Summary
Background:
Results from the phase 3 CLEAR study showed that lenvatinib plus pembrolizumab improved progression-free survival and overall survival compared with sunitinib in patients with advanced renal cell carcinoma. We aimed to assess the health-related quality-of-life (HRQOL) outcomes from the CLEAR study.
Methods:
This open-label, randomised, phase 3 study was done across 200 hospitals and cancer centres in 20 countries. Patients were required to be 18 years or older, with advanced clear-cell renal cell carcinoma, and a Karnofsky performance status of 70% or higher. Patients who had received previous systemic anticancer therapy for renal cell carcinoma were not eligible. Patients were randomly assigned (1:1:1) to lenvatinib (oral 20 mg per day) plus pembrolizumab (intravenous 200 mg every 21 days), lenvatinib (oral 18 mg per day) plus everolimus (oral 5 mg per day) in 21-day cycles, or sunitinib (oral 50 mg per day, 4 weeks on followed by 2 weeks off). Patients were assigned to treatments with a computer-generated randomisation scheme and were stratified by geographical region and Memorial Sloan Kettering Cancer Center prognostic groups. The primary endpoint, previously reported, was progression-free survival, and HRQOL was a secondary endpoint. Most HRQOL analyses were done in patients who underwent randomisation, received at least one dose of study treatment, and had any HRQOL data. Completion and compliance analyses were done in the full analysis set. Functional Assessment of Cancer Therapy Kidney Symptom Index-Disease-Related Symptoms (FKSI-DRS), European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30), and the EQ-5D-3 Level (EQ-5D-3L) preference questionnaire were administered at baseline and on day 1 of each subsequent 21-day cycle. This study is registered with ClinicalTrials.gov, NCT02811861, and is closed to new participants.
Findings:
Between Oct 13, 2016, and July 24, 2019, 355 patients were randomly assigned to the lenvatinib plus pembrolizumab group, 357 to the lenvatinib plus everolimus group, and 357 to the sunitinib group. Median follow-up for HRQOL analyses was 12·9 months (IQR 5·6–22·3). Because of the promising efficacy and safety results of lenvatinib plus pembrolizumab in the first-line setting, we focus the HRQOL results in this report on that combination versus sunitinib. Mean change from baseline in the lenvatinib plus pembrolizumab group compared with the sunitinib group was −1·75 (SE 0·59) versus −2·19 (0·66) for FKSI-DRS, −5·93 (0·86) versus −6·73 (0·94) for EORTC QLQ-C30 global health status/quality of life (GHS/QOL), and −4·96 (0·85) versus −6·64 (0·94) for the EQ-5D visual analogue scale (VAS). Median time to first deterioration in the lenvatinib plus pembrolizumab group compared with the sunitinib group was 9·14 weeks (95% CI 6·43–12·14) versus 12·14 weeks (9·14–15·29; HR 1·13 [95% CI 0·94–1·35], log-rank p=0·20) for FKSI-DRS, 12·00 weeks (7·29–15·14) versus 9·14 weeks (6·29–12·14; 0·88 [0·74–1·05], log-rank p=0·17) for EORTC QLQ-C30 GHS/QOL, and 9·43 weeks (6·43–12·29) versus 9·14 weeks (6·29–12·00; 0·83 [0·70–0·99], log-rank p=0·041) for the EQ-5D VAS. Median time to definitive deterioration in the lenvatinib plus pembrolizumab group compared with the sunitinib group was 134·14 weeks (95% CI 120·00–not estimable) versus 117·43 weeks (90·14–131·29; HR 0·70 [95% CI 0·53–0·92], log-rank p=0·0081) for FKSI-DRS, 114·29 weeks (102·14–153·29) versus 75·14 weeks (57·29–105·14; 0·60 [0·47–0·77], log-rank p<0·0001) for EORTC QLQ-C30 GHS/QOL, and 124·86 weeks (94·71–134·57) versus 74·86 weeks (54·14–96·00; 0·67 [0·53–0·85], log-rank p=0·0012) for the EQ-5D VAS. No outcomes on any of the instruments significantly favoured sunitinib over lenvatinib plus pembrolizumab. Most HRQOL comparisons of lenvatinib plus everolimus versus sunitinib were similar or favoured sunitinib.
Interpretation:
These HRQOL results demonstrate that patients given lenvatinib plus pembrolizumab treatment had similar or favourable scores compared with patients given sunitinib, particularly with respect to time to definitive deterioration. These results support the efficacy and safety profile of lenvatinib plus pembrolizumab as first-line therapy for patients with advanced renal cell carcinoma.
Funding:
Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
INTRODUCTION
Lenvatinib, a multityrosine kinase inhibitor, has previously shown activity as a monotherapy and in combination with everolimus in a phase 2 study of patients with advanced renal cell carcinoma following one previous antiangiogenic therapy.1 Pembrolizumab is a PD-1 inhibitor that has shown preliminary efficacy in the treatment of renal cell carcinoma, as a monotherapy and in combination with lenvatinib.2,3 Lenvatinib plus everolimus and lenvatinib plus pembrolizumab more recently showed benefit versus sunitinib in the first-line treatment of patients with advanced renal cell carcinoma in the phase 3 CLEAR study (Study 307/KEYNOTE-581),4 and lenvatinib plus pembrolizumab is recommended by recognised international guidelines for the treatment of renal cell carcinoma.5,6 Lenvatinib plus pembrolizumab demonstrated improvements in progression-free survival, overall survival, and objective response rate versus sunitinib, whereas lenvatinib plus everolimus demonstrated improvements in progression-free survival and objective response rate versus sunitinib, but not in overall survival.4 The safety profiles of both combinations were consistent with each drug’s known profile,7,8 and adverse events were generally manageable through dose modifications, as needed.4
Patients with renal cell carcinoma often have associated signs and symptoms that can affect their quality of life, including flank pain, haematuria, bone pain, coughing, palpable renal mass, and manifestations of paraneoplastic syndrome.9,10 Moreover, several common adverse events associated with kinase inhibitors and immunotherapies are known to negatively affect quality of life, including rash, nausea, diarrhoea, fatigue, and musculoskeletal pain.11–15 Adverse events can lead to patients temporarily or permanently stopping treatment or reducing dosage to the point of affecting efficacy.11 Therefore, it is important to determine the effect of treatments on health-related quality of life (HRQOL) to optimize patients’ wellbeing during therapy and outcomes. Using data from the CLEAR study, we aimed to investigate patient-reported outcomes comparing the effect of lenvatinib plus pembrolizumab or everolimus versus sunitinib on HRQOL, including functional measures and disease-specific symptoms.
METHODS
Study Design and Participants
This open-label, randomised, phase 3 study was done across 200 hospitals and cancer centres in 20 countries (appendix pp 19–23). Eligibility criteria have previously been published.4 Briefly, patients were required to be 18 years or older, with a Karnofsky performance status of 70% or higher, with adequate organ function, and to have histological or cytological confirmation of advanced renal cell carcinoma with a clear-cell component with at least one target lesion measurable by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Patients who had received any previous systemic anticancer therapy for renal cell carcinoma were excluded.
The study was done in accordance with the International Council for Harmonization Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki 2013. Institutional review boards or independent ethics committees approved the protocol and appropriate related documents; all patients provided written, informed consent.
Randomisation and Masking
Patients were randomly assigned 1:1:1 to one of three treatment arms (lenvatinib plus everolimus, lenvatinib plus pembrolizumab, or sunitinib alone) with a computer-generated randomisation scheme that was reviewed and approved by an independent statistician. The randomization scheme was stratified by geographic region (western Europe and North America vs other) and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic groups (favorable, intermediate, and poor risk). As the study was an open-label design, participants and study personnel were not masked to treatment.
Procedures
For patients in the lenvatinib plus pembrolizumab group, lenvatinib was administered orally at a starting dose of 20 mg per day in 21-day cycles and pembrolizumab was administered at a dose of 200 mg intravenously on day 1 of each 21-day cycle.4 In the lenvatinib plus everolimus group, lenvatinib was administered at a starting dose of 18 mg per day and everolimus was administered orally at a dose of 5 mg per day in 21-day cycles. Sunitinib was administered orally at a dose of 50 mg per day (4 weeks on treatment followed by 2 weeks off treatment). Dose reductions of lenvatinib, everolimus, or sunitinib due to toxicity were allowed; dose reductions of lenvatinib occurred in succession based on the previous dose level (14 mg, 10 mg, or 8 mg per day, when in combination with everolimus or pembrolizumab), everolimus could be reduced to 5 mg every other day, and sunitinib could be reduced to 37·5 mg per day and then to 25 mg per day, still using the 4 weeks on, 2 weeks off schedule. Dose reductions of pembrolizumab were not permitted. Tumour assessments (CT [chest] and CT or MRI [abdomen, pelvis, and other known or suspected sites of disease]) by RECIST 1.1 were done every 8 weeks from the date of randomisation. Laboratory assessments were done according to the protocol,4 and adverse events were graded according to Common Terminology Criteria for Adverse Events (version 4.03) and monitored throughout. Patients continued to receive study treatment until confirmed disease progression by an independent review committee, development of unacceptable toxicity, patient request, withdrawal of consent, or study termination by the sponsor. If patients were deemed by the investigator to have received clinical benefit and were tolerating the study treatment, they were permitted to continue to receive study treatment beyond disease progression assessed according to RECIST 1.1. Patients could be removed from the study at any time for safety or administrative reasons, or due to patient choice.
The HRQOL instruments were administered before study drug administration (when feasible) at baseline and on day 1 of each subsequent 21-day cycle starting with cycle 2, for patients remaining on study treatment. An off-treatment visit occurred within 30 days of treatment discontinuation (appendix p 1).
The Functional Assessment of Cancer Therapy Kidney Symptom Index-Disease-Related Symptoms (FKSI-DRS) consists of nine items previously prioritised by patients with kidney cancer and classified as primarily disease-related by clinical experts.16 The total score ranges from 0 to 36, with higher scores corresponding to better symptom status. The European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) comprises five functional scales, nine symptom scales, and a global health status/quality of life (GHS/QOL) score.17 Scores for all scales range from 0 to 100. For the GHS/QOL and functional scales, a higher score corresponds to better HRQOL; for symptom scales, a higher score represents worsening of symptoms. EQ-5D-3 Levels (EQ-5D-3L) is a general preference-based HRQOL instrument developed to assess health outcomes for a wide range of interventions on a common scale, on which patients rank their perceived health on five dimensions.18 The index component applies preference weights, on a scale of 0 to 1, with 1 representing perfect health. The visual analogue scale (VAS) component is on a scale of 0 to 100, with 100 representing optimal health.
Outcomes
The primary endpoint of CLEAR was progression-free survival by independent review. Key secondary endpoints were overall survival and objective response rate. Other secondary endpoints were safety, the proportion of patients who discontinued treatment due to toxicity, time to treatment failure due to toxicity, progression-free survival on next line of therapy, progression-free survival by investigator assessment, model-predicted clearance and area under the curve (AUC) for lenvatinib (in both lenvatinib groups), model-predicted clearance and AUC for everolimus in the lenvatinib plus everolimus group, and HRQOL. Results from the primary and key secondary endpoints have previously been published.4 HRQOL was assessed by three patient-reported outcome instruments measuring HRQOL and disease-specific symptoms that were completed by patients on paper forms: the FKSI-DRS, the EORTC QLQ-C30, and the EQ-5D-3L instruments. These instruments were selected because they are widely used in the published literature, both for kidney-specific conditions (FKSI-DRS) and across indications (EORTC QLQ-C30 and EQ-5D-3L).19–22
Statistical Analysis
Detailed statistical methods for the primary and key secondary endpoints have previously been published.4 No formal power calculation was done for the HRQOL analyses. In this HRQOL assessment, any statistical tests and CIs have an associated α level of 0·05 unless otherwise specified. According to the analysis plan, no adjustments for multiple testing or estimation were used, so all p values and CIs are nominal and descriptive in nature, and nominally significant refers to significant without adjustment for multiplicity. No imputations were done; missing data (ie, questionnaire items that were not answered or scores that could not be computed because of missing questionnaire items) were excluded, and variables were analysed using the available observed data. Completion and compliance rates for HRQOL instruments were computed based on the full analysis set, which included all patients who were randomly assigned to treatment. All other HRQOL analyses were based on the quality-of-life analysis set, which consisted of all patients in the safety population (those who were randomly assigned to, and received at least one dose of, study treatment) with any HRQOL data. Patients who had missing data at baseline were excluded from longitudinal analyses; intermittently missing data in the longitudinal mixed-model analyses were assumed to be missing at random.
Completion rates were defined as the percentage of patients who completed any HRQOL instrument among all patients who were enrolled in the full analysis set at baseline and were assessed for each of the HRQOL outcomes by assessment timepoint and treatment group. Compliance rates were defined as the percentage of patients who completed any instrument among all patients who were still enrolled in the study and on study treatment at a particular post-baseline timepoint and were, therefore, expected to complete the instrument. Compliance rates were summarised for each of the HRQOL instruments by assessment timepoint and treatment group.
To assess the effect of treatment on HRQOL outcomes, mixed models with random coefficients were fitted using the change from baseline for each respective HRQOL score as the response variable. Each model included treatment, time (as a continuous variable), a time-by treatment interaction term, the adjustment factors of baseline HRQOL score and the two randomization stratification variables (ie, geographical region and MSKCC prognostic group), and patient-specific random intercept and slope terms. The covariance matrix for these random effects was assumed to be unstructured. We estimated the least-squares model-adjusted mean change from baseline for each treatment group and the difference between treatment groups at each timepoint, along with an overall least-squares mean difference estimated at the mean HRQOL follow-up time (ie, week 46, cycle 15). We also estimated the differences in least-squares means between each lenvatinib treatment group (lenvatinib plus pembrolizumab and lenvatinib plus everolimus) and sunitinib, along with associated 95% CIs and p values.
A deterioration event for any individual HRQOL outcome was defined as a detrimental change in score relative to baseline that met or exceeded a prespecified decline value for that score; death was considered a deterioration event if it occurred within 30 days of the last HRQOL assessment, regardless of the start date of any new anticancer treatment. Patients without a deterioration event at the analysis cutoff date were censored at the date of the last HRQOL assessment. We evaluated time to deterioration using the Kaplan-Meier method to estimate the distribution of time to deterioration and median time to deterioration value for each treatment group. Deterioration events were defined as detrimental changes in score relative to baseline that exceed the minimally important difference thresholds. Minimally important differences were a decrease of 3 or more points for the FKSI-DRS;16,23 a decrease of 10 or more points for the EORTC QLQ-C30 functional and GHS/QOL scores;24 an increase of 10 or more points for the EORTC QLQ-C30 symptom scores;24 a decrease of 0·08 or more points for the EQ-5D-3L index; and a decrease of 7 or more points for the EQ-5D-3L VAS.25
We used log-rank tests to compare the distributions for lenvatinib plus pembrolizumab versus sunitinib and lenvatinib plus everolimus versus sunitinib. Cox models stratified by the randomisation stratification variables were fitted for each score, and hazard ratios (HRs) and associated 95% CIs were estimated to compare each lenvatinib treatment group with the sunitinib treatment group. We assessed both time to first deterioration (number of weeks between randomisation and the first deterioration event during the treatment period) and time to definitive deterioration (the number of weeks between randomisation and the earliest deterioration event during the treatment period with no subsequent recovery above the deterioration threshold or no subsequent HRQOL assessment data).
We did post-hoc subgroup analyses to compare outcomes for MSKCC and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups (favourable versus intermediate or poor). For this analysis, we used standard assumptions for HRQOL methods; mixed models required the assumption that missing data were missing at random and time-to-deterioration analyses assumed that right-censoring was not informative and that the hazards for the treatment groups were proportional.
HRQOL data were collected and analysed for all three groups of this study. Because of the promising efficacy (including a benefit in overall survival) and safety results of lenvatinib plus pembrolizumab in the first-line setting, the main body of this report focuses on that combination versus sunitinib.
All analyses were done with SAS (version 9.4 or higher). Safety and efficacy data were monitored by an independent data monitoring committee. This study is registered with ClinicalTrials.gov, NCT02811861. This trial is closed to new participants.
Role of the funding source
The funders of the study contributed to the study design; collection, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
RESULTS
Between Oct 13, 2016, and July 24, 2019, 355 patients were randomly assigned to receive lenvatinib plus pembrolizumab, 357 to receive lenvatinib plus everolimus, and 357 to receive sunitinib, with a median follow-up time for the HRQOL analyses of 12·9 months (IQR 5·6–22·3). Overall disposition details have been published,4 and disposition at selected timepoints is shown in figure 1. Baseline patient characteristics are shown in the table.
Figure 1. Trial Profile.
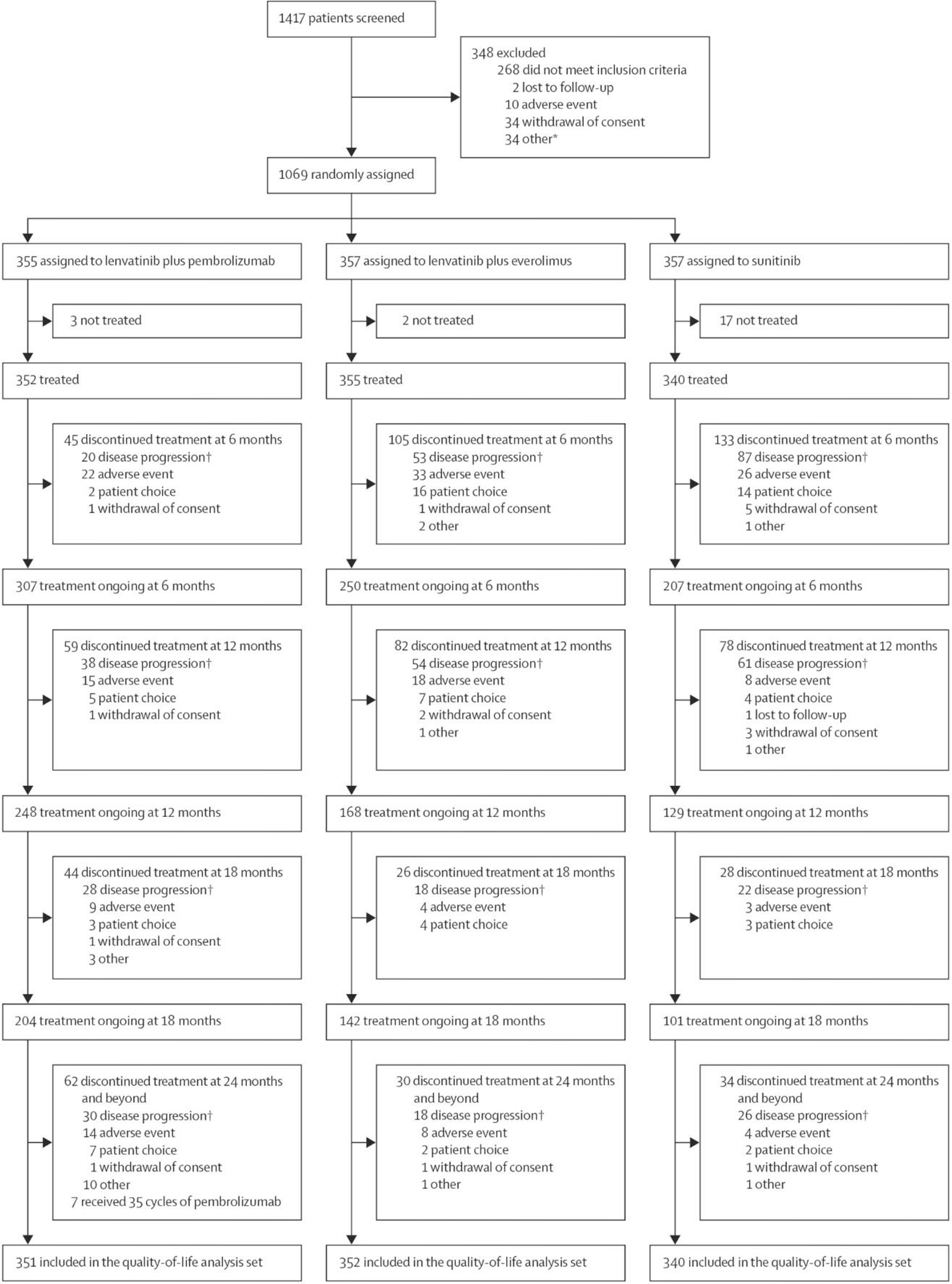
*Other reasons are listed in the appendix (p 18).
†Includes radiological and clinical disease progression.
Table.
Summary of Selected Baseline Characteristics
| Parameter | Lenvatinib plus pembrolizumab group (n = 355) | Sunitinib group (n = 357) | Lenvatinib plus everolimus group (n = 357) |
|---|---|---|---|
| Age, years | 64 (56–70) | 61 (54–69) | 62 (55–69) |
| Sex | |||
| Male | 255 (72%) | 275 (77%) | 266 (75%) |
| Female | 100 (28%) | 82 (23%) | 91 (25%) |
| Race | |||
| White | 263 (74%) | 270 (76%) | 254 (71%) |
| Black or African American | 2 (1%) | 3 (1%) | 1 (<1%) |
| Asian | 81 (23%) | 67 (19%) | 77 (22%) |
| American Indian or Alaskan native | 0 | 0 | 1 (<1%) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 1 (<1%) |
| Other | 4 (1%) | 7 (2%) | 7 (2%) |
| Missing | 5 (1%) | 10 (3%) | 16 (4%) |
| Geographic region | |||
| Western Europe or North America | 198 (56%) | 199 (56%) | 200 (56%) |
| Rest of the world | 157 (44%) | 158 (44%) | 157 (44%) |
| MSKCC prognostic risk group | |||
| Favorable | 96 (27%) | 97 (27%) | 98 (27%) |
| Intermediate | 227 (64%) | 228 (64%) | 227 (64%) |
| Poor | 32 (9%) | 32 (9%) | 32 (9%) |
| IMDC prognostic risk group | |||
| Favorable | 110 (31%) | 124 (35%) | 114 (32%) |
| Intermediate | 210 (59%) | 192 (54%) | 195 (55%) |
| Poor | 33 (9%) | 37 (10%) | 42 (12%) |
| Could not be evaluated | 2 (1%) | 4 (1%) | 6 (2%) |
Data are median (IQR) or n (%) unless otherwise stated. Percentages might not sum to 100 as result of rounding.
Adapted from Motzer and colleagues.4 Copyright © (2021) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
IMDC=International Metastatic Renal Cell Carcinoma Database Consortium. MSKCC=Memorial Sloan Kettering Cancer Center.
Most patients completed the HRQOL instruments at baseline (338 [95%] of 355 patients in the lenvatinib plus pembrolizumab group, 343 [96%] of 357 in the Lenvatinib plus everolimus group, and 328 [92%] of 357 in the sunitinib group), and compliance rates were greater than 90% in all three groups during the early cycles of treatment (appendix p 2). Completion rates decreased over time as patients discontinued from the study. At the off-treatment visit, all of the HRQOL instruments were completed by 142 (40%) of 355 patients in the lenvatinib plus pembrolizumab group, 160 (45%) of 357 patients in the lenvatinib plus everolimus group, and 199 (56%) of 357 patients in the sunitinib group (appendix p 2). At the off-treatment visit, compliance rates for all HRQOL instruments were high (HRQOL instruments completed by 142 [80%] of 177 patients in the lenvatinib plus pembrolizumab group, 160 [79%] of 203 patients in the lenvatinib plus everolimus group, and 199 [84%] of 237 patients in the sunitinib group; appendix p 2). Although completion rates decreased as patients discontinued treatment over the course of the study period, compliance rates remained relatively stable.
The median duration of treatment was 17·0 months (IQR 9·4–25·4) in the lenvatinib plus pembrolizumab group, 11·0 months (5·0–20·7) in the lenvatinib plus everolimus group, and 7·8 months (3·7–17·8) in the sunitinib group.4 According to the study protocol, patients were permitted to continue study drug treatment beyond RECIST 1.1-defined disease progression if they were considered by the investigator to be receiving benefit with acceptable toxicity; treatment beyond progression occurred in 82 (23%) of 352 patients in the lenvatinib plus pembrolizumab group, 71 (20%) of 355 patients in the lenvatinib plus everolimus group, and 75 (22%) of 340 patients in the sunitinib group. The percentages of patients who completed at least one HRQOL assessment after disease progression were similar across treatment groups (123 [35%] of 355 in the lenvatinib plus pembrolizumab group, 134 [38%] of 357 in the lenvatinib plus everolimus group, and 147 [41%] of 357 in the sunitinib group).
HRQOL scores at baseline were similar across the three treatment groups for all instruments and scales (appendix p 4). Least-squares mean change at mean follow-up (46 weeks, cycle 15) from baseline in the lenvatinib plus pembrolizumab group compared with the sunitinib group was –1·75 (SE 0·59) versus –2·19 (0·66) for FKSI-DRS, –5·93 (0·86) versus –6·73 (0·94) for EORTC QLQ-C30 GHS/QOL, and –4·96 (0·85) versus –6·64 (0·94) for the EQ-5D visual analogue scale (VAS; figure 2A). The lenvatinib plus pembrolizumab group was nominally significantly favoured versus the sunitinib group on four EORTC QLQ-C30 scales: physical functioning, fatigue, dyspnoea, and constipation, but not for any other scales evaluated (figure 2A; appendix p 6). The sunitinib group was not favoured (nominal p<0·05) versus the lenvatinib plus pembrolizumab group for any scale (figure 2A; appendix p 6). Post-hoc subgroup analyses of MSKCC and IMDC risk groups included a more granular perspective on these data (appendix p 7).
Figure 2. Least-Squares Mean Differences for Change From Baselinea.
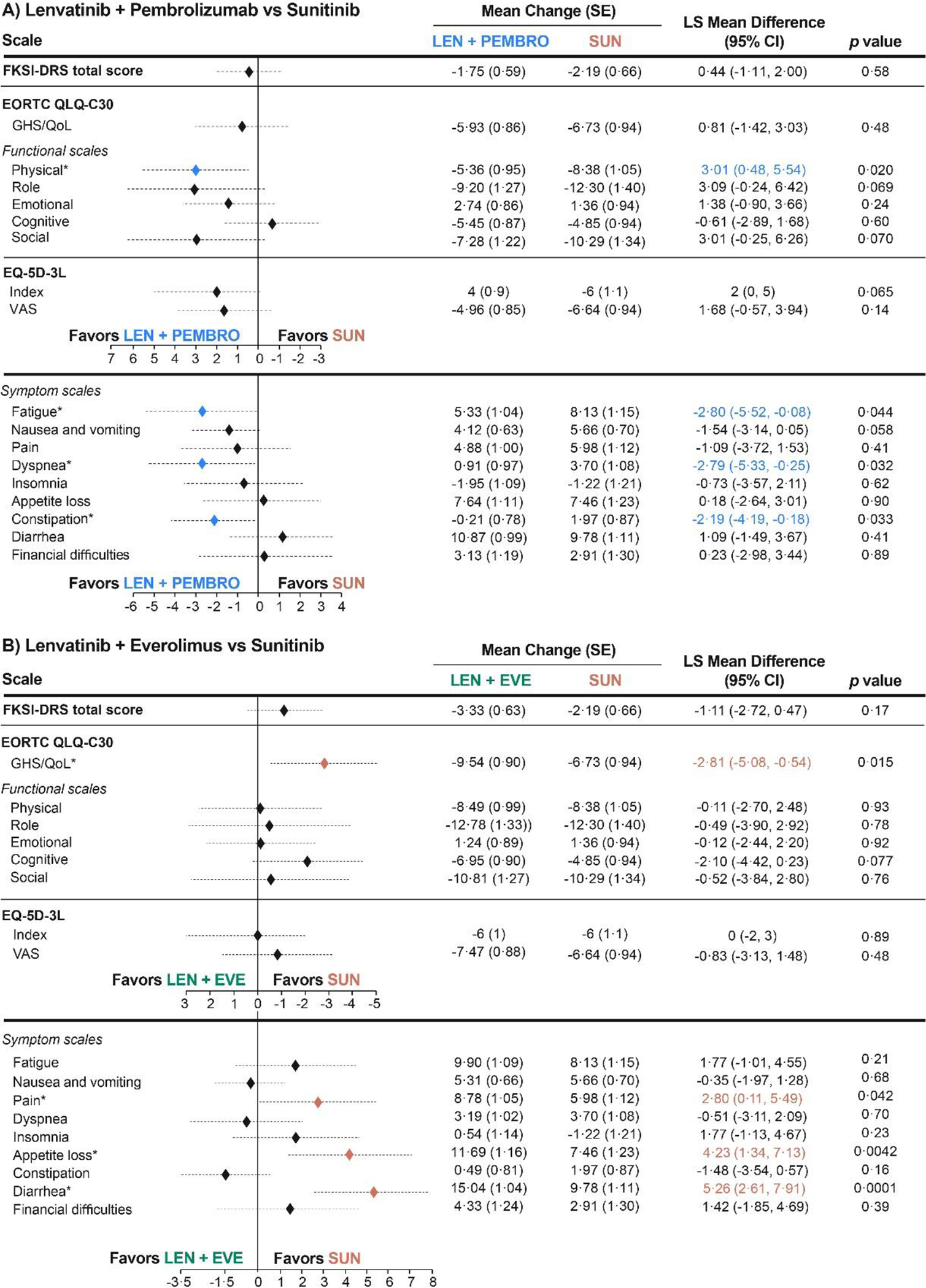
aThe overall least-squares mean difference was estimated at mean follow-up (46 weeks, cycle 15). For presentation, scores from the FKSI-DRS and EQ-5D-3L Index instruments were transformed to the scale of 0–100 (FKSI-DRS transformed score = (raw score/36)*100; EQ-5D-3L Index transformed score = (raw score)*100). For the FKSI-DRS total score, EORTC QLQ-C30 GHS/QoL and functional scales, and EQ-5D-3L scales, a higher score corresponds to better HRQoL. For EORTC QLQ-C30 symptom scales, a higher score represents worse symptoms *Statistically significant difference (P < 0·05).
CI, confidence interval; EORTC QLQ-C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EQ-5D-3L, EuroQol 5 Dimensions 3 Levels; EVE, everolimus; FKSI-DRS, Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease-related Symptoms; GHS/QoL, global health status/quality of life; HRQoL, health-related quality of life; LEN, lenvatinib; LS, least squares; PEMBRO, pembrolizumab; SUN, sunitinib.
For the comparison of lenvatinib plus everolimus versus sunitinib, mean changes were similar or favoured sunitinib. There were no nominally significant differences in favour of lenvatinib plus everolimus versus sunitinib; nominally significant differences in favour of sunitinib were found for EORTC QLQ-C30 GHS/QOL, and for the EORTC QLQ-C30 pain, appetite loss, and diarrhoea scales (figure 2B; appendix p 6).
Analyses of time to first deterioration showed a similar outcome between the lenvatinib plus pembrolizumab and sunitinib treatment groups across most instruments (figure 3). Median time to first deterioration in the lenvatinib plus pembrolizumab group compared with the sunitinib group was 9·14 weeks (95% CI 6·43–12·14) versus 12·14 weeks (9·14–15·29; HR 1·13 [95% CI 0·94–1·35]; log-rank p=0·20) for FKSI-DRS, 12·00 weeks (7·29–15·14) versus 9·14 weeks (6·29–12·14; 0·88 [0·74–1·05]; log-rank p=0·17) for EORTC QLQ-C30 GHS/QOL, and 9·43 weeks (6·43–12·29) versus 9·14 weeks (6·29–12·00; 0·83 [0·70–0·99]; log-rank p=0·041) for the EQ-5D VAS. Times to first deterioration were nominally significantly in favour of lenvatinib plus pembrolizumab versus sunitinib for EORTC QLQ-C30 physical functioning (median 15·29 weeks [95% CI 12·29–21·43] vs 12·71 weeks [9·29–18·14]; HR 0·81 [95% CI 0·68–0·98], log-rank p=0·034), EORTC QLQ-C30 dyspnoea (39·29 weeks [24·43–51·00] vs 21·14 weeks [15·43–32·71]; 0·79 [0·64–0·97], log-rank p=0·023), EORTC QLQ-C30 appetite loss (18·29 weeks [15·14–21·71] vs 9·14 weeks [6·29–15·14]; 0·82 [0·68–0·98], log-rank p=0·028), and EQ-5D-3L VAS, but not for any other scales evaluated (figure 3; figure 4). Post-hoc subgroup analyses of MSKCC and IMDC risk groups are shown in the appendix (pp 9–10).
Figure 3. Time to First Deterioration: Lenvatinib + Pembrolizumab vs Sunitinib.
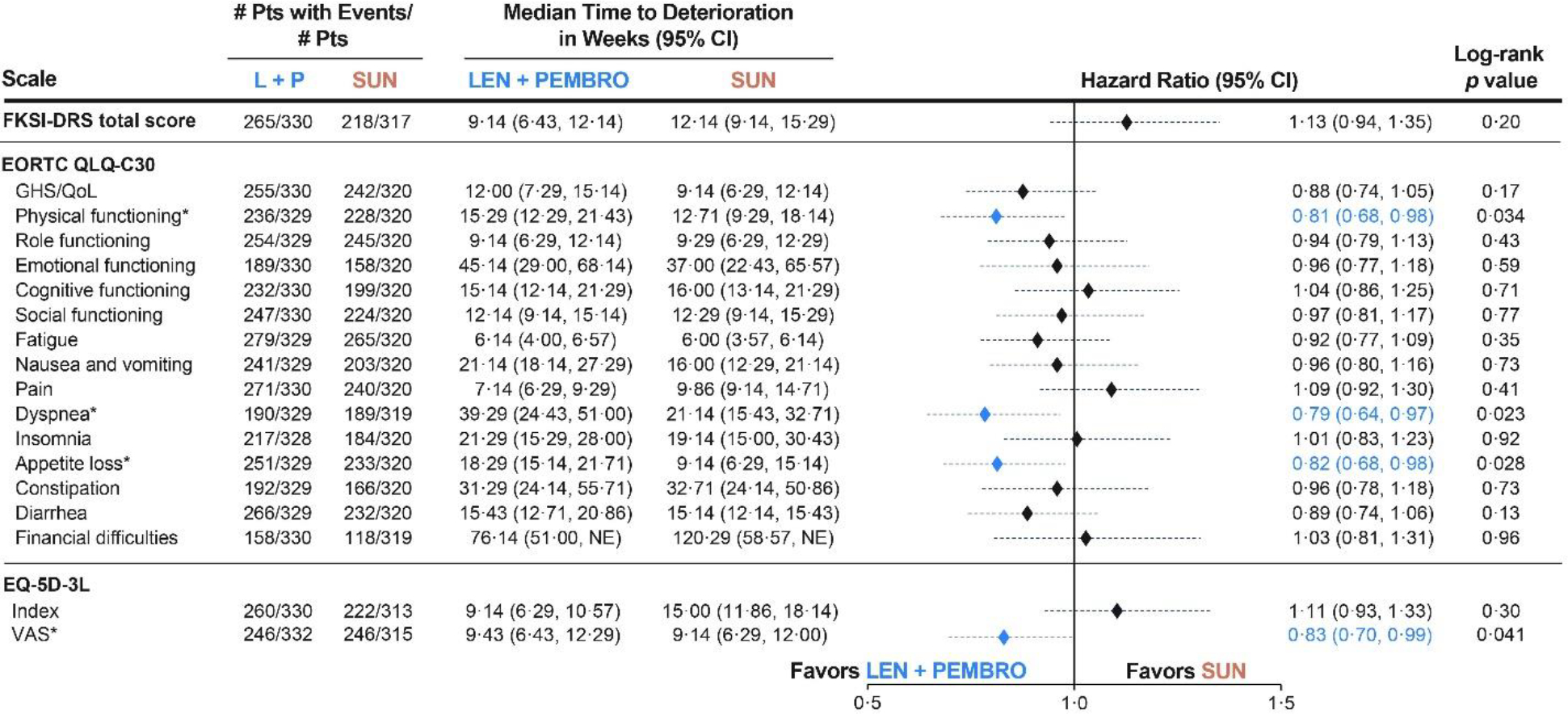
*Denotes a statistically significant log-rank difference (P < 0·05) on the distributions of lenvatinib + pembrolizumab vs sunitinib.
This analysis includes all patients in the HRQoL analysis set (n=351 in the lenvatinib + pembrolizumab arm, n=340 in the sunitinib arm.
CI, confidence interval; EORTC QLQ-C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EQ-5D-3L, EuroQol 5 dimensions 3 levels; FKSI-DRS, Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease related Symptoms; GHS/QoL, global health status/quality of life; LEN, lenvatinib; PEMBRO, pembrolizumab; SUN, sunitinib; VAS, visual analog scale.
Figure 4. Kaplan-Meier Plots of Nominally Significant Scales for Time to First Deterioration: Lenvatinib + Pembrolizumab vs Sunitinib.
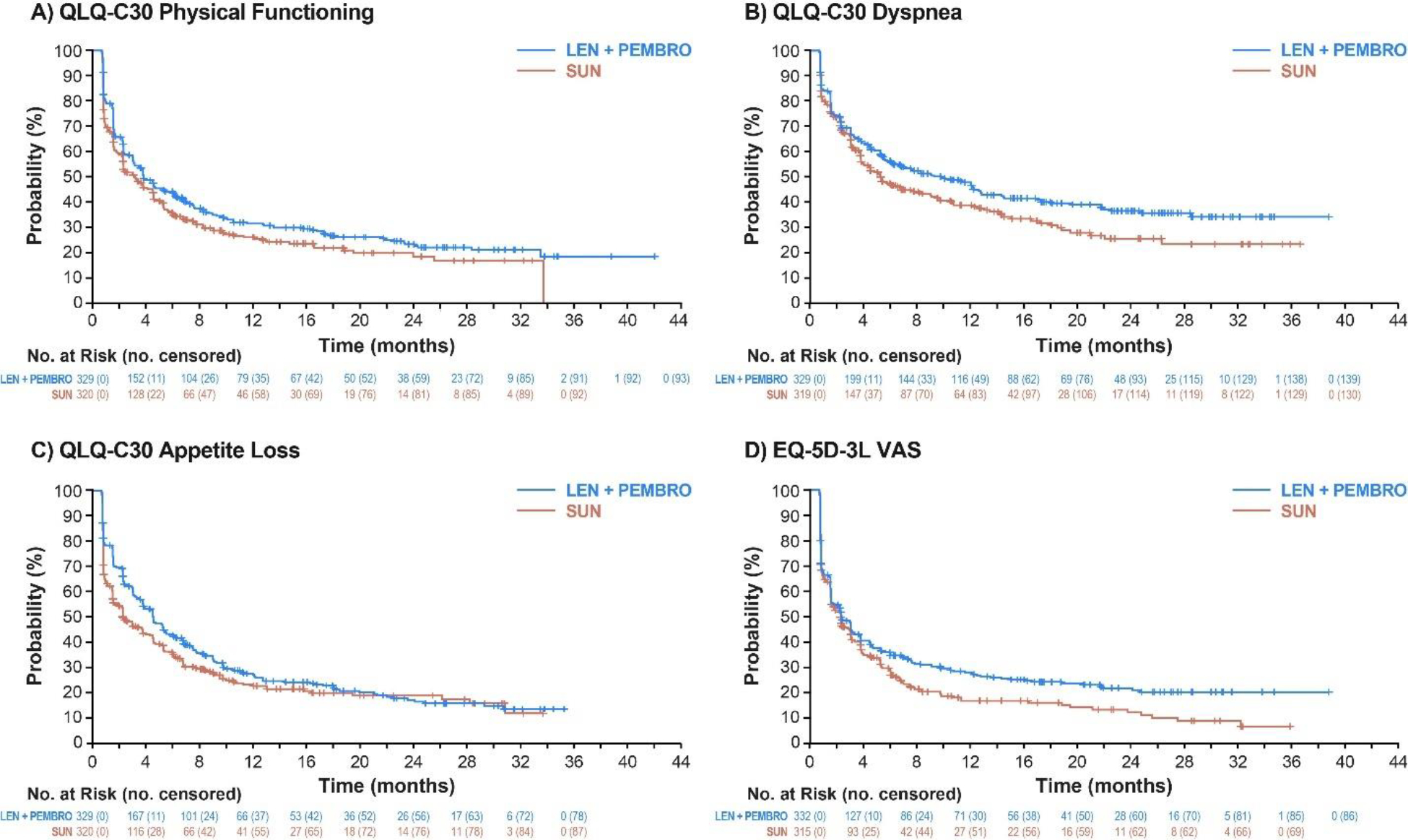
QLQ-C-30, Quality of Life Questionnaire; EQ-5D-3L, EuroQol 5 Dimensions 3 Levels; LEN, lenvatinib; PEMBRO, pembrolizumab; SUN, sunitinib; VAS, visual analog scale.
In the overall population, no scales had nominally significant differences in favour of sunitinib over lenvatinib plus pembrolizumab in time to first deterioration (as assessed by HR). By contrast, results for time to first deterioration with lenvatinib plus everolimus versus sunitinib were similar or favoured (nominally significant) sunitinib (FKSI-DRS, EORTC QLQ-C30 pain, EORTC QLQ-C30 diarrhoea, and EQ-5D-3L index; appendix pp 11–12). Although time to first deterioration included deaths and deterioration events, the number of deaths made up a small proportion of the events across all groups and instruments (<10% in all scales); data from selected instruments are in the appendix (p 5).
Time to definitive deterioration was longer with lenvatinib plus pembrolizumab versus sunitinib in nearly all scales assessed (figure 5). Median time to definitive deterioration in the lenvatinib plus pembrolizumab group compared with the sunitinib group was 134·14 weeks (95% CI 120·00–not estimable) versus 117·43 weeks (90·14–131·29; HR 0·70 [95% CI 0·53–0·92], log-rank p=0·0081) for FKSI-DRS, 114·29 weeks (102·14–153·29) versus 75·14 weeks (57·29–105·14; 0·60 [0·47–0·77], log-rank p<0·0001) for EORTC QLQ-C30 GHS/QOL, and 124·86 weeks (94·71–134·57) versus 74·86 weeks (54·14–96·00; 0·67 [0·53–0·85], log-rank p=0·0012) for the EQ-5D VAS. The only scales that did not demonstrate a nominally significant difference (as assessed by HR) were cognitive functioning and financial difficulties (figure 5). Kaplan-Meier plots for time to definitive deterioration for all scales that showed a significant difference between lenvatinib plus pembrolizumab and sunitinib can be seen in the appendix (p 13). Time-to-definitive-deterioration data for the post-hoc analyses of MSKCC and IMDC risk subgroups are shown in the appendix (pp 15–16). No significant differences between lenvatinib plus everolimus versus sunitinib were found with respect to time to definitive deterioration when assessed by HR (appendix p 17). Similar to the results for time to first deterioration, the number of deaths made up a small proportion of definitive deterioration events in all three groups (data from selected instruments are in the appendix p 5).
Figure 5. Time to Definitive Deterioration: Lenvatinib + Pembrolizumab vs Sunitinib.
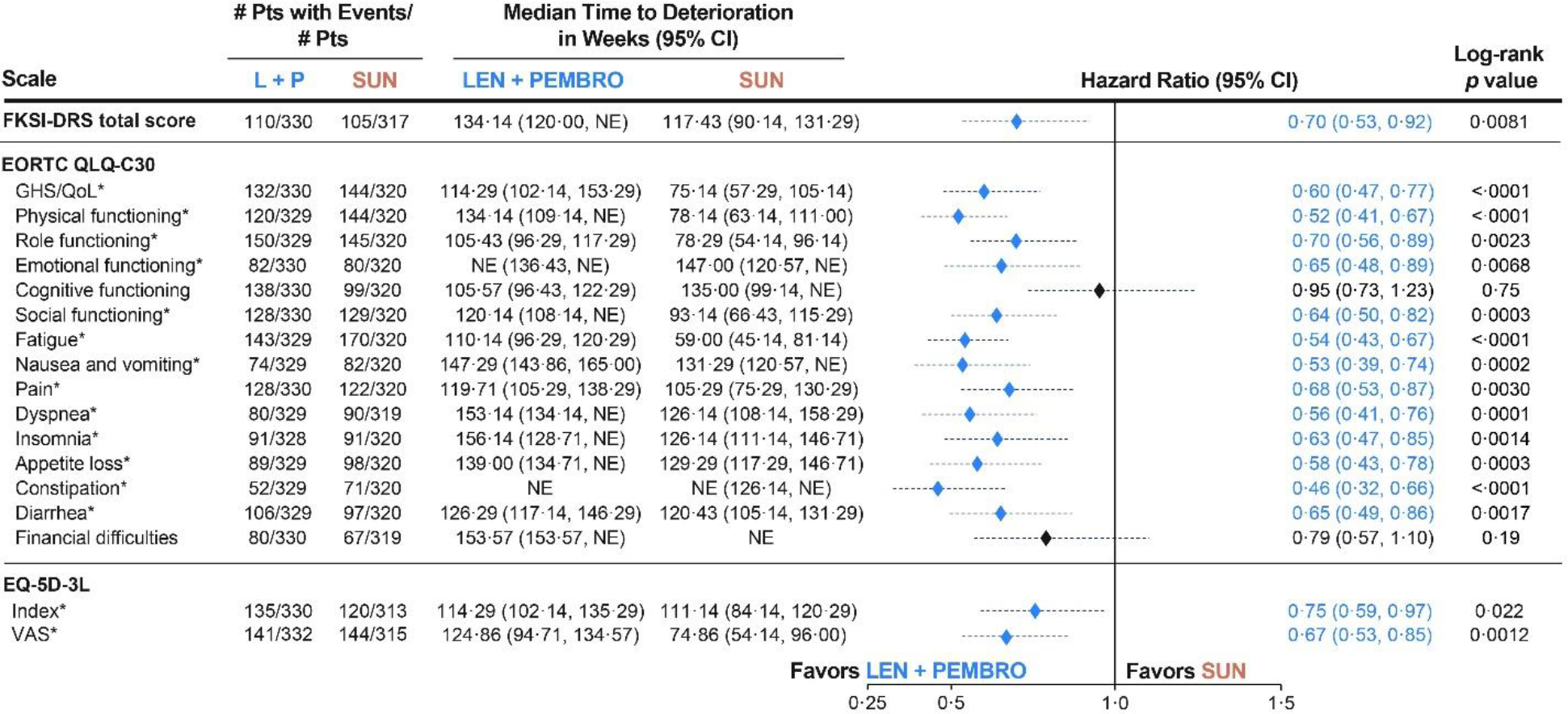
*Denotes a statistically significant log-rank difference (P < 0·05) on the distributions of lenvatinib + pembrolizumab vs sunitinib.
This analysis includes all patients in the HRQoL analysis set (n=351 in the lenvatinib + pembrolizumab arm, n=340 in the sunitinib arm.
CI, confidence interval; EORTC QLQ-C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EQ-5D-3L, EuroQol 5 Dimensions 3 Levels; FKSI-DRS, Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease related Symptoms; GHS/QoL, global health status/quality of life; LEN, lenvatinib; PEMBRO, pembrolizumab; NE, not estimable; SUN, sunitinib; VAS, visual analog scale.
DISCUSSION
HRQOL results in patients given lenvatinib plus pembrolizumab, combined with the efficacy previously reported,4 support this combination as a first-line treatment for patients with advanced renal cell carcinoma. Patients in the lenvatinib plus pembrolizumab group had similar or modestly improved HRQOL and disease-related symptom mean scores compared with those in the sunitinib group when assessing longitudinal changes from baseline (EORTC QLQ-C30 scales of physical functioning, fatigue, dyspnoea, and constipation). Additionally, time to first deterioration on several scales (EORTC QLQ-C30 physical functioning, EORTC QLQ-C30 dyspnoea, EORTC QLQ-C30 appetite loss, and EQ-5D-3L VAS) also favoured lenvatinib plus pembrolizumab versus sunitinib. Notably, time to definitive deterioration favoured lenvatinib plus pembrolizumab versus sunitinib for almost all outcomes (including FKSI-DRS total, EQ-5D-3L index, EQ-5D-3L VAS, EORTC QLQ-C30 GHS/QOL, EORTC QLQ-C30 physical functioning, and all EORTC QLQ-C30 symptom scales except financial difficulties). Moreover, this pattern was seen in patients in both the MSKCC and IMDC intermediate-risk or poor-risk subgroups, whereas results for patients in the favourable-risk subgroups favoured lenvatinib plus pembrolizumab in fewer outcomes. The patient numbers in each subgroup were small, with 27·0% of patients in the trial considered favourable risk by MSKCC and 31·0% by IMDC,4 and the analyses were done post hoc; the data should therefore be interpreted with caution. However, one explanation for these findings could be that patients in favourable-risk subgroups experienced decreases in HRQOL mostly because of drug-induced toxicity with fewer disease-related symptoms than those with intermediate-risk or poor-risk features, who were experiencing symptoms related to more extensive disease. Results from CLEAR indicate that patients who received lenvatinib plus pembrolizumab had longer maintenance of HRQOL and symptom control than those in the sunitinib group. As patients in the lenvatinib plus pembrolizumab group were also able to remain on treatment for longer, the data from this HRQOL analysis support the long-term tolerability of the lenvatinib plus pembrolizumab combination in comparison with sunitinib.
The general stability of HRQOL scores throughout the study probably reflects success in ameliorating adverse events via lenvatinib dose adjustments, thereby maintaining HRQOL over time. Specifically, adverse events led to dose reductions of lenvatinib in 68·8% of patients in the lenvatinib plus pembrolizumab group,4 a programmatic strategy used to maximise early drug exposure and therapeutic benefit while maintaining tolerability of lenvatinib. Combined with the longitudinal analysis, these data demonstrate that dose reductions of Lenvatinib and supportive care measures can be administered as needed without negatively affecting HRQOL.
One feature of this trial was that patients could continue to receive study drugs beyond initial disease progression if the investigator considered the patient to be receiving clinical benefit and tolerating treatment. Approximately 20% of patients in each group did continue to receive study drugs following confirmed progression. The effect of the treatment-beyond-progression trial design feature might have contributed to the favourable outcome for lenvatinib plus pembrolizumab compared with sunitinib in the time-to-definitive-deterioration analyses. Although modest differences were seen between the lenvatinib plus pembrolizumab and sunitinib groups in the time to first deterioration, results were more striking when examining time to definitive deterioration. An additional explanation for the higher degree of benefit for lenvatinib plus pembrolizumab compared with sunitinib in time to definitive deterioration versus time to first deterioration could be that some patients in the lenvatinib plus pembrolizumab group who initially had deterioration (possibly due to an adverse event) recovered because they remained on treatment. Time to definitive deterioration differs from time to first deterioration in that it is defined as the number of weeks between randomisation and the earliest deterioration event during the treatment period without subsequent recovery above the deterioration threshold (including additional assessments after the first deterioration score was recorded). This recovery reflected in the time-to-definitive-deterioration scores might have been facilitated by dose modification, supportive care, or both, which allowed patients to remain on treatment and continue to receive therapeutic benefits from lenvatinib plus pembrolizumab. These results are further supported by the fact that a large majority of deterioration events (first and definitive) were decreases in patient-reported outcome scores rather than patient deaths.
The HRQOL data should be interpreted in the context of the CLEAR phase 3 trial efficacy and safety results. Lenvatinib plus pembrolizumab demonstrated robust and clinically meaningful improvements compared with sunitinib in progression-free survival, overall survival, and objective response rate.4 Median progression-free survival (the primary endpoint) was 23·9 months (95% CI 20·8–27·7) in the lenvatinib plus pembrolizumab group versus 9·2 months (95% CI 6·0–11·0) in the sunitinib group (HR 0·39, 95% CI 0·32–0·49, p<0·001). Most importantly, comparison of overall survival was significantly in favour of lenvatinib plus pembrolizumab over sunitinib (median overall survival was not reached in both groups due to insufficient follow-up time), and objective response rate was 71·0% (95% CI 66·3–75·7) in the lenvatinib plus pembrolizumab group versus 36·1% (95% CI 31·2–41·1) in the sunitinib group (relative risk 1·97, 95% CI 1·69–2·29).4 Overall survival benefits with lenvatinib plus pembrolizumab were also seen in patients in the MSKCC intermediate-risk subgroup (HR 0·66, 95% CI 0·47–0·94) and poor-risk subgroup (0·50, 0·23–1·08), as well as the IMDC intermediate-risk subgroup (0·72, 0·50–1·05) and poor-risk subgroup (0·30, 0·14–0·64). Moreover, 16·1% of patients in the lenvatinib plus pembrolizumab group had a complete response, compared with 4·2% of patients in the sunitinib group.4 In a follow-up analysis (data cutoff date March 31, 2021), overall survival outcomes remained favourable for lenvatinib plus pembrolizumab over sunitinib (HR 0·72, 95% CI 0·55–0·93).26 Median duration of treatment was 17·0 months (range 0·1–39·1) in the lenvatinib plus pembrolizumab group compared with 7·8 months (0·1–37·0) in the sunitinib group. Adverse events of grade 3 or worse occurred in 82·4% of patients in the lenvatinib plus pembrolizumab group and 71·8% of patients in the sunitinib group.4 These results coupled with the HRQOL results provide strong support for lenvatinib plus pembrolizumab treatment as a first-line treatment for patients with advanced renal cell carcinoma.
Lenvatinib plus everolimus also demonstrated improvements in progression-free survival and objective response rate compared with sunitinib.4 Median progression-free survival was 14·7 months (95% CI 11·1–16·7) in the lenvatinib plus everolimus group and 9·2 months (6·0–11·0) in the sunitinib group (HR 0·65, 95% CI 0·53–0·80, p<0·001). Comparison of overall survival for lenvatinib plus everolimus versus sunitinib was not significant (HR 1·15, 95% CI 0·88–1·50, p=0·30). Median duration of treatment was 11·0 months (range 0·1–40·0) in the lenvatinib plus everolimus group. Grade 3 or worse adverse events were observed in 83·1% of patients in the lenvatinib plus everolimus group.4 Although lenvatinib plus everolimus showed improved progression-free survival and objective response rate compared with sunitinib, HRQOL results were similar or favoured sunitinib.
In addition to CLEAR, other phase 3 studies have investigated immune-based or kinase inhibitor-based therapies for renal cell carcinoma, and several have included HRQOL assessments. The CheckMate 214 study assessed HRQOL in patients with IMDC intermediate-risk or poor-risk disease who were treated with either sunitinib or the immunotherapy combination of nivolumab plus ipilimumab.20 Results from CheckMate 214 showed that HRQOL scores were maintained or improved from baseline with nivolumab plus ipilimumab, and that better HRQOL scores were observed with this combination than with sunitinib. In the CheckMate 9ER study, which compared nivolumab plus cabozantinib with sunitinib in patients with previously untreated advanced renal cell carcinoma, patients reported better HRQOL with nivolumab plus cabozantinib than with sunitinib.21 The KEYNOTE-426 study compared pembrolizumab plus axitinib with sunitinib in patients with advanced clear-cell renal cell carcinoma27 and found no clinically meaningful differences in change from baseline between groups using the EORTC QLQ-C30 GHS/QOL; however, time to true deterioration in the FKSI-DRS favoured sunitinib.22,28 Although these results should not be directly compared as a result of different analysis instruments being used in different studies, these kinase inhibitor-plus-immunotherapy combinations do appear to show some variation in resultant patient HRQOL profiles. Nevertheless, the results of these trials demonstrated that compared with sunitinib, immunotherapy-based combinations yield better efficacy and often improved patient-reported outcomes among patients with renal cell carcinoma.
This analysis of HRQOL in CLEAR had several limitations. Specifically, this was an open-label study in which treatment groups were not blinded, which could have led to a preference to stay on either of the two investigational treatment groups. Moreover, in the statistical analysis, the α value was not adjusted for multiple comparisons. In addition, although minimally important difference thresholds in this study were selected on the basis of published literature sources, uniformity surrounding cutoff points is absent in the literature. To address this issue, we used within-patient thresholds, which have greater variance than within-group thresholds and, thus, are larger. Although differences between groups were observed for the EORTC QLQ-C30 financial difficulties scale, all study drugs were provided free of charge during this trial, and thus financial difficulties are challenging to control for across groups. There might be bias in favour of sunitinib because of the timing of HRQOL assessments for patients in the sunitinib group, because those conducted at odd-numbered cycles coincided with the ends of the sunitinib 2 week off-treatment periods (ie, after cycle 3 [week 6] and cycle 5 [week 12]) when patients in the sunitinib group might have been expected to have had better HRQOL than those in the lenvatinib plus everolimus and lenvatinib plus pembrolizumab groups.
These HRQOL analyses from CLEAR (Study 307/KEYNOTE-581) demonstrated similar or favourable scores for lenvatinib plus pembrolizumab compared with sunitinib. By contrast, HRQOL scores for sunitinib were similar or favourable versus lenvatinib plus everolimus. Overall, the results of these analyses, combined with the established efficacy and safety results,4 support lenvatinib plus pembrolizumab as a standard first-line therapy for patients with advanced renal cell carcinoma.
Supplementary Material
Research in Context:
Evidence before this study
We searched PubMed on Oct 26, 2021, using the terms “renal cell carcinoma” AND “tyrosine kinase inhibitor” AND “immunotherapy” AND “patient-reported outcomes”, with no language restrictions. This search yielded three reports, all of which were review articles. When we revised the search criteria to “advanced renal cell carcinoma,” removed “immunotherapy,” and included clinical studies, the search yielded five results. All five results were phase 2 analyses or study design reports. The authors were also aware of a few phase 3 studies in renal cell carcinoma with health-related quality-of-life data that help to put this analysis in context: the CheckMate 214 study of nivolumab plus ipilimumab versus sunitinib and the CheckMate 9ER study of nivolumab plus cabozantinib versus sunitinib. Results from both studies showed better health-related quality-of-life outcomes in patients who received combination treatment compared with patients who received sunitinib.
Added value of this study
The combination of lenvatinib plus pembrolizumab demonstrated promising efficacy versus sunitinib in patients with advanced renal cell carcinoma in the phase 3 CLEAR study (Study 307/KEYNOTE-581). Health-related quality-of-life data from CLEAR indicate that lenvatinib plus pembrolizumab is similar or favourable to sunitinib in patient-reported outcomes when assessing change from baseline, time to first deterioration, and time to definitive deterioration, using the Functional Assessment of Cancer Therapy Kidney Symptom Index-Disease-Related Symptoms, European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30, and EQ-5D-3 Level assessment instruments. These results demonstrate a positive risk–benefit profile for lenvatinib plus pembrolizumab versus sunitinib in the treatment of patients with advanced renal cell carcinoma. Health-related quality-of-life data are a valuable tool to assist clinicians in selecting anticancer therapy for their patients; these data from CLEAR provide further support for administration of lenvatinib plus pembrolizumab in the first-line setting to patients with advanced renal cell carcinoma.
Implications of all the available evidence
In combination with the previously published efficacy data, results from these analyses indicate that combination lenvatinib plus pembrolizumab treatment provides both survival benefits and health-related quality-of-life benefits when compared with sunitinib in the first-line treatment of patients with advanced renal cell carcinoma.
Acknowledgments of research support for the study/role of the funding source:
This study was sponsored by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Medical writing support was provided by Heather A. Mitchell, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Declaration of Interests
Robert Motzer: Research funding (institutional) from Bristol Myers Squibb, Eisai, Exelixis, Genentech/Roche, Merck, Pfizer; consulting fees from AstraZeneca, Aveo Pharmaceuticals, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly, Merck, Novartis, Pfizer.
Camillo Porta: Consulting fees and honoraria from Angelini Farma, AstraZeneca, Bristol Myers Squibb, Eisai, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer; expert testimony for EUSA Pharma, Pfizer; protocol steering committee member for BMS, Eisai, EUSA Pharma, MSD; travel support from Roche.
Boris Alekseev: Support for the present manuscript from Eisai; grants or contracts from Amgen, AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, Roche; consulting fees and honoraria from Amgen, AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, Roche; expert testimony for Amgen, AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, Roche; travel support from AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, Roche; participation on a data safety monitoring board or advisory board for Astellas, Ipsen, Janssen.
Sun Young Rha: Grants or contracts from Eisai; honoraria from Eisai; participation on a data safety monitoring board or advisory board for Eisai.
Toni K. Choueiri: Support for the present manuscript from Eisai and Merck; research funding (institutional) from AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, and Takeda; patents filed, royalties or other intellectual properties (no income as of current date) related to biomarkers of immune checkpoint blockers and ctDNA; consulting/honoraria or advisory role for Alexion, Analysis Group, Aravive, AstraZeneca, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Cerulean, Corvus, Eisai, EMD Serono, Exelixis, Foundation Medicine Inc., Genentech, GlaxoSmithKline, Heron Therapeutics, Infinity Pharma, Ipsen, Janssen Oncology, IQVIA, Lilly, Merck, NCCN, NiKang, Novartis, Nuscan, Peloton, Pfizer, Prometheus Labs, Roche, Sanofi/Aventis, Surface Oncology, Tempest, Up-to-Date, CME-related events (e.g.: OncLive, PVI, MJH Life Sciences); NCI GU Steering Committee, ASCO, ESMO, NCCN; travel in relation to meetings, lectures and advisory boards; stock ownership from Pionyr, Tempest.
Maria Jose Mendez-Vidal: Consulting fees from Astellas Pharma, Bristol Myers Squibb, EUSA Pharma, Ipsen, EISAI, Janssen-Cilag, Novartis, Pfizer, Roche, Sanofi; honoraria from Astellas Pharma, Bristol Myers Squibb, Ipsen, EUSA Pharma, Janssen-Cilag, Pfizer, Roche; support for attending meetings and/or travel from Astellas Pharma, Bristol Myers Squibb, Ipsen, Janssen-Cilag, Pfizer, Roche.
Sung-Hoo Hong: Nothing to declare.
Anil Kapoor: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Bristol Myers Squibb, Ipsen, Eisai, Janssen, AbbVie. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Vice Chair, Kidney Cancer Canada. Stock or stock options from Verity Pharma.
Jeffrey C. Goh: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, MSD Australia (Merck), Ipsen, Bristol Myers Squibb. Participation on a Data Safety Monitoring Board or Advisory Board for Bristol Myers Squibb, Janssen (Johnson & Johnson). Stock or stock options from ICON Cancer Care, Australia.
Masatoshi Eto: Research funding from Kissei, Sanofi, Astellas, ONO, Takeda, and Bayer; honoraria for lectures from MSD, ONO, Chugai, Novartis, Pfizer, Bristol Myers Squibb, Takeda, Janssen, and Merck.
Lee Bennett: Employee of RTI Health Solutions, which was contracted by Eisai, Inc., to perform statistical analyses reported in this manuscript.
Jinyi Wang: Employee of RTI Health Solutions, which was contracted by Eisai, Inc., to perform statistical analyses reported in this manuscript.
Janice Pan: Full time employee of Eisai Inc.
Todd L. Saretsky: Paid employee of Merck & Co., Inc.; shareholder of Merck & Co., Inc.
Rodolfo F. Perini: Employee of Merck & Co., Inc.; stock owner of Merck & Co., Inc.
Cixin Steven He: Full time employee of Eisai Inc.
Kalgi Mody: Employee of Eisai Inc.
David Cella: Consulting fees (personal) from Eisai, Bristol Myers Squibb, Pfizer, Merck, Novartis, Ipsen, Evidera. President of FACIT.org.
Contributor Information
Robert Motzer, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Camillo Porta, Interdisciplinary Department of Medicine, University of Bari ‘A Moro’, Bari, Italy; Department of Biomedical Sciences and Human Oncology, University of Pavia, Pavia, Italy.
Boris Alekseev, Department of Oncourology, P A Herzen Moscow Oncological Research Institute, Moscow, Russia.
Sun Young Rha, Yonsei Cancer Center, Yonsei University Health System, Seoul, South Korea.
Toni K. Choueiri, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Maria Jose Mendez-Vidal, Maimonides Institute for Biomedical Research of Cordoba Hospital, Universitario Reina Sofía, Córdoba, Spain.
Sung-Hoo Hong, Seoul St Mary’s Hospital, The Catholic University of Korea, Seoul, South Korea.
Anil Kapoor, McMaster University, Hamilton, ON, Canada.
Jeffrey C. Goh, ICON Research, South Brisbane and Queensland University of Technology, Brisbane, QLD, Australia.
Masatoshi Eto, Kyushu University, Fukuoka, Japan.
Lee Bennett, RTI Health Solutions, Research Triangle Park, NC, USA.
Jinyi Wang, RTI Health Solutions, Research Triangle Park, NC, USA.
Jie Janice Pan, Eisai, Nutley, NJ, USA.
Todd L. Saretsky, Merck & Co, Kenilworth, NJ, USA.
Rodolfo F. Perini, Merck & Co, Kenilworth, NJ, USA.
Cixin Steven He, Eisai, Nutley, NJ, USA.
Kalgi Mody, Eisai, Nutley, NJ, USA.
David Cella, Department of Medical Social Sciences, Northwestern University, Chicago, IL, USA.
Data sharing statement
The data are commercially confidential and will not be available for sharing; however, Eisai will consider written requests to share the data on a case-by-case basis. Data requests should be made to Kalgi Mody at Kalgi_Mody@eisai.com.
References
- 1.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015; 16: 1473–82. [DOI] [PubMed] [Google Scholar]
- 2.McDermott DF, Lee JL, Bjarnason GA, et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol 2021; 39: 1020–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021; 22: 946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384: 1289–300. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20: 71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Albiges L, Bex A, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol 2021; 32: 1511–19. [DOI] [PubMed] [Google Scholar]
- 7.Merck Sharp & Dohme. Keytruda® (pembrolizumab) package insert. Whitehouse Station, NJ, USA: Merck Sharp & Dohme, 2021. [Google Scholar]
- 8.Eisai. Lenvima® (lenvatinib) package insert. Nutley, NJ, USA: Eisai, 2021. [Google Scholar]
- 9.Padala SA, Kallam A. Clear cell renal carcinoma. StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 10.Song XD, Tian YN, Li H, Liu B, Zhang AL, Hong Y. Research progress on advanced renal cell carcinoma. J Int Med Res 2020; 48: 300060520924265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kekäle M, Peltoniemi M, Airaksinen M. Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on per oral tyrosine kinase inhibitor treatment. Patient Prefer Adherence 2015; 9: 1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu ST, Ge JN, Luo JY, Wei ZG, Sun BH, Lei ST. Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: a systematic review and meta-analysis. Cancer Manag Res 2019; 11: 1525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabanillas ME, Takahashi S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin Oncol 2019; 46: 57–64. [DOI] [PubMed] [Google Scholar]
- 14.Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus 2020; 12: e6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou K, Fountzilas C. Outcomes and quality of life of systemic therapy in advanced hepatocellular carcinoma. Cancers (Basel) 2019; 11: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health 2007; 10: 285–93. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76. [DOI] [PubMed] [Google Scholar]
- 18.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–43. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol 2022; 23: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella D, Grünwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019; 20: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021; 384: 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedke J, Rini B, Plimack E, et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). European Association of Urology (EAU20) Virtual Congress; July 17–26, 2020. [Google Scholar]
- 23.Cella D, Li JZ, Cappelleri JC, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. J Clin Oncol 2008; 26: 3763–69. [DOI] [PubMed] [Google Scholar]
- 24.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–44. [DOI] [PubMed] [Google Scholar]
- 25.Pickard AS, De Leon MC, Kohlmann T, Cella D, Rosenbloom S. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care 2007; 45: 259–63. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab versus sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (the CLEAR study). Kidney Cancer Research Summit (KCRS21); Oct 7–8, 2021. [Google Scholar]
- 27.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–27. [DOI] [PubMed] [Google Scholar]
- 28.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Assessement report, procedure no. EMEA/H/C/003820/II/0069. July 25, 2019. https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-3820-ii-0069-epar-assessment-report-variation_en.pdf (accessed Nov 8, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are commercially confidential and will not be available for sharing; however, Eisai will consider written requests to share the data on a case-by-case basis. Data requests should be made to Kalgi Mody at Kalgi_Mody@eisai.com.


