Abstract
Environmental changes along an altitudinal gradient can facilitate the differentiation of life-history features in ectothermic species, but little attention has been devoted to the reciprocal influence of altitude and alpine slope directionality on life-history variation. According to life-history theory, increased environmental stress causes a change in reproductive allocation from number to quality of offspring, as well as a stronger trade-off between size and number of offspring. To clarify the influence of environmental pressures on the life-history features of the Qinghai toad-headed lizard Phrynocephalus vlangalii along an altitudinal cline, we surveyed late pregnant females from 3 populations of low (2,600 m), middle (3,400 m), and high (3,600 m) elevations in the Dangjin Mountain of Gansu, China from July to October 2019, and compared their inter-population differences in maternal body size, reproductive characteristics, offspring growth, and locomotor performance. Because of lower temperatures, higher humidity, and lower light intensity caused by slope aspect and altitude, the middle-altitude region experienced stronger environmental stress than the high- and low-altitude regions. Our results showed that females were larger at middle- and high-altitude sites and smaller at the low-altitude site, following Bergmann’s rule. We also found that females from low-altitude population gave birth earlier than those from the middle and high altitudes. Our results showed a shift in the offspring size-number trade-off of P. vlangalii in response to colder and harsher environments, with lizards from the alpine steppe (i.e. the middle- and high-altitude habitats) producing fewer but larger offspring than those from the warm steppe (i.e. the low-altitude habitat). Low-altitude juveniles grew faster than high-altitude ones, but at the same rates as middle-altitude juveniles. This result demonstrates that the growth of P. vlangalii was associated with temperature and light intensity. Our findings contribute to enhancing our understanding of the altitudinal variation in life-history features of plateau ectotherms and their phenotypic plasticity or local adaptation.
Keywords: altitudinal gradient, growth, life-history, Phrynocephalus vlangalii, plateau lizard, trade-off
Among-population changes in life-history features, such as body size, reproduction, and offspring growth, are common in animals that have wide latitudinal or altitudinal distribution ranges (Fitch 1985; Forsman and Shine 1995; Wapstra and Swain 2001; Ji et al. 2002; Iraeta et al. 2013; Lu et al. 2018b). Environmental stress can be a major driver of life-history variation, which may be the consequence of species resistance and adaptation to specific stress (Einum and Fleming 1999; Rasanen et al. 2008). Because environmental stresses in various habitats lead to disparities in maternal disposable resources, life-history theory suggests that females must optimally allocate their resources between present and future reproduction, as well as between reproduction, growth, and maintenance (Williams 1965; Stearns 1992). Consequently, variation in life-history features is significant to understanding how animals respond to different environmental stresses.
One of the important topics in studies of animal life history is the trade-off between size and number of offspring, which may influence the maternal reproductive output determined by allocable energy resources (Stearns 1992; Roff 1993; Charnov and Downhower 1995). Life-history theory predicts that animals often give birth to fewer but larger offspring, indicating a trade-off of offspring size vs. number, to cope with increasing environmental pressure (Charnov and Downhower 1995; Ljungstrom et al. 2016). Because of the reproductive advantage over smaller females, natural selection favors larger females (Braña 1996; Olsson et al. 2002). Females also tend to produce as many offspring of high quality or large size as possible to increase their reproductive output, because larger offspring have a greater chance of surviving in harsh environments (Stearns 1992; Liao et al. 2014). However, females cannot increase both the number and size of offspring because the amount of resources at their disposal is always limited (Roff 1993). Thus, females choose to produce larger but fewer offspring, or smaller but more offspring, which largely reflects the strategies adopted under different environmental stresses (Ji and Wang 2005). To date, how shifts in the trade-off of offspring size-number among different populations are driven still remain a question worthy of further investigation, especially under different environmental pressures.
For juveniles, growth rate is a critical component of their life-history (Stearns 1992), and strongly influences their size at maturity and survival rates (Sorci et al. 1996; Clobert et al. 2000; Du et al. 2012). However, the growth of some lizards is indeterminate and requires alternative strategies to evolve, because it is vulnerable to proximal environmental forces (Dunham 1978; Sinervo and Adolph 1994; Lorenzon et al. 2001). For example, food shortages may promote the growth of lizards in the presence of food, or reduce their activities in harsh environments (Iraeta et al. 2006, 2008). Thus, variation in juvenile growth rates has been interpreted as an adaptive strategy in different populations of the same species (Lorenzon et al. 2001; Niewiarowski 2001). Although cold conditions or food scarcity can inhibit juvenile growth, some species may evolve genetically-based phenotypes that grow quickly in order to compensate for this deficiency (Blanckenhorn 1991; Arnett and Gotelli 1999; Jonassen et al. 2000; Ficetola and De Bernardi 2005). The reasons for variation in juvenile growth are not well understood, so it is necessary to study the juvenile growth rate in different populations of the same species.
Life-history evolution under stressful circumstances can be better understood by studying populations at different altitudes (Kozlowska 1971; Howard and Wallace 1985). For instance, owing to environmental conditions like lower temperatures, stronger seasonality, shorter reproductive seasons, and more volatile food resources, ectotherms at high altitudes tend to increase investment in each offspring, as a tactic to improve the survival rate of offspring (Berven 1982; Morrison and Hero 2003). In fact, among diverse reptiles, high-altitude populations are characterized by fewer and larger offspring, while low-altitude populations have more and smaller offspring (Jin and Liu 2007; Li et al. 2014). However, some research has shown that high-altitude lizards produce more and smaller offspring than low-altitude ones (Tang et al. 2012). Furthermore, some studies have found that reptile juveniles grow faster at high altitudes (Iraeta et al. 2006, 2013; Lu et al. 2018a, 2018b; Hu et al. 2019), whereas others have found that high-altitude juveniles grow slower (Sinervo and Adolph 1989; Sorci et al. 1996). Notably, these research results on growth of juvenile across altitudes are also inconsistent. Meanwhile, light-environment is an important component of lizard thermoregulation which is different from temperature, and lower light can prevent lizards from achieving optimal body temperature (Sievert and Hutchison 1988, 1989; Refsnider et al. 2018). Therefore, comprehensive data sets from different populations of ectothermic reptiles are essential for comprehending the common pattern of covariation among life-history features, and the relationship between life-history features and ecological factors.
The Qinghai toad-headed lizard Phrynocephalus vlangalii, a viviparous agamid endemic to China, is widely distributed in the northeastern Qinghai-Tibetan Plateau, with a broad altitudinal distribution range of 2,000–4,500 m (Wake et al. 1994; Zhao 1997). Altitudinal-related changes in life-history features in this lizard species have attracted increasing attention from researchers (Jin and Liu 2007; Jin et al. 2007; Li et al. 2014; Lu et al. 2018a). A previous study reported that the body size of adult P. vlangalii decreased with increasing altitude (Jin et al. 2007), but 3 other recent studies showed that females at high altitudes were larger than those at low altitudes (Jin and Liu 2007; Li et al. 2014; Lu et al. 2018a). Two studies reported that females of P. vlangalii at high altitudes produced fewer and larger offspring than those at low altitudes (Jin and Liu 2007; Li et al. 2014), but Lu et al. (2018a) also found that newborns of high-altitude females were smaller than those of low-altitude females. Obviously, the results of these studies are inconsistent. We noticed that there was not only a large altitudinal gradient but also a large latitudinal gradient between the geographical localities of these lizard populations selected for the studies. Because of the controversy in altitudinal patterns of the life-history features, the underlying proximate causes of the altitudinal change remain elusive for this plateau lizard. Patterns of variation in life-history features of a plateau ectothermic lizard might be further clarified among geographically close altitudinal populations.
In this study, we captured late pregnant females of the Qinghai toad-headed lizard at 3 altitudinal sites on a high-elevation mountain, and reared them at each collection site. We compared their inter-population differences in maternal body size, reproductive characteristics, offspring growth and locomotor performance. We hypothesized that geographically close populations of the Qinghai toad-headed lizard in a high mountain would show different reproductive strategies along an altitudinal gradient because of large environmental changes. We then predicted that females of P. vlangalii at high altitudes would be larger, and produce fewer but larger offspring, than those at low altitudes due to colder and harsher environments. The objective was to clarify the variation of life-history features in a Qinghai-Tibet Plateau lizard along an altitudinal cline.
Materials and Methods
Study site and species
This study was conducted at 3 sites of low (2,600 m), middle (3,400 m), and high (3,600 m) elevations in the middle Dangjin Mountain (39°18ʹ–25ʹN, 94°14ʹ–16ʹE) with an altitudinal range of 2,300–3,750 m, Aksay County, Gansu Province, China, during July–October of 2019. The low- and middle-altitude sites are located in the warm steppe area at the mountain’s northern foot and the alpine steppe area near the mountain’s northern peak respectively, whereas the high-altitude site is located in the alpine steppe area near the mountain’s southern peak. The warm steppe vegetation is mainly composed of Achnatherum splendens, Carex spp., Ephedra sinica, Haloxylon ammodendron, Leymus secalinus, Poa annua, Stipa capillata, S. breviflora, Sympegma regelii, while the alpine steppe vegetation consists mainly of A. splendens, Krascheninnikovia compacta, Leontopodium nanum, Rhodiola quadrifida, Roegneria nutans, S. capillata, S. purpurea. The mean annual temperature is < 3.9 °C with an average minimum of −9 to −20 °C in January and an average maximum of 11 to 16 °C in July. Annual precipitation ranges from 19 to 176 mm, with most rain falling between May and September (Han et al. 1999).
The Qinghai toad-headed lizard generally inhabits arid and semi-arid grasslands with sparse vegetation. Sexual dimorphism of this lizard species in morphological traits is evident in adults, the largest male being 70.2-mm snout–vent length (SVL) and the largest female being 82.8 mm SVL (Zhang et al. 2005). The females produce a single litter per breeding season from June to August, with a litter size of 2–6 young (Zhang et al. 2005; Li et al. 2014). The life-history characteristics of different populations of this species have great variability. In terms of reproductive characteristics, populations at different altitudes also make corresponding changes due to changes in ecological factors like as temperature and food resources (Jin et al. 2007; Li et al. 2014; Lu et al. 2018b).
Sampling and breeding
According to previous research, female P. vlangalii give birth from 25 July to 28 August; populations from lower altitudes have litters earlier than those from higher altitudes (Li et al. 2014). In the field, the lizards were palpated around the abdomen to determine their pregnancy status. We collected 170 late-pregnant females of the 3 lizard populations in the low (69♀), middle- (30♀) and high- (71♀) altitude sites from 21 July to 15 August, and then transferred each lizard to plastic storage bins (67 × 49 × 40 cm) installed at the local altitude site in the field. After being caught, female lizards were measured by experimental instruments, and immediately marked and numbered on their backs with a black marker pen. Females were then toe-clipped for permanent individual identification (Hao et al. 2021). Any females that shed their skin were marked again for better visual identification. Each bin housed 1 gravid female. Half of the plastic storage bin was covered with 10- to 15-cm-thick sandy soil, and the other half was covered with eggs paper trays, so that the lizard could adjust its body temperature autonomously by natural sunlight. One half of the box lid was removed and replaced with bird nets to ensure air circulation and defense against predators. After the litters were born, they stayed in the same bin with their mother. The lizards were fed approximately 5–6 mealworms and drinking water every day, and newborns were fed with small mealworms ad libitum.
Data collection
Each captured female lizard was measured for SVL using digital vernier calipers (PD-151, Pro’sKit) accurate to 0.01mm, and the body mass (BM) was measured with an electronic balance (ES-08B, Hochoice) accurate to 0.01 g. Plastic storage bins were checked at least twice per day to record any newly born offspring. When newborns were found, we recorded the approximate litter time of their mothers and measured postpartum BM and SVL. Then we measured the neonate BM and SVL, and recorded litter size and litter mass.
When offspring were 2 weeks old, we measured their BM and SVL again. Sprint speed is often used as a proxy of animal fitness (Hoskins et al. 2017; González-Morales et al. 2021a). Then we measured their sprint speeds on a wooden track (100 cm × 15 cm × 20 cm) in the field. The bottom of the track was a rough cork board, which can provide suitable traction for the lizards. The wooden track is divided into eight 10 cm long sections separated by horizontal strips of contrasting colors. During a sprint trial, the lizard was introduced from 1 end, and the researcher used a brush to touch the tail and stimulate the lizard to run away. Sprint trials were recorded with a digital video camera (DCR-SR220E, SONY). Subsequently, we used the software PotPlayer to analyze the videos, and took the fastest speed of each subject running through a 20 cm interval as the maximum sprint speed. Before each lizard was tested, we used an electronic thermometer (UNT T325, Shenzhen Meter Instruments) to measure their cloacal temperature.
In addition, we recorded the temperature, humidity, and light intensity of the environment every half hour at the 3 altitudinal sites by Onset HOBO Temperature/Relative Humidity/Light/External Data Logger (U12-012, HOBO) from 23 July to 15 September.
Statistical analyses
In this study, we used Julian Day to represent the litter time for data analysis (January 1st of the current year is the first day of Julian Day, arranged in recursive order). Relative litter mass was an indicator of the lizard’s reproductive investment, calculated as the ratio of litter mass to maternal post-partum BM (Dupoué and Lourdais 2014). We used Shapiro-Wilk tests, Bartlett tests and the glht function in the multcomp package to determine normality, homogeneity of variance, and homogeneity of the slope of the data. For the analysis of environmental factors, we used Kruskal tests to compare differences in temperature, humidity, and light intensity among the 3 altitudinal sites. The PMCMRplus package was used for each pairwise comparison test between environmental levels. We used chi-square tests to analyze the inter-population difference in the reproductive success rate of gravid females. The reproductive success rate is calculated as the percentage of lizard mothers giving birth to normal litters and gravid females collected. One-way ANOVAs were used to compare the inter-population differences in female SVL and BM, litter time, and relative litter mass. One-way ANCOVA was used to determine inter-population difference in litter mass with female SVL as a covariate. The Permutation test method in the lmPerm package was used to compare the inter-population differences in litter size with female SVL as a covariate. Tukey HSD tests were used for each pairwise comparison test between levels. Growth rate of offspring SVL and BM was calculated using ln(measurement2/measurement1)/(date2 − date1). Linear mixed models, with maternal identity as a random factor, were used to compare the inter-population differences in neonate SVL and neonate BM with maternal SVL as a covariate, offspring growth rates with initial SVL as a covariate, and offspring locomotor performance with initial SVL and cloacal temperature as covariates. All data were expressed as mean ± SE. All data was analyzed in R (v.3.6.3).
Results
Environmental factors
Among the 3 sites, there were significant differences in mean daily temperature (χ2 = 509.01, P < 0.001), humidity (χ2 = 256.18, P < 0.001), and light intensity (χ2 = 40.92, P < 0.001). The low-altitude site had higher temperature and lower humidity than the high- and middle-altitude sites, with no difference between the middle-altitude site of the north slope of the mountain and the high-altitude site of the south slope (Figure 1). The low-altitude site was noticeably warmer and drier compared to the middle- and high-altitude sites. However, the light intensity at the middle-altitude site of mountain’s north was generally weaker than that at the low-altitude site of mountain’s north and the high-altitude site of mountain’s south (Figure 1).
Figure 1.
Daily average temperature, humidity and light intensity at the 3 sites of low (2,600 m), middle (3,400 m), and high (3,600 m) elevations in the Dangjin Mountain of Gansu, China. Mean values with different letters after the names of the 3 altitudinal sites in the legends are statistically significantly different (Kruskal test).
Female body size and reproductive traits
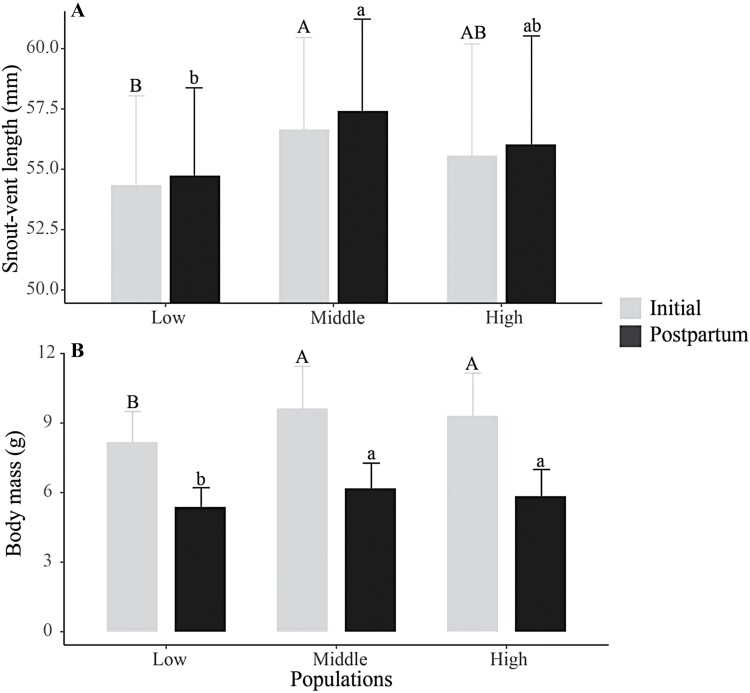
In total, there were 50 (72.5%), 23 (76.7%), and 61 (85.9%) females giving birth in the low-, middle-, and high-altitude populations, respectively, showing no interpopulation difference in the reproductive success rate (χ2 = 3.895, df = 2, P = 0.143). There were significant differences in the female body size measured by SVL (Initial: F2,131 = 3.67, P < 0.05; post-partum: F2,131 = 5.00, P < 0.05) and BM (Initial: F2,131 = 8.75, P < 0.001; post-partum: F2,131 = 5.47, P < 0.05) among the 3 altitudinal populations of P. vlangalii (Figure 2, Table 1). Specifically, the SVL was larger in middle- than low-altitude populations (Figure 2A), and the BM was heavier in middle- and high-altitude populations than in low-altitude population (Figure 2B).
Figure 2.
Initial and postpartum snout-vent length and body mass of adult female Qinghai toad-headed lizards Phrynocephalus vlangalii, from the 3 populations along an altitudinal gradient of low (2,600 m), middle (3,400 m), and high (3,600 m) in the Dangjin Mountain of Gansu, China. Data are showed as means ± 1SE. Mean values with different capital or small letters above the error bars are statistically significantly different (Tukey’s test). Sample sizes for the low-, middle-, and high-altitude populations were 50, 23, and 61, respectively.
Table 1.
Life-history features of female Phrynocephalus vlangalii from the three populations along an altitudinal gradient of low (2,600 m), middle (3,400 m), and high (3,600 m) in the study area
| Variable | Altitudinal populations | Statistical analyses | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Initial maternal SVL (mm) | 54.35 ± 0.44b (50) | 56.66 ± 0.66a (23) | 55.57 ± 0.51ab (61) | F 2,131 = 3.67, P = 0.028 |
| Post-partum SVL (mm) | 54.74 ± 0.43b (50) | 57.42 ± 0.66a (23) | 56.03 ± 0.50ab (61) | F 2,131 = 5.00, P = 0.008 |
| Initial maternal BM (g) | 8.18 ± 0.19b (50) | 9.63 ± 0.38a (23) | 9.31 ± 0.24a (61) | F 2,131 = 8.75, P < 0.001 |
| Post-partum BM (g) | 5.39 ± 0.12b (50) | 6.18 ± 0.23a (23) | 5.85 ± 0.15a (61) | F 2,131 = 5.47, P = 0.005 |
| Litter size | 3.00 ± 0.14a (50) | 2.52 ± 0.19b (23) | 2.92 ± 0.11a (61) | F 2,130 = 5.64, P = 0.005 |
| Litter mass (g) | 2.36 ± 0.12 (50) | 2.41 ± 0.17 (23) | 2.47 ± 0.11 (61) | F 2,130 = 0.93, P = 0.398 |
| Relative litter mass | 0.42 ± 0.02 (50) | 0.45 ± 0.02 (23) | 0.40 ± 0.03 (61) | F 2,130 = 1.81, P = 0.167 |
| Neonate SVL (mm) | 24.41 ± 0.15c (74) | 25.70 ± 0.16a (45) | 25.13 ± 0.18b (55) | F 2,94.902 = 8.55, P < 0.001 |
| Neonate BM (g) | 0.79 ± 0.01c (74) | 0.95 ± 0.02a (45) | 0.88 ± 0.02b (55) | F 2,98.45 = 9.42, P < 0.001 |
| Offspring growth in SVL (mm/d) | 0.0067 ± 0.0005a (74) | 0.0041 ± 0.0004ab (45) | 0.0038 ± 0.0005b (55) | F 2,87.60 = 4.68, P = 0.012 |
| Offspring growth in BM (g/d) | 0.0153 ± 0.0011 (74) | 0.0110 ± 0.0011 (45) | 0.0131 ± 0.0010 (55) | F 2,86.84 = 2.54, P = 0.085 |
| Offspring sprint speed (m/s) | 0.63 ± 0.03 (66) | 0.34 ± 0.02 (36) | 0.42 ± 0.02 (56) | F 2,84.28 = 0.90, P = 0.409 |
Values are showed as mean ± 1SE, sample size is shown in parentheses. Comparisons among the 3 populations were performed using one-way ANOVA for maternal SVL (snout-vent length) and BM (body mass), relative litter mass, Permutation tests for litter size, and one-way ANCOVA for litter mass with maternal SVL as the covariate. Linear mixed models, with maternal identity as a random factor, were used to detect the inter-population differences in neonate SVL and neonate BM with maternal SVL as a covariate, offspring growth rates with initial SVL as a covariate, and offspring sprint speed with initial SVL and cloacal temperature as covariates. Mean values matching different variables with different superscript letters are statistically different (Tukey’s test).
Litter periods of the low-, middle-, and high-altitude populations of P. vlangalii lasted 29 d (from 30 July to 27 August), 25 d (from 28 August to 21 September), and 44 d (from 22 August to 4 October), respectively. There was a significant difference in litter time among 3 populations (F2,131 = 246.9, P < 0.001). Low-altitude females gave birth earlier than middle- and high-altitude females (Figure 3).
Figure 3.
Number of birthing events per day for pregnant females from each of the 3 populations of Phrynocephalus vlangalii along an altitudinal gradient of low (2,600 m), middle (3,400 m), and high (3,600 m) in the study area. Mean values with different letters after the names of the altitudinal populations in the legends are statistically significantly different (Tukey’s test).
After the influences of maternal size were statistically removed, the litter size differed significantly among the 3 populations of P. vlangalii (P < 0.01), with fewer litters produced by females from the middle-altitude population than in low- and high-altitude populations; in contrast, there was no difference among populations in litter mass (P = 0.398; Table 1). Consequently, the reproductive effort (measured as relative litter mass, RLM) also did not differ among the 3 altitudinal populations (P = 0.167; Table 1). After the influences of maternal identity and female body size were statistically removed, we discovered that neonate body size measured by both SVL and BM was largest for the middle-altitude population, and smallest for the low-altitude population, with the high-altitude population in between (Table 1). Therefore, females of P. vlangalii from the middle-altitude population gave birth to larger and fewer offspring than those from the low- and high-altitude populations. Additionally, females from the high-altitude population gave birth to larger offspring than those from the low-altitude population, although their litter size was not significantly fewer than that of the low-altitude population (Table 1).
Growth rate and locomotive performance of offspring
Initial body size of newborn lizards differed among the 3 altitudinal populations (Table 1). Their growth rates by body length (SVL) also significantly differed among the 3 populations (F2,87.60 = 4.68, P < 0.05), but there was no difference in growth rate by mass (BM) (F2,86.84 = 2.54, P = 0.085; Figure 4). The SVL growth rates of offspring in the low-altitude population were significantly faster than offpsring from the high-altitude population (Figure 4, Table 1). However, offpsring locomotive performance measured by sprint speed did not differ among the 3 populations (F2,84.28 = 0.90, P = 0.409, Figure 4, Table 1).
Figure 4.
Growth and locomotor performance of the offspring of Qinghai toad-headed lizards Phrynocephalus vlangalii, from the 3 populations along an altitudinal gradient of low (2,600 m), middle (3,400 m), and high (3,600 m) in the Dangjin Mountain of Gansu, China. Data are showed as means ± 1SE. Mean values with different letters above the frame are statistically significantly different (Tukey’s test). Sample sizes of offspring measured for body growth from the low-, middle-, and high-altitude populations were 74, 45, and 55, and for locomotor performance were 66, 36, and 56, respectively.
Discussion
Variation in maternal body size is common among ectotherm populations inhabiting different elevations, although the magnitude and pattern of these differences changes between species (Ashton and Feldman 2003; Pincheira-Donoso et al. 2008). High-altitude populations have larger body size than low-altitude ones in some species (Chown and Klok 2003; Zamora-Camacho et al. 2014a), while the opposite is also true in others (Mousseau 1997; Jin and Liu 2007; Jin et al. 2007; Bock et al. 2009; González-Morales et al. 2021b). Consistent with our prediction, our results indicated that adult female P. vlangalii had larger body size at the middle- and high- than low-altitude areas due to colder and harsher environments in the former (Figures 1 and 2). One possible interpretation for this pattern (i.e. larger body size in colder environments) is that females from high altitudes may be under selection to grow bigger to optimize fecundity (Charnov and Downhower 1995; Braña 1996; Cox et al. 2003). However, our data do not support this view. High- and middle-altitude females had larger body sizes than low-altitude females (Figure 2), but there was no significant difference in their reproductive investments (measured as RLM; Table 1). Another possibility is that the ratio of surface area to volume decreases with an increase in body weight, thus slowing heat exchange with the environment. Bergmann’s rule explains why large-scale patterns of change in body size develop as a result of environmental influences (Bergmann 1847). Altitudinal change in female body size among the 3 lizard populations in this study follows Bergmann’s rule, and this theory has also received support in ectotherms (Sacchi et al. 2007; Pincheira-Donoso et al. 2008; Zamora-Camacho et al. 2014a).
Temperature affects embryonic development. At least up to a certain point, embryonic growth is faster at high rather than low temperatures (Muth 1980; Shine 1983). We found that Qinghai toad-headed lizards inhabiting the low-altitude area gave birth earlier than those in the middle- and high-altitude areas (Figure 3). As a result of the lower ambient temperatures, embryos in high latitudes or altitudes grow at a slower rate than those at lower latitudes or altitudes (Mathies and Andrews 1995), and our results follow this rule. On the other hand, at lower altitudes, the date of emergence from hibernation is earlier for reptiles, and the time between emergence and ovulation can influence their egg-laying or parturition dates (Ji and Wang 2005; Lin et al. 2012). In this study, we also noticed that low-altitude lizards emerged from hibernation around mid-April, while high-altitude lizards emerged around mid-May, which is consistent with the above situation.
Life-history theory predicts that animals should optimally allocate limited resources between number and size of offspring (Stearns 1992; Roff 1993). With increasing environmental stress, females should produce fewer but larger offspring to improve their chances of surviving in harsh environments (Charnov and Downhower 1995; Liao et al. 2014; Ljungstrom et al. 2016). Our results showed a shift in the offspring size-number trade-off of P. vlangalii in response to colder and harsher environments, with lizards from the alpine steppe (i.e. the middle- and high-altitude habitats) producing fewer but larger offspring than those from the warm steppe (i.e. the low-altitude habitat) (Table 1). The observed variation in the trade-offs of offspring size-number among populations may be the result of female resource allocation influenced by environmental factors such as temperature, humidity and light intensity (Roff 2002). Middle- and high-altitude females thus produced larger offspring than low-altitude females to enhance the survival chances of offspring under higher environmental stress caused by lower temperatures and higher humidity (Figure 1 and Table 1). Furthermore, because of lower light intensity, middle-altitude females of P. vlangalii had higher environmental stress than high-altitude females, resulting in fewer but larger offspring (Figure 1 and Table 1).
Our results also showed that the fitness (measured by sprint speed) of P. vlangalii offspring did not differ among the 3 populations (Figure 4), indicating that all their offspring have the same good performance of adapting to local environments after the offspring size-number trade-off. The sprint speed of lizards is affected by biotic and abiotic factors, such as limb length, body color, temperature and so on (Zamora-Camacho et al. 2014b; González-Morales et al. 2021a). High-elevation lizards in a low thermal-quality habitat can achieve high optimum body temperatures through a number of thermoregulatory strategies, thus maximizing their speed performance (Zamora-Camacho et al. 2015). Whether the Qinghai toad-headed lizards at high altitude have similar thermoregulatory strategies to maximize their speed performance is worthy of further study.
Our study suggests that the growth of P. vlangalii was associated with temperature and light intensity, with the offspring in the low-altitude population having faster growth rate than that in the high-altitude population, with no difference between the middle-altitude population of the mountain’s north slope and the high-altitude population of the mountain’s south slope (Figures 1 and 4). The most important ecological factors that influence lizard growth are considered to be temperature and food quality (Iraeta et al. 2006). Due to thermal constraints on body temperatures, the length of time during which a lizard’s body temperature is adequate for activity should be positively associated with its growth. The growth rate of lizards can be improved by increasing the total time of lizards at the temperatures of supporting activities (Sinervo and Adolph 1994; Sears 2005). Higher ambient temperatures at the lower altitude areas may result in longer daily and seasonal activities of lizards, and therefore likely faster growth rates and longer growing periods (Sears 2005; Zamora-Camacho et al. 2013). In our study, high- and low-altitude juveniles of P. vlangalii follow this feature, but not for middle-altitude juveniles (Figures 1 and 4). Solar radiation increases with increasing elevation (Heinl et al. 2013). However, the light intensity at the middle-altitude region in this study was weaker than that at low- and high-altitude regions (Figure 1), and was caused by the slope aspect of the mountainous terrain. Thus, middle-altitude region experienced greater environmental stress due to lower temperature, higher humidity and lower light intensity. Although we did not explicitly investigate the availability of food resources in the 3 regions, we may assume from temperature and light intensity that primary productivity is lower in the middle-altitude region. Under directional selection, different environmental pressures may favor different genotypes (Irschick and Meyers 2007). Fast-growing genotypes are commonly discovered in those resource-poor environments causing slow growth (Blanckenhorn 1991; Arnett and Gotelli 1999; Jonassen et al. 2000; Ficetola and De Bernardi 2005). Temperate juveniles of the eastern water skink Eulamprus quoyii, for instance, grow quicker than tropical counterparts under same laboratory conditions (Caley and Schwarzkopf 2004). Therefore, middle-altitude lizards may have evolved fast-growing genotypes that resulted in faster growth rates in offspring under conditions of enough artificial food.
In the end, our results support the idea that the inter-population change in life-history features of P. vlangalii is an adaptive response to environmental stress. The environmental pressure gradually increases with the increase of altitude at a mountain, but its inverse gradient may also be affected by the slope aspect of mountain. Due to the difference of altitude and slope aspect, the middle-altitude region in this study experienced more severe environmental stress than the high- and low-altitude regions. Our results indicated that female P. vlangalii had larger body size at the middle- and high- than low-altitude areas due to colder and harsher environments. Our results also showed a shift in the offspring size-number trade-off of P. vlangalii in response to colder and harsher environments, with lizards from the alpine steppe producing fewer but larger offspring than those from the warm steppe. In addition, neonates in the middle-altitude regions with harsher environment had higher potential SVL growth rate. The findings of this study can contribute to enhancing our understanding of the altitudinal variation in life-history features of plateau ectotherms and their phenotypic plasticity or local adaptation.
Acknowledgments
We thank Weiguo DU for his advice regarding experimental design and manuscript preparation. We also thank Kun WANG for their assistance in the field.
Contributor Information
Wei Yu, College of Wildlife Resources, Northeast Forestry University, Harbin 150040, China; Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China.
Zeyu Zhu, College of Wildlife Resources, Northeast Forestry University, Harbin 150040, China; Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China.
Xiaolong Zhao, Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; The Key Laboratory of Zoological Systematics and Application, School of Life Science, Institute of Life Science and Green Development, Hebei University, Baoding 071002, Hebei, China.
Shuang Cui, College of Wildlife Resources, Northeast Forestry University, Harbin 150040, China.
Zhensheng Liu, College of Wildlife Resources, Northeast Forestry University, Harbin 150040, China; Key Laboratory of Conservation Biology, State Forestry Administration, Harbin 150040, China.
Zhigao Zeng, Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China.
Author Contributions
Z.G.Z. and Z.S.L. conceived and designed the studies. W.Y., Z.Y.Z., X.L.Z., and S.C. collected and analyzed data; W.Y. and Z.G.Z. wrote the manuscript, and all authors contributed to revisions.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050201) and the National Natural Science Fund of China (31861143023).
Conflict of Interest
All authors declare no conflict of interest.
Ethical Approval
The collection and handling of lizards in this study were approved by the Animal Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences (IOZ14001).
References
- Arnett AE, Gotelli NJ, 1999. Geographic variation in life-history traits of the ant lion Myrmeleon immaculatus: Evolutionary implications of Bergmann’s rule. Evolution 53(4):1180–1188. [DOI] [PubMed] [Google Scholar]
- Ashton KG, Feldman CR, 2003. Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57(5):1151–1163. [DOI] [PubMed] [Google Scholar]
- Bergmann C, 1847. Über die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Göttinger Studien 3:595–708. [Google Scholar]
- Berven KA, 1982. The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. an experimental analysis of life history traits. Evolution 36(5):962–983. [DOI] [PubMed] [Google Scholar]
- Blanckenhorn WU, 1991. Life-history differences in adjacent water strider populations: Phenotypic plasticity or heritable responses to stream temperature? Evolution 45(6):1520–1525. [DOI] [PubMed] [Google Scholar]
- Bock BC, Ortega AM, Zapata AM, Paez VP, 2009. Microgeographic body size variation in a high elevation Andean anole (Anolis mariarum; Squamata, Polychrotidae). Rev Biol Trop 57(4):1253–1262. [DOI] [PubMed] [Google Scholar]
- Braña F, 1996. Sexual dimorphism in lacertid lizards: Male head increase vs female abdomen increase? Oikos 75(3):511–523. [Google Scholar]
- Caley MJ, Schwarzkopf L, 2004. Complex growth rate evolution in a latitudinally widespread species. Evolution 58(4):862–869. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Downhower JF, 1995. A trade-off-invariant life-history rule for optimal offspring size. Nature 376(6539):418–419. [DOI] [PubMed] [Google Scholar]
- Chown SL, Klok CJ, 2003. Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography 26(4):445–455. [Google Scholar]
- Clobert J, Oppliger A, Sorci G, Ernande B, Swallow JGet al. , 2000. Trade-offs in phenotypic traits: Endurance at birth, growth, survival, predation and susceptibility to parasitism in a lizard, Lacerta vivipara. Funct Ecol 14(6):675–684. [Google Scholar]
- Cox RM, Skelly SL, John-Alder HB, 2003. A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 57(7):1653–1669. [DOI] [PubMed] [Google Scholar]
- Du WG, Warner DA, Langkilde T, Robbins TR, Shine R, 2012. The roles of pre- and post-hatching growth rates in generating a latitudinal cline of body size in the eastern fence lizard Sceloporus undulatus. Biol J Linn Soc 106(1):202–209. [Google Scholar]
- Dunham AE, 1978. Food availability as a proximate factor influencing individual growth rates in the iguanid lizard Sceloporus merriami. Ecology 59(4):770–778. [Google Scholar]
- Dupoué A, Lourdais O, 2014. Relative reproductive effort drives metabolic changes and maternal emaciation during pregnancy in a viviparous snake. J Zool 293(1):49–56. [Google Scholar]
- Einum S, Fleming IA, 1999. Maternal effects of egg size in brown trout Salmo trutta: Norms of reaction to environmental quality. Proc R Soc B-Biol Sci 266(1433):2095–2100. [Google Scholar]
- Ficetola GF, De Bernardi F, 2005. Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Anim Conserv 8(1):33–40. [Google Scholar]
- Fitch HS, 1985. Variation in clutch and litter size in new world reptiles. Univ Kansas Mus Nat Hist Misc Publ 76:1–76. [Google Scholar]
- Forsman A, Shine R, 1995. Parallel geographic variation in body shape and reproductive life history within the Australian scincid lizard Lampropholis delicata. Funct Ecol 9(6):818–828. [Google Scholar]
- González-Morales JC, Rivera-Rea J, Moreno-Rueda G, Bastiaans E, Castro-López Met al. , 2021a. Fast and dark: The case of Mezquite lizards at extreme altitude. J Therm Biol 102(1):103115. [DOI] [PubMed] [Google Scholar]
- González-Morales JC, Rivera-Rea J, Moreno-Rueda G, Bastiaans E, Díaz-Albiter Het al. , 2021b. To be small and dark is advantageous for gaining heat in mezquite lizards Sceloporus grammicus (Squamata: Phrynosomatidae). Biol J Linn Soc 132:93–103. [Google Scholar]
- Han LX, Wu ZL, He YH, 1999. Summer avifauna survey in Aksay Kazakzu Autonomous County Gansu Province. Territ Nat Resour Study (4):65–67. [Google Scholar]
- Hao X, Zou TT, Han XZ, Zhang FS, Du WG, 2021. Grow fast but don’t die young: Maternal effects mediate life-history trade-offs of lizards under climate warming. J Anim Ecol 96(6):1550–1559. [DOI] [PubMed] [Google Scholar]
- Heinl M, Leitinger G, Tappeiner U, 2013. Diurnal surface temperature regimes in mountain environments. Phys Geogr 33(4):344–359. [Google Scholar]
- Hoskins AJ, Hare KM, Miller KA, Schumann N, Chapple DG, 2017. Repeatability, locomotor performance and trade-offs between performance traits in two lizard species, Oligosoma alani and O. smithi. Biol J Linn Soc 122(4):850–859. [Google Scholar]
- Howard JH, Wallace RL, 1985. Life-history characteristics of populations of the long-toed salamander Ambystoma macrodactylum from different altitudes. Am Midl Nat 113(2):361–373. [Google Scholar]
- Hu YC, Lu HL, Cheng KM, Luo LG, Zeng ZG, 2019. Thermal dependence of feeding performance and resting metabolic expenditure in different altitudinal populations of toad-headed lizards. J Therm Biol 80:16–20. [DOI] [PubMed] [Google Scholar]
- Iraeta P, Monasterio C, Salvador A, Diaz JA, 2006. Mediterranean hatchling lizards grow faster at higher altitude: a reciprocal transplant experiment. Funct Ecol 20(5):865–872. [Google Scholar]
- Iraeta P, Salvador A, Diaz JA, 2008. A reciprocal transplant study of activity, body size and winter survivorship in juvenile lizards from two sites at different altitude. Ecoscience 15:298–304. [Google Scholar]
- Iraeta P, Salvador A, Diaz JA, 2013. Life-history traits of two Mediterranean lizard populations: A possible example of countergradient covariation. Oecologia 172(1):167–176. [DOI] [PubMed] [Google Scholar]
- Irschick DJ, Meyers JJ, 2007. An analysis of the relative roles of plasticity and natural selection in the morphology and performance of a lizard Urosaurus ornatus. Oecologia 153(2):489–499. [DOI] [PubMed] [Google Scholar]
- Ji X, Huang H, Hu X, Du W, 2002. Geographic variation in female reproductive characteristics and egg incubation of Eumeces chinensis. Ying Yong Sheng Tai Xue Bao 13(6):680–684. [PubMed] [Google Scholar]
- Ji X, Wang ZW, 2005. Geographic variation in reproductive traits and trade-offs between size and number of eggs of the Chinese cobra Caja atra. Biol J Linn Soc 85(1):27–40. [Google Scholar]
- Jin YT, Liu NF, 2007. Altitudinal variation in reproductive strategy of the toad-headed lizard Phrynocephalus vlangalii in North Tibet Plateau (Qinghai). Amphib Reptil 28(4):509–515. [Google Scholar]
- Jin YT, Liu NF, Li JL, 2007. Elevational variation in body size of Phrynocephalus vlangalii in the North Qinghai-Xizang (Tibetan) Plateau. Belg J Zool 137(2):197–202. [Google Scholar]
- Jonassen TM, Imsland AK, Fitzgerald R, Bonga SW, Ham EVet al. , 2000. Geographic variation in growth and food conversion efficiency of juvenile Atlantic halibut related to latitude. J Fish Biol 56(2):279–294. [Google Scholar]
- Kozlowska M, 1971. Differences in the reproductive biology of mountain and lowland common frogs Rana temporaria L. Acta Biol Cracov 14:17–32. [Google Scholar]
- Li JQ, Zhou R, Liu NF, 2014. Life-history variation among three populations of the toad-headed lizard Phrynocephalus vlangalii along an elevation gradient on the northeastern Tibetan Plateau. Herpetol J 24(1):17–23. [Google Scholar]
- Liao WB, Lu X, Jehle R, 2014. Altitudinal variation in maternal investment and trade-offs between egg size and clutch size in the Andrew’s toad. J Zool 293(2):84–91. [Google Scholar]
- Lin LH, Mao F, Chen C, Ji X, 2012. Reproductive traits of the gray ratsnake Ptyas korros from three geographically distinct populations. Curr Zool 58(6):820–827. [Google Scholar]
- Ljungstrom G, Stjernstedt M, Wapstra E, Olsson M, 2016. Selection and constraints on offspring size-number trade-offs in sand lizards Lacerta agilis. J Evol Biol 29(5):979–990. [DOI] [PubMed] [Google Scholar]
- Lorenzon P, Clobert J, Massot M, 2001. The contribution of phenotypic plasticity to adaptation in Lacerta vivipara. Evolution 55(2):392–404. [DOI] [PubMed] [Google Scholar]
- Lu HL, Xu CX, Jin YT, Hero JM, Du WG, 2018a. Proximate causes of altitudinal differences in body size in an agamid lizard. Ecol Evol 8(1):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HL, Xu CX, Zeng ZG, Du WG, 2018b. Environmental causes of between-population difference in growth rate of a high-altitude lizard. BMC Ecol 18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies T, Andrews RM, 1995. Thermal and reproductive biology of high and low elevation populations of the lizard Sceloporus scalaris: implications for the evolution of viviparity. Oecologia 104(1):101–111. [DOI] [PubMed] [Google Scholar]
- Morrison C, Hero JM, 2003. Geographic variation in life-history characteristics of amphibians: a review. J Anim Ecol 72(2):270–279. [Google Scholar]
- Mousseau TA, 1997. Ectotherms follow the converse to Bergmann’s rule. Evolution 51(2):630–632. [DOI] [PubMed] [Google Scholar]
- Muth A, 1980. Physiological ecology of desert iguana Dipsosaurus dorsalis eggs: Temperature and water relations. Ecology 61(6):1335–1343. [Google Scholar]
- Niewiarowski PH, 2001. Energy budgets, growth rates, and thermal constraints: toward an integrative approach to the study of life-history variation. Am Nat 157(4):421–433. [DOI] [PubMed] [Google Scholar]
- Olsson M, Shine R, Wapstra E, Uivari B, Madsen T, 2002. Sexual dimorphism in lizard body shape: the roles of sexual selection and fecundity selection. Evolution 56(7):1538–1542. [DOI] [PubMed] [Google Scholar]
- Pincheira-Donoso D, Hodgson DJ, Tregenza T, 2008. The evolution of body size under environmental gradients in ectotherms: Why should Bergmann’s rule apply to lizards? BMC Evol Biol 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasanen K, Soderman F, Laurila A, Merila J, 2008. Geographic variation in maternal investment: Acidity affects egg size and fecundity in Rana arvalis. Ecology 89(9):2553–2562. [DOI] [PubMed] [Google Scholar]
- Refsnider JM, Qian SS, Streby HM, Carter SE, Clifton ITet al. , 2018. Reciprocally transplanted lizards along an elevational gradient match light environment use of local lizards via phenotypic plasticity. Funct Ecol 32:1227–1236. [Google Scholar]
- Roff DA, 1993. The Evolution of Life Histories: Theory and Analysis. New York: Chapman and Hall. [Google Scholar]
- Roff DA, 2002. Life History Evolution. Sunderland: Sinauer Associates, Inc. [Google Scholar]
- Sacchi R, Pupin F, Pellitteri-Rosa D, Fasola M, 2007. Bergmann’s rule and the Italian Hermann’s tortoises Testudo hermanni: latitudinal variations of size and shape. Amphib Reptil 28(1):43–50. [Google Scholar]
- Sears MW, 2005. Geographic variation in the life history of the sagebrush lizard: The role of thermal constraints on activity. Oecologia 143(1):25–36. [DOI] [PubMed] [Google Scholar]
- Shine R, 1983. Reptilian viviparity in cold climates: Testing the assumptions of an evolutionary hypothesis. Oecologia 57(3):397–405. [DOI] [PubMed] [Google Scholar]
- Sievert LM, Hutchison VH, 1988. Light versus heat: Thermoregulatory behavior in a nocturnal lizard Gekko gecko. Herpetologica 44(3):266–273. [Google Scholar]
- Sievert LM, Hutchison VH, 1989. Influences of season, time of day, light and sex on the thermoregulatory behavior of Crotaphytus collaris. J Therm Biol 14(3):159–165. [Google Scholar]
- Sinervo B, Adolph SC, 1989. Thermal sensitivity of growth rate in hatchling Sceloporus lizards: Environmental, behavioral and genetic aspects. Oecologia 78(3):411–419. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Adolph SC, 1994. Growth plasticity and thermal opportunity in Sceloporus lizards. Ecology 75(3):776–790. [Google Scholar]
- Sorci G, Clobert J, Belichon S, 1996. Phenotypic plasticity of growth and survival in the common lizard Lacerta vivipara. J Anim Ecol 65(6):781–790. [Google Scholar]
- Stearns SC, 1992. The Evolution of Life Histories. Oxford: Oxford University Press. [Google Scholar]
- Tang XL, Yue F, Yan XF, Zhang DJ, Xin Yet al. , 2012. Effects of gestation temperature on offspring sex and maternal reproduction in a viviparous lizard Eremias multiocellata living at high altitude. J Therm Biol 37(6):438–444. [Google Scholar]
- Wake DB, Zhao EM, Adler K, 1994. Herpetology of China. Copeia 1994(4):1065. [Google Scholar]
- Wapstra E, Swain R, 2001. Geographic and annual variation in life-history traits in a temperate zone Australian skink. J Herpetol 35(2):194–203. [Google Scholar]
- Williams GC, 1965. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690. [Google Scholar]
- Zamora-Camacho FJ, Reguera S, Moreno-Rueda G, 2014a. Bergmann’s rule rules body size in an ectotherm: Heat conservation in a lizard along a 2200-metre elevational gradient. J Evol Biol 27(12):2820–2828. [DOI] [PubMed] [Google Scholar]
- Zamora-Camacho FJ, Reguera S, Moreno-Rueda G, Pleguezuelos JM, 2013. Patterns of seasonal activity in a Mediterranean lizard along a 2200m altitudinal gradient. J Therm Biol 38(2):64–69. [Google Scholar]
- Zamora-Camacho FJ, Reguera S, Rubiño-Hispán MV, Moreno-Rueda G, 2014b. Effects of limb length, body mass, gender, gravidity, and elevation on escape speed in the lizard Psammodromus algirus. J Evol Biol 41(4):509–517. [Google Scholar]
- Zamora-Camacho FJ, Rubiño-Hispán MV, Reguera S, Moreno-Rueda G, 2015. Thermal dependence of sprint performance in the lizard Psammodromus algirus along a 2200-meter elevational gradient: Cold-habitat lizards do not perform better at low temperatures. J Therm Biol 52:90–96. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Ji X, Luo LG, Gao JF, Zhang L, 2005. Sexual dimorphism and female reproduction in the Qinghai toad-headed lizard Phrynocephalus vlangalii. Acta Zool Sin 51(6):1006–1012. [Google Scholar]
- Zhao K, 1997. Toad-headed agamids in China. Chin J Zool 32:15–19. [Google Scholar]