Abstract
“Differences of Sexual Development (DSD),” individuals with rearranged Y chromosome breaks in their 46,XY cells are reported with male and female gender phenotypes and differences in germ cell tumour (GCT) risk. This raised the question of whether male or female gender and GCT risk depends on the site of the break and/or rearrangement of the individual’s Y chromosome. In this paper, we report molecular mapping of the breakpoint on the aberrant Y chromosome of 22 DSD individuals with a 45,X/46,XY karyotype reared with a different gender. Their Y chromosome breaks are found at different sites on the long and short Y arms. Our data indicate that gender rearing is, neither dependent on the site of Y breakage, nor on the amount of 45,X0 cells in the individuals’ leukocytes. Most prominent are secondary rearrangements of the Y chromosome breaks forming di-centric Y-structures (“dic-Y”). Duplications of the short Y arm and the proximal part of the long Y arm are the results. A putative GCT risk has been analysed with immunohistochemical experiments on some dysgenetic gonadal tissue sections. With specific antibodies for OCT3/4 expression, we marked the pluripotent germ cell fraction being potential tumour precursor cells. With specific antibodies for DDX3Y, TSPY, and UTY we analyzed their putative Gonadoblastoma Y (GBY) tumour susceptibility function in the same specimen. We conclude GBY expression is only diagnostic for GCT development in the aberrant germ cells of these DSD individuals when strong OCT3/4 expression has marked their pluripotency.
Keywords: 45X/46XY mosaic DSD, Germ cell tumour risk, OCT3/4 expression GBY expression, di-centric Y chromosome
Introduction
The development of male and female gonad cells is controlled by the constitution of the sex chromosomes (X and Y) in the surrounding somatic cells. Testicles are formed in the case of a 46,XY karyotype, and ovaries in case of a 46,XX karyotype in these cells. Both sexual phenotypes represent a stable developmental condition (Capel 2017).
In mice, large-scale transcriptome analyses performed for the gonads’ cells during early development reveal that only a few genes with sexually dimorphic expression rates are active at the beginning of the gonadal sex determination pathway (Nef et al. 2005; Stévant et al. 2018). Surprisingly, many of these genes are located on the X and on the Y chromosome.
The putatively genetic nosology of dysgenetic early gonad formation was first reported more than 60 years ago with the detection of sex chromosome anomalies in men with Klinefelter syndrome (47,XXY; Jacobs and Strong 1959) and in women with Turner syndrome (45,X0; Ford et al. 1959). Sex chromosome mosaics (45,X/46,XX/46,XY) are diagnosed first in the leukocytes of children born with intersexual phenotypes: hypospadias in males and clitoris hypertrophy in females (Sohval 1964; Pfeiffer et al. 1968).
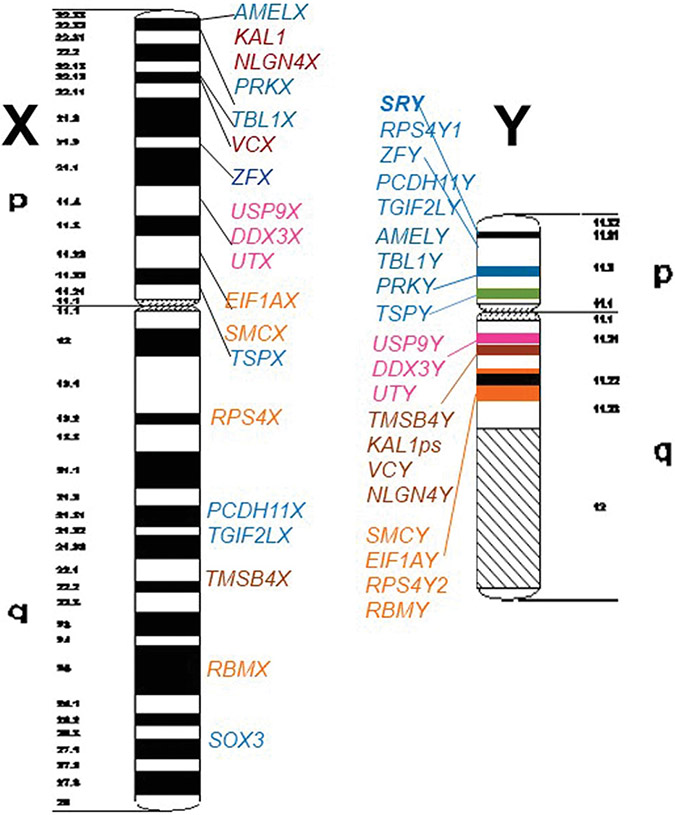
After complete sequence analyses of the human X (Ross et al. 2005) and Y chromosome (Skaletsky et al. 2003), 18 functional homologous X-Y gene pairs have been found along the short and long arms of both sex chromosomes (Figure 1). Three of these Y genes, DDX3Y, TSPY, and UTY, are located in a genomic Y region in the proximal part of the short and long Y arm designated as “Gonadoblastoma Y-linked (GBY)” germ cell tumor susceptibility region (Page 1987). It suggests that they are involved in the development of germ cell tumours (GCTs) occurring in the aberrant germ cells of the dysgenetic gonads of individuals with “Differences of Sexual Development” (DSD) and with a Y chromosome in their karyotype (Honecker et al. 2004; Cools et al. 2006; 2011; Vogt et al. 2019, 2021). The pre-malignant cellular phenotypes are coined “Gonadoblastoma” (GB) cells, when they are surrounded by granulosa cells (Verp and Simpson 1987), respectively, “Germ Cell Neoplasia In Situ” (GCNIS) cells when they are surrounded by Sertoli Cells (Hersmus et al. 2008). Both cell types are pluripotent because they are also marked by OCT3/4 expression (Cheng et al. 2007).
Figure 1. Schematic view on GIEMSA staining pattern of human X and Y chromosomes to present location of the 18 X-Y functional homologous genes identified by genomic sequence analysis of the human sex chromosomes.
Colour code of the Y genes on the short Y arm (blue colour), in AZFa in proximal Yq11 (pink colour), between the AZFa and AZFb interval (brownish colour) and in AZFb (orange colour) should help to distinguish their distinct locations on the X chromosome. The sex-determining gene SRY is marked with bold letters. The coloured stripes on the Y chromosome scheme should indicate X-Y homology blocks from putatively distinct evolutionary strata.
In human male germ cells, the same Y genes, DDX3Y, TSPY, UTY, are involved in controlling the proliferation rate of early fetal and adult spermatogonia (Honecker et al. 2004; Lau et al. 2011; Gueler et al. 2012; Vogt et al. 2021). These germ cells have left their state of pluripotency; accordingly, OCT3/4 expression is absent.
“Sex chromosome DSD” individuals with mosaic 45,X/46,XY cells in their leukocyte karyotypes are reared with distinct gender phenotypes. However, only in individuals with pronounced female sexual ambiguity, a high tumor risk is reported for this “DSD” subgroup (Gravholt et al. 2000; Cools et al. 2006; Lindhardt Johansen et al. 2012). The clinical decision process for the sexual rearing of these individuals after birth is, therefore, a major issue of genetic counseling in children’s hospitals.
Most variable is the risk of gonadal tumor development (5–48%) when the Y chromosome in the “Sex chromosome DSD,” 46,XY karyotypes are broken, respectively, rearranged (Coyle et al. 2016). We, therefore, wanted to explore whether the location of the breakpoint on the Y chromosome of these individuals might have some impact on the clinical decision of gender rearing, respectively, on the putative tumor risk of the aberrant germ cells in their dysgenetic gonads. For this purpose, we mapped the Y chromosome breakpoints of 22 individuals reared with different gender phenotypes and explored a putative GCT risk in their dysgenetic gonads by immunohistochemical expression analyses with (i) specific OCT3/4 antibodies marking the fraction of pluripotent germ cells and (ii) with specific antibodies for the Y encoded proteins, DDX3Y, TSPY, and UTY, located in the GBY called “germ cell tumor susceptibility region” on the same gonadal tissue sections.
Results
Molecular mapping of Y chromosome break sites in “sex chromosome DSD” individuals with mosaic 45,X/46,XY karyotypes
We collected genomic DNA samples of 22 “Sex chromosome DSD” individuals with male (11 individuals) and female (11 individuals) gender (Supplementary Table S1) diagnosed with a 45,X/46,XY mosaic karyotype and where the Y chromosome was found to be broken, and/or rearranged by cytogenetic analyses. We note that GBY3 and GBY11 were reared with female gender although their gonads are clinically described with small immature testis tubules (hypotrophy) and GBY102 was reared with male gender although the clinical description of his external genitalia contained the term “clitoris hypertrophy” (Supplementary Table S1).
Molecular deletion analyses on their Y chromosomes were performed with 4 PCR multiplex assays containing Y gene STS markers for 16 Y genes located in Yp11 and Yq11, respectively (Vogt and Bender 2012). These included the sex determination SRY gene and the 3 Y genes of the putative GBY tumour susceptibility region, DDX3Y, TSPY, and UTY, respectively. We also analyzed the presence of the Y centromere region by a specific PCR assay (for more details see: Material & Methods chapter)
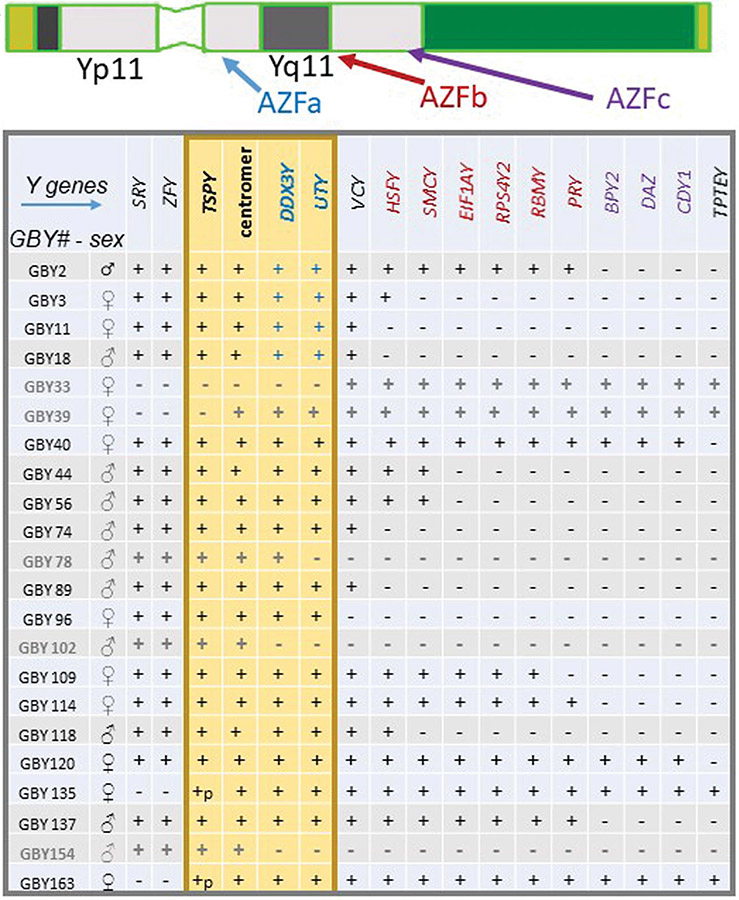
We found that the Y chromosome of these DSD individuals was broken and/or arranged at distinct sites on the short and long Y arm (Figure 2). Individuals with male gender displayed mainly a break in Yq11; distal to the GBY region and near or in the proximal AZFb interval (Figure 2: GBY18, GBY44, GBY56, GBY74, GBY89, GBY118). Three DNA samples (GBY78, GBY102; GBY154) were identified with deletion of the GBY candidate genes in the AZFa interval in proximal Yq11 and two DNA samples (GBY2, GBY137) displayed their Y breakpoints in or distal to the AZFc interval in the distal part of the long Y arm (Figure 2).
Figure 2. Molecular Y chromosome breakpoint mapping on the Y chromosome of 22 “Sex chromosome DSD” individuals with rearranged Y structure in a karyotype.
Each DSD individual is listed with a specific GBY number code and her/his gender decided for rearing at birth in the clinic (♀: female rearing; ♂: male rearing) in the first two columns at the left of this table. The Y genes analyzed for presence (“+”) or absence (“−”) in Yp11 and Yq11 are listed along the first line of this table. Their colour code point to their location in AZFa (marked blue), in AZFb (marked red) and in AZFc (marked violet), respectively. The putative GBY germ cell tumour susceptibility region is highlighted by a yellow box including also the Y centromere. Two samples, GBY135, GBY163 are broken in the cluster of the repetitive TSPY genes in proximal Yp11 (here marked + p(artial). For further details see the main text.
Breakpoint sites on the Y chromosome of the 11 DSD individuals with female gender were more spread. SRY deletions in Yp11 with distinct distal Y extensions were found in four of them: GBY135 and GBY163 were broken in the TSPY gene cluster in proximal Yp11.1; Y deletion of GBY39 included the complete short Y arm; deletion of the complete GBY region in proximal Yp11 and Yq11 was found on the Y chromosome of GBY33 (Figure 2). Notably, eight DSD individuals with female gender contained a Y chromosome in their leukocytes with the presence of the SRY gene and the complete short Y arm, i.e., including completely the male sex-determining region: GBY96 displayed a break on the long Y arm in Yq11 distal to GBY and the GBY3, GBY11 female individuals displayed Yq11 breakpoints similar as six individuals with male gender, i.e., in the proximal AZFb interval (Figure 2). Five individuals with female gender displayed a break in the distal Yq11 region of AZFb (GBY2; GBY109; GBY114), respectively, a break in distal AZFc (GBY40; GBY120).
Immunohistochemical analyses for putative germ cell tumour risks in gonadal tissue sections of “sex chromosome DSD” individuals with Y chromosome breaks
Seven of the 22 DSD individuals with Y chromosome breakage analyzed in this study were gonadectomized before puberty (GBY3; GBY18; GBY74, GBY89, GBY96; GBY102, GBY137), and five (GBY11; GBY40; GBY109; GBY154; GBY163) after puberty. Some of them agreed by written consent to also use some tissue sections from their gonadal biopsies for analysis of a putative tumour risk of their aberrant germ cells.
We usually analyzed at least 10 distinct gonadal tissue sections from each specimen and performed on these immunohistochemical expression analyses in two steps as suggested recently (Vogt et al. 2019). First, we analysed OCT3/4 expression to mark their fraction of pluripotent germ cells and secondly, we analysed the 3 GBY candidate proteins DDX3Y, TSPY, and UTY, with specific antisera for their expression levels in the visible pre-malignant GB/GCNIS cells (for more details see Materials & Methods section).
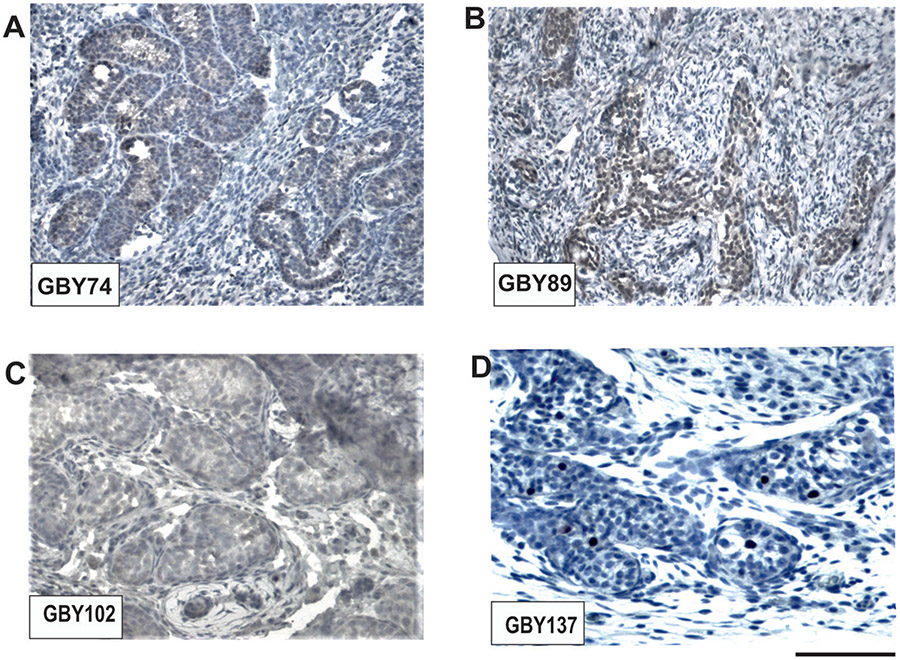
The analyzed prepubertal samples with male gender, GBY74, GBY89, GBY102 and GBY137, contained only a low number of germ cells in their testicular tubules as expected. In serial sections of the dysgenetic gonads of GBY74, GBY89, GBY102, and GBY137, we found significant OCT3/4 expression only in the samples of GBY74 and GBY89, no expression in the GBY102 sample and expression in only single cells was found in the GBY137 left testis tissue sections (Figure 3). These left GBY137 testis tubules already had been diagnosed with a Juvenile Granulosa Cell Tumour (JGCT) after birth. This is a benign neoplasm accounting for 1–5% of all prepubertal testis tumors (Kao et al. 2015) and might explain the low OCT3/4 expression level. Unfortunately, tissue sections of the right testis could not yet analyzed because they were not available.
Figure 3. Immunohistochemical OCT3/4 expression analyses in dysgenetic gonads of prepubertal “Sex chromosome DSD” male patients:
GBY74 (A), GBY89 (B), GBY102 (C), and GBY137 (D), respectively. Significant OCT3/4 expression was only found in GBY74 and GBY89. GBY102 does not indicate any OCT3/4 staining, whereas only single germ cells of GBY137 displayed visible OCT3/4 expression. Scale bar lengths given under D corresponds to 100 μm.
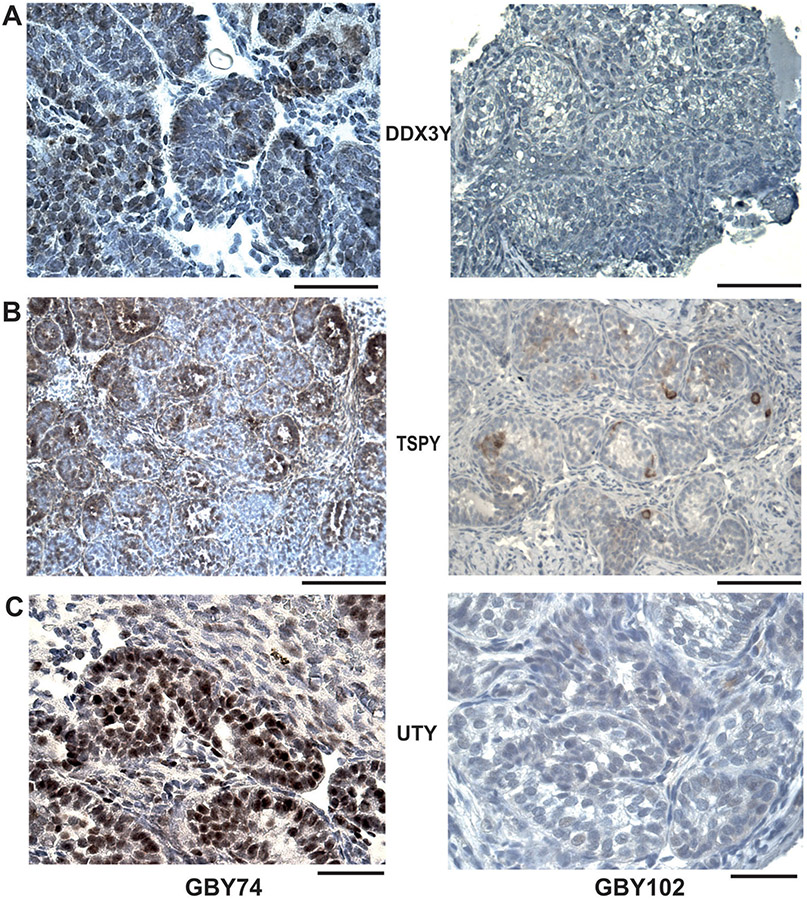
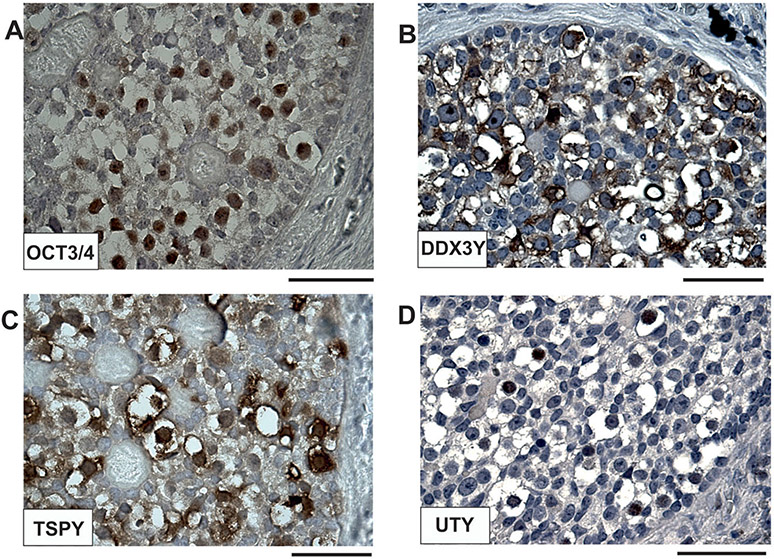
In order to evaluate -comparatively- the expression levels of the GBY candidate proteins, DDX3Y, TSPY, UTY, in one sample with strong OCT3/4 against one sample with an absence of OCT3/4 expression, we performed immunohistochemical analysis of their expression profiles in the dysgenetic gonads of GBY74 and GBY102, respectively (Figure 4). Only GBY74 contains the 3 GBY candidate genes on his Y chromosome, whereas GBY102 contains only the TSPY gene cluster in Yp11 (Figure 2). Significant expression of all three GBY candidate proteins was only found in the aberrant germ cells of GBY74, whereas GBY102, with an absence of OCT3/4 expression, displayed only low TSPY expression. These data confirm the specificity of the three GBY antisera because the expression of DDX3Y and UTY was found to be absent as expected due to the absence of these Y genes on his di-centric Y chromosome (Figure 2).
Figure 4. Immunohistochemical expression analyses of GBY candidates, DDX3Y, TSPY, and UTY in dysgenetic gonads of GBY74 and GBY102.
Comparison of immunohistochemical staining pattern for DDX3Y (A), TSPY (B), UTY (C) in serial tissue sections of GBY74 with positive OCT3/4 staining pattern (left) and of GBY102 with the absence of OCT3/4 staining pattern (right). Since the Y chromosome of GBY102 is deleted for the GBY candidate genes, DDX3Y and UTY (see Figure 2), only TSPY expression is found in some of the aberrant prepubertal germ cells of GBY102. For further discussion see the main text. Scale bar lengths given in A and B corresponds to 100 μm; in C to 50 μm, respectively.
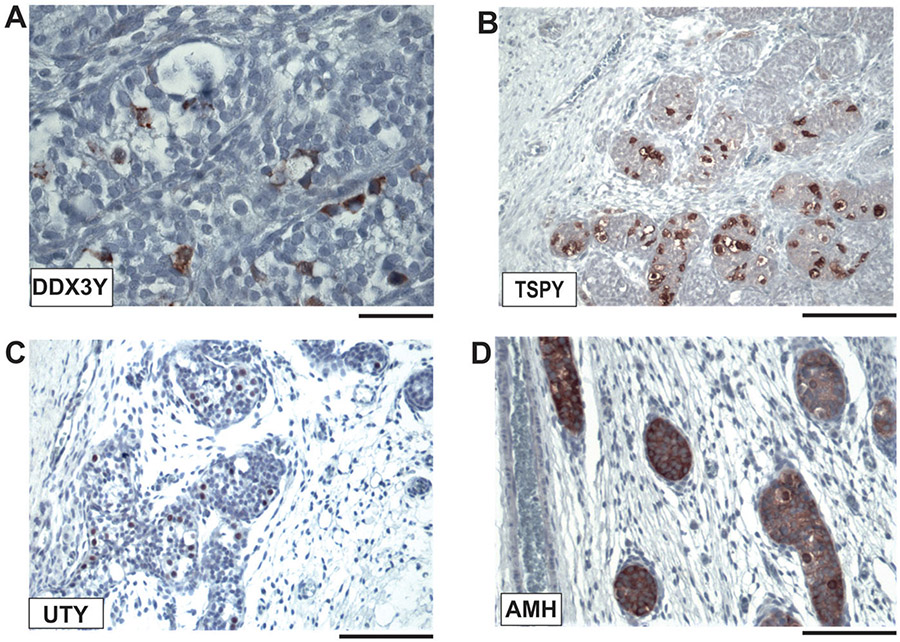
Only low OCT3/4 expression was also found in the postnatal (1 month) germ cells of GBY137 (Figure 3) along with significantly the expression of all three GBY candidate proteins (Figure 5). Most of these germ cells display a Germ Cell Neoplasia in Situ (GCNIS) phenotype with large cytoplasm areals. GCNIS cells are described as pre-malignant male germ cells surrounded by Sertoli cells (Hersmus et al.2008). The presence of Sertoli cells is shown by strong expression of the Anti-Muellerian-Hormone (AMH), a specific marker for immature Sertoli cells (Aksglaede et al. 2010), on the same gonadal tissue sections (Figure 5D). It confirms that these tissue sections indeed also contain JGCT typed somatic tumour cells; they originate from immature Sertoli cells (Kao et al. 2015).
Figure 5. Immunohistochemical diagnostic of a prepubertal testicular tissue section of GBY137 diagnosed in the clinic with Juvenile Granulosa Cell Tumor (JGCT) cells in his left testis at birth (Table S1).
The significant staining pattern of the 3 GBY candidate proteins, DDX3Y (A), TSPY (B), and UTY (C) is observed in the aberrant germ cells of which many display a Germ Cell Neoplasia in Situ (GCNIS) phenotype. (D): Staining serial tissue sections with AMH (Anti Muellerian Hormone) antibodies marking specifically only immature Sertoli cells confirmed the origin of JGCT cells from immature Sertoli cells. For further discussion see the main text. Scale bars in A correspond to 50 μm; in B-D to 100 μm, respectively.
Similar immunohistochemical experiments with serial gonadal tissue sections of the postpubertal samples of GBY40 and GBY109 demonstrate the predominant presence of Gonadoblastoma (GB) cells in the dysgenetic gonads of GBY40. Strong expression of OCT3/4 and of the three GBY candidate proteins, DDX3Y, TSPY, UTY is shown in the aberrant germ cells of the serial gonadal tissue sections (Figure 6). Obviously, the streak gonads of GBY40 still contain some germ cell nests in the undifferentiated gonadal tissue (“UGT”) areals, described first by Cools et al. (2006). GBY109 did not display any gonadoblastoma histology in her aberrant germ cells and also no streak gonads but ovarian atrophy without follicles (Supplementary Table S1). Accordingly, neither significant OCT3/4 expression nor of any of the three GBY candidate proteins could be observed in serial sections of her dysgenetic gonads (data not shown).
Figure 6. Immunohistochemical analyses of the aberrant germ cells with Gonadoblastoma (GB) in GBY40 gonadal tissue sections with female sexual phenotype.
Strong expression of OCT3/4 (A) and of all three GBY candidate proteins, DDX3Y (B), TSPY (C), UTY (D) confirms the pre-malignant morphology of the patient aberrant germ cell nests present in the so-called “Undifferentiated Gonadal Tissue” (“UGT”) regions of the streak gonads as first described by Cools et al. (2006). Scale bars correspond to 50 μm.
Discussion
Sexual gender rearing of “sex chromosome DSD” individuals with Y chromosome breaks in mosaic 45,X/46,XY karyotypes does not depend on Y break site
In human, the 45,X/46,XY karyotype can be associated with Turner syndrome (TS), with mixed gonadal dysgenesis (MGD), earlier also called male pseudohermaphroditism (MPH) and with apparently a normal male phenotype, although infertile (Simpson 1978). Today, these patient groups are summarized under an umbrella coined: “Differences of Sexual Development” (DSD) individuals (Hughes et al. 2006). In the subgroup of “Sex chromosome DSD” individuals with rearrangements or break events on the Y chromosome, sexual gender assignment in the clinic after birth is challenging (Vidal et al. 2010). Current guidelines, therefore, propose child’s/adolescent’s sex assignment first when she/he is able to express autonomously her/his perception of gender identity (Weidler et al. 2019).
In this paper, we report that molecular mapping of the break sites on the Y chromosomes of 22 “Sex chromosome DSD” individuals does not reveal any gender-specific break site region. Remarkably, eight DSD individuals reared with female gender contained a Y chromosome with the male sex-determining SRY gene and the complete short Y arm. The most prominent example was GBY40 with a di-centric Y chromosome structure harboring two copies of the male determining SRY gene and all AZF-Y genes functional for spermatogenesis (Vogt et al. 2008; Krausz and Casamonti 2017).
Dicentric Y chromosome (dic-Y) structures with two Y centromeres and two complements of the short Y arm are indeed most prominent in the karyotypes of “Sex chromosome DSD” individuals (Yang and Hao 2019). Accordingly, 13 dic-Y samples were found in the 22 individuals analyzed in this study (Supplementary Table S1).
Induction of the first Y chromosome breakage and fusion event in these individuals occurred most likely already early during embryonic development. Two daughter mosaic cell lines, one with 47,Xdic-Y, and one with 45,X0 karyotype are the consequence. If two active Y centromeres are present in the dicentric Y structure they are mitotically unstable. Consequently, they break again, thereby extending the first Y deletion and increasing the amount of 45,X0 cells in the gonads (Patsalis et al. 2005).
Accordingly, comparative cytogenetic analyses of lymphocytes, fibroblasts and gonadal tissue biopsies isolated from monozygotic twin (MZT) samples discordant for sex phenotype revealed that the percentage of 45,X0 and 46,XY cells can be significantly different in each cell/tissue analyzed (Costa et al. 1998).
However, routinely collecting a gonadal tissue sample of the DSD individuals is not allowed in the outpatient clinic. Medical and ethical guidelines permit experimental use of human tissues biopsies only after the diagnosis of a significant pathology like the indication of tumour cells. Moreover, culturing of metaphase chromosomes in gonad cells required for karyotyping is not possible for technical reasons. If a fresh and unfixed gonadal tissue biopsy can be requested from the patient, marking the chromosomes required appropriate FISH (Fluorescent In Situ Hybridisation) assays with specific fluorescent chromosome markers in the interphase nuclei of the gonadal tissue sections (Mazen et al. 2018).
Molecular Y chromosomal breakpoint mapping with genomic DNA samples extracted from fresh gonadal tissue aliquots is however only possible when all cell types in the gonad (i.e., Sertoli cells, Leydig cells, interstitial cells and germ cells) will have the same Y chromosomal deletion event.
From our patient collection, GBY40 consented to gonadectomy in the clinic because of the high tumour risk already detected during her clinical routine diagnostics schedule. Accordingly, the gonadal chromosomes of GBY40 were analyzed by FISH with a Y specific centromere probe (DYZ3). 15% dic-Y cells and 85% X0 cells could be identified (data not shown). In leukocytes, the same mosaic sex chromosome composition was rather different reporting 90% dic-Y cells (Supplementary Table S1). Obviously, these data point to an inherent weakness of the routine clinical analysis using only blood cells for DSD-XY chromosome diagnostics. GBY40 gonadal chromosome composition can explain the patient’s external female genitalia much better than her leukocyte karyotype.
Putative tumour development in the germ cells of dysgenetic gonads of “sex chromosome DSD” individuals with Y chromosome breaks is marked by OCT3/4 expression
Significant risk for tumour development in the aberrant germ cells of DSD individuals with a Y chromosome in their karyotype was found first by Scully (1970). It is generally rather variable (Verp and Simpson 1987). In patients with DSD and Turner syndrome features, it can range between 4 and 60% (Gravholt et al. 2000). Recently, we observed that risk for tumour cells in dysgenetic gonads can be revealed best by immunohistochemical analysis with OCT3/4 specific antibodies on appropriate gonadal tissue sections; the presence of OCT3/4 expression in the germ cells marks their pluripotency, a putative prerequisite for further tumour development (Vogt et al. 2019). If present, further support can be gained by strong expression of the recently identified GBY candidate proteins, DDX3Y, TSPY and UTY, if their karyotype includes a Y chromosome with the GBY tumour susceptibility region (Lau et al. 2011; Vogt et al. 2019; 2021). The data reported in this paper, therefore, indicates a significant tumour risk in the aberrant germ cells of GBY74, but not of GBY102 with only low TSPY expression (Figures 3 and 4).
However, we have to keep in mind that DDX3Y, TSPY and UTY proteins are also expressed in the male germline in early spermatogonia during their fetal and adult proliferation phase (TSPY: Honecker et al. 2004; Lau et al. 2011; DDX3Y: Gueler et al. 2012; UTY: Vogt et al. 2021). It is therefore challenging to judge any active tumour susceptibility function of the same Y genes in the aberrant germ cells of the dysgenetic gonads of DSD-XY individuals only by the intensity of their immunohistochemical staining patterns. An example presented in this paper is GBY137 with low OCT3/4 expression but significantly expressing the 3 GBY candidate proteins (Figure 5). Since most of these germ cells displayed a Germ Cell Neoplasia in Situ (GCNIS) the phenotype of these germ cells are pre-malignant (Hersmus et al. 2008).
In the testis tubules of normal male germ cells, the GBY associated Y genes start their expression after the fetal gonocytes have lost their state of pluripotency (Lau et al. 2011; Gueler et al. 2012; Vogt et al. 2021). Consequently, we proposed that their GBY tumor susceptibility function must be associated with the presence of OCT3/4 expression in the same germ cells marking those germ cells as pluripotent (Vogt et al. 2019). However, we do not yet know about possible exceptions from this rule as suggested here from our GBY137 data.
Strong support for our current view of the reliability of our diagnostic schedule to assess putative germ cell tumor risk in the aberrant germ cells of DSD individuals with a Y chromosome in their karyotype (Vogt et al. 2019; 2021) as exemplified in this paper by the data of GBY40. Predominantly, pre-malignant gonadoblastoma (GB) cells were found in the complete dysgenetic gonad, presenting the strong expression of OCT3/4 and all 3 GBY candidate proteins (Figure 6). These data indicate a strong risk for the subsequent development of dysgerminoma tumor cells in accord with Verp and Simpson (1987).
Material & methods
“Sex chromosome DSD” 45,X/46,XY patient group selection for blood and tissue sampling
Blood samples of 22 DSD patients from the subgroup of “Sex chromosome DSD” individuals with a mosaic 45,X/46,XY karyotype in their leukocytes containing rearranged Y chromosomes with chromosome breaks were routinely collected in the outpatient clinic of the children and women hospital, University of Heidelberg, respectively. Gonadal tissue sampling was performed from patients who agreed to gonadectomy because of increased germ cell tumour risk. Their clinical data are listed with their external and internal gonad pathology in Supplementary Table S1 and subdivided into a prepubertal age group: 0–13 years, and post-puberal age group: 14–35 years, respectively.
Blood and tissue samples were collected only after written patient consent for DNA extractions and for diagnostic immunohistochemical experiments according to the “Declaration of Helsinki”. The study was approved by the local ethical committee of the University of Heidelberg (approval code: S-406/2015). Fresh gonadal biopsies isolated during surgical treatment in the paediatric surgical department or in the university women’s hospital were fixed in buffered formaldehyde or with Bouin’s fixative solution. Pathologic assessment of gonadal histology was performed using current terminology (Ulbright and Young 2014).
Molecular PCR assays for mapping centromere and breakpoints on the human Y chromosome of the 22 “sex chromosome DSD” individuals
“Sex chromosome DSD” mosaic 45,X/46,XY patients were first selected for putative broken or rearranged Y chromosomes by cytogenetic routine analyses. Low amounts of 46,XY cells in 45,X0 Turner samples were analyzed for presence or absence of the Y centromere region by a sensitive PCR assay described in detail by Knauer-Fischer et al. (2015). Diagnostic analyses of the putative Y chromosome breakpoint regions on the Y chromosome of the 22 patient samples were performed by a PCR multiplex assay with a panel of Y gene-specific sequence-tagged sites (STS) from 16 Y genes as described by Vogt and Bender (2012). These Y genes are located in the euchromatic region of the short and long Y arm (Yp11-Yq11) including all Y genes of the GBY region overlapping with the AZFa genes in proximal Yq11 and the AZFb and AZFc interval, respectively. For experimental details and set up of the PCR multiplex format, see Vogt and Bender (2012).
Immunohistochemical analyses of “sex chromosome DSD” gonadal serial tissue sections
Immunohistochemical experiments with specific antibodies for protein expression of the GBY candidate genes DDX3Y, TSPY and UTY were performed with serial sections of the paraffin-embedded gonadal tissue specimen. In parallel, we analyzed protein expression of the pluripotent germ cell marker OCT3/4 on the same tissue sections to identify the pluripotent germ cell fraction. We used 4–5μm sections from tissue samples fixed in buffered formaldehyde, or in Bouin’s fixative and subsequently embedded in paraffin. All paraffin-embedded fixed tissue sections were first dewaxed, then rehydrated in decreasing concentrations of ethanol. Immunohistochemistry staining was carried out as described by Gueler et al. (2012). In short: antigen retrieval was achieved by incubating the slides overnight with 0.2 M boric acid, pH 7 at 60 °C, respectively, with 10 mM Citrat buffer pH6 (AMH) in a microwave. After washing in permeabilization buffer (0.1 M Tris, 0.1 M NaCl, 0.1% Triton X-100; pH 7.4), endogenous peroxidase was quenched by incubation in 3% v/v hydrogen peroxide in methanol for 10 min at room temperature. Sections were incubated overnight at 4 °C with the polyclonal antisera, DBY-10, TSPY, and UTY-1, the origin of which has been described recently (Vogt et al. 2019; 2021); using the following dilutions: DBY-10 (1:500), TSPY (1:3000), UTY-1 (1:60). The presence of pluripotent gonocytes in the same dysgenetic gonad tissue samples was analyzed with a monoclonal OCT3/4 antiserum (Santa Cruz; sc-5279; 1:300 v/v) marking the OCT4A protein variant expressed only in still pluripotent fetal germ cells (Cheng et al. 2007). For staining AMH expression in JGCT cells (GBY137) we used the polyclonal antibodies: sc-6886 (AMH: 1:400 v/v) from Santa Cruz. A secondary biotinylated goat-anti-rabbit antibody (TSPY), goat-anti-mouse antibody (DDX3Y; OCT3/4) was applied followed by an avidin-biotin complex (Vector Laboratories, Burlingame, CA, USA or Zymed, San Francisco, CA, USA). Finally, slides were stained with DAB (3,3’-diamino-benzidine tetra-hydrochloride), respectively AEC (aminoethyl carbazole) counterstained with Mayer’s hematoxylin and mounted in Immuno-Mount.
Acknowledgments
We thank all DSD patients counselled in our outpatient clinics for their willingness to fill in the detailed clinical questionnaires and for the blood and gonadal tissue samples to study genomic extensions of the putative Y chromosome deletions in their lymphocytes and a putative germ cell tumour risk of their dysgenetic gonads by OCT3/4 and GBY candidate proteins# immuno-histochemical expression analyses. All clinical colleagues in the outpatient clinics not included on the co-author list, especially U. Heinrich, I. Inta, C. Kneppo, G. Krauch in the children’s hospital and A. Germeyer, S. Rösner, S. Wallwiener in the women’s hospital, are thanked for their most valuable support in collecting the patient samples. Gonadal tissue sections were provided by the tissue bank of the National Centre for Tumour Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the tissue bank and the approval of the ethics committee of Heidelberg University.
Funding
This study was funded by grant 01GM0627 of the BMBF (Bundesministerium für Bildung und Forschung) to PHV and BB and by a clinical grant to T.S. Y-FCL is a Research Career Scientist (5IK6BX004854) and supported by a Merit grant (5IO1BX004446) from the US Department of Veterans Affairs.
Abbreviations:
- AZF
Azoospermia Facto
- CGD
Complete Gonadal Dysgenesis
- DDX3Y
Deadbox RNA helicase 3, Y linked
- DSD
Differences of Sexual Development
- FISH
Fluorescent In Situ Hybridisation
- FOXL2
Forkhead transcription factor L2
- GB
Gonadoblastoma
- GBY
Gonadoblastoma Y-linked
- GC
Granulosa Cells
- GCT
Germ Cell Tumour
- GCNIS
Germ Cell Neoplasia In Situ
- JGCT
Juvenile Granulosa Cell Tumour
- OCT3/4
OCtamer-binding Transcription factor 3/4
- PGD
Partial Gonadal Dysgenesis
- RSPOI
Respondin family gene member 1
- SRY
Sex Determining Region Gene
- SOX9
SRY-related HMG box gene 9
- STS
Sequence Tagged Site
- TSPY
Testis Specific Protein Y
- UTY
Ubiquitous Transcribed Y
- WT4
Willms Tumour gene 4
Footnotes
Ethics approval
The study was approved by the local ethical commission of the University of Heidelberg (approval code: S-406/2015). Patients’ blood and tissue samples were collected only after their written informed consent.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
All data presented in this manuscript are freely available at the personal request
References
- Aksglaede L, Sørensen K, Boas M, Mouritsen A, Hagen CP, Jensen RB, Petersen JH, Linneberg A, Andersson AM, Main KM, et al. 2010. Changes in anti-Mullerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 95(12):5357–5364. [DOI] [PubMed] [Google Scholar]
- Capel B 2017. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat Rev Genet. 18(11):675–689. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. 2007. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 211(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Cools M, Pleskacova J, Stoop H, Hoebeke P, Van Laecke E, Drop SLS, Lebl J, Oosterhuis JW, Looijenga LHJ, Wolffenbuttel KP. 2011. Gonadal pathology and tumor risk in relation to clinical characteristics in patients with 45,X/46,XY mosaicism. J. Clin. Endocrinol. Metab. 96(7): E1171–1180. [DOI] [PubMed] [Google Scholar]
- Cools M, Stoop H, Kersemaekers AM, Drop SL, Wolffenbuttel KP, Bourguignon JP, Slowikowska-Hilczer J, Kula K, Faradz SM, Oosterhuis JW, et al. 2006. Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab. 91(6):2404–2413. [DOI] [PubMed] [Google Scholar]
- Costa T, Lambert M, Teshima I, Ray PN, Richer CL, Dallaire L. 1998. Monozygotic twins with 45,X/46,XY mosaicism discordant for phenotypic sex. Am. J. Med. Genet. 6:40–44. [PubMed] [Google Scholar]
- Coyle D, Kutasy B, Han Suyin K, Antao B, Lynch SA, McDermott MB, ÓConnell SM, Quinn F. 2016. Gonadoblastoma in patients with 45,X/46,XY mosaicsm: a 16-year experience. J. Pediatric Urol. 12(5):283.e1–283.e7. [DOI] [PubMed] [Google Scholar]
- Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH. 1959. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). Lancet. 273(7075):711–713. [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Fedder J, Naerraa RW, Mueller J. 2000. Occurrence of Gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study. J. Clin. Endocrinol, Metab. 85:3199–3202. [DOI] [PubMed] [Google Scholar]
- Gueler B, Sonne SB, Zimmer J, Hilscher B, Hilscher W, Graem N, Rajpert-De Meyts E, Vogt PH. 2012. AZFa protein DDX3Y is differentially expressed in human male germ cells during development and in testicular tumours: new evidence for phenotypic plasticity of germ cells. Hum Reprod. 27(6):1547–1555. [DOI] [PubMed] [Google Scholar]
- Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M, et al. 2008. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J Pathol. 215(1):31–38. [DOI] [PubMed] [Google Scholar]
- Honecker F, Stoop H, de Krijger RR, Lau Y-FC, Bokemeyer C, Looijenga LH. 2004. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 203(3):849–857. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA. 2006. Consensus statement on management of intersex disorders. J Pediatr Urol. 2(3):148–162. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Strong JA. 1959. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature. 183(4657):302–303. [DOI] [PubMed] [Google Scholar]
- Kao C-S, Cornejo KM, Ulbright TM, Young RH. 2015. Juvenile granulosa cell tumors of the testis: a clinicopathologic study of 70 cases with emphasis on its wide morphologic spectrum. Am J Surg Pathol. 39(9): 1159–1169. [DOI] [PubMed] [Google Scholar]
- Knauer-Fischer S, Besikoglu B, Inta I, Kneppo C, Vogt PH, Bettendorf M. 2015. Analyses of Gonadoblastoma Y (GBY)-locus and of Y centromere in Turner syndrome patients. Exp Clin Endocrinol Diabetes. 123(1):61–65. [DOI] [PubMed] [Google Scholar]
- Krausz C, Casamonti E. 2017. Spermatogenic failure and the Y chromosome. Hum Genet. 136(5):637–655. [DOI] [PubMed] [Google Scholar]
- Lau Y-FC, Li Y, Kido T. 2011. Role of the Y-located putative gonadoblastoma gene in human spermatogenesis. Syst Biol Reprod Med. 57(1–2):27–34. [DOI] [PubMed] [Google Scholar]
- Lindhardt Johansen M, Hagen CP, Rajpert-De Meyts E, Kjaergaard S, Petersen BL, Skakkebaek NE, Main KM, Juul A. 2012. 45,X/46,XY mosaicism: phenotypic characteristics, growth, and reproductive function-a retrospective longitudinal study. J Clin Endocrinol Metab. 97(8): E1540–E1549. [DOI] [PubMed] [Google Scholar]
- Mazen IM, Mekkawy MK, Ibrahim HM, Kamel AK, Hamza RT, Elaidy AA. 2018. Clinical and cytogenetic study of egyptian patients with sex chromosome disorders of sex development. Sex Dev. 12(5):211–217. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti J-L, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. 2005. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 287(2):361–377. [DOI] [PubMed] [Google Scholar]
- Page DC. 1987. Hypothesis: a Y-chromosomal gene causes gonadoblastoma in dysgenetic gonads. Development. 101(Supplement):151–155. [DOI] [PubMed] [Google Scholar]
- Patsalis PC, Skordis N, Sismani C, Kousoulidou L, Koumbaris G, Eftychi C, Stavrides G, Ioulianos A, Kitsiou-Tzeli S, Galla-Voumvouraki A, et al. 2005. Identification of high frequency of Y chromosome deletions in patients with sex chromosome mosaicism and correlation with the clinical phenotype and Y-chromosome instability. Am J Med Genet A. 135(2):145–149. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RA, Lambertz B, Friederiszick FK, Distel H, Pawlowitzki IH, Nicole R, Ober KG, Ruckes J. 1968. The nosologic place of the X0/XY mosaicism. Arch Gynak. 206(4):369–410. [DOI] [PubMed] [Google Scholar]
- Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, Platzer M, Howell GR, Burrows C, Bird CP, et al. 2005. The DNA sequence of the human X chromosome. Nature. 434(7031):325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully RE. 1970. Gonadoblastoma. A review of 74 cases. Cancer. 25(6):1340–1356. [DOI] [PubMed] [Google Scholar]
- Simpson JL. 1978. Male pseudohermaphroditism: genetics and clinical delineation. Hum Genet. 44(1):1–49. [DOI] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, et al. 2003. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 423(6942):825–837. [DOI] [PubMed] [Google Scholar]
- Sohval AR. 1964. Hermaphroditism with “atypical” or “mixed” gonadal dysgenesis. RELATIONSHIP TO GONADAL NEOPLASM. Am J Med. 36:281–291. [DOI] [PubMed] [Google Scholar]
- Stévant I, Papaioannou MD, Nef S. 2018. A brief history of sex determination. Mol Cell Endocrinol. 468:3–10. [DOI] [PubMed] [Google Scholar]
- Ulbright TM, Young RH. 2014. Gonadoblastoma and selected other aspects of gonadal pathology in young patients with disorders of sex development. Semin Diagn Pathol. 31(5):427–440. [DOI] [PubMed] [Google Scholar]
- Verp MS, And, Simpson JL. 1987. Abnormal sexual differentiation and neoplasia. Cancer Genet Cytogenet. 25(2): 191–218. [DOI] [PubMed] [Google Scholar]
- Vidal I, Gorduza DB, Haraux E, Gay CL, Chatelain P, Nicolino M, Mure PY, Mouriquand P. 2010. Surgical options in disorders of sex development (DSD) with ambiguous genitalia. Best Pract Res Clin Endocrinol Metab. 24(2):311–324. [DOI] [PubMed] [Google Scholar]
- Vogt PH, Bender U. 2012. Human Y chromosome microdeletion analysis by PCR multiplex protocols identifying only clinically relevant AZF microdeletions. In: Meth. Mol. Biol.”: Spermatogenesis and spermiogenesis: (Carrell DT & Aston KI, eds; New York, Heidelberg: Springer. 927: p. 187–204. [DOI] [PubMed] [Google Scholar]
- Vogt PH, Besikoglu B, Bettendorf M, Frank-Herrmann P, Zimmer J, Bender U, Knauer-Fischer S, Choukair D, Sinn P, Lau Y-FC, et al. 2019. Gonadoblastoma Y locus genes expressed in germ cells of individuals with dysgenetic gonads and a Y chromosome in their karyotypes include DDX3Y and TSPY. Hum Reprod. 34(4):770–779. [DOI] [PubMed] [Google Scholar]
- Vogt PH, Falcao CL, Hanstein R, Zimmer J. 2008. The AZF proteins. Int J Androl. 31(4):383–394. [DOI] [PubMed] [Google Scholar]
- Vogt PH, Zimmer J, Bender U, Strowitzki T. 2021. AZFa candidate gene UTY and its X homologue UTX are expressed in human germ cells. Reprod Fertil. 2(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidler EM, Pearson M, van Leeuwen K, Garvey E. 2019. Clinical management in mixed gonadal dysgenesis with chromosomal mosaicism: considerations in newborns and adolescents. Semin Pediatr Surg. 28(5):150841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hao W. 2019. Clinical, cytogenetic, and molecular findings of isodicentric Y chromosomes. Mol. Cytogenet. 27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this manuscript are freely available at the personal request