ABSTRACT
Autophagosomes are crucial components of the cellular recycling machinery that form at endoplasmic reticulum (ER)-associated sites. As the autophagosome membrane is largely devoid of transmembrane proteins, autophagosome biogenesis is thought to be largely regulated by lipid transfer and lipid modifications, as well as membrane-associated proteins. While the membrane origin of autophagosomes and their lipid composition are still incompletely understood, previous studies have found the autophagosome membrane to be enriched in unsaturated fatty acids and have little cholesterol, suggesting that cholesterol removal is an integral step during autophagosome biogenesis. In our study, we demonstrate that short term cholesterol depletion leads to a rapid induction of autophagy and identify the ER-localized cholesterol transport protein GRAMD1C as a negative regulator of starvation-induced macroautophagy/autophagy. Abbreviations: ATG: autophagy related; ccRCC: clear cell renal cell carcinoma; ER: endoplasmic reticulum; GRAM: glucosyltransferases, RAB-like GTPase activators and myotubularins; GRAMD: GRAM domain containing; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MCBD: methyl-cyclodextrin; MTOR: mechanistic target of rapamycin kinase; VASt: VAD1 analog of StAR-related lipid transfer.
KEYWORDS: Aster, autophagy, ccRCC, cholesterol, GRAMD1C, VASt
Cholesterol is a key component of mammalian membranes that regulates membrane fluidity. Cholesterol is synthesized intracellularly, but also imported from the extracellular environment, and is redistributed to various intracellular organelles by sterol transfer proteins. One such family of cholesterol transport proteins, the GRAMD1 family proteins (also known as Aster proteins), consisting of GRAMD1A, GRAMD1B, and GRAMD1C, are ER-localized transmembrane proteins that contain two critical domains; a lipid-binding PH-like GRAM domain and a sterol binding VASt domain. The role of cholesterol in autophagy is not completely understood, and we therefore asked whether cholesterol is directly involved in regulation of autophagosome membrane biogenesis [1].
Cholesterol depletion from U2OS cell membranes using methyl-b-cyclodextrin (MCBD) for 1 h results in significant upregulation of autophagy, as measured by increased MAP1LC3B/LC3B lipidation and LC3B puncta in both fed and starved cells. Furthermore, ATG13 and ATG16L1 puncta representing the early autophagy machinery proteins are increased upon MCBD treatment in both starved and fed cells. Importantly, we demonstrated that short-term cholesterol depletion promotes formation of puncta containing a membrane curvature reporter consisting of the ATG14 BATS domain fused with eGFP. Together, this indicates that cholesterol depletion can contribute toward the formation of autophagosome-related curved membranes upon starvation (Figure 1).
Figure 1.
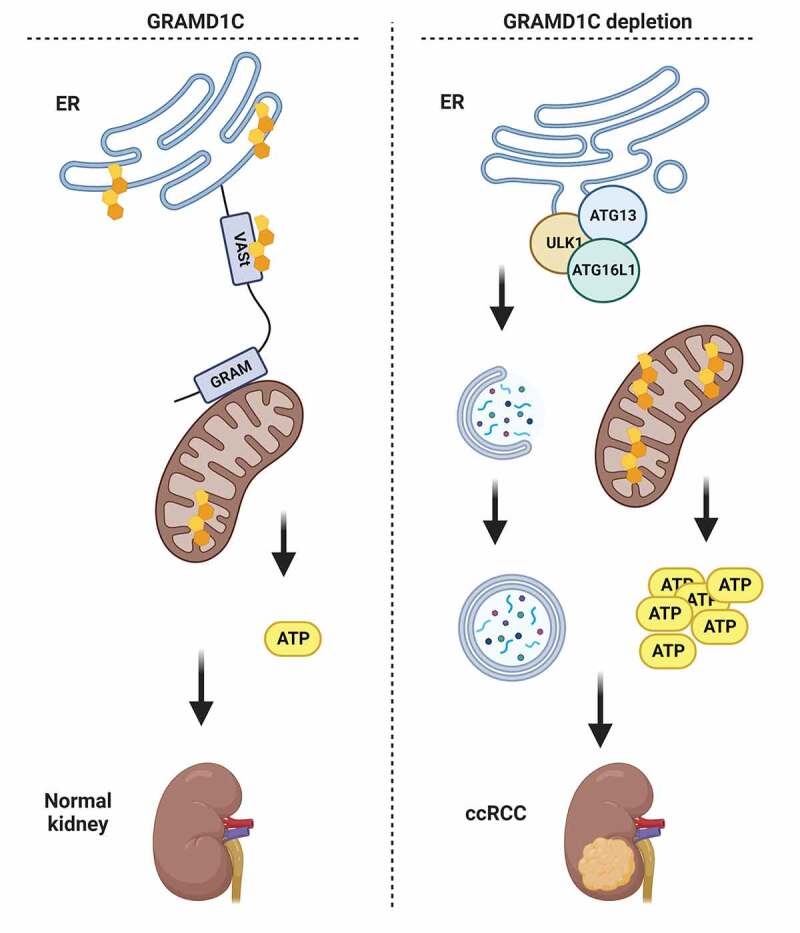
GRAMD1C is an ER cholesterol transfer protein that interacts with mitochondria via its GRAM domain to facilitate mitochondria-ER cholesterol transfer. Cells lacking GRAMD1C expression show increased autophagic flux and a concomitant increase in mitochondrial cholesterol levels and mitochondrial respiration. By maintaining cholesterol levels at ER membranes, GRAMD1C likely contributes to the suppression of autophagosome biogenesis and regulation of mitochondrial bioenergetics, potentially contributing to the decreased survival of ccRCC patients with low GRAMD1C levels. Figure generated with Biorender.
In order to better understand the temporal dynamics of autophagosome biogenesis during cholesterol depletion, we analyzed autophagy initiation events at early time points (15 and 30 min) in MCBD-treated cells, as well as the activity of MTOR, a kinase that functions as a negative regulator of autophagy and is regulated by cholesterol. Importantly, cholesterol depletion promotes autophagy initiation at an earlier time point than MTOR inactivation, suggesting an additional MTOR-independent role of cholesterol in autophagy initiation.
As autophagosomes form at ER-associated sites, we set out to identify possible ER-localized cholesterol transfer proteins that might directly regulate autophagosome biogenesis. Given an already reported involvement of GRAMD1A in autophagy regulation, we investigated the role of other GRAMD1 family proteins by knocking down separately all GRAMD1 proteins in U2OS cells stably expressing mCherry-EGFP-LC3B. Our initial results showed significant autolysosome formation in starved GRAMD1C knockdown cells. This was further validated by measuring of the turnover of radioactively labeled long-lived proteins and LC3B western blot analysis. Intriguingly, the increased autophagic flux seen in GRAMD1C knockout cells can be rescued with wild-type GRAMD1C, but not using GRAMD1C mutants lacking either the GRAM domain (ΔGRAM) or the VASt domain (ΔVASt), suggesting a crucial role of the cholesterol transporter GRAMD1C as a negative regulator of starvation-induced autophagy. Similar to our observations on the negative regulatory role of cholesterol in autophagosome initiation, we detected an increased number of puncta of the early autophagic markers ATG13, ATG16L1, and the PtdIns3P effector protein WIPI2B in starved GRAMD1C-depleted cells. These observations, together with colocalization of ATG13 and ATG16L1 with GRAMD1C-positive ER sites suggest a role of GRAMD1C in the regulation of autophagosome biogenesis by suppressing membrane curvature and recruitment of early autophagic markers to ER-associated initiation sites.
Given that autophagosome biogenesis takes place at ER–mitochondrial contact sites, we asked if GRAMD1C might facilitate cholesterol transfer at the ER mitochondria interface. As the lipid-binding GRAM domain is responsible for targeting the protein to a specific membrane, we created cell lines stably expressing GRAMD1C-EGFP, ΔGRAM-EGFP and EGFP-GRAM domain only. Using live microscopy, we observed a dynamic interaction of EGFP-GRAM to mitochondrial structures, suggesting that GRAMD1C interacts with mitochondria via its GRAM domain. Indeed, EGFP-GRAM is detected in isolated mitochondria and immunoprecipitation assays confirm its interaction with the outer mitochondrial membrane protein TOMM70/TOMM70A. Additionally, mass spectrometric analysis of the interactome of GRAMD1C demonstrated that GRAMD1C interacts with other GRAMD1 proteins and also with RHOT2 and ACSL4, an outer mitochondrial membrane protein and an ER-mitochondrial contact site marker, respectively.
To investigate GRAMD1Cs role in cholesterol transfer we utilized an assay that involves a cholesterol binding reporter, mCherry-D4, that binds to purified mitochondria and can be quantified by western blotting. We found decreased binding of the reporter to MBCD-treated mitochondria, as well as increased binding to mitochondria from GRAMD1C KO cells. Moreover, SREBF2/SREBP2 target genes are upregulated in GRAMD1C-depleted cells, suggesting a positive role of GRAMD1C in ER cholesterol levels. Mitochondrial bioenergetics analysis shows increased ATP production-linked respiration and respiratory capacity in GRAMD1C knockout cells that can be rescued with the full-length GRAMD1C, but not with GRAMD1C lacking the cholesterol transporting VASt domain. These results suggest that GRAMD1C functions as a negative regulator of autophagy and mitochondrial ATP production.
Finally, based on a previously reported correlation of GRAMD1C with clear cell renal cell carcinoma (ccRCC) immune infiltration and patient survival, we investigated the role of the different GRAMD family members in ccRCC. Low GRAMD1A and GRAMD1B expression are associated with improved patient survival in ccRCC, in line with in vitro colony formation assay where depletion of GRAMD1A and GRAMD1B significantly decrease the ability of ccRCC 786-O cells to form colonies.
In conclusion, our results point toward a role of the ER cholesterol transfer protein GRAMD1C as a negative regulator of starvation-induced autophagy. We propose that GRAMD1C regulates cholesterol levels at autophagosome initiation sites by interacting with mitochondria to facilitate mitochondria-ER cholesterol transport and that dysregulation of GRAMD1C plays a critical role in autophagosome biogenesis, mitochondrial bioenergetics, and cancer.
Acknowledgments
We would like to thank the other co-authors who contributed to the original manuscript; Ana Lapao, Sakshi Singh, Laura Trachsel-Moncho, Sebastian W. Schultz, Sigve Nakken and Michael J. Munson. This work was funded by the Research Council of Norway through its Centres of Excellence funding scheme (Project: 262652) and FRIPRO grants (Project: 249753 and 314684) and by the Norwegian Cancer Society (Project: 171318).
Funding Statement
This work was supported by the Research Council of Norway [262652, 249753 and 314684]; Norwegian Cancer Society [171318].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Ng MYW, Charsou C, Lapao A, et al. Simonsen A. The cholesterol transport protein GRAMD1C regulates autophagy initiation and mitochondrial bioenergetics. Nat Commun. 2022 Oct 21;13(1):6283. PMID: 36270994 [DOI] [PMC free article] [PubMed] [Google Scholar]


