Abstract
Background
Safe and effective treatments are needed to prevent severe outcomes in individuals with coronavirus disease 2019 (COVID-19). We report results from STAMP, a phase 2/3, multicenter, double-blind, randomized, placebo-controlled trial of adintrevimab, an extended half-life monoclonal antibody, for treatment of high-risk ambulatory patients with mild to moderate COVID-19.
Methods
Nonhospitalized, unvaccinated participants aged ≥12 years with mild to moderate COVID-19 and ≥1 risk factor for disease progression were randomized to receive a single intramuscular injection of 300 mg adintrevimab or placebo. Enrollment was paused due to the global emergence of the Omicron BA.1/BA1.1 variants, against which adintrevimab showed reduced activity in vitro. The primary efficacy endpoint was COVID-19–related hospitalization or all-cause death through day 29 in participants with COVID-19 due to laboratory-confirmed or suspected non-Omicron severe acute respiratory syndrome coronavirus 2 variants.
Results
Between 8 August 2021 and 11 January 2022, 399 participants were randomized to receive adintrevimab (n = 198) or placebo (n = 201), including 336 with COVID-19 due to non-Omicron variants. COVID-19–related hospitalization or all-cause death through day 29 occurred in 8 of 169 (4.7%) participants in the adintrevimab group and 23 of 167 (13.8%) participants in the placebo group, a 66% relative risk reduction in favor of adintrevimab (standardized risk difference, −8.7% [95% confidence interval, −14.71% to −2.67%]; P = .0047). Incidence of treatment-emergent adverse events (TEAEs) was similar between treatment groups (33.9% for adintrevimab and 39.5% for placebo). No adintrevimab-related serious TEAEs were reported.
Conclusions
Treatment with a single intramuscular injection of adintrevimab provided protection against severe outcomes in high-risk ambulatory participants with COVID-19 due to susceptible variants, without safety concerns.
Clinical Trial Registration. NCT04805671.
Keywords: adintrevimab, COVID-19, monoclonal antibody, SARS-CoV-2, treatment
In the STAMP trial, a single intramuscular injection of adintrevimab provided a 66% relative risk reduction in COVID-19–related hospitalization or all-cause death through day 29 in high-risk ambulatory participants with mild or moderate COVID-19 due to non-Omicron lineage variants.
With >6 million coronavirus disease 2019 (COVID-19)–related deaths reported worldwide through 2022 [1], additional safe and effective treatments are needed to prevent severe outcomes in high-risk individuals with COVID-19 [2]. Vulnerable populations like those presenting with advanced age, diabetes, obesity, and immunosuppression have a higher risk of severe outcomes, including hospitalization and death, than the general population [3–5]. COVID-19–related hospitalizations are associated with substantial mortality, long inpatient stays, and high medical and societal costs [6].
Although treatment strategies such as antivirals and monoclonal antibodies (mAbs) have become widely available, there is still an area of unmet need. Some patients may not benefit from antiviral therapy, particularly those at risk for serious drug–drug interactions with currently available oral treatment agents [7–10]. Furthermore, the effectiveness of most mAbs authorized for emergency use has been limited by the rapid emergence and global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variants with mutations exhibiting enhanced transmissibility and/or evasion from natural or acquired immunity [11–15].
Adintrevimab (ADG20) is a fully human immunoglobulin G1 (IgG1) mAb derived from a survivor of the 2003 SARS-CoV epidemic [16, 17] and engineered to have improved potency and broad neutralization against SARS-CoV, SARS-CoV-2, and other SARS-like coronaviruses with pandemic potential [13, 16]. The crystallizable fragment region of adintrevimab contains two amino acid modifications designed to extend half-life without impacting typical IgG1 effector functions [16, 18]. Adintrevimab binds to a distinct epitope in the receptor-binding domain of the spike glycoprotein of SARS-CoV-2 that partially overlaps the angiotensin-converting enzyme 2 binding site [16]. Consistent with strong epitope conservation, adintrevimab has demonstrated broad and potent in vitro neutralizing activity against most authentic SARS-CoV-2 variants (including Alpha, Beta, Gamma, and Delta) [13, 19]; however, the Omicron variant (BA.1) introduced specific mutations within the epitope targeted by adintrevimab, resulting in loss of binding and neutralization of Omicron compared with previous variants. In vitro, adintrevimab has shown reduced or no activity against the Omicron variant and its sublineages [20].
In a phase 1 study, a single dose of adintrevimab, up to 1200 mg intramuscular (IM) or 4500 mg intravenous (IV), was well tolerated by healthy adults with no reported study drug–related adverse events (AEs) [21, 22]. Based on quantitative systems pharmacology/population-based pharmacokinetics model predictions [23, 24] and preliminary pharmacokinetic data from the phase 1 study, a single 300-mg IM injection of adintrevimab was selected for evaluation in the STAMP trial for the treatment of mild or moderate COVID-19 in high-risk ambulatory participants. Given limited neutralization of Omicron BA.1 by adintrevimab, primary analyses were limited to participants with COVID-19 due to laboratory-confirmed or suspected non-Omicron SARS-CoV-2 variants.
Here, we report the efficacy and safety results of the STAMP trial.
METHODS
Trial Design
STAMP was a multicenter, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier NCT04805671). Participants were enrolled at 68 sites in 11 countries—Argentina, Brazil, Bulgaria, Germany, Hungary, Greece, Republic of Moldova, Romania, Poland, Ukraine, and South Africa.
The first participant was randomized on 8 August 2021; however, due to the emergence and subsequent global spread of the Omicron BA.1/BA.1.1 variants, enrollment was paused on 11 January 2022. Pharmacokinetic/pharmacodynamic analyses incorporating the reduced in vitro activity of adintrevimab against the BA.1/BA.1.1 variants suggested that the 300-mg IM dose under study might not provide clinically meaningful protection against disease caused by this variant. Prior to data analysis and unblinding, the statistical analysis plan was adjusted to evaluate the primary efficacy endpoint as a preplanned analysis in participants with COVID-19 due to laboratory-confirmed or suspected non-Omicron SARS-CoV-2 variants. Here, we report data for the key primary, secondary, and exploratory efficacy endpoints at the primary data cutoff of 28 March 2022. Safety results are reported up to a data cutoff of 8 August 2022, when all participants had completed ≥6 months of safety follow-up. The trial was terminated on 3 November 2022, as continued participation was not expected to yield any additional safety information.
Participants
Eligible participants were nonhospitalized adults aged ≥18 years or adolescents aged 12–17 years (inclusive) weighing ≥40 kg at screening who had a SARS-CoV-2–positive antigen test, reverse-transcription polymerase chain reaction (RT-PCR), or other locally approved molecular diagnostic assay and initial onset of 1 or more self-reported COVID-19–related signs or symptoms within 5 days before randomization. Participants must also have had mild or moderate COVID-19 not requiring oxygen supplementation (severity categorized per US Food and Drug Administration guidance [25], detailed in the Supplementary Appendix) on the day of randomization and at least 1 risk factor associated with high risk of disease progression, including age ≥55 years, obesity (body mass index [BMI] ≥30 kg/m2), and comorbidities (as shown in Table 1). Adolescents were eligible for enrollment after an independent data monitoring committee had reviewed pharmacokinetic and safety data for the first 200 adult participants.
Table 1.
Baseline Characteristics (Non-Omicron Modified Full Analysis Set)
| Characteristic | Adintrevimab (n = 169) |
Placebo (n = 167) |
Overall (N = 336) |
|---|---|---|---|
| Age, y | |||
| Median (range) | 58 (22–93) | 56 (18–90) | 57 (18–93) |
| >65 | 47 (27.8) | 46 (27.5) | 93 (27.7) |
| >70 | 31 (18.3) | 23 (13.8) | 54 (16.1) |
| Female sex | 99 (58.6) | 85 (50.9) | 184 (54.8) |
| Race | |||
| White | 167 (98.8) | 164 (98.2) | 331 (98.5) |
| Black or African American | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Other | 1 (0.6) | 2 (1.2) | 3 (0.9) |
| Country | |||
| Brazil | 0 | 1 (0.6) | 1 (0.3) |
| Bulgaria | 95 (56.2) | 92 (55.1) | 187 (55.7) |
| Germany | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Greece | 8 (4.7) | 10 (6.0) | 18 (5.4) |
| Poland | 12 (7.1) | 12 (7.2) | 24 (7.1) |
| Romania | 16 (9.5) | 13 (7.8) | 29 (8.6) |
| South Africa | 3 (1.8) | 3 (1.8) | 6 (1.8) |
| Ukraine | 34 (20.1) | 35 (21.0) | 69 (20.5) |
| BMI, kg/m2, mean ± SD | 29.3 ± 4.8 | 30.6 ± 5.0 | 29.9 ± 4.9 |
| BMI ≥30 kg/m2 | 96 (56.8) | 98 (58.7) | 194 (57.7) |
| SARS-CoV-2 antibody serology status | |||
| Overalla | |||
| Negative | 123 (72.8) | 119 (71.3) | 242 (72.0) |
| Positive | 43 (25.4) | 48 (28.7) | 91 (27.1) |
| Missing | 3 (1.8) | 0 | 3 (0.9) |
| Qualitative assayb | |||
| Negative | 147 (87.0) | 143 (85.6) | 290 (86.3) |
| Positive | 19 (11.2) | 23 (13.8) | 42 (12.5) |
| Missing | 3 (1.8) | 1 (0.6) | 4 (1.2) |
| IgG assay | |||
| Negative | 125 (74.0) | 129 (77.2) | 254 (75.6) |
| Positive | 41 (24.3) | 38 (22.8) | 79 (23.5) |
| Missing | 3 (1.8) | 0 | 3 (0.9) |
| IgA assay | |||
| Negative | 149 (88.2) | 146 (87.4) | 295 (87.8) |
| Positive | 17 (10.1) | 21 (12.6) | 38 (11.3) |
| Missing | 3 (1.8) | 0 | 3 (0.9) |
| Disease severity | |||
| Mild | 97 (57.4) | 76 (45.5) | 173 (51.5) |
| Moderate | 72 (42.6) | 91 (54.5) | 163 (48.5) |
| Risk factors for disease progression | |||
| Obesity (BMI ≥30 kg/m2) | 96 (56.8) | 98 (58.7) | 194 (57.7) |
| Age ≥55 y | 94 (55.6) | 90 (53.9) | 184 (54.8) |
| Cardiac disease | 23 (13.6) | 26 (15.6) | 49 (14.6) |
| Diabetes (type 1 or type 2) | 23 (13.6) | 21 (12.6) | 44 (13.1) |
| Chronic lung disease | 14 (8.3) | 16 (9.6) | 30 (8.9) |
| Stroke or cerebrovascular disease | 4 (2.4) | 9 (5.4) | 13 (3.9) |
| Other immunodeficiency | 5 (3.0) | 4 (2.4) | 9 (2.7) |
| Substance use disorder | 2 (1.2) | 1 (0.6) | 3 (0.9) |
| Chronic kidney disease | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Days from symptom onset to drug administration | |||
| Mean ± SD | 2.4 ± 1.3 | 2.5 ± 1.3 | 2.4 ± 1.3 |
| 0–3 | 133 (78.7) | 128 (76.6) | 261 (77.7) |
| 4–5 | 33 (19.5) | 39 (23.4) | 72 (21.4) |
| Missing | 3 (1.8) | 0 | 3 (0.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; IgA, immunoglobulin A; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Overall baseline serologic status was positive if any of the 3 component assays (qualitative, IgG, or IgA) were positive, missing if all 3 component assays were missing, and negative otherwise.
Baseline serology qualitative assay measured total antibodies to SARS-CoV-2 nucleocapsid (N) antigen.
Serology testing was not required for study eligibility. Participants were excluded if they had a known history of a positive SARS-CoV-2 antibody serology test, were hospitalized, had a known active coinfection, and/or had received a SARS-CoV-2 vaccine or mAb, or plasma from a person who recovered from COVID-19 any time before participation in the trial.
Randomization, Blinding, and Intervention
Eligible participants were randomly assigned in a 1:1 ratio, by an interactive response technology system, to receive a single 300-mg IM dose of adintrevimab or placebo. Randomization was stratified by age (12–17, 18–65, and >65 years) and by country. Limited personnel, including the study pharmacist, designated clinical research associates, and a clinical trial manager independent of the clinical team, were not blinded to study drug assignment. Select sponsor personnel were unblinded after the database lock of the day 29 primary efficacy and interim safety data analyses. All participants, investigators, other site staff, and sponsor personnel (or designees) working directly with the clinical sites were blinded to the study drug assignment until the final database lock.
Viral Load and Viral Sequencing
A validated quantitative RT-PCR (RT-qPCR) assay was used to measure viral load, as described previously [26]. Virology testing and confirmation of SARS-CoV-2 variants by whole genome sequencing (WGS) were performed at Eurofins Viracor BioPharma (Lenexa, Kansas) using a nasopharyngeal or saliva sample collected at baseline or the earliest available postbaseline sample. Participants without WGS data were classified as having either a suspected non-Omicron or Omicron variant based on their randomization date (either before or after the date of the first WGS-confirmed Omicron participant enrolled from the same country or the date of Omicron emergence in the country).
Analysis Populations
The full analysis set (FAS) included all randomized participants, regardless of whether they received study drug. The non-Omicron modified FAS (mFAS) included all randomized participants with COVID-19 due to WGS-confirmed or suspected non-Omicron SARS-CoV-2 variants, regardless of whether they received study drug. The Omicron mFAS included all randomized participants with COVID-19 due to WGS-confirmed or suspected Omicron SARS-CoV-2 variants, regardless of whether they received study drug. The safety set included all participants who received any amount of study drug.
Endpoints
The primary efficacy endpoint was the incidence of COVID-19–related hospitalization or all-cause death through day 29 in the non-Omicron mFAS. Hospitalization was defined as ≥24 hours of acute care in a hospital or acute-care facility (including emergency rooms, intensive care units, acute-care facilities created for COVID-19 pandemic hospitalization needs, or other acute-care facilities).
Prespecified secondary efficacy endpoints were the incidence of severe/critical COVID-19 or all-cause death through day 29, change from baseline in SARS-CoV-2 viral load (log10 copies/mL) assessed by RT-qPCR from saliva samples (days 3, 5, 7, 11, 14, 21, and 29), and time to sustained resolution of COVID-19 symptoms through day 29 (defined as time from the first dose date to the first date when applicable symptoms were scored as absent with no symptom recurrence or new symptoms, except cough, fatigue, and headache, which may have been mild or absent). Exploratory endpoints included duration of COVID-19–related hospitalization and frequency of postacute sequelae of SARS-CoV-2 infection. A prespecified exploratory analysis evaluated the incidence of COVID-19–related hospitalization or all-cause death through day 29 in the Omicron mFAS.
Safety monitoring, including assessment of treatment-emergent AEs (TEAEs), serious AEs (SAEs), and changes from baseline in laboratory tests and vital signs, was conducted throughout the trial. Solicited injection-site reactions were recorded through day 4.
Statistical Analysis
The primary endpoint was estimated via a population-level standardized estimator [27] from a logistic regression model fitted to the binary outcome predicted by treatment and adjusted for the following prognostic factors: age (continuous), sex (categorical), baseline serostatus (categorical), BMI (continuous), and baseline viral load (continuous as log10 copies/mL). Missing status on COVID-19–related hospitalization or all-cause death was imputed as not having a COVID-19 hospitalization or all-cause death. The same method was applied to the analysis of the other secondary clinical endpoints with a binary outcome. Time to sustained resolution of COVID-19 symptoms through day 29 was analyzed using the Kaplan-Meier method and a stratified Cox proportional hazards model including age (12–17, 18–65, and >65 years) as stratification and adjusted for the other prognostic factors as covariates. The change from baseline in SARS-CoV-2 viral load (log10 copies/mL) was analyzed using a mixed model for repeated measures with treatment group and adjusted for the prognostic factors as covariates. The exploratory and safety endpoints were analyzed descriptively.
The protocol version under which the patients were enrolled and statistical analysis plan are available in the Supplementary material. Additional details on protocol amendments and study methodology, including full inclusion/exclusion criteria, definitions, SARS-CoV-2 variant determination, and sample size calculations, are provided in the Supplementary Appendix.
Participant Consent Statement
The trial was conducted in accordance with the International Conference for Harmonisation guideline on Good Clinical Practice, the principles of the Declaration of Helsinki, and all applicable regulations. The protocol and amendments were approved by an ethics committee at each site. Written informed consent was obtained from all participants.
RESULTS
Participants
A total of 399 participants were randomly assigned to receive adintrevimab (n = 198) or placebo (n = 201) (Supplementary Figure 1). Six participants in the adintrevimab group and 1 in the placebo group did not receive the study drug and were excluded from the safety population (n = 392). A total of 336 participants with COVID-19 due to WGS-confirmed or suspected non-Omicron variants (non-Omicron mFAS) comprised the primary efficacy population (169 in the adintrevimab group and 167 in the placebo group). Sixty-three participants with COVID-19 due to WGS-confirmed or suspected Omicron variants comprised the Omicron mFAS population.
Viral sequencing data available for 361 participants confirmed that the Delta B.1.617.2–like SARS-CoV-2 variant was the most prevalent (64.3%), followed by the Delta AY.4–like (16.9%) and Omicron BA.1–like (12.7%) variants (Supplementary Table 1).
Baseline characteristics in the primary efficacy non-Omicron mFAS were generally well balanced between treatment groups (Table 1). The median age was 57 years (range, 18–93 years), 27.7% of participants were aged >65 years, 27.1% had evidence of preexisting immunity to SARS-CoV-2 (ie, seropositivity), and 48.5% of participants had moderate COVID-19 severity. There was a mean of 2 days (range, 0–5 days) from symptom onset to study drug administration. In the Omicron mFAS, the median age was 38 years (range, 15–91 years) and 1 participant in the adintrevimab group was aged <18 years (Supplementary Table 2).
Efficacy
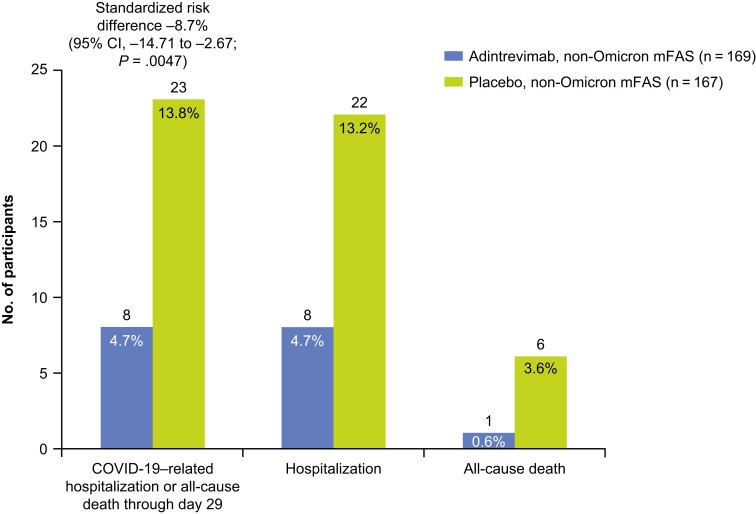
In the primary efficacy analysis (non-Omicron mFAS), COVID-19–related hospitalization or all-cause death through day 29 occurred in 8 of 169 (4.7%) participants in the adintrevimab group and 23 of 167 (13.8%) participants in the placebo group, a 66% relative risk reduction (RRR) in favor of adintrevimab, with a standardized risk difference of −8.7% (95% confidence interval [CI], −14.71% to −2.67%; P = .0047) (Figure 1). There was 1 (0.6%) death in the adintrevimab group and 6 (3.6%) deaths in the placebo group. Other than those events that qualified for the primary endpoint, there were no additional COVID-19–related events of emergency room visits, hospitalizations <24 hours, or severe/critical COVID-19 through day 29; therefore, these additional secondary endpoints are not presented.
Figure 1.
Coronavirus disease 2019–related hospitalization or all-cause death through day 29 (non-Omicron modified full analysis set). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; mFAS, modified full analysis set.
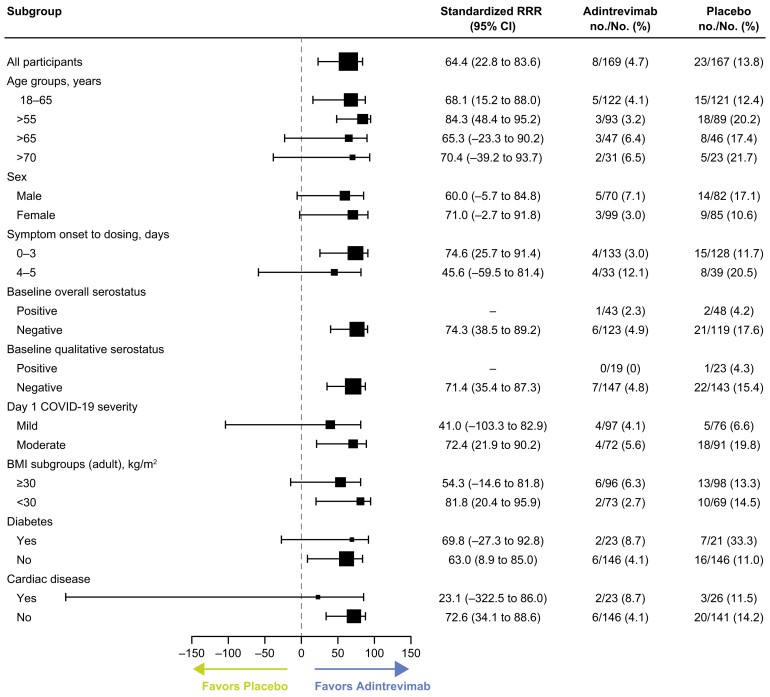
Consistent with the primary analysis, the incidence of COVID-19–related hospitalization or all-cause death through day 29 was lower with adintrevimab versus placebo across all prespecified subgroups in the non-Omicron mFAS, regardless of age, sex, BMI, baseline serology status, disease severity, or coexisting conditions (Figure 2).
Figure 2.
Coronavirus disease 2019–related hospitalization or all-cause death through day 29 across key subgroups (non-Omicron modified full analysis set). Abbreviations: BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; RRR, relative risk reduction.
Among the 63 participants (29 adintrevimab, 34 placebo) with COVID-19 due to confirmed or suspected Omicron variant, there were no events of COVID-19–related hospitalization or all-cause death through day 29 in the adintrevimab group and 2 events (both hospitalizations) in the placebo group.
Additional secondary and exploratory efficacy analyses in the non-Omicron mFAS are shown in Supplementary Tables 3–7. An analysis by number of risk factors at baseline showed that the RRR with adintrevimab was 72.6% among participants with only 1 risk factor for disease progression at baseline (nominal P = .0386), 61.4% among those with >1 risk factor (nominal P = .0633), and 83.3% among those with >2 risk factors (nominal P = .0144) (Supplementary Table 3). In a subgroup of 261 participants who received study drug within 3 days of symptom onset, COVID-19–related hospitalization or all-cause death through day 29 occurred in 4 of 133 (3.0%) participants who received adintrevimab and 15 of 128 (11.7%) who received placebo (Supplementary Table 4), a 74% RRR in favor of adintrevimab, with a standardized risk difference of −8.0% (95% CI, −14.11 to −1.86; nominal P = .0106).
Overall, in the non-Omicron mFAS, median time to sustained COVID-19 resolution was numerically shorter with adintrevimab than placebo (13 vs 16 days; hazard ratio [HR], 1.255; P = .0781), primarily driven by those with moderate disease at baseline (13 vs 22 days; HR, 1.432; nominal P = .0664), with no numerical difference observed among those with mild disease. In participants who received the drug within 3 days of symptom onset, median time to sustained COVID-19 resolution was 11 versus 15 days, respectively (HR, 1.346; nominal P = .0420) (Supplementary Table 5).
In additional exploratory analyses, the duration of hospitalization among those with any COVID-19–related hospital stay without death (n = 7 adintrevimab; n = 17 placebo) was numerically shorter with adintrevimab (mean ± standard deviation, 10 ± 4 days vs 18 ± 15 days) (Supplementary Table 6). Postacute sequelae of SARS-CoV-2 infection were reported numerically by a lower percentage of participants treated with adintrevimab than placebo (3.6%, 2.4%, and 0.6% vs 7.8%, 6.6%, and 3.0% at the day 60, day 90, and month 6 visits, respectively) (Supplementary Table 7).
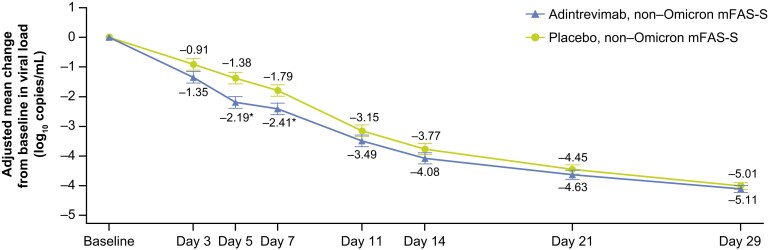
Viral Load
Participants with saliva samples at both baseline and a postbaseline visit through day 29 (the non-Omicron mFAS–saliva) were assessed for changes in viral load (Figure 3). A significantly greater reduction in SARS-CoV-2 viral load from baseline was observed in the adintrevimab group than the placebo group at day 5 that was maintained at day 7 (P < .05 at both timepoints). The adjusted least squares mean difference at day 5 was −0.81 (95% CI, −1.33 to −.30; nominal P = .0020). In the subgroup of participants who received study drug within 3 days of symptom onset, the reduction in SARS-CoV-2 viral load from baseline was significantly greater with adintrevimab than placebo at days 3, 5, and 7 (P < .05 at all timepoints). The adjusted least squares mean difference at day 5 was −0.97 (95% CI, −1.55 to −.40; nominal P = .0010) (Supplementary Figure 2).
Figure 3.
Adjusted mean change from baseline in severe acute respiratory syndrome coronavirus 2 viral load (log10 copies/mL) assessed by quantitative reverse-transcription polymerase chain reaction from saliva samples (non-Omicron modified full analysis set–saliva). Only participants with saliva samples and a value at both baseline visit and the specific postbaseline visit were included (determination made after imputation). The bars represent the standard error. *P < .05. Further detail on methodology provided in Supplementary Figure 2. Abbreviation: mFAS-S, modified full analysis set–saliva.
Safety
The safety analysis included 192 participants who received adintrevimab and 200 participants who received placebo (Table 2). The median duration of follow-up was 258 days for adintrevimab and 253 days for placebo. TEAEs were reported in 65 (33.9%) participants in the adintrevimab group and 79 (39.5%) in the placebo group. The most frequently reported TEAEs were injection-site pain (adintrevimab 12.0%, placebo 8.0%), COVID-19 pneumonia (adintrevimab 4.2%, placebo 12.0%), and injection-site erythema (adintrevimab 1.6%, placebo 3.0%). Solicited injection-site reactions were reported in 13.0% and 9.5% of participants in the adintrevimab and placebo groups, respectively. There were no hypersensitivity reactions, nor were there any trends in laboratory values or vital signs indicative of specific safety risks for adintrevimab.
Table 2.
Safety Summary (Safety Population)
| Adverse Event Category | Adintrevimab (n = 192) |
Placebo (n = 200) |
|---|---|---|
| Any TEAE | 65 (33.9) | 79 (39.5) |
| Unsolicited TEAE | 50 (26.0) | 64 (32.0) |
| Solicited TEAE (ISRs)a | 25 (13.0) | 19 (9.5) |
| Hypersensitivity reactionb | 0 | 0 |
| Most common TEAEs (≥1% of participants) | ||
| Injection-site pain | 23 (12.0) | 16 (8.0) |
| COVID-19 pneumonia | 8 (4.2) | 24 (12.0) |
| Injection-site erythema | 3 (1.6) | 6 (3.0) |
| COVID-19 | 5 (2.6) | 0 |
| Back pain | 4 (2.1) | 3 (1.5) |
| Neck pain | 3 (1.6) | 0 |
| Dizziness | 2 (1.0) | 2 (1.0) |
| Diabetes mellitus | 2 (1.0) | 1 (0.5) |
| Hyperglycemia | 2 (1.0) | 1 (0.5) |
| Hypertension | 2 (1.0) | 1 (0.5) |
| ALT increase | 2 (1.0) | 0 |
| Pneumonia (bacterial) | 2 (1.0) | 0 |
| Abdominal pain (upper) | 1 (0.5) | 3 (1.5) |
| Arthralgia | 1 (0.5) | 2 (1.0) |
| Syncope | 1 (0.5) | 2 (1.0) |
| Toothache | 1 (0.5) | 2 (1.0) |
| Anemia | 0 | 2 (1.0) |
| Hepatic enzyme increase | 0 | 2 (1.0) |
| Insomnia | 0 | 2 (1.0) |
| Pneumonia (Klebsiella pneumoniae) | 0 | 2 (1.0) |
| Upper respiratory tract infection | 0 | 2 (1.0) |
| Pain in extremity | 0 | 2 (1.0) |
| Any SAEc | 13 (6.8) | 29 (14.5) |
| Study drug–related SAEd | 0 | 0 |
| Non-COVID-19 SAE | 6 (3.1) | 8 (4.0) |
| COVID-19 pneumonia SAE | 8 (4.2) | 24 (12.0) |
| Any TEAE leading to death | 2 (1.0) | 7 (3.5) |
| Any study drug–related TEAE leading to deathd | 0 | 0 |
Data are presented as No. (%). Safety data are reported for the data cutoff (8 August 2022). Adverse events were coded using MedDRA version 24.0.
Abbreviations: ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; ISR, injection-site reaction; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
All solicited TEAEs (ISRs) were assumed to be related to study drug.
Hypersensitivity reactions are defined in the Supplementary material.
Solicited adverse events (ISRs) do not have seriousness assigned and were therefore not included in this category.
Missing relationship to study drug was imputed as “related.”
SAEs, including cases of worsening of COVID-19 that met SAE criteria, were recorded in 13 (6.8%) participants in the adintrevimab group and 29 (14.5%) in the placebo group (Table 2). Non-COVID-19–related SAEs occurred with similar incidence in both groups (adintrevimab, 3.1%, placebo, 4.0%). There were 2 TEAEs leading to death in the adintrevimab group and 7 TEAEs leading to death in the placebo group. COVID-19 was the primary cause of death in 6 participants (1 adintrevimab, 5 placebo); the 3 other deaths were attributed to cerebral stroke (adintrevimab), sudden death at home (placebo), and multiorgan failure in a participant hospitalized for COVID-19 who experienced subsequent bacterial infection (placebo). None of the SAEs or deaths in either treatment group were considered related to study drug by the investigators.
DISCUSSION
The results of the STAMP trial demonstrated the efficacy and safety of adintrevimab for the treatment of mild to moderate COVID-19 due to non-Omicron SARS-CoV-2 in a population of high-risk ambulatory participants. A single 300-mg IM injection of adintrevimab provided a statistically and clinically significant (66%) reduction in the relative risk of COVID-19–related hospitalization or all-cause death through day 29 versus placebo. Adintrevimab was well tolerated, and no study drug-related SAEs were reported.
Additional analyses showed that the reduced risk of COVID-19–related hospitalization or all-cause death through day 29, the reduction in viral load, and the more rapid time to sustained COVID-19 resolution were most notable in those who started adintrevimab therapy within 3 days of symptom onset. As peak SARS-CoV-2 viral replication occurs in the upper respiratory tract in presymptomatic and early symptomatic phases of infection [28], these findings support previous research indicating that administration of a therapeutic intervention (eg, mAb or antiviral) is most beneficial in the early stages of symptomatic disease, especially for patients with comorbidities who are at high risk of disease progression and prolonged resolution [29]. Likewise, rapid reduction in viral load is associated with reduced risk of hospital admission and mortality [30].
The results are comparable with those of other SARS-CoV-2 mAbs that have demonstrated efficacy in high-risk COVID-19 patients with disease due to earlier SARS-CoV-2 variants (eg, Alpha, Gamma) in the outpatient treatment setting [31–35]. The participants included in STAMP had disease predominantly due to the Delta variant and related sublineages, which have been associated with more severe disease, including a higher risk of hospitalization and/or death, compared with earlier variants [36–38]. The risk of severe COVID-19 due to the Delta variant is particularly elevated in high-risk participants lacking prior immunity, such as those enrolled in STAMP, underscoring the favorable results seen with adintrevimab in this population.
A limitation of the STAMP trial was that the numbers were too small to allow for meaningful analysis of the exploratory endpoints. There was also a lack of racial diversity and limited number of immunocompromised individuals enrolled, limiting extrapolation of results to these key patient populations who are disproportionately impacted by the COVID-19 pandemic. Likewise, as only unvaccinated participants were enrolled, the utility of adintrevimab in high-risk vaccinated patients is unknown.
The efficacy assessment period of the STAMP trial spanned the emergence and global spread of the SARS-CoV-2 variants Delta and Omicron BA.1/BA1.1. However, because the study was suspended early, insufficient data were obtained in participants with COVID-19 due to Omicron variants. In vitro studies have demonstrated a loss in the neutralizing activity of adintrevimab against the later Omicron BA.2, BA.3, BA.4, and BA.5 sublineages, and adintrevimab is unlikely to provide sufficient activity to reduce the risk of disease progression in the treatment of COVID-19 caused by Omicron.
Strengths of the STAMP trial include a population with high risk of COVID-19 progression based on unvaccinated status, advanced age (∼30% of the participants were aged >65 years), and comorbidities, which is reflective of patients who are in most need of effective and safe therapies to prevent severe outcomes [2]. Furthermore, although a known history of SARS-CoV-2–positive serology was an exclusion criterion, 91 participants in the non-Omicron mFAS population (27%) were seropositive for SARS-CoV-2 at baseline. Of this subgroup, only 3 participants (1 adintrevimab; 2 placebo) had a COVID-19–related hospitalization or all-cause death through day 29, potentially reflective of increased immunity levels from prior infection. The evaluation of IM dosing, which is potentially a more readily accessible route of administration in the outpatient setting compared with IV infusion utilized by most other COVID-19 mAbs, is another strength of the trial. The results support continued exploration of alternative routes of antibody administration that have the potential to facilitate early intervention and ease of access.
CONCLUSIONS
In the STAMP trial, treatment with a single IM injection of adintrevimab was well tolerated and provided statistically and clinically significant protection against severe outcomes in high-risk ambulatory participants with COVID-19 due to non-Omicron SARS-CoV-2 variants, predominantly Delta. These data support the continued development of mAbs for the treatment of COVID-19, particularly for vulnerable populations with limited options. Adintrevimab demonstrated clinically meaningful results in global phase 3 clinical trials for both the prevention and treatment of COVID-19. The adintrevimab clinical data may have the potential to support accelerated development of future mAbs engineered from adintrevimab or utilizing the adintrevimab antibody scaffold, including VYD222. VYD222 was engineered from adintrevimab, has demonstrated in vitro neutralizing activity against currently circulating variants of concern including XBB.1.5, and is currently being studied in a phase 1 clinical trial (NCT05791318) [39].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Michael G Ison, Respiratory Diseases Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, Rockville, Maryland, USA.
Myra Popejoy, Invivyd, Inc, Waltham, Massachusetts, USA.
Nikolay Evgeniev, Complex Oncological Center, Ruse Pltd, Ruse, Bulgaria.
Maria Tzekova, Department of Propedeutics of Internal Diseases, Medical University, Pleven, Bulgaria.
Kathryn Mahoney, Invivyd, Inc, Waltham, Massachusetts, USA.
Natalia Betancourt, Invivyd, Inc, Waltham, Massachusetts, USA.
Yong Li, Invivyd, Inc, Waltham, Massachusetts, USA.
Deepali Gupta, Invivyd, Inc, Waltham, Massachusetts, USA.
Kristin Narayan, Invivyd, Inc, Waltham, Massachusetts, USA.
Ellie Hershberger, Invivyd, Inc, Waltham, Massachusetts, USA.
Lynn E Connolly, Invivyd, Inc, Waltham, Massachusetts, USA.
Ilker Yalcin, Invivyd, Inc, Waltham, Massachusetts, USA.
Anita F Das, Invivyd, Inc, Waltham, Massachusetts, USA.
John Genge, Invivyd, Inc, Waltham, Massachusetts, USA.
Michelle Smith, Invivyd, Inc, Waltham, Massachusetts, USA.
Ed Campanaro, Invivyd, Inc, Waltham, Massachusetts, USA.
Pamela Hawn, Invivyd, Inc, Waltham, Massachusetts, USA.
Pete Schmidt, Invivyd, Inc, Waltham, Massachusetts, USA.
for the STAMP Study Group:
Heloísa Costa Ravagnani Muniz, Maria Tzekova, Kiril Palaveev, Vasil Tsenov, Lilia Pekova, Antoaneta Hadzhieva, Roza Mitreva, Nikolay Nikolaev, Elena Gyuzeleva, Marc Oliver Kornmann, Olaf Schmidt, Garyfalia Poulakou, Charalampos Milionis, Diamantis Kofteridis, Meletios-Athanasios Dimopoulos, Ilias Skopelitis, Anastasia Kotanidou, Sotirios Tsiodras, Grzegorz Kania, Dagmara Grenik, Tomasz Zajac, Anca Streinu-Cercel, Larisha Pillay-Ramaya, Mohamed Mookadam, Lerato Mohapi, Johan George Geldenhuys, Yacoob Vahed, Chantelle Holmgren, Martha Mekebeb-Reuter, William Brumskine, Douwe De Jong, Natasha Joseph, Kirsten McHarry, Shahid Wadvalla, Olena Kobrynska, Igor Kireyev, Kyrylo Lebed, Pavlo Logoida, Olga Barna, Olga Gyrina, Bogdan Gundertaylo, and Viktoriia Rodionova
Notes
Author contributions. Trial design: M. P., P. S., Y. L., K. M., E. H., K. N., I. Y., L. E. C., and A. F. D. Protocol development: M. P., P. S., K. M., N. B., M. S., Y. L., K. N., D. G., A. F. D., L. E. C., and I. Y. Principal investigators: M. T. and N. E. Conducting the trial and medical monitoring: M. P., K. M., N. B., J. G., and E. C. All authors contributed to data interpretation and were involved in drafting and critically revising the manuscript, and all authors approved the final version and are accountable for the accuracy and integrity of the manuscript. All authors had final responsibility for the decision to submit for publication. Y. L., D. G., and N. B. have verified the data.
Acknowledgments. The authors thank the STAMP Study Group members and the participants and their families. We thank the independent data monitoring committee for their review of safety data. Writing assistance for the protocol and the statistical analysis plan was provided by PPD (Wilmington, North Carolina), part of Thermo Fisher Scientific. Writing assistance for the manuscript was provided by Georgiana Manica, PhD, and Jean Turner of Parexel, and was funded by Invivyd, Inc.
Financial support. This work was supported by Invivyd, Inc.
References
- 1. Coronavirus Resource Center . Global deaths. Available at: https://coronavirus.jhu.edu. Accessed 17 January 2023.
- 2. Havers FP, Pham H, Taylor CA, et al. COVID-19–associated hospitalizations among vaccinated and unvaccinated adults 18 years or older in 13 US states, January 2021 to April 2022. JAMA Intern Med 2022; 182:1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 2021; 21:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singson JRC, Kirley PD, Pham H, et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19—COVID-NET, 10 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trujillo-Rodriguez M, Muñoz-Muela E, Serna-Gallego A, et al. Clinical, laboratory data and inflammatory biomarkers at baseline as early discharge predictors in hospitalized SARS-CoV-2 infected patients. PLoS One 2022; 17:e0269875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shrestha SS, Kompaniyets L, Grosse SD, et al. Estimation of coronavirus disease 2019 hospitalization costs from a large electronic administrative discharge database, March 2020–July 2021. Open Forum Infect Dis 2021; 8:ofab561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abraham S, Nohria A, Neilan TG, et al. Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week. J Am Coll Cardiol 2022; 80:1912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deb S, Reeves AA, Hopefl R, Bejusca R. ADME and pharmacokinetic properties of remdesivir: its drug interaction potential. Pharmaceuticals (Basel) 2021; 14:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross SB, Bortolussi-Courval É, Hanula R, Lee TC, Goodwin Wilson M, McDonald EG. Drug interactions with nirmatrelvir-ritonavir in older adults using multiple medications. JAMA Netw Open 2022; 5:e2220184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institutes of Health . Drug–drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir-paxlovid-/paxlovid-drug-drug-interactions/. Accessed 17 January 2023.
- 11. Caniels TG, Bontjer I, van der Straten K, et al. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci Adv 2021; 7:eabj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022; 185:467–484.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 2021; 184:2939–2954.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med 2022; 386:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rappazzo CG, Tse LV, Kaku CI, et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science 2021; 371:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wec AZ, Wrapp D, Herbert AS, et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 2020; 369:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker LM, Pu X, Gong J, Hawn P, Schmidt P. Validation of a predictive model to correlate neutralization titers and efficacy for the prevention of COVID-19 [Poster P-208]. In: International Society for Influenza and Other Respiratory Virus Diseases, Belfast, United Kingdom, 26–29 September 2022. [Google Scholar]
- 19. Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021; 184:4220–4236.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaku CI, Narayan K, Schmidt P, Engler F, Li Y, Walker LM. ADG20, a half-life-extended monoclonal antibody in development for the prevention and treatment of COVID-19, demonstrated broad in vitro neutralisation against SARS-CoV-2 variants [Poster P2161]. In: 32nd European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, 23–26 April 2022.
- 21. Schmidt P, Gong J, Narayan K, et al. Safety, pharmacokinetics, serum neutralizing titers, and immunogenicity of adintrevimab, a monoclonal antibody targeting SARS-CoV-2: a randomized, double-blind, placebo-controlled, phase 1 dose-escalation study in healthy adults. Infect Dis Ther 2023; 12:1365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pu X, Gong J, Campanaro E, et al. Higher doses of adintrevimab, an extended half-life monoclonal antibody, for the treatment and prevention of COVID-19: preliminary results from a phase 1 single ascending-dose study [Poster 226]. In: IDWeek, Washington, DC, 19 October–23 October 2022.
- 23. Rubino CM, Ambrose PG, Connolly LE, Pu X. Population pharmacokinetics of ADG20, an extended-half-life monoclonal antibody being developed for the treatment and prevention of COVID-19 [Poster P2162]. In: 32nd European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, 23–26 April 2022.
- 24. Tarbell ED, Van Wart SA, Shah DK, et al. A whole-body quantitative system pharmacology physiologically based pharmacokinetic model to support dose selection of ADG20: an extended half-life monoclonal antibody being developed for the treatment of coronavirus disease (COVID-19) [Poster 1088]. In: IDWeek, Virtual, 29 September–3 October 2021.
- 25. US Food and Drug Administration . COVID-19: developing drugs and biological products for treatment or prevention. Guidance for industry. Available at: https://www.fda.gov/media/137926/download. Accessed 17 January 2023.
- 26. Schmidt P, Narayan K, Li Y, et al. Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers. Sci Transl Med 2023; 15:eadg2783. [DOI] [PubMed] [Google Scholar]
- 27. Ge M, Durham LK, Meyer RD, Xie W, Thomas N. Covariate-adjusted difference in proportions from clinical trials using logistic regression and weighted risk differences. Drug Inf J 2011; 45:481–93. [Google Scholar]
- 28. Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect 2020; 81:357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gueguen J, Colosio C, Del Bello A, et al. Early administration of anti-SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients. Kidney Int Rep 2022; 7:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Souverein D, van Stralen K, van Lelyveld S, et al. Initial severe acute respiratory syndrome coronavirus 2 viral load is associated with disease severity: a retrospective cohort study. Open Forum Infect Dis 2022; 9:ofac223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 33. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10:985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Earnest R, Uddin R, Matluk N, et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. Cell Rep Med 2022;3100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 Delta (B.1.617.2) compared with Alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis 2022; 22:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. West B, Wec AZ, Doyle M, et al. NVD200 potently neutralises Omicron and its sublineages [Poster P2623]. In: 33rd European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 15–18 April 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.