Abstract
Background
Between October 2022 and January 2023, influenza A(H1N1)pdm09, A(H3N2) and B/Victoria viruses circulated in Europe with different influenza (sub)types dominating in different areas.
Aim
To provide interim 2022/23 influenza vaccine effectiveness (VE) estimates from six European studies, covering 16 countries in primary care, emergency care and hospital inpatient settings.
Methods
All studies used the test-negative design, but with differences in other study characteristics, such as data sources, patient selection, case definitions and included age groups. Overall and influenza (sub)type-specific VE was estimated for each study using logistic regression adjusted for potential confounders.
Results
There were 20,477 influenza cases recruited across the six studies, of which 16,589 (81%) were influenza A. Among all ages and settings, VE against influenza A ranged from 27 to 44%. Against A(H1N1)pdm09 (all ages and settings), VE point estimates ranged from 28% to 46%, higher among children (< 18 years) at 49–77%. Against A(H3N2), overall VE ranged from 2% to 44%, also higher among children (62–70%). Against influenza B/Victoria, overall and age-specific VE were ≥ 50% (87–95% among children < 18 years).
Conclusions
Interim results from six European studies during the 2022/23 influenza season indicate a ≥ 27% and ≥ 50% reduction in disease occurrence among all-age influenza vaccine recipients for influenza A and B, respectively, with higher reductions among children. Genetic virus characterisation results and end-of-season VE estimates will contribute to greater understanding of differences in influenza (sub)type-specific results across studies.
Keywords: influenza, vaccine effectiveness, multicentre study, test-negative design, Europe
Key public health message.
What did you want to address in this study?
Different types (A or B) or subtypes (e.g. A(H3N2), A(H1N1)pdm09)) of influenza viruses exist. In Europe several virus (sub)types have been co-circulating in the 2022/23 influenza season. We wanted to understand how well the influenza vaccine for this season has protected people so far. Because people’s settings, the virus (sub)types they encounter and their age might all influence vaccine effectiveness, these potential factors were considered.
What have we learnt from this study?
In primary care, emergency and hospital settings, interim influenza vaccine effectiveness estimations for 2022/23 indicated some protection by the vaccine. Regardless of setting, all-age vaccine effectiveness against influenza A(H3N2) and A(H1N1)pdm09 virus subtypes ranged from 2 to 46%; this was higher for influenza B (≥ 50%). Vaccine effectiveness point estimates in <18-year-old children were higher (49–95%) than adults across all (sub)types.
What are the implications of your findings for public health?
While this report presents interim results, the findings support that influenza vaccination should be continued according to national guidelines as the influenza season unfolds. Further characterisations of circulating influenza viruses and updated vaccine effectiveness estimates at the end of the season will enhance the understanding of the protection conferred by the vaccine in a European context, supporting preparation for future seasons.
Introduction
In European Union (EU) countries and the United Kingdom (UK), seasonal influenza vaccine is recommended for older adults (mainly considered as those aged ≥ 60 years or ≥ 65 years, depending on the country) and those at increased risk of influenza complications and severe disease (e.g. those with chronic conditions) [1]. Moreover, routine childhood influenza vaccination programmes have been introduced in some World Health Organization (WHO) European Region countries, including in the UK since 2013/14, in Ireland since 2020/21, and in Denmark in 2–6-year-olds only, since 2021/22 [2,3].
The WHO recommended that the 2022/23 northern hemisphere influenza season trivalent influenza vaccine strains to be included in egg-based vaccines should be an A/Victoria/2570/2019 (H1N1)pdm09-like virus, an A/Darwin/9/2021 (H3N2)-like virus and a B/Austria/1359417/2017-like virus (B/Victoria lineage). For non-egg-based vaccines (i.e. cell culture- or recombinant-based vaccines), WHO recommended inclusion of an A/Wisconsin/588/2019 (H1N1)pdm09-like virus, an A/Darwin/6/2021 (H3N2)-like virus and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus. In both egg- and cell-culture-based quadrivalent vaccines, WHO recommended to also include a B/Phuket/3073/2013 virus (B/Yamagata lineage) [4].
The influenza season for 2022/23 started early in most of the 53 WHO European Region countries, with activity crossing the epidemic threshold of 10% sentinel specimen positivity in week 45 2022 and high seasonal influenza virus circulation reported from 29 of the 37 influenza-reporting countries by the first week in January 2023 [5]. In primary care sentinel specimens, during the period covered by this study, which goes up to the end of January (week 4) 2023, influenza A(H3N2) subtypes were initially dominant, with influenza A(H1N1)pdm09 subtypes subsequently dominating from week 2 2023, although there was substantial heterogeneity in influenza A subtype distribution by country [6]. Influenza B virus was also reported. For hospitalised patients, (mostly untyped) influenza A viruses were detected in urgent care wards, while specimens from patients with severe acute respiratory illness (SARI) were predominantly influenza A(H1N1)pdm09 [7]. Other respiratory viruses, particularly severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial virus (RSV) were also co-circulating during the 2022/23 influenza season, the latter at high levels [7].
The European Centre for Disease Prevention and Control (ECDC)’s Vaccine Effectiveness, Burden and Impact Studies (VEBIS) project began measuring influenza vaccine effectiveness (VE) in the 2022/23 season through multicentre studies in primary care and hospital settings. Previously, many VEBIS study sites were included in the Influenza – Monitoring Vaccine Effectiveness in Europe (I-MOVE) network, which measured influenza VE annually from 2008/09 to 2021/22. The UK and Denmark were I-MOVE partners until 2021/22 and have been estimating influenza VE in single-country studies since 2006 and 2009, respectively.
We report interim influenza VE estimates for the 2022/23 season from six studies (four single- and two multi-country), including out-patient (primary care), in-patient (hospital) and emergency care settings, in order to inform measures of influenza prevention and control for the remaining season.
Methods
Study setting
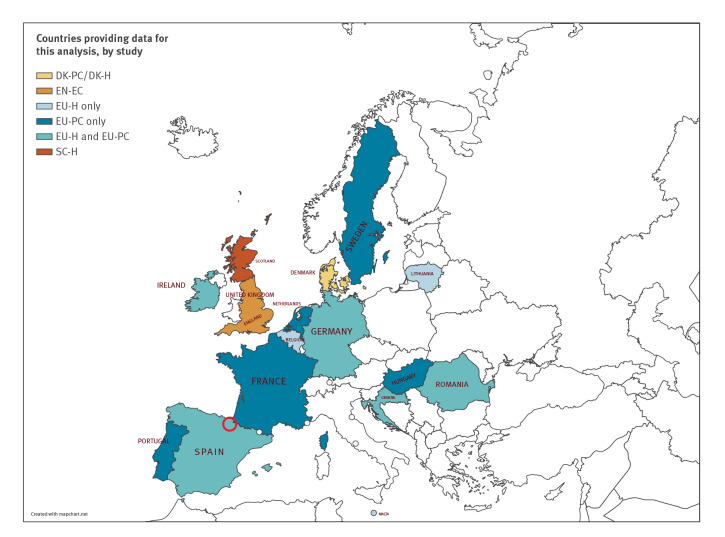
The two primary care studies were conducted in Denmark (Danish primary care study; DK-PC) and in several EU countries (EU primary care study; EU-PC) through the ECDC VEBIS multi-country network (Table 1). All 10 participating countries in this network had available data for interim analysis; one country, Spain, includes two study sites: Navarra region as one, and 11 other regions combined as the other. The single study at hospital emergency care department level was conducted in England (English emergency care study; EN-EC), with 76% (9,867/13,058) of patients subsequently admitted to hospital. The three studies in the hospital setting were in Denmark (DK-H), Scotland (SC-H) and across several EU countries through the ECDC VEBIS multi-country network (EU-H; Table 1). Seven of 14 participating countries in this network provided data for interim analysis; one country, Spain, has two study sites: one being Navarra and the second comprising 12 other regions combined (Figure 1). A total of 16 European countries (with England and Scotland counted as two countries) contributed data to these interim influenza VE results.
Table 1. Summary of methods for the six European interim influenza vaccine effectiveness studies, influenza season 2022/23.
| Study characteristics | Study | |||||
|---|---|---|---|---|---|---|
| DK-PC | EU-PC | EN-EC | DK-H | EU-H | SC-H | |
| Study period | 1 Nov 2022 to 29 Jan 2023 | 3 Oct 2022 to 31 Jan 2023 | 9 Oct 2022 to 8 Jan 2023 | 1 Nov 2022 to 29 Jan 2023 | 10 Oct 2022 to 30 Jan 2023 | 3 Oct 2022 to 22 Jan 2023 |
| Setting | Non-hospitalised patientsa | Primary care | Emergency care | Hospital | Hospital | Hospital |
| Location | DK | HR, FR, DE, HU, IE, NL, PT, RO, ES (Navarra region), ES (11 regions combined, excluding Navarra) and SE | EN | DK | 29 hospitals in BE, HR, DE, LT, MT, RO, ES (Navarra region) and ES (12 regions combined, excluding Navarra) | SC |
| Study design | TND | TND | TND | TND | TND | TND |
| Data source | Data linkage of Danish Microbiology Database, the Danish Vaccination Register and the Danish National Patient Register | Physicians and laboratory, in some sites data linkage to electronic health records | Data linkage of sentinel laboratory surveillance (Respiratory DataMart), the National Immunisations Management System (NIMS), and the Emergency Care DataSet (ECDS) | Data linkage of Danish Microbiology Database, the Danish Vaccination Register and the Danish National Patient Register | Hospital charts, vaccine registers, interviews with patients, laboratory records | EAVE-II national patient-level dataset, Electronic Communication of Surveillance in SC (ECOSS; all virology testing national database), Rapid Preliminary Inpatient Data (RAPID; Scottish hospital admissions data), National Records of Scotland (NRS; death certification), National Clinical Data Store (NCDS; vaccination events in SC) |
| Age groups of study population | All ages | All agesb | All ages | All ages | All agesb | Adults ≥ 18 years old |
| Case definition for patient recruitment | Sudden onset of symptoms with feverc, myalgia and respiratory symptomsd | EU ARIe
or EU ILI (sudden onset of symptoms AND ≥ 1 of: feverc or feverishness, malaise, headache, myalgia AND ≥ 1 of cough, sore throat, shortness of breath) |
Patients with an influenza swab test 14 days before to 7 days after an emergency care visit compatible with influenza infection or its complications | Sudden onset of symptoms with feverc, myalgia and respiratory symptoms among patients admitted to hospital for at least 12 hours | EU SARI (hospitalised person with ≥ 1 of feverc/feverishness, malaise, headache, myalgia, deterioration of general condition (asthenia, weight loss, anorexia, confusion/dizziness) AND ≥ 1 respiratory symptom (cough, sore throat or shortness of breath) at admission or within 48 hours after admission) | Patients with EU ARIe symptoms and clinician’s judgement that there is an infectionf and limited to emergency care where the influenza test occurs 14 days before admission or within 48 hours after admission |
| Selection of patients | At practitioner's/ clinician's judgement | Systematic | At practitioner's/ clinician's judgement | At practitioner's/ clinician's judgement | Exhaustive (DE, HR, LT, MT, NA, RO) Systematic (BE, ES; some hospitals in BE: exhaustive on either 1 or 2 days per week, depending on workload) |
At practitioner's/ clinician's judgement |
| Vaccine types used nationally or in the studyg,h | 100% QIV (children 2–6 years of age are offered a LAIV as nasal spray) |
In the study among controls: among the seven study sites providing information on influenza vaccine brand, the brand was unknown in 19% of vaccinated controls; among the rest, 8% used a QIVc, 2% a LAIV, 12% an egg-propagated trivalent and 78% a QIVe | In the study among controls: ages 2–17 years (90% LAIV, 7% QIVc, 1% QIVe, 2% unknown); ages 18–64 years (74% QIVc, 14% QIVe, 2% QIVr, 5% aQIV, 5% unknown); ages ≥ 65 years (4% QIVc, 1% QIVr, 91% aQIV, 5% unknown) | 100% QIV (children 2–6 years of age are offered a LAIV as nasal spray) |
In the study among controls: in the only site providing influenza vaccine brand information, 77% were QIV; the remaining 23% unknown (all countries participating use QIV nationally) | 22% QIVc; 78% aQIV |
| Variables of adjustment | Age group, sexi, presence of chronic conditions, calendar time as month (Nov–Jan) (if possible, week) | Age (modelled as RCS, age group or linear term depending on analysis), sexi, presence of chronic conditions, onset date (RCS) and study site | Age group, region, clinically extremely vulnerable, COVID-19 vaccination status, calendar time as week (spline) | Age group, sexi, presence of chronic conditions, calendar time as month (Nov–Jan) (if possible, week) | Age (modelled as RCS, age group or linear term depending on analysis), sexi, presence of chronic conditions, time (onset date as RCS or month of swab as categorical term) and study site | Age (spline), sexi, number of QCOVIDj clinical risk groups (0,1,2,3,4, ≥ 5)j, time (days, spline), setting (community or hospital) and deprivation quintile (SIMD) |
ARI: acute respiratory infection; aQIV: adjuvanted QIV; BE: Belgium; DE: Germany; DK: Denmark; DK-H: DK hospital study; DK-PC: DK primary care study; EN: England; EN-EC: EN emergency care study; ES: Spain; EU: European Union; EU-H: EU hospital multicentre VEBIS study; EU-PC: EU primary care multicentre VEBIS study; FR: France; GP: general practitioner; HR: Croatia; HU: Hungary; IE: Ireland; ILI: influenza-like illness; LAIV4: quadrivalent live attenuated influenza vaccine; LT: Lithuania; LRI: lower respiratory infection; MT: Malta; NL: Netherlands; PT: Portugal; QIV: quadrivalent inactivated influenza vaccine; QIVc: cell-grown QIV; QIVe: egg-grown QIV; QIVr: recombinant QIV; RCS: restricted cubic spline; RO: Romania; SARI: severe acute respiratory infection; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SC: Scotland; SC-H: SC hospital study; SIMD: Scottish Index of Multiple Deprivation; SE: Sweden; TIV: trivalent inactivated influenza vaccine; TND: test-negative design; VE: vaccine effectiveness; VEBIS: VE, Burden and Impact Studies.
a Patients are seen by the GP, but also in emergency care.
b Patients < 6 months of age should have been excluded from the study, however the age group is specified as ‘all ages’ as age in months could not be verified from the data.
c For EU-PC and EU-H, there is no temperature threshold reported for fever, which can be measured or self-reported. For DK-PC and DK-H there is no temperature threshold for fever given in the guidance to practitioners.
d This is the case definition for patient recruitment for all GPs within DK-PC. Sentinel GPs within DK-PC follow the EU-ILI case definition (sudden onset of symptoms, AND ≥1 of: fever >38°C, feverishness, malaise, headache, myalgia AND ≥1 of: cough, sore throat, shortness of breath).
e The EU-ARI definition is sudden onset of symptoms AND ≥ 1 of cough, sore throat, shortness of breath or coryza AND a clinician’s judgement that the illness is due to an infection.
f Varies according to SARS-CoV-2/influenza testing practices by Health Board.
g Vaccines were prepared from egg-propagated vaccine viruses, non-adjuvanted and administered intramuscularly unless otherwise specified.
h Where indicated, vaccine coverage among controls were used as representative of the source population from which the cases arose.
i For all studies ‘sex’ is used in the statistical model for estimating VE as a binary variable: male/female.
j The QCOVID risk groups are defined as the number of generic comorbidity conditions of a patient, and are used as a measure of comorbidity. The list of conditions is found in the study of Clift et al [26].
Figure 1.
Countries providing interim influenza vaccine effectiveness results, Europe, influenza season 2022/23 (n = 16)
DK-PC/DK-H: Denmark primary care and hospital studies; EN-EC: England emergency care study; EU-PC/EU-H: European Union primary care-based and hospital-based multi-country VEBIS studies; SC-H: Scotland hospital study; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
Red circle: Navarra site (EU-PC and EU-H).
Study design
The methods for all six studies have been described elsewhere [8-12]. All studies used the test-negative design [13], although some differed in how they recruited patients and/or in their data collection (Table 1). Briefly, four studies obtained data through electronic database linkage (DK-H, DK-PC, EN-EC, SC-H) and two predominantly used prospective patient recruitment (EU-PC and EU-H). For primary care studies, nasopharyngeal (or saliva specimens, in France) were collected from patients with influenza-like illness (ILI) or acute respiratory infection (ARI) symptoms. For the emergency care setting, patients were recruited if they had an influenza swab test from 14 days before to 7 days after an emergency care visit compatible with influenza infection or its complications. In the hospital setting, patients admitted with severe ARI (SARI) symptoms were swabbed. In SC-H, all patients entering hospital as an emergency admission and tested for influenza were assumed to have an ARI symptom.
Two studies used an exhaustive or systematic selection of patients to swab/include (EU-PC and EU-H), while physicians' discretion was used to select patients for swabbing in the other four (DK-H, DK-PC, EN-EC and SC-H). Samples were tested by reverse transcription (RT)-PCR for influenza virus detection, type A subtyping and type B lineage determination. We defined cases as patients with positive results by influenza virus (sub)type. We defined controls as those testing RT-PCR negative for influenza virus. Most studies recruited patients among all ages. In SC-H, analyses are restricted to patients aged ≥ 18 years, as not all National Health Service Health Boards in Scotland submit patient level data for vaccination events in children. In EU-H, a few hospitals in some study sites only recruit patients aged ≥ 18 years.
Vaccinated patients were defined as those having had the 2022/23 influenza vaccine at least 14 days before onset of symptoms. Those vaccinated less than 14 days before symptom onset, or with unknown date of vaccination, were excluded. In EN-EC children vaccinated within 20 days were excluded from the analysis to avoid live attenuated influenza vaccine (LAIV)-related infections.
Many study countries (eight from EU-PC; three from EU-H; and Denmark) selected all or a random sample of influenza virus-positive specimens for haemagglutinin genome segment and/or whole genome sequencing, where technically feasible. In SC-H, the selection was based on other criteria (including vaccination status, antiviral use and being from an area with other cases) and cannot be considered a random sample. Sequencing was followed by phylogenetic analysis to determine clade distribution for potential impact on VE. Sequencing results were provided for both studies in Denmark together (DK-PC and DK-H).
Statistical analysis
We computed VE in each study as one minus the adjusted ratio of the odds of vaccination in cases and controls, as a percentage: VE = (1 − ORa) × 100. We applied logistic regression to adjust for measured potential confounding variables (Table 1). We estimated study-specific VE overall and, where possible, by age group and target population (as defined locally in the various studies and study sites) against influenza A and B combined (not in DK-PC or DK-H, as this country decided to only present A and B types separately due to the heterogeneity across influenza type-specific estimates), influenza A overall, A(H1N1)pdm09, A(H3N2), and influenza B. We defined small sample size analyses as those having fewer than 10 cases or controls per parameter. For these, a sensitivity analysis was performed using Firth’s method of penalised logistic regression (PLR) to assess small sample bias [14,15]. We considered a difference of > 10% between the original estimate and that obtained using PLR to be an indication of small sample bias; none of these estimates are shown.
Results
From 3 October 2022 to 31 January 2023, for the primary care setting we included 6,097 cases and 30,957 controls in the DK-PC study; 3,977 and 10,184 in EU-PC. For the emergency care setting there were 3,760 cases and 9,298 controls. In the hospital setting, there were 1,520 cases and 32,581 controls in DK-H; 488 and 2,620 in EU-H; 4,635 and 29,442 in SC-H.
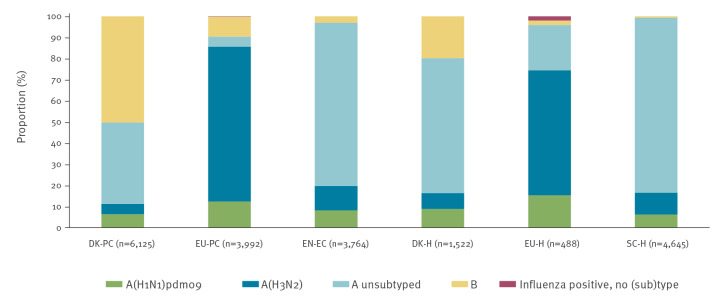
Overall, 81% (16,604/20,536) of confirmed infections were influenza A virus-positive and 19% (3,913/20,536) were influenza B virus-positive (the remaining 19 (< 1%) being untyped), noting that there were 20,536 infections among 20,477 cases. There were 44 influenza A and B co-infections and 15 influenza A(H1N1)pdm09 and A(H3N2) co-infections. The proportion of subtyped influenza A viruses was 95% in EU-PC, 78% in EU-H, and 17–23% in DK-PC, DK-H, EN-EC and SC-H. Most subtyped influenza A viruses were influenza A(H3N2) (58–85%) in EN-EC, EU-PC, EU-H and SC-H; this subtype comprised 43–46% in DK-PC and DK-H (Figure 2). The proportion of influenza B among all influenza viruses ranged from 1 to 9% in EN-EC, EU-PC, EU-H and SC-H to 20–50% in DK-H and DK-PC (Figure 2).
Figure 2.
Proportion of influenza virus (sub)type infections, six European studies, interim influenza season 2022/23 (n = 20,536)a
DK-H: Denmark hospital study; DK-PC: Denmark primary care study; EC: Emergency care; EN-EC: England emergency care study; EU: European Union; EU-H: EU hospital multicentre VEBIS study; EU-PC: EU primary care multicentre VEBIS study; PC: primary care; SC-H: Scottish hospital study; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
a Includes 28 A and B co-infections in DK-PC, two in DK-H, four in EN-EC, 10 in EU-PC; includes five A(H1N1)pdm09 and A(H3N2) co-infections in EU-PC and 10 in EU-H.
All influenza (A and B)
The overall VE estimates for influenza types A and B together were not presented for DK-PC and DK-H due to difference in VE across influenza types.
Primary care and emergency care settings
The VE against all influenza among children aged 0–17 years was 55% (95% confidence interval (CI): 31 to 71) in EU-PC; 61% (95% CI: 50 to 70) in EN-EC for those aged 2–17 years. For adults, VE varied between 28% (95% CI: 16 to 38) among those aged ≥ 65 years in EN-EC and 43% (95% CI: 29 to 54) among those aged 18–64 years in EU-PC (Table 2).
Table 2. Interim adjusted vaccine effectiveness (VE) against all laboratory-confirmed influenza, influenza A, A(H1N1)pdm09, A(H3N2) and B, by age group, target group for vaccination and by study, six European studies, influenza season 2022/23.
| Influenza (sub)type and study | Setting | Study populationa |
Cases | Controls | VEb | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Vaccinated | % | All | Vaccinated | % | ||||||
| All influenza (A and B)c | |||||||||||
| EU-PC | PC | All ages | 3,977 | 275 | 7 | 10,184 | 1,582 | 16 | 44 | 34 to 52 | |
| 0–17 years | 1,679 | 36 | 2 | 2,892 | 96 | 3 | 55 | 31 to 71 | |||
| 18–64 years | 2,067 | 123 | 6 | 5,598 | 524 | 9 | 43 | 29 to 54 | |||
| ≥ 65 years | 231 | 116 | 50 | 1,694 | 962 | 57 | 33 | 8 to 51 | |||
| Target groupd | 1,005 | 220 | 22 | 4,112 | 1,367 | 33 | 38 | 24 to 49 | |||
| EN-EC | EC | All ≥ 18 years | 3,046 | 1,158 | 38 | 7,797 | 3,722 | 48 | 30 | 21 to 38 | |
| 2–17 years | 714 | 116 | 16 | 1,501 | 389 | 26 | 61 | 50 to 70 | |||
| 18–64 years | 1,667 | 315 | 19 | 2,954 | 798 | 27 | 36 | 22 to 48 | |||
| ≥ 65 years | 1,379 | 843 | 61 | 4,843 | 2,924 | 60 | 28 | 16 to 38 | |||
| EU-H | H | All ages | 488 | 170 | 35 | 2,620 | 1,249 | 48 | 27 | 6 to 44 | |
| 18–64 years | 157 | 25 | 16 | 500 | 135 | 27 | 17 | −44 to 52 | |||
| ≥ 65 years | 279 | 143 | 51 | 1,597 | 1,086 | 68 | 29 | 4 to 47 | |||
| Target groupd | 378 | 161 | 43 | 1,959 | 1,212 | 62 | 31 | 9 to 48 | |||
| SC-H | H | All ≥ 18 years | 4,635 | 2,317 | 50 | 29,442 | 14,709 | 50 | 29 | 24 to 35 | |
| 18–64 years | 1,987 | 492 | 25 | 10,166 | 2,561 | 25 | 34 | 26 to 42 | |||
| ≥ 65 years | 2,648 | 1,825 | 69 | 19,276 | 12,148 | 63 | 27 | 19 to 34 | |||
| Influenza A (all) | |||||||||||
| DK-PC | PC | All ages | 3,048 | 612 | 20 | 30,957 | 9,996 | 32 | 44 | 37 to 50 | |
| 2–6 years | 339 | 18 | 5 | 2,368 | 399 | 17 | 77 | 62 to 86 | |||
| 0–17 years | 898 | 25 | 3 | 8,804 | 474 | 5 | 61 | 40 to 74 | |||
| 18–64 years | 1,702 | 226 | 13 | 14,189 | 3,065 | 22 | 49 | 41 to 56 | |||
| ≥ 65 years | 448 | 361 | 81 | 7,964 | 6,457 | 81 | 19 | −4 to 37 | |||
| EU-PC | PC | All ages | 3,602 | 265 | 7 | 10,174 | 1,578 | 16 | 40 | 30 to 49 | |
| 0–17 years | 1,492 | 35 | 2 | 2,890 | 96 | 3 | 50 | 22 to 68 | |||
| 18–64 years | 1,883 | 116 | 6 | 5,592 | 522 | 9 | 40 | 25 to 52 | |||
| ≥ 65 years | 227 | 114 | 50 | 1,692 | 960 | 57 | 32 | 7 to 51 | |||
| Target groupd | 943 | 214 | 23 | 4,107 | 1,363 | 33 | 35 | 21 to 47 | |||
| EN-EC | EC | All ≥ 18 years | 2,969 | 1,152 | 39 | 7,797 | 3,722 | 48 | 29 | 20 to 37 | |
| 2–17 years | 680 | 114 | 17 | 1,501 | 389 | 26 | 60 | 48 to 69 | |||
| 18–64 years | 1,596 | 312 | 20 | 2,954 | 798 | 27 | 35 | 20 to 46 | |||
| ≥ 65 years | 1,373 | 840 | 61 | 4,843 | 2,924 | 60 | 27 | 16 to 38 | |||
| DK-H | H | All ages | 1,221 | 620 | 51 | 32,581 | 18,513 | 57 | 33 | 23 to 42 | |
| 2–6 years | 49 | 1 | 2 | 708 | 115 | 16 | 90 | 23 to 99 | |||
| 0–17 years | 142 | 2 | 1 | 3,116 | 145 | 5 | 80 | 16 to 95 | |||
| 18–64 years | 356 | 87 | 24 | 8,390 | 2,316 | 28 | 36 | 17 to 50 | |||
| ≥ 65 years | 723 | 531 | 73 | 21,075 | 16,052 | 76 | 29 | 16 to 40 | |||
| EU-H | H | All ages | 468 | 166 | 35 | 2,611 | 1,245 | 48 | 27 | 6 to 44 | |
| 18–64 years | 146 | 24 | 16 | 498 | 134 | 27 | 12 | −53 to 50 | |||
| ≥ 65 years | 272 | 140 | 51 | 1,590 | 1,083 | 68 | 28 | 2 to 47 | |||
| Target groupd | 363 | 157 | 43 | 1,951 | 1,208 | 62 | 30 | 7 to 47 | |||
| SC-H | H | All ≥ 18 years | 4,601 | 2,310 | 50 | 29,381 | 14,671 | 50 | 29 | 23 to 34 | |
| 18–64 years | 1,961 | 489 | 25 | 10,141 | 2,552 | 25 | 34 | 25 to 42 | |||
| ≥ 65 years | 2,640 | 1,821 | 69 | 19,240 | 12,119 | 63 | 26 | 18 to 33 | |||
| Influenza A(H1N1)pdm09 | |||||||||||
| DK-PC | PC | All ages | 394 | 74 | 19 | 30,957 | 9,996 | 32 | 46 | 26 to 60 | |
| 2–6 years | 42 | 2 | 5 | 2,368 | 399 | 17 | 79 | 14 to 95 | |||
| 0–17 years | 122 | 2 | 2 | 8,804 | 474 | 5 | 77 | 6 to 94 | |||
| 18–64 years | 224 | 36 | 16 | 14,189 | 3,065 | 22 | 35 | 6 to 55 | |||
| ≥ 65 years | 48 | 36 | 75 | 7,964 | 6,457 | 81 | 37 | −22 to 67 | |||
| EU-PC | PC | All agese | 485 | 55 | 11 | 9,569 | 1,488 | 16 | 28 | 0 to 50 | |
| 18–64 years | 328 | 26 | 8 | 5,261 | 494 | 9 | 42 | 9 to 64 | |||
| Target groupd | 168 | 50 | 30 | 3,896 | 1,285 | 33 | 8 | −39 to 39 | |||
| EN-EC | EN | All ≥ 18 years | 244 | 94 | 39 | 2,357 | 1,116 | 47 | 26 | −9 to 50 | |
| 2–17 years | 65 | 9 | 14 | 660 | 134 | 20 | 49 | −17 to 77 | |||
| 18–64 years | 133 | 30 | 23 | 995 | 271 | 27 | 21 | −44 to 57 | |||
| ≥ 65 years | 111 | 64 | 58 | 1,362 | 845 | 62 | 28 | −21 to 57 | |||
| DK-H | H | All ages | 135 | 64 | 47 | 32,581 | 18,513 | 57 | 34 | 1 to 56 | |
| 18–64 years | 45 | 13 | 29 | 8,390 | 2,316 | 28 | 22 | −51 to 60 | |||
| ≥ 65 years | 73 | 50 | 68 | 21,075 | 16,052 | 76 | 42 | 5 to 65 | |||
| SC-H | H | All ≥ 18 years | 290 | 116 | 40 | 29,435 | 14,707 | 50 | 42 | 24 to 56 | |
| 18–64 years | 142 | 23 | 16 | 10,161 | 2,561 | 25 | 56 | 19 to 73 | |||
| ≥ 65 years | 148 | 93 | 63 | 19,274 | 12,146 | 63 | 31 | −1 to 52 | |||
| Influenza A(H3N2) | |||||||||||
| DK-PCc | PC | All ages | 297 | 80 | 27 | 30,957 | 9,996 | 32 | 23 | −7 to 45 | |
| 18–64 years | 163 | 24 | 15 | 14,189 | 3,065 | 22 | 36 | 1 to 59 | |||
| EU-PC | PC | All agese | 2,913 | 193 | 7 | 9,879 | 1,544 | 16 | 44 | 32 to 54 | |
| 0–17 years | 1,291 | 25 | 2 | 2,874 | 95 | 3 | 62 | 37 to 78 | |||
| 18–64 years | 1,455 | 86 | 6 | 5,379 | 512 | 10 | 39 | 22 to 53 | |||
| ≥ 65 years | 167 | 82 | 49 | 1,626 | 937 | 58 | 39 | 11 to 57 | |||
| Target groupd | 719 | 150 | 21 | 3,961 | 1,333 | 34 | 41 | 26 to 54 | |||
| EN-EC | EC | All ≥ 18 years | 288 | 104 | 36 | 2,357 | 1,116 | 47 | 37 | 12 to 55 | |
| 2–17 years | 144 | 17 | 12 | 660 | 134 | 20 | 70 | 46 to 84 | |||
| 18–64 years | 145 | 28 | 19 | 995 | 271 | 27 | 42 | −5 to 68 | |||
| ≥ 65 years | 111 | 76 | 68 | 1,362 | 845 | 62 | 42 | 10 to 62 | |||
| DK-H | H | All ages | 115 | 65 | 57 | 32,581 | 18,513 | 57 | 2 | −53 to 37 | |
| EU-H | H | All agesf | 288 | 120 | 42 | 2,575 | 1,242 | 48 | 27 | 1 to 46 | |
| ≥ 65 years | 191 | 103 | 54 | 1,574 | 1,080 | 69 | 28 | −3 to 49 | |||
| Target groupd | 238 | 114 | 48 | 1,929 | 1,205 | 62 | 26 | −3 to 47 | |||
| SC-H | H | All ≥ 18 years | 479 | 152 | 32 | 29,381 | 14,703 | 50 | 32 | 16 to 45 | |
| 18–64 years | 188 | 42 | 22 | 10,164 | 2,559 | 25 | 36 | 7 to 56 | |||
| ≥ 65 years | 291 | 203 | 70 | 19,272 | 12,144 | 63 | 33 | 12 to 49 | |||
| Influenza B | |||||||||||
| DK-PC | PC | All ages | 3,077 | 94 | 3 | 30,957 | 9,996 | 32 | 85 | 82 to 88 | |
| 2–6 years | 270 | 3 | 1 | 2,368 | 399 | 17 | 95 | 85 to 99 | |||
| 0–17 years | 1,511 | 11 | 1 | 8,804 | 474 | 5 | 90 | 82 to 95 | |||
| 18–64 years | 1,532 | 62 | 4 | 14,189 | 3,065 | 22 | 86 | 82 to 89 | |||
| ≥ 65 years | 34 | 21 | 62 | 7,964 | 6,457 | 81 | 66 | 33 to 83 | |||
| EU-PC | PC | All agese | 368 | 10 | 3 | 8,601 | 1,388 | 16 | 64 | 32 to 83 | |
| 0–17 years | 190 | 1 | 1 | 2,638 | 93 | 4 | 90 | 52 to 100 | |||
| 18–64 years | 177 | 8 | 5 | 4,553 | 448 | 10 | 51 | 1 to 79 | |||
| EN-EC | EC | All ≥ 18 years | 80 | 6 | 8 | 7,797 | 3,722 | 48 | 78 | 44 to 92 | |
| 2–17 years | 35 | 2 | 6 | 1,501 | 389 | 26 | 88 | 47 to 97 | |||
| 18–64 years | 73 | 3 | 4 | 2,954 | 798 | 27 | 84 | 43 to 95 | |||
| DK-H | H | All ages | 301 | 39 | 13 | 32,581 | 18,513 | 57 | 73 | 61 to 82 | |
| 2–6 years | 35 | 1 | 3 | 708 | 115 | 16 | 87 | 6 to 98 | |||
| 0–17 years | 132 | 1 | 1 | 3,116 | 145 | 5 | 89 | 19 to 98 | |||
| 18–64 years | 125 | 11 | 9 | 8,390 | 2,316 | 28 | 78 | 59 to 88 | |||
| ≥ 65 years | 44 | 27 | 61 | 21,075 | 16,052 | 76 | 58 | 22 to 77 | |||
| SC-H | PC | All ≥ 18 years | 34 | 7 | 21 | 29,410 | 14,702 | 50 | 50 | −36 to 82 | |
CI: confidence interval; DK-H: Denmark hospital study; DK-PC: Denmark primary care study; EC: Emergency care; EN-EC: England emergency care study; EU: European Union; EU-H: EU hospital multicentre VEBIS study; EU-PC: EU primary care multicentre VEBIS study; PC: primary care; SC-H: Scottish hospital study; VE: vaccine effectiveness; VEBIS: VE, Burden and Impact Studies.
a Age-specific or target group-specific VE was not included for overall or (sub)type-specific VE in some study sites, where sample size did not allow estimation of VE. Additionally for EU-PC: three study sites with < 10 A(H1N1)pdm09 cases were excluded from A(H1N1)pdm09 VE analysis (11 cases); two study sites with < 10 A(H3N2) cases were dropped from A(H3N2) VE analysis (8 cases); five study sites with < 10 B cases were not included in B VE analysis (8 cases).
b For details of adjustment variables, see Table 1.
c All influenza (A and B) estimates were not presented for DK-PC and DK-H due to difference in VE across influenza types.
d Groups targeted by seasonal influenza vaccination as defined locally in the studies and study sites.
e Three study sites with < 10 A(H1N1)pdm09 cases were excluded from A(H1N1)pdm09 VE analysis (11 cases); two study sites with < 10 A(H3N2) cases were dropped from A(H3N2) VE analysis (8 cases); five study sites with < 10 B cases were not included in B VE analysis (8 cases).
f Two study sites with < 10 influenza A(H3N2) cases were dropped from A(H3N2) VE analysis (10 cases).
Hospital inpatient settings
For older adults (aged ≥ 65 years), VE against all laboratory-confirmed hospitalised influenza was 29% (95% CI: 4 to 47) in EU-H and 27% (95% CI: 19 to 34) in SC-H. In the EU-H target group for influenza vaccination, VE was 31% (95% CI: 9 to 48).
Influenza A overall
For all ages and regardless of setting, VE against influenza A ranged from 27% (95% CI: 6 to 44) in EU-H to 44% (95% CI: 37 to 50) in DK-PC.
Primary care and emergency care settings
Among 18–64-year-olds, VE against laboratory-confirmed influenza A ranged from 35% (20 to 46) in EN-EC to 49% (41 to 56) in DK-PC. The VE against influenza A among people ≥ 65 years old ranged from 19% (95% CI: −4 to 37) in DK-PC to 32% (95% CI: 7 to 51) in EU-PC. In children < 18 years old, VE ranged from 50% (95% CI: 22 to 68) in EU-PC (0–17-year-olds), to 77% (95% CI: 62 to 86) in DK-PC (2–6-year-olds) (Table 2).
Hospital inpatient settings
For all ages, VE against laboratory-confirmed hospitalised influenza A ranged from 27% (95% CI: 6 to 44) in EU-H to 33% (95% CI: 23 to 42) in DK-H. For children, VE was between 80% (95% CI: 16 to 95) in those aged 0–17 years in DK-H and 90% (95% CI: 23 to 99) among those aged 2–6 years in DK-H. For adults aged 18–64 years, VE ranged from 12% in EU-H (95%CI: −53 to 50) to 36% in DK-H (95%CI: 17 to 50). For adults ≥ 65 years of age, VE ranged between 26% (95% CI: 18 to 33) in SC-H and 29% (95% CI: 16 to 40) in DK-H.
Influenza A(H1N1)pdm09
For all ages and regardless of setting, VE against A(H1N1)pdm09 ranged from 28% (95% CI: 0 to 50) in EU-PC to 46% (95% CI: 26 to 60) in DK-PC.
Primary care and emergency care settings
The VE against laboratory-confirmed influenza A(H1N1)pdm09 among children < 18 years of age was 49% (95% CI: −17 to 77) in EN-EC (2–17 years) and 77% (95% CI 6 to 94) in DK-PC (0–17 years). Among adults < 65 years old, VE ranged between 21% (95% CI: −44 to 57) in EN-EC and 42% (95% CI: 9to 64) in EU-PC. VE for people ≥ 65 years old was 28% (95% CI: −21 to 57) in the EN-EC study and 37% (95% CI: −22 to 67) in the DK-PC study. Target groups in the EU-PC had VE of 8% (95% CI: −39 to 39) against influenza A(H1N1)pdm09.
Hospital inpatient settings
For hospitalised patients aged 18–64 years, VE against A(H1N1)pdm09 was 22% (95% CI: −51 to 60) in DK-H and 56% (95% CI: 19 to 73) in SC-H. Among adults aged ≥ 65 years old, VE was 31% (95% CI: −1 to 52) in SC-H and 42% (95% CI: 5 to 65) in the DK-H study (Table 2). Sample size was too small to calculate VE estimates against influenza A(H1N1)pdm09 for the EU-H study.
Virological results
Among the 175 A(H1N1)pdm09 viruses sequenced, all but one 99% (n = 174) belonged to genetic clade 6B.1A.5a.2 (Table 3), the same as the vaccine virus. Among these 6B.1A.5a.2 viruses, 59/80 (74%) and 57/74 (77%) were A/Sydney/5/2021 A(H1N1)pdm09-like viruses in EU-PC and DK-H/DK-PC, respectively. These viruses had undergone the K54Q, A186T, Q189E, E224A, R259K and K308R amino acid changes compared with the vaccine virus A/Victoria/2570/2019. All 20 SC-H, 23% (n = 17) of the 74 DK-H/DK-H and 26% (n = 21) of the 81 EU-PC viruses were A/Norway/25089/2022 A(H1N1)pdm09-like viruses, characterised by the amino acid mutations P137S, K142R, D260E and T277A, compared to the vaccine strain. Study-specific virological data were not available in EN-EC, but from English national virological surveillance data, ca 80% of influenza A(H1N1)pdm09 viruses were A/Norway/25089/2022-like and ca 20% of viruses are A/Sydney/5/2021-like (Maria Zambon, personal communication, March 2023).
Table 3. Influenza viruses characterised by clade, amino acid substitutions and study site, six European studies, interim influenza season 2022/23 (n = 806).
| Characterised viruses | Clade | DK-H/DK-PCa | EU-PCb | EU-H | SC-H | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | N | % | ||
| Influenza A(H1N1)pdm09 (n = 175) | n = 74 | n = 81 | n = 0 | n = 20 | |||||
| A/Guangdong Maonan/SWL1536/2019 | 6B.1A.5a.1 | 0 | NC | 1 | 1 | 0 | NC | 0 | NC |
| A/Victoria/2570/2019 | 6B.1A.5a.2 | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| A/Sydney/5/2021c | 6B.1A.5a.2 | 57 | 77 | 59 | 73 | 0 | NC | 0 | NC |
| A/Norway/25089/2022d | 6B.1A.5a.2 | 17 | 23 | 21 | 26 | 0 | 0 | 20 | NC |
| Influenza A(H3N2) (n = 570) | n = 93 | n = 444 | n = 18 | n = 15 | |||||
| A/Denmark/3264/2019 | 3C.2a1b.1a | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| A/Cambodia/e0826360/2020 | 3C.2a1b.2a.1 | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| A/Darwin/9/2021 | 3C.2a1b.2a.2 | 8 | 9 | 0 | NC | 0 | NC | 0 | NC |
| A/Slovenia/8720/2022e | 3C.2a1b.2a.2 | 40 | 43 | 113 | 25 | 4 | NC | 0 | NC |
| A/Bangladesh/4005/2020f | 3C.2a1b.2a.2 | 45 | 48 | 331 | 75 | 14 | NC | 15 | NC |
| Group (i) S156H + others | NA | 45 | NC | 296 | 89 | 13 | NC | 0 | NC |
| Group (ii) D53N + others | NA | 0 | NC | 35 | 11 | 1 | NC | 0 | NC |
| Influenza B/Victoria (n = 82) | n = 22 | n = 60 | n = 0 | n = 0 | |||||
| B/Washington/02/2019 | V1A.3 | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| B/Netherlands/11267/2022 | V1A.3 | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| B/Cote d'Ivoire/948/2020 | V1A.3a.1 | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| B/Austria/1359417/2021 | V1A.3a.2 | 22 | NC | 60 | 100 | 0 | NC | 0 | NC |
DK-H: Denmark hospital study; DK-PC: Denmark primary care study; EU-H: European Union hospital multicentre VEBIS study; EU-PC: European Union primary care multicentre VEBIS study; NA: not applicable; NC: not calculated (percentages not shown where denominators < 60); SC-H: Scotland hospital study; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
a DK-H and DK-PC samples are combined.
b At time of writing, issues between linkage of epidemiological record identification (ID) numbers and sequencing ID numbers resulted in a low proportion of viruses included from two study sites within EU-PC.
c Harbouring the K54Q, A186T, Q189E, E224A, R259K and K308R amino acid mutations compared with the vaccine virus A/Victoria/2570/2019.
d Harbouring the A/Sydney/5/2021-like amino acid changes, and additionally P137S, K142R, D260E and T277A amino acid mutations compared with the vaccine virus A/Victoria/2570/2019.
e Harbouring the D53G, D104G and K276R amino acid mutations compared with the vaccine virus A/Darwin/9/2021.
f Harbouring the S156H amino acid mutation among others or the D53N amino acid mutation among others compared with the vaccine virus A/Darwin/9/2021.
Influenza A(H3N2)
For all ages and regardless of setting, VE against A(H3N2) ranged from 2% (95% CI: −53 to 37) in DK-H to 44% (95% CI: 32 to 54) in EU-PC.
Primary care and emergency care settings
For children aged 0–17 years in EU-PC, VE against influenza A(H3N2) was 62% (95% CI: 37 to 78). For children aged 2–17 years in the EN-EC, VE was 70% (95% CI: 46 to 84). For those aged 18–64 years, VE was between 36% (95% CI: 1 to 59) in DK-PC and 42% (95% CI: −5 to 68) in EN-EC. VE was 39% (95% CI: 11 to 57) in EU-PC and 42% (95% CI: 10 to 62) in EN-EC in those aged ≥ 65 years (Table 2).
Hospital inpatient settings
VE among hospitalised patients of all ages was 2% (95% CI: −53 to 37) in DK-H, 27% (95% CI: 1 to 46) in EU-H and 32% (95% CI: 16 to 45) in SC-H, the latter among those aged ≥ 18 years. Among adults aged ≥ 65 years, VE against influenza A(H3N2) was 28% (95% CI: −3 to 49) in EU-H and 33% (95% CI: 12 to 49) in SC-H (Table 2).
Virological results
Of the 570 influenza A(H3N2) viruses sequenced, all belonged to the same clade as the vaccine strain (3C.2a1b.2a.2). In DK-PC/DK-H, 43% (40/93), in EU-PC 25% (113/444), and in EU-H 22% (4/18) of the viruses belonged to A/Slovenia/8720/2022-like viruses, harbouring the specific amino acid mutations D53G, D104G and K276R, while no such viruses were sequenced in SC-H. In DK-PC/DK-H 48% (45/93), in EU-PC 75% (331/444), in EU-H 78% (14/18) and in SC-H 100% (15/15) belonged to A/Bangladesh/4005/2020-like viruses. Among these, where known, 100% (n = 45) in DK-PC/DK-H, 89% (296/331) in EU-PC and 93% (13/14) in EU-H belonged to group (i), harbouring the S156H amino acid mutation among others. In EU-PC 11% (35/331) and in EU-H 7% (1/14) belonged to group (ii), harbouring the amino acid mutation D53N among others. In DK-PC/DK-H 9% (8/93) belonged to the northern hemisphere vaccine strain A/Darwin/9/2021-like virus (Table 3).
Influenza B
Primary care and emergency care settings
Among children, VE against laboratory-confirmed influenza B ranged from 88% (95% CI: 47 to 97) in EN-EC among those aged 2–17 years to 95% (95% CI: 85 to 99) in DK-PC for those aged 2–6 years. For those aged 18–64 years, VE was 51% in EU-PC (95% CI: 1 to 79) and 86% (95% CI: 82 to 89) in DK-PC. The VE among those aged ≥ 65 years was 66% (95% CI: 33 to 83%) in DK-PC (Table 2).
Hospital settings
The VE against influenza B was 50% (95% CI: −1 to 52) in SC-H among those aged ≥ 18 years. In DK-H, the VE was 78% (95% CI: 59 to 88) among those aged 18–64 years and 58% (95% CI: 22 to 77) among those aged ≥ 65 years. Among children aged 0–17 years, the VE in DK-H was 89% (95% CI: 19 to 98) (Table 2). Sample size was too small to calculate VE estimates against influenza B for the EU-H study.
Virological results
Of the 82 influenza B/Victoria viruses sequenced, all belonged to the V1A.3a.2 clade, represented by B/Austria/1359417/2021, which is also the vaccine virus (Table 3).
Sensitivity analyses
Results with small sample sizes were subject to sensitivity analyses, most of which gave similar results (absolute difference < 10%). Results from the three estimates with absolute difference ≥ 10% (evidence of small sample bias) were not presented.
Discussion
In six well-established influenza studies across Europe during the 2022/23 influenza season, interim VE against influenza A (all subtypes) (all ages; primary care, emergency care and hospital settings) ranged from 27% to 44%. All interim VE against influenza B was ≥ 50%, among overall and age-stratified estimates. The proportions of influenza A and B and influenza A subtypes circulating differed by country and setting.
Influenza A (all subtypes) point estimates for VE were higher among children (50–90%), compared with adults (12–49%). Against influenza A(H1N1)pdm09, VE point estimates among all ages ranged from 28% to 46%. The VE point estimates were higher among children at 49% and 77% in EN-EC and DK-PC, respectively (21–56% among those aged 18–64 years). VE against influenza A(H3N2) ranged from 2 to 44% among all ages (36–42% among 18–64-year-olds). Children had higher VE point estimates at 62–70%. Against laboratory-confirmed influenza B, VE in children < 18 years old was between 88 and 90% (87–95% in those aged 2–6 years old).
The proportion of subtyped influenza A viruses varied by study site (between 17% and 95%). While the lack of subtyping may have affected the precision of subtype-specific estimates, descriptive analyses at study site level indicated that those subtyped are likely to belong to a representative sample of all viruses.
In the EN-EC and EU-PC studies, for which the end-of-season 2021/22 influenza A(H1N1)pmd09 VE are available, the 2022/23 interim season estimates were lower: 26% (among ≥ 18-year-olds) vs 76% (among ≥ 50-year-olds) in EN-EC and 28% vs 75% (among all ages) EU-PC [16,17]. The influenza vaccine component remained the same between these two seasons; however, circulating strains differed. While post-infection ferret antisera raised against the vaccine strain A/Victoria/2570/2019 had good recognition to circulating viruses, post-vaccination human sera showed lower reactivity [18]. The 2022/23 end-of-season overall results, as well as clade/genetic variant-specific results and birth cohort-specific VE, may help unravel the differences between these two seasons. Additionally, around 25% of all sequenced influenza A(H1N1)pdm09 viruses in DK-PC/DK-H and in EU-PC, and all 20 sequenced viruses in SC-H belonged to the A/Norway/25089/2022-like viruses.
The VE point estimates against influenza A(H3N2) among the three primary care and emergency care studies (DK-PC, EU-PC and EN-EC) over adult ages, at 36–42%, were slightly lower than the VE point estimates from Canada (58–59%) [19]. Among children, the primary care and emergency care study results presented here were higher at 62–70% compared with those in the Canadian study (47%). Authors in Canada noted a high proportion of T135K substitutions among those ≤ 25 years of age. Position 135 is a haemagglutinin (HA) glycosylation site associated with potential antigenic change [20]. Information on substitutions at this position is not available from all studies, but only two of the 444 sequenced EU-PC viruses and only one of 93 from the DK-H/PC studies, also in an individual aged < 25 years, harboured the T135K substitution. In EU-PC, 11% (51/444) of sequenced samples had a T135A substitution, which also involves the loss of the HA glycosylation site. All A(H3N2) viruses with available genetic information from the studies presented here belonged to the 3C.2a1b.2a.2 clade, but with varying genetic diversity within this clade.
In the DK-PC, DK-H, EN-EC and EU-PC studies, for which end-of-season 2021/22 influenza A(H3N2) VE estimates were available, the overall 2022/23 interim results against influenza A(H3N2) were higher for EN-EC and EU-PC studies (37% vs 28% and 44% vs 29%, for EN-EC and EU-PC, respectively, noting a difference in the reported age cohort for EN-EC) [16,17]. For DK-PC and DK-H, 2021/22 influenza A(H3N2) varied considerably by age group, particularly above and below 45 years of age, and cannot be directly compared with the interim 2022/23 A(H3N2) VE results in these studies [3]. However, VE was generally low (23% and 2% for DK-PC and DK-H, respectively), although sample size was also low. As the 2021/22 season was a long and late season in Europe, the 2021/22 A(H3N2) VE may have declined with time since vaccination, as reported by the EU-PC study, rendering the overall 2021/22 season A(H3N2) estimates not comparable to 2022/23 interim estimates. While reports suggest a good antigenic match between circulating and vaccine strains for A(H3N2) in 2022/23, there was considerable genetic diversity within the 3C.2a1b.2a.2 clade, and end-of-season and clade/genetic variant-specific results may help understand differences between sites.
Influenza B virus has had little circulation in Europe since the 2019/20 season. The observed VE against influenza B was high at ≥ 50%, with estimates among children at ≥ 87%. Influenza B VE is often high, as seen in the 2019/20 season in Canada, the United States, Denmark, Spain and in the EU-PC study [21-23]. All sequenced viruses belonged to the V1A.3a.2 clade and, as expected, no influenza B/Yamagata was detected among sequenced viruses. Recent B/Victoria viruses harbour substitutions at positions resulting in a phenotypic ‘reversion’ to viruses with similar antigenic properties to viruses circulating ≥ 50 years before [24]. Potential imprinting effects may explain differing VE by birth cohort and could be explored further if end-of-season sample size allows.
In general, across influenza (sub)types, particularly for influenza A(H3N2) and B, VE point estimates were high in children. While some confidence intervals overlapped between children’s and adults’ estimates, the point estimates were consistently higher among children across all influenza (sub)types in each study. LAIV is part of the routine childhood immunisation schedule in the UK and has been introduced in Ireland and Denmark in recent years. The use of LAIV could contribute to the age-specific differences in VE and these results indicate good performance of LAIV in this season. A further contribution to age-specific differences in VE is that routine childhood immunisation is targeted towards all children, including healthy children. In contrast, in young adults, immunisation is indicated mainly for those with underlying medical conditions, who may be at greater risk of influenza infection/hospitalisation.
The early start of the season in most European countries included in these six studies [5-7] resulted in higher incidence and greater precision for interim VE estimates than in other interim season estimates. However, due to the different circulation of influenza viruses across Europe, some studies had lower sample size for some subgroups in this interim analysis and results should be interpreted with caution. Each study used their own specific criteria to define whether a sample size was too small to attempt VE estimation. Sensitivity analyses were used to address potential small sample bias where appropriate. These studies are all observational in nature and residual confounding and bias may potentially be present.
Conclusion
Vaccination remains a successful means of influenza prevention. Interim results from six European studies during the 2022/23 influenza season indicate a ≥ 27% and ≥ 50% reduction in disease occurrence among all-age influenza vaccine recipients for influenza A and B, respectively. Influenza VE point estimates were ≥ 50% against all influenza (sub)types in children, indicating a successful LAIV campaign. Influenza vaccination should continue to be promoted according to national guidelines in all European countries with ongoing influenza virus circulation.
Findings of the current study were presented as part of the Global Influenza Vaccine Effectiveness (GIVE) report to the WHO Vaccine Strain Selection Committee, held on 20–23 February 2023. In this meeting, the WHO recommendations for the 2023/24 Northern Hemisphere influenza vaccine viruses did not change for influenza B/Victoria, B/Yamagata or A(H3N2) [25]. For influenza A(H1N1)pdm09, the recommendation for the 2023–24 influenza vaccines changed to A/Victoria/4897/2022 (H1N1)pdm09-like virus for egg-based vaccines and A/Wisconsin/67/2022 (H1N1)pdm09-like viruses for cell-based vaccines.
End-of-season influenza VE and genetic analyses may help understand observed differences in age as well as study-specific VE.
European IVE group members
BELGIUM, EU-H study, Belgium SARI Surveillance Network (BelsariNet): Arne Witdouck (Department of Microbiology and Infection control, UZ Brussel, Brussels), Benedicte Delaere (Department of Infectious Diseases, CHU UCL Namur, Université catholique de Louvain, Yvoir), Benédicte Lissoir (Service of Clinical Biology, Grand Hôpital de Charleroi, Charleroi), Caroline Wylock (Department of Microbiology and Infection control, UZ Brussel, Brussels), Catherine Sion (Service of Infectiology, Grand Hôpital de Charleroi, Charleroi), Cyril Barbezange (National Influenza Centre, Sciensano, Brussels), Door Jouck (Department of Microbiology, Jessa Ziekenhuis, Hasselt), Els Van Nedervelde (Department of Microbiology and Infection control, UZ Brussel, Brussels), Evelyn Petit (Department of Laboratory Medicine, Medical Microbiology, Algemeen Ziekenhuis Sint-Jan, Brugge-Oostende AV), François Dufrasne (National Influenza Centre, Sciensano, Brussels), Isabelle Thomas (Epidemiology of Infectious Diseases, Sciensano, Brussels), Koen Magerman (Department of Microbiology, Jessa Ziekenhuis, Hasselt), Lucie Seyler (Department of Infectiology, UZ Brussel, Brussels), Marc Bourgeois (Department of Infectious Diseases, CHU UCL Namur, Université catholique de Louvain, Yvoir), Marc Hainaut (Pediatrics Department, CHU Saint-Pierre, Université Libre de Bruxelles (ULB), Brussels), Marieke Bleyen (Department of Microbiology, Jessa Ziekenhuis, Hasselt), Marijke Reynders (Department of Laboratory Medicine, Medical Microbiology, Algemeen Ziekenhuis Sint-Jan, Brugge-Oostende AV), Melissa Vermeulen (Epidemiology of Infectious Diseases, Sciensano, Brussels), Nathalie Bossuyt (Epidemiology of Infectious Diseases, Sciensano, Brussels), Nicolas Dauby (Department of Infectious Diseases, Centre hospitalier Universitaire Saint-Pierre, Brussels), Sarah Denayer (National Influenza Centre, Sciensano, Brussels), Sebastien Fierens (Epidemiology of Infectious Diseases, Sciensano, Brussels), Siel Daelemans (Department of Microbiology and Infection control, UZ Brussel, Brussels), Thomas Demuyser (Department of Microbiology and Infection control, UZ Brussel, Brussels), Virgini Van Buggenhout (Department of Microbiology and Infection control, UZ Brussel, Brussels), Xavier Holemans (Service of Infectiology, Grand Hôpital de Charleroi, Charleroi). CROATIA, EU-H and EU-PC studies: Vesna Višekruna Vučina (Croatian Institute of Public Health, Zagreb), Maja Ilić (Croatian Institute of Public Health, Zagreb), Goranka Petrović (Croatian Institute of Public Health, Zagreb), Ivan Mlinarić (Croatian Institute of Public Health, Zagreb), Sanja Kurečić Filipović (Croatian Institute of Public Health, Zagreb), Bernard Kaić (Croatian Institute of Public Health, Zagreb), Iva Pem Novosel (Croatian Institute of Public Health, Zagreb), Ivana Ferenčak (Croatian Institute of Public Health, Zagreb), Irena Tabain (Croatian Institute of Public Health, Zagreb), Katica Čusek Adamić (Institute of Public Health, Varaždin County), Mirjana Lana Kosanović Ličina (“Dr. Andrija Štampar” Teaching Institute of Public Health, Zagreb), Danijela Lakošeljac (Teaching Institute of Public Health, Primorje-Gorski kotar County), Ivana Mihin Huskić (Teaching Institute of Public Health, Osijek-Baranja County), Diana Nonković (Teaching Institute of Public Health, Split-Dalmatia County). DENMARK, DK-H and DK-PC studies: Jens Nielsen (Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, Copenhagen. FRANCE, EU-PC study: Noémie Sève (Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris), Ana-Maria Vilcu (Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris), Caroline Guerrisi (Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris), Thierry Blanchon (Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris), Titouan Launay (Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris), Alessandra Falchi (Laboratoire de Virologie, Université de Corse-Inserm, Corte), Shirley Masse (Laboratoire de Virologie, Université de Corse-Inserm, Corte), Sylvie van der Werf (Institut Pasteur, Université Paris Cité, Unité de Génétique Moléculaire des Virus à ARN, UMR 3569 CNRS, Paris), Vincent Enouf (Institut Pasteur, Université Paris Cité, Unité de Génétique Moléculaire des Virus à ARN, UMR 3569 CNRS, Paris), Bruno Lina (Laboratoire de Virologie, CNR des virus des infections respiratoires, Institut des Agents Infectieux, Groupement Hospitalier Nord des HCL, Lyon), Martine Valette (Laboratoire de Virologie, CNR des virus des infections respiratoires, Institut des Agents Infectieux, Groupement Hospitalier Nord des HCL, Lyon); Epiconcept, France, EU-H and EU-PC studies: Anthony Nardone (Epiconcept, Paris); EU-H study: Ruoran Li (Epiconcept, Paris). EUROPEAN CENTRE FOR DISEASE PREVENTION AND CONTROL, SWEDEN, EU-PC and EU-H study: Marlena Kaczmarek (ECDC, Stockholm), Nathalie Nicolay (ECDC, Stockholm), Sabrina Bacci (ECDC, Stockholm). GERMANY, EU-PC and EU-H study: Silke Buda (Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute, Berlin), Luise Goerlitz (Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute, Berlin), Kristin Tolksdorf (Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute, Berlin), Ute Preuss (Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute, Berlin), Ralf Duerrwald (National Reference Center for Influenza, Robert Koch Institute, Berlin), Marianne Wedde (National Reference Center for Influenza, Robert Koch Institute, Berlin), Barbara Biere (National Reference Center for Influenza, Robert Koch Institute, Berlin), Janine Reiche (National Reference Center for Influenza, Robert Koch Institute, Berlin). HUNGARY, EU-PC study: Gergő Túri (National Laboratory for Health Security, Epidemiology and Surveillance Centre, Semmelweis University, Budapest), Judit Krisztina Horváth (National Laboratory for Health Security, Epidemiology and Surveillance Centre, Semmelweis University, Budapest), Beatrix Oroszi (National Laboratory for Health Security, Epidemiology and Surveillance Centre, Semmelweis University, Budapest), Katalin Kristóf (Institute of Laboratory Medicine, Semmelweis University, Budapest). IRELAND, EU-PC and EU-H studies: Lisa Domegan (HSE-Health Protection Surveillance Centre, Dublin), Joan O’Donnell (HSE-Health Protection Surveillance Centre, Dublin), Róisín Duffy (HSE-Health Protection Surveillance Centre, Dublin), Adele McKenna (HSE-Health Protection Surveillance Centre, Dublin), Charlene Bennett (National Virus Reference Laboratory, University College Dublin), Jeff Connell (National Virus Reference Laboratory, University College Dublin), Joanne Moran (National Virus Reference Laboratory, University College Dublin), Michael Joyce (Irish College of General Practitioners, Dublin). LITHUANIA, EU-H study: Ligita Jančorienė (Clinic of Infectious Diseases and Dermatovenerology, Institute of Clinical Medicine, Vilnius University, Vilnius), Birutė Zablockienė (Clinic of Infectious Diseases and Dermatovenerology, Institute of Clinical Medicine, Vilnius University, Vilnius), Ieva Kubiliūtė (Clinic of Infectious Diseases and Dermatovenerology, Institute of Clinical Medicine, Vilnius University, Vilnius), Fausta Majauskaitė (Clinic of Infectious Diseases and Dermatovenerology, Institute of Clinical Medicine, Vilnius University, Vilnius), Rolandas Zabockis (Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Vilnius University, Vilnius), Goda Šlekytė (Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Vilnius University, Vilnius), Giedrė Cincilevičiūtė (Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Vilnius University, Vilnius), Auksė Mickienė (Department of Infectious Diseases, Lithuanian University of Health Sciences, Kaunas), Monika Kuliešė (Department of Infectious Diseases, Lithuanian University of Health Sciences, Kaunas), Roberta Vaikutytė (Department of Infectious Diseases, Lithuanian University of Health Sciences, Kaunas). MALTA, EU-H study: Stephen Abela (Infectious Disease Prevention and Control Unit (IDCU), Health Promotion and Disease Prevention, Msida), Aušra Džiugytė (Infectious Disease Prevention and Control Unit (IDCU), Health Promotion and Disease Prevention, Msida), Maria-Louise Borg (Infectious Disease Prevention and Control Unit (IDCU), Health Promotion and Disease Prevention, Msida), John-Paul Cauchi (Infectious Disease Prevention and Control Unit (IDCU), Health Promotion and Disease Prevention, Msida), Tanya Melillo (Infectious Disease Prevention and Control Unit (IDCU), Health Promotion and Disease Prevention, Msida). THE NETHERLANDS, EU-PC study: Adam Meijer (National Institute for Public Health and the Environment (RIVM), Bilthoven), Marit de Lange (National Institute for Public Health and the Environment (RIVM), Bilthoven), Frederika Dijkstra (National Institute for Public Health and the Environment (RIVM), Bilthoven), Mariëtte Hooiveld (Nivel (the Netherlands Institute for Health Services Research), Utrecht), Rianne van Gageldonk (National Institute for Public Health and the Environment (RIVM), Bilthoven). PORTUGAL, EU-PC study: Ana Paula Rodrigues (Departamento de Epidemiologia, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Ausenda Machado (Departamento de Epidemiologia, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Irina Kislaya (Departamento de Epidemiologia, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Verónica Gomez (Departamento de Epidemiologia, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Aryse Melo (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Camila Henriques (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Inês Costa (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Licínia Gomes (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Miguel Lança (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon), Nuno Verdasca (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge), Raquel Guiomar (Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisbon). ROMANIA, EU-PC and EU-H studies: Mihaela Lazar (“Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Maria Elena Mihai (“Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Alina Ivanciuc (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Catalina Pascu (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Iulia Bistriceanu (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Sorin Dinu (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Mihaela Oprea (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest); EU-H study: Odette Popovici (National Institute of Public Health, Bucharest), Isabela Loghin (Clinical Hospital of Infectious Diseases “Sf Parascheva”, Iasi), Elena Duca (Clinical Hospital of Infectious Diseases “Sf Parascheva”, Iasi), Mihaela Catalina Luca (Clinical Hospital of Infectious Diseases “Sf Parascheva”, Iasi), Carmen Mihaela Dorobat (Clinical Hospital for Infectious Diseases, “Sf Parascheva”, Iasi), Corneliu-Petru Popescu (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest), Gratiela Tardei (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest), Alexandru Marin (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest), Alma-Gabriela Tudor (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest), Simin-Aysel Florescu (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest), Emanoil Ceausu (Clinical Hospital of Infectious Diseases “Dr Victor Babes”, Bucharest); EU-PC study: Rodica Popescu (National Institute of Public Health, Bucharest), Olivia Timnea (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest), Adrian Jidovu (National Influenza Centre, “Cantacuzino” National Military-Medical Institute for Research and Development, Bucharest). SPAIN, EU-PC and EU-H studies: Virtudes Gallardo García (Dirección General de Salud Pública y Ordenación Farmacéutica, Junta de Andalucía), Irene Pedrosa Corral (Servicio de Microbiología, Hospital Universitario Virgen de las Nieves; Instituto de Investigación Biosanitaria, Granada), Silvia Martínez (Dirección General de Salud Pública, Departamento de Sanidad, Gobierno de Aragón), Ana Milagro (Laboratorio de Microbiología, Hospital Universitario Miguel Servet, Zaragoza), Ana Fernández Ibañez (Servicio de Vigilancia Epidemiológica (DGSP), Consejeria de Salud, Asturias), Marta Huerta Huerta (Servicio de Vigilancia Epidemiológica (DGSP), Consejeria de Salud, Asturias), Jordi Reina (Servicio de Microbiología, Hospital Universitario Son Espases, IdISBa, Palma de Mallorca), Jaume Giménez (Servicio de Epidemiología, Consellería de Salut, Gobierno de las Islas Baleares, IdISBa), Nieves López González-Coviella (Servicio de Epidemiología y Prevención, Dirección General de Salud Pública, Canarias), Eva Rivas Wagner (Servicio de Epidemiología y Prevención, Dirección General de Salud Pública, Canarias), Luis Viloria (Sección d Epidemiología. Consejería de Sanidad, Trabajo y Servicios Sociales. Cantabria), Tomás Vega Alonso (Dirección General de Salud Pública, Junta de Castilla y León), José Eugenio Lozano Alonso (Dirección General de Salud Pública, Junta de Castilla y León), María Socorro Fernández Arribas (Dirección General de Salud Pública, Junta de Castilla y León), Ana Martínez (Subdirección General de Vigilancia y Respuesta a Emergencias de Salud Pública, Agencia de Salud Pública de Catalunya), Luca Basile (Subdirección General de Vigilancia y Respuesta a Emergencias de Salud Pública, Agencia de Salud Pública de Catalunya), Francesc Botella Quijal (Direcció general salut pública i addiccions, Comunitat Valenciana), Aurora López Maside (Direcció general salut pública i addiccions, Comunitat Valenciana), Ana Sofía Lameiras Azevedo (Direcció general salut pública i addiccions, Comunitat Valenciana), Juan Antonio Linares Dopido (Subdirección de Epidemiología, Dirección General de Salud Pública, Servicio Extremeño de Salud, Extremadura), Cecilia Gordillo (Subdirección de Epidemiología, Dirección General de Salud Pública, Servicio Extremeño de Salud, Extremadura), Olaia Pérez Martínez (Servizo de Epidemioloxía, Dirección Xeral de Saúde Pública, Consellería de Sanidade, Xunta de Galicia), Rosa María García Álvarez (Servicio de Medicina Preventiva, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela), Luis García Comas (Subdirección General de Vigilancia en Salud Pública, Comunidad de Madrid), Mercedes Rumayor (Subdirección General de Vigilancia en Salud Pública, Comunidad de Madrid), María Isabel Barranco (Servicio de Epidemiología, Dirección General de Salud Pública, Consejería de Salud, Región de Murcia), Judith Huete Obispo (Servicio de Epidemiología, Dirección General de Salud Pública, Consejería de Salud, Región de Murcia), Ana Isabel Rivas Pérez (Servicio de Epidemiología de la Consejería de Sanidad, Consumo y Gobernación de la Ciudad Autónoma de Ceuta), Violeta Ramos Marín (Servicio de Epidemiología de la Consejería de Sanidad, Consumo y Gobernación de la Ciudad Autónoma de Ceuta), Daniel Castrillejo Pérez (Servicio de Epidemiología, Dirección General de Salud Pública y Consumo, Melilla), Sergio Román Soto (Sección de Microbiología, Hospital Comarcal de Melilla, Melilla), Gloria Pérez-Gimeno (CNE, CIBERESP, ISCIII, Madrid), Clara Mazagatos (CNE, CIBERESP, ISCIII, Madrid), Amparo Larrauri (CNE, CIBERESP, ISCIII, Madrid); Spain – Navarra, EU-PC and EU-H studies: Iván Martínez-Baz (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Itziar Casado (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Jesús Castilla (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Aitziber Echeverría (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Nerea Egüés (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Guillermo Ezpeleta (Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona), Ana Navascués (Hospital Universitario de Navarra – IdiSNA, Pamplona), Ana Miqueleiz (Hospital Universitario de Navarra – IdiSNA, Pamplona), Carmen Ezpeleta (Hospital Universitario de Navarra – IdiSNA, Pamplona). SWEDEN, EU-PC study: Neus Latorre-Margalef (The Public Health Agency of Sweden, Stockholm), Åsa Wiman (The Public Health Agency of Sweden, Stockholm), Annasara Carnahan (The Public Health Agency of Sweden, Stockholm). UNITED KINGDOM, EN-EC study: Katie Hassell (UK Health Security Agency, London), Julia Stowe (UK Health Security Agency, London), Hongxin Zhao (UK Health Security Agency, London), Catherine Quinot (UK Health Security Agency, London), Nick Andrews (UK Health Security Agency, London), Nick Richardson (UK Health Security Agency, London), Katja Hoschler (UK Health Security Agency, London), Angie Lackenby (UK Health Security Agency, London), Catherine Thompson (UK Health Security Agency, London), Maria Zambon (UK Health Security Agency, London); SC-H study: Chris Robertson (Public Health Scotland, Glasgow; University of Strathclyde, Glasgow), Josie Evans (Public Health Scotland, Glasgow), Naoma William (Public Health Scotland, Glasgow), Mark Hamilton (Public Health Scotland, Glasgow).
Ethical statement
The planning, conduct and reporting of the studies was in line with the Declaration of Helsinki [27]. Some countries/studies did not require official ethical approval or patient consent as they are part of routine care/surveillance: DK-H, DK-PC, EN-EC, EU-H (Ireland, Malta and Spain), EU-PC (Ireland, Spain), SC-H. In EU-PC (the Netherlands), as the data are initially collected through surveillance, no formal ethical approval was necessary. Verbal informed consent from patients for participation in the national respiratory surveillance, however, is required. In addition, patients have the option to object against participation in any further research (including VE studies). In SC-H, the EAVE-II study in Scotland was granted ethical approval by the National Research Ethics Service Committee (Southeast Scotland 02; reference number 12/SS/0201), and the approval for data linkage was granted by the Public Benefit and Privacy Panel for Health and Social Care (reference number 1920–0279). Other study sites received local ethical approval from a national or regional review board: EU-H (Belgium: the fifth amendment of ethical approval No. 12/310, B.U.N. 143201215671 was approved on 12 October 2022; Croatia: approved by the Ethics Committee of the Croatian Institute of Public Health (class: 030-02/22-01/2, 20 June 2022); Germany: approved by Charité Universitätsmedizin Berlin Ethical Board: references EA2/126/11 and EA2/218/19; Lithuania: approved 03 July 2020 by the Lithuanian Biomedical Research Ethics Committee No.: L-20-3/1-2; updated 25 July 2022 and 25 January 2023; Romania: CE236/2022; Spain/Navarra: approved by the Navarra Ethics Committee, Ethical Committee for Clinical Research (PI2020/45), which waived the requirement of obtaining informed consent); EU-PC (Croatia: approved by the Ethics Committee of the Croatian Institute of Public Health (class: 030-02/22-01/4, 27 September 2022); France: 471393; Germany: EA2/126/11; Ireland: ICGP2019.4.04; Hungary: approved by the National Scientific and Ethical Committee (IV/1885-5/2021/EKU); Navarra: as for EU-H; Portugal: approved 18 January 2012 by the Ethics Committee of Instituto Nacional de Saúde Doutor Ricardo Jorge, no registration number given; Romania: CE199/2022; Sweden: 2006/1040–31/2 revised Drn 2021-02791).
Funding statement
ECDC funded the study sites and coordination of the EU-PC and EU-H VEBIS multi-country studies.
Acknowledgements
All study teams are very grateful to all patients, general practitioners, paediatricians, hospital teams, laboratory teams, and regional epidemiologists who have contributed to the studies.
Special thanks from study teams to each of the following for their substantial contributions to the studies. In DK-PC and DK-H: The influenza team at Statens Serum Institut. In EN-EC: Shahjian Miah, Jade Cogdale, Respiratory Virus Unit, Virus Reference Department, UK Health Security Agency and Melanie Amphlett, Specialised Microbiology and Laboratories, UK Health Security Agency for technical assistance. In EU-H, for Lithuania: Akvilė Rudėnaitė, Tomas Masilionis, Ieva Bradūnienė; for Romania: Violeta Melinte, Elena Nedu, Cojanu Filofteia, Bianca Voinescu, Cristiana Cristea, Olivia Burcos, Delia Stanciu, Adelina Dogaru, Anca Hotescu, Nicoleta Mirea, Claudia Leulescu, Amalia Dascalu, Corina Oprisa, and Andreea Toderan. In EU-PC, for the Netherlands, National Institute for Public Health and the Environment (RIVM): Mariam Bagheri, Sharon van den Brink, Sacha van Deemter, Gabriel Goderski, Chantal Herrebrugh, Liz Jenniskens, Wesley Jones, John Sluimer, Daphne Reukers, Tara Sprong, Anne Teirlinck, Eddie Vierklau, Lisa Wijsman, molecular and virus culture pool technicians; for the Netherlands, Nivel, Utrecht: Nivel Primary Care Database – Sentinel Practices team (Iris Haitsma, Ruben van der Burgh, Cathrien Kager, Eline Baarda, Marloes Riethof, Mayra Klinkhamer, Bart Knottnerus, Daan van Kooten, Nienke Veldhuijzen) and participating sentinel general practices and their patients; for Hungary: The Hungarian study team works as part of the National Laboratory for Health Security Hungary (RRF-2.3.1-21-2022-00006) supported by the National Research, Development and Innovation Office (NKFIH). The Hungarian study team in addition thanks Annamária Ferenczi, Katalin Krisztalovics and Krisztina Mucsányiné Juhász for their hard work; for Sweden, Public Health Agency of Sweden: Elin Arvesen, Nora Nid, Eva Hansson-Pihlainen, Tove Samuelsson-Hagey. In EU-PC and EU-H: We gratefully acknowledge Marta Valenciano and Alain Moren for all their support to the primary care and hospital networks across the seasons. We thank the SIVIRA surveillance and vaccine effectiveness group for their important contributions. In SC-H: Kimberly Marsh, Kevin Wilson-Smith, Shivani Karanwal, Masoumeh Rezaie, Khaled Boukhari, Stefanie Wilson, Fiona Johnston, Louise Shaw Primrose, Eddie McArdle, Lorraine McGee, Melissa Llano, Laura Walsh, Angela Stockton, Georgia Ladbury, Damilola Mokogwu, John Wood, Jonathan Wells, Fatima Sadiq, Dan McPhail, Mirza Amir Baig, April Went, Anneke van Belle, Nick Young, Leonardo I Green, Amanda Weir, Lesley Wallace, Kat Karacaoglu, Esme Wright, Jen Bishop, Jennifer Weir, Samantha Shepherd, Rory Gunson and Aziz Sheikh on behalf of the EAVE-II collaborators.
Participating laboratories submitted their sequences to GISAID (www.gisaid.org) for easy sharing with the central laboratory in Madrid.
Test results for influenza virus were obtained from the Danish Microbiology Database (MiBa, http://miba.ssi.dk), which contains all electronic reports from departments of clinical microbiology in Denmark since 2010, and we acknowledge the collaboration with the MiBa Board of Representatives.
Conflict of interest: None declared.
Authors’ contributions: Esther Kissling: coordination of VEBIS primary care network, study design, interpretation of results, manuscript writing. Angela Rose: coordination of VEBIS hospital network, study design, analysis of hospital data, interpretation of results, manuscript writing. Both authors contributed equally to the study and manuscript. Amanda Bolt Botnen, Hanne-Dorthe Emborg, Beth Findlay, Ciaran Harvey, Jim McMenamin, Ramona Trebbien, Conall Watson and Heather Whitaker: coordination of their respective studies, data analysis and interpretation of results, read, contributed to and approved the final version of the manuscript. Francisco Pozo: coordinated the virological analysis of the primary care study, read, contributed to and approved the final version of the manuscript. Marine Maurel: analysis of primary care data, interpretation of results, contribution to manuscript writing. Jennifer Howard: data management for hospital data, interpretation of results, contribution to manuscript writing. European IVE group: (i) Primary care and hospital sites at national/regional level: data collection, data validation, results interpretation, review of manuscript. (ii) Laboratories: virological data collection, validation and analysis, genetic characterisation, interpretation of results, review of manuscript. (iii) ECDC and Epiconcept co-authors: study design, interpretation of results, review of manuscript.
References
- 1.European Centre for Disease Prevention and Control (ECDC). Seasonal influenza vaccines. Stockholm: ECDC. [Accessed 05 May 2023]. Available from: https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/seasonal-influenza-vaccines
- 2.Hakin B, Cosford P, Harvey F. The flu immunisation programme 2013/14 – extension to children. London: Department of Health; 2013. [Accessed 05 May 2023]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/225360/Children_s_flu_letter_2013.pdf
- 3.Emborg HD, Vestergaard LS, Botnen AB, Nielsen J, Krause TG, Trebbien R. A late sharp increase in influenza detections and low interim vaccine effectiveness against the circulating A(H3N2) strain, Denmark, 2021/22 influenza season up to 25 March 2022. Euro Surveill. 2022;27(15):2200278. . 10.2807/1560-7917.ES.2022.27.15.2200278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2022-2023 northern hemisphere influenza season. Geneva: WHO. [Accessed 3 Feb 2023]. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022-2023-northern-hemisphere-influenza-season
- 5.European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Flu News Europe. Summary week 01/2023. Stockholm: ECDC and Copenhagen: WHO/Europe; Jan 2023. Available from: https://flunewseurope.org/Archives
- 6.European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Flu News Europe. Summary week 02/2023. Stockholm: ECDC and Copenhagen: WHO/Europe; Jan 2023. Available from: https://flunewseurope.org/Archives
- 7.European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Flu News Europe. Summary week 04/2023. Stockholm: ECDC and Copenhagen: WHO/Europe; Jan 2023. Available from: https://flunewseurope.org/Archives
- 8.European Union (EU). Epiconcept. I-MOVE + Protocol for hospital-based test negative case control studies to measure seasonal influenza vaccine effectiveness against influenza laboratory confirmed SARI hospitalisation among the elderly across the European Union and European Economic Area Member States. Brussels: EU; 2016. Available from: https://docs.google.com/viewer?a=v&pid=sites&srcid=ZXBpY29uY2VwdC5mcnxpbW92ZXBsdXN8Z3g6Njg0NjQwM2QxZDRlM2JmMw
- 9.European Union (EU). Generic protocol for the test negative design case control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States. Brussels: EU; 2015. Available from: https://drive.google.com/file/d/0Byv9pYYPpY4PM25qSXczQ3g4T0E/view
- 10.Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016;21(38):30348. 10.2807/1560-7917.ES.2016.21.38.30348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emborg HD, Krause TG, Nielsen L, Thomsen MK, Christiansen CB, Skov MN, et al. Influenza vaccine effectiveness in adults 65 years and older, Denmark, 2015/16 - a rapid epidemiological and virological assessment. Euro Surveill. 2016;21(14). 10.2807/1560-7917.ES.2016.21.14.30189 [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control (ECDC). Core protocol for ECDC VEBIS studies of COVID-19 vaccine effectiveness against hospitalisation with Severe Acute Respiratory Infection laboratory-confirmed with SARS-CoV-2 or seasonal influenza - Version 2.0. Stockholm: ECDC; 2023 Jan. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-vaccine-effectiveness-sari-protocol-version-2.pdf
- 13.Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017;35(36):4796-800. 10.1016/j.vaccine.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503-10. 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 15.Covenay J. FIRTHLOGIT: Stata module to calculate bias reduction in logistic regression. Boston: Boston College Department of Economics; 2015. [Google Scholar]
- 16.Influenza surveillance section, Immunisation and Vaccine-Preventable Diseases Division, UK Health Security Agency. Surveillance of influenza and other seasonal respiratory viruses in winter 2021 to 2022. London: UK Health Security Agency; Jun 2022. Available from: https://www.gov.uk/government/statistics/annual-flu-reports/surveillance-of-influenza-and-other-seasonal-respiratory-viruses-in-winter-2021-to-2022
- 17.Kissling E, Pozo F, Martínez-Baz I, Buda S, Vilcu AM, Domegan L, et al. I-MOVE study team . Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021-2022 I-MOVE primary care multicentre study. Influenza Other Respir Viruses. 2023;17(1):e13069. . 10.1111/irv.13069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control (ECDC). Influenza virus characterisation, Summary Europe,December 2022. Stockholm: ECDC; Jan 2023. [Accessed 3 Feb 2023]. Available from: https://www.ecdc.europa.eu/en/publications-data/influenza-virus-characterization-summary-europe-december-2022
- 19.Skowronski DM, Chuang ES, Sabaiduc S, Kaweski SE, Kim S, Dickinson JA, et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Euro Surveill. 2023;28(5):2300043. . 10.2807/1560-7917.ES.2023.28.5.2300043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koel BF, Burke DF, Bestebroer TM, van der Vliet S, Zondag GCM, Vervaet G, et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science. 2013;342(6161):976-9. 10.1126/science.1244730 [DOI] [PubMed] [Google Scholar]
- 21.Skowronski DM, Zou M, Sabaiduc S, Murti M, Olsha R, Dickinson JA, et al. Interim estimates of 2019/20 vaccine effectiveness during early-season co-circulation of influenza A and B viruses, Canada, February 2020. Euro Surveill. 2020;25(7):2000103. 10.2807/1560-7917.ES.2020.25.7.2000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawood FS, Chung JR, Kim SS, Zimmerman RK, Nowalk MP, Jackson ML, et al. Interim Estimates of 2019-20 Seasonal Influenza Vaccine Effectiveness - United States, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(7):177-82. 10.15585/mmwr.mm6907a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose A, Kissling E, Emborg HD, Larrauri A, McMenamin J, Pozo F, et al. European IVE group . Interim 2019/20 influenza vaccine effectiveness: six European studies, September 2019 to January 2020. Euro Surveill. 2020;25(10):2000153. . 10.2807/1560-7917.ES.2020.25.10.2000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosu ME, Lexmond P, Bestebroer TM, Hauser BM, Smith DJ, Herfst S, et al. Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc Natl Acad Sci USA. 2022;119(42):e2211616119. 10.1073/pnas.2211616119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2023-2024 northern hemisphere influenza season. Geneva: WHO. [Accessed 14 Mar 2023]. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2023-2024-northern-hemisphere-influenza-season
- 26.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. 10.1136/bmj.m3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]