ABSTRACT
Ischemia may be the most common pathological occurrence to restrict nutrient availability and induce macroautophagy/autophagy. As a self-digestive process, autophagy helps sustain nutrient/energy and restrict damages in short-term scenarios, but it switches to a self-destructive process leading to cell death in long-term scenarios. Notably, ischemia has been used as one clinical application to treat cancer, particularly transarterial embolization (TAE) and chemoembolization (TACE) as the first-line treatments of intermediate-stage hepatocellular carcinoma (HCC, the predominant type of liver cancer). Partly due to the induced autophagy together with hypoxia-induced angiogenesis, TAE/TACE is not successful to treat HCC in many cases. Our recent work demonstrated that simultaneous treatments with sorafenib (a first-line therapeutic agent for advanced HCC) can sensitize HCC cells to cell death induced by glucose starvation via impairing mitophagy, a mitochondria-specific form of autophagy. Moreover, we identified SIAH1 as an important E3 ubiquitin ligase for mitophagic induction in HCC cells.
KEYWORDS: HCC, mitophagy, sorafenib, glucose restriction, canagliflozin
On the one hand, deprivation of glucose, rather than glutamine and other amino acids or FBS, is sufficient to sensitize sorafenib-induced cell death. On the other hand, sorafenib, rather than other HCC chemotherapeutic agents such as lenvatinib, doxorubicin and cisplatin, is sufficient to sensitize cell death induced by glucose starvation. Sorafenib alone can induce mitophagy, removing damaged mitochondria and promoting survival in HCC cells (Figure 1A). PINK1 (PTEN induced kinase 1)-PRKN/parkin are part of a classic mitophagy pathway. However, PRKN is downregulated or absent in almost 50% of HCC tissues and in most established HCC cell lines. Among the tested E3 ubiquitin ligases, we found that SIAH1, rather than MUL1, STUB1 or SMURF, is essential for sorafenib-induced mitophagy in HCC cells [1].
Figure 1.
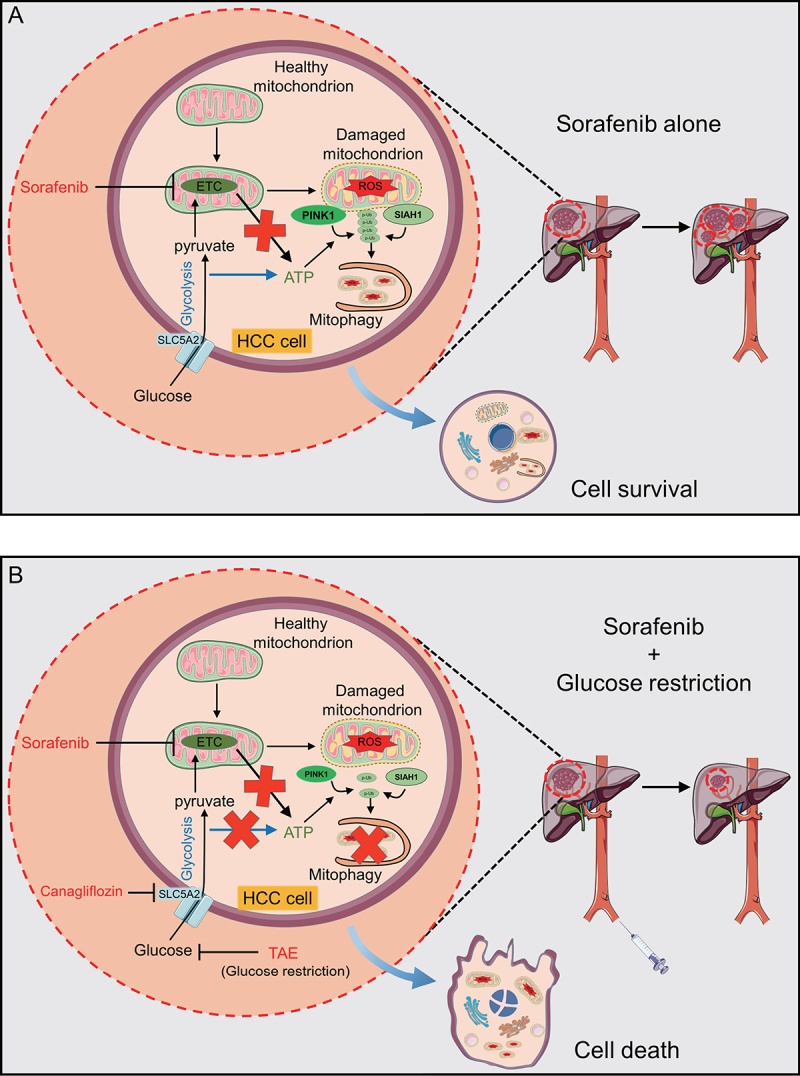
Concept map. (A) Sorafenib alone induces mitophagy to eliminate damaged mitochondria and restrict ROS accumulation. Although sorafenib inhibits activities of ETC complexes, glycolysis-involved ATP production is sufficient for PINK1 activation and subsequent mitophagy induction. SIAH1 cooperates with PINK1 to generate poly-phospho-Ub (p-Ub) chains at the mitochondria membrane surface as “eat me” signals for phagophore recognition. (B) With glucose restriction (via TAE) or inhibition of glucose import by canagliflozin treatment, HCC cells lack energy sources to produce ATP and thus are unable to sustain mitophagy; HCC cells then succumb to cell death.
Mechanistically, sorafenib inhibits activities of electron transport chain (ETC) complex II and III, whereas glucose deprivation abolishes glycolysis, both of which completely deplete ATP production and abolish PINK1 activation (the latter depends on ATP availability). The results further show that metformin (an inhibitor of ETC complex I) or the well-studied mitochondrial poisons oligomycin (complex V) and antimycin A (complex III) exhibit similar effects as sorafenib to sensitize HCC cell death. As a result, the synergistic effect was found to be dependent on both disruption of mitochondrial energy production and impairment of mitophagy.
Because HCC cells overexpress glucose transporter SLC5A2/SGLT2, it is thus feasible to administer canagliflozin (a clinically-available drug for type 2 diabetes) to repress glucose import into cells and then sensitize HCC cells to cell death induced by ETC complex inhibitors (Figure 1B). More importantly, co-administration of canagliflozin and sorafenib can markedly inhibit HCC xenograft in vivo. Due to the concern of undesired cytotoxicity of the systemic administration of the two drugs, it may be more practical to perform transarterial sorafenib-embolization (TASE) that combines TAE with intra-arterial administration of sorafenib. Nevertheless, this strategy warrants further pre-clinical and clinical validations.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81972291, 81672370, 32160160]; Guangxi Natural Science Foundation Key Grant [2018GXNSFDA050006]; Guangxi Hundred-Talent Program [2016]; Guangxi Medical University Training Program for Distinguished Young Scholars [2017]; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education [GKE-ZZ202131].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Zhou J, Feng J, Wu Y, et al. Simultaneous treatment with sorafenib and glucose restriction inhibits hepatocellular carcinoma in vitro and in vivo by impairing SIAH1-mediated mitophagy. Exp Mol Med. 2022. Nov 16;54(11):2007–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


