal R, Martinez FA, et al. Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 203.Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Young JB, Dunlap ME, Pfeffer MA, et al. Circulation. 2004;110:2618–2626. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 204.The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 205.Effect of enalapril on the progression of chronic renal failure. A randomized controlled trial. Kamper AL, Strandgaard S, Leyssac PP. Am J Hypertens. 1992;5:423–430. doi: 10.1093/ajh/5.7.423. [DOI] [PubMed] [Google Scholar]
- 206.Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Maschio G, Alberti D, Janin G, et al. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 207.Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. Brenner BM, Cooper ME, de Zeeuw D, et al. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 208.The FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC) trial. Yoo TH, Hong SJ, Kim S, et al. Hypertens Res. 2022;45:2008–2017. doi: 10.1038/s41440-022-01028-6. [DOI] [PubMed] [Google Scholar]
- 209.Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. Lewis EJ, Hunsicker LG, Clarke WR, et al. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 210.Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. Henriksen EJ, Jacob S. J Cell Physiol. 2003;196:171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- 211.Effects of angiotensin-converting enzyme inhibitors on glucose uptake. Kudoh A, Matsuki A. Hypertension. 2000;36:239–244. doi: 10.1161/01.hyp.36.2.239. [DOI] [PubMed] [Google Scholar]
- 212.ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Shiuchi T, Cui TX, Wu L, et al. Hypertension. 2002;40:329–334. doi: 10.1161/01.hyp.0000028979.98877.0c. [DOI] [PubMed] [Google Scholar]
- 213.Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Hansson L, Lindholm LH, Niskanen L, et al. Lancet. 1999;353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 214.Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Yusuf S, Ostergren JB, Gerstein HC, et al. Circulation. 2005;112:48–53. doi: 10.1161/CIRCULATIONAHA.104.528166. [DOI] [PubMed] [Google Scholar]
- 215.Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. Heart Outcomes Prevention Evaluation Study I, Yusuf S, Sleight P, et al. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 216.Olmesartan inhibits cardiac hypertrophy in mice overexpressing renin independently of blood pressure: its beneficial effects on ACE2/Ang(1-7)/Mas axis and NADPH oxidase expression. Tanno T, Tomita H, Narita I, et al. J Cardiovasc Pharmacol. 2016;67:503–509. doi: 10.1097/FJC.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 217.Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 218.Effect of olmesartan and amlodipine on serum angiotensin-(1-7) levels and kidney and vascular function in patients with type 2 diabetes and hypertension. Kim K, Moon JH, Ahn CH, Lim S. Diabetol Metab Syndr. 2023;15:43. doi: 10.1186/s13098-023-00987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Angiotensin-converting enzyme inhibition reduces food intake and weight gain and improves glucose tolerance in melanocortin-4 receptor deficient female rats. Mul JD, Seeley RJ, Woods SC, Begg DP. Physiol Behav. 2013;121:43–48. doi: 10.1016/j.physbeh.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. de Kloet AD, Krause EG, Kim DH, Sakai RR, Seeley RJ, Woods SC. Endocrinology. 2009;150:4114–4123. doi: 10.1210/en.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Physiol Behav. 2009;98:192–197. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 222.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. Pitt B, Zannad F, Remme WJ, et al. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 223.Eplerenone in patients with systolic heart failure and mild symptoms. Zannad F, McMurray JJ, Krum H, et al. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 224.Cardiovascular events with finerenone in kidney disease and type 2 diabetes. Pitt B, Filippatos G, Agarwal R, et al. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 225.Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. Bakris GL, Agarwal R, Anker SD, et al. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 226.Phase 2 trial of baxdrostat for treatment-resistant hypertension. Freeman MW, Halvorsen YD, Marshall W, et al. N Engl J Med. 2023;388:395–405. doi: 10.1056/NEJMoa2213169. [DOI] [PubMed] [Google Scholar]