Background:
Smoking is associated with carotid intima-media thickness (C-IMT). However, knowledge about how genetics may influence this association is limited. We aimed to perform nonhypothesis driven gene-smoking interaction analyses to identify potential genetic variants, among those included in immune and metabolic platforms, that may modify the effect of smoking on carotid intima-media thickness.
Methods:
We used baseline data from 1551 men and 1700 women, aged 55 to 79, included in a European multi-center study. Carotid intima-media thickness maximum, the maximum of values measured at different locations of the carotid tree, was dichotomized with cut point values ≥75, respectively. Genetic data were retrieved through use of the Illumina Cardio-Metabo- and Immuno- Chips. Gene-smoking interactions were evaluated through calculations of Synergy index (S). After adjustments for multiple testing, P values of <2.4×10−7 for S were considered significant. The models were adjusted for age, sex, education, physical activity, type of diet, and population stratification.
Results:
Our screening of 207 586 SNPs available for analysis, resulted in the identification of 47 significant gene-smoking synergistic interactions in relation to carotid intima-media thickness maximum. Among the significant SNPs, 28 were in protein coding genes, 2 in noncoding RNA and the remaining 17 in intergenic regions.
Conclusions:
Through nonhypothesis-driven analyses of gene-smoking interactions, several significant results were observed. These may stimulate further research on the role of specific genes in the process that determines the effect of smoking habits on the development of carotid atherosclerosis.
Keywords: carotid intima-media thickness; epidemiologic studies; gene-environment interaction; polymorphism, single nucleotide; smoking
Subclinical atherosclerosis is an asymptomatic, chronic condition that is easily undiagnosed until a clinical event occurs, such as myocardial infarction or stroke.1 Carotid intima-media thickness (C-IMT), assessed with B-mode ultrasound, a noninvasive method, has been shown to be a valid surrogate marker for subclinical atherosclerosis,2 and a predictor for future cardiovascular disease (CVD).3,4
Previous studies indicate that genetic susceptibility plays an important role in the pathogenesis of atherosclerosis.5–8 The reported proportions of heritability of carotid atherosclerosis vary between 2% and 78%.8 Part of this heritability is likely to be explained by gene-environment interactions.8 There are hopes from the scientific community and healthcare that personalized medicine, such as knowledge of how genetic background can interact with modifiable factors and thereby influence cardiovascular risk, will be able to contribute to improved prevention of CVD.
Among the risk factors for premature atherosclerosis, smoking has been identified as a major determinant of atherosclerotic development.9–11 Studies have shown that smoking exposure and duration of smoking cessation can affect carotid artery structure in all phases of atherosclerosis.12,13 In an earlier investigation based on data from a European multi-center study IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population), smoking was found to be a major determinant of C-IMT.14
Previous studies that have investigated gene-smoking interactions behind carotid atherosclerosis were generally performed with a candidate gene approach, and the results are inconclusive.15–33 Only 2 studies evaluated gene-smoking interactions with an explorative approach, using the whole genome, one based on 669 Hispanics, mainly women, residing in New York,34 and the other based on 1776 men from West Africa.35 These studies are insufficient to detect all important gene-smoking interactions due to their limited sample size. In addition, it is doubtful whether the results can be generalized to populations of other ancestries.
Hence, we aimed to explore gene-smoking interactions behind carotid subclinical atherosclerosis in a multi-center study including men and women of European ancestry. We limited the search for interactions to include genetic variants available via platforms for genetic studies of cardiovascular, metabolic, and immune traits.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The Institutional review board at each recruitment center (Karolinska Institutet, Stockholm, Sweden; University of Milan, Milan, Italy; University of Kuopio and Kuopio Research Institute of Exercise Medicine, Kuopio, Finland; University Hospital Groningen, Groningen, The Netherlands; University of Perugia, Perugia, Italy; Groupe Hôpital Pitie-Salpetriere, Paris, France) approved the study. Written informed consents for general participation and for the genotyping were provided by all participants. The study was performed in accordance with the Helsinki Declaration.
Full materials and methods are available in Supplemental Materials.
Results
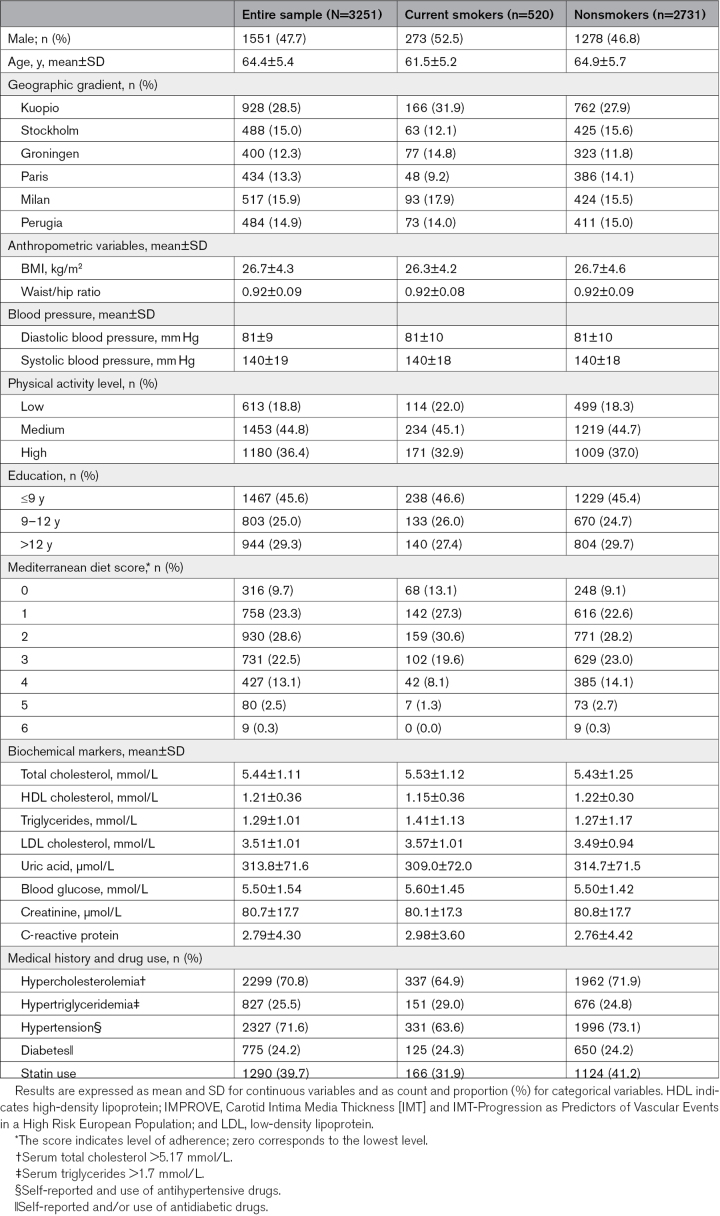
Baseline characteristics of all study participants and by their smoking status are presented in Table 1. The current smokers were younger, less physically active, and educated than nonsmokers. Smokers had also higher levels of total cholesterol, triglycerides, LDL-C (low-density lipoprotein cholesterol), blood glucose, and High-sensitivity C-reactive protein. However, their level of uric acid and creatinine were lower than in nonsmokers.
Table 1.
Characteristics of the IMPROVE Study Participants by Smoking Status
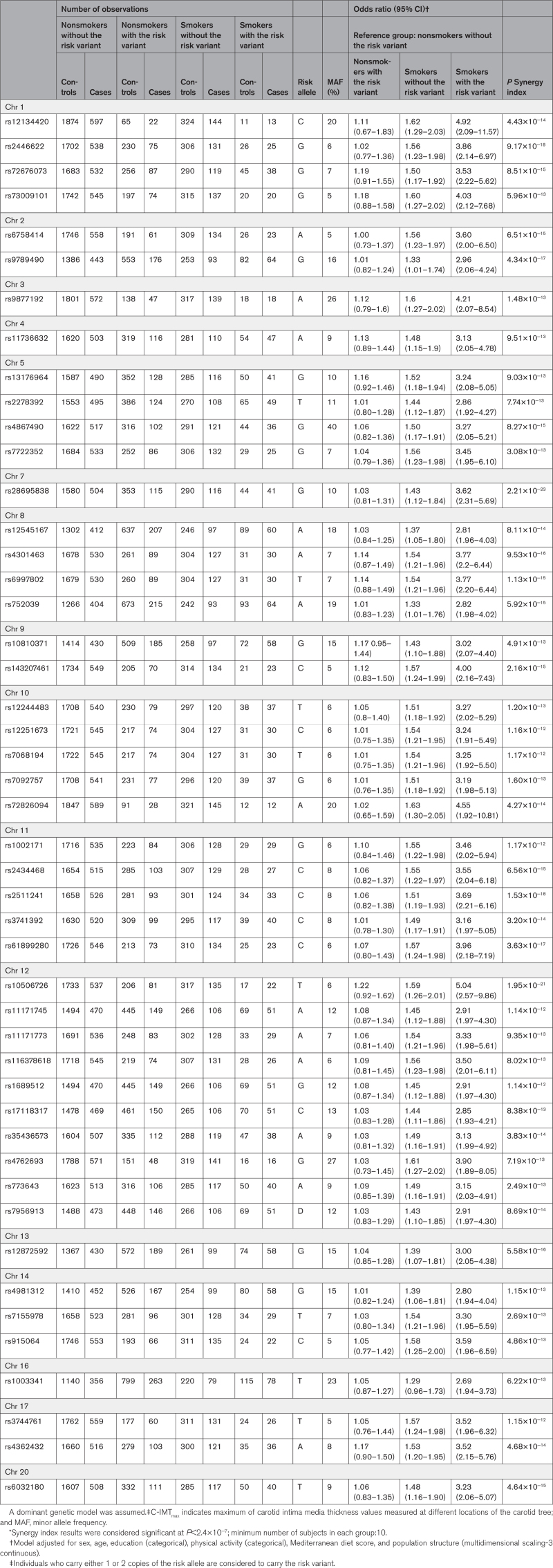
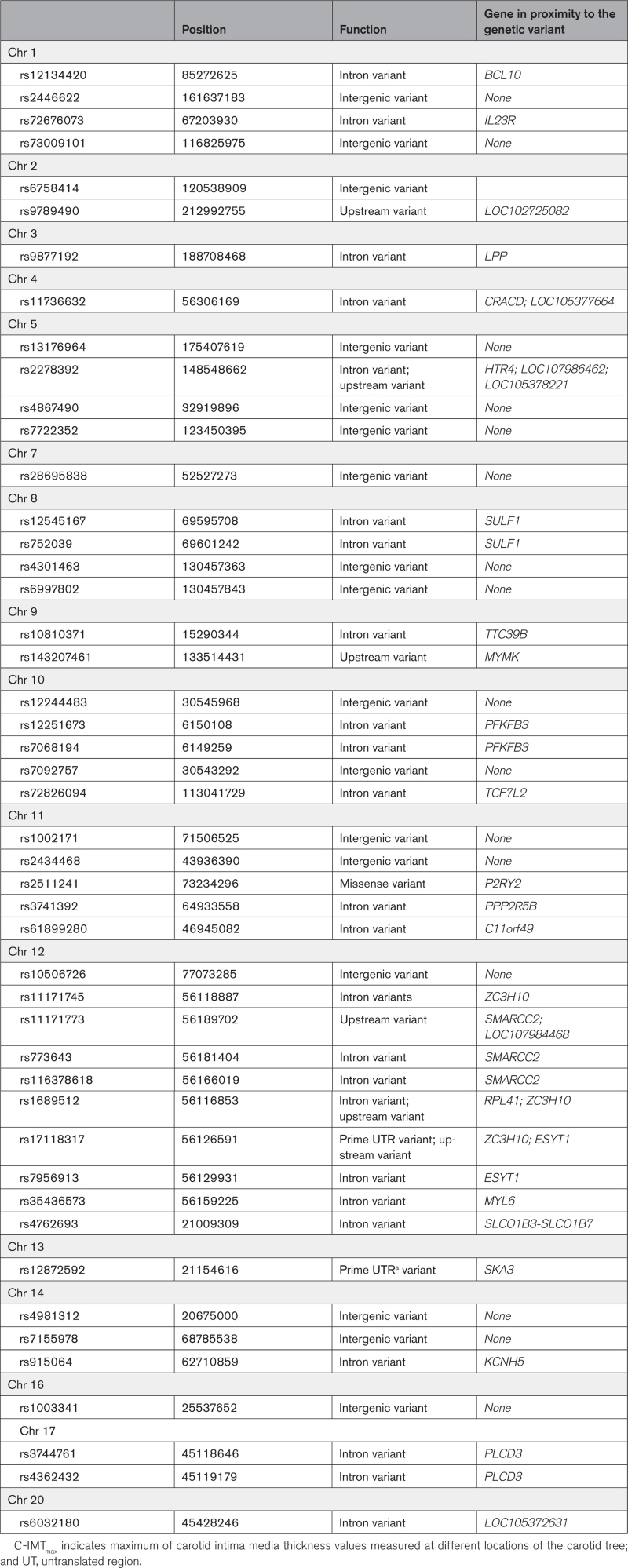
In total, 207 586 genetic variants were available for analyses. Results from the main analysis investigating gene-smoking interaction in relation to C-IMTmax cut off at the 75th percentile are shown in Table 2. We found 47 single nucleotide polymorphisms (SNPs) significant (P for Synergy index <2.4×10−7) after Bonferroni correction. All the aforementioned interaction results were synergistic, with Synergy index point estimates in the range between 3.3 and 5.8 (Table S1). Compared with the reference group of nonsmokers without the risk variant, the odds for having C-IMT >75th percentile associated with smoking and having the risk variant were ≈3 to 4-fold higher (Table 2). Of the 47 significant SNPs, 28 were in protein coding genes, 2 in noncoding RNA and the remaining 17 in intergenic regions (Table 3). None of the 47 SNPs involved in the interactions identified in our study were among the published quantitative trait locus data included in the Genotype-Tissue Expression (accessed March 25, 2022).
Table 2.
Significant Gene-Smoking Interaction Results* After Bonferroni Adjustment for Multiple Testing in Relation to C-IMTmax With Cutoff at the 75th Percentile
Table 3.
Genes in Proximity to the Genetic Variants Included in the Significant Gene-Smoking Interaction Results Observed for C-IMTmax With Cutoff at the 75th Percentile
Additional analysis that used C-IMTmax cutoff at the 50th percentile resulted in the identification of 146 SNPs for which a significant synergistic interaction with smoking was observed (Table S2). Among those SNPs, 75 were in protein coding genes, 21 in noncoding RNA, and the remaining 50 in intergenic regions (Table S3). Two of these significant SNPs (rs6032180 in LOC105372631 and rs3744761 in PLCD3) were found both when using the 75th and the 50th percentile C-IMTmax cutoff values.
Analyses of gene-smoking interactions that also considered data where the number of observations for each of the possible combinations of the exposures considered are <10 resulted in the identification of additional significant results for the C-IMTmax, cutoff 75th percentile (Table S4), and for C-IMTmax, cutoff 50th percentile (Table S5). All the observed interactions were synergistic. Of the SNPs that appeared in these results, 130 are located in protein coding genes, 43 in long noncoding RNA, and 84 in intergenic regions (Table S6).
We observed no significant results of interaction on the multiplicative scale.
Discussion
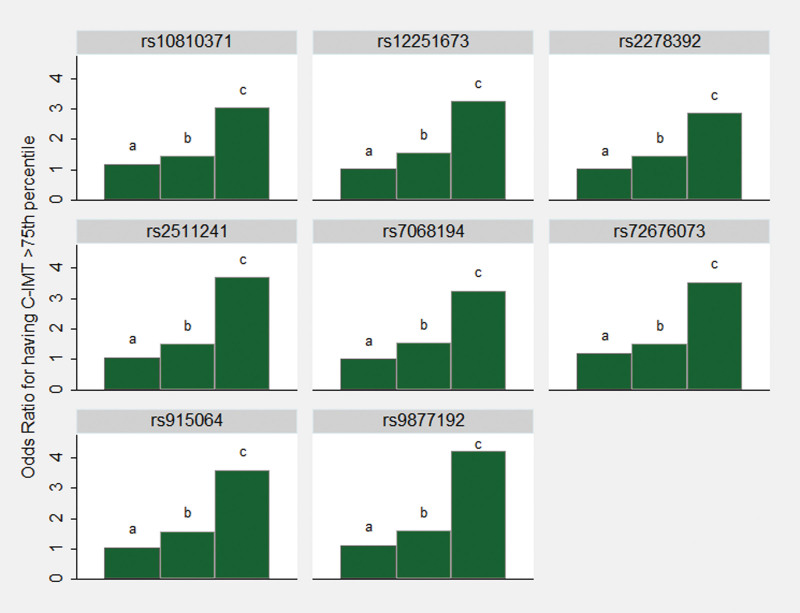
In this population of European descent at high risk of CVD but free of clinical manifestations of CVD, our nonhypothesis-based analyses of gene-smoking interactions resulted in the identification of several genetic variants that may have a role in the process behind the effects of smoking on the development of carotid atherosclerosis. Among the 47 SNPs identified in the main analyses, 8 SNPs (Figure 1) are located in any of 7 coding genes that in previous research have been linked to atherosclerosis development: rs72676073 in the interleukin 23 receptor (IL23R), rs9877192 in the LIM (Lin-11, Islet-1, and Mec-3) domain containing preferred translocation partner in lipoma (LPP), rs2278392 in the 5-hydroxytryptamine receptor 4 (HTR4), rs10810371 in the tetratricopeptide repeat domain 39B (TTC39B), rs7068194 and rs12251673 in the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB3), rs2511241 in the purinergic receptor P2Y2 (P2RY2), and rs915064 in the potassium voltage-gated channel subfamily H member 5 (KCNH5).36–42 None of these coding genes were identified in 2 previous studies that evaluated gene-smoking interactions with an explorative approach in relation to carotid atherosclerosis.34,35 These 2 studies were based on the whole genome and assessed interaction on the multiplicative scale only; significant findings of interaction with smoking were observed for a few genetic variants (rs112017404; rs144170770; rs4941649; rs1192824; rs77461169; rs3751383)34,35 that were not available in the Cardio-Metabo- and Immuno-Chips.
Figure 1.
Visualization of 8 selected significant results from interaction analyzes. The bars show odds ratio point estimates for the risk of having carotid intima-media thickness (C-IMT) above the 75th percentile associated with (a) the genetic risk variant without the presence of smoking, (b) smoking without the presence of the genetic risk variant, and (c) the genetic risk variant in combination with smoking. Reference category is nonsmoking without the presence of the genetic risk variant. These 8 SNPs are located in coding genes previously linked to the development of atherosclerosis.
Scientific support for relevance of the IL23R gene seems to be emerging; it encodes for a protein, interleukin 23 receptor, involved in the cascade of proinflammatory mediators which may in turn play a role in the development of atherosclerosis.36 Further, the IL23R gene has been previously related to autoimmune disease43,44 and smoking behavior.45 It has been found to synergically interact with smoking in relation to sarcoidosis, an autoimmune disease, in a Swedish population-based case-control study.43 The HTR4 and P2RY2 genes may also possibly be of particular interest. These proteins belong to the family of serotonin and purinergic receptors, respectively. The activation of extracellular nucleotide purinergic receptors, such as ATP, has been suggested to stimulate inflammatory mediators46 and regulate the expression of vascular cell adhesion molecule, which is thought to be important for the pathogenesis of atherosclerosis.39 The HTR4 gene has been noted to associate to C-IMT in a previous study based on the IMPROVE study material using a candidate gene approach.41
Among the 47 SNPs identified in our main analysis of interaction as well as in our additional analyses that used the 50th percentile cutoff, there is a SNP (rs3744761), located in a protein coding gene, the phospholipase C delta 3 (PLCD3) gene, which may be of particular interest due to its link to hypertension. This gene has been identified in the Global Blood Pressure Genetics Consortium genome-wide association study (GWAS) including >34 000 study participants, as one of 8 genes linked to hypertension.47 Hypertension, in turn, has been consistently associated with increased C-IMT in several studies including the IMPROVE.14,48 The identification of the PLCD3 gene in the Global Blood Pressure Genetics was not confirmed in a later larger GWAS: the International Consortium for Blood Pressure (≈200 000 study participants including also Global Blood Pressure Genetics participants).49 A possible explanation for this lack of replication may relate to underlying gene-smoking interaction.
Among the 146 significant interaction results generated from analyses that used the 50th percentile C-IMTmax cutoff, 75 are in protein coding genes. Among those, perhaps the most interesting finding involves the APOB (apolipoprotein B) gene (rs550619 and rs570877). The APOB gene encodes for the well-known APOB protein involved in the transportation and metabolism of lipids such as LDL-C, which in turn seems to play a fundamental role in CVD pathophysiology.50 Findings from recent Mendelian randomization studies suggest APOB as the predominant lipoprotein trait that accounts for a causal mechanism that links LDL-C to CVD.51,52 Also, levels of APOB have been noted to increase in relation to smoking tobacco,53 however, not consistently.54
The remaining significant results (not discussed above) from analyses based on the C-IMTmax 75th or 50th percentile cutoffs, involve SNPs located in genes previously discussed in relation to: (1) regulation of cardiometabolic factors and related diseases such as obesity, hypertension and diabetes (eg, COBLL1; HFM1, CXCR1; COL21A1, DOCK3; DGKB, BMP1; IDE; KCNQ1; and KCNQ1-AS1, ZC3H10),55–64 (2) endothelial inflammation and dysfunction (eg, TNFAIP8L1, CCNY, GSE1),65–67 (3) vascular smooth muscle cell proliferation (eg, VEGFA),68 (4) inflammatory diseases (eg, PSORS1C1),69 (5) risk of CVD hard end point such as atrial fibrillation and venous thromboembolism (eg, ZFPM2; LMO7),70,71 and (6) addiction behavior including nicotine dependence (eg, SP140L, THSD7B).72,73
From the results of our analyses restricted to cell counts of 10 or below, the identification of a SNP located in the PIN2/TERF1 interacting, telomerase inhibitor 1 (PINX1) gene is potentially interesting, because this gene was previously identified in GWAS of subclinical atherosclerosis6 and carotid plaque.7 However, it was not found to interact with smoking in a previous study on C-IMT using a candidate gene approach.33 The study addressed multiplicative interactions only.
An important advantage of our study is that we did not limit the gene-smoking interaction analyzes to involve SNPs identified in previous GWAS of C-IMT. It is possible that a gene itself is not associated with C-IMT but becomes important only when smoke exposure occurs. Interestingly, none of the SNPs we have identified as significantly involved in smoking interaction are among the significant findings reported in previous GWAS in relation to C-IMT or smoking behavior.5,7
Limitations
Our study, just like other exploratory studies, cannot determine which findings are truly positive, and as to whether there are other true effects we did not detect. However, we used the most conservative approach available to adjust for multiple testing, which increases the likelihood that reported findings are true positive. Further, to our knowledge, our study is the largest to date investigating gene-smoking interaction in relation to subclinical atherosclerosis with an explorative approach. Interactions we may have failed to identify should be of a smaller magnitude than those we have identified. Concerning our positive findings, replication analyses using an external study material would have been a good complement. However, no suitable material for replication analyses was available. Another study limitation is that our genetic data were extracted from genetic chips which do not encompass the whole genome; our results are thus limited to genes related to cardiovascular and immunologic traits which means that some of the relevant SNPs related to smoking predisposition may not have been included. An additional limitation is that our results may not be generalized to populations other than those with European ancestry and at high risk of CVD. Finally, there is also a limitation linked to the fact that our chosen method for interaction analyses requires dichotomization of exposure variables; the results may have been diluted because we included former smokers in the same category as the current smokers. However, smoking cessation is considered a risk factor for CVD.74 Further, studies on the relation between smoking cessation and C-IMT have not shown any clear decreased risk of C-IMT progression.75
Conclusions
In this European population at high risk of CVD, we identified several significant gene-smoking interactions in relation to C-IMT. Further research in this field is urged to build strong scientific evidence that may open new possibilities for improving cardiovascular prevention through personalized recommendations or drug development.
Article Information
Acknowledgments
The authors thank all the study participants of the IMPROVE study (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population). The authors also thank Max Vikström and Paolo Frumento for their statistical support.
Sources of Funding
The IMPROVE study (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) was supported by the European Commission (contract number: QLG1- CT- 2002- 00896; to Drs Tremoli, Baldassarre, Giral, Kurl, Pirro); Ministero della Salute Ricerca Corrente, Italy (2017; 2018; to Dr Baldassarre). The IMPROVE was also funded by the Swedish Research Council (8691 and 0593), European Commission (contract number: QLG1- CT- 2002- 00896), the Swedish Heart-Lung Foundation, the Swedish Foundation for Strategic Research, the Stockholm County Council (project 562183), and the British Heart Foundation (RG2008/008). The present study was supported by the Swedish Research Council (project 2016-02815 to Dr Leander). Dr Strawbridge is supported by United Kingdom Research and Innovation-Health Data Research United Kingdom Fellowship (MR/S003061/1).
Disclosures
None.
Supplemental Material
Supplemental Methods
Tables S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- APOB
- apolipoprotein B
- C-IMT
- carotid intima-media thickness
- C-IMTmax
- maximum of C-IMT values measured at different locations of the carotid tree
- GWAS
- genome-wide association study
- IMPROVE
- Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population
- SNP
- single nucleotide polymorphism
B. Maitusong and F. Laguzzi contributed equally.
A list of all IMPROVE study group members is given in the Supplemental Material.
For Sources of Funding and Disclosures, see page 245.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.122.003710.
Circulation: Genomic and Precision Medicine is available at www.ahajournals.org/journal/circgen
Contributor Information
Buamina Maitusong, Email: amina_ki@163.com.
Rona J. Strawbridge, Email: rona.strawbridge@glasgow.ac.uk.
Damiano Baldassarre, Email: damiano.baldassarre@unimi.it.
Fabrizio Veglia, Email: fveglia@gvmnet.it.
Steve E. Humphries, Email: steve.humphries@ucl.ac.uk.
Kai Savonen, Email: kai.savonen@uef.fi.
Sudhir Kurl, Email: sudhir.kurl@uef.fi.
Matteo Pirro, Email: matteo.pirro@unipg.it.
Andries J. Smit, Email: a.j.smit@umcg.nl.
Philippe Giral, Email: philippe.giral@aphp.fr.
Angela Silveira, Email: angela.silveira@ki.se.
Elena Tremoli, Email: etremoli@gvmnet.it.
Anders Hamsten, Email: anders.hamsten@gmail.com.
Ulf de Faire, Email: ulf.defaire@ki.se.
Bruna Gigante, Email: bruna.gigante@ki.se.
Karin Leander, Email: karin.leander@ki.se.
References
- 1.Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P, Garcia MJ, Gregson J, Pocock S, Falk E, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65:1065–1074. doi: 10.1016/j.jacc.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 2.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, Giral P, Kurl S, Rauramaa R, Mannarino E, et al. ; IMPROVE Study Group. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol. 2012;60:1489–1499. doi: 10.1016/j.jacc.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 3.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, Ballantyne CM. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183–190. doi: 10.1093/eurheartj/ehr192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strawbridge RJ, Ward J, Bailey MES, Cullen B, Ferguson A, Graham N, Johnston KJA, Lyall LM, Pearsall R, Pell J, et al. Carotid intima-media thickness: novel loci, sex-specific effects, and genetic correlations with obesity and glucometabolic traits in UKB. Arterioscler Thromb Vasc Biol. 2020;40:446–461. doi: 10.1161/ATVBAHA.119.313226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bis JC, Kavousi M, Franceschini N, Isaacs A, Abecasis GR, Schminke U, Post WS, Smith AV, Cupples LA, Markus HS, et al. ; CARDIoGRAM Consortium. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43:940–947. doi: 10.1038/ng.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschini N, Giambartolomei C, de Vries PS, Finan C, Bis JC, Huntley RP, Lovering RC, Tajuddin SM, Winkler TW, Graff M, et al. ; MEGASTROKE Consortium. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat Commun. 2018;9:5141. doi: 10.1038/s41467-018-07340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forgo B, Medda E, Hernyes A, Szalontai L, Tarnoki DL, Tarnoki AD. Carotid artery atherosclerosis: a review on heritability and genetics. Twin Res Hum Genet. 2018;21:333–346. doi: 10.1017/thg.2018.45 [DOI] [PubMed] [Google Scholar]
- 9.Sugiura T, Dohi Y, Takagi Y, Yokochi T, Yoshikane N, Suzuki K, Tomiishi T, Nagami T, Iwase M, Takase H, et al. Close association between subclinical atherosclerosis and pulmonary function in middle-aged male smokers. J Atheroscler Thromb. 2020;27:1230–1242. doi: 10.5551/jat.55996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Mason AM, Back M, Klarin D, Damrauer SM, Million Veteran P, Michaelsson K, Burgess S. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J. 2020;41:3304–3310. doi: 10.1093/eurheartj/ehaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41:4057–4070. doi: 10.1093/eurheartj/ehaa460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kweon SS, Lee YH, Shin MH, Choi JS, Rhee JA, Choi SW, Ryu SY, Kim BH, Nam HS, Jeong SK, et al. Effects of cumulative smoking exposure and duration of smoking cessation on carotid artery structure. Circ J. 2012;76:2041–2047. doi: 10.1253/circj.cj-11-1353 [DOI] [PubMed] [Google Scholar]
- 13.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010;16:247–253. doi: 10.2119/molmed.2009.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldassarre D, Nyyssonen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ, Mannarino E, Humphries SE, Giral P, Grossi E, et al. ; IMPROVE study group. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J. 2010;31:614–622. doi: 10.1093/eurheartj/ehp496 [DOI] [PubMed] [Google Scholar]
- 15.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Jr, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 16.Wang XL, Greco M, Sim AS, Duarte N, Wang J, Wilcken DE. Effect of CYP1A1 MspI polymorphism on cigarette smoking related coronary artery disease and diabetes. Atherosclerosis. 2002;162:391–397. doi: 10.1016/s0021-9150(01)00723-7 [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan SR, Li S, Chen W, Tang R, Bond MG, Boerwinkle E, Berenson GS. Q192R polymorphism of the paraoxanase 1 gene and its association with serum lipoprotein variables and carotid artery intima-media thickness in young adults from a biracial community. The Bogalusa Heart Study. Atherosclerosis. 2004;177:167–174. doi: 10.1016/j.atherosclerosis.2004.06.013 [DOI] [PubMed] [Google Scholar]
- 18.Rosner SA, Ridker PM, Zee RY, Cook NR. Interaction between inflammation-related gene polymorphisms and cigarette smoking on the risk of myocardial infarction in the Physician’s Health Study. Hum Genet. 2005;118:287–294. doi: 10.1007/s00439-005-0052-6 [DOI] [PubMed] [Google Scholar]
- 19.Rios DL, D’Onofrio LO, Souza JK, Queiroz AM, Raduy-Maron L, Silva-Neto N, Carvalho HG, Santos-Filho A, Galvao-Castro B. Smoking-dependent and haplotype-specific effects of endothelial nitric oxide synthase gene polymorphisms on angiographically assessed coronary artery disease in Caucasian- and African-Brazilians. Atherosclerosis. 2007;193:135–141. doi: 10.1016/j.atherosclerosis.2006.05.041 [DOI] [PubMed] [Google Scholar]
- 20.Payne JR, Dhamrait SS, Toor IS, Cooper J, Jones A, Miller GJ, Humphries SE, Montgomery HE. The -344T>C promoter variant of the gene for aldosterone synthase (CYP11B2) is not associated with cardiovascular risk in a prospective study of UK healthy men. Atherosclerosis. 2004;174:81–86. doi: 10.1016/j.atherosclerosis.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Olshan AF, Li R, Pankow JS, Bray M, Tyroler HA, Chambless LE, Boerwinkle E, Pittman GS, Bell DA. Risk of atherosclerosis: interaction of smoking and glutathione S-transferase genes. Epidemiology. 2003;14:321–327. doi: 10.1097/01.EDE.0000059229.74889.CF [PubMed] [Google Scholar]
- 22.North KE, Carr JJ, Borecki IB, Kraja A, Province M, Pankow JS, Wilk JB, Hixson JE, Heiss G; Investigators FHS. QTL-specific genotype-by-smoking interaction and burden of calcified coronary atherosclerosis: the NHLBI Family Heart Study. Atherosclerosis. 2007;193:11–19. doi: 10.1016/j.atherosclerosis.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 23.Malin R, Loimaala A, Nenonen A, Mercuri M, Vuori I, Pasanen M, Oja P, Bond G, Koivula T, Lehtimaki T. Relationship between high-density lipoprotein paraoxonase gene M/L55 polymorphism and carotid atherosclerosis differs in smoking and nonsmoking men. Metabolism. 2001;50:1095–1101. doi: 10.1053/meta.2001.25641 [DOI] [PubMed] [Google Scholar]
- 24.Lee CR, North KE, Bray MS, Avery CL, Mosher MJ, Couper DJ, Coresh J, Folsom AR, Boerwinkle E, Heiss G, et al. NOS3 polymorphisms, cigarette smoking, and cardiovascular disease risk: the Atherosclerosis Risk in Communities study. Pharmacogenet Genomics. 2006;16:891–899. doi: 10.1097/01.fpc.0000236324.96056.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karvonen J, Kauma H, Kervinen K, Ukkola O, Rantala M, Paivansalo M, Savolainen MJ, Kesaniemi YA. Apolipoprotein E polymorphism affects carotid artery atherosclerosis in smoking hypertensive men. J Hypertens. 2002;20:2371–2378. doi: 10.1097/00004872-200212000-00015 [DOI] [PubMed] [Google Scholar]
- 26.Jerrard-Dunne P, Sitzer M, Risley P, Buehler A, von Kegler S, Markus HS. Inflammatory gene load is associated with enhanced inflammation and early carotid atherosclerosis in smokers. Stroke. 2004;35:2438–2443. doi: 10.1161/01.STR.0000144681.46696.b3 [DOI] [PubMed] [Google Scholar]
- 27.Inamoto N, Katsuya T, Kokubo Y, Mannami T, Asai T, Baba S, Ogata J, Tomoike H, Ogihara T. Association of methylenetetrahydrofolate reductase gene polymorphism with carotid atherosclerosis depending on smoking status in a Japanese general population. Stroke. 2003;34:1628–1633. doi: 10.1161/01.STR.0000075769.09092.82 [DOI] [PubMed] [Google Scholar]
- 28.Djousse L, Myers RH, Province MA, Hunt SC, Eckfeldt JH, Evans G, Peacock JM, Ellison RC. Influence of apolipoprotein E, smoking, and alcohol intake on carotid atherosclerosis: National Heart, Lung, and Blood Institute Family Heart Study. Stroke. 2002;33:1357–1361. doi: 10.1161/01.str.0000014325.54063.1a [DOI] [PubMed] [Google Scholar]
- 29.Viiri LE, Viiri KM, Ilveskoski E, Huhtala H, Maki M, Tienari PJ, Perola M, Lehtimaki T, Karhunen PJ. Interactions of functional apolipoprotein E gene promoter polymorphisms with smoking on aortic atherosclerosis. Circ Cardiovasc Genet. 2008;1:107–116. doi: 10.1161/CIRCGENETICS.108.791764 [DOI] [PubMed] [Google Scholar]
- 30.Fan M, Raitakari OT, Kahonen M, Juonala M, Hutri-Kahonen N, Porsti I, Viikari J, Lehtimaki T. The association between cigarette smoking and carotid intima-media thickness is influenced by the -930A/G CYBA gene polymorphism: the Cardiovascular Risk in Young Finns Study. Am J Hypertens. 2009;22:281–287. doi: 10.1038/ajh.2008.349 [DOI] [PubMed] [Google Scholar]
- 31.Luo S, Wang F, Li Z, Deng J. Effect of the +781C/T polymorphism in the interleukin-8 gene on atherosclerotic cerebral infarction, and its interaction with smoking and drinking. PLoS One. 2013;8:e80246. doi: 10.1371/journal.pone.0080246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemiec P, Nowak T, Iwanicki T, Krauze J, Gorczynska-Kosiorz S, Grzeszczak W, Ochalska-Tyka A, Zak I. The -930A>G polymorphism of the CYBA gene is associated with premature coronary artery disease. A case-control study and gene-risk factors interactions. Mol Biol Rep. 2014;41:3287–3294. doi: 10.1007/s11033-014-3191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Chen W, Jiang F, Simino J, Srinivasan SR, Berenson GS, Mei H. Genetic association and gene-smoking interaction study of carotid intima-media thickness at five GWAS-indicated genes: the Bogalusa Heart Study. Gene. 2015;562:226–231. doi: 10.1016/j.gene.2015.02.078 [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Rundek T, Beecham A, Hudson B, Blanton SH, Zhao H, Sacco RL, Dong C. Genome-wide interaction study identifies RCBTB1 as a modifier for smoking effect on carotid intima-media thickness. Arterioscler Thromb Vasc Biol. 2014;34:219–225. doi: 10.1161/ATVBAHA.113.302706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boua PR, Brandenburg JT, Choudhury A, Hazelhurst S, Sengupta D, Agongo G, Nonterah EA, Oduro AR, Tinto H, Mathew CG, et al. Novel and known gene-smoking interactions with cIMT identified as potential drivers for atherosclerosis risk in West-African Populations of the AWI-Gen Study. Front Genet. 2019;10:1354. doi: 10.3389/fgene.2019.01354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Chang C, Hu H, Yang H. Interleukin-23: a new atherosclerosis target. J Interferon Cytokine Res. 2018;38:440–444. doi: 10.1089/jir.2018.0006 [DOI] [PubMed] [Google Scholar]
- 37.Jin L, Hastings NE, Blackman BR, Somlyo AV. Mechanical properties of the extracellular matrix alter expression of smooth muscle protein LPP and its partner palladin; relationship to early atherosclerosis and vascular injury. J Muscle Res Cell Motil. 2009;30:41–55. doi: 10.1007/s10974-009-9173-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh J, Koseki M, Molusky MM, Yakushiji E, Ichi I, Westerterp M, Iqbal J, Chan RB, Abramowicz S, Tascau L, et al. TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis. Nature. 2016;535:303–307. doi: 10.1038/nature18628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Qian S, Hoggatt A, Tang H, Hacker TA, Obukhov AG, Herring PB, Seye CI. Endothelial cell-specific deletion of P2Y2 receptor promotes plaque stability in atherosclerosis-susceptible ApoE-null mice. Arterioscler Thromb Vasc Biol. 2017;37:75–83. doi: 10.1161/ATVBAHA.116.308561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poels K, Schnitzler JG, Waissi F, Levels JHM, Stroes ESG, Daemen M, Lutgens E, Pennekamp AM, De Kleijn DPV, Seijkens TTP, et al. Inhibition of PFKFB3 hampers the progression of atherosclerosis and promotes plaque stability. Front Cell Dev Biol. 2020;8:581641. doi: 10.3389/fcell.2020.581641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabater-Lleal M, Malarstig A, Folkersen L, Soler Artigas M, Baldassarre D, Kavousi M, Almgren P, Veglia F, Brusselle G, Hofman A, et al. Common genetic determinants of lung function, subclinical atherosclerosis and risk of coronary artery disease. PLoS One. 2014;9:e104082. doi: 10.1371/journal.pone.0104082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Donnell CJ, Cupples LA, D’Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, et al. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI’s Framingham Heart Study. BMC Med Genet. 2007;8:S4. doi: 10.1186/1471-2350-8-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera NV, Patasova K, Kullberg S, Diaz-Gallo LM, Iseda T, Bengtsson C, Alfredsson L, Eklund A, Kockum I, Grunewald J, et al. A gene-environment interaction between smoking and gene polymorphisms provides a high risk of two subgroups of sarcoidosis. Sci Rep. 2019;9:18633. doi: 10.1038/s41598-019-54612-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karami J, Aslani S, Jamshidi A, Garshasbi M, Mahmoudi M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene. 2019;702:8–16. doi: 10.1016/j.gene.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 45.Doecke JD, Simms LA, Zhao ZZ, Roberts RL, Fowler EV, Croft A, Lin A, Huang N, Whiteman DC, Florin TH, et al. Smoking behaviour modifies IL23r-associated disease risk in patients with Crohn’s disease. J Gastroenterol Hepatol. 2015;30:299–307. doi: 10.1111/jgh.12674 [DOI] [PubMed] [Google Scholar]
- 46.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886 [DOI] [PubMed] [Google Scholar]
- 47.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, et al. ; Wellcome Trust Case Control Consortium. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira JP, Girerd N, Bozec E, Machu JL, Boivin JM, London GM, Zannad F, Rossignol P. Intima-media thickness is linearly and continuously associated with systolic blood pressure in a population-based cohort (STANISLAS Cohort Study). J Am Heart Assoc. 2016;5:e003529. doi: 10.1161/JAHA.116.003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, et al.; International Consortium for Blood Pressure Genome-Wide Association S. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, et al.; Cholesterol Treatment Trialists C. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 51.Zuber V, Gill D, Ala-Korpela M, Langenberg C, Butterworth A, Bottolo L, Burgess S. High-throughput multivariable Mendelian randomization analysis prioritizes apolipoprotein B as key lipid risk factor for coronary artery disease. Int J Epidemiol. 2021;50:893–901. doi: 10.1093/ije/dyaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, Holmes MV. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubin JH, Couper D, Lutsey PL, Yatsuya H. Synergistic and non-synergistic associations for cigarette smoking and non-tobacco risk factors for cardiovascular disease incidence in the Atherosclerosis Risk In Communities (ARIC) study. Nicotine Tob Res. 2017;19:826–835. doi: 10.1093/ntr/ntw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain RB. Impact of smoking on the observed levels of apolipoprotein B: data from NHANES 2007-2012. Environ Toxicol Pharmacol. 2017;53:227–233. doi: 10.1016/j.etap.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 55.Perez-Montarelo D, Madsen O, Alves E, Rodriguez MC, Folch JM, Noguera JL, Groenen MA, Fernandez AI. Identification of genes regulating growth and fatness traits in pig through hypothalamic transcriptome analysis. Physiol Genomics. 2014;46:195–206. doi: 10.1152/physiolgenomics.00151.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraja AT, Chasman DI, North KE, Reiner AP, Yanek LR, Kilpelainen TO, Smith JA, Dehghan A, Dupuis J, Johnson AD, et al. ; Cross Consortia Pleiotropy Group. Pleiotropic genes for metabolic syndrome and inflammation. Mol Genet Metab. 2014;112:317–338. doi: 10.1016/j.ymgme.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coulson DJ, Bakhashab S, Latief JS, Weaver JU. MiR-126, IL-7, CXCR1/2 receptors, inflammation and circulating endothelial progenitor cells: the study on targets for treatment pathways in a model of subclinical cardiovascular disease (type 1 diabetes mellitus). J Transl Med. 2021;19:140. doi: 10.1186/s12967-021-02785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiu YF, Chung RH, Lee CY, Kao HY, Hou L, Hsu FC. Identification of rare variants for hypertension with incorporation of linkage information. BMC Proc. 2014;8:S109. doi: 10.1186/1753-6561-8-S1-S109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, Grarup N, Sim X, Barnes DR, Witkowska K, et al. ; CHARGE-Heart Failure Consortium. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016;48:1151–1161. doi: 10.1038/ng.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benn M, Tybjaerg-Hansen A, McCarthy MI, Jensen GB, Grande P, Nordestgaard BG. Nonfasting glucose, ischemic heart disease, and myocardial infarction: a Mendelian randomization study. J Am Coll Cardiol. 2012;59:2356–2365. doi: 10.1016/j.jacc.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerjee S, Andrew RJ, Duff CJ, Fisher K, Jackson CD, Lawrence CB, Maeda N, Greenspan DS, Kellett KAB, Hooper NM. Proteolysis of the low density lipoprotein receptor by bone morphogenetic protein-1 regulates cellular cholesterol uptake. Sci Rep. 2019;9:11416. doi: 10.1038/s41598-019-47814-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borges DO, Patarrao RS, Ribeiro RT, de Oliveira RM, Duarte N, Belew GD, Martins M, Andrade R, Costa J, Correia I, et al. Loss of postprandial insulin clearance control by Insulin-degrading enzyme drives dysmetabolism traits. Metabolism. 2021;118:154735. doi: 10.1016/j.metabol.2021.154735 [DOI] [PubMed] [Google Scholar]
- 63.Chiou J, Zeng C, Cheng Z, Han JY, Schlichting M, Miller M, Mendez R, Huang S, Wang J, Sui Y, et al. Single-cell chromatin accessibility identifies pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nat Genet. 2021;53:455–466. doi: 10.1038/s41588-021-00823-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Audano M, Pedretti S, Ligorio S, Gualdrini F, Polletti S, Russo M, Ghisletti S, Bean C, Crestani M, Caruso D, et al. Zc3h10 regulates adipogenesis by controlling translation and F-actin/mitochondria interaction. J Cell Biol. 2021;220:e202003173. doi: 10.1083/jcb.202003173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shao J, Li Y, Zhou C, Geng M, Zhang G, Zhang N, Jin G, Zhang L, Gao C, Liu S. TIPE1 accelerates atherogenesis by inducing endothelial dysfunction in response to oxidative stress. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165578. doi: 10.1016/j.bbadis.2019.165578 [DOI] [PubMed] [Google Scholar]
- 66.Kyselova A, Siragusa M, Anthes J, Solari FA, Loroch S, Zahedi RP, Walter U, Fleming I, Randriamboavonjy V. Cyclin Y is expressed in platelets and modulates integrin outside-in signaling. Int J Mol Sci. 2020;21:8239. doi: 10.3390/ijms21218239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng ML, Du XP, Zhao L, Yang XC. Expression profile of circular RNAs in epicardial adipose tissue in heart failure. Chin Med J (Engl). 2020;133:2565–2572. doi: 10.1097/CM9.0000000000001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, Mao W, Wang L, Lu L, Pang Y. Circular RNA circLMF1 regulates PDGF-BB-induced proliferation and migration of human aortic smooth muscle cells by regulating the miR-125a-3p/VEGFA or FGF1 axis. Clin Hemorheol Microcirc. 2022;80:167–183. doi: 10.3233/CH-211166 [DOI] [PubMed] [Google Scholar]
- 69.Butt SA, Jeppesen JL, Torp-Pedersen C, Sam F, Gislason GH, Jacobsen S, Andersson C. Cardiovascular manifestations of systemic sclerosis: a Danish Nationwide Cohort Study. J Am Heart Assoc. 2019;8:e013405. doi: 10.1161/JAHA.119.013405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klarin D, Emdin CA, Natarajan P, Conrad MF, Consortium I, Kathiresan S. Genetic analysis of venous thromboembolism in UK biobank identifies the ZFPM2 locus and implicates obesity as a causal risk factor. Circ Cardiovasc Genet. 2017;10:e001643. doi: 10.1161/CIRCGENETICS.116.001643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li MY, Chen HX, Hou HT, Wang J, Liu XC, Yang Q, He GW. Biomarkers and key pathways in atrial fibrillation associated with mitral valve disease identified by multi-omics study. Ann Transl Med. 2021;9:393. doi: 10.21037/atm-20-3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Loukola A, Gillespie NA, Peterson R, Jia P, Riley B, Maes H, Dick DM, Kendler KS, Damaj MI, et al. Genome-wide meta-analyses of FTND and TTFC phenotypes. Nicotine Tob Res. 2020;22:900–909. doi: 10.1093/ntr/ntz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, Malone S, Oetting WS, Iacono WG. A genome-wide association study of behavioral disinhibition. Behav Genet. 2013;43:363–373. doi: 10.1007/s10519-013-9606-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duncan MS, Freiberg MS, Greevy RA, Jr, Kundu S, Vasan RS, Tindle HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. 2019;322:642–650. doi: 10.1001/jama.2019.10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansen K, Ostling G, Persson M, Nilsson PM, Melander O, Engstrom G, Hedblad B, Rosvall M. The effect of smoking on carotid intima-media thickness progression rate and rate of lumen diameter reduction. Eur J Intern Med. 2016;28:74–79. doi: 10.1016/j.ejim.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 76.Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, Smit AJ, Giral P, Kurl S, Mannarino E, et al. Progression of carotid intima-media thickness as predictor of vascular events: results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013;33:2273–2279. doi: 10.1161/atvbaha.113.301844 [DOI] [PubMed] [Google Scholar]
- 77.Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, Smit AJ, Kurl S, Ravani A, Frigerio B, Sansaro D, et al. ; IMPROVE study group. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis. 2017;263:412–419. doi: 10.1016/j.atherosclerosis.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x [DOI] [PubMed] [Google Scholar]
- 79.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189. doi: 10.1016/j.echo.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 80.Voight BF, Kang HM, Ding J, Palmer CD, Sidore C, Chines PS, Burtt NP, Fuchsberger C, Li Y, Erdmann J, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, Bakker SF, Bardella MT, Bhaw-Rosun L, Castillejo G, et al. ; Spanish Consortium on the Genetics of Coeliac Disease (CEGEC). Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet. 2011;43:1193–1201. doi: 10.1038/ng.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rothman KJ GS, Lash TL. Modern Epidemiology. Lippncott William and Wilkins; 2008. [Google Scholar]
- 84.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012 [DOI] [PubMed] [Google Scholar]
- 85.Veglia F, Baldassarre D, de Faire U, Kurl S, Smit AJ, Rauramaa R, Giral P, Amato M, Di Minno A, Ravani A, et al. A priori-defined Mediterranean-like dietary pattern predicts cardiovascular events better in north Europe than in Mediterranean countries. Int J Cardiol. 2019;282:88–92. doi: 10.1016/j.ijcard.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding B, Kallberg H, Klareskog L, Padyukov L, Alfredsson L. GEIRA: gene-environment and gene-gene interaction research application. Eur J Epidemiol. 2011;26:557–561. doi: 10.1007/s10654-011-9582-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.