Abstract
In this report, we describe a simple and accurate method to analyze restriction fragments using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The two complementary strands of restriction fragments are separated through hybridization to a capture probe, which is a single-stranded undigested fragment. Using the biotin–streptavidin linkage, the hybrid is immobilized on streptavidin-coated magnetic beads. After conditioning the captured restriction fragments, they are eluted from the probe and their molecular weights are determined. The proposed method greatly improves the quality, and reduces the complexity of the mass spectrum by analyzing only one of the complementary strands of restriction fragments.
INTRODUCTION
During the last decade, the soft ionization techniques for mass spectrometry (MS) have revolutionized the analysis of nucleic acids (1–3). The unparalleled accuracy of MS for determining molecular weights of nucleic acids and the multiplexing capability of MS without the requirement of any reporting label have proven to be superior to the conventional gel-based methods (4,5). Recently, a high-throughput platform for various types of DNA analysis using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS has been described (6).
The current upper limit of ultraviolet MALDI MS is ∼500 nucleotides (nt) (7). In the case of infrared MALDI MS, this limit has been extended ∼4-fold (8). However, the resolution of large DNA fragments (>100 nt) has remained low. Hence, in order to attain the highest resolution, it is desirable to have smaller DNA fragments. For creating small DNA fragments (<100 nt), the most straightforward approach is to digest a fragment into smaller pieces. In general, DNA fragments can be split by either chemical or enzymatic methods (9). The specificity of enzymatic reactions is usually comparatively high, especially in the case of restriction endonucleases. With the availability of many different varieties of commercial software, the selection of suitable restriction endonucleases can be easily carried out. Additionally, for multiple digests, the information on buffer compatibility of different restriction endonucleases is often available from the manufacturers.
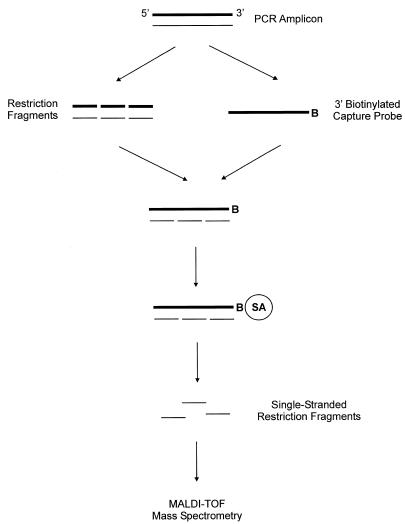
In the proposed method (Fig. 1), a PCR amplicon (199 bp) was digested into six different fragments. One of the complementary strands of an undigested amplicon, which has been biotinylated, was used as a capture probe. Through the hybridization with the capture probe, the complementary strands of restriction fragments were physically separated. The capture probe–restriction fragment hybrid was subsequently immobilized on streptavidin-coated magnetic beads. Washing the beads effectively desalted the restriction fragments that were captured on the probe and thereby conditioned them for MS analysis. The single-stranded restriction fragments were subsequently eluted from the probe under an alkaline condition, and their molecular weights were determined by MALDI-TOF MS.
Figure 1.
Schematic presentation of the proposed method to analyze a set of restriction fragments. The restriction fragments are prepared by digesting a PCR amplicon (199 bp) with restriction endonucleases as described in Materials and Methods. A single-stranded capture probe (sense strand) is prepared from an undigested amplicon, which has been biotinylated at the 3′ end (B = biotin). Without purifying the restriction fragments, the anti-sense strand (thin lines) of the restriction fragments is hybridized to the probe. After the hybridization, the capture probe–restriction fragment hybrid is immobilized on streptavidin-coated magnetic beads (SA = streptavidin). The captured fragments are conditioned by washing the beads, and subsequently eluted from the probe. This is followed by the analysis of the restriction fragments using MALDI-TOF MS.
MATERIALS AND METHODS
Preparation of restriction fragments
Restriction fragments were prepared by digesting a PCR amplicon (199 bp) into six different fragments, which were abbreviated as I–VI (Table 1). The amplicon was prepared by amplifying the human β-globin gene (3′ end of intron 1 to 5′ end of exon 2). The PCR amplification was performed in a total volume of 50 µl consisting of GeneAmp 1× PCR Buffer II (10 mM Tris–HCl, pH 8.3, and 50 mM KCl, Perkin Elmer, Foster City, CA), 2 mM MgCl2, 0.2 mM dNTP mix, 10 pmol of each primer (forward primer 5′-ACTGGGCATGTGGAG-ACAG-3′ and reverse primer 5′-GCACTTTCTTGCCATG-AG-3′), 2 U of AmpliTaq Gold (Perkin Elmer) and ~200 ng of human genomic DNA. The template was denatured at 94°C for 8 min. Thermal cycling was continued with a touch-down program that included 11 cycles of 20 s at 94°C, 30 s at 64°C, 1 min at 72°C; 36 cycles of 20 s at 94°C, 30 s at 56°C, 1 min at 72°C; and a final extension of 5 min at 72°C. The quality of the amplicon was examined by using 1.5% agarose gel electrophoresis and ethidium bromide staining (10). Before the digestion, the amplicon was purified by using an UltraClean™ PCR clean-up kit (MO BIO Laboratories, Solana Beach, CA) according to the manufacturer’s instructions. The concentration of purified amplicon was determined by scanning an ethidium bromide-stained agarose gel, in which the amplicon was electrophoresed in one lane and a known amount (2 µg) of linear DNA markers (φX174 DNA digested with HaeIII, Promega, Madison, WI) in an adjacent lane, with a Fluorimager 595 (excitation filter = 514 nm, emission filter = 610 nm, Molecular Dynamics, Sunnyvale, CA). The digital images of the DNA markers were used to construct a calibration curve, from which the concentration of purified amplicon was calculated. The amplicon was double digested with DdeI and HinfI (New England Biolabs, Beverly, MA), which have been selected by using an online software called Webcutter. Typically, 1 µg of amplicon was incubated at 37°C for 90 min with 2 U of each endonuclease in 30 µl of 1× Buffer 2 (10 mM Tris–HCl, pH 7.9, 10 mM MgCl2, 50 mM NaCl, 1 mM dithiothreitol, New England Biolabs). Complete digestion was confirmed by the separation pattern of restriction fragments in a non-denaturing 15% polyacrylamide gel, which had been stained with ethidium bromide.
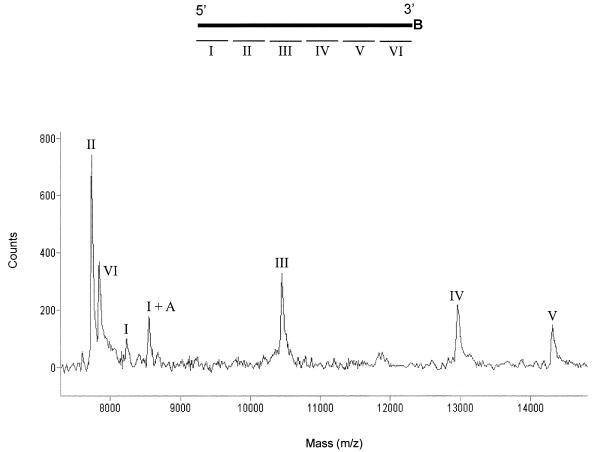
Table 1. Physical parameters of the restriction fragments being analyzed in this study, and the results obtained from their mass spectroscopic measurements.
| Restriction fragment | Size (nt) | Percentage of GC | Nearest neighbour Tm (°C) | Calculated mass (Da) | Measured mass (Da) | Peak height (counts) |
|---|---|---|---|---|---|---|
| I | 27 | 51.9 | 75.2 | 8217 | 8217 | 101 |
| I + A | 28 | – | – | 8530 | 8532 | 177 |
| II | 25 | 48.0 | 70.5 | 7724 | 7725 | 736 |
| III | 33 | 45.5 | 80.3 | 10 438 | 10 441 | 326 |
| IV | 42 | 57.1 | 93.7 | 12 951 | 12 955 | 220 |
| V | 46 | 52.2 | 95.7 | 14 297 | 14 305 | 145 |
| VI | 26 | 53.8 | 78.5 | 7833 | 7834 | 368 |
The size and calculated mass of the fragments refer to their anti-sense strand. The nearest neighbor melting temperature (Tm) is calculated by using the Oligo primer analysis software version 5.0 (National Biosciences Inc.) under the standard conditions of 100 pM oligonucleotide and 1 M salt. The peak height is measured by using PerSeptive GRAMS/386 version 3.01c (Galactic Industries Corp.).
Preparation of capture probe
The capture probe was also prepared by PCR as described in the previous section, except the reverse primer was biotinylated at the 5′ end. After the amplification, the free biotinylated primer was removed by using the UltraClean™ PCR clean-up kit. The concentration of biotinylated amplicon was determined as described above. For preparing the capture probe, the biotinylated amplicon was first immobilized on streptavidin-coated magnetic beads (Dynabeads M-280, Dynal, Lake Success, NY). Typically, 2.6 mg of streptavidin-coated magnetic beads was used to immobilize 60 pmol of amplicon according the manufacturer’s instructions. After the immobilization, the beads were washed once with 2× B/W buffer (10 mM Tris–HCl, pH 7.5, 2 M NaCl and 1 mM EDTA). The immobilized amplicon was subsequently denatured by incubating the beads in 200 µl of 0.2 M NaOH at room temperature for 20 min. By removing the beads from the solution, the sense strand of the amplicon, which had not been biotinylated, was separated from the biotinylated anti-sense strand. The solution containing the sense strand was then purified by gel filtration (Chroma Spin–10 column, Clontech, Palo Alto, CA). In order to reduce the volume, the purified sense strand was precipitated with ethanol, and the pellet was redissolved in TE buffer (10 mM Tris–HCl, pH 7.6, and 1 mM EDTA). The sense strand was then enzymatically labeled with a biotin at the 3′ end using terminal transferase. The labeling reaction was performed in 20 µl containing 1× reaction buffer (100 mM cacodylate, pH 6.8, 1 mM CoCl2 and 0.1 mM dithiothreitol, Promega), 30 µM biotin-ddUTP (Boehringer Mannheim, Germany), 20 U of terminal transferase (Promega), and the sense strand. The reaction was carried out at 37°C for 1 h. Before using as a capture probe, the biotinylated sense strand was purified by gel filtration as before.
Hybridization of restriction fragments with capture probe
Without purification, 2.5 pmol of restriction fragments was hybridized with 6.25 pmol of capture probe in 35 µl of hybridization buffer containing 200 mM NaCl, 20 mM sodium citrate and 1% blocking reagent (Boehringer Mannheim). The reaction mixture was heated to 95°C for 5 min and cooled to room temperature over 30 min by using a thermal cycler (PTC-200 DNA engine, MJ Research, Waltham, MA). Then the capture probe–restriction fragment hybrid was immobilized on 140 µg of streptavidin-coated magnetic beads. The beads were subsequently washed three times with 70 mM ammonium citrate. The captured single-stranded restriction fragments were eluted by heating to 80°C for 5 min in 5 µl of 50 mM ammonium hydroxide.
Mass spectrometry
Matrix stock solution containing 0.7 M 3-hydroxypicolinic acid (Sigma, St Louis, MO) and 0.07 M ammonium citrate in 1:1 water and acetonitrile was diluted 2.5-fold with water. Diluted matrix solution (0.15 µl) was pipetted onto a sample target and allowed to crystallize. Then 0.15 µl of eluted restriction fragments was added. A linear PerSeptive Voyager DE mass spectrometer, operated in positive ion mode, was used for the measurements. The target and middle plate were kept at +18.2 kV for 400 ns after each laser shot, and the target voltage was then raised to +20 kV. The ion guide wire in the flight tube was kept at –2 V. Approximately 250 laser shots were accumulated. The original spectrum was digitized at 500 MHz. Using in-house developed software, the spectrum was smoothed, and the baseline was corrected.
RESULTS AND DISCUSSION
Under normal circumstances, the genetic information in each of the complementary strands of DNA is theoretically equivalent. In this report, we present a method for analyzing one of the complementary strands of restriction fragments. Using a single capture probe, the proposed method (Fig. 1) can selectively capture a set of restriction fragments. It also allows the conditioning of restriction fragments to be done easily in comparison to phenol/chloroform extraction and ethanol precipitation (11). Furthermore, the proposed method simplifies the complexity of mass spectroscopic measurements of multiple restriction fragments. This is because the complementary strands are physically separated prior to the measurements. As a result, the number of peaks in the spectrum is reduced by half. In many cases, the measurements of both complementary strands are difficult because their molecular weights can be very similar.
In an initial study, the anti-sense strand of biotinylated amplicon, which was immobilized on streptavidin-coated magnetic beads, was used as a capture probe, instead of the sense strand as described in Materials and Methods. The intended benefit of this approach was to simplify the procedure used to prepare the capture probe. The hybridization method described in Materials and Methods was employed, except the restriction fragments were denatured separately by heating at 95°C for 5 min and immediately chilled on ice prior to the hybridization with the capture probe, which has been pre-heated to 55°C. However, no restriction fragments were detected. In an attempt to improve the hybridization reaction, the surface density of the immobilized capture probe was reduced by half. This was achieved by using twice the amount of streptavidin-coated magnetic beads for the immobilization of biotinylated amplicon. Again, no signal was obtained. Further decreasing the surface density of immobilized capture probe by using even more streptavidin-coated magnetic beads would eventually lower the concentration of eluted restriction fragments, because a larger volume of effluent would be required.
When the hybridization of restriction fragments to the capture probe (sense strand) was carried out prior to its immobilization on streptavidin-coated magnetic beads as described in Materials and Methods, the mass spectrum revealed seven different peaks as shown in Figure 2. According to their measured masses (Table 1), six of them are identified as the expected restriction fragments (I–VI). The extra peak (I + A) is identified as the 3′ end fragment (I) of the amplicon with an additional adenosine at its 3′ end. This is a result of the template-independent activity of Taq DNA polymerase, which adds an adenosine to the 3′ hydroxyl terminus of blunt-ended double-stranded DNA substrate (12). According to a recent report on quantitative analysis of oligonucleotides using MALDI-TOF MS (13), the peak height should be proportionally related to the concentration of oligonucleotide. Hence, based on the peak heights of I and I + A (Table 1), ∼60% of PCR amplicon contained an extra adenosine at the 3′ end. When comparing the peak heights in Figure 2, the peaks for fragments I and VI are relatively small. This contradicts the expected decrease on the intensity of the signal as the molecular weight increases, which is due to the lower efficiency of desorption and detection of relatively heavy ions. By comparing the percentage of GC and the nearest neighbor melting temperature (Table 1), the binding of fragments I and VI to the capture probe are expected to be as strong as the fragments II and III. The smaller peaks for fragments I and VI may be explained by the loss of those fragments from the capture probe during washing the beads at a stringent condition as described in Materials and Methods. The two fragments both hybridize to the ends of the capture probe (Fig. 2). The stacking interaction of these with the adjacent fragments is, therefore, lower (14,15). In order to maximize the possibility for stacking interactions between each of the individual fragments, the molar ratio of capture probe to restriction fragments in the hybridization reaction was kept below 2.5.
Figure 2.
Mass spectrum of restriction fragments. In the diagram above the spectrum, the upper thicker line represents a 3′ biotinylated capture probe (B = biotin) and the smaller lines below, which are labeled from I to VI, represent the anti-sense strand of restriction fragments.
In conclusion, we have developed a simple and accurate method to analyze one of the complementary strands of a set of restriction fragments. In principle, the proposed method can be used to analyze either one of the complementary strands by using the appropriate undigested fragment as the capture probe. The use of this method to detect both known and unknown single nucleotide polymorphisms is currently being investigated.
REFERENCES
- 1.Crain P.F. and McCloskey,J.A. (1998) Curr. Opin. Biotechnol., 9, 25–34. [DOI] [PubMed] [Google Scholar]
- 2.Graber J.H., O’Donnell,M.J., Smith,C.L. and Cantor,C.R. (1998) Curr. Opin. Biotechnol., 9, 14–18. [DOI] [PubMed] [Google Scholar]
- 3.Nordhoff E., Kirpekar,F. and Roepstorff, P. (1996) Mass Spectrom. Rev., 15, 67–138. [DOI] [PubMed] [Google Scholar]
- 4.Koster H., Tang,K., Fu,D.J., Braun,A., Boom,D.V.D., Smith,C.L., Cotter,R.J. and Cantor,C.R. (1996) Nat. Biotechnol., 14, 1123–1128. [DOI] [PubMed] [Google Scholar]
- 5.Ross P., Hall,L., Smirnov,I., and Haff,L. (1998) Nat. Biotechnol., 16, 1347–1351. [DOI] [PubMed] [Google Scholar]
- 6.Little D.P., Braun,A., O’Donnell,M.J. and Koster,H. (1997) Nat. Med., 3, 1413–1416. [DOI] [PubMed] [Google Scholar]
- 7.Tang K., Taranenko,N.I., Allman,S.L., Chang,L.Y. and Chen,C.H. (1994) Rapid Commun. Mass Spectrom., 8, 727–730. [DOI] [PubMed] [Google Scholar]
- 8.Berkenkamp S., Kirpekar,F. and Hillenkamp,F. (1998) Science, 281, 260–262. [DOI] [PubMed] [Google Scholar]
- 9.Bashkin J.K. (1998) Chemical Rev., 98, 937–938. [DOI] [PubMed] [Google Scholar]
- 10.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning. A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 11.Wada Y. (1998) J. Mass Spectrom., 33, 187–192. [DOI] [PubMed] [Google Scholar]
- 12.Clark J.M. (1988) Nucleic Acids Res., 16, 9677–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruenner B.A., Yip,T.T. and Hutchens,T.W. (1996) Rapid Commun. Mass Spectrom., 10, 1797–1801. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson P., O’Meara,D., Edebratt,F., Persson,B., Uhlen,M., Lundeberg,J. and Nygren,P. (1999) Anal. Biochem., 269, 155–161. [DOI] [PubMed] [Google Scholar]
- 15.Cantor C.R. and Smith,C.L. (1999) Genomics . The Science and Technology Behind The Human Genome Project. John Wiley & Sons, Inc., NY. [Google Scholar]