Abstract
Background & objectives:
FOLFIRINOX and gemcitabine plus nab-paclitaxel (GN) are the most commonly used regimens in advanced pancreatic ductal adenocarcinomas (PDACs). As there is limited data on comparison of these two regimens, the present study was aimed to compare survivals and tolerance for both regimens through a match-pair analysis.
Methods:
The data of 350 patients with metastatic and locally advanced PDAC, treated between January 2013 and December 2019, were retrieved. A 1:1 matching, using age and performance status, without replacement was performed by using nearest neighbour matching method.
Results:
A total of 260 patients (130 modified FOLFIRINOX and 130 GN) were matched. The median overall survival (OS) was 12.98 months [95% confidence interval (CI) 7.257-8.776 months] in modifications of FOLFIRINOX (mFOLFIRINOX) cohort and 12.06 months (95% CI 6.690-8.88 months) in GN group (P=0.080). The incidence of grade 3 and 4 infections, diarrhoea, oral mucositis, and fatigue was higher with mFOLFIRINOX. Patients who received second line therapy had improved OS as compared to those who did not (14.06 vs. 9.07 months, P<0.001).
Interpretation & conclusions:
GN and mFOLFIRINOX appear to have similar survival outcomes in an unselected match paired patient population with advanced PDAC. A markedly increased incidence of non-myelosuppressive grade 3 and grade 4 side-effects and lack of survival improvements suggest a need for nuanced use of the mFOLFIRINOX regimen. Administration of second-line chemotherapy improves OS in patients with advanced PDAC.
Keywords: Advanced pancreatic cancer, gemcitabine-nab-paclitaxel, match-pair, modified FOLFIRINOX, survival, toxicity
Pancreatic ductal adenocarcinomas (PDACs) predominantly present in the metastatic or locally advanced pancreatic adenocarcinoma (LAPC) stages of disease, where the primary management strategy revolves around systemic chemotherapy1. Although a small proportion of patients with LAPC undergo resection of the primary tumour in case of downstaging with chemotherapy, the mainstay of management remains initiation of effective systemic chemotherapy2,3.
The two most effective and commonly used regimens for advanced PDAC are FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin) and gemcitabine plus nanoparticle albumin-bound paclitaxel (GN) in the current era4,5. Modifications of FOLFIRINOX (mFOLFIRINOX) are commonly used to ameliorate the increased rates of grade 3 and grade 4 adverse events associated with the regimen6,7. Although randomized evidence comparing the two treatment options is not available, FOLFIRINOX is considered the more efficacious regimen in terms of response rates, local downstaging, progression-free survival (PFS) and overall survival (OS). However, patient accrued in clinical trials may differ markedly from patients in clinical practice with advanced PDAC. Older age, presence of comorbidities, suboptimal nutrition status and borderline ECOG (Eastern Cooperative Oncology Group) performance status (PS) are usually present concurrently with a diagnosis of advanced PDAC and entail consideration for the use of the better tolerated GN regimen8. The superior tolerance profile of GN makes it a safer choice in advanced PDAC, though there is a potential trade-off in terms of reduced efficacy when using cross-trial comparisons.
The present study evaluated and compared the performance of mFOLFIRINOX and GN in terms of response rates, tolerance and survival in an unselected consecutive patients with advanced PDAC. The study was aimed to provide comparative evidence regarding the preferred regimen through a match pair analysis, pending availability of a randomized comparative trial in advanced PDAC.
Material & Methods
Patient selection: Patients diagnosed with adenocarcinoma of the pancreas between June 1, 2013 and December 31, 2019 in the department of Gastrointestinal Medical Oncology, Tata Memorial Hospital were evaluated as part of this retrospective study. The study was conducted according to the principles of the Helsinki Declaration and good clinical practice guidelines. The study was approved by the Institution Review Board at Advanced Centre for Treatment, Research & Education in Cancer (ACTREC), Tata Memorial Centre, Kharghar, Navi Mumbai (IEC 900655), with a waiver of requirement for patient consent considering the minimal risk to the patients and lack of adverse effects on the rights and welfare of the patients.
Inclusion criteria: Patients with Metastatic or LAPC, ECOG PS 0-2, and who received at least one cycle of either mFOLFIRINOX or GN were included in the study. Patients underwent standard pre-chemotherapy workup including assessment of ECOG PS, end-organ function and CA 19-9 levels (hospital reference range: 0-37 U/ml).
Details of therapy: The mFOLFIRINOX regimen was administered every 14 days with Oxaliplatin 65 mg/m2 intravenous (IV) over 2 h, Irinotecan 135mg/m2 IV over 90 mins, leucovorin 300 mg/m2 IV over 2 h and 5 FU – 1800 mg/m2 IV over 46 h continuous infusion. Granulocyte colony-stimulating factor (G-CSF) or pegylated G-CSF was a mandatory part of the mFOLFIRINOX protocol.
The GN regimen was administered every 28 days with nab-paclitaxel 125 mg/m2 IV over 1 h and gemcitabine 1000 mg/m2 IV over 1 h weekly for three weeks followed by a break of one week before the next cycle.
Dose modifications during treatment were as per treating physician decision. Treatment with chemotherapy was continued assuming absence of significant treatment-related toxicity, disease progression as per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.19 or patient choice.
Response criteria and toxicity assessment: All patients included in the study underwent a baseline contrast enhanced computed tomography (CT) scan of the thorax, abdomen and pelvis or [fluorine-18]fluoro-D-glucose positron emission tomography-CT (18F-FDG-PET-CT) scan before treatment and CT scans were repeated every 2-3 months for response assessment. RECIST criteria version 1.1 were used for classifying responses9. Response rates (RR) were calculated by combining complete response (CR) and partial response (PR) rates, while clinical benefit rate was reported by summing percentages of CR, PR and stable disease. Grade 3 and 4 toxicities were recovered from medical records and reported as per National Cancer Institute (NCI)- common terminology criteria for adverse events (CTCAE) version 4.0 (NIH CTEP; https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40). Maximal grade of toxicities is reported in the current study.
Statistical analysis: Baseline demographic and clinical variables were compared between patients receiving either of the 2 regimens. The primary endpoint of this analysis was comparison of median overall survival (OS). OS was calculated from the date of diagnosis of PDAC to date of death or loss to follow up using the Kaplan–Meier method. The primary analysis was on an intent-to-treat basis. OS was compared using the log-rank test. A stratified cox proportional hazards regression model was used to evaluate the association of pre-treatment clinical variables with OS. Clinical factors that had a P<0.1 on univariate analysis were included in the multivariate analysis.
A matched pair analysis was planned using factors identified on multivariate analysis predicting for OS. A 1:1 matching without replacement was performed using nearest neighbour matching method. The absolute standard difference (ASD) for the factors used to compute the propensity score was evaluated before and after the match. An ASD lower than 0.1 would suggest a substantial matching between the 2 regimens. Based on the matched data sets, median OS was compared using the Kaplan–Meier method with. Factors that had P<0.10 on univariable analysis were included in the multivariate analysis.
Secondary endpoints included comparison of median PFS, response rates and grade 3 and 4 toxicities between mFOLFIRINOX and GN groups. PFS was calculated from the date of diagnosis to PDAC to date of clinical and/or radiological progression or death (in case disease had not progressed) or loss to follow up. OS and PFS were calculated using Kaplan-Meier survival analysis and compared with the log rank test. Statistical analyses were performed using SPSS 21.0 software for windows (SPSS Inc., Chicago, IL, USA) and statistical software for data science (STATA version 14, https://www.stata.com/). Subgroups explored for effect of treatment are detailed in Table I.
Table I.
Baseline clinical profile of all included patients receiving first-line chemotherapy
| Characteristics | All patients (%) | mFOLFIRINOX (%) | GN (%) | P |
|---|---|---|---|---|
| Number of patients | 350 | 152 | 198 | |
| Median age (yr) (range) | 55 (22-80) | 53 (22-75) | 58 (23-80) | |
| >60 | 113 | 26 (17) | 87 (44) | <0.001 |
| ≤60 | 237 | 126 (83) | 111 (56) | |
| Sex | ||||
| Male | 227 (65) | 108 (71) | 119 (60) | 0.033 |
| Female | 123 (35) | 44 (29) | 79 (40) | |
| Comorbidities | ||||
| Diabetes mellitus | 145 (41) | 75 (49) | 70 (35) | 0.008 |
| Hypertension | 148 (42) | 40 (26) | 108 (55) | <0.001 |
| Presence of baseline obstructive jaundice | 83 (24) | 44 (29) | 39 (20) | <0.044 |
| ECOG PS | ||||
| 0/1 | 320 (91) | 145 (95) | 175 (88) | 0.035 |
| 2 | 30 (9) | 7 (5) | 23 (12) | |
| Tumour site | ||||
| Head | 154 (44) | 75 (49) | 79 (40) | 0.078 |
| Others | 196 (56) | 77 (51) | 119 (60) | |
| Raised CA 19.9 levels | 259 (74) | 105 (69) | 154 (78) | 0.066 |
| Radiological disease status | ||||
| Locally advanced | 114 (33) | 71 (46) | 43 (22) | <0.001 |
| Metastatic | 236 (67) | 81 (54) | 155 (78) | |
| Sites of metastases | ||||
| Hepatic | 169 (48) | 64 (42) | 105 (53) | <0.001 |
| Pulmonary | 32 (9) | 10 (7) | 22 (11) | <0.001 |
| Peritoneal | 67 (19) | 20 (13) | 47 (24) | <0.001 |
| Response rates | ||||
| Complete response | 4 (1) | 3 (2) | 1 (<1) | - |
| Partial response | 132 (38) | 46 (30) | 86 (43) | - |
| Response rates | 136 (39) | 49 (32) | 87 (44) | 0.026 |
| Stable disease | 120 (34) | 69 (45) | 51 (26) | - |
| Clinical benefit rate | 256 (73) | 118 (78) | 138 (70) | 0.097 |
| Progressive disease | 68 (19) | 23 (15) | 45 (23) | - |
| Not assessable | 26 (7) | 11 (7) | 15 (7) | - |
| Treatment related grade 3/4 adverse events | ||||
| Neutropenia | 56 (16) | 19 (13) | 37 (19) | 0.118 |
| Thrombocytopenia | 20 (6) | 11 (7) | 9 (5) | 0.282 |
| Anaemia | 35 (10) | 8 (5) | 27 (14) | 0.01 |
| Febrile neutropenia | 9 (3) | 3 (2) | 6 (3) | 0.536 |
| Infections | 45 (13) | 32 (21) | 13 (7) | <0.001 |
| Nausea and vomiting | 14 (4) | 14 (9) | 0 | <0.001 |
| Diarrhoea | 31 (9) | 25 (16) | 6 (3) | <0.001 |
| Oral mucositis/stomatitis | 13 (4) | 13 (9) | 0 | <0.001 |
| Characteristics | All patients (%) | mFOLFIRINOX (%) | GN (%) | P |
| Fatigue (grade 2 and 3) | 76 (22) | 49 (32) | 27 (14) | <0.001 |
| Hand-foot-syndrome (grade 3) | 7 (2) | 7 (5) | 0 | 0.002 |
| Peripheral neuropathy (grade 2 and 3) | 68 (19) | 20 (13) | 48 (24) | 0.009 |
| Requirement for dose modifications | ||||
| Yes | 116 (33) | 40 (26) | 76 (38) | 0.017 |
| No | 234 (67) | 112 (74) | 122 (62) | |
| Second- line therapy received | 167 (48) | 78 (51) | 89 (45) | 0.28 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan and oxaliplatin; GN, gemcitabine-nab-paclitaxel; CA 19-9, carbohydrate antigen 19-9
Results
A total of 350 patients were included in the analysis, of whom 198 patients (57%) received GN and 152 patients (43%) received mFOLFIRINOX regimens (Fig. 1).
Fig. 1.
Consort diagram.
Baseline clinical characteristics: Baseline clinical profile of all 350 patients is detailed in Table I. Patients in the mFOLFIRINOX group were younger at diagnosis (53 vs. 58 yr, P<0.001), predominantly male (71 vs. 60%, P=0.033), had better ECOG PS (ECOG PS 0/1 – 95 vs. 88%, P=0.035) and had a greater proportion of patients with unresectable non-metastatic disease as opposed to metastatic disease (46 vs. 22%, P<0.001).
Response rates and treatment related adverse events: Patients receiving GN had greater response rates than patients receiving mFOLFIRINOX (44 vs. 32%, P=0.001), though clinical benefit rates were not statistically different (70 vs. 78%, P=0.097) (Table II).
Table II.
Response rates, treatment related adverse events and second line therapy of all patients
| Characteristics | All patients (%) | mFOLFIRINOX (%) | GN (%) | P |
|---|---|---|---|---|
| Response rates | ||||
| CR | 4 (1) | 3 (2) | 1 (<1) | |
| PR | 132 (38) | 46 (30) | 86 (43) | |
| RR | 136 (39) | 49 (32) | 87 (44) | 0.026 |
| SD | 120 (34) | 69 (45) | 51 (26) | |
| CBR | 256 (73) | 118 (78) | 138 (70) | 0.097 |
| PD | 68 (19) | 23 (15) | 45 (23) | |
| Not assessable | 26 (7) | |||
| Treatment related grade 3/4 adverse events | ||||
| Neutropenia | 56 (16) | 19 (13) | 37 (19) | 0.118 |
| Thrombocytopenia | 20 (6) | 11 (7) | 9 (5) | 0.282 |
| Anaemia | 35 (10) | 08 (5) | 27 (14) | 0.01 |
| Febrile neutropenia | 9 (3) | 3 (2) | 6 (3) | 0.536 |
| Infections | 45 (13) | 32 (21) | 13 (7) | <0.001 |
| Nausea and vomiting | 14 (4) | 14 (9) | 0 (0) | <0.001 |
| Diarrhoea | 31 (9) | 25 (16) | 6 (3) | <0.001 |
| Oral mucositis/stomatitis | 13 (4) | 13 (9) | 0 (0) | <0.001 |
| Fatigue (grade 2/3) | 76 (22) | 49 (32) | 27 (14) | <0.001 |
| Hand-foot-syndrome (grade 3) | 7 (2) | 7 (5) | 0 (0) | 0.002 |
| Peripheral neuropathy (grade 2 and 3) | 68 (19) | 20 (13) | 48 (24) | 0.009 |
| Requirement for dose modifications | ||||
| Yes | 116 (33) | 40 (26) | 76 (38) | 0.017 |
| No | 234 (67) | 112 (74) | 122 (62) | |
| Second line therapy received | 167 (48) | 78 (51) | 89 (45) | 0.28 |
Patients receiving mFOLFIRINOX had a statistically significant increase in grade 3 and 4 vomiting (9 vs. 0%, P<0.001), diarrhoea (16 vs. 3%, P<0.001), oral mucositis (9 vs. 0%, P<0.001), hand-foot-syndrome (5 vs. 0%, P=0.002) and infections (21 vs. 7%, P<0.001) while patients in the GN cohort had a greater incidence of grade 3 and 4 anaemia (14 vs. 5%, P=0.01) and neuropathy (24 vs. 13%, P=0.009). A greater proportion of patients receiving GN required dose modification (38 vs. 26%, P=0.017).
Resection rates in locally advanced pancreatic adenocarcinoma (LAPC) patient population: Of the 114 patients with LAPC, 71 patients were treated with mFOLFIRINOX, while 43 patients received GN. Six of the 71 patients (9%) receiving mFOLFIRINOX and one out of the 43 patient (2%) treated with GN, underwent pancreatectomy.
Survival data and prognostic factors of 350 patients: The median OS for the entire patient population was 12.12 months [95% confidence interval (CI): 11.18-13.26]. Patients who received mFOLFIRINOX had a superior OS when compared to patients receiving GN (median OS: 12.95 vs. 11.73 months, P=0.010) (Supplementary Fig. 1 (70.8KB, tif) ), while elderly age >60 yr (10.22 vs. 12.95 months, P=0.001) and ECOG PS 2 at presentation (9.17 vs. 12.42 months, P=0.06) predicted for inferior OS on univariate analysis. Elderly age (HR 1.46, 95% CI: 1.12-1.91, P=0.005) and ECOG PS 2 (HR 1.64, 95% CI: 1.05-2.55, P=0.027) maintained prognostic value while receipt of mFOLFIRINOX (HR 1.25, 95% CI: 0.95-1.65, P=0.118) did not retain statistical significance on multivariate analysis (Table III).
Table III.
Univariate and multivariate cox proportional hazards regression analysis of overall survival for all patients
| Characteristic | Univariable | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| Median overall survival (months) | P | HR (95% CI) | P | |
| Age (yr) | ||||
| >60 | 10.22 | 0.001 | 1.46 (1.12-1.91) | 0.005 |
| ≤60 | 12.95 | |||
| ECOG PS ≥2 | ||||
| Yes | 9.17 | 0.006 | 1.61 (1.05-2.55) | 0.027 |
| No | 12.42 | |||
| Head of pancreas primary | ||||
| Yes | 11.23 | 0.264 | - | - |
| No | 12.61 | |||
| Metastatic stage | ||||
| Yes | 11.66 | 0.301 | - | - |
| No | 12.98 | |||
| Raised CA 19.9 levels | ||||
| Yes | 12.12 | 0.116 | - | - |
| No | 12.26 | |||
| mFOLFIRINOX | ||||
| Yes | 12.95 | 0.010 | 1.25 (0.95-1.65) | 0.118 |
| No | 11.73 | |||
ECOG PS, Eastern Cooperative Oncology Group Performance Status; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan and oxaliplatin; CI, confidence interval; HR, hazard ratio; CA 19-9, carbohydrate antigen 19-9
The median PFS for the entire cohort was 7.13 months (95% CI: 6.33 - 7.92). The median PFS with mFOLFIRINOX was superior compared to the median PFS with GN (8.35 vs. 6.87 months, P=0.001)
Match pair analysis and outcomes in matched cohort of patients: Since age > 60 yr and ECOG PS 2 were factors prognostic for OS, these were used for the purpose of matching. The ASDs for age and ECOG PS before and after propensity matching are detailed in Supplementary Figure 2 (45.2KB, tif) . A propensity-matched cohort of 260 patients was generated with 130 patients each being treated with mFOLFIRINOX and GN, respectively.
The clinical characteristics and treatment related details of the 260 patients in the matched cohort are presented in Supplementary Table II. Similar trends were seen in terms of baseline clinical variables, response rates and treatment-related adverse events in the matched cohort of 260 patients as compared to the entire cohort of 350 patients.
Supplementary Table II.
Univariate and multivariate cox proportional hazards regression analysis of 260 matched patients
| Characteristic | Univariable | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| Median overall survival (months) | P | HR (95% CI) | P | |
| Head of pancreas primary | ||||
| Yes | 11.24 | 0.185 | - | - |
| No | 12.95 | |||
| Metastatic stage | ||||
| Yes | 12.12 | 0.356 | - | - |
| No | 12.98 | |||
| Raised CA 19.9 levels | ||||
| Yes | 12.45 | 0.169 | - | - |
| No | 12.26 | |||
| mFOLFIRINOX | ||||
| Yes | 12.98 | 0.086 | - | - |
| No | 12.06 | |||
mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan and oxaliplatin; CI, confidence interval; HR, hazard ratio; CA 19-9, carbohydrate antigen 19-9
Supplementary Table I.
Clinical profile and treatment related characteristics of 260 matched patients
| Characteristics | mFOLFIRINOX (%) | GN (%) | P |
|---|---|---|---|
| Sex | |||
| Male | 89 (69) | 73 (56) | 0.041 |
| Female | 41 (31) | 57 (44) | |
| Comorbidities | |||
| Diabetes mellitus | 64 (49) | 45 (35) | 0.017 |
| Hypertension | 37 (29) | 71 (55) | <0.001 |
| Tumour site | |||
| Head | 65 (50) | 54 (42) | 0.171 |
| Others | 65 (50) | 76 (58) | |
| Obstructive jaundice at baseline | 36 (28) | 26 (20) | 0.146 |
| Radiological disease status | |||
| Locally advanced | 57 (44) | 26 (20) | <0.001 |
| Metastatic | 73 (56) | 104 (80) | |
| Raised CA 19.9 | 89 (69) | 100 (77) | 0.126 |
| Hepatic metastases | 58 (45) | 71 (55) | <0.001 |
| Response rates | |||
| Complete response | 3 (2) | 0 | - |
| Partial response | 41 (32) | 60 (46) | - |
| Response rates | 44 (34) | 60 (46) | - |
| Stable disease | 55 (42) | 29 (22) | 0.043 |
| Clinical benefit rate | 99 (76) | 89 (69) | - |
| Progressive disease | 21 (16) | 30 (23) | 0.166 |
| Not assessable | 10 (8) | 11 (9) | - |
| Grade 3 and Grade 4 toxicities | |||
| Neutropenia | 18 (14) | 27 (21) | 0.140 |
| Thrombocytopenia | 8 (6) | 6 (5) | 0.583 |
| Anaemia | 8 (6) | 16 (12) | 0.087 |
| Febrile neutropenia | 3 (2) | 4 (3) | 0.702 |
| Infections | 27 (21) | 6 (5) | <0.001 |
| Nausea and vomiting | 11 (9) | 0 | 0.001 |
| Diarrhoea | 22 (17) | 4 (3) | <0.001 |
| Oral mucositis/stomatitis | 11 (9) | 0 | 0.001 |
| Hand-foot-syndrome | 7 (5) | 0 | 0.007 |
| Fatigue (Grade 2 and Grade 3) | 39 (30) | 14 (11) | <0.001 |
| Peripheral neuropathy (Grade 2 and Grade 3) | 14 (11) | 28 (22) | 0.018 |
| Dose modifications | 34 (26) | 47 (36) | 0.082 |
| Second-line therapy received | 63 (48) | 58 (45) | 0.31 |
mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan and oxaliplatin; GN, gemcitabine-nab-paclitaxel; CA 19-9, carbohydrate antigen 19-9
There was no significant difference in median OS between the mFOLFIRINOX and GN regimens (median OS: 12.98 vs. 12.06 months, P=0.086) in the matched cohort of 260 patients (Fig. 2 and Supplementary Table II).
Fig. 2.
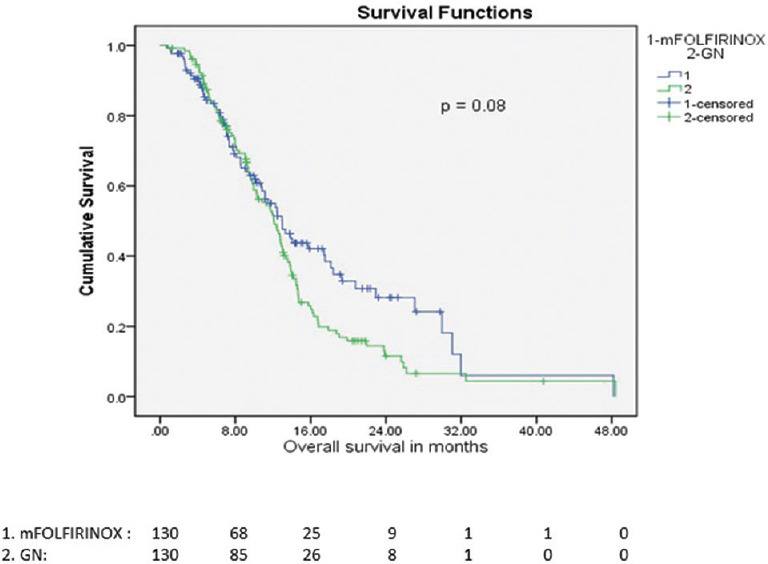
Overall survival of mFOLFIRINOX vs. Gemcitabine and nab-Paclitaxel in match pair cohort.
Second-line treatment and impact on survival: One hundred and sixty seven patients (48%) received second-line chemotherapy (CT2). Seventy eight patients (51.3%) receiving first-line mFOLFIRINOX and 89 patients (45.4%) receiving first-line GN were administered CT2, with no significant differences in proportion of patients receiving CT2 (P=0.28). CT2 used commonly post mFOLFIRINOX included GN, and gemcitabine based, while capecitabine-irinotecan (CAPIRI)/FOLFIRI based regimen were used in patients post progression on GN.
Patients who received CT2 had a superior OS as compared to patients who did not receive CT2 (14.46 vs. 9.17 months, P<0.001; Supplementary Fig. 3 (85.1KB, tif) ). A similar difference in outcomes was noted in the matched cohort for patients receiving CT2 (14.06 vs. 9.07 months, P<0.001).
Discussion
Survivals and quality of life (QOL) have improved slowly in PDAC across stages, with the initial advances based on identifying nuances in surgical technique and the later improvements due to more effective systemic therapies10,11. As a majority of PDAC present with advanced/metastatic stage, most of the data with regard to improving survival has been seen initially in this group of patients with further sequential evaluation in patients with resectable or borderline resectable cancers. FOLFIRINOX and gemcitabine plus nanoparticle albumin-bound paclitaxel (GN) are two such regimens which are successfully used in advanced PDAC with emerging data in more resectable PDACs3,12. There is no randomized phase II/III data comparing the two regimens. This has led to the choice of regimens based on fitness, ECOG PS and the need for ‘conversion’ to resectability13. Such a bias is reflected in the present study as well, where younger patients with better ECOG PS and faint possibility of resection (non-metastatic PDAC) preferentially received mFOLFIRINOX. Contrary to expectations, increased response rates were seen with GN, though clinical benefit rates were similar between the two cohorts. Such a discordance in RRs is known and is a reflection of a greater proportion of patients with non-metastatic unresectable PDAC receiving mFOLFIRINOX; the dense fibrotic stroma surrounding pancreatic primaries entails low response rates compared to response rates at metastatic sites14,15.
Significantly, ECOG PS 2 and elderly age predicted for inferior survival outcomes in the entire patient cohort and with a greater proportion of such patients receiving GN, it would be expected that there would be a significantly lower survival in patients administered GN. However, our analysis reveals that there no statistically significant difference in OS on multivariable analysis in the unmatched patient population. Since the key clinically relevant factors of age and ECOG PS predicting for inferior outcomes were significantly different in the mFOLFIRINOX and GN cohorts, we used these two factors for the match-pair analysis. On comparing outcomes in the matched patients, a similar trend was seen, with no difference in survivals between patients receiving mFOLFIRINOX and GN. There are two important take aways from these results. First, in patients with advanced PDAC, GN may perform as well as mFOLFIRINOX in a real-world patient cohort as opposed to available cross compared trial results where FOLFIRINOX shows superior outcomes. This is of paramount importance, especially when the tolerance and adverse events are considered. GN was markedly better tolerated with a significantly lesser incidence of grade 3 and grade 4 non-myelosuppressive side-effects such as vomiting, diarrhoea, mucositis, hand foot syndrome (HFS), fatigue and infections, despite use of a modified dose-reduced schedule of FOLFIRINOX. While the current study does not have data on QOL assessments, an adverse impact of such an increased incidence of adverse effects on patient QOL may be seen. Coupled with a potentially increased need for hospitalizations and resource utilization due to such side-effects, the purportedly increased benefits of mFOLFIRINOX need to be examined closely. Conversely, the cumulative dose-dependent neuropathy with GN needs close monitoring for patients on therapy. There is increasing data on the use of a biweekly regimen that has a lower incidence of neuropathy and such an approach may assist in amelioration of this particular side-effect16,17.
Second, based on the relative lack of superior efficacy of mFOLFIRINOX over GN in terms of survival in this dataset, even patients with LAPC should be carefully selected for mFOLFIRINOX. A recently published match-pair analysis evaluating ‘localized’ PDAC showed an increased partial response rate and pancreatectomy rate with FOLFIRINOX, but no improvement in survival end-points18. Available evidence points to PDAC being a systemic disease from the outset and using endpoints like resectability rates may not translate into an appropriate management strategy for patients with LAPC. Another pointer in this direction is the lack of survival difference in patients with LAPC versus advanced metastatic PDAC. Such a lack of difference may be explained by the small number of patients with LAPC or an artefact of selecting patients with predominantly arterial involvement by PDAC. However, balancing survival goals and tolerance with a greater stress on reducing toxicities and improving patient-related outcomes is probably a more pragmatic way forward. Greater use of novel strategies like the addition of losartan and selective use of stereotactic body radiotherapy (SBRT) are additional steps in this direction19,20.
Commonly recommended second-line (CT2) regimens in advanced PDAC have been shown to modestly improve survivals in advanced PDAC21,22. The current study shows that patients who received second-line therapy (CT2) survived longer than those who did not. The proportion of patients receiving CT2 in this study was slightly higher than usual, though in equal proportions between the mFOLFIRINOX and GN cohorts. Preserving ECOG PS and organ function, limiting side-effects as well as increasing patient acceptance for CT2 are important considerations when planning initial systemic therapy for advanced PDAC. Careful management of these factors may allow increased use of efficacious regimens as CT2, further underlying the importance of appropriate treatment sequencing in advanced PDAC.
The present study had a number of limitations, besides being single institution based and retrospective in nature. A small proportion of patients with ECOG PS 2 received FOLFIRINOX and this would be the exception rather than the norm in clinical practice. A modified schedule of FOLFIRINOX (dose reduced by 25% as compared to the original schedule) was used and this may have contributed to inferior outcomes, especially in the LAPC cohort. However, survival data seen in patients with metastatic PDAC receiving mFOLFIRINOX in this study appear comparable to outcomes seen with full dose FOLFIRINOX in the seminal Phase III trial, implying that this dose modification may not be significant. The rates of locoregional treatment, especially resection, in the LAPC cohort were low as compared to previously published data3,23. However, we have previously published data from our institution wherein a majority of patients with LAPC had arterial encasement/involvement, thereby almost precluding surgery2. The early and late mortality rates associated with arterial resections as well as lack of potential benefits are known, despite recent ongoing improvements in surgical techniques24,25. It is also in line with our understanding that LAPCs should be treated on the lines of metastatic PDAC with a very careful selection of patients for locoregional therapy.
In conclusion, GN and mFOLFIRINOX appear to have similar survival outcomes in this unselected patient population with advanced PDAC on multivariable analysis. A similar trend in survival was noted on matching the clinically significant variables of elderly age and ECOG PS. The lack of improved survival outcomes and markedly increased incidence of non-myelosuppressive grade 3 and 4 side-effects with mFOLFIRINOX suggests a need for careful selection of patients when administering this regimen. Patients receiving second-line chemotherapy survive longer than patients who do not and this should be considered while sequencing treatment options in patients with advanced PDAC.
Overall survival of mFOLFIRINOX vs. Gemcitabine and nab-Paclitaxel in the entire patient cohort.
Absolute standard difference for age and ECOG PS before and after propensity matching.
Impact of second-line chemotherapy on overall survival.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaswamy A, Jandyal S, Ostwal V, Engineer R, Lewis S, Bose S, et al. Nontrial, real-world outcomes in unresectable locally advanced pancreatic cancer: Chemotherapy and chemoradiation is the standard while surgery is uncommon. Indian J Cancer. 2017;54:530–4. doi: 10.4103/ijc.IJC_377_17. [DOI] [PubMed] [Google Scholar]
- 3.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–10. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong H, Fan Z, Liu B, Lu T. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: A systematic review and meta-analysis. Sci Rep. 2018;8:8666. doi: 10.1038/s41598-018-26811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZQ, Zhang F, Deng T, Zhang L, Feng F, Wang FH, et al. The efficacy and safety of modified FOLFIRINOX as first-line chemotherapy for Chinese patients with metastatic pancreatic cancer. Cancer Commun (Lond) 2019;39:26. doi: 10.1186/s40880-019-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williet N, Saint A, Pointet AL, Tougeron D, Pernot S, Pozet A, et al. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: A comparative propensity score study. Therap Adv Gastroenterol. 2019;12:1756284819878660. doi: 10.1177/1756284819878660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Acher AW, Bleicher J, Cannon A, Scaife C. Advances in surgery for pancreatic cancer. J Gastrointest Oncol. 2018;9:1037–43. doi: 10.21037/jgo.2018.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 12.Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285–94. doi: 10.1016/S2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 13.Chiorean EG, Cheung WY, Giordano G, Kim G, Al-Batran SE. Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: A systematic review. Ther Adv Med Oncol. 2019;11:1758835919850367. doi: 10.1177/1758835919850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–43. doi: 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, et al. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut. 2019;68:159–71. doi: 10.1136/gutjnl-2018-316451. [DOI] [PubMed] [Google Scholar]
- 16.Ahn DH, Krishna K, Blazer M, Reardon J, Wei L, Wu C, et al. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: A retrospective analysis. Ther Adv Med Oncol. 2017;9:75–82. doi: 10.1177/1758834016676011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostwal V, Sahu A, Zanwar S, Nayak L, Shrikhande SV, Shetty N, et al. Experience with non-cremophor-based paclitaxel-gemcitabine regimen in advanced pancreatic cancer: Results from a single tertiary cancer centre. Indian J Med Res. 2018;148:284–90. doi: 10.4103/ijmr.IJMR_249_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, et al. Response and survival associated with first-line FOLFIRINOX vs. gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 2020;155:832–9. doi: 10.1001/jamasurg.2020.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol. 2019;5:1020–7. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toesca DAS, Koong AJ, Poultsides GA, Visser BC, Haraldsdottir S, Koong AC, et al. Management of borderline resectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2018;100:1155–74. doi: 10.1016/j.ijrobp.2017.12.287. [DOI] [PubMed] [Google Scholar]
- 21.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 22.Citterio C, Baccini M, Orlandi E, Di Nunzio C, Cavanna L. Second-line chemotherapy for the treatment of metastatic pancreatic cancer after first-line gemcitabine-based chemotherapy: A network meta-analysis. Oncotarget. 2018;9:29801–9. doi: 10.18632/oncotarget.25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggino L, Malleo G, Marchegiani G, Viviani E, Nessi C, Ciprani D, et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 2019;154:932–42. doi: 10.1001/jamasurg.2019.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, et al. Arterial resection during pancreatectomy for pancreatic cancer: A systematic review and meta-analysis. Ann Surg. 2011;254:882–93. doi: 10.1097/SLA.0b013e31823ac299. [DOI] [PubMed] [Google Scholar]
- 25.Rebelo A, Michalski CW, Ukkat J, Kleeff J. Pancreatic cancer surgery with vascular resection: Current concepts and perspectives. J Pancreatol. 2019;2:1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall survival of mFOLFIRINOX vs. Gemcitabine and nab-Paclitaxel in the entire patient cohort.
Absolute standard difference for age and ECOG PS before and after propensity matching.
Impact of second-line chemotherapy on overall survival.


