Abstract
Aims
Icosapent ethyl (IPE) significantly reduced ischaemic events in statin-treated patients with atherosclerosis or diabetes and elevated triglycerides in REDUCE-IT, including large reductions in myocardial infarction and elective, urgent, and emergent coronary revascularization. However, the mechanisms driving this clinical benefit are not fully known. The EVAPORATE trial demonstrated that IPE significantly reduced plaque burden. No study to date has assessed the impact of IPE on coronary physiology. Fractional flow reserve (FFR) derived from coronary computed tomography angiography (CTA) data sets (FFRCT) applies computational fluid dynamics to calculate FFR values in epicardial coronary arteries. Our objective was to assess the impact of IPE on coronary physiology assessed by FFRCT using imaging data from EVAPORATE.
Methods and results
A total of 47 patients and of 507 coronary lesions at baseline, 9 months, and 18 months with coronary CTA and FFRCT were studied in a blinded core lab. The pre-specified primary endpoint was the FFRCT value in the distal coronary segment from baseline to follow-up in the most diseased vessel per patient using IPE compared with placebo. The pre-specified secondary endpoint was the change in translesional FFRCT (ΔFFRCT) across the most severe (minimum 30% diameter stenosis) coronary lesion per vessel. Baseline FFRCT was similar for IPE compared with placebo (0.83 ± 0.08 vs. 0.84 ± 0.08, P = 0.55). There was significant improvement in the primary endpoint, as IPE improved mean distal segment FFRCT at 9- and 18-month follow-up compared with placebo (0.01 ± 0.05 vs. −0.05 ± 0.09, P = 0.02, and −0.01 ± 0.09 vs. −0.09 ± 0.12, P = 0.03, respectively). ΔFFRCT in 140 coronary lesions was improved, although not statistically significant, with IPE compared with placebo (−0.06 ± 0.08 vs. −0.09 ± 0.1, P = 0.054).
Conclusion
Icosapent ethyl demonstrated significant benefits in coronary physiology compared with placebo. This early and sustained improvement in FFRCT at 9- and 18-month follow-up provides mechanistic insight into the clinical benefit observed in the REDUCE-IT trial. Furthermore, this is the first assessment of FFRCT to determine drug effect.
Keywords: icosapent ethyl, fractional flow reserve, coronary computed tomography angiography, computational fluid dynamics
Graphical Abstract
Graphical Abstract.
See the editorial comment for this article ‘Icosapent ethyl and plaque regression: insights from the EVAPORATE-FFRCT study’, by Robert Sykes and Richard McFarlane, https://doi.org/10.1093/ehjci/jead086.
Introduction
Patients with elevated triglyceride (TG) levels are at increased risk for ischaemic events.1–5 Icosapent ethyl (IPE) significantly reduced the burden of first, subsequent, and total ischaemic events among statin-treated patients with cardiovascular disease or diabetes and elevated triglycerides in REDUCE-IT.6–9 However, the mechanisms driving this marked clinical benefit are not fully known.
The CHERRY study demonstrated that IPE in addition to statin therapy significantly reduced coronary plaque volume assessed by intravascular ultrasound compared with statin therapy alone in a post-acute coronary syndrome population.10 However, intravascular ultrasound is an invasive technique employed in the catheterization laboratory. The Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE, NCT02926027) trial demonstrated that in statin-treated patients, IPE significantly reduced plaque burden measured by serial coronary computed tomography angiography (CTA) compared with placebo.11 However, no study to date has assessed the impact of IPE on coronary physiology. Haemodynamic forces, plaque vulnerability, and the interaction between these factors may cause subsequent cardiovascular events.12
Among non-invasive anatomic tests for coronary artery disease (CAD), coronary CTA has emerged as a novel imaging modality that is capable of providing high-resolution images of coronary artery lesions.13 Over the past decade, there has been a strong interest in computing fractional flow reserve (FFR) non-invasively derived from coronary CTA data sets.14 FFR derived from coronary CTA data sets (FFRCT) applies computational fluid dynamics to calculate FFR values in all epicardial coronary arteries without the need for additional medications, imaging, or change in imaging protocol.15,16 The combination of high-resolution anatomic definition of CAD via CTA with FFRCT in a single test provides a comprehensive non-invasive anatomic and functional assessment of epicardial CAD which is supported by the American College of Cardiology and American Heart Association guidelines.17,18 We hypothesized that IPE would improve coronary physiology. This study assessed the impact of IPE compared with placebo on coronary haemodynamics assessed by FFRCT using imaging data from the EVAPORATE clinical trial.
Methods
A total of 47 patients and 507 coronary lesions at baseline, 9 months, and 18 months with coronary CTA and FFRCT were studied in core laboratories blinded to clinical information, symptom status, and outcomes (Lundquist/Harbor-UCLA, Los Angeles, CA, USA, and Loyola University Medical Center, Maywood, IL, USA).
Study endpoints
The purpose of this study was to assess whether IPE at 4 g/day, as an adjunct to diet and statin therapy, in patients with fasting triglycerides between 135 and 499 mg/dL at randomization affected coronary physiology at follow-up. The pre-specified primary endpoint was the FFRCT value in the distal coronary segment from baseline to follow-up in the most diseased vessel per patient using IPE compared with placebo. If the evaluable distal FFRCT segment was different, the proximal one was used as the reference for comparison.
The pre-specified secondary endpoint comparing baseline to follow-up using IPE compared with placebo was the change in translesional FFRCT (ΔFFRCT) across the most severe (minimum 30% diameter stenosis) coronary lesion per vessel.
Study population
The study population for EVAPORATE has been published previously.19 Briefly, EVAPORATE included individuals between 30 and 85 years with CAD (one or more angiographic stenoses with ≥20% narrowing), elevated fasting triglycerid levels (135–499 mg/dL), and low-density lipoprotein levels (LDL-C) ranging from 40 to 115 mg/dL. Patients were on stable statin therapy, with or without ezetimibe, and adhered to diet and exercise for ≥4 weeks prior to study entry.
Study design
The study design and rationale for EVAPORATE have been published previously.19 Briefly, EVAPORATE was a multicenter, randomized, double-blind, placebo-controlled trial that evaluated the effect of IPE 4 g/day on coronary plaque progression determined by coronary CTA compared with placebo. Patients were randomized 1:1 to IPE or placebo to evaluate plaque volume progression rates using coronary CTA. Participants underwent coronary CTA at baseline, 9 months, and 18 months.
The study was approved by the institutional review board at each site and was conducted in accordance with the principles of Good Clinical Practice, and the trial conformed to the principles outlined in the Declaration of Helsinki. All patients provided written informed consent prior to randomization.
Computation of FFR from coronary CTA
FFRCT analysis was performed by HeartFlow Inc. (Mountain View, CA, USA) as previously described.15 First, semi-automated segmentation of the coronary arteries and determination of left ventricular mass were performed. Then, calculations of FFRCT were achieved by computational fluid dynamic modeling and determined for all vessels of diameter >1.8 mm. ΔFFRCT was derived by determining the presence of stenosis on the FFRCT 3D model in multiple projections. The FFRCT interactive model was co-localized with the coronary CTA data set using branch points and bifurcations as landmarks. The 3D model was used to pin proximal and distal reference points at regions immediately adjacent to the stenosis which appeared free of stenosis. ΔFFRCT was defined as the difference of FFRCT values between the proximal and distal points.20 If the evaluable distal FFRCT segment was different, the proximal one was used as the reference for comparison. An analysable baseline coronary CTA for FFRCT was needed. In the event a vessel was not analysed for FFRCT secondary to an artefact, when possible, another vessel with baseline and follow-up FFRCT was used.
Statistical analyses
Baseline characteristics were compared between the two arms using Student’s t test and χ2 statistic. Results are presented as counts and frequencies (per cent) for discrete variables and mean and standard deviations or median for continuous variables. FFRCT changes between baseline, 9 months, and 18 months were compared between the two arms using a random effects model with baseline value, arm, visit, and the interaction between visit and arm as fixed effects and a random term for the intercept. Intra-observer and inter-observer reproducibility testing was performed by having both observers repeat measurements in random order for 10 randomly selected coronary vessels. Intra-observer reproducibility was reported using the coefficient of variance (COV), which measures relative dispersion, and intraclass correlation coefficient (ICC). Inter-observer variability was measured by inter-rater variability (kappa statistic).
All statistical analyses report two-sided P-values for the outcomes. A P < 0.05 was considered significant for the outcomes. No adjustment for multiplicity of testing was performed. All analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Cary, NC), and intention to treat, with study patients analysed by treatment group assigned regardless of study drug adherence.
Results
Population characteristics
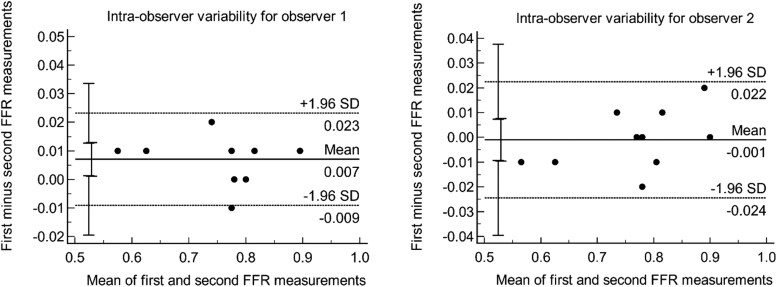
Of the 68 patients completing the 18-month visit and having a coronary CTA at baseline and the 18-month visit, a total of 47 patients had interpretable FFRCT analyses at baseline and follow-up (Figure 1). Coronary CTA image quality was not acceptable for FFRCT analysis due to motion artefact, calcification, and misregistration. The mean age of the participants was 57 ± 9 years, with 51% being male. Baseline characteristics of the study participants, stratified by arm (IPE group, n = 22, and placebo group, n = 25), are demonstrated in Table 1. Baseline demographics and risk factors (including age, BMI, hypertension, diabetes, hyperlipidaemia, and smoking prevalence) were similar between the IPE and placebo groups (Table 1). Bland–Altman plots for the assessment of intra-observer variability are shown in Figure 2. Overall, intra-observer reproducibility was excellent as the COV was 1.03% [confidence interval (CI): 0.69–1.38] and ICC was 99%. Limits of agreement (LOA) were 0.03 and 0.04 for the first and second observers, respectively. There was a very good inter-observer reproducibility as well, with a weighted kappa of 0.88 (CI: 0.81–0.95).
Figure 1.
Flow diagram.
Table 1.
Baseline characteristics of the EVAPORATE-FFRCT cohort
| Characteristic | Total | Icosapent ethyl | Placebo | P |
|---|---|---|---|---|
| (n = 47) | (n = 22) | (n = 25) | ||
| Mean (SD) or count (%) | Mean (SD) or count (%) | Mean (SD) or count (%) | ||
| Age, years | 57.1 (9.3) | 56.5 (9.9) | 57.6 (8.9) | 0.67 |
| Sex, male | 24 (51.1%) | 11 (50.0%) | 13 (52.0%) | 0.89 |
| Body mass index, kg/m2 | 32.0 (5.0) | 31.8 (4.5) | 32.2 (5.5) | 0.78 |
| Ethnicity, Hispanic | 25 (53.2%) | 11 (50.0%) | 14 (56.0%) | 0.68 |
| Race, white | 40 (85.1%) | 19 (86.4%) | 21 (84.0%) | 0.88 |
| Diabetes mellitus | 29 (61.7%) | 13 (59.1%) | 16 (64.0%) | 0.73 |
| Family history | 14 (29.8%) | 6 (27.3%) | 8 (32.0%) | 0.72 |
| Hypertension | 32 (68.1%) | 15 (68.2%) | 17 (68.0%) | 0.99 |
| Past smoking | 17 (36.2%) | 8 (36.4%) | 9 (36.0%) | 0.98 |
| Aspirin use | 20 (42.6%) | 9 (40.9%) | 11 (44.0%) | 0.83 |
Figure 2.
Bland–Altman plots for intra-observer reproducibility.
Changes with therapy
Baseline FFRCT was similar for IPE compared with placebo (0.83 ± 0.08 vs. 0.84 ± 0.08, P = 0.55). Coronary physiology changes between groups were significantly different (Table 2). There was significant improvement in the primary endpoint as IPE improved distal segment FFRCT at 9- and 18-month follow-up compared with placebo (0.01 ± 0.05 vs. −0.05 ± 0.09, P = 0.02, and −0.01 ± 0.09 vs. −0.09 ± 0.12, P = 0.03, respectively). Compared with placebo, IPE significantly improved the primary endpoint FFRCT by 0.06 and 0.08 at 9- and 18-month follow-up [0.06 (95% CI: 0.01–0.11; P = 0.02) and 0.08 (95% CI: 0.01–0.15; P = 0.03), respectively]. ΔFFRCT in 140 coronary lesions was improved with IPE compared with placebo (−0.06 ± 0.08 vs. −0.09 ± 0.1, P = 0.05; Table 3). Representative case examples comparing IPE to placebo on coronary physiology are illustrated in Figures 3 and 4.
Table 2.
FFRCT change by treatment group
| Characteristic | Overall (n = 47) | Icosapent ethyl (n = 22) | Placebo (n = 25) | Difference (95% CI) | P-value |
|---|---|---|---|---|---|
| Baseline FFRCT | |||||
| Mean ± SD (n) | 0.84 ± 0.08 (44) | 0.83 ± 0.08 (20) | 0.84 ± 0.08 (24) | −0.02 (−0.06, 0.03) | 0.55 |
| Median and range | 0.86 (0.62–0.96) | 0.86 (0.67–0.94) | 0.86 (0.62–0.96) | ||
| IQR | 0.79–0.89 | 0.76–0.89 | 0.8–0.9 | ||
| FFRCT 9 months | |||||
| Mean ± SD (n) | 0.81 ± 0.11 (39) | 0.83 ± 0.07 (19) | 0.79 ± 0.13 (20) | 0.04 (−0.03, 0.11) | 0.23 |
| Median and range | 0.84 (0.5–0.93) | 0.85 (0.73–0.93) | 0.84 (0.5–0.92) | ||
| IQR | 0.77–0.89 | 0.77–0.9 | 0.76–0.88 | ||
| FFRCT 18 months | |||||
| Mean ± SD (n) | 0.78 ± 0.13 (38) | 0.81 ± 0.11 (18) | 0.75 ± 0.14 (20) | 0.06 (−0.02, 0.14) | 0.14 |
| Median and range | 0.79 (0.5–0.93) | 0.81 (0.5–0.93) | 0.78 (0.5–0.92) | ||
| IQR | 0.74–0.89 | 0.76–0.9 | 0.68–0.87 | ||
| FFRCT baseline change to 9 months | |||||
| Mean ± SD (n) | −0.02 ± 0.08 (39) | 0.01 ± 0.05 (19) | −0.05 ± 0.09 (20) | 0.06 (0.01, 0.11) | 0.02 |
| Median and range | −0.01 (−0.28 to 0.11) | 0.01 (−0.09 to 0.11) | −0.04 (−0.28 to 0.09) | ||
| IQR | −0.04 to 0.03 | −0.01 to 0.04 | −0.08 to 0.01 | ||
| FFRCT baseline change to 18 months | |||||
| Mean ± SD (n) | −0.06 ± 0.11 (38) | −0.01 ± 0.09 (18) | −0.09 ± 0.12 (20) | 0.08 (0.01, 0.15) | 0.03 |
| Median and range | −0.03 (−0.41 to 0.12) | −0.01 (−0.25 to 0.12) | −0.05 (−0.41 to 0.04) | ||
| IQR | −0.1 to 0.01 | −0.04 to 0.02 | −0.13 to 0.01 |
FFRCT, fractional flow reserve derived from coronary computed tomography angiography data sets.
Table 3.
Translesional FFRCT (ΔFFRCT) across the most severe coronary lesion per vessel
| Characteristic | Overall (n = 140) | Icosapent ethyl (n = 78) | Placebo (n = 62) | Difference (95% CI) | P-value |
|---|---|---|---|---|---|
| ΔFFRCT | |||||
| Mean ± SD (n) | −0.08 ± 0.09 (140) | −0.06 ± 0.08 (78) | −0.09 ± 0.1 (62) | 0.0298 (−0.0006, 0.0601) | 0.054 |
| Median and range | −0.04 (−0.48 to 0) | −0.04 (−0.48 to 0) | −0.05 (−0.46 to 0) | ||
| IQR | −0.1 to 0.02 | −0.09 to 0.02 | −0.13 to 0.02 |
FFRCT, fractional flow reserve derived from coronary computed tomography angiography data sets.
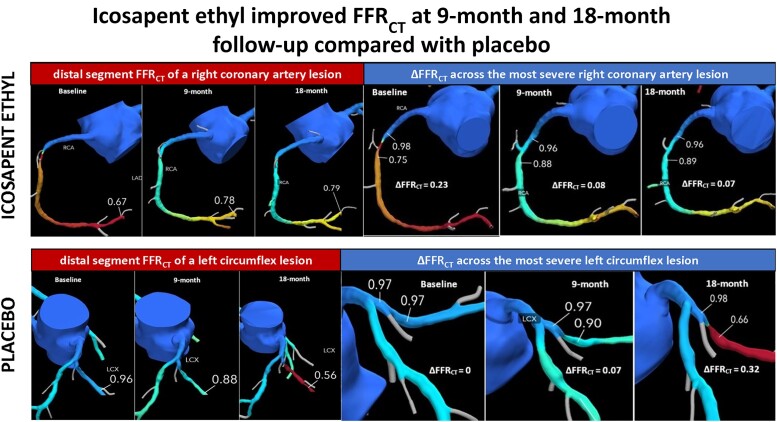
Figure 3.
Icosapent ethyl (IPE) group. Top: example of a patient from the IPE arm showing distal segment FFRCT of a right coronary artery lesion at baseline, 9 months, and 18 months. Bottom: example of the same patient from the IPE arm showing ΔFFRCT across the most severe right coronary artery lesion at baseline, 9 months, and 18 months. FFRCT, fractional flow reserve derived from coronary computed tomography angiography data sets; ΔFFRCT, translesional fractional flow reserve derived from coronary computed tomography angiography data sets.
Figure 4.
Placebo group. Top: example of a patient from the placebo arm showing distal segment FFRCT of a left circumflex lesion at baseline, 9 months, and 18 months. Bottom: example of the same patient from the placebo arm showing ΔFFRCT across the most severe left circumflex lesion at baseline, 9 months, and 18 months. FFRCT, fractional flow reserve derived from coronary computed tomography angiography data sets; ΔFFRCT, translesional fractional flow reserve derived from coronary computed tomography angiography data sets.
Discussion
The REDUCE-IT trial demonstrated that IPE significantly reduced ischaemic events in statin-treated patients with atherosclerosis or diabetes and elevated triglycerides, including large reductions in myocardial infarction (MI) and elective, urgent, and emergent coronary revascularization.6–9,21–24 However, the mechanisms driving this clinical benefit are not fully known. The EVAPORATE trial was designed to be a mechanistic study with similar inclusion and exclusion criteria to parallel REDUCE-IT and demonstrated that IPE significantly reduced plaque burden.11 This is the first study to assess the impact of IPE on coronary physiology. We demonstrated significant benefits in coronary physiology with IPE compared with placebo. This early improvement in FFRCT and ΔFFRCT at 9 months was sustained at longer term 18-month follow-up and provides mechanistic insight into the clinical benefits observed in the REDUCE-IT trial. Moreover, this is the first assessment of FFRCT to determine drug effect, which has potentially important implications in utilizing FFRCT to predict treatment response.
Based on the evidence, the American Heart Association and American College of Cardiology, along with multiple other medical societies, elevated coronary CTA as a Class 1A non-invasive test and recognized FFRCT as a Class 2a with Level B evidence in the Guideline for the Evaluation and Diagnosis of Chest Pain.17,18 In fact, non-invasive coronary haemodynamics derived from coronary CTA and computational fluid dynamics have been shown to predict high-risk plaque and downstream clinical outcomes.
The international Assessing Diagnostic Value of Non-Invasive FFRCT in Coronary Care (ADVANCE, NCT02499679) registry of over 5000 patients with CAD who underwent a diagnostic strategy of coronary CTA and FFRCT demonstrated significantly lower cardiovascular death or MI in patients with higher FFRCT values at 1-year follow-up.25 Cardiovascular death or MI occurred more in patients with an FFRCT ≤0.80 compared with patients with an FFRCT > 0.80 (relative risk [RR]: 4.22; 95% CI: 1.28–13.95; P = 0.01), and major adverse cardiovascular events (MACE), all-cause death or MI, and cardiovascular death or MI increased as FFRCT values decreased. Our findings demonstrate significant improvement in the primary endpoint as IPE improved distal segment FFRCT at 9- and 18-month follow-up compared with placebo (0.01 ± 0.05 vs. −0.05 ± 0.09, P = 0.02, and −0.01 ± 0.09 vs. −0.09 ± 0.12, P = 0.03, respectively) which may shed light on the underlying mechanisms driving the marked clinical benefit observed in the REDUCE-IT trial, including early revascularization. Results from the REDUCE-IT REVASC study demonstrated significant benefit soon after randomization and achieved statistical significance by only 11 months.21
ΔFFRCT has emerged as an imaging biomarker to predict cardiovascular outcomes.26 The Exploring the Mechanism of the Plaque Rupture in Acute Myocardial Infarction (EMERALD, NCT02374775) multinational, multicenter trial assessed haemodynamic predictors of acute coronary syndrome (ACS) using computational fluid dynamics.27 Compared with non-culprit lesions, culprit lesions had significantly lower FFRCT and higher ΔFFRCT, axial plaque, and wall shear stress. Non-invasive computational fluid dynamic–derived haemodynamic parameters were better at identifying culprit lesions causing ACS than diameter stenosis or adverse plaque characteristics. In fact, ΔFFRCT was shown to be the greatest predictor of ACS.
Furthermore, in a study of 4730 patients from the ADVANCE registry, ΔFFRCT remained an independent predictor for early revascularization [odds ratio per 0.05 increase (95% CI), 1.31 (1.26–1.35); P < 0.001] after adjusting for risk factors, stenosis features, and lesion-specific FFRCT.20 ΔFFRCT improved the discrimination of patients who underwent early revascularization compared with a standard diagnostic strategy of coronary CTA with FFRCT. In our study, ΔFFRCT in coronary lesions was improved with IPE compared with placebo, which may partially explain the robust clinical benefit in ischaemic events observed in the REDUCE-IT trial.
FFRCT has been associated with various clinical outcomes, such as the safe deferral of invasive coronary angiography, cardiovascular death or MI, and revascularization.14,25 Considering this correlation between FFRCT and clinical outcomes, assessing for improvement in coronary physiology using FFRCT over time with pharmacologic therapy may serve as a surrogate for treatment response, cardiovascular outcomes, and drug efficacy and warrants further investigation. In addition to its high diagnostic performance and prognostic ability, this trial paves the way for FFRCT to determine drug effect and has implications in utilizing FFRCT and other non-invasive haemodynamic metrics derived from computational fluid dynamics to assess treatment response.
Limitations
The study’s primary limitation is the small sample size. Despite this, we demonstrated improvement in the primary and secondary endpoints with IPE compared with placebo. EVAPORATE did not assess for long-term outcomes; rather, it was designed as a mechanistic study to accompany the REDUCE-IT randomized outcome trial with similar study design, inclusion and exclusion criteria, and interventions. Notably, this is the first study to demonstrate improvement in coronary physiology and associate improved coronary haemodynamics with improvement in outcomes shown in a clinical trial. It would be interesting to study the impact of IPE on the microcirculation. However, with the current generation of FFRCT utilized in this analysis, assessment of the microcirculation is not possible. Twenty-one of 68 patients did not have interpretable baseline and follow-up coronary CT scans for FFRCT analysis. Guideline-directed coronary CTA acquisition methods, including adequate heart rate control and nitroglycerin administration, optimize image quality. Adherence to these methods may improve acceptance rates for FFRCT.
Conclusion
Icosapent ethyl demonstrated significant benefits in coronary physiology. This early and sustained improvement in FFRCT at 9- and 18-month follow-up provides mechanistic insight into the clinical benefit observed in the REDUCE-IT trial. Furthermore, this is the first assessment of FFRCT to determine drug effect.
Contributor Information
Mark G Rabbat, Department of Medicine, Division of Cardiology, Loyola University Medical Center, 2160 S. 1st Avenue, Maywood, IL 60153, USA; Department of Medicine, Division of Cardiology, Edward Hines Jr. VA Hospital, 5000 South 5th Avenue, Hines, IL, USA.
Suvasini Lakshmanan, Department of Medicine, Lundquist Institute at Harbor-UCLA Medical Center, 1124 W Carson Street, Torrance, CA 90502, USA.
Mina M Benjamin, Department of Medicine, Division of Cardiology, Loyola University Medical Center, 2160 S. 1st Avenue, Maywood, IL 60153, USA.
Gheorghe Doros, Department of Biostatistics, Baim Institute for clinical research, Boston University, 930 Commonwealth Ave #3, Boston, MA 02215, USA.
April Kinninger, Department of Medicine, Lundquist Institute at Harbor-UCLA Medical Center, 1124 W Carson Street, Torrance, CA 90502, USA.
Matthew J Budoff, Department of Medicine, Lundquist Institute at Harbor-UCLA Medical Center, 1124 W Carson Street, Torrance, CA 90502, USA.
Deepak L Bhatt, Mount Sinai Heart, Icahn School of Medicine at Mount Sinai, 1 Gustave Levy Place, Box 1030, New York, NY 10029, USA.
Funding
EVAPORATE was funded by Amarin Pharma, Inc. (Bridgewater, NJ, USA). As an investigator-initiated study, the company had no input in analysis, endpoint adjudication, or study performance or measures.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J 2015;36:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klempfner R, Erez A, Sagit B-Z, Goldenberg I, Fisman E, Kopel E, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes 2016;9:100–108. [DOI] [PubMed] [Google Scholar]
- 3. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab 2018;103:3019–3027. [DOI] [PubMed] [Google Scholar]
- 4. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes Metab 2019;21:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc 2018;7:e008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum TB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 7. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Jiao L, et al. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol 2019;74:1159–1161. [DOI] [PubMed] [Google Scholar]
- 9. Gaba P, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Prevention of cardiovascular events and mortality with icosapent ethyl in patients with prior myocardial infarction. J Am Coll Cardiol 2022;79:1660–1671. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017;70:537–544. [DOI] [PubMed] [Google Scholar]
- 11. Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 2020;41:3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pontone G, Rabbat MG. The new era of computational fluid dynamics in CT angiography: far beyond the FFR number. JACC Cardiovasc Imaging 2017;10:674–676. [DOI] [PubMed] [Google Scholar]
- 13. Baessato F, Guglielmo M, Muscogiuri G, Baggiano A, Fusini L, Scafuri S, et al. The incremental role of coronary computed tomography in chronic coronary syndromes. J Clin Med 2020;9:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabbat M, Leipsic J, Bax J, Kauh B, Verma R, Doukas D, et al. Fractional flow reserve derived from coronary computed tomography angiography safely defers invasive coronary angiography in patients with stable coronary artery disease. J Clin Med 2020;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61(22):2233–2241. [DOI] [PubMed] [Google Scholar]
- 16. Rabbat MG, Berman DS, Kern M, Raff G, Chinnaiyan K, Koweek L, et al. Interpreting results of coronary computed tomography angiography-derived fractional flow reserve in clinical practice. J Cardiovasc Comput Tomogr 2017;11:383–388. [DOI] [PubMed] [Google Scholar]
- 17. Writing Committee Members, Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2021;78:e187–e285. [DOI] [PubMed] [Google Scholar]
- 18. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 19. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol 2018;41:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takagi H, Leipsic JA, McNamara N, Martin I, Fairbairn TA, Akasaka T, et al. Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry. J Cardiovasc Comput Tomogr 2022;16:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson BE, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Reduction in revascularization with icosapent ethyl: insights from REDUCE-IT revascularization analyses. Circulation 2021;143:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verma S, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Icosapent ethyl reduces ischemic events in patients with a history of previous coronary artery bypass grafting: REDUCE-IT CABG. Circulation 2021;144:1845–1855. [DOI] [PubMed] [Google Scholar]
- 23. Peterson BE, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Treatment with icosapent ethyl to reduce ischemic events in patients with prior percutaneous coronary intervention: insights from REDUCE-IT PCI. J Am Heart Assoc 2022;11:e022937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatt RD, Libby P, Verma S, Mason RP, Bhatt DL. The role of eicosapentaenoic acid in reducing important cardiovascular events, including coronary revascularization. Prog Cardiovasc Dis 2021;69:3–10. [DOI] [PubMed] [Google Scholar]
- 25. Patel MR, Nørgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry. JACC Cardiovasc Imaging 2020;13:97–105. [DOI] [PubMed] [Google Scholar]
- 26. Park J, Lee JM, Koo B-K, Choi G, Hwang D, Rhee T-M, et al. Relevance of anatomical, plaque, and hemodynamic characteristics of non-obstructive coronary lesions in the prediction of risk for acute coronary syndrome. Eur Radiol 2019;29(11):6119–6128. [DOI] [PubMed] [Google Scholar]
- 27. Lee JM, Choi G, Koo B-K, Hwang D, Park J, Zhang J, et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc Imaging 2019;12:1032–1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.