Abstract
Organophosphate esters (OPEs) are used extensively as flame retardants and plasticizers and are found ubiquitously in the environment and human matrices. Previous studies suggested that exposure to some of these chemicals may disrupt the homeostasis of female sex hormones and have detrimental effects on female fertility. Here, we determined the effects of OPEs on the function of KGN ovarian granulosa cells. We hypothesized that OPEs alter the steroidogenic ability of these cells by dysregulating the expression of transcripts involved in steroid and cholesterol biosynthesis. KGN cells were exposed for 48 hours to 1 of 5 OPEs (1-50μM): triphenyl phosphate (TPHP), tris(methylphenyl) phosphate (TMPP), isopropylated triphenyl phosphate (IPPP), tert-butylphenyl diphenyl phosphate (BPDP), and tributoxyethyl phosphate (TBOEP), or to a polybrominated diphenyl ether flame retardant, 2,2′,4,4′ tetrabromodiphenyl ether (BDE-47), in the presence or absence of Bu2cAMP. OPEs increased the basal production of progesterone (P4) and 17β-estradiol (E2) and had either no effect or inhibited Bu2cAMP-stimulated P4 and E2 synthesis; exposure to BDE-47 had no effect. Quantitative real-time polymerase chain reaction (qRT-PCR) analyses revealed that OPEs (≥5μM) increased the basal expression of critical genes (STAR, CYP11A1, CYP19A1, HSD3B2, and NR5A1) involved in steroidogenesis; upon stimulation, the expression of all genes tested was downregulated. An overall inhibition in cholesterol biosynthesis was induced by OPEs, characterized by a downregulation in HMGCR and SREBF2 expression. TBOEP consistently showed the least effect. Therefore, OPEs perturbed steroidogenesis in KGN granulosa cells by targeting the expression of steroidogenic enzymes and cholesterol transporters; these effects may have an adverse impact on female reproduction.
Keywords: organophosphate esters, ovarian granulosa cells, steroidogenesis
Since the phase-out of polybrominated diphenyl ether (PBDE) flame retardants, organophosphate esters (OPEs) have been used frequently as their replacements (1, 2). OPEs are also used as plasticizers and lubricants in a wide variety of industrial and consumer products (2, 3). The significantly increased production of OPEs as a consequence of restrictions to the use of PBDEs in many jurisdictions has led to temporal trends of increase in their environmental concentrations and in the exposure of wildlife and humans (4‐8). Human exposures occur through incidental ingestion or inhalation of house dust, dermal exposure, and consumption of contaminated food or water (1, 2, 9, 10). Indeed, OPEs have been detected in a wide range of human biological samples (1, 3, 11‐16). The urinary concentrations of OPE metabolites have increased dramatically (up to 15 times) in samples collected between 2002 and 2015 in the United States (7). This alarming trend in exposure to these chemicals has raised concerns about their potential environmental and health impacts.
Although a number of studies have linked exposure to OPEs to neurodevelopmental alterations (17‐19) and thyroid dysfunction (13, 20), their potential reproductive toxicity is less clearly defined. Epidemiological studies have revealed that higher urinary concentrations of OPE metabolites in women undergoing in vitro fertilization were associated with negative pregnancy outcomes, such as reduced fertilization and implantation rates, more frequent pregnancy loss, and preterm birth (21‐24). Other studies reported that OPE exposures were associated with altered levels of testosterone and estradiol in serum samples collected from adolescents and adults of both sexes (3, 25).
Studies in animal models have provided evidence that exposure to some OPEs (tri-o-cresyl phosphate, tris(methylphenyl) phosphate, and butylated triphenyl phosphate) alters the concentrations of circulating progesterone (P4) and estradiol (E2) and disrupts folliculogenesis and estrous cyclicity, contributing to impaired fertility (reviewed by Wang et al, 2022 (26)). Cell-based and high-throughput screening studies have provided evidence that OPEs affect the activities of a range of nuclear hormone receptors, including estrogen, androgen, and glucocorticoid receptors (27‐30). Together, data from these studies suggest that OPE exposures may alter the actions of circulating sex hormones and adversely affect female reproductive health. Given that the ovaries are primary sites for sex hormone production in females, they are considered to be potential targets of OPEs (31, 32). OPEs affect other steroid-producing sites, such as adrenocortical cells and testicular Leydig cells, through dysregulation of the expression of genes involved in steroidogenesis (33, 34). However, the molecular mechanisms by which OPE exposures may disrupt the production of hormones in ovarian steroidogenic cells have yet to be investigated. OPEs may also act as endocrine disruptors through perturbing cholesterol homeostasis, since all steroid hormones are derived from cholesterol (35, 36). In a previous study, we reported that exposure to several prevalent OPEs was associated with major increases in lipid droplets in KGN ovarian granulosa cells (37) which may be indicative of their effects on steroidogenesis and cholesterol homeostasis. In line with this observation, the results of a few in vivo studies indicate that exposure to OPEs causes lipid accumulation in ovarian interstitial tissues of the exposed animals (26).
The objective of this study was to determine the effects of commonly used OPEs on steroidogenesis in ovarian granulosa cells. We hypothesized that exposure to OPEs will alter the production of sex hormones by granulosa cells and modulate their responses to a steroidogenic stimulus. We also investigated whether the expression of key transcripts involved in steroidogenesis and cholesterol biosynthesis was affected. Among the established human granulosa cell lines, KGN cells preserve most of the granulosa cell features, including P4 and E2 synthesis, responsiveness to follicle-stimulating hormone and cAMP, expression of functional aromatase, and an absence of endogenous production of androgens (38, 39). Hence, the KGN human granulosa cell line is a suitable model to study ovarian steroidogenesis. We tested the effects of 5 OPEs that have been detected frequently in both human and environmental matrices: triphenyl phosphate (TPHP), tris(methylphenyl) phosphate (TMPP), isopropylated triphenyl phosphate (IPPP), tert-butylphenyl diphenyl phosphate (BPDP), and tris(2-butoxyethyl) phosphate (TBOEP) (3, 15, 40, 41). A predominant congener of the legacy polybrominated diphenyl ether flame retardant, 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), was used as a reference compound throughout the study.
Materials and Methods
Chemicals
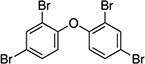
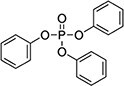
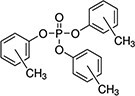
The names of the chemicals tested in this study, their CAS numbers, and structures, are provided in Table 1. BDE-47, TPHP, TMPP (a mixture of ortho-, meta-, para-isomers), and TBOEP were kindly provided by Dr. Nicole Kleinstreuer from the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). IPPP was provided by Dr. Michael G. Wade (Health Canada, Ottawa, Ontario, Canada). BPDP was a gift from Dr. Heather M. Stapleton (Duke University, Durham, North Carolina, USA). All chemicals were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, Missouri, USA).
Table 1.
List of chemicals tested
| Name | CAS | Structure | Purity |
|---|---|---|---|
| 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) | 5436-43-1 |
|
98% |
| Triphenyl phosphate (TPHP) | 115-86-6 |
|
99.9% |
| Tris(methylphenyl) phosphate (TMPP) | 1330-78-5 |
|
98.6% |
| Isopropylated triphenyl phosphate (IPPP)a | 68937-41-7 |
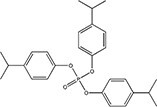
|
N/A |
| tert-Butylphenyl diphenyl phosphate (BPDP)a | 56803-37-3 |
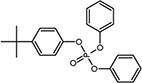
|
N/A |
| Tris(2-butoxyethyl) phosphate (TBOEP) | 78-51-3 |
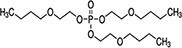
|
93.6% |
IPPP and BPDP are mixtures that contain varying amounts of TPHP, and/or mono-, di-, and tri-substituted compounds; the purity is not available (N/A).
Cell Culture
KGN human granulosa cells were a generous gift from Drs. Christopher Price and Bruce Murphy (Université de Montréal, Montréal, Canada); authorization to use these cells was granted by Dr. Toshihiko Yanase (Fukuoka University, Japan). Cells were cultured in phenol red-free DMEM/F-12 medium (with L-glutamine, 15 mM HEPES) obtained from Gibco BRL (Burlington, Ontario, Canada). The medium was supplemented with 10% charcoal-stripped fetal bovine serum (Wisent Bioproducts, Montreal, Quebec, Canada) and 0.5% 100X penicillin-streptomycin (Wisent). Cells were maintained in T-75 or T-175 Corning culture flasks (virgin polystyrene) at 37°C in a humidified atmosphere of 5% CO2.
Assessment of Effects of OPEs and BDE-47 on the Production of Progesterone and 17β-Estradiol Under Basal and Stimulated Conditions
KGN cells were allowed to adhere for 24 hours at an initial density of 60 000 cells per well in 96-well plates coated with 0.2% rat tail collagen I (Gibco BRL, Burlington, Ontario, Canada). After this acclimation period, the cells were exposed to 200 μL of complete medium containing 0.5% DMSO (vehicle control), or specified concentrations (1μM to 50μM) of BDE-47 or an OPE for 48 hours with or without co-treatment with 1 mM N6,2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate sodium (Bu2cAMP), a cell-permeable analog of the secondary messenger cyclic AMP (cAMP) (Sigma-Aldrich). We selected the highest concentration of each chemical that caused a significant increase in intracellular lipid droplets in KGN cells, while maintaining cell survival at levels above 70% (37). We also used concentrations 10 to 20 times lower to study concentration-dependent responses. The final concentration of DMSO was adjusted to 0.5% in each treatment condition. Cells were supplemented with 10μM androstenedione (Sigma-Aldrich) as a substrate to determine the ability of the cells to synthesize 17β-estradiol (E2). At the end of exposure, the conditioned media in duplicate wells were pooled and stored at −80 °C until use. Cells from these experiments were stained with Hoechst 33 342 (Invitrogen, Waltham, Massachusetts, USA) for 30 minutes. No washing was done to prevent cell loss prior to plate screening with the PerkinElmer Operetta Imaging System (at 10X magnification, all fields were screened). The numbers of Hoechst-positive cells were quantified with the Columbus Image Data Storage and Analysis System (Perkin Elmer) (Supplementary Methods (42)) and used for data normalization. The total cell counts after exposure are shown in Supplementary Fig. S1 (42). Concentrations of progesterone (P4) and E2 were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (progesterone: catalog #IB79105, AB_2892151; estradiol: catalog #RE52041, AB_2934323) according to the manufacturer's instructions; each sample was measured in duplicate. The ELISA plates were read at a wavelength of 450 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices, San Jose, CA, USA). The final hormone concentrations are presented as nanograms (for P4) or picograms (for E2) per 1 million cells. The analytical sensitivities of the P4 and E2 ELISA assays were 0.045 ng/mL and 10.6 pg/mL, respectively. The average intra-assay and inter-assay coefficients of variation were 5.5% and 14.0% for the P4 assay and 5.5% and 12.7% for the E2 assay.
RNA Extraction
KGN cells were seeded in collagen-coated 6-well plates at a density of 360 000 cells/well in 1 mL of complete culture medium. After 24 hours of acclimation, the cells were treated for 48 hours with 0.5% DMSO (control), BDE-47 or an OPE (5µM or 20µM) in duplicate in the absence or presence of Bu2cAMP (1 mM). Total RNA was extracted using the RNeasy Plus Mini Kit (QIAGEN, Mississauga, Ontario, Canada) as per the manufacturer's instructions with slight modifications (Supplementary Methods (42)). The extracted RNA was eluted in 50 µL of RNase-free water and run on a NanoDrop 2000 spectrophotometer (Thermofisher, Waltham, Massachusetts, USA) to assess the concentration and purity of the samples.
Quantitative Real-time Polymerase Chain Reaction
Primers for steroidogenic acute regulatory protein (STAR, QT00091959), translocator protein (TSPO, QT00997731), cholesterol side-chain cleavage enzyme (CYP11A1, QT00040117), 3-beta-hydroxysteroid dehydrogenase (HSD3B2, QT00000490), steroidogenic factor 1 (NR5A1, QT00088018), HMG-CoA reductase (HMGCR, QT00004081), sterol regulatory element binding transcription factor 2 (SREBF2, QT00052052), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, QT00079247) were purchased from QuantiTect Primer Assays (QIAGEN). Primers for CYP19A1 were customized from Integrated DNA Technologies (Coralville, Iowa, USA) using sequences published by Colombe et al (F: 5′-TGCAAAGCACCCTAATGTTG-3′; R: 5′-CATGACCAAGTCCACGACAG-3′) (43) and prepared in 1X Tris-EDTA buffer (Sigma-Aldrich) to a working concentration of 12.5µM. The concentration of RNA was diluted to 2 ng/µL in RNase-free water. For transcripts that had a moderate to low expression level in KGN cells (ie, CYP19A1 and HSD3B2), the working concentration of RNA was optimized to 10 ng/µL. Power SYBR Green RNA-to-CT 1-Step Kits (Applied Biosystems, Foster City, California) were used to quantify the transcripts of interest. Each 20 µL of PCR reaction was run in triplicate and was composed of 10 µL of SYBR Green Master Mix, 2 µL of primer, 0.16 µL of reverse transcriptase, 2.84 µL of RNase-free water, 5 µL of RNA samples or sterile water (negative control). The PCR plates were run on the StepOne Real-Time PCR System (Applied Biosystems) or the ViiA7 Real-Time PCR System (Applied Biosystems) with the following thermocycling conditions: 48 °C for 30 minutes, 95 °C for 10 minutes, followed by 40 cycles at 95 °C for 15 seconds, 55 °C for 30 seconds, and 72 °C for 30 seconds, a melt curve stage (65–95 °C heating, 0.6 °C/s with continuous fluorescence reading). Cycle threshold (CT) values were determined. Triplicate data were manually checked and outliers (>0.2 CT values deviating from the other 2) were excluded. The PCR data were analyzed using StepOnePlus Software (version 2.3) or QuantStudio Real Time PCR Software (version 1.3). The expression levels were normalized to those of the housekeeping gene GAPDH (Delta CT). Relative quantification results were plotted as fold changes compared to the basal controls (2−DDCT).
Statistics
Data are displayed as means ± SEM or means ± 95% CI and analyzed using GraphPad Prism (version 9.4.1, GraphPad Software Inc., La Jolla, California). Two-way repeated measure analysis of variance (ANOVA), followed by Dunnett post hoc tests, was done to determine significant differences from controls under the same condition. P < .05 was considered as statistically significant. For qRT-PCR data, the minimum level of significance was set to P < .01. To compare chemical potencies, the concentrations (EC10) that induced a 10% change in hormone levels from the lowest test concentration were calculated. [Agonist] vs response − Find ECanything model was used for all nonlinear regression analyses. For analyses of the basal datasets, the F value was constrained to 10 (ie, EC10); the top response was set as the highest observed response in all datasets; all curves were set to have a standard slope (Hill slope = 1). For analyses of the stimulated datasets, the F value was set as 90 (ie, IC10) with Hill slope = −1; the bottom response was constrained to a constant value of 0. Each experiment was repeated independently 5 to 8 times.
Results
Effects of Exposure to BDE-47 or to an OPE on Sex Hormone Secretion by KGN Cells
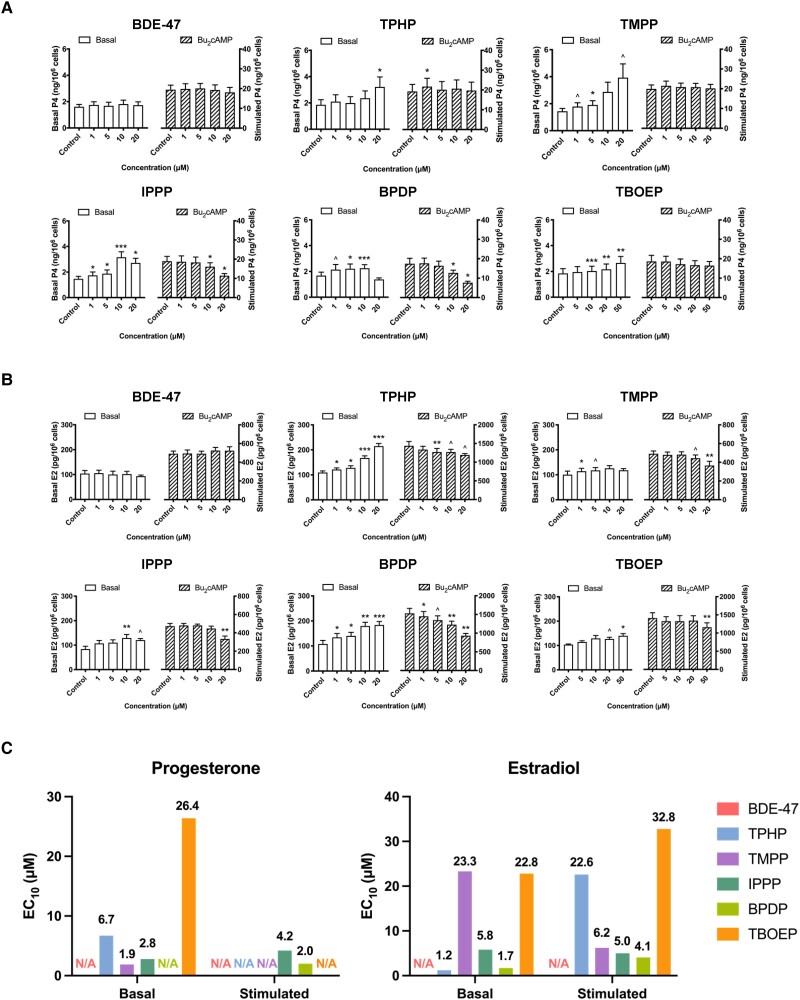
The effects of exposure to BDE-47 or to an OPE on sex hormones secretion by KGN cells were assessed to determine if the overall steroidogenic activities of these cells were affected (Fig. 1). The average baseline production of P4 or E2 per 1 million KGN cells over a 48-hour period was 1.7 ± 0.3 ng and 101.7 ± 10.1 pg, respectively. Exposure to BDE-47 did not affect the basal production of P4 (Fig. 1A). All 5 OPEs significantly increased the basal concentrations of P4. At 10µM, TBOEP caused the smallest increase (11%) and IPPP caused more than a doubling of P4 secretion (116%) compared to controls (Fig. 1A). The presence of Bu2cAMP, a steroidogenic stimulus, increased the concentrations of P4 produced by KGN cells approximately 9-fold; the presence of BDE-47 did not affect this induction. After Bu2cAMP stimulation, an increase above control was observed after 1µM TPHP exposure, whereas there was a decrease after exposure to 10µM or 20µM IPPP or BPDP; neither TMPP nor TBOEP affected P4 production in response to this stimulus.
Figure 1.
OPEs, but not BDE-47, impaired hormone production in KGN cells. Cells were exposed to the chemicals (1μM to 50μM) for 48 hours in the absence or presence of Bu2cAMP (1mM). To measure the production of estradiol, the cells were additionally supplemented with 10 μM androstenedione. The numbers of cells were quantified by Hoechst 33342 staining and high content imaging (10X magnification). Bar graphs show the effects of BDE-47 and 5 OPEs on the basal (open bars, left Y axis) and the Bu2cAMP-stimulated (striped bars, right Y axis) production of (A) progesterone (ng per 106 cells) or (B) estradiol (pg per 106 cells). Values represent means ± SEM; n = 6-8. (C) Estimated concentrations (EC10 values) of chemicals that induced a 10% response from the lowest test concentration were calculated by nonlinear regression analyses. Two-way repeated measure ANOVA followed by Dunnett's tests was conducted to determine significant differences from controls under the same condition: ^0.09 ≤P ≤ .05, *P < .05, **P < .01, ***P < .001. N/A: not available.
The culture medium was supplemented with androstenedione as an exogenous androgen substrate to assess the ability of KGN cells to produce E2. BDE-47 exposure did not affect basal or stimulated E2 production (Fig. 1B). In contrast, under basal conditions, all 5 of the OPEs tested induced an increase in E2 secretion, with 20µM TPHP causing the largest increase (98%) in E2 production relative to control. Exposure to all of the OPEs decreased the secretion of E2 in response to Bu2cAMP stimulation.
To compare chemical potencies, nonlinear regression analysis was done to estimate the EC10 values (Supplementary Figure S2 (42)). A 10% threshold has been commonly used in the literature and recommended by the U.S. Environmental Protection Agency to assess the risk of chemicals (44). With respect to the increase in basal P4 secretion, TMPP (EC10: 1.9µM) was the most potent OPE, followed by IPPP (EC10: 2.8µM), TPHP (EC10: 6.7µM), and TBOEP (EC10: 26.4µM) (Fig. 1C); the EC10 value for BPDP was not calculated due to the absence of a concentration-dependent effect. After stimulation, IPPP (EC10: 4.2µM) and BPDP (EC10: 2µM) were the only 2 OPEs that decreased P4 secretion (Fig. 1C). There was a significant (P < .05) concentration-dependent increase in KGN cell E2 production under basal conditions after exposure to TPHP or BPDP (Supplementary Figure S2 (42)); trends toward an increase were also observed after exposure to the other 3 OPEs tested. TPHP (EC10: 1.2µM) had the most prominent effect on basal E2 secretion (Fig. 1C); after Bu2cAMP stimulation, the inhibition of E2 secretion was greatest after exposure to BPDP (EC10: 4.1µM) or IPPP (EC10: 5.0µM). Collectively, OPEs, but not BDE-47, altered the synthesis of female sex hormones in KGN cells and impaired the responses of these cells to steroidogenic stimuli.
OPEs Altered the Expression of Cholesterol Transporters in KGN Cells
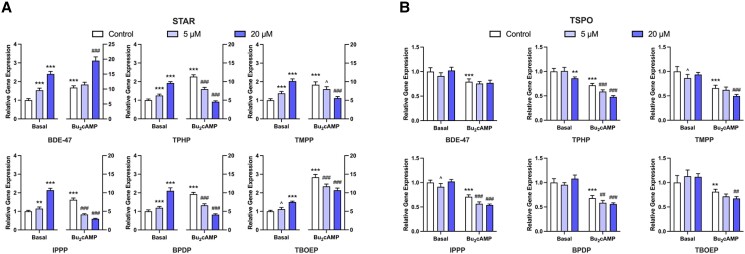
We further determined whether OPEs interfere with steroidogenesis in KGN cells at the transcriptional level by altering the expression of key transcripts involved in this pathway. First, we measured the mRNA expression of 2 cholesterol transporters, STAR and TSPO, following 48 hours of exposure to BDE-47 or an OPE with or without stimulation. Bu2cAMP alone significantly upregulated the relative expression of STAR (Fig. 2A). Interestingly, although exposure to BDE-47 did not affect P4 or E2 secretion, it increased both the basal and stimulated levels of STAR transcripts in KGN cells (Fig. 2A). Under basal conditions, treatment with OPEs caused a concentration-dependent increase in the expression of STAR transcripts (1.1-fold to 2.1-fold). In contrast, the expression of STAR was decreased in cells treated with OPEs after Bu2cAMP stimulation (Fig. 2A). Notably, exposure to 20µM TPHP, IPPP, or BPDP downregulated Bu2cAMP-induced expression of STAR by more than 50%. (Fig. 2A). These findings closely parallel those seen for steroid secretion by KGN cells.
Figure 2.
qRT-PCR results displaying the effects of BDE-47 and 5 OPEs (TPHP, TMPP, IPPP, BPDP, and TBOEP) on the mRNA expression of cholesterol transporters. KGN cells were exposed to vehicle control, 5μM, or 20μM BDE-47 or 1 of the 5 OPEs for 48 hours under basal or Bu2cAMP-stimulated conditions. Bar graphs display the effects test compounds on the relative expression of (A) STAR and (B) TSPO. Data represent means ± 95% CI, n = 5. Two-way repeated measure ANOVA was conducted to determine significant changes relative to controls: *P < .01, **P < .001, ***P < .0001 vs basal control; #P < .01, ##P < .001, ###P < .0001 vs stimulated control; ^0.05 ≤ P ≤ .01 vs the corresponding controls.
Under basal conditions, BDE-47, BPDP, and TBOEP had no effect on TSPO expression and the other OPEs showed minimal effects; only TPHP at 20µM significantly decreased TSPO expression (Fig. 2B). The overall impact of Bu2cAMP stimulation on the expression of TSPO mRNA tended to be downregulation, in contrast to the response observed for STAR. A further decrease in the relative levels of TSPO transcripts was observed after exposure to all 5 OPEs, but not BDE-47 (Fig. 2B). In conclusion, OPEs may interfere with P4 and E2 production in KGN cells by affecting the transport of cholesterol across the mitochondrial membrane. This effect is most likely to be mediated by effects on STAR, rather than by TSPO.
OPEs Altered the Expression of Transcripts Related to Steroidogenesis
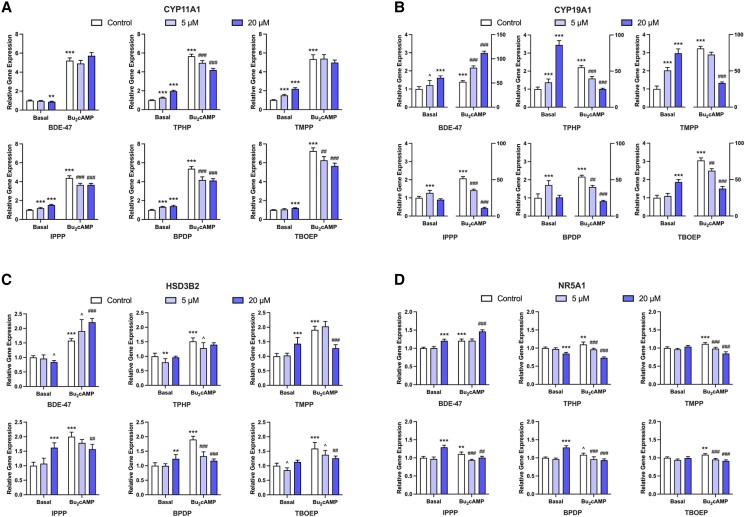
To test whether the alterations in steroid synthesis are associated with direct changes in the expression of steroidogenic enzymes, we assessed effects on the expression of CYP11A1, CYP19A1, HSD3B2, and an upstream regulator of the pathway, NR5A1 (Fig. 3). BDE-47 displayed different effects than OPEs on the expression of these genes. BDE-47, at a concentration of 20µM, induced a slight but significant decrease in the basal expression of CYP11A1; in contrast, all of the OPEs increased the expression of CYP11A1 by 19% (TBOEP) to 120% (TMPP) relative to basal controls (Fig. 3A). Under stimulated conditions, BDE-47 had no effect on CYP11A1 expression; in contrast, TPHP, IPPP, BPDP, and TBOEP (≥5µM) downregulated the expression of CYP11A1 to 74% to 83% of the Bu2cAMP control (Fig. 3A).
Figure 3.
qRT-PCR results displaying the effects of BDE-47 and OPEs on the mRNA expression of key steroidogenic enzymes and an upstream regulator. KGN cells were exposed to vehicle control, 5μM, or 20μM BDE-47 or 1 of the 5 OPEs (TPHP, TMPP, IPPP, BPDP, and TBOEP) for 48 hours under basal or Bu2cAMP-stimulated conditions. Bar graphs display the effects of test compounds on the relative expression of steroidogenic enzymes (A) CYP11A1, (B) CYP19A1, (C) HSD3B2, and the upstream regulator (D) NR5A1. Data represent means ± 95% CI, n = 5. Two-way repeated measure ANOVA was conducted to determine significant changes relative to controls: *P < .01, **P < .001, ***P < .0001 vs basal control; #P < .01, ##P < .001, ###P < .0001 vs stimulated control; ^0.05 ≤ P ≤ .01 vs the corresponding controls.
The baseline levels of CYP19A1 were significantly increased by exposure to BDE-47 (1.6-fold) and by all of the OPEs (1.3-fold to 3.5-fold) compared to the unstimulated controls (Fig. 3B). IPPP and BPDP increased basal CYP19A1 expression at 5µM. Of note, the upregulation induced by 20µM TPHP or TMPP was triple that of basal control (Fig. 3B). After Bu2cAMP stimulation, BDE-47 increased the expression of CYP19A1 by 55% to 113%, whereas all of the OPEs significantly decreased its expression with IPPP causing the largest (79%) and TBOEP the smallest decrease (51%) compared to the Bu2cAMP control (Fig. 3B).
Exposure to BDE-47 induced a small but significant decrease in basal HSD3B2 expression only at 20µM; exposure to TPHP and TBOEP decreased basal HSD3B2 expression at 5µM (Fig. 3C). In contrast, exposure to 20µM TMPP, IPPP, or BPDP increased basal HSD3B2 expression by 24% to 62% compared to control (Fig. 3C). The expression of HSD3B2 was significantly increased in the presence of Bu2cAMP. BDE-47 further increased HSD3B2 expression whereas the addition of all 5 OPEs decreased the HSD3B2 transcripts (Fig. 3C).
All chemicals altered the expression of the steroidogenesis regulator, NR5A1. BDE-47 increased the relative level of NR5A1 transcripts under both basal and stimulated conditions (Fig. 3D). TPHP induced a decrease in basal NR5A1 expression at 20µM, whereas IPPP and BPDP induced an increase at the same concentration (Fig. 3D). Consistent with its effects on other genes, BDE-47 increased Bu2cAMP-stimulated expression of NR5A1; in contrast, all of the OPEs reduced the expression of NR5A1 (Fig. 3D).
In summary, we observed chemical-specific and transcript-specific effects on the basal expression of critical genes involved in steroidogenesis. When the cells were stimulated by Bu2cAMP, an overall upregulation was induced by BDE-47, while a downregulation was induced by OPEs.
OPEs Disrupt Cholesterol Homeostasis in KGN Cells
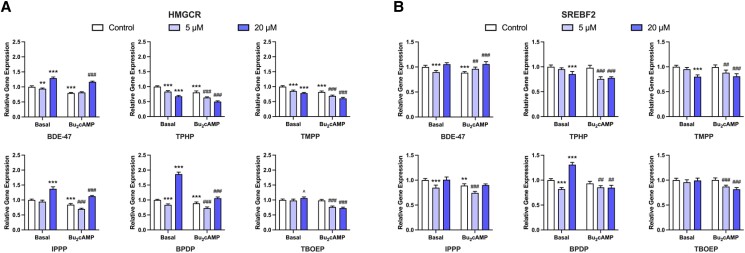
Since the intracellular cholesterol pool is closely related to steroidogenesis, we also measured the mRNA expression of the rate-limiting enzyme (HMGCR) and the main upstream regulator (SREBF2) of the cholesterol biosynthesis pathway following BDE-47 and OPE exposures. Under basal conditions, BDE-47 and BPDP induced biphasic transcriptional changes: exposure to 5µM concentrations downregulated and 20µM upregulated HMGCR transcription (Fig. 4A). At both test concentrations, TPHP and TMPP significantly decreased basal HMGCR levels, by 15% to 32% compared to the control. In contrast, IPPP at 20µM increased HMGCR expression to 1.4-fold of the basal control. TBOEP had no significant effect (Fig. 4A). In the presence of Bu2cAMP, exposure to 20µM BDE-47 upregulated HMGCR transcription by 47% compared to the stimulated control. TPHP, TMPP, TBOEP decreased stimulated HMGCR levels in a concentration-dependent manner (Fig. 4A). It is worth noting that the effects of TPHP and TMPP are highly similar under basal and stimulated conditions; two-way ANOVA statistical analysis also revealed no interaction between treatment and stimulation, indicating that the effects of TPHP and TMPP on HMGCR expression were independent of the stimulation status of cells. IPPP and BPDP first induced a decrease in stimulated HMGCR expression at 5µM, and then an increase at 20µM; the increase was lower in magnitude compared to their effects under the basal condition (Fig. 4A).
Figure 4.
qRT-PCR results displaying the effects of OPEs on the expression of transcripts related to cholesterol biosynthesis. KGN cells were exposed to vehicle control, 5μM, or 20μM BDE-47 or 1 of the 5 OPEs (TPHP, TMPP, IPPP, BPDP, and TBOEP) for 48 hours under basal or Bu2cAMP-stimulated conditions. Bar graphs display the effects of test compounds on the relative expression of the rate-limiting enzyme (A) HMGCR, and the upstream regulator of cholesterol biosynthesis (B) SREBF2. Data represent means ± 95% CI, n = 5. Two-way repeated measure ANOVA was conducted to determine significant changes relative to controls: *P < .01, **P < .001, ***P < .0001 vs basal control; #P < .01, ##P < .001, ###P < .0001 vs stimulated control; ^0.05 ≤ P ≤ .01 vs the corresponding controls.
BDE-47 and some OPEs affected SREBF2 transcripts in a biphasic manner (Fig. 4B). BDE-47, IPPP, and BPDP at 5µM caused a decrease in SREBF2 expression in cells in the basal state; this decrease disappeared after exposure to 20µM (BDE-47 and IPPP) or switched to an increase (BPDP, ∼1.3-fold of the control) (Fig. 4B). TPHP and TMPP at 20µM decreased basal SREBF2 expression by 15% to 20% relative to control, whereas TBOEP showed no effects at either test concentration (Fig. 4B). No apparent induction of SREBF2 expression was elicited by Bu2cAMP. Upon stimulation, although BDE-47 significantly increased SREBF2 transcription, the stimulated levels were comparable to those of the basal control (Fig. 4B). The impact of OPEs on stimulated SREBF2 expression was similar. All of the OPEs decreased the relative levels of SREBF2 by about 9% to 23%, starting at 5µM (Fig. 4B). Together, our data suggest that OPEs may affect HMGCR expression through the regulation of its upstream regulator SREBF2. Some of the effects appeared to be independent of stimulation by Bu2cAMP; an overall inhibition in the pathway was induced by OPEs under both basal and stimulated conditions.
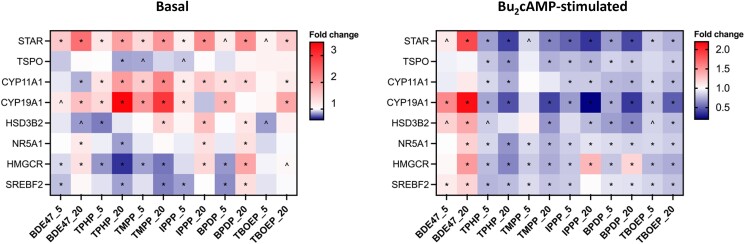
A heatmap of relative gene expression is shown in Fig. 5, summarizing our qRT-PCR results. Both BDE-47 and OPEs had clear concentration-dependent effects, but their effects differed significantly from each other; the difference is more striking under the stimulated condition, with BDE-47 causing a stimulation and OPEs causing an overall inhibition in the steroidogenic and cholesterol biosynthesis pathways. Several transcripts (STAR, CYP11A1, CYP19A1, HMGCR) were more sensitive than the others. Overall, TBOEP had relatively fewer effects on transcript expression compared to other OPEs.
Figure 5.
Heatmaps showing the mean fold change of gene expression compared to controls under the same condition. Cells were exposed to vehicle control, 5μM, or 20μM BDE-47 or 1 of the 5 OPEs for 48 hours in the absence or presence of Bu2cAMP. Two-way repeated measure ANOVA followed by Dunnett's tests was conducted to determine significant changes (n = 5): *P < .01, ^0.05 ≤ P ≤ .01. An upregulation appears red, and a downregulation appears blue.
Discussion
Despite the widespread presence of OPEs, our current knowledge regarding their effects on the function of granulosa cells is limited. Previously, we reported that some OPEs induced an accumulation of lipid droplets in KGN granulosa cells, suggesting disrupted lipid homeostasis and potential endocrine effects (37). In this study, we determined the impact of exposure to OPEs on steroidogenesis in KGN cells. The 4 triaryl-OPEs that we studied, ie, TPHP, TMPP, IPPP, BPDP, had the highest potencies to induce phenotypic changes in KGN cells (37). TBOEP, the one nontriaryl-OPE we investigated, also induced lipid accumulation but had relatively fewer effects on other parameters that were assessed (37). Here, we found that the production of both P4 and E2 was affected by all 5 of these OPEs; the effects varied depending on whether the cells were stimulated or not. These 2 female sex hormones have essential roles in the maintenance of physiological functions in reproductive organs and in other target tissues, such as skeleton, skin, and brain (45‐49). Thus, exposure to OPEs may have profound effects on health.
To the best of our knowledge, the current study is the first to demonstrate the effects of OPEs on steroidogenesis in cells of the female reproductive system. In line with these data, several OPEs, including TPHP, TMPP, and TBOEP, at concentrations of ∼ 0.5µM to 25µM, increased the basal production of E2 by H295R human adrenal cells without causing apparent cell death (33). Similarly, TMPP, BPDP, IPPP, and 2 diphenyl phosphate compounds at 10µM were shown to increase basal P4 synthesis in MA-10 mouse Leydig cells (34). In the same study, TPHP, TMPP, and BPDP had no effects on steroid production after Bu2cAMP or luteinizing hormone stimulation, whereas IPPP further increased stimulated P4 concentrations (34). These data suggest that the endocrine disrupting effects of OPEs are chemical and cell-type specific.
For purposes of comparing the effects of OPEs to legacy flame retardants, we also assessed the effects of a major polybrominated diphenyl ether flame retardant, BDE-47. A 48-hour exposure to 50 μM of BDE-47 did not affect the secretion of either P4 or E2 by KGN cells, regardless of the presence of Bu2cAMP. Thus, our data show that OPEs are more prone to altering ovarian steroidogenesis than some of the legacy flame retardants that are regulated. In contrast to the lack of research on the effects of OPEs on ovarian steroidogenesis, there are previous in vitro and in vivo studies focusing on BDE-47 (50‐54). Studies using a co-culture model of theca and granulosa cells or porcine ovarian follicles, reported that exposure to BDE-47, or its hydroxylated metabolites, resulted in altered production of P4 and E2, but there was no consistent direction of change in these studies (50, 52, 53). The discrepancy in experimental models used may be a factor that contributes to the different observations made.
TBOEP consistently showed fewer effects on P4 or E2 production than the triaryl compounds. This is concordant with our previous observation that nontriaryl-OPEs were less potent than triaryl-OPEs in altering cell phenotypes (37). Interestingly, we noted significant alterations in steroid production at the NOAEL (ie, 1 μM, no-observed-adverse-effect level) determined based on phenotypic effects (37). Therefore, it is important to consider the effects on both phenotypes and functions of cells to fully evaluate the toxicity of emerging chemicals.
Here we report that the effects of OPEs on steroid hormones are reflected by changes in the mRNA expression of several genes responsible for steroidogenesis in KGN granulosa cells. There is a distinct pattern in the effects of BDE-47 and OPEs on gene expression. Although BDE-47 had no effect on the concentrations of P4 or E2 in the medium, it was able to modulate the expression of several of the genes tested. This could be partly attributed to the involvement of other pathways or posttranscriptional events that neutralize the transcriptional changes. For instance, exposure to BDE-47 was found to inhibit the activity of aromatase in porcine ovarian cells (52) and accelerate the metabolism of steroid hormones (55, 56). As a net outcome, the balance of P4 and E2 may remain unaffected.
Alterations in the transcript levels of STAR and CYP11A1 best explain the changes in both basal and stimulated release of P4 observed after exposure to OPEs. STAR is one of the major components of a protein complex that facilitates the delivery of cholesterol across mitochondrial membranes for the initiation of sex steroid production (57). This translocation process is considered pivotal in acute steroidogenic responses owing to the sterol-poor characteristics of the inner mitochondrial membranes (36). The transcription and activity of CYP11A1, the enzyme that catalyzes the first step in progesterone biosynthesis, are important determinants of the steroidogenic capacity of cells (36). Aromatase is the rate-limiting enzyme in estrogen biosynthesis. We observed strong responses of CYP19A1 to OPE exposures. These changes provide an explanation for the effects of TPHP, IPPP, and TBOEP on E2 production, but not for those of 20μM TMPP or BPDP under basal conditions. These OPEs may have additional targets that contribute to the alterations in E2. For example, in H295R adrenal cells, TMPP, TPHP, and TBOEP inhibited the expression of 2 transcripts related to estradiol metabolism, SULT1E1 and SULT2A1 (33). Together, our studies have demonstrated that OPEs target multiple rate-limiting steps of steroid synthesis in KGN cells. Several OPE compounds have the ability to upregulate the basal expression of these genes with a potency comparable to that of Bu2cAMP, a potent steroidogenic stimulus. In agreement with our data, Liu et al reported that TPHP, TMPP, TBOEP, and 3 other OPEs stimulated the basal synthesis of E2 and testosterone by upregulating the mRNA expression of steroidogenic enzymes such as CYP11A1 and CYP19A1 in H295R adrenal cells (33). Our results indicate that several OPEs may affect steroidogenesis by targeting HSD3B2 expression, ie, expression of the enzyme that synthesizes P4 from pregnenolone (35). While HSD3B2 transcription did not appear to be a major target of TPHP, 3 other OPEs (TMPP, IPPP, and BPDP) increased the basal expression of HSD3B2 and reduced the magnitude of stimulation induced by Bu2cAMP. Schang et al also reported an inhibitory effect of IPPP and tri-o-cresyl phosphate on the expression of HSD3B2 in Bu2cAMP or luteinizing hormone–stimulated MA-10 Leydig cells (34). In KGN cells, TBOEP had an effect only under stimulated conditions.
To investigate if the observed changes were mediated by the master regulator of steroidogenesis, SF-1, we measured the expression of NR5A1 transcripts. Although the overall patterns of changes were similar, alterations in NR5A1 mRNA levels alone cannot fully account for changes in the expression of other steroidogenic enzymes. Despite the essential role of SF-1 in the regulation of most steroidogenic genes, the specific expression of each gene is also under the control of many other transcription factors that may act independently or in concert with SF-1 (58). In addition, we observed that Bu2cAMP alone caused only a minor stimulation on NR5A1 transcription in KGN cells. Previous studies have suggested that follicle-stimulating hormone or cAMP alone may primarily regulate the activation and recruitment of SF-1 and its interaction with other regulatory cofactors, rather than affecting its mRNA and protein expression (58). Indeed, only the combination of activin and follicle-stimulating hormone may effectively increase the expression of SF-1 (59). Given the strong interplay between SF-1 and the cAMP signaling transduction, it is interesting that some OPEs exhibited an effect on NR5A1 only when the cells were stimulated (60). Further studies are required to unravel how OPE exposures may interfere with the upstream regulators of steroidogenic genes.
Cholesterol is the primary type of lipid stored in steroidogenic cells. In light of our previous observation that OPEs induced lipid droplet accumulation in KGN cells, we investigated the effects of OPEs on the expression of the rate-limiting enzyme (HMGCR) and the main regulator (SREBF2) of cholesterol biosynthesis (61, 62). We observed a consistent downregulation of these 2 genes following OPE exposures, with the exception of IPPP and BPDP at 20µM; this inhibition appeared to be independent of the presence of the stimulus. It is possible that exposure to OPEs causes an initial rise in intracellular cholesterol which subsequently triggers negative feedback in KGN cells (36). Previous studies support this hypothesis. TPHP and 2-ethylhexyl diphenyl phosphate (EHDPP) were reported to antagonize the activity of liver X receptor (LXR), which counteracts cholesterol overload by increasing cholesterol export and decreasing cholesterol uptake (63, 64). As a result, these 2 OPEs significantly reduced the rate of cholesterol efflux and induced foam-cell-like phenotype in RAW264.7 macrophages (63). Notably, human exposure to TPHP and EHDPP was associated with higher levels of total cholesterol and triglyceride in plasma (65). Exposure to TPHP and BPDP increased the protein expression of low-density lipoprotein receptor (LDLR) in coronary artery smooth muscle cells, suggesting an elevated uptake of LDL-bound cholesterol (29). Moreover, cholesterol insufficiency was associated with an acute increase (within the first 6-12 hours) in pregnenolone production by MA-10 Leydig cells, Y-1 adrenocortical cells, and BeWo placental choriocarcinoma cells (66), suggesting a role for cholesterol availability in the regulation of steroidogenesis. Treatment of granulosa-lutein cells with lipoproteins enhanced basal and Bu2cAMP-stimulated steroidogenesis by delivering cholesterol as a substrate (67). Our results indicate that OPEs may modulate the intracellular cholesterol pool in KGN cells via multiple mechanisms. It will be interesting to assess if the effects of OPEs on cholesterol homeostasis directly contribute to their effects on steroidogenesis.
Conclusions
Our data reveal that the pervasive OPEs that are used as flame retardants and plasticizers have major endocrine-active effects on human ovarian granulosa cells. In comparison to the effects of a legacy brominated flame retardant, these alternative organophosphate compounds displayed higher potencies in perturbing hormone synthesis by KGN cells. The transcription of key transporters, enzymes, and regulators involved in the steroidogenic pathway is recognized as an important mediator for the bioactivities of OPEs. The effects of OPEs on P4 and E2 production were mostly consistent with those on STAR, CYP11A1, and CYP19A1 transcripts; several OPEs also targeted the transcription of TSPO, HSD3B2, and NR5A1. We also observed a structure-dependent potency of OPEs with respect to their impact on steroidogenesis; the 4 triaryl-OPEs consistently showed higher bioactivity than TBOEP. Moreover, our results strongly suggest that OPEs can induce alterations in the intracellular cholesterol pool, which functions as a readily accessible source of substrate for steroidogenesis. Thus, the endocrine disrupting effects of OPEs may occur not only through direct induction of transcriptional changes but also through modulation of the availability of steroidogenic substrates. This study provides insight into the mechanism by which OPEs induce a steroidogenic disruption in granulosa cells and highlights the importance of assessing endocrine function as a toxicity endpoint when evaluating emerging chemicals.
Acknowledgments
The authors acknowledge Dr. Christopher Price and Dr. Bruce Murphy (Université de Montréal, Montréal, Canada) and Dr. Toshihiko Yanase (Fukuoka University, Japan) for providing and allowing the use of KGN cells. We thank Dr. Nicole Kleinstreuer (National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods [NICEATM]), Dr. Michael G. Wade (Health Canada), and Dr. Heather M. Stapleton (Duke University) for providing test chemicals. Image acquisition (Operetta) and analysis (Columbus), qRT-PCR experiments were performed with the McGill University Imaging and Molecular Biology Platform (IMBP) equipment. We thank Dr. Nicolas Audet (IMBP), Trang Luu (McGill), and Lama Iskandarani (McGill) for technical support.
Glossary
Abbreviations
- ANOVA
analysis of variance
- BDE-47
2,2′,4,4′-tetrabromodiphenyl ether
- BPDP
tert-butylphenyl diphenyl phosphate
- Bu2cAMP
dibutyryladenosine-3′,5′-cyclic monophosphate sodium
- cAMP
cyclic AMP
- CYP11A1
cholesterol side-chain cleavage enzyme
- CYP19A1
aromatase
- DMSO
dimethyl sulfoxide
- E2
17β-estradiol
- ELISA
enzyme-linked immunosorbent assay
- HMGCR
HMG-CoA reductase
- HSD3B2
3-beta-hydroxysteroid dehydrogenase
- IPPP
isopropylated triphenyl phosphate
- NR5A1
steroidogenic factor 1
- OPE
organophosphate ester
- P4
progesterone
- PBDE
polybrominated diphenyl ether
- qRT-PCR
quantitative real-time polymerase chain reaction
- SREBF2
sterol regulatory element binding transcription factor 2
- STAR
steroidogenic acute regulatory protein
- TBOEP
tris(2-butoxyethyl) phosphate
- TMPP
tris(methylphenyl) phosphate
- TPHP
triphenyl phosphate
- TSPO
translocator protein
Contributor Information
Xiaotong Wang, Department of Pharmacology & Therapeutics, McGill University, Montreal, QC, H3G 1Y6, Canada.
Elaine Lee, Department of Pharmacology & Therapeutics, McGill University, Montreal, QC, H3G 1Y6, Canada.
Barbara F Hales, Department of Pharmacology & Therapeutics, McGill University, Montreal, QC, H3G 1Y6, Canada.
Bernard Robaire, Department of Pharmacology & Therapeutics, McGill University, Montreal, QC, H3G 1Y6, Canada; Department of Obstetrics & Gynecology, McGill University, Montreal, QC, H3G 1Y6, Canada.
Funding
Canadian Institutes of Health Research (CIHR) Institute for Population and Public Health team Grant (FRN IP3-150711), Canadian Institutes of Health Research (CIHR) Project Grant FRN 156239, and McGill University. X.W. is the recipient of scholarships from McGill University and the Macau Government. B.F.H. and B.R. are James McGill Professors.
Author Contributions
X.W., B.F.H., and B.R. were responsible for the experimental design, data interpretation, and manuscript preparation. X.W. and E.L. were responsible for data acquisition and analyses. All authors approved the final version of the article.
Disclosures
The authors have nothing to disclose. The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability
All data are available upon request. Supplemental data are available at https://doi.org/10.5683/SP3/59VOBT
References
- 1. van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119‐1153. [DOI] [PubMed] [Google Scholar]
- 2. Chokwe TB, Abafe OA, Mbelu SP, Okonkwo JO, Sibali LL. A review of sources, fate, levels, toxicity, exposure and transformations of organophosphorus flame-retardants and plasticizers in the environment. Emerg Contam. 2020;6:345‐366. [Google Scholar]
- 3. Siddique S, Farhat I, Kubwabo C, et al. Exposure of men living in the greater Montreal area to organophosphate esters: association with hormonal balance and semen quality. Environ Int. 2022;166:107402. [DOI] [PubMed] [Google Scholar]
- 4. Greaves AK, Letcher RJ. A review of organophosphate esters in the environment from biological effects to distribution and fate. Bull Environ Contam Toxicol. 2017;98(1):2‐7. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Xiong S, Hao Y, et al. Organophosphate esters in Arctic air from 2011 to 2019: concentrations, temporal trends, and potential sources. J Hazard Mater. 2022;434:128872. [DOI] [PubMed] [Google Scholar]
- 6. Dodson RE, Perovich LJ, Covaci A, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46(24):13056‐13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffman K, Butt CM, Webster TF, et al. Temporal trends in exposure to organophosphate flame retardants in the United States. Environ Sci Tech Let. 2017;4(3):112‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choo G, Ekpe OD, Park KW, Chung D, Lee J, Oh J-E. Temporal and spatial trends of chlorinated paraffins and organophosphate flame retardants in black-tailed gull (Larus crassirostris) eggs. Sci Total Environ. 2022;803:150137. [DOI] [PubMed] [Google Scholar]
- 9. Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiology. 2008;18(1):2‐19. [DOI] [PubMed] [Google Scholar]
- 10. Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Persp. 2015;123(2):160‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubwabo C, Kosarac I, Lalonde K. Determination of selected perfluorinated compounds and polyfluoroalkyl phosphate surfactants in human milk. Chemosphere. 2013;91(6):771‐777. [DOI] [PubMed] [Google Scholar]
- 12. Yang J, Zhao Y, Li M, Du M, Li X, Li Y. A review of a class of emerging contaminants: the classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int J Mol Sci. 2019;20(12):2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruis MT, Rock KD, Hall SM, Horman B, Patisaul HB, Stapleton HM. PBDEs concentrate in the fetal portion of the placenta: implications for thyroid hormone dysregulation. Endocrinology. 2019;160(11):2748‐2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Health Canada . Report on human biomonitoring of environmental chemicals in pooled samples (2020). Accessed January 18, 2023. https://www.canada.ca/en/health-canada/services/environmental-workplace-health/environmental-contaminants/human-biomonitoring-environmental-chemicals/report-pooled-samples.html
- 15. Siddique S, Harris SA, Kosarac I, Latifovic L, Kubwabo C. Urinary metabolites of organophosphate esters in women and its relationship with serum lipids: an exploratory analysis. Environ Pollut. 2020;263(6):114110. [Google Scholar]
- 16. Lefèvre PLC, Nardelli TC, Son W-Y, et al. Polybrominated diphenyl ethers in human follicular fluid dysregulate mural and cumulus granulosa cell gene expression. Endocrinology. 2021;162(6):bqab00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doherty BT, Hoffman K, Keil AP, et al. Prenatal exposure to organophosphate esters and cognitive development in young children in the pregnancy, infection, and nutrition study. Environ Res. 2019;169:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vuong AM, Yolton K, Cecil KM, Braun JM, Lanphear BP, Chen A. Flame retardants and neurodevelopment: an updated review of epidemiological literature. Curr Epidemiol Rep. 2020;7(4):220‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patisaul HB, Behl M, Birnbaum LS, et al. Beyond cholinesterase inhibition: developmental neurotoxicity of organophosphate ester flame retardants and plasticizers. Environ Health Persp. 2021;129(10):105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Percy Z, Vuong AM, Xu Y, et al. Maternal urinary organophosphate esters and alterations in maternal and neonatal thyroid hormones. Am J Epidemiol. 2021;190(9):kwab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carignan CC, Mínguez-Alarcón L, Butt CM, et al. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ Health Persp. 2017;125(8):087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carignan CC, Mínguez-Alarcón L, Williams PL, et al. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ Int. 2018;111:232‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messerlian C, Williams PL, Mínguez-Alarcón L, et al. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil Steril. 2018;110(6):1137‐1144.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffman K, Stapleton HM, Lorenzo A, et al. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ Int. 2018;116:248‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo K, Liu J, Wang Y, et al. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ Int. 2020;136:105461. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Hales BF, Robaire B. Effects of flame retardants on ovarian function. Reprod Toxicol. 2021;102:10‐23. [DOI] [PubMed] [Google Scholar]
- 27. Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology. 2013;314(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Zhang R, Song C, Crump D. Computational evaluation of interactions between organophosphate esters and nuclear hormone receptors. Environ Res. 2019;182:108982. [DOI] [PubMed] [Google Scholar]
- 29. United States Environmental Protection Agency (U.S EPA), Tox21/ToxCast database, CompTox chemicals dashboard. Accessed January 18, 2023. https://comptox.epa.gov/dashboard/
- 30. Young AS, Zoeller T, Hauser R, et al. Assessing indoor dust interference with human nuclear hormone receptors in cell-based luciferase reporter assays. Environ Health Persp. 2021;129(4):047010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foster WG, Gannon AM, Furlong HC. Chapter 29 - Environmental Contaminants and Ovarian Toxicity. In: Leung PCK, Adashi EY, eds. The ovary. Academic Press;2019:485‐491. [Google Scholar]
- 33. Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012;114-115:173‐181. [DOI] [PubMed] [Google Scholar]
- 34. Schang G, Robaire B, Hales BF. Organophosphate flame retardants act as endocrine-disrupting chemicals in MA-10 mouse tumor Leydig cells. Toxicol Sci. 2016;150(2):499‐509. [DOI] [PubMed] [Google Scholar]
- 35. Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295‐318. [DOI] [PubMed] [Google Scholar]
- 36. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52(12):2111‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Luu T, Beal MA, Barton-Maclaren TS, Robaire B, Hales BF. The effects of organophosphate esters used as flame retardants and plasticizers on granulosa, Leydig, and spermatogonial cells analyzed using high-content imaging. Toxicol Sci. 2022;186(2):269‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishi Y, Yanase T, Mu Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437‐445. [DOI] [PubMed] [Google Scholar]
- 39. Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228(1-2):67‐78. [DOI] [PubMed] [Google Scholar]
- 40. Fan X, Kubwabo C, Rasmussen PE, Wu F. Simultaneous determination of thirteen organophosphate esters in settled indoor house dust and a comparison between two sampling techniques. Sci Total Environ. 2014;491–492:80‐86. [DOI] [PubMed] [Google Scholar]
- 41. Kubwabo C, Fan X, Katuri GP, Habibagahi A, Rasmussen PE. Occurrence of aryl and alkyl-aryl phosphates in Canadian house dust. Emerg Contam. 2021;7:149‐159. [Google Scholar]
- 42. Wang X, Lee E, Hales BF, Robaire B. 2023. Supplemental Materials_Wang et al Organophosphate Esters Disrupt Steroidogenesis in KGN Human Ovarian Granulosa Cells. V1. Borealis. Published May 10, 2023. 10.5683/SP3/59VOBT [DOI] [PMC free article] [PubMed]
- 43. Colombe S, Houllier L, Fleurot E, et al. Syndecan 1 represses cell growth and FSH responsiveness in human granulosa cells. Reproduction. 2017;153(6):797‐808. [DOI] [PubMed] [Google Scholar]
- 44. United States Environmental Protection Agency (U.S. EPA) . 2012. Benchmark Dose Technical Guidance. Washington, DC 20460: Risk Assessment Forum, U.S. EPA Report EPA/100/R-12/001. https://www.epa.gov/risk/benchmark-dose-technical-guidance
- 45. McNatty KP, Makris A, DeGrazia C, Osathanondh R, Ryan KJ. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J Clin Endocrinol Metabolism. 1979;49(5):687‐699. [DOI] [PubMed] [Google Scholar]
- 46. Hsueh AJW, Adashi EY, Jones PBC, Welsh TH. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5(1):76‐127. [DOI] [PubMed] [Google Scholar]
- 47. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Gene Dev. 1995;9(18):2266‐2278. [DOI] [PubMed] [Google Scholar]
- 48. Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144(8):3329‐3337. [DOI] [PubMed] [Google Scholar]
- 49. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biology. 2003;86(3-5):225‐230. [DOI] [PubMed] [Google Scholar]
- 50. Gregoraszczuk EŁ, Rak A, Kawalec K, Ropstad E. Steroid secretion following exposure of ovarian follicular cells to single congeners and defined mixture of polybrominateddibenzoethers (PBDEs), p, p′-DDT and its metabolite p, p′-DDE. Toxicol Lett. 2008;178(2):103‐109. [DOI] [PubMed] [Google Scholar]
- 51. Talsness CE, Kuriyama SN, Sterner-Kock A, et al. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Persp. 2008;116(3):308‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karpeta A, Rak-Mardyła A, Jerzak J, Gregoraszczuk EL. Congener-specific action of PBDEs on steroid secretion, CYP17, 17β-HSD and CYP19 activity and protein expression in porcine ovarian follicles. Toxicol Lett. 2011;206(3):258‐263. [DOI] [PubMed] [Google Scholar]
- 53. Karpeta A, Barc J, Ptak A, Gregoraszczuk EL. The 2,2′,4,4′-tetrabromodiphenyl ether hydroxylated metabolites 5-OH-BDE-47 and 6-OH-BDE-47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology. 2013;305:65‐70. [DOI] [PubMed] [Google Scholar]
- 54. Zhu Y, Tan YQ, Leung LK. Exposure to 2,2′,4,4′-tetrabromodiphenyl ether at late gestation modulates placental signaling molecules in the mouse model. Chemosphere. 2017;181:289‐295. [DOI] [PubMed] [Google Scholar]
- 55. Karpeta A, Warzecha K, Jerzak J, Ptak A, Gregoraszczuk EL. Activation of the enzymes of phase I (CYP2B1/2) and phase II (SULT1A and COMT) metabolism by 2,2′,4,4′-tetrabromodiphenyl ether (BDE47) in the pig ovary. Reprod Toxicol. 2012;34(3):436‐442. [DOI] [PubMed] [Google Scholar]
- 56. Lefevre PLC, Wade M, Goodyer C, Hales BF, Robaire B. A mixture reflecting polybrominated diphenyl ether (PBDE) profiles detected in human follicular fluid significantly affects steroidogenesis and induces oxidative stress in a female human granulosa cell line. Endocrinology. 2016;157(7):2698‐2711. [DOI] [PubMed] [Google Scholar]
- 57. Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26(6):771‐790. [DOI] [PubMed] [Google Scholar]
- 58. Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol Baltim Md. 2010;24(7):1322‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hunzicker-Dunn M, Maizels ET. FSH Signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sugawara T, Saito M, Fujimoto S. Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology. 2000;141(8):2895‐2903. [DOI] [PubMed] [Google Scholar]
- 61. Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35‐46. [DOI] [PubMed] [Google Scholar]
- 62. Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: A delicate balance. Cell Metab. 2008;8(6):512‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hu W, Jia Y, Kang Q, et al. Screening of house dust from Chinese homes for chemicals with liver X receptors binding activities and characterization of atherosclerotic activity using an in vitro macrophage cell line and ApoE−/− mice. Environ Health Persp. 2019;127(11):117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lund EG, Menke JG, Sparrow CP. Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(7):1169‐1177. [DOI] [PubMed] [Google Scholar]
- 65. Zhao F, Li Y, Zhang S, Ding M, Hu J. Association of aryl organophosphate flame retardants triphenyl phosphate and 2-ethylhexyl diphenyl phosphate with human blood triglyceride and total cholesterol levels. Environ Sci Tech Let. 2019;6(9):532‐537. [Google Scholar]
- 66. Bassi G, Sidhu SK, Mishra S. The intracellular cholesterol pool in steroidogenic cells plays a role in basal steroidogenesis. J Steroid Biochem Mol Biology. 2022;220(1-2):106099. [DOI] [PubMed] [Google Scholar]
- 67. Ragoobir J, Abayasekara D, Bruckdorfer K, Michael A. Stimulation of progesterone production in human granulosa-lutein cells by lipoproteins: evidence for cholesterol-independent actions of high-density lipoproteins. J Endocrinol. 2002;173(1):103‐111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request. Supplemental data are available at https://doi.org/10.5683/SP3/59VOBT