Abstract
Introduction:
MEK inhibitors (MEKi) have shown efficacy in pediatric low-grade glioma as well as plexiform neurofibroma. MEKi have been associated with acute cardiac dysfunction in adults. Cardiac consequences in children are unknown.
Material and methods:
We performed a single center retrospective cohort study evaluating cardiac function by echocardiography (echo) in children and young adults <21 years receiving MEKi between October 2013 and May 2018. Blinded assessment of left ventricular function by fractional shortening (FS) and ejection fraction (EF) was performed on all available echocardiograms performed before, during, and following therapy, as well as after re-initiation of therapy.
Results:
Twenty-six patients underwent MEKi therapy with echo follow-up during the study period. Twenty-four of these had complete echo data. Median follow-up was 12 months. Borderline EF (EF 53–57.9%) occurred in 12 (50%) patients; and 3 (12.5%) progressed to abnormal EF (EF <53%). Cardiac dysfunction, when it occurred, was mild (lowest documented EF was 45%, and lowest FS was 24.4%). EF abnormalities typically fluctuated during therapy, resolved off therapy, and recurred with MEKi re-initiation. No clinical or demographic differences were detected between those who maintained normal cardiac function and those who developed borderline or overt cardiac dysfunction. Symptomatic heart failure did not occur.
Conclusion:
In this cohort of children and young adults, MEKi use was associated with a high (50%) incidence of borderline or mildly decreased left ventricular function. There was no evidence of permanent cardiac dysfunction. Further evaluation in larger prospective trials is needed.
Keywords: Echocardiography, ventricular dysfunction, MEK inhibitor, pediatrics, low-grade glioma
Introduction
The RAS-RAF-MEK-ERK pathway is one of the most commonly activated signal transduction pathways in cancer; and is known to be a primary driver of pediatric low grade gliomas, both sporadic and neurofibromatosis type 1 (NF1)-associated, as well as of other NF1-associated tumors [1, 2]. MEK inhibitors (MEKi), a relatively new class of small molecule agents, have demonstrated efficacy in both pediatric low-grade gliomas and NF1-associated plexiform neurofibromas [3–6]. Recently, MEKi selumetinib became the first drug approved in the United States for treatment of NF1-associated plexiform neurofibromas in children [7].
MEKi are associated with various cardiovascular toxicities in adult patients, including arterial hypertension, pulmonary embolism, cardiomyopathy, and decreased ejection fraction [8–10]. Despite the increasingly wide-spread clinical use of MEKi in children, the incidence of cardiac abnormalities in children and adolescents treated with MEKi has, to our knowledge, not yet been described.
The primary objective of this single institution retrospective cohort study was to describe the incidence of cardiac dysfunction in children and young adults less than 21 years old treated with MEKi. Exploratory objectives included assessment of association of cardiac changes with age, diagnosis, and clinical features. We also assessed the reversibility and recovery of cardiac function after MEKi discontinuation in children with MEKi-associated cardiac dysfunction.
Material and methods
The Children’s Hospital Los Angeles (CHLA) Institutional Review Board approved this retrospective cohort study. Patients who were followed at CHLA, treated with MEKi before their twenty-first birthday, and followed with serial echocardiography (echo) were eligible. We included all patients with at least 2 echocardiograms performed at CHLA before May 1, 2018 and available for review, at least one of which was obtained after initiation of MEKi therapy. Patients without echocardiograms, or with echocardiograms obtained at outside institutions and not available for direct imaging review, were excluded. Clinical data, including demographic characteristics, diagnosis, age at MEKi treatment, duration of treatment, MEKi drug and dose, prior tumor-directed treatment (including prior radiation or anthracycline therapy), and concomitant medications, was extracted by the investigators (MJF and NJR) directly from patients’ electronic medical records and entered into a study database. Cardiac functional measurements were recorded as described below.
Echocardiography.
Transthoracic echocardiographic evaluation was performed using a cardiac ultrasound imaging system (Philips Medical Systems, Andover, MA). All studies were clinically indicated and by protocol included measurement of left ventricular (LV) dimensions via M-mode measurements, systolic shortening, ejection fraction (EF), and diastolic parameters. M-mode measurements included LV end-diastolic and end-systolic dimensions (LVIDd and LVIDs), used for calculation of fractional shortening (FS).
Two cardiologists with expertise in echocardiographic interpretation (JDM and JAS) jointly interpreted the studies using the original DICOM images of all studies and calculated the FS and EF using Philips IntelliSpace software. The cardiologists were blinded to each patient’s identity and clinical status during the image analysis. FS ≥ 29% was considered normal, FS < 27% was considered abnormal, and FS ≥ 27 and < 29% was considered “borderline.” Systolic function assessed by EF was calculated via the Biplane Simpson’s Method. EF ≥ 58% was considered normal, EF < 53% was considered overtly abnormal, and EF ≥ 53 and < 58% was considered “borderline” [11]. Left ventricular measurements were considered normal if they fell within two standard deviations (SD) of their mean for body surface area (Boston Z-score within ±2). Diastolic measurements were inconsistently recorded, mostly including mitral inflow E and A wave peaks; therefore, we did not analyze this data. Borderline and/or cardiac dysfunction status was assessed using all available imaging after the patient initiated MEKi therapy.
Statistical analysis.
In a planned exploratory analysis, T-tests, χ2, or Fisher’s exact tests were utilized to compare the distribution of demographics across patients with and without borderline or cardiac dysfunction. Time to borderline or abnormal EF and/or FS was defined as the minimum time between the start of MEKi treatment and date of subsequent ECHO resulting in borderline or abnormal EF and/or FS. Patients with borderline EF at baseline were excluded from time to borderline EF survival analysis. Patients with normal cardiac function throughout follow up were censored at the last available imaging. Kaplan-Meier survival curves were provided for each borderline and abnormal EF and/or FS status with the 2-year survival estimates. The survival estimates included 95%-point wide confidence intervals which were calculated using the log-log transformation and Greenwood’s formula. All analyses utilized two-sided tests, with significance level set at p<0.05 and were completed using Stata Statistical Software: Release 14 (StataCorp LP, College Station, TX).
Results
Twenty-six children and young adults who initiated MEKi treatment at age < 21 years in our institution between October 2013 and May 2018 were identified. Two patients did not have echos available for review and were excluded, leaving 24 subjects for inclusion in this study. The median age at first MEKi exposure was 10.1+/−4.86 years (range 3–20 years). Patient characteristics are shown in Table 1. Six patients were treated on dose escalation studies at doses less than the current recommended pediatric phase II dose; the remaining 18 patients were treated at current standard pediatric starting doses. Duration of primary treatment course (defined as time from MEKi initiation to last dose, time to treatment interruption if drug held for > 1 month, or time to last follow-up if still on drug) was 0.9 to 24.4 months (mean 13, median 12 months). Seven patients underwent a second course of MEKi; this includes patients who resumed therapy after treatment interruption > 1 month. Mean follow-up, defined as time from MEKi initiation to last echo, was 15.4 months (SD 13.2, range 0.8 to 52.4 months). No patients in this cohort received craniospinal radiation, thoracic radiation, or radiation with scatter to the thorax. One patient with juvenile xanthogranuloma and a previous history of acute lymphoblastic leukemia had received previous anthracycline therapy as per CCG-1961 five to six years prior to MEKi initiation; this patient’s cumulative doxorubicin isotoxic dose was 175mg/m2, and the cardiac function was normal at the time of MEKi initiation.
TABLE 1.
Patient characteristics
| Variable | Median (Range) |
|---|---|
| Age at first MEK inhibitor treatment (years) | 10.1 (1.7, 20.5) |
| Duration of follow up (months) | 12.0 (0.9, 52.4) |
| Number (%) n=24 | |
| Female | 12 (50) |
| Race/Ethnicity | |
| White/Non-Hispanic | 12 (50) |
| Hispanic | 9 (38) |
| Other | 3 (13) |
| Diagnosis | |
| Low-grade Glioma | 16 (67) |
| High-grade glioma | 1 (4) |
| Other brain tumors | 3 (13) |
| Plexiform neurofibroma | 3 (13) |
| Juvenile xanthogranuloma | 1 (4) |
| Neurofibromatosis type 1 | 4 (17) |
| MEK inhibitor received | |
| Binimetinib | 8 (33) |
| Selumetinib | 7 (29) |
| Trametinib | 9 (38) |
| Concurrent BRAF inhibitor | |
| None | 19 (79) |
| Dabrafenib | 3 (13) |
| Vemurafenib | 2 (8) |
Borderline EF, but not FS, was seen in 6 patients (25%) on baseline echo prior to therapy initiation. During treatment, borderline EF was seen in 12 patients (50%), four of whom had borderline EF at baseline, and borderline FS in 6 patients (25%) on at least one echo. With only three exceptions, borderline EF and FS were not coincident: borderline EF was typically seen in isolation without corresponding borderline FS, and vice versa. Three patients progressed to both abnormal EF and FS, none of whom had borderline findings at pre-treatment baseline. Abnormal cardiac function resolved without dose interruption or reduction in 2 of these patients, one of whom had recurrence of dysfunction during a subsequent course of therapy and received ACE inhibition (lisinopril) (Fig. 1a). Follow-up echo after initial development of abnormal cardiac function was not available for the third patient. MEKi therapy was interrupted in one patient for borderline cardiac dysfunction that subsequently resolved; this patient again developed borderline cardiac dysfunction upon re-initiation of therapy (Fig. 1b). No patient developed abnormal EF without abnormal FS, or abnormal FS without abnormal EF. The lowest measured EF in any patient was 45%, and lowest FS was 24.4%. Symptomatic heart failure did not occur, and no patient required inotropic therapy or hospital admission for cardiac failure.
Fig. 1. Case examples of patients with borderline or decreased cardiac function.

(a) A 19-year-old female with tectal glioma, undergoing treatment with a single agent MEK inhibitor, experienced asymptomatic decrease in left ventricular function during the first 7 months of treatment. This resolved without dose interruption or modification. Decreased left ventricular function reoccurred two and a half years later; lisinopril was prescribed. (b) A 12-year-old male with BRAF V600E-mutated anaplastic astroblastoma, treated with dual agent MEK and BRAF inhibitors, experienced asymptomatic decrease in left ventricular shortening fraction after one month of therapy. MEKi treatment was interrupted and then resumed at the same dose. BRAF inhibitor was continued without interruption.
Grey dotted and dashed lines represent lower limits of normal and lower limits of borderline values, respectively, for both EF and FS.
M, MEK inhibitor therapy. EF, left ventricular ejection fraction. FS, left ventricular fractional shortening
Kaplan-Meier estimates of freedom from borderline and abnormal cardiac function are shown in Fig. 2. No clinical or demographic differences were detected between those who maintained normal cardiac function, and those who developed borderline or overt cardiac dysfunction in response to MEKi (Supplementary Tables). Notably, the one patient with prior anthracycline exposure had a transiently borderline ejection fraction but did not experience overt cardiac dysfunction during 12 months of MEKi therapy.
Fig. 2. Freedom from borderline and overt cardiac dysfunction.
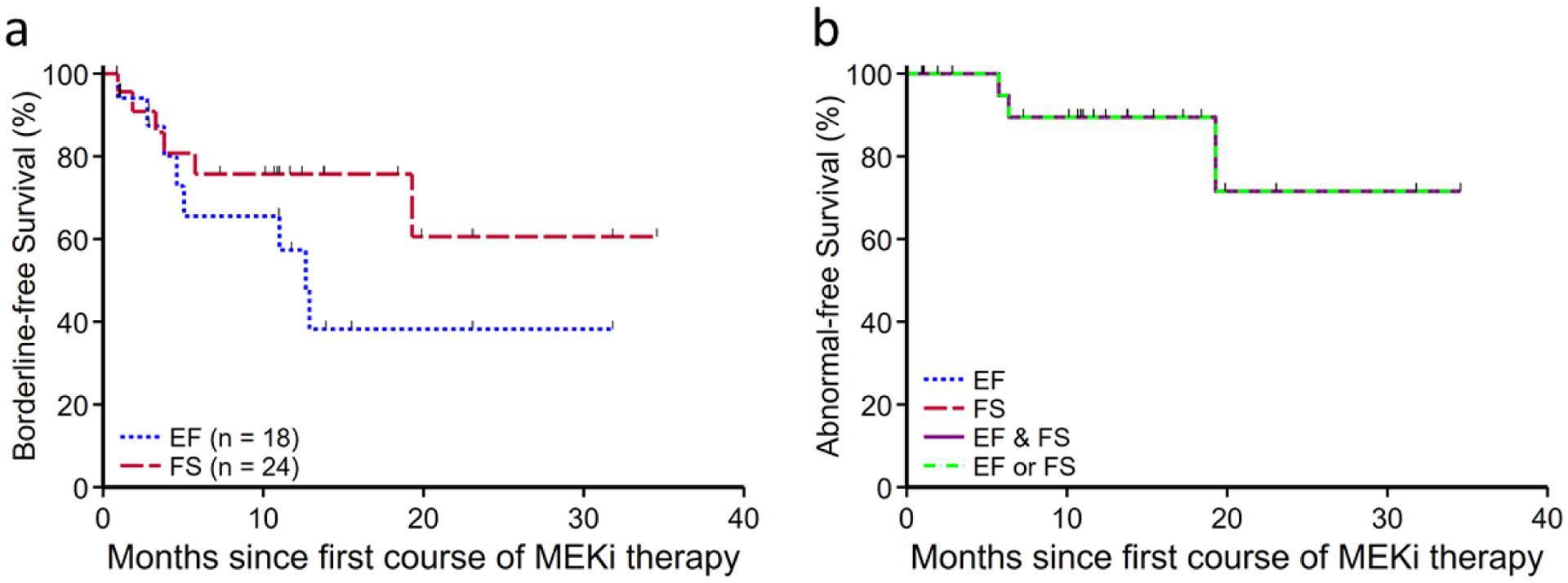
(a) Kaplan-Meier estimate of freedom from borderline cardiac function at 2 years from MEKi initiation was 38.2% (95% confident interval [CI] 12.9–63.7%) for EF and 60.6% (95% CI 25.5–83.2%) for FS. For this survival analysis, borderline cardiac function was defined as left ventricular FS <29% or EF<58%. Patients with borderline EF at baseline were excluded from borderline EF survival analysis. (b) Kaplan-Meier estimate of freedom from abnormal cardiac function at 2 years was 71.6% (95% CI 26.1–92.0%). Abnormal cardiac function was defined as FS<27% or EF < 53%. Abnormal FS and EF always occurred synchronously in this cohort, thus only a single abnormal-free survival curve is shown..
EF, left ventricular ejection fraction. FS, left ventricular shortening fraction. MEKi, MEK inhibitor.
Discussion
MEKi treatment has been associated with a number of cardiac effects in adults, including decreased LV EF, as well blood pressure elevation and decreased heart rate [12–14]. We note in our pediatric population that MEKi is associated with a high (50%) incidence of borderline cardiac dysfunction (by EF and/or FS). Importantly, these events were mild and self-resolved even with continued therapy in most cases. Symptomatic heart failure was not seen, nor did patients suffer morbidity of cardiac dysfunction.
The Ras-Raf-MEK-ERK pathway plays a key role in cell proliferation, differentiation, survival, and death; its specific role within the heart is complex, and perhaps not fully understood [15–17]. There is evidence that the pathway plays a cytoprotective role within the heart, including protecting cardiac myocytes from apoptotic death and oxidative stress, and maintaining cardiac progenitor cells [9, 18–22]. On the other hand, inhibition of the MEK-ERK axis has been shown to be cardioprotective in pressure overload-induced cardiac hypertrophy in human cardiac tissue samples and murine models [16, 23–25]. How and whether these findings relate to the changes in cardiac function shown in our study is unclear. Assuming a causal relationship, the rapid recovery seen in affected subjects may argue against a cardiac progenitor cell-mediated effect, and instead suggests a possible metabolic/energy depletion etiology.
Several patients in this cohort received dual therapy with a MEKi and a first-generation BRAF inhibitor (BRAFi). Decreased toxicity with combined MEKi and BRAFi compared to monotherapy with either drug alone has been described, putatively due to mutually cancelling effects of pathway inhibition and paradoxical pathway activation in non-tumor cells by MEKi and BRAFi, respectively [12, 26, 27]. While the sample size precludes an adequately powered comparison between dual and single agent therapy in this cohort, it is noteworthy that borderline EF and FS were seen in dual therapy patients. This is similar to findings in adult studies, and suggests that dual therapy may not be protective against MEKi-induced cardiac effects [10].
The population included in this study was diverse and reflects the ethnic and racial diversity represented in patients treated at our institution. Similarly, the range of diagnoses, including patients with and without brain tumors, and with and without NF1, reflects the diversity of children likely to be treated with MEKi.
Our study has several important limitations. Although the experience reported is large for a single pediatric center, the relatively small sample size precludes evaluation of rare events, or a well-powered assessment of association between patient characteristics and cardiac risks. Similarly, the relatively small sample size precludes adequate assessment of potential confounders or effect modifiers, including prior therapy. An adequately powered prospective study is needed to identify potential risk factors for MEKi-associated cardiac function. A minority of patients in the cohort were treated at starting dose less than the standard or recommended phase 2 pediatric doses, which could result in an underestimate of a dose-related cardiac effect. Similarly, duration of therapy was heterogeneous, and several patients had interrupted courses of MEKi therapy over the study period. As a retrospective study, we also are confined to existing clinically ordered echocardiographic data, which was often limited in advanced heart function assessments in this population of patients without clinically significant heart dysfunction; serum markers of cardiac dysfunction were not available for similar reasons. This would be better addressed in a prospective, protocolized study. Perhaps most importantly, since MEKi have only recently been introduced into pediatric use and long-term follow-up is not yet available, the study did not evaluate possible long-term or late-onset cardiac effects of MEKi therapy in childhood.
It is perhaps unconventional to use both EF and FS as endpoints for systolic function analysis, and to delineate a borderline function category for each. However, the authors felt that this description of the data best illuminated the mild nature of the cardiac function changes seen in this data set. The high incidence of transiently borderline cardiac function, especially borderline EF, reflects the inherent limitations of echocardiography.
This study is unique because it describes specific cardiac effects of MEKi in children. We have demonstrated that MEKi treatment is associated with varying degrees of left ventricular dysfunction in children, seemingly comparable to that in older adults. This confirms the importance of cardiac monitoring for children treated with MEKi, whether in a research or clinical practice setting. Findings were reassuring, however, in that borderline or mildly decreased left ventricular function in this cohort was not a harbinger of more serious or progressive acute cardiac dysfunction, and in that no patient developed symptomatic or irreversible heart failure.
In conclusion, we report that minor cardiac dysfunction is common in young patients receiving MEKi. In our cohort, dose adjustment or treatment interruption was rarely required, and abnormalities self-resolved without treatment interruption in many cases. Heart failure was not seen, and there was no evidence of permanent cardiac systolic dysfunction. Overall, our findings are reassuring, and preliminarily suggest that children with minor MEKi-associated cardiac dysfunction may be able to safely continue MEKi therapy. However, prospective evaluation in larger cohorts with long-term follow-up is needed.
Supplementary Material
Acknowledgments:
The authors would like to thank Ashley S. Margol, Tom Belle Davidson, Anat Erdreich-Epstein, Kasey Rangan, Kaaren Waters, and Kimberly Bira for their intellectual contributions and assistance with data collection. The authors would also like to thank Cecilia Patino-Sutton, Todd Alonzo, and Yueh-Yun Chi for their editorial review of the manuscript drafts.
MJF was a participant in the University of Southern California/CHLA Summer Oncology Research Fellowship (SORF) Program.
Footnotes
Note: Preliminary data were presented under the title “Cardiac Effects of MEK Inhibitors in Children” at the American Heart Association Scientific Sessions, Nov 2019, Philadelphia; meeting abstract published in Circulation. 2019;140:A12608.
Competing interest: The authors declare that they have no financial or non-financial conflict of interest.
Ethical approval and consent to participate: The study was approved by the Children’s Hospital Los Angeles Institutional Review Board (IRB), with waiver of consent.
Data availability:
Raw data supporting the findings of this study are available from the corresponding authors upon request.
References
- 1.Garcia MA, Solomon DA, Haas-Kogan DA (2016) Exploiting molecular biology for diagnosis and targeted management of pediatric low-grade gliomas. Future Oncol 12:1493–1506. 10.2217/fon-2016-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker JA, Upadhyaya M (2018) Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin Ther Targets 22:419–437. 10.1080/14728222.2018.1465931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dombi E, Baldwin A, Marcus LJ, et al. (2016) Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N Engl J Med 375:2550–2560. 10.1056/NEJMoa1605943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee A, Jakacki RI, Onar-Thomas A, et al. (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncol 19:1135–1144. 10.1093/neuonc/now282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. (2019) Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20:1011–1022. 10.1016/S1470-2045(19)30277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross AM, Wolters PL, Dombi E, et al. (2020) Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med 382:1430–1442. 10.1056/NEJMoa1912735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Office of the Commissioner (2020) FDA Approves First Therapy for Children with Debilitating and Disfiguring Rare Disease. In: FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed 15 May 2020 [Google Scholar]
- 8.Abdel-Rahman O, ElHalawani H, Ahmed H (2015) Risk of Selected Cardiovascular Toxicities in Patients With Cancer Treated With MEK Inhibitors: A Comparative Systematic Review and Meta-Analysis. J Glob Oncol 1:73–82. 10.1200/JGO.2015.000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks M, Crowell K, Proctor A, Jensen BC (2017) Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol 17:487–493. 10.1007/s12012-017-9425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mincu RI, Mahabadi AA, Michel L, et al. (2019) Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open 2:e198890–e198890. 10.1001/jamanetworkopen.2019.8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buccheri S, Costanzo L, Tamburino C, Monte I (2015) Reference Values for Real Time Three-Dimensional Echocardiography-Derived Left Ventricular Volumes and Ejection Fraction: Review and Meta-Analysis of Currently Available Studies. Echocardiogr Mt Kisco N 32:1841–1850. 10.1111/echo.12972 [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KT, Infante JR, Daud A, et al. (2012) Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N Engl J Med 367:1694–1703. 10.1056/NEJMoa1210093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaherty KT, Robert C, Hersey P, et al. (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114. 10.1056/NEJMoa1203421 [DOI] [PubMed] [Google Scholar]
- 14.Patnaik A, Tolcher A, Papadopoulos KP, et al. (2016) Phase 1 study to evaluate the effect of the MEK inhibitor trametinib on cardiac repolarization in patients with solid tumours. Cancer Chemother Pharmacol 78:491–500. 10.1007/s00280-016-3090-y [DOI] [PubMed] [Google Scholar]
- 15.Chang F, Steelman LS, Shelton JG, et al. (2003) Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int J Oncol 22:469–480. 10.3892/ijo.22.3.469 [DOI] [PubMed] [Google Scholar]
- 16.Khalilimeybodi A, Daneshmehr A, Sharif-Kashani B (2018) Investigating β-adrenergic-induced cardiac hypertrophy through computational approach: classical and non-classical pathways. J Physiol Sci 68:503–520. 10.1007/s12576-017-0557-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezatabar S, Karimian A, Rameshknia V, et al. (2019) RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol 234:14951–14965. 10.1002/jcp.28334 [DOI] [PubMed] [Google Scholar]
- 18.Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: The gp130-STAT3 axis. Basic Res Cardiol 102:393–411. 10.1007/s00395-007-0674-z [DOI] [PubMed] [Google Scholar]
- 19.Aikawa R, Komuro I, Yamazaki T, et al. (1997) Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest 100:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vajravelu BN, Hong KU, Al-Maqtari T, et al. (2015) C-Kit Promotes Growth and Migration of Human Cardiac Progenitor Cells via the PI3K-AKT and MEK-ERK Pathways. PLOS ONE 10:e0140798. 10.1371/journal.pone.0140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia P, Liu Y, Cheng Z (2016) Signaling Pathways in Cardiac Myocyte Apoptosis. BioMed Res Int 2016:. 10.1155/2016/9583268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue T-L, Wang C, Gu J-L, et al. (2000) Inhibition of Extracellular Signal–Regulated Kinase Enhances Ischemia/Reoxygenation–Induced Apoptosis in Cultured Cardiac Myocytes and Exaggerates Reperfusion Injury in Isolated Perfused Heart. Circ Res 86:692–699. 10.1161/01.RES.86.6.692 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhang X-F, Gao L, et al. (2014) Growth/differentiation factor 1 alleviates pressure overload-induced cardiac hypertrophy and dysfunction. Biochim Biophys Acta BBA - Mol Basis Dis 1842:232–244. 10.1016/j.bbadis.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 24.Bisserier M, Berthouze-Duquesnes M, Breckler M, et al. (2015) Carabin Protects Against Cardiac Hypertrophy by Blocking Calcineurin, Ras, and Ca2+/Calmodulin-Dependent Protein Kinase II SignalingCLINICAL PERSPECTIVE. Circulation 131:390–400. 10.1161/CIRCULATIONAHA.114.010686 [DOI] [PubMed] [Google Scholar]
- 25.Cipolletta E, Rusciano MR, Maione AS, et al. (2015) Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy. PLoS ONE 10:. 10.1371/journal.pone.0130477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cichowski K, Jänne PA (2010) Drug discovery: Inhibitors that activate. Nature 464:358–359. 10.1038/464358a [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Spevak W, Zhang Y, et al. (2015) RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 526:583–586. 10.1038/nature14982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data supporting the findings of this study are available from the corresponding authors upon request.


