Abstract
Aims
Left ventricular remodelling occurs during the chronic course of aortic regurgitation (AR) and aortic stenosis (AS), leading to myocardial hypertrophy and fibrosis. Several studies have shown that extracellular volume fraction (ECV) and indexed extracellular volume (iECV) are important surrogate markers of diffuse myocardial fibrosis (MF). Postoperative data on these cardiovascular magnetic resonance (CMR) extracellular expansion parameters for either AS or AR are scarce. This study aimed to demonstrate the postoperative changes that occur in diffuse MF, and the influence of preoperative MF on the reversal of LV remodelling, in patients with AR or AS.
Methods and results
Patients with severe AR or AS and indications for surgery were prospectively enrolled. Patients underwent pre- and postoperative CMR, and ECV and iECV were quantified. Data from 99 patients were analysed (32 with AR and 67 with AS). After surgery, the left ventricle mass index decreased in both groups (AR: 110 vs. 91 g/m2; AS: 86 vs. 68 g/m2, both P < 0.001). The late gadolinium enhancement fraction (AR: preoperative 1.9% vs. postoperative 1.7%, P = 0.575; AS: preoperative 2.4% vs. postoperative 2.4%, P = 0.615) and late gadolinium enhancement mass (AR: preoperative 3.8 g vs. postoperative 2.5 g, P = 0.635; AS: preoperative 3.4 g vs. postoperative 3.5 g, P = 0.575) remained stable in both groups. Preoperative iECV and ECV were greater in the AR group (iECV: 30 mL/m2 vs. 22 mL/m2, P = 0.001; ECV: 28.4% vs. 27.2%, P = 0.048). Indexed extracellular volume decreased after surgery in both groups (AR: 30–26.5 mL/m2, AS: 22–18.2 mL/m2, both P < 0.001); it was still greater in the AR group (AR: 26.5 mL/m2 vs. AS: 18.2 mL/m2, P < 0.001). Postoperative ECV remained stable in the AR group (preoperative 28.4% vs. postoperative 29.9%; P = 0.617) and increased in the AS group (preoperative 27.2% vs. postoperative 28.6%; P = 0.033).
Conclusion
Patients with both AR or AS presented reduction in iECV after surgery, unfolding the reversible nature of diffuse MF. In contrast to patients with AS, those with AR developed postoperative iECV regression with stable ECV, suggesting a balanced reduction in both intracellular and extracellular myocardial components.
Keywords: aortic valve insufficiency, aortic valve stenosis, magnetic resonance imaging, fibrosis, heart valve diseases, myocardium
Graphical Abstract
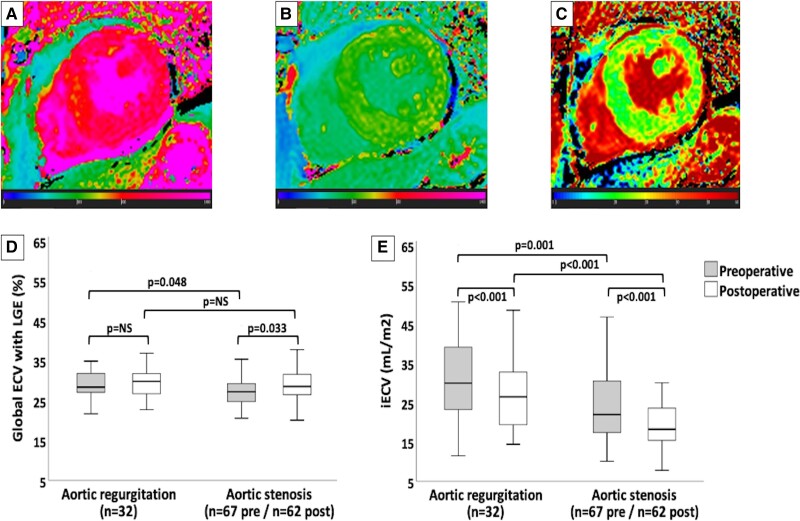
Graphical Abstract.
Diffuse myocardial fibrosis measures and changes in ECV and iECV. Panels A, B, and C demonstrate automatic native T1, post-contrast T1, and extracellular volume (ECV) measures, respectively, in the postoperative cardiovascular magnetic resonance of a patient with severe aortic stenosis (Global ECV with LGE = 26.1%; iECV = 17.4 mL/m2). Graphics D and E show comparisons between the pre- and postoperative measures of the global ECV with late gadolinium enhancement (LGE) and the indexed extracellular volume (iECV), respectively, in aortic valvular heart diseases. Solid horizontal lines indicate median values; boxes indicate p25 and p75; and vertical lines indicate the highest and lowest values. P values indicate differences between measures (significant if <0.05). NS, non-significant.
See the editorial comment for this article ‘Imaging biomarkers in aortic valve disease: it is time to shift the focus to the myocardium’, by Deborah Kwon and Emmanuel Akintoye, https://doi.org/10.1093/ehjci/jead095.
Introduction
Progressive left ventricular remodelling occurs during the chronic course of aortic valvular heart disease (VHD). Aortic stenosis (AS) induces left ventricle (LV) pressure overload,1 while aortic regurgitation (AR) causes pressure and volume overload,2,3 activating different intracellular signalling pathways4–6 and leading to different patterns of myocyte hypertrophy and fibrosis.7 Myocardial fibrosis (MF) has been previously quantified histopathologically.8–10 More recently, cardiovascular magnetic resonance (CMR) with late gadolinium enhancement (LGE) sequences have enabled the non-invasive detection and quantification of regional replacement fibrosis.11,12 Over the last decade, several studies have shown the importance of the quantification of extracellular volume fraction (ECV) and indexed extracellular volume (iECV) as surrogate markers of diffuse MF in patients with VHD, including the clinical prognostic impact of these measures when preoperatively evaluated.13–15
In contrast to a reasonable number of studies on AS, data on CMR extracellular expansion parameters for AR are scarce and, until recently, have included a very limited number of patients.16,17 In 2021, Senapati et al.15 published the largest study on diffuse MF in patients with AR. Most of their population, however, had predominantly mild-to-moderate AR, and only 28% of their patients underwent surgery. In addition, CMR data were limited to the preoperative period. To date, postoperative CMR diffuse fibrosis parameters are still limited (for AS)18,19 or missing (for AR), leaving postoperative changes in AR and the results of comparisons between both groups after surgery undetermined. Furthermore, it is still unknown whether postoperative fibrosis influences the long-term prognosis. Better knowledge of myocardial structure and postoperative changes may lead to the development of better treatment targets and a more accurate definition of the timing for intervention.
This study aimed to demonstrate pre- to postoperative changes in regional and diffuse MF in patients with AR or AS. We also studied the potential influence of preoperative MF on the reversal of LV hypertrophy after surgery. Considering the differences in LV remodelling patterns in both diseases, we performed a comparative analysis between the two groups.
Methods
Patient selection
Patients with isolated severe AS or AR (in clinical, echocardiographic, and if needed, haemodynamic evaluation) and indications for their first cardiac surgery were prospectively enrolled between 2016 and 2019. The classification of valvular compromise and indications for surgery were in accordance with VHD guidelines.20,21 The exclusion criteria were as follows: concomitant moderate or severe aortic or mitral VHD; conditions that contraindicate CMR or compromise image quality for analysis (e.g. haemodynamic instability, claustrophobia, CMR non-compatible pacemaker or implantable defibrillator, creatinine clearance < 40 mL/min determined using the Cockcroft–Gault formula,22 and atrial fibrillation); previous or current known obstructive coronary artery disease (> 50%),23,24 and insulin-dependent diabetes.25 This study protocol was approved by the research ethics committee of our hospital (approval number 982.296), and all patients provided written informed consent.
After protocol inclusion, a medical visit was scheduled for the assessment of symptom status, medications, physical examination, routine laboratory and echocardiographic data of patients, and programming of CMR and protocol lab exams.
Cardiovascular magnetic resonance
Patients underwent CMR 90 days prior to and 6–9 months after surgery. Images were acquired using a 1.5-T Phillips scanner equipped with a cardiovascular coil. Standard morphologic and functional images were regularly assessed using steady-state free precession (SSFP) during a period of apnoea. Left ventricle volumes and dimensions, ejection fraction (EF), and myocardial mass were determined using short-axis end-systolic and end-diastolic images.
In this study, the different patterns of remodelling were defined as follows: concentric remodelling is characterized by an increase in both LV mass index and mass/volume ratio, while eccentric remodelling is characterized by an increased LV mass index and a dilated LV, with a normal mass/volume ratio. The mass/volume ratio is represented by the relative wall thickness that is calculated as: (2 × lateral wall thickness)/LV end-diastolic diameter. It is increased when > 0.42.26,27 In this study, the postoperative decreases in the LV mass index and/or in the indexed LV end-diastolic volume were considered as markers of LV reverse remodelling. Likewise, an increase in LV mass index after surgery was considered as LV adverse remodelling.
Phase-contrast images were acquired using commercially available 2D pulse sequence available in the scanner with standard parameters, with high temporal resolution and carefully adjusting the velocity encoding to avoid aliasing. The severity of AR was measured by direct flow assessment using through-plane velocity mapping performed just above the aortic valve (2D phase-contrast imaging: 2D-PC) in a plane perpendicular to the direction of blood flow. The specialized post-processing software generates a flow curve, which allows calculation of the aortic forward flow, aortic regurgitant volume (area under the backward flow curve during the diastolic phase of the cardiac cycle), and regurgitant fraction, calculated as aortic regurgitant volume/aortic forward flow. A regurgitant fraction > 33% or regurgitant volume > 42 mL were used as the CMR thresholds for severe AR.28 For quantification of aortic valve gradient, we used the peak velocity derived from phase-contrast imaging and calculated peak gradient using simplified Bernoulli equation (peak gradient = 4 × peak velocity squared). Aortic valve area was measured as the maximum opening area of the aortic valve by planimetry in cine-MR images acquired in aortic valve plane, using SSFP pulse sequence.
Late gadolinium enhancement sequences were obtained ∼20 min after a 0.15 mmol/kg intravenous infusion of gadolinium-diethylenetriamine penta-acetic acid (gadopentate dimeglumine, Magnevist, Bayer Health Care, Pharmaceuticals, Wayne, NJ, USA). Sequence pulses were synchronized to the electrocardiogram to increase the signal-to-noise ratio and improve image quality. Late gadolinium enhancement areas were quantified as previously described11,12 and expressed as myocardial mass and percentage. In case of small or equivocal LGE regions in the short-axis slices, a perpendicular long-axis slice was used to confirm the presence of LGE and to avoid inclusion of image artefacts.
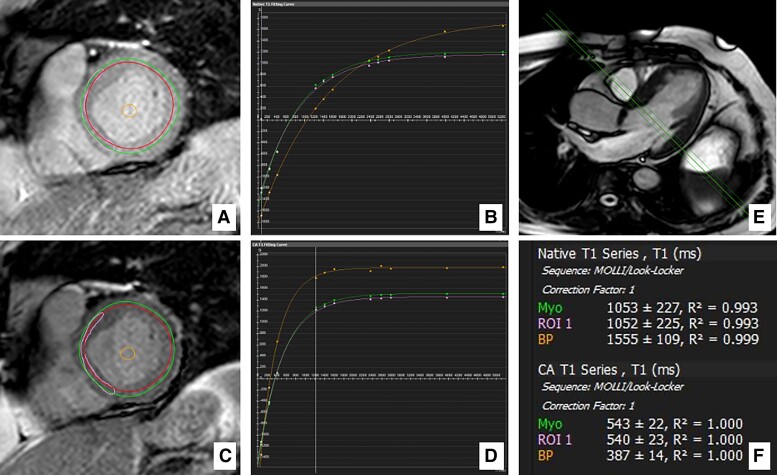
T1 measurements were acquired before (native T1) and 20 min after gadolinium administration (post-contrast T1), using the modified look locker inversion (MOLLI) recovery sequence protocol29 synchronized to the electrocardiogram, according to T1 mapping guidelines.30 Images were obtained from basal, mid, and apical LV short-axis levels using a 3(3)3(3)5 sampling scheme at the end-diastole of the cardiac cycle and a single-shot SSFP combined with sensitivity encoding (SENSE).31 The delay time was 400 ms, the flip angle 40°, and the slice thickness 10 mm. The total apnoea time was 11 s. The resultant mean T1 values were used for analyses. In patients with detected LGE, analyses were performed including and then excluding LGE areas. The regions of interest were selected at each short-axis level (Figure 1). Endocardial fat or cavity blood pool areas were manually excluded, if necessary. Additionally, a single region of interest in the basal septal myocardium was selected for T1 measurement, which had been shown improved reproducibility.32
Figure 1.
Regions of interest and T1 measures. Example of basal-level short-axis region of interest selection and T1 measurements in postoperative cardiovascular magnetic resonance in a patient with severe aortic stenosis (global ECV with LGE = 26.1%; iECV = 17.4 mL/m2). (A and B) native regions of interest and T1 maps. (C and D) post-contrast regions of interest and T1 maps. (E) long-axis view showing the corresponding basal position of the (A and B) short-axis slices. (F) automatic measurement of native and post-contrast T1 times. Myo, global level selection; ROI 1, septal selection; BP, blood pool selection.
Extracellular volume fraction measurements were derived from a previously described formula using pre- and post-contrast myocardium and blood pool T1 times and haematocrit;33 they are expressed as percentages. Representing the absolute amount of myocardial extracellular space, iECV was calculated by multiplying global ECV by the indexed LV end-diastolic myocardial volume; it is expressed in millilitres per metre squared (mL/m2).34,35 The indexed cellular mass was calculated as: LV mass index × (1−absolute global ECV value).
Cardiovascular magnetic resonance analyses were performed using post-processing analysis software CVI42 version 5.12 (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada).
Protocol lab exams
On the day CMR was performed, blood samples were collected for analysis of haematocrit, creatinine, B-type natriuretic peptide (BNP), and high-sensitivity troponin I. B-type natriuretic peptide was quantified using the ADVIA Centaur® assay (Siemens Medical Solutions Diagnostic, Los Angeles, CA, USA). Troponin I was quantitatively determined using the ADVIA Centaur® XP high-sensitivity troponin I assay (Siemens Healthineers, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA), with a limit of detection of 6 ng/L and a percentile 99 of 40 ng/L.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges and categorical variables as absolute or relative frequencies. The Mann–Whitney U and Wilcoxon tests were used to compare numerical variables, while Pearson’s chi-square and Fisher’s exact tests were used to compare categorical variables, as appropriate. Bivariable correlations were evaluated using Spearman’s coefficient. Linear regression analysis was used to define predictors of postoperative iECV decrease using a stepwise method in multivariable regression. Linear regression analysis and binary logistic regression were used to define the potential role of preoperative MF parameters as predictors of LV reverse remodelling after surgery. Analyses were performed using SPSS software, version 25 (IBM, Armonk, NY, USA). The tests were two-tailed, and a P value < 0.05 was considered statistically significant.
Results
Study group
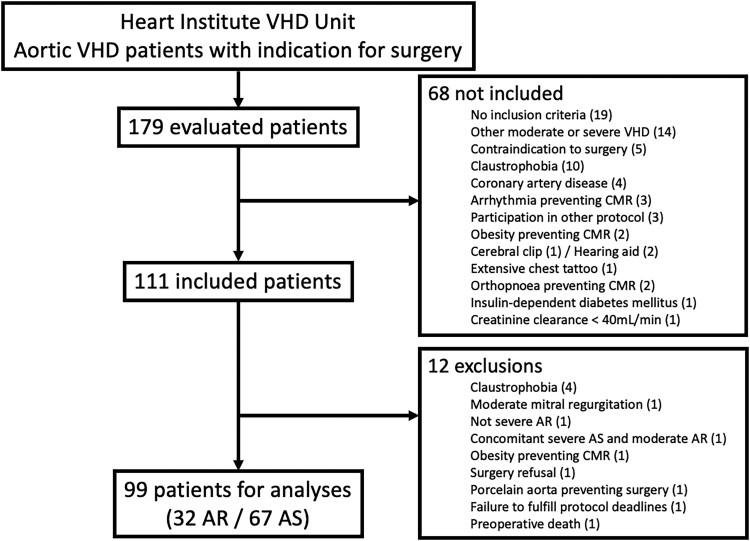
One hundred and seventy-nine patients were evaluated for possible inclusion in this study; after applying the inclusion and exclusion criteria, 111 of them were enrolled. Twelve patients were excluded, while 99 (32 with AR and 67 with AS) remained for analysis (Figure 2).
Figure 2.
Patient flowchart. Flowchart for aortic regurgitation (AR) and aortic stenosis (AS) patient selection. CMR, cardiovascular magnetic resonance; VHD, valvular heart diseases.
Baseline clinical characteristics of the patients are summarized in Table 1. Patients with AR were younger (AR: 57 [45–65] vs. AS: 65 [60–71] years, P = 0.001) and had a greater proportion of men, a lower prevalence of diabetes mellitus or angina, and a lower Society of Thoracic Surgeons (STS) score. They also took more vasodilators and beta-blockers while awaiting surgery and were more frequently considered for surgery while asymptomatic.
Table 1.
Baseline clinical characteristics and laboratorial data of patients
| Total n = 99 |
Aortic regurgitation | Aortic stenosis | P value | |
|---|---|---|---|---|
| n = 32 (32%) | n = 67 (68%) | |||
| Male sex | 58 (58.6%) | 24 (75.0%) | 34 (50.7%) | 0.022 |
| Age (years) | 63 (55–69) | 57 (45–65) | 65 (60–71) | 0.001 |
| VHD aetiology | < 0.001 | |||
| Degenerative | 45 (45.5%) | 8 (25.0%) | 37 (55.2%) | |
| Bicuspid aortic valve | 35 (35.4%) | 7 (21.9%) | 28 (41.8%) | |
| Rheumatic | 7 (7.1%) | 5 (15.6%) | 2 (3.0%) | |
| Aortic or annular dilation | 6 (6.1%) | 6 (18.8%) | 0 (0.0%) | |
| Others | 6 (6.1%) | 6 (18.8%) | 0 (0.0%) | |
| Hypertension | 70 (70.7%) | 25 (78.1%) | 45 (67.2%) | 0.262 |
| Diabetes mellitus | 21 (21.2%) | 3 (9.4%) | 18 (26.9%) | 0.046 |
| Obstructive coronary artery disease (> 50%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| ACE inhibitors/ARBs | 66 (66.7%) | 27 (84.4%) | 39 (58.2%) | 0.010 |
| Other vasodilators | 30 (30.3%) | 14 (43.8%) | 16 (23.9%) | 0.044 |
| Beta-blockers | 17 (17.2%) | 10 (31.3%) | 7 (10.4%) | 0.010 |
| Diuretics | 63 (63.6%) | 22 (68.8%) | 41 (61.2%) | 0.465 |
| Symptoms for intervention | 93 (94%) | 26 (81%) | 67 (100%) | 0.001 |
| Dyspnoea | 86 (86.9%) | 25 (78.1%) | 61 (91.0%) | 0.110 |
| Functional class (NYHA) | 0.242 | |||
| I | 13 (13.1%) | 7 (21.9%) | 6 (9.0%) | |
| II | 37 (37.4%) | 13 (40.6%) | 24 (35.8%) | |
| III | 44 (44.4%) | 11 (34.4%) | 33 (49.3%) | |
| IV | 5 (5.1%) | 1 (3.1%) | 4 (6.0%) | |
| Angina | 41 (41.4%) | 6 (18.8%) | 35 (52.2%) | 0.002 |
| Syncope | 19 (19.2%) | 5 (15.6%) | 14 (20.9%) | 0.533 |
| Euroscore II (%) | 1.12 (0.87–1.54) | 1.13 (0.76–1.35) | 1.12 (0.91–1.62) | 0.224 |
| STS mortality score (%) | 0.91 (0.66–1.25) | 0.69 (0.57–0.89) | 1.09 (0.74–1.35) | < 0.001 |
| Haematocrit (%) | 41 (39–44) | 40 (37–44) | 41 (39–44) | 0.328 |
| CrCl (CG) (mL/min) | 76 (61–92) | 82 (61–98) | 73 (61–88) | 0.255 |
Values are expressed as n (%) or median (p25–p75). Bold P values indicate statistical significance.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CrCl, creatinine clearance; CG, Cockcroft–Gault; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; VHD, valvular heart diseases.
Preoperative cardiovascular magnetic resonance data
Preoperative CMR data are presented in Table 2 and Supplementary data online, Table S1. Patients with AR presented with eccentric LV remodelling, and in 37.5% of cases, LV EF was < 50%. Indexed mass and volumes were greater in patients with AR than in those with AS [indexed mass: 110 (91–134) vs. 86 (71–104) g/m2; indexed diastolic volume: 153 (125–194) vs. 71 (64–88) mL/m2, indexed systolic volume: 75 (43–90) vs. 24 (19–31) mL/m2; all P ≤ 0.001]. Cardiovascular magnetic resonance regurgitant volume [65 (45–96) mL] and fraction [50 (40–63)%] indicated that patients had severe AR.36 Likewise, CMR aortic valve area [0.7 (0.6–0.8) cm2] and peak gradient [69 (58–93) mmHg] were well correlated with echocardiographic measures (Supplementary data online, Figure S1 and Table S2) and compatible with severe AS.37,38 Absolute [AR: 3.8 (2.7–5.8) g vs. AS: 3.4 (1.5–9.6) g, P = 0.586] and proportional [AR: 1.9 (1.1–2.2)% vs. AS: 2.5 (1.0–5.2) %, P = 0.463] quantities of LGE were similar in both groups, with predominantly non-ischaemic patterns (AR vs. AS: 90% vs. 82%; P = 1.000). Extracellular volume fraction and iECV were significantly higher among patients with AR (Figure 3 and Graphical Abstract).
Table 2.
Preoperative cardiovascular magnetic resonance data
| Total n = 99 |
Aortic regurgitation | Aortic stenosis | P value | |
|---|---|---|---|---|
| n = 32 (32%) | n = 67 (68%) | |||
| CMR to surgery (days) | 50 (33–70) | 55 (36–86) | 49 (28–67) | 0.251 |
| Indexed LA (mm/m2) | 43 (38–52) | 45 (40–57) | 42 (36–50) | 0.107 |
| Indexed LV end-diastolic volume (mL/m2) | 81 (66–141) | 153 (125–194) | 71 (64–88) | < 0.001 |
| Indexed LV end-systolic volume (mL/m2) | 30 (20–56) | 75 (43–90) | 24 (19–31) | < 0.001 |
| LV EF (%) | 63 (57–71) | 57 (45–61) | 66 (58–75) | < 0.001 |
| LV EF <50% | 15 (15.2%) | 12 (37.5%) | 3 (4.5%) | < 0.001 |
| LV mass index (g/m2) | 92 (76–118) | 110 (91–134) | 86 (71–104) | 0.001 |
| Regurgitant volume (mL) | 65 (45–96) | |||
| Regurgitant fraction (%) | 50 (40–63) | |||
| Aortic valve area (cm2) | 0.7 (0.6–0.8) | |||
| Peak gradient (mmHg) | 69 (58–93) | |||
| Presence of LGE | 38 (38.4%) | 10 (31.3%) | 28 (41.8%) | 0.313 |
| LGE mass (g) | 3.8 (2.0–8.3) | 3.8 (2.7–5.8) | 3.4 (1.5–9.6) | 0.586 |
| LGE fraction (%) | 2.0 (1.0–3.8) | 1.9 (1.1–2.2) | 2.5 (1.0–5.2) | 0.463 |
| Septal ECV w/o LGE (%) | 28.4 (26.0–32.6) | 30.2 (27.3–34.5) | 27.9 (24.7–30.6) | 0.015 |
| Global ECV w/o LGE (%) | 27.3 (24.9–29.6) | 28.2 (26.5–31.6) | 26.8 (24.5–28.8) | 0.019 |
| Septal ECV with LGE (%) | 28.4 (26.1–33.4) | 31.9 (27.7–35.3) | 27.9 (25.5–31.4) | 0.010 |
| Global ECV with LGE (%) | 27.9 (25.0–30.7) | 28.4 (27.0–31.9) | 27.1 (24.7–29.3) | 0.048 |
| Indexed cellular mass (g/m2) | 65.8 (55.0–82.8) | 79.8 (65.8–92.5) | 61.0 (51.9–74.4) | 0.001 |
| iECV (mL/m2) | 24.2 (18.8–33.6) | 30.0 (22.8–39.6) | 22.0 (17.2–30.5) | 0.001 |
Values are expressed as n (%) or median (p25–p75). Bold P values indicate statistical significance.
CMR, cardiovascular magnetic resonance; ECV, extracellular volume fraction; EF, ejection fraction; iECV, indexed extracellular volume; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; w/o, without.
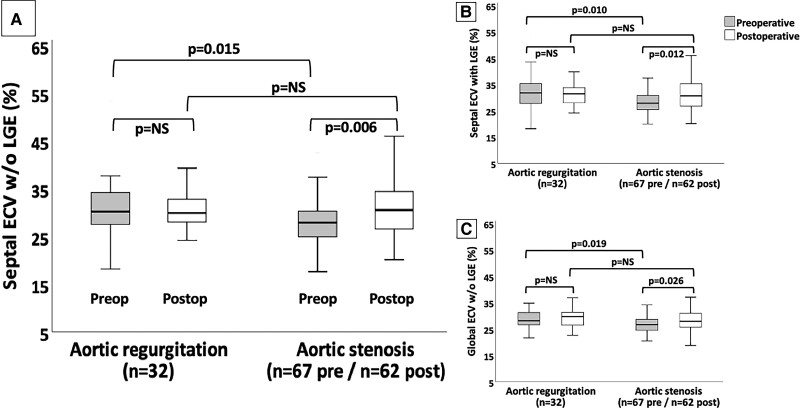
Figure 3.
Different ECV measures in patients with aortic valvular heart disease. Comparison between three different extracellular volume (ECV) measures in aortic regurgitation and stenosis on pre- and postoperative cardiovascular magnetic resonance. (A, B, and C) show the septal ECV without (w/o) late-gadolinium enhancement (LGE), septal ECV with LGE, and global ECV without LGE, respectively. Solid horizontal lines indicate median values; boxes indicate p25 and p75; and vertical lines indicate the highest and lowest values. P values indicate differences between measures (significant if < 0.05). NS, non-significant. Preop, preoperative. Postop, postoperative.
Postoperative cardiovascular magnetic resonance data
Surgical data are summarized in Supplementary data online, Table S3. Five patients with AS did not undergo postoperative CMR: two died within 30 days of surgery due to refractory cardiogenic and septic shock, one was diagnosed with metastatic sigmoid colon cancer during treatment for early prosthetic infective endocarditis (caused by Streptococcus gallolyticus) 3 months after surgery and died of cancer evolution, and two had CMR non-compatible pacemakers implanted 55 and 117 days after surgery. All other patients showed LV hypertrophy regression, with lower postoperative LV indexed mass and volumes in patients with AR [indexed mass: 110 (91–134) vs. 91 (74–109) g/m2; indexed end-diastolic volume: 153 (125–194) vs. 95 (77–128) mL/m2; indexed end-systolic volume: 75 (43–90) vs. 42 (30–76) mL/m2; all P < 0.001] (Supplementary data online, Table S4) and lower postoperative LV indexed mass and indexed end-diastolic volume in those with AS [indexed mass: 86 (71–104) vs. 68 (58–84) g/m2, P < 0.001; indexed end-diastolic volume: 71 (64–88) vs. 66 (57–76) mL/m2, P = 0.002] (Supplementary data online, Table S5). Besides, there was a decrease in the indexed cellular mass in both groups [AR: preoperative 79.8 (65.8–92.5) vs. postoperative 65.3 (54.2–75.6) g/m2, P < 0.001; AS: preoperative 61.0 (51.9–74.4) vs. postoperative 48.5 (38.9–59.5) g/m2, P < 0.001].
Postoperative MF CMR data are presented in Table 3 and Supplementary data online, Table S6. In the AR group, there was no difference between pre- and postoperative absolute [3.8 (2.7–5.8) g vs. 2.5 (1.6–6.1) g, P = 0.635] or proportional [1.9 (1.1–2.2)% vs. 1.7 (0.8–2.7)%, P = 0.575] LGE quantities. There was a decrease in iECV [preoperative: 30.0 (22.8–39.6) mL/m2 vs. postoperative: 26.5 (19.3–33.2) mL/m2, P < 0.001] and ECV measurements stabilized (all P > 0.60).
Table 3.
Postoperative cardiovascular magnetic resonance data
| Total n = 94 |
Aortic regurgitation | Aortic stenosis | P value | |
|---|---|---|---|---|
| n = 32 (34%) | n = 62 (66%) | |||
| Surgery to CMR (days) | 188 (185–213) | 189 (185–210) | 187 (185–220) | 0.519 |
| Indexed LA (mm/m2) | 38 (31–44) | 39 (33–44) | 38 (30–45) | 0.546 |
| Indexed LV end-diastolic volume (mL/m2) | 72 (62–89) | 95 (77–128) | 66 (57–76) | < 0.001 |
| Indexed LV end-systolic volume (mL/m2) | 25 (19–38) | 42 (30–76) | 22 (15–29) | < 0.001 |
| LV EF (%) | 62 (54–72) | 54 (38–65) | 67 (60–77) | < 0.001 |
| LV EF <50% | 13 (13.8%) | 11 (34.4%) | 2 (3.2%) | < 0.001 |
| LV mass index (g/m2) | 74 (61–95) | 91 (74–109) | 68 (58–84) | < 0.001 |
| Delta LV mass indexa (g/m2) | 16.0 (7.0–31.0) | 19.0 (5.3–38.3) | 15.5 (8.5–27.0) | 0.786 |
| Presence of LGE | 41 (43.6%) | 14 (43.8%) | 27 (43.5%) | 0.985 |
| LGE mass (g) | 3.4 (1.9–6.3) | 2.5 (1.6–6.1) | 3.5 (1.9–6.5) | 0.842 |
| LGE fraction (%) | 2.3 (1.1–3.4) | 1.7 (0.8–2.7) | 2.4 (1.4–3.7) | 0.135 |
| Septal ECV w/o LGE (%) | 30.5 (26.8–33.5) | 29.9 (28.0–33.1) | 30.5 (26.5–34.4) | 0.676 |
| Global ECV w/o LGE (%) | 28.5 (25.8–31.2) | 29.8 (26.5–31.4) | 28.0 (25.8–31.0) | 0.571 |
| Septal ECV with LGE (%) | 31.0 (26.9–34.8) | 31.5 (28.0–34.1) | 30.7 (26.7–35.3) | 1.000 |
| Global ECV with LGE (%) | 29.2 (26.6–31.6) | 29.9 (26.7–31.9) | 28.6 (26.4–31.6) | 0.534 |
| Indexed cellular mass (g/m2) | 53.0 (44.7–66.6) | 65.3 (54.2–74.6) | 48.5 (38.9–59.5) | < 0.001 |
| iECV (mL/m2) | 19.9 (16.5–26.8) | 26.5 (19.3–33.2) | 18.2 (15.3–23.8) | < 0.001 |
| Delta iECVa (mL/m2) | 4.1 (0.1–8.0) | 5.0 (1.4–9.5) | 3.9 (–0.4–7.5) | 0.363 |
| Haematocrit (%) | 41 (38–44) | 41 (38–44) | 41 (39–44) | 0.959 |
Values are expressed as n (%) or median (p25–p75). Bold P values indicate statistical significance.
CMR, cardiovascular magnetic resonance; ECV, extracellular volume fraction; EF, ejection fraction; iECV, indexed extracellular volume; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; w/o, without.
Delta was calculated as preoperative value minus postoperative value.
In the AS group, ECV measurements increased postoperatively (all P < 0.05), with a concomitant decrease in postoperative iECV [preoperative: 22.0 (17.3–30.7) mL/m2 vs. postoperative: 18.2 (15.3–23.8) mL/m2, P < 0.001]. The absolute [preoperative: 3.4 (1.5–9.5) g vs. postoperative: 3.5 (1.9–6.5) g, P = 0.575] and proportional [preoperative: 2.4 (1.0–4.7)% vs. postoperative: 2.4 (1.4–3.7)%, P = 0.615] LGE quantities stabilized, maintaining the predominantly non-ischaemic pattern (AR vs. AS: 85.7% vs. 77.8%, P = 0.692). Comparing the AR and AS groups, we found no differences in ECV after surgery (all P > 0.50) because of the increase in ECV measurements in the AS group. In contrast, iECV remained higher in patients with AR on postoperative CMR [AR vs. AS: 26.5 (19.3–33.2) vs. 18.2 (15.3–23.8) mL/m2; P < 0.001] (Figure 3 and Graphical Abstract).
Laboratory
From pre- to postoperative exams, BNP levels remained stable in both groups [AR: preoperative: 46 (26–81) pg/mL vs. postoperative: 61 (32–98) pg/mL, P = 0.274; AS: preoperative: 72 [42–118] pg/mL vs. postoperative: 66 (47–105) pg/mL, P = 0.790]. Troponin I levels remained stable in patients with AR [preoperative: 18 (5–34) ng/L vs. postoperative: 16 (5–26) ng/L, P = 0.382] and decreased in those with AS [preoperative: 17 (5–34) ng/L vs. postoperative: 6 (5–15) ng/L; P < 0.001]. Comparing groups, preoperative BNP was lower in the AR group [AR vs. AS: 46 (26–81) pg/mL vs. 72 (42–118) pg/mL; P = 0.016], while troponin I was similar between the groups [AR vs. AS: 18 (5–34) ng/L vs. 17 (5–34) ng/L; P = 0.949]. After surgery, BNP levels were similar between the AR and the AS groups [AR vs. AS: 61 (32–98) pg/mL vs. 66 (47–105) pg/mL; P = 0.265], while troponin I levels were lower in the AS group [AR vs. AS: 16 (5–26) ng/L vs. 6 (5–15) ng/L; P = 0.022].
Correlations with myocardial fibrosis measures
Supplementary data online, Tables S7 and S8 present the correlations between preoperative fibrosis measures and clinical, CMR, and laboratory parameters in patients with AR and AS, respectively. There was a correlation between baseline iECV and male sex in both valvulopathies (AR: r = 0.500, P = 0.004; AS: r = 0.444, P < 0.001).
In the AR group, preoperative iECV correlated to regurgitant volume (r = 0.589, P < 0.001) and to all other LV structural parameters analysed, including a negative correlation with LV EF (r = −0.450, P = 0.021). The presence of LGE and iECV correlated with greater postoperative LV indexed mass (LGE: r = 0.581, P < 0.001; iECV: r = 0.789, P < 0.001), while all analysed preoperative fibrosis measures correlated with greater postoperative iECV (LGE: r = 0.577, P = 0.001; ECV: r = 0.546, P = 0.001; iECV: r = 0.839, P < 0.001). Regarding biomarkers, the presence of LGE correlated with pre- and postoperative troponin I (preoperative: r = 0.620, P < 0.001; postoperative: r = 0.511, P = 0.003), and iECV correlated with preoperative troponin I (r = 0.616, P < 0.001) and BNP (r = 0.548, P = 0.001).
In the AS group, there was a more frequent correlation between fibrosis measures and LV structural parameters. Notably, iECV correlated with both pre- and postoperative indexed masses (pre: r = 0.916, P < 0.001; post: r = 0.742, P < 0.001), and with preoperative troponin I levels (r = 0.547, P < 0.001). Postoperative BNP levels did not correlate with preoperative fibrosis measures in neither group of patients.
Predictors of postoperative decrease in indexed extracellular volume
Analysing the decrease in iECV after surgery, different variables showed predictive value in AR and AS on linear regression analysis (Tables 4 and 5 and Supplementary data online, Tables S9 and S10). In the AR group, the univariable predictors for a decrease in iECV were echocardiographic LV volumes and diastolic diameter, CMR LV septum, lateral wall, indexed volumes, LGE mass and fraction, ECV measures, baseline BNP and prosthesis size, and low CMR LV EF. In the AS group, the positive predictors on univariable analysis were male sex, echocardiographic LV end-systolic volume and systolic diameter, CMR LV indexed volumes, ECV measures (except septal ECV with LGE), baseline BNP, and prosthesis size, while the negative predictors were age, baseline functional class, echocardiographic and CMR LV EF, and LGE mass and fraction.
Table 4.
Univariable and multivariable predictors for decrease in iECVa after aortic valve replacement in aortic regurgitation
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | |
| Echocardiographic parameters | ||||
| Echo SV (mL) | 0.627 (0.089 to 0.276) | < 0.001 | NS | |
| Echo indexed SV (mL) | 0.488 (0.069 to 0.452) | 0.010 | NS | |
| Echo EDV (mL) | 0.598 (0.032 to 0.112) | 0.001 | NS | |
| Echo ESV (mL) | 0.464 (0.016 to 0.137) | 0.015 | NS | |
| Echo LV diastolic diameter (mm) | 0.400 (0.065 to 0.824) | 0.023 | NS | |
| Baseline CMR parameters | ||||
| Ventricular septum (mm) | 0.448 (0.356 to 2.429) | 0.010 | NS | |
| LV lateral wall (mm) | 0.574 (1.029 to 3.365) | 0.001 | NS | |
| CMR EF (%) | −0.383 (−0.523 to −0.028) | 0.031 | NS | |
| Indexed LV EDV (mL/m2) | 0.508 (0.025 to 0.109) | 0.003 | NS | |
| Indexed LV ESV (mL/m2) | 0.575 (0.042 to 0.136) | 0.001 | NS | |
| Indexed mass (g/m2) | 0.732 (0.119 to 0.245) | < 0.001 | 0.230 (0.012 to 0.140) | 0.030 |
| LGE mass (g) | 0.849 (0.382 to 1.343) | 0.004 | NS | |
| LGE fraction (%) | 0.798 (0.975 to 5.007) | 0.010 | NS | |
| Septal ECV w/o LGE (%) | 0.595 (0.384 to 1.260) | 0.001 | 0.862 (1.036 to 1.640) | <0.001 |
| Global ECV w/o LGE (%) | 0.731 (0.732 to 1.538) | < 0.001 | NS | |
| Septal ECV with LGE (%) | 0.500 (0.221 to 1.117) | 0.005 | NS | |
| Global ECV with LGE (%) | 0.699 (0.629 to 1.406) | < 0.001 | NS | |
| iECV (mL/m2) | 0.863 (0.365 to 0.568) | < 0.001 | NS | |
| Laboratorial parameter | ||||
| Baseline BNP (pg/mL) | 0.667 (0.017 to 0.041) | < 0.001 | NS | |
| Surgical parameter | ||||
| Prosthesis size (mm) | 0.417 (0.380 to 4.042) | 0.020 | NS | |
Bold P values indicate statistical significance.
BNP, B-type natriuretic peptide; CI, confidence interval; CMR, cardiovascular magnetic resonance; ECV, extracellular volume fraction; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; iECV, indexed extracellular volume; LGE, late gadolinium enhancement; LV, left ventricle; NS, non-significant; SV, stroke volume; w/o, without.
Preoperative iECV minus postoperative iECV.
Table 5.
Univariable and multivariable predictors for decrease in iECVa after aortic valve replacement in aortic stenosis
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | |
| Clinical parameters | ||||
| Age (years) | −0.304 (−0.374 to −0.04) | 0.016 | NS | |
| Male sex | 0.354 (1.311 to 6.93) | 0.005 | NS | |
| Baseline functional class (NYHA) | −0.329 (−4.533 to −0.674) | 0.009 | NS | |
| Echocardiographic parameters | ||||
| Echo EF (%) | −0.403 (−0.599 to −0.156) | 0.001 | −0.370 (−0.623 to −0.012) | 0.043 |
| Echo ESV (mL) | 0.282 (0.010 to 0.197) | 0.030 | NS | |
| Echo LV systolic diameter (mm) | 0.349 (0.138 to 0.765) | 0.005 | NS | |
| Baseline CMR parameters | ||||
| CMR EF (%) | −0.415 (−0.320 to −0.089) | 0.001 | NS | |
| Indexed LV EDV (mL/m2) | 0.457 (0.056 to 0.169) | <0.001 | NS | |
| Indexed LV ESV (mL/m2) | 0.472 (0.070 to 0.200) | < 0.001 | NS | |
| Indexed mass (g/m2) | 0.537 (0.070 to 0.165) | < 0.001 | NS | |
| LGE mass (g) | −0.509 (−0.515 to −0.080) | 0.009 | NS | |
| LGE fraction (%) | −0.558 (−1.367 to −0.299) | 0.004 | NS | |
| Septal ECV w/o LGE (%) | 0.255 (0.002 to 0.599) | 0.049 | NS | |
| Global ECV w/o LGE (%) | 0.437 (0.305 to 1.010) | < 0.001 | NS | |
| Global ECV with LGE (%) | 0.429 (0.280 to 0.947) | < 0.001 | NS | |
| iECV (mL/m2) | 0.612 (0.274 to 0.548) | < 0.001 | 0.528 (0.136 to 0.702) | 0.006 |
| Laboratorial parameter | ||||
| Baseline BNP (pg/mL) | 0.321 (0.002 to 0.014) | 0.011 | NS | |
| Surgical parameter | ||||
| Prosthesis size (mm) | 0.305 (0.180 to 1.827) | 0.018 | NS | |
Bold P values indicate statistical significance.
BNP, B-type natriuretic peptide; CI, confidence interval; CMR, cardiovascular magnetic resonance; ECV, extracellular volume fraction; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; iECV, indexed extracellular volume; LGE, late gadolinium enhancement; LV, left ventricle; NYHA, New York Heart Association; NS, non-significant; SV, stroke volume; w/o, without.
Preoperative iECV minus postoperative iECV.
On multivariable regression analysis, independent predictors for decrease in iECV were baseline CMR-indexed mass and septal ECV without LGE in the AR group, and echocardiographic LV EF and baseline CMR iECV in the AS group.
Predictors of left ventricle reverse remodelling
The Supplementary data online, Tables S11−S16 summarize the analyses of the predictors of LV reverse remodelling.
Including all patients, nested models have shown both LV mass index and global ECV without LGE as independent predictors for LV reverse remodelling (LV mass index P < 0.001, global ECV without LGE P = 0.039, P = 1.000 for interaction), with similar results using global ECV with LGE. When only the group of patients with AR was included, only LV mass index was maintained as a predictor (LV mass index P < 0.001, global ECV without LGE P = 0.415).
Of 94 patients who underwent postoperative CMR, 12 patients (5 with AR and 7 with AS) had LV adverse remodelling. A higher preoperative iECV was associated with a lower chance of developing adverse LV remodelling (Beta 0.911, 95% CI 0.836–0.993, P = 0.034).
Analysing the groups of patients separately in univariable linear regression, the predictors of LV reverse remodelling for the patients with AR were iECV, presence of LGE, regurgitant volume, and regurgitant fraction. For the AS group, the positive predictors were male sex, iECV, and septal ECV without LGE and global ECV without LGE, while negative predictors were age, hypertension, CMR LV EF, and LGE fraction.
Discussion
The main findings of our study are as follows: (i) patients with AR have greater preoperative ECV and iECV than those with AS; (ii) after surgery, patients with AR and AS show iECV regression and LGE stability, but unlike in patients with AS, those with AR do not show a significant increase in ECV; and (iii) iECV is the CMR fibrosis parameter better correlated to LV structural parameters and biomarkers in both valvulopathies.
Aortic stenosis, mainly degenerative or bicuspid, has received increasing attention from the scientific community in recent years, as its increasing prevalence is associated with population aging, and less invasive treatment methods are being developed. Aortic regurgitation, although less prevalent than AS,39 involves a younger population with a more extensive number of causes (including rheumatic disease and aortopathies); it may lead to premature morbidity and the need for medical assistance. A better understanding of the postoperative changes in myocardial structure will deepen the knowledge on the pathophysiology of aortic VHD and may lead to the development of better treatment targets and a more accurate definition of the timing for intervention. While the long-term prognostic relevance of preoperative diffuse MF in aortic valvulopathies has been demonstrated,14,15 information regarding diffuse fibrosis changes after surgery remain scarce (for AS) or missing (for AR).
Myocardial fibrosis
Our results showed increased preoperative ECV and iECV, suggesting that diffuse fibrosis is a significant component of myocardial remodelling in aortic VHD.14,15 This has been previously demonstrated using both histopathology9,40,41 and LGE.11,12,42 The greater diffuse fibrosis seen in patients with AR may reflect the different signalling pathways that are activated when both pressure and volume overload occur, as indicated by Olsen’s experimental study.4 The longer asymptomatic course of AR permits the development of myocardial extracellular matrix alterations until surgery is needed and may contribute to the greater diffuse MF observed in this group in our study.
To our knowledge, this is the first time that CMR diffuse fibrosis parameters (ECV/iECV) are directly compared in both aortic valvulopathies. Likewise, our present data originally parallel the postoperative resemblances and differences between the MF parameters in patients with AS and AR, when measured by CMR.
In agreement with previous studies on AS indicating regional fibrosis irreversibility over time, our two groups maintained similar LGE postoperatively, strengthening the idea that regional fibrosis is most likely a non-reversible myocardial alteration.18,19 Everett et al.18 suggested that in patients with AS, prompt aortic valve replacement at the first sign of LGE or just before its development might improve long-term outcomes. Ongoing studies will be able to generate information on the possible validation of this strategy [EVoLVeD (Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients with Severe AS), NCT03094143]. Senapati et al.15 found LGE in only 7% of patients with AR and suggested that this lower prevalence, compared with that in AS, was due to the lower propensity for ischaemia for the same level of LV hypertrophy in patients with both pressure and volume overload and to differences in signalling pathways. However, in our study, LGE areas were present in 31% of patients with AR, mostly non-ischaemic (90%), suggesting that replacement fibrosis may not be uncommon in severe AR. Our data are in line with a previous publication from our group11 and a recent large cohort study43 that showed even higher prevalence of LGE in patients with moderate-to-severe AR: 69% and 51%, respectively.
Regarding diffuse MF in AR, our patients had similar ECV before and after surgery. This stability suggests balanced postoperative reductions in myocyte mass and extracellular matrix and is in accordance with the histopathologic data published by Krayenbuehl et al.8 In that study, the authors analysed the percentage of interstitial fibrosis in ventricular biopsies collected prior to and 18 (range, 9–28) months after aortic valve replacement and found similar values in AR. In our study, patients with AS had an increase in pre- to postoperative ECV measurements concomitant with a decrease in both iECV and indexed cellular mass, confirming previous data suggesting that in AS, reverse remodelling begins with a more prominent involution of myocardial cellular components, allowing an early increase in ECV.8,18,19,44 In Treibel’s study, postoperative ECV in patients with AS increased from 28.2 ± 2.9% to 29.9 ± 4.0% (P < 0.001), and Everett et al. estimated an ECV yearly increase of 1.2% (0.4–2.2%).
Postoperative iECV was lower than preoperative iECV in both groups, indicating that total absolute diffuse MF decreases in both concentric and eccentric LV remodelling. To our knowledge, this is the first study to elucidate this change in AR after surgery. The three previous publications15–17 that studied CMR diffuse fibrosis parameters in patients with AR focused on preoperative data. Compared with the present study, only one of them included a greater number of patients that underwent surgical treatment (49 patients).15 Since both valvulopathies showed similar reductions in iECV, this parameter remained significantly greater in patients with AR compared with patients with AS. This sustained difference in fibrosis was accompanied by greater indexed mass and volumes in postoperative CMR, in patients with AR.
Associations between fibrosis and other relevant parameters
In both aortic VHD, we found an association between iECV and male sex. While previous studies on patients with AS reported controversial data regarding the association between fibrosis parameters and sex,45,46 Senapati et al. found male sex to be a significant predictor of iECV in AR. These sex-based differences require confirmation in further studies; however, they could be explained by sex-specific differences in cellular signalling pathways leading to different responses to pressure and volume overload.
Although somewhat different between the groups, the positive associations of LGE, ECV, and iECV with preoperative myocardial volumes and mass and their negative association with LV EF indicate that fibrosis is most likely related to more advanced myocardial alterations in both aortic VHD. This is also indicated by the correlations of preoperative LGE, ECV, and iECV with a greater postoperative septum, lateral wall (mainly in AS), and indexed mass and postoperative fibrosis parameters.
By revealing different MF parameters as predictors of LV reverse remodelling, our study presents additional information to the data of Treibel et al.19 who have shown that ECV had a predictive impact in patients with AS. This predictive role for MF parameters is now also shown for patients with AR, and may be used in future studies to better define the timing for aortic valve intervention.
Previous echocardiographic data47–49 demonstrate a steeper decline in LV mass in both AS and AR in the first 12–24 months after surgery. Those data show a long-term reversal in myocardial remodelling that can continue up to 5 years after the procedure. Also, the group of patients with AR had a greater echocardiographic LV mass in the preoperative exams and developed a proportional more pronounced early decline in LV mass. Our CMR analyses show similar data, with greater LV mass index in the group of patients with AR both in pre- and postoperative exams, and demonstrate that the LV mass regression can be detected as early as 6 months after surgery in both AS and AR.
B-type natriuretic peptide and troponin are associated with adverse events in AS50; however, little is known regarding their importance in AR.51–53 Studies on the association between BNP or troponin and fibrosis parameters are scarce. In our study, we found higher baseline BNP and positive correlations of BNP and troponin with regional and diffuse MF in patients with AS, similar to previous data.19,42,54 For patients with AR, the correlations of BNP with LGE and with iECV and troponin I with LGE, ECV, and iECV are presented for the first time. The relationships between all these biomarkers point to the existence of common pathophysiologic stimuli, in both AR and AS, that concomitantly induce myocyte injury, increased ventricular pressures, and MF. Also, the type of LV hypertrophy presented by the patients with AR, with a chronic development of eccentric remodelling, probably better accommodates the increase of cavity filling pressures, what could explain the lower preoperative BNP values in this group of patients. Possibly, the unchanged levels of BNP and troponin I seen after surgery in patients with AR are associated to the greater degree of LV remodelling that occurs until the surgery is indicated and performed. In this case, a longer postoperative time may be needed until a decrease in the levels of these biomarkers can be detected. On the other hand, in patients with AS, this time length seems to be shorter, and a decrease in the troponin I levels could already be observed 6 months after surgery.
In addition to being the CMR MF parameter better correlated with BNP and troponin I, iECV showed a decrease on postoperative CMR in the AR and AS groups. When analysing its predictors, we observed that the greater indexed mass in AR and lower LV EF in AS were independently associated with greater decreases in iECV, unfolding the plastic and reversible nature of diffuse fibrosis, even in more advanced stages of aortic VHD.
This study provides a better understanding of the similarities and distinctions in MF between patients with AR and those with AS, including their postoperative changes. Furthermore, our data answer the important question of how diffuse MF CMR parameters evolve after surgery in AR, as recently stated by Salerno and Patel,55 and reinforce the need for multicentre studies that can further test and validate the clinical and prognostic applications of pre- and postoperative MF CMR measurements in aortic VHD.
Study limitations
Our study has some limitations. First, this was a single-centre study, with a relatively small number of patients. Second, because the prevalence of AS is greater than that of AR in the population,39 our study included a greater number of patients with AS. The smaller AR sample size in our manuscript might have impacted the robustness of these specific statistical comparisons and should be confirmed in larger cohorts in the future. Nonetheless, it is the largest study to analyse postoperative diffuse MF in AR and the first to compare the similarities and differences in postoperative diffuse MF between patients with AR and AS. Furthermore, since our hospital is a tertiary medical centre, there may be both referral and surgical biases, as illustrated by the disease severity in our patients. The strict inclusion and exclusion criteria applied in this study may have limited external validation of scenarios involving comorbidities such as diabetes and coronary artery disease. In addition, all CMR examinations were performed using the same 1.5 T scan. Nevertheless, these restrictions minimized confounding variables and strengthened the hypothesis that aortic VHD was the aetiology of the fibrosis encountered, thus reinforcing internal validation.
Conclusions
The amount of preoperative diffuse fibrosis observed was greater in patients with AR than in those with AS. After surgery, patients with both aortic VHD showed iECV regression (indicating a decrease in total absolute diffuse MF); however, greater iECV values were maintained in the AR group compared with the AS group, consistent with the greater LV structural changes observed in the AR group. Our results suggest a balanced postoperative reduction in intracellular and extracellular myocardial components in AR, in contrast to AS. The results also reinforce the plastic nature of diffuse fibrosis and irreversible character of replacement fibrosis in both aortic VHD, indicating that LGE is a marker of a more advanced myocardial fibrotic stage. Finally, some CMR parameters of MF are predictors of LV reverse remodelling in both AS and AR. Future studies are needed to determine how MF evolution may impact the clinical outcomes and timing of aortic valve intervention in both aortic VHD.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowledgements
Authors would like to thank Rute Ribeiro and Monica Udo for their valuable support during all phases of the study. This work was performed at the Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil.
Contributor Information
Lucas T Pires, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Vitor E E Rosa, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Thamara C Morais, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Juliana H S M Bello, Hospital do Coracao, Sao Paulo, SP, Brazil.
Joao R C Fernandes, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Antonio de Santis, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Mariana P Lopes, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Paulo S Gutierrez, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Carlos E Rochitte, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Cesar H Nomura, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Pablo M A Pomerantzeff, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Roney O Sampaio, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Flávio Tarasoutchi, Instituto do Coracao, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Funding
Research support process 2015/01269-9, Sao Paulo Research Foundation (FAPESP).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour Aet al. . Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carabello BA. Aortic regurgitation. A lesion with similarities to both aortic stenosis and mitral regurgitation. Circulation 1990;82:1051–3. [DOI] [PubMed] [Google Scholar]
- 3. Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation 2005;112:125–34. [DOI] [PubMed] [Google Scholar]
- 4. Olsen NT, Dimaano VL, Fritz-Hansen T, Sogaard P, Chakir K, Eskesen Ket al. . Hypertrophy signaling pathways in experimental chronic aortic regurgitation. J Cardiovasc Transl Res 2013;6:852–60. [DOI] [PubMed] [Google Scholar]
- 5. Truter SL, Catanzaro DF, Supino PG, Gupta A, Carter J, Ene ARet al. . Fibronectin gene expression in aortic regurgitation: relative roles of mitogen-activated protein kinases. Cardiology 2009;113:291–8. [DOI] [PubMed] [Google Scholar]
- 6. Borer JS, Truter S, Herrold EM, Falcone DJ, Pena M, Carter JNet al. . Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation 2002;105:1837–42. [DOI] [PubMed] [Google Scholar]
- 7. Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner Aet al. . Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 2005;112:1136–44. [DOI] [PubMed] [Google Scholar]
- 8. Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989;79:744–55. [DOI] [PubMed] [Google Scholar]
- 9. Oldershaw PJ, Brooksby IA, Davies MJ, Coltart DJ, Jenkins BS, Webb-Peploe MM. Correlations of fibrosis in endomyocardial biopsies from patients with aortic valve disease. Br Heart J 1980;44:609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schoen FJ, Lawrie GM, Titus JL. Left ventricular cellular hypertrophy in pressure- and volume-overload valvular heart disease. Hum Pathol 1984;15:860–5. [DOI] [PubMed] [Google Scholar]
- 11. Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio ROet al. . Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–87. [DOI] [PubMed] [Google Scholar]
- 12. Nigri M, Azevedo CF, Rochitte CE, Schraibman V, Tarasoutchi F, Pommerantzeff PMet al. . Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: correlation with quantitative histopathology. Am Heart J 2009;157:361–8. [DOI] [PubMed] [Google Scholar]
- 13. Podlesnikar T, Delgado V, Bax JJ. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int J Cardiovasc Imaging 2018;34:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh Aet al. . Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol 2020;75:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senapati A, Malahfji M, Debs D, Yang EY, Nguyen DT, Graviss EAet al. . Regional replacement and diffuse interstitial fibrosis in aortic regurgitation: prognostic implications from cardiac magnetic resonance. J Am Coll Cardiol Img 2021;14:2170–82. [DOI] [PubMed] [Google Scholar]
- 16. Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol 2006;187:W630–5. [DOI] [PubMed] [Google Scholar]
- 17. de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior Jet al. . Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from modified look-locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson 2015;17:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everett RJ, Tastet L, Clavel MA, Chin CWL, Capoulade R, Vassiliou VSet al. . Progression of hypertrophy and myocardial fibrosis in aortic stenosis: A multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging 2018;11:e007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva ANet al. . Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol 2018;71:860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarasoutchi F, Montera MW, Grinberg M, Piñeiro DJ, Sánchez CR, Bacelar ACet al. . [Brazilian guidelines for valve disease - SBC 2011/I guideline inter-American valve disease - 2011 SIAC]. Arq Bras Cardiol 2011;97(5 Suppl 1):1–67. [DOI] [PubMed] [Google Scholar]
- 21. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RAet al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:e521–643. [DOI] [PubMed] [Google Scholar]
- 22. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 23. Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2003;5:353–9. [DOI] [PubMed] [Google Scholar]
- 24. Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JPet al. . Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med 2007;58:34–40. [DOI] [PubMed] [Google Scholar]
- 25. Salvador D, Gamba M, Gonzalez-Jaramillo N, Gonzalez-Jaramillo V, Raguindin P, Minder Bet al. . Diabetes and myocardial fibrosis. J Am Coll Cardiol Img 2022;15:796–806. [DOI] [PubMed] [Google Scholar]
- 26. Pozo E, Kanwar A, Deochand R, Castellano JM, Naib T, Pazos-López Pet al. . Cardiac magnetic resonance evaluation of left ventricular remodelling distribution in cardiac amyloidosis. Heart 2014;100:1688–95. [DOI] [PubMed] [Google Scholar]
- 27. Butcher SC, Pio SM, Kong WKF, Singh GK, Ng ACT, Perry Ret al. . Left ventricular remodelling in bicuspid aortic valve disease. Eur Heart J Cardiovasc Imaging 2022;23:1669–79. [DOI] [PubMed] [Google Scholar]
- 28. Myerson SG, d'Arcy J, Mohiaddin R, Greenwood JP, Karamitsos TD, Francis JMet al. . Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation 2012;126:1452–60. [DOI] [PubMed] [Google Scholar]
- 29. Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081–6. [DOI] [PubMed] [Google Scholar]
- 30. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander Met al. . Myocardial T1 mapping and extracellular volume quantification: a society for cardiovascular magnetic resonance (SCMR) and CMR working group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–62. [PubMed] [Google Scholar]
- 32. Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel Eet al. . Standardization of T1 measurements with MOLLI in differentiation between health and disease–the ConSept study. J Cardiovasc Magn Reson 2013;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AAet al. . Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012;126:1206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson Get al. . Myocardial fibrosis and cardiac decompensation in aortic stenosis. J Am Coll Cardiol Img 2017;10:1320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosa VEE, Ribeiro HB, Sampaio RO, Morais TC, Rosa MEE, Pires LTet al. . Myocardial fibrosis in classical low-flow, low-gradient aortic stenosis. Circ Cardiovasc Imaging 2019;12:e008353. [DOI] [PubMed] [Google Scholar]
- 36. Guglielmo M, Rovera C, Rabbat MG, Pontone G. The role of cardiac magnetic resonance in aortic stenosis and regurgitation. J Cardiovasc Dev Dis 2022;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bohbot Y, Renard C, Manrique A, Levy F, Maréchaux S, Gerber BLet al. . Usefulness of cardiac magnetic resonance imaging in aortic stenosis. Circ Cardiovasc Imaging 2020;13:e010356. [DOI] [PubMed] [Google Scholar]
- 38. Garcia J, Kadem L, Larose E, Clavel MA, Pibarot P. Comparison between cardiovascular magnetic resonance and transthoracic Doppler echocardiography for the estimation of effective orifice area in aortic stenosis. J Cardiovasc Magn Reson 2011;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonow RO, Leon MB, Doshi D, Moat N. Management strategies and future challenges for aortic valve disease. Lancet 2016;387:1312–23. [DOI] [PubMed] [Google Scholar]
- 40. Elias N, Tarasoutchi F, Spina GS, Sampaio RO, Pomerantzeff PM, Laurindo FRet al. . Myocardial fibrosis and ventricular remodeling in severe chronic aortic regurgitation. Arq Bras Cardiol 2009;92:63–7. [DOI] [PubMed] [Google Scholar]
- 41. Taniguchi K, Kawamaoto T, Kuki S, Masai T, Mitsuno M, Nakano Set al. . Left ventricular myocardial remodeling and contractile state in chronic aortic regurgitation. Clin Cardiol 2000;23:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulz O, Rudolph A, Scheiner S, Mut H, Schulz-Menger J, Berghoefer Get al. . Influence of acute and chronic myocardial loading conditions, function, structural changes and extracardiac factors on NT-proBNP in asymptomatic patients with preserved ejection fraction. Clin Res Cardiol 2011;100:57–65. [DOI] [PubMed] [Google Scholar]
- 43. Zheng Y, Yang K, Chen X, Li R, Su G, Yin Get al. . Prognostic significance of myocardial fibrosis and CMR characteristics in bicuspid aortic valve with moderate and severe aortic insufficiency. Eur Radiol 2021;31:7262–72. [DOI] [PubMed] [Google Scholar]
- 44. Hess OM, Ritter M, Schneider J, Grimm J, Turina M, Krayenbuehl HP. Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement. Circulation 1984;69:855–65. [DOI] [PubMed] [Google Scholar]
- 45. Tastet L, Kwiecinski J, Pibarot P, Capoulade R, Everett RJ, Newby DEet al. . Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. J Am Coll Cardiol Img 2020;13:699–711. [DOI] [PubMed] [Google Scholar]
- 46. Singh A, Musa TA, Treibel TA, Vassiliou VS, Captur G, Chin Cet al. . Sex differences in left ventricular remodelling, myocardial fibrosis and mortality after aortic valve replacement. Heart 2019;105:1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monrad ES, Hess OM, Murakami T, Nonogi H, Corin WJ, Krayenbuehl HP. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation 1988;77:1345–55. [DOI] [PubMed] [Google Scholar]
- 48. Une D, Mesana L, Chan V, Maklin M, Chan R, Masters RGet al. . Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation 2015;132:741–7. [DOI] [PubMed] [Google Scholar]
- 49. Vollema EM, Singh GK, Prihadi EA, Regeer MV, Ewe SH, Ng ACTet al. . Time course of left ventricular remodelling and mechanics after aortic valve surgery: aortic stenosis vs. aortic regurgitation. Eur Heart J Cardiovasc Imaging 2019;20:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. White M, Baral R, Ryding A, Tsampasian V, Ravindrarajah T, Garg Pet al. . Biomarkers associated with mortality in aortic stenosis: a systematic review and meta-analysis. Med Sci (Basel) 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Onishi H, Naganuma T, Izumo M, Ouchi T, Yuki H, Mitomo Set al. . Prognostic relevance of B-type natriuretic peptide in patients with moderate mixed aortic valve disease. ESC Heart Fail. 2022. [DOI] [PMC free article] [PubMed]
- 52. Sharma V, Stewart RA, Lee M, Gabriel R, Van Pelt N, Newby DEet al. . Plasma brain natriuretic peptide concentrations in patients with valvular heart disease. Open Heart 2016;3:e000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, Szymański P. The usefulness of selected biomarkers in aortic regurgitation. Cardiol J 2019;26:477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Treibel TA, López B, González A, Menacho K, Schofield RS, Ravassa Set al. . Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salerno M, Patel T. Assessing cardiac remodeling in aortic regurgitation using indexed extracellular volume: more than meets the “i”? J Am Coll Cardiol Img 2021;14:2183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.