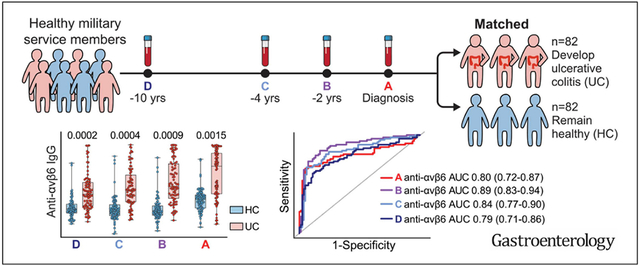
Abstract
BACKGROUND & AIMS:
Better biomarkers for prediction of ulcerative colitis (UC) development and prognostication are needed. Anti-integrin αvβ6 (anti-αvβ6) autoantibodies have been described in patients with UC. We tested for the presence of anti-αvβ6 antibodies in the preclinical phase of UC and studied their association with disease-related outcomes after diagnosis.
METHODS:
Anti-αvβ6 autoantibodies were measured in 4 longitudinal serum samples collected from 82 subjects who later developed UC and 82 matched controls from a Department of Defense preclinical cohort (PREDICTS [Proteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects]). In a distinct, external validation cohort (Crohn’s and Colitis Canada Genetic Environmental Microbial project cohort), we tested 12 pre-UC subjects and 49 matched controls. Furthermore, anti-αvβ6 autoantibodies were measured in 2 incident UC cohorts (COMPASS [Comprehensive Care for the Recently Diagnosed IBD Patients], n = 55 and OSCCAR [Ocean State Crohn’s and Colitis Area Registry], n = 104) and associations between anti-αvβ6 autoantibodies and UC-related outcomes were defined using Cox proportional hazards model.
RESULTS:
Anti-αvβ6 autoantibodies were significantly higher among individuals who developed UC compared with controls up to 10 years before diagnosis in PREDICTS. The anti-αvβ6 autoantibody seropositivity was 12.2% 10 years before diagnosis and increased to 52.4% at the time of diagnosis in subjects who developed UC compared with 2.7% in controls across the 4 time points. Anti-αvβ6 autoantibodies predicted UC development with an area under the curve of at least 0.8 up to 10 years before diagnosis. The presence of anti-αvβ6 autoantibodies in preclinical UC samples was validated in the GEM cohort. Finally, high anti-αvβ6 autoantibodies was associated with a composite of adverse UC outcomes, including hospitalization, disease extension, colectomy, systemic steroid use, and/or escalation to biologic therapy in recently diagnosed UC.
CONCLUSIONS:
Anti-integrin αvβ6 autoantibodies precede the clinical diagnosis of UC by up to 10 years and are associated with adverse UC-related outcomes.
Keywords: Inflammatory Bowel Disease, Ulcerative Colitis, Anti-Integrin αvβ6, Autoantibodies, Biomarkers
Graphical Abstract
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders that primarily affect the gastrointestinal tract with shared genetic and environmental risk factors.1-3 Analogous to other immune-mediated diseases, there is an increasing appreciation that IBD may be preceded by subclinical immune perturbations,4-6 and there is a growing interest in biomarkers that can predict the occurrence of IBD.7 In addition, identification of biomarkers to predict disease course is a major unmet need due to the considerable variability of IBD disease progression.7 In this context, noninvasive blood-based biomarkers are appealing because of their ease of collection and application in clinical practice. The identification of predictive biomarkers has been more successful in CD,4,5,8,9 with some being used to risk stratify patients in clinical trials.10 In contrast, discovery of predictive biomarkers in UC remains elusive.
Recent studies have highlighted a novel autoantibody against integrin αvβ6 in the serum of patients diagnosed with UC.11-13 In a study from Japan, anti-integrin αvβ6 (anti-αvβ6) autoantibodies had a sensitivity of 92.0% and a specificity of 94.8% for diagnosing UC in adult patients compared with non-IBD subjects.11 These results were further confirmed in a Swedish cohort and extended to a Japanese pediatric population.12,13 Integrin αvβ6 is an epithelium-associated heterodimer that interacts with the extracellular matrix14 and enables activation of latent transforming growth factor–β, which is thought to be its primary role in vivo.15,16 Accordingly, critical homeostatic roles, such as maintenance of epithelial barrier integrity and suppression of epithelial inflammation, have been ascribed to integrin αvβ6.17-19
Recognizing that loss of epithelial barrier integrity is a major and perhaps early feature of disease pathogenesis,20 we hypothesized that onset of UC may be preceded by the appearance of anti-αvβ6 autoantibodies and, thus, may serve as a preclinical biomarker. Furthermore, due to the important physiological role of integrin αvβ6, we explored the performance of anti-αvβ6 autoantibodies to predict adverse clinical outcomes in recently diagnosed UC.
Our study leverages 2 unique preclinical cohorts of UC. In our primary preclinical cohort— PREDICTS (Proteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects)—longitudinal serum samples were obtained up to 10 years before disease diagnosis. The Crohn’s and Colitis Canada Genetic Environmental Microbial (CCC-GEM) project served as an independent external validation preclinical cohort. Finally, we also studied 2 well-characterized incident IBD cohorts to better understand the association between anti-αvβ6 autoantibodies and UC-related disease outcomes.
Methods
Clinical Cohorts
Proteomic Evaluation and Discovery in an Inflammatory Bowel Disease Cohort of Tri-service Subjects cohort, primary preclinical cohort.
We studied longitudinal serum samples from 82 individuals who eventually developed UC and 82 healthy controls (HCs) matched by age, sex, and race from the PREDICTS cohort, which was created in collaboration with the Department of Defense Serum Repository.21 This study was approved by the Institutional Review Board of the Naval Medical Research Center, Silver Spring, MD (NMRC.2014.0019) in compliance with all federal regulations governing the protection of human volunteers, and this research was performed under a Cooperative Research and Development Agreement (NMR 17-10209). Incident cases of UC between 1998 and 2013 within the Defense Medical Surveillance System were identified by having 2 or more medical encounters with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)22 code for UC (556 [including all subgroup codes] and linked to the Department of Defense Serum Repository). For each subject, 4 serum samples were retrieved: 1 from the time of diagnosis (±1 year) (sample A) and 3 other samples preceding diagnosis (samples B, C, and D). HCs were required to have no medical encounter with evidence of IBD, rheumatoid arthritis, celiac disease, or colon cancer (based on ICD-9-CM codes) and available serum at the time of sample A for subjects with UC (±1 year). Controls were matched on the basis of age, sex, and race. Sample B was at a median of −2.01 years before diagnosis (sample A) for UC and −2.03 years (before sample A) for HC; sample C was at a median of −4.06 years for UC and −3.99 years for HC; and sample D, the earliest available sample, was at a median of −10.03 years for UC and −10.52 years for HC. Measurement of anti-integrin αvβ6 IgG autoantibodies and total IgG was performed with blinding to diagnosis and sample time point. From sample D, 1 serum sample from an HC subject was excluded due to a discrepancy in serum identification discovered after unblinding. ICD-9-CM codes, considering the code representing the highest disease extent, were used to determine the disease extent of the patients with UC according to the Montreal classification.4,9,23 Specifically, the extent of disease was defined by the following 3 subgroups: E1, proctitis only (disease limited to the rectum); E2, left-sided colitis (disease distal to the splenic flexure); and E3, extensive colitis (disease beyond the splenic flexure).23
Crohn’s and Colitis Canada Genetic Environmental Microbial project cohort, validation preclinical cohort.
We evaluated 61 sera samples that were collected as part of the prospective CCC-GEM Project. As described else-where,5,24 this is a prospective study that recruited asymptomatic first-degree relatives of patients with CD between 2008 and 2017. Subjects were between the age of 6 and 35 years at the time of recruitment. The study was approved by the Mount Sinai Hospital Research Ethics Board (Toronto Managing Center) and local recruitment centers. All subjects were contacted every 6 months via telephone and if a subject reported being diagnosed with UC, this was confirmed by their treating physician on the basis of clinical, endoscopic, radiographic, and/or histologic reports. The present study represents a nested case-control within the CCC-GEM, including 12 individuals who developed UC (cases) matched to at least 4 controls per subject by age, sex, geographic location (using postal and country codes), and follow-up duration. As with the PREDICTS cohort samples, all samples from the GEM cohort were blinded during sample testing.
Comprehensive Care for the Recently Diagnosed Inflammatory Bowel Disease Patients cohort.
The Comprehensive Care for the Recently Diagnosed IBD Patients (COMPASS) cohort is a prospective cohort of recently diagnosed patients with IBD who are enrolled in a registry at The Mount Sinai Hospital (NewYork,NY) and approved by the Institutional Review Board (STUDY-17-01304). All patients are enrolled within 18 months of diagnosis and disease diagnosis was confirmed on the basis of standard criteria.25 Baseline demographic and clinical variables were obtained via patient questionnaires and standardized data abstraction from the medical record, including age; sex; race and ethnicity; baseline medications; laboratory data, including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR); and disease extent (following the Montreal classification23). Outcomes were determined by means of chart review by a gastroenterologist to determine whether the patient had IBD-related hospitalization, disease extension (defined as E1 disease extending to either E2 or E3, or E2 disease extending to E3 disease), IBD-related surgery, systemic steroid use (defined as oral prednisone or intravenous steroid formulations), or required biologic therapy. Serum samples from the time of enrollment were available on 55 patients with UC and were used in this study. Non-IBD control subjects were defined as subjects without an IBD diagnosis who were seen at The Mount Sinai Hospital gastroenterology clinic for endoscopy and had serum or plasma samples available for testing.
Ocean State Crohn’s and Colitis Area Registry cohort.
The Ocean State Crohn’s and Colitis Area Registry (OSCCAR) is a community-based prospective inception cohort established in the state of Rhode Island (Sands Med Health Rhode Island), as described previously.26,27 Enrollment was between January 2008 and January 2018. Patients were included if they had a new diagnosis of IBD confirmed by means of endoscopic, pathologic, or radiographic findings according to the criteria of the National Institute of Diabetes and Digestive and Kidney Diseases IBD Genetics Consortium2 and were a resident of Rhode Island at the time of diagnosis. Patients were excluded if they had received a diagnosis of IBD previously or were unwilling to provide informed consent. Baseline and longitudinal data, including medication prescriptions, IBD-related hospitalizations, IBD-related surgery, and endoscopic disease extent (based on the Montreal classification23), were collected by means of annual structured interview, self-completion of validated questionnaires, and standardized central data abstraction from the medical record. In addition, select laboratory data were available, including CRP and ESR, as well as perinuclear anti-neutrophil cytoplasmic antibodies (pANCAs) at baseline. pANCA testing was performed at Prometheus Laboratories (San Diego, CA). In this cohort, there were 151 patients with UC. Two patients were eliminated because they did not have follow-up data and 1 additional patient was removed because diagnosis was more than 6 months from enrollment. Therefore, analysis was performed on the remaining 148 samples. Of these 148 subjects, 143 had pANCA data to analyze and 104 had serum available for measurement of anti-integrin αvβ6 IgG autoantibodies.
Enzyme-Linked Immunosorbent Assay for Measurement of Total IgG and Anti-Integrin αvβ6 IgG Autoantibodies
Total serum IgG was measured using Mabtech human IgG enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s protocol. For detection of IgG against integrin αvβ6, all serum samples were diluted to a concentration of 10 μg/mL IgG for subsequent testing for anti-integrin αvβ6 IgG, as detailed below. MaxiSorp immuno microtiter plates (Thermo Fisher Scientific) were coated with 100 μL per well of 1.5 μg/mL human recombinant integrin αvβ6 (R&D Systems) diluted in coating buffer from the ELISA Starter Accessory Kit (Bethyl Laboratories) and incubated at room temperature for 60 minutes. Next, 1 mM MgCl2 and CaCl2 were added to the incubation buffers to stabilize the αvβ6 integrin heterodimer, as described by Kuwada et al.11 The plates were subsequently washed with ELISA washing buffer and blocked with blocking buffer for 30 minutes at room temperature (all buffers were purchased from Bethyl Laboratories). The plates were then washed and incubated with 100 μL per well of samples prepared as described above and with mouse anti-human αvβ6 (Millipore, MAB2077Z) from 312.5 ng/mL to 1.22 ng/mL to produce the standard curve for 60 minutes at room temperature. Plates were then washed and incubated with secondary antibodies. For the human sera samples, anti-human IgG secondary conjugated to horseradish peroxidase (Invitrogen, 31410) diluted 1:4000 was used, and for the standards, antimouse IgG secondary conjugated to horseradish peroxidase (Invitrogen, 62-6520) diluted 1:2000 was used. Plates were washed and then developed by means of incubating with 100 μL per well of 3,3′,5,5′-tetramethylbenzidine for 4 minutes, at which time the reaction was stopped with 100 μL per well of 0.18 M H2SO4 and immediately read on the POLARstar Omega plate reader (BMG LABTECH).
In a subset of patients with established UC (n = 6) and HCs (n = 2), plasma samples were assayed for anti-integrin αvβ6 IgG at a 1:200 starting dilution and 7 additional 3-fold serial dilutions from which the area under the curve (AUC) was calculated. In addition, we measured anti-αvβ6 IgG as described above with samples diluted to a concentration of 10 μg/mL IgG (IgG normalized measurement). The IgG normalized measurement strongly correlated with the AUC from the dilution curves (Pearson correlation r = 0.97, P < .0001) (Supplementary Figure 1A and B).
Statistical Analysis
Descriptive statistics were performed in GraphPad Prism, version 9.3.0. The anti-αvβ6 IgG was treated as a continuous variable and binary variable. As a binary variable the cutoff for positivity was defined as greater than the mean HC plus 3 SD.11 For the univariate analyses of continuous variables, we used the Mann-Whitney test (for 2 group analyses) or Kruskal-Wallis with Dunn’s multiple comparisons test (for >2 group analyses). For categorical comparisons, Fisher exact test or χ2 test were used. For comparing the anti-αvβ6 IgG OD450 between pre-UC and matched HC (or asymptomatic) subjects in the PREDICTS cohort and the GEM cohort, we performed conditional logistic regressions using the R function clogit from the survival package.28 Linear regression using glm function in R was used to model anti-αvβ6 as a function of the disease extent in the PREDICTS cohort.29 Spearman correlations were used to determine strength and direction of the associations between 2 variables.
In order to assess the predictive performance of anti-αvβ6 IgG in the PREDICTS cohort, 10-fold cross-validation was performed. For each fold, the model was trained on the remaining 9 sets of samples and the predictive performance of the estimated model was applied to predict the outcome of samples in the validation set. For this analysis, UC status was modeled via logistic regression as a function of anti-αvβ6. Predictive performance was evaluated on the basis of receiver operating characteristic curve (ROC) and AUC. Logistic regressions were estimated using the R function glm.29 The 95% CIs of AUC were evaluated on the basis of 10,000 bootstrap iterations using function ci.auc from the pROC package30 available in R.
For the COMPASS and OSCCAR cohort analyses, multivariable regressions was performed using the R function glm29 and ROC curves created in R using the pROC package30 and graphed in GraphPad Prism. The 95% CIs of AUC were evaluated as described above, with 10,000 bootstrap iterations. Cox proportional hazards models were used to define associations between anti-αvβ6 and UC-related outcomes and visualized using Kaplan-Meier curves. For these analyses, we used a composite of disease-related adverse outcomes, including the need for biologic therapy, disease extension, systemic steroid use, UC hospitalization, and/or surgery. In the COMPASS model, we used age and extensive disease (E3) at baseline as covariates. In the OSCCAR model, we included the clinical risk factors for complicated disease course, including age younger than 40 years at diagnosis, extensive disease (E3), ESR/CRP elevation, systemic steroid use, focal ulcers, and history of UC-related hospitalization.31
Results
Anti-Integrin αvβ6 Autoantibodies Are Detected up to 10 Years Before Diagnosis of Ulcerative Colitis
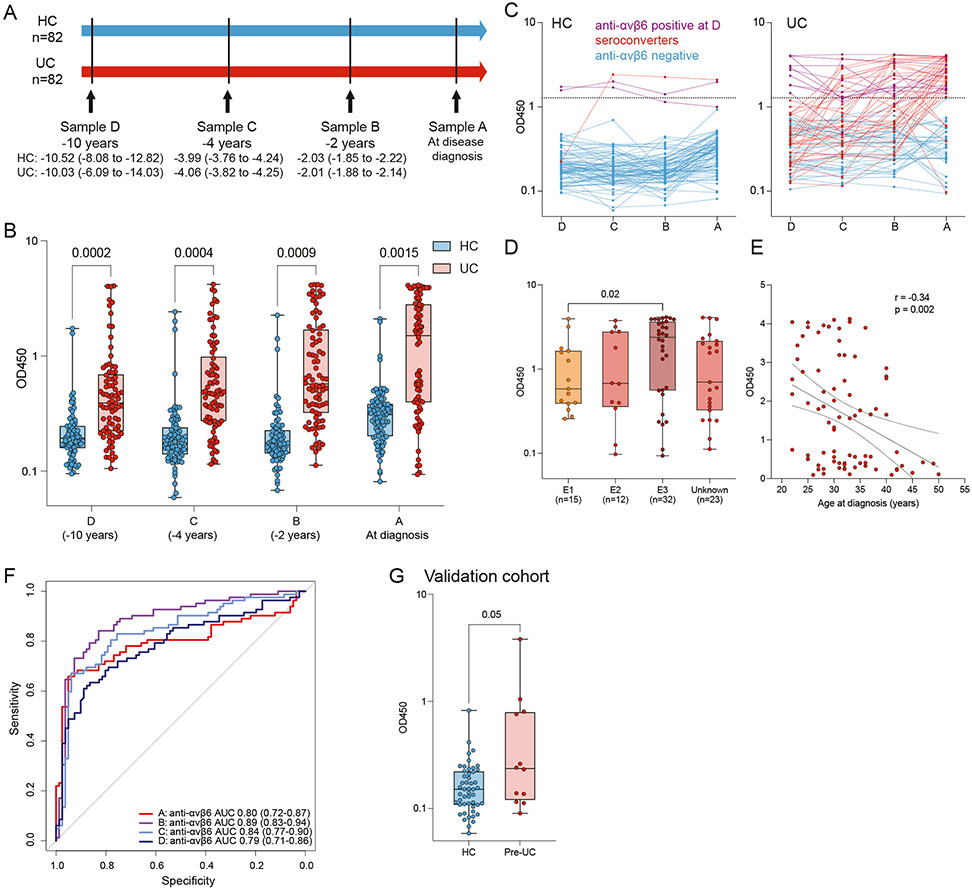
We hypothesized that anti-αvβ6 autoantibodies would predate UC diagnosis and analyzed longitudinal samples predating UC diagnosis by up to 10 years in 82 subjects who developed UC matched by age, sex, and race with 82 subjects who did not develop IBD (HCs) (Figure 1A and Table 1). Anti-αvβ6 levels were significantly higher in sera from patients who developed UC compared with the controls at all time points tested (P < .001, samples A–C; P = .0015, sample D) (Supplementary Table 1, Figure 1B and C). During the preclinical phase (sample D–A), anti-αvβ6 seropositive subjects increased from 12.2% (sample D) to 20.7% (sample C) to 30.5% (sample B) to 52.4% (sample A) of subjects who developed UC (χ2 test for trend P < .0001), compared with a mean of 2.7% in the HC group across the 4 time points (Figure 1C).
Figure 1.
Anti-αvβ6 autoantibodies in preclinical subjects with UC. (A) Timeline of sample collection in the PREDICTS cohort from 82 subjects who developed UC and 82 matched controls (HC). Sample A was collected at the time of diagnosis for subjects with UC and samples B, C, and D were collected before diagnosis. Median (interquartile range [IQR]) time in years for samples A, B, C, and D are detailed for both UC and HC. (B) Anti-αvβ6 autoantibody absorbance values (OD450) determined by means of ELISA in UC (n = 82, shown in red) and HC (n = 82, shown in blue) sera obtained before diagnosis (samples B, C, and D) and at the time of diagnosis (sample A) from the PREDICTS cohort. In sample D, 1 HC sample was excluded due to an identification issue. For all boxplots, the box represents the IQR, the center line represents the median, and the whiskers indicate the minimum to maximum value. Statistical significance determined by means of conditional logistic regressions at each time point comparing subjects who developed UC with matched controls. (C) Dynamics of anti-αvβ6 in each HC or UC subject across the 4 longitudinal samples in the PREDICTS cohort. Purple lines represent samples that are positive for anti-αvβ6 in sample D, red lines indicate samples that seroconvert between sample D and A, and blue lines indicate subjects that are negative at all sample time points. (D) Anti-αvβ6 autoantibody levels as a function of UC disease extent as defined by Montreal classification (E1: proctitis only, n = 15; E2: left-sided disease, n = 12; E3: extensive disease, n = 32; unknown, n = 23) and (E) by age at diagnosis, shown as scatterplot in the PREDICTS cohort. Statistical significance determined by means of linear regression and Spearman correlation, respectively. (F) Predictive performance of anti-αvβ6 autoantibodies based on 10-fold cross-validation and the 95% CI of the AUC based on 10,000 bootstrap iterations in the PREDICTS cohort. Sample A (time of diagnosis) is in red, sample B (−2 years) is in purple, sample C (−4 years) is in light blue, and sample D (−10 years) is in dark blue. (G) Anti-αvβ6 autoantibody absorbance values (OD450) determined by means of ELISA in 12 pre-UC subjects and 49 matched control subjects from the GEM cohort. Statistical significance determined by conditional logistic regression comparing pre-UC subjects with matched control subjects.
Table 1.
Demographic and Clinical Characteristics of PREDICTS Cohort
| Characteristic | HCs | UC |
|---|---|---|
| No. of individuals | 82 | 82 |
| No. of samples | 327a | 328 |
| Age at diagnosis or sample A, y, mean ± SD | 32.6 ± 5.6 | 32.0 ± 6.4 |
| Male/female, n | 79/3 | 79/3 |
| Race, n | ||
| White | 62 | 62 |
| Black | 18 | 18 |
| Other | 2 | 2 |
| Disease extent at diagnosis, n | ||
| E1: proctitis | NA | 15 |
| E2: left-sided colitis | NA | 12 |
| E3: extensive | NA | 32 |
| Unknown | NA | 23 |
NA, not applicable.
Sample D from 1 HC subject excluded due to an identification discrepancy.
Next, we examined disease-related factors that were associated with anti-αvβ6 autoantibodies (in sample A, at diagnosis), and determined that anti-αvβ6 autoantibodies were associated with extensive (E3) disease (odds ratio [OR], 2.76; 95% CI, 1.21–6.33; Figure 1D) and inversely associated with age of diagnosis (Spearman correlation r = −0.34, P = .002; Figure 1E).
After identifying the presence of anti-αvβ6 autoantibodies in UC samples before diagnosis, we sought to determine the performance of this autoantibody as a predictive biomarker. The predictive performance of anti-αvβ6 autoantibodies was assessed via ROC curves and AUC based on 10-fold cross-validation. The AUC was 0.80 at the time of diagnosis (sample A). Notably, the AUC remained high in all 3 prediagnostic samples, with AUC of 0.89, 0.84, and 0.79 at samples B, C, and D, respectively (Figure 1F and Table 2). Furthermore, the lower bound of the 95% CI of AUCs calculated via bootstrapping was >0.71 for samples A, B, C, and D; confirming the excellent predictive performance of anti-αvβ6 autoantibodies in all prediagnostic groups of samples (Figure 1F and Table 2).
Table 2.
Predictive Model for UC Development in PREDICTS Samples
| 95% CIa |
|||
|---|---|---|---|
| Sample | AUCa | Lower | Upper |
| A: anti-αvβ6 | 0.80 | 0.72 | 0.87 |
| B: anti-αvβ6 | 0.89 | 0.83 | 0.94 |
| C: anti-αvβ6 | 0.84 | 0.77 | 0.90 |
| D: anti-αvβ6 | 0.79 | 0.71 | 0.86 |
Based on 10-fold cross-validation.
To confirm our findings in a second independent cohort, we tested sera collected as part of the CCC-GEM Project. In this cohort of more than 5000 first-degree relatives of patients with CD, 12 subjects had developed UC since recruitment (median time from recruitment to diagnosis was 4.2 years [range, 0.4–8.5 years]). We tested these pre-UC samples and compared them with 49 matched subjects who remained asymptomatic (HCs) since recruitment (Table 3). The pre-UC samples had higher anti-αvβ6 autoantibody levels compared with controls (conditional logistic regression P = .05) (Figure 1G). In the pre-UC group, 4 of the 12 subjects (33%) were positive for anti-αvβ6 autoantibodies (defined as mean + 3 SD of asymptomatic subjects from the GEM cohort) and 1 of the 49 controls (2%) were anti-αvβ6 autoantibody–positive (Fisher exact test, P = .004).
Table 3.
Demographic and Clinical Characteristics of the CCC-GEM Cohort
| Characteristic | Subjects that remained asymptomatic (HC) |
Subjects that developed UC (pre-UC) |
|---|---|---|
| No. of individuals | 49 | 12 |
| Age at recruitment, y, mean ± SD | 17.7 ± 7.1 | 17.3 ± 7.5 |
| Male/female, n | 24/25 | 6/6 |
| Country recruitment, n | ||
| Canada | 40 | 10 |
| Israel | 5 | 1 |
| United States | 4 | 1 |
| Relation to proband, n | ||
| Offspring | 23 | 1 |
| Sibling | 26 | 11 |
| Time from recruitment to diagnosis, y, mean (range) | NA | 4.2 (0.4–8.5) |
NA, not applicable.
Anti-αvβ6 Autoantibodies Are Associated With Adverse Disease-Related Outcomes
Due to the physiological roles ascribed to αvβ6 autoantibodies in mucosal homeostasis, we hypothesized that anti-αvβ6 autoantibodies would be associated with adverse clinical outcomes. To test this hypothesis, we studied anti-αvβ6 autoantibodies in 2 inception cohorts of patients with UC—COMPASS (n = 55) and OSCCAR (n = 104)—and compared them with non-IBD controls (non-IBD) (n = 54) (Table 4). With the exception of the patients with UC being younger than controls (P < .0001, COMPASS; P = .0011, OSCCAR), cases and controls shared similar characteristics. Ninety-one percent of COMPASS patients and 96% of OSCCAR patients were naïve to any biologic therapies.
Table 4.
Demographic Characteristics of 2 UC Inception Cohorts and Non-IBD Controls
| Characteristic | Non-IBD controls |
COMPASS UC |
OSCCAR UC |
|---|---|---|---|
| No. of individuals | 54 | 55 | 104 |
| No. of samples | 54 | 55 | 104 |
| Age at diagnosis, y, mean ± SD | 46.3 ± 15.9 | 31.3 ± 12.4 | 36.8 ± 20.1 |
| Male/female, n | 31/23 | 28/27 | 42/62 |
| Race, n | |||
| White | 19 | 35 | 92 |
| Black | 14 | 4 | 4 |
| Asian | 3 | 5 | 0 |
| Other | 18 | 11 | 8 |
| Disease extent at diagnosis, n | |||
| E1: proctitis | NA | 7 | 27 |
| E2: left-sided colitis | NA | 24 | 66 |
| E3: extensive | NA | 24 | 44 |
| Mayo Endoscopic score, n | |||
| 0 | NA | 5 | NA |
| 1 | NA | 15 | NA |
| 2 | NA | 16 | NA |
| 3 | NA | 14 | NA |
| Unknown | NA | 5 | 104 |
| Biologic-naïve (at enrollment/blood draw), n (%) | 54 (100) | 50 (90.9) | 100 (96.2) |
NA, not applicable or not available.
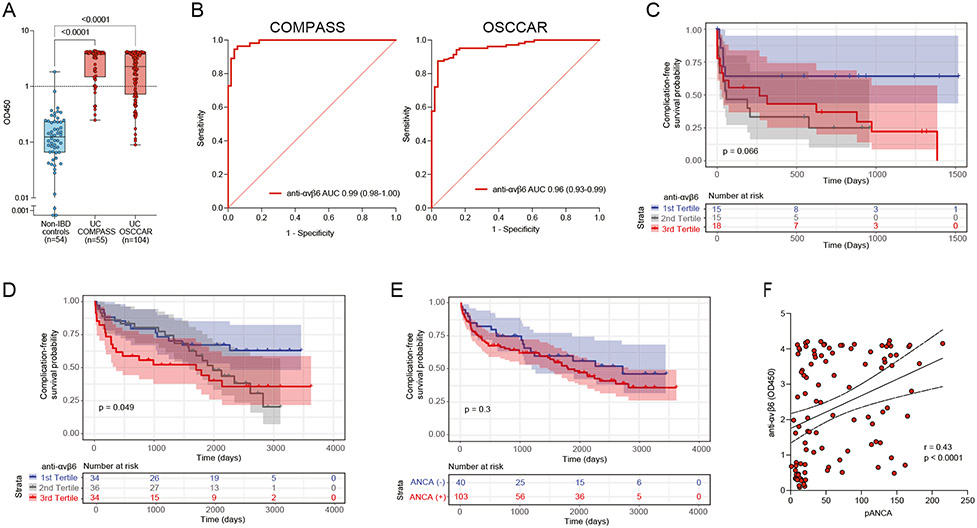
Anti-αvβ6 autoantibody levels were significantly higher in both the COMPASS and OSCCAR patients with UC compared with non-IBD controls (P < .0001) (Figure 2A), which was consistent with published data.11,13 ROC analysis revealed an AUC of 0.99 (95% CI, 0.97–1.00) for COMPASS and AUC of 0.96 (95% CI, 0.93–0.98) for OSCCAR (Figure 2B). With a cutoff of 0.99 (mean non-IBD + 3 SD), the sensitivity of anti-αvβ6 autoantibodies for distinguishing UC from non-IBD controls was 85.5% and 70.2% for the COMPASS cohort and for the OSCCAR cohort, respectively, with specificity of 98.1%. Anti-αvβ6 remained significantly associated with UC diagnosis in a multivariable model, including age, sex, and race (OR, 64.05; 95% CI, 7.41–553.74; P = .0002 for COMPASS and OR, 156.29; 95% CI, 12.53–1949.04; P = .0001 for OSCCAR) (Supplementary Tables 2 and 3).
Figure 2.
Anti-αvβ6 autoantibodies in patients with newly diagnosed UC and their association with adverse disease-related outcomes. (A) Anti-αvβ6 autoantibody absorbance values (OD450) determined by means of ELISA in non-IBD controls (n = 54, shown in blue), in COMPASS patients with UC (n = 55, shown in red), and in OSCCAR patients with UC (n = 104, shown in red). (B) ROC analysis of anti-αvβ6 autoantibodies in both COMPASS patients with UC (left) and OSCCAR patients with UC (right) compared with the non-IBD controls. (C, D) Kaplan-Meier curve for composite outcome of IBD hospitalization, proximal disease extension, need for surgery, systemic steroid use, and/or requiring new biologic therapy in the COMPASS cohort (C) and OSCCAR cohort (D) stratified by anti-αvβ6 titer tertiles. The blue line represents the first tertile (lowest), the gray line represents the second tertile, and the red line the third (highest) tertile. (E) Kaplan-Meier curve for the same composite outcomes in the OSCCAR cohort stratified according to the presence or absence of pANCA. (F) Spearman correlation between pANCA and anti-αvβ6 titers.
Next, we examined whether anti-αvβ6 autoantibodies were associated with any UC-related adverse outcomes representing more complicated disease, defined as a composite of need for biologic therapy, disease extension, systemic steroid use, IBD-related hospitalization, and/or surgery. In the COMPASS cohort, we found that anti-αvβ6 autoantibodies were significantly associated with the composite of the above disease-related outcomes (hazard ratio [HR], 1.39; 95% CI, 1.03–1.89, with inclusion of disease extent–adjusted HR, 1.35; 95% CI, 0.99–1.85) (Figure 2C, Supplementary Table 4). In the OSCCAR cohort, we confirmed these findings and again found that subjects with higher autoantibody levels were more likely to have a more complicated course, as defined by the same composite outcome even when correcting for baseline clinical risk factors for complications, including age younger than 40 years at diagnosis, extensive disease (E3), elevated CRP or ESR, use of systemic steroids or hospitalization at diagnosis, and ulcers on baseline endoscopy31 (adjusted HR, 1.24; 95% CI, 1.01–1.53) (Figure 2D, Supplementary Table 5). In contrast, pANCA, an established UC-associated biomarker,32,33 was not associated with disease outcomes (HR, 0.75; 95% CI, 0.36–1.54) (Figure 2E, Supplementary Table 6); however, we did note a moderate positive correlation between anti-αvβ6 and pANCA (r = 0.43, P < .0001) (Figure 2F).
Discussion
We identified anti-αvβ6 autoantibodies as a new preclinical biomarker that precedes the diagnosis of UC by up to 10 years. Furthermore, we provide evidence in support of anti-αvβ6 autoantibodies as a prognostic biomarker associated with adverse UC-related outcomes in 2 well-characterized incident cohorts of patients recently diagnosed with IBD.
Prior work had shown that anti-αvβ6 autoantibodies were present in 92% of patients with established UC and had a high sensitivity and specificity for the diagnosis of UC.11 The present study reported that anti-αvβ6 autoantibodies are associated with the preclinical phase of UC. Importantly, these data were replicated in an independent external validation cohort.
The predictive performance of anti-αvβ6 autoantibodies as assessed via AUROC with 10-fold cross-validation was at least 0.8 at all time points and remained predictive even up to 10 years before diagnosis. Furthermore, the number of seropositive patients significantly increased before the development of clinically overt disease. The increasing prevalence of anti-αvβ6 autoantibodies with time is in contrast to other autoantibodies, such as anti-Saccharomyces cerevisiae antibodies and pANCA, which remained relatively stable before diagnosis.4 The predictive performance of anti-αvβ6 autoantibodies is superior to that of pANCA, an established UC-associated diagnostic biomarker.4 Specifically, pANCA was found to be positive in a small subset of preclinical subjects with UC (2 of 8 [25%] in Israeli et al),34 but had poor predictive performance, with AUCs in the range of 0.59–0.61.4 The combination of antimicrobial antibodies and pANCAs also had a low predictive performance for UC, with AUCs ranging from 0.57 at 5 years to 0.61 at 1 year before diagnosis in a prior study using samples derived from the PREDICTS cohort. In the same study, 1129 proteins were measured using the SomaLogic platform and predictive performances using select disease-associated markers also were suboptimal, with AUCs ranging from 0.49 at 5 years before diagnosis to 0.68 at diagnosis.4 In the EPIC (European Prospective Investigation into Cancer and Nutrition) study, which included a single prediagnostic sample from 54 subjects who developed UC a mean of 4.4 years after collection, a combination of antimicrobial antibodies (including pANCA, anti-CBir1, anti-OmpC, and as anti-Saccharomyces cerevisiae antibody IgA) had an AUC <0.70.35 In addition, in the Nurses’ Health Study cohort, subjects with the highest quintile of serum interleukin-6 and high-sensitivity CRP were more likely to develop UC (OR, 3.43; 95% CI, 1.44–8.15 and OR, 1.79; 95% CI, 0.80–3.99, respectively); however, these associations were only present at the highest quintiles.36 Finally, in a population cohort from Sweden, 6 proteins (MMP10, CXCL9, CCL11, SLAMF1, CXCL11, and MCP-1) were increased in patients who later developed UC compared with those who remained healthy, with a high predictive performance (AUC 0.92); however, the predictive performance of these proteins was assessed in an incident cohort rather than in a preclinical cohort.6 Thus, to date, anti-αvβ6 autoantibodies have at least as high (or higher) a predictive performance as all existing UC-associated biomarkers and may accurately identify patients at risk for developing UC. The presence of anti-αvβ6 autoantibodies before UC diagnosis further suggests that a preclinical phase, potentially amenable to therapeutic intervention, may indeed predate UC diagnosis.
Next, we examined anti-αvβ6 autoantibodies in 2 well-characterized cohorts of recently diagnosed IBD, COMPASS, and OSCCAR, and found a significant association of this autoantibody with adverse UC-related outcomes that included disease extension, escalation to biologic therapy, need for systemic steroids, and UC-related surgery, and/or hospitalization. Consistent with prior data,37,38 pANCA was not associated with these adverse clinical outcomes. Another potentially promising noninvasive biomarker is a panel of serum proteins that was associated with the need for treatment escalation in both UC and CD.39 This serum protein panel is now being investigated in the Nordic IBD Treatment Strategy Trial (NORDTREAT), which includes patients with UC as well as CD. Notably, the 5-protein model with the highest predictive accuracy included ITGAV and EpCAM.39 Interestingly, EpCAM is an epithelial cell-associated adhesion molecule40 and ITGAV is the integrin subunit αV, which is part of the αvβ6 integrin heterodimer that is targeted by anti-αvβ6 autoantibodies.11,14 ITGAV has also been identified as an IBD risk allele in genome-wide association studies.41 Altogether, after confirmation in larger data sets, anti-αvβ6 can potentially serve to risk-stratify patients with UC to identify those who may benefit from earlier introduction of targeted immunosuppressive therapy and can be studied in a “biomarker-stratified” trial similar to the PROFILE (Predicting Outcomes for Crohn’s Disease Using a Molecular Biomarker) trial in CD.10
Considering the strong predictive value of anti-αvβ6 autoantibodies for UC, understanding the pathophysiological role of these autoantibodies could shed light on some of the early pathophysiological events associated with this disease. For example, we recently identified colonic plasma cells specific to integrin αvβ6 in a patient with UC,42 providing evidence that these autoantibodies originate from intestinal plasma cells. The αvβ6 integrin expression is low in the adult intestinal epithelium, but increases dramatically in the setting of epithelial injury, which possibly leads to increased exposure of this autoantigen.16,43,44 In addition, in vitro studies from Kuwada et al11 found that anti-αvβ6 autoantibodies inhibit binding of integrin αvβ6 to fibronectin and, therefore, may disrupt homeostatic epithelial–stromal interactions, culminating in impaired barrier integrity.19 Other potential mechanisms of action of anti-αvβ6 may be mediated through blockade of the activation of transforming growth factor–β, resulting in increased inflammation15 and antibody-dependent cellular cytotoxicity, an important mechanism of action of autoantibodies in other autoimmune conditions, such as vitiligo.45
Our study has several strengths, including the detection of anti-αvβ6 in 2 independent preclinical UC cohorts. In our primary preclinical cohort, PREDICTS, multiple longitudinal prediagnostic samples allowed us to study the evolution of anti-αvβ6 autoantibodies over time. In addition, we have provided evidence of anti-αvβ6 autoantibodies as a potential prognostic biomarker using 2 independent incident cohorts. It is possible that subjects had unrecognized symptoms of UC before the clinical diagnosis in the PREDICTS cohort. However, we believe this is limited, given the routine medical evaluations and physical rigor required of active component military populations. Furthermore, this potential limitation does not apply to samples collected up to 10 years before diagnosis and subjects in our preclinical validation cohort (GEM), who were required to be asymptomatic at enrollment. Finally, we were unable to perform functional studies due to limited sample availability.
In conclusion, anti-αvβ6 autoantibodies are a novel biomarker associated with the preclinical phase of UC and are a prognostic biomarker associated with development of complicated disease in patients recently diagnosed with UC. These findings further highlight potential key early events in the development of UC, providing opportunities for diagnostic and therapeutic interventions.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Biomarkers for predicting the development of ulcerative colitis (UC) and disease course are currently limited.
NEW FINDINGS
In samples derived from 2 independent cohorts, we found autoantibodies directed against integrin αvβ6 among individuals who go on to develop UC, which were not present in healthy controls. In addition, in 2 independent incident cohorts, we found an association between anti-integrin αvβ6 autoantibodies and disease-related adverse outcomes.
LIMITATIONS
Subjects enrolled in the preclinical PREDICTS cohort may have had symptoms before the clinical diagnosis of UC. However, we believe this is limited, given the routine medical evaluations and physical rigor required of active military personnel and the disturbing character of UC manifestations. Furthermore, this limitation would not apply to samples collected up to 10 years before diagnosis and in the preclinical validation cohort (GEM), as subjects were required to be asymptomatic at enrollment.
CLINICAL RESEARCH RELEVANCE
In patients with UC, anti-integrin αvβ6 autoantibodies are a novel biomarker associated with disease development and disease-related adverse outcomes.
BASIC RESEARCH RELEVANCE
Given the presence of anti-integrin αvβ6 autoantibodies before diagnosis and their physiologic role in intestinal epithelial homeostasis, these autoantibodies may play a role in disease pathogenesis, which will require more detailed functional studies to further elaborate.
Acknowledgments
The authors acknowledge the contributions of the members of the CCC-GEM (Crohn’s and Colitis Canada Genetic Environmental Microbial) project Research Consortium, as well as the OSCCAR (Ocean State Crohn’s and Colitis Area Registry) Consortium. The CCC-GEM Project Research Consortium is composed of Maria Abreu, Paul Beck, Charles Bernstein, Kenneth Croitoru, Leo Dieleman, Brian Feagan, Anne Griffiths, David Guttman, Kevan Jacobson, Gilaad Kaplan, Denis O. Krause (deceased), Karen Madsen, John Marshall, Paul Moayyedi, Mark Ropeleski, Ernest Seidman (deceased), Mark Silverberg, Scott Snapper, Andy Stadnyk, Hillary Steinhart, Michael Surette, Dan Turner, Thomas Walters, Bruce Vallance, Guy Aumais, Alain Bitton, Maria Cino, Jeff Critch, Lee Denson, Colette Deslandres, Wael El-Matary, Hans Herfarth, Peter Higgins, Hien Huynh, Jeff Hyams, David Mack, Jerry McGrath, Anthony Otley, and Remo Panancionne. The CCC-GEM Project recruitment site directors include Maria Abreu, Guy Aumais, Robert Baldassano, Charles Bernstein, Maria Cino, Lee Denson, Colette Deslandres, Wael El-Matary, Anne M. Griffiths, Charlotte Hedin, Hans Herfarth, Peter Higgins, Seamus Hussey, Hien Hyams, Kevan Jacobson, David Keljo, David Kevans, Charlie Lees, David Mack, John Marshall, Jerry McGrath, Sanjay Murthy, Anthony Otley, Remo Panaccione, Nimisha Parekh, Sophie Plamondon, Graham Radford-Smith, Mark Ropeleski, Joel Rosh, David Rubin, Michael Schultz, Ernest Seidman (deceased), Corey Siegel, Scott Snapper, Hillary Steinhart, and Dan Turner. The OSCCAR Consortium includes Jason Shapiro, Samir Shah, and Neal S. Leleiko. We also acknowledge the contribution of Jill Gregory for the graphical abstract.
Funding
This work was supported by the following grants: anonymous donor (to Saurabh Mehandru and Jean-Frederic Colombel), National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 123749 (to Saurabh Mehandru). Alexandra Livanos was supported by a philanthropic donation from the Segal family. In addition, Francesca Petralia and Jean-Frederic Colombel were supported by the Kenneth-Rainin Foundation (grant 20210021), Alexandra Dunn was supported by the Digestive Disease Research Foundation, Manasi Agrawal was supported by the NIDDK (K23DK129762-01), Ryan C. Ungaro was supported by NIH K23 Career Development Award K23KD111995-01A1, and Sacha Gnjatic was supported by NIH grants CA224319, DK124165, CA196521, and by a Helmsley Charitable Trust award. This study was also supported by grants from Crohn’s and Colitis Canada grant CCC-GEMIII, Canadian Institutes of Health Research (CIHR) grant CMF108031, and the Helmsley Charitable Trust. Williams Turpin is a former recipient of a Postdoctoral Fellowship Research Award from the CIHR Fellowship/Canadian Association of Gastroenterology/Ferring Pharmaceuticals Inc. Williams Turpin and Sun-Ho Lee are recipients of fellowships from the Department of Medicine, Mount Sinai Hospital, Toronto. Kenneth Croitoru is recipient of a Canada Research Chair in Inflammatory Bowel Diseases.
Abbreviations used in this paper:
- anti-αvβ6
anti-integrin αvβ6
- AUC
area under the curve
- CCC-GEM
Crohn’s and Colitis Canada Genetic Environmental Microbial project cohort
- CD
Crohn’s disease
- COMPASS
Comprehensive Care for the Recently Diagnosed IBD Patients
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- ESR
erythrocyte sedimentation rate
- HC
healthy control
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- OR
odds ratio
- OSCCAR
Ocean State Crohn’s and Colitis Area Registry
- pANCA
perinuclear anti-neutrophil cytoplasmic antibody
- PREDICTS
Proteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects
- ROC
receiver operating characteristic curve
- UC
ulcerative colitis
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://dx.doi.org/10.1053/j.gastro.2022.12.042.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the US Government. This is a US Government work. There are no restrictions on its use. Chad K. Porter is an employee of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
CRediT Authorship Contributions
Alexandra Livanos, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Alexandra Dunn, BA (Data curation: Supporting; Investigation: Supporting).
Jeremy Fischer, BS (Data curation: Supporting; Investigation: Supporting).
Ryan C. Ungaro, MD, MS (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting).
Williams Turpin, PhD (Validation: Supporting; Writing – review & editing: Supporting).
Sun-Ho Lee, MD, PhD (Formal analysis: Supporting; Validation: Supporting; Writing – review & editing: Supporting).
Shumin Rui, MS (Formal analysis: Supporting).
Diane Marie Del Valle, MS (Data curation: Supporting; Project administration: Supporting).
Julia J. Jougon, MD (Project administration: Supporting).
Gustavo Martinez-Delgado, PhD (Investigation: Supporting; Project administration: Supporting).
Mark S. Riddle, MD (Data curation: Supporting; Resources: Supporting; Writing – review & editing: Supporting).
Joseph A. Murray, MD (Writing – review & editing: Supporting).
Renee M. Laird, PhD, PMP (Project administration: Supporting; Writing – review & editing: Supporting).
Joana Torres, MD, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting).
Manasi Agrawal, MD, MS (Writing – review & editing: Supporting).
Jared S. Magee, DO, MPH (Project administration: Supporting; Resources: Supporting; Writing – review & editing: Supporting).
Thierry Dervieux, PharmD, PhD (Data curation: Supporting; Writing – review & editing: Supporting).
Sacha Gnjatic, PhD (Resources: Supporting; Writing – review & editing: Supporting).
Dean Sheppard, MD (Writing – review & editing: Supporting).
Bruce E. Sands, MD, MS (Resources: Supporting; Writing – review & editing: Supporting).
Chad K. Porter, PhD, MPH (Data curation: Supporting; Resources: Supporting; Writing – review & editing: Supporting).
Kenneth Croitoru, MDCM FRCPC (Validation: Supporting; Writing – review & editing: Supporting).
Francesca Petralia, PhD (Formal analysis: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting).
Jean-Frederic Colombel, MD (Conceptualization: Equal; Resources: Supporting; Writing – original draft: Equal; Writing – review & editing: Supporting).
Saurabh Mehandru, MD (Conceptualization: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
Conflicts of interest
These authors disclose the following: Saurabh Mehandru reports receiving research grants from Genentech and Takeda; receiving payment for lectures from Takeda, Genentech, and Morphic; and receiving consulting fees from Takeda, Morphic, Ferring, and Arena Pharmaceuticals. Jean-Frederic Colombel reports receiving research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, BMS, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Genentech, Glaxo Smith Kline, Janssen Pharmaceuticals, Kaleido Biosciences, Imedex, Immunic, Iterative Scopes, Merck, Microbia, Novartis, PBM Capital, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, Vifor; and holds stock options in Intestinal Biotech Development. Ryan C. Ungaro has served as an advisory board member or consultant for AbbVie, Bristol Myer Squibb, Janssen, Pfizer, and Takeda; research support from AbbVie, Boehringer Ingelheim, Eli Lily, and Pfizer. Thierry Dervieux is an employee of Prometheus Laboratories and hold stock options. Joseph A. Murray reports receiving grants from Nexpep/ImmusanT, National Institutes of Health, Immunogenix, Takeda Pharmaceutical, Allakos, Oberkotter, Cour; and consultancy fees from Bionix, Lilly Research Laboratory, Johnson & Johnson, Dr. Schar USA, UCB Biopharma, Celimmune, Intrexon Corporation, Dren Bio, Reistone pharma, Chugai Pharma, Kanyos, Boehringer Ingelheim, Equillium, and Torax Medical. Sacha Gnjatic reports other research funding from Genentech, Boehringer-Ingelheim, EMD Serono, Takeda, and Regeneron. Joana Torres received grants from AbbVie and Janssen, payment for lectures from Janssen, AbbVie, and Pfizer, and consulting fees from Janssen, AbbVie, Pfizer, and BMS. Dean Sheppard is a founder of Pliant Therapeutics, receives research funding from AbbVie, and is on the Scientific Review Board for Genentech and Amgen. The remaining authors disclose no conflicts.
Data Availability
All requests for raw and analyzed data and materials will be promptly reviewed by the corresponding authors and study team. The authors will provide source data files for all of the figures. Additional raw data are provided in the Supplementary Material.
Code Availability
The R code developed for analyses in this study will be made available.
References
- 1.Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology 2003;124:521–536. [DOI] [PubMed] [Google Scholar]
- 2.NIDDK IBD Genetics Consortium Phenotype Operating Manual. Published May 10, 2006. Accessed February 1, 2023. https://repository.niddk.nih.gov/media/studies/ibd/ibd_phenotyping-manual.pdf.
- 3.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres J, Petralia F, Sato T, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 2020;159:96–104. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Turpin W, Espin-Garcia O, et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 2021;161:1540–1551. [DOI] [PubMed] [Google Scholar]
- 6.Bergemalm D, Andersson E, Hultdin J, et al. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology 2021;161:1526–1539.e9. [DOI] [PubMed] [Google Scholar]
- 7.Verstockt B, Parkes M, Lee JC. How do we predict a patient’s disease course and whether they will respond to specific treatments? Gastroenterology 2022;162:1383–1395. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest 2011;121:4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choung RS, Princen F, Stockfisch TP, et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol Ther 2016;43:1300–1310. [DOI] [PubMed] [Google Scholar]
- 10.Parkes M, Noor NM, Dowling F, et al. PRedicting Outcomes For Crohn’s dIsease using a moLecular biomarkEr (PROFILE): protocol for a multicentre, randomised, biomarker-stratified trial. BMJ Open 2018;8:e026767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwada T, Shiokawa M, Kodama Y, et al. Identification of an anti-integrin αvβ6 autoantibody in patients with ulcerative colitis. Gastroenterology 2021;160:2383–2394.e21. [DOI] [PubMed] [Google Scholar]
- 12.Muramoto Y, Nihira H, Shiokawa M, et al. Anti-integrin αvβ6 antibody as a diagnostic marker for pediatric patients with ulcerative colitis. Gastroenterology 2022;163:1094–1097.e14. [DOI] [PubMed] [Google Scholar]
- 13.Rydell N, Ekoff H, Hellstrom PM, et al. Measurement of serum IgG anti-integrin αvβ6 autoantibodies is a promising tool in the diagnosis of ulcerative colitis. J Clin Med 2022;11:1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem 1992;267:5790–5796. [PubMed] [Google Scholar]
- 15.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 16.Koivisto L, Bi J, Hakkinen L, et al. Integrin alphavbeta6: structure, function and role in health and disease. Int J Biochem Cell Biol 2018;99:186–196. [DOI] [PubMed] [Google Scholar]
- 17.Blanco-Mezquita JT, Hutcheon AE, Stepp MA, et al. αVβ6 integrin promotes corneal wound healing. Invest Ophthalmol Vis Sci 2011;52:8505–8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang XZ, Wu JF, Cass D, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996;133:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y, Chen S, Lu GF, et al. Alphavbeta6 is required in maintaining the intestinal epithelial barrier function. Cell Biol Int 2014;38:777–781. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999;116:301–309. [DOI] [PubMed] [Google Scholar]
- 21.Porter CK, Riddle MS, Gutierrez RL, et al. Cohort profile of the PRoteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects (PREDICTS) study: Rationale, organization, design, and baseline characteristics. Contemp Clin Trials Commun 2019;14:100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Classification of Diseases, Ninth Revision, Clinical Modification. Centers for Disease Control and Prevention. Accessed January 24, 2023. https://www.cdc.gov/nchs/icd/icd9cm.htm. [Google Scholar]
- 23.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 24.Turpin W, Lee SH, Raygoza Garay JA, et al. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology 2020;159:2092–2100.e5. [DOI] [PubMed] [Google Scholar]
- 25.Lennard-Jones JE, Shivananda S. Clinical uniformity of inflammatory bowel disease a presentation and during the first year of disease in the north and south of Europe. EC-IBD Study Group. Eur J Gastroenterol Hepatol 1997;9:353–359. [DOI] [PubMed] [Google Scholar]
- 26.Shah S, Leleiko N, Lidofsky S, et al. Ocean State Crohn’s and Colitis Area Registry (OSCCAR): incidence of Crohn’s disease and ulcerative colitis in a prospective, population-based inception cohort in Rhode Island: 1172. Am J Gastroentrol 2010;105:S425–S426. [Google Scholar]
- 27.Cohen BL, Zoega H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther 2014;39:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau TM. A package for survival analysis in R. Accessed November 20, 2022. https://CRAN.R-project.org/package=survival. [Google Scholar]
- 29.McCullagh P, Nelder JA. Generalized Linear Models. Chapman and Hall, 1989. [Google Scholar]
- 30.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dassopoulos T, Cohen RD, Scherl EJ, et al. Ulcerative colitis care pathway. Gastroenterology 2015;149:238–245. [DOI] [PubMed] [Google Scholar]
- 32.Duerr RH, Targan SR, Landers CJ, et al. Antineutrophil cytoplasmic antibodies in ulcerative-colitis. Comparison with other colitides diarrheal illnesses. Gastroenterology 1991;100:1590–1596. [DOI] [PubMed] [Google Scholar]
- 33.Peeters M, Joossens S, Vermeire S, et al. Diagnostic value of anti-Saccharomyces cerevisiae and anti-neutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol 2001;96:730–734. [DOI] [PubMed] [Google Scholar]
- 34.Israeli E, Grotto I, Gilburd B, et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005;54:1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Schaik FD, Oldenburg B, Hart AR, et al. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut 2013;62:683–688. [DOI] [PubMed] [Google Scholar]
- 36.Lochhead P, Khalili H, Ananthakrishnan AN, et al. Association between circulating levels of C-reactive protein and interleukin-6 and risk of inflammatory bowel disease. Clin Gastroenterol Hepatol 2016;14:818–824.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kevans D, Waterman M, Milgrom R, et al. Serological markers associated with disease behavior and response to anti-tumor necrosis factor therapy in ulcerative colitis. J Gastroenterol Hepatol 2015;30:64–70. [DOI] [PubMed] [Google Scholar]
- 38.Hoie O, Aamodt G, Vermeire S, et al. Serological markers are associated with disease course in ulcerative colitis. A study in an unselected population-based cohort followed for 10 years. J Crohns Colitis 2008;2:114–122. [DOI] [PubMed] [Google Scholar]
- 39.Kalla R, Adams AT, Bergemalm D, et al. Serum proteomic profiling at diagnosis predicts clinical course, and need for intensification of treatment in inflammatory bowel disease. J Crohns Colitis 2021;15:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trzpis M, McLaughlin PM, de Leij LM, et al. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol 2007;171:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Lange KM, Moutsianas L, Lee JC, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet 2017;49:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uzzan M, Martin JC, Mesin L, et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med 2022;28:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995;108(Pt 6):2241–2251. [DOI] [PubMed] [Google Scholar]
- 44.Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets 2009;10:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris DA, Kissinger RM, Naughton GM, et al. Evidence for immunologic mechanisms in human vitiligo: patients’ sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol 1988;90:783–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All requests for raw and analyzed data and materials will be promptly reviewed by the corresponding authors and study team. The authors will provide source data files for all of the figures. Additional raw data are provided in the Supplementary Material.
The R code developed for analyses in this study will be made available.