Abstract
Objectives
The risk of severe COVID-19 in children with a solid organ transplant (SOT) is not well established. We compare the relative risk of severe COVID-19 infection between pediatric SOT and non-SOT children.
Methods
The newly constructed K-COV-N cohort (Korea Disease Control and Prevention Agency-COVID-19-National Health Insurance Service) was used. Children with COVID-19 (<18 years old) who underwent SOT between January 2008–January 2022 were included. Non-SOT children with COVID-19 were selected in a ratio of 1:4 using propensity score matching. Three definitions of severe COVID-19 were established based on their requirement of respiratory support: Severe I (requiring respiratory support above a high-flow nasal cannula or prolonged hospitalization ≥6 days), Severe II (requiring any oxygen supplement), and Severe III (requiring any oxygen supplement or prolonged hospitalization ≥6 days).
Results
Among 2,957,323 children with COVID-19, 206 pediatric SOTRs were identified and included in the analysis along with 803 matched non-SOT children. Most infections (96.6%) occurred during the Omicron period; no cases of mortality were reported. Pediatric SOTR had a 3.6-fold (95% CI=1.1–11.7, P=0.03) higher risk of Severe I, and a 4.9-fold (95% CI=1.6–15.0, P=0.006) higher risk of Severe III than non-SOT children. No cases of Severe II occurred in the non-SOT children. Although not statistically significant, no severe COVID-19 cases were reported in the vaccinated SOT group (0.0% vs 5.7%, P=0.09 in Severe III).
Conclusions
Pediatric SOTRs have a significantly higher risk of severe COVID-19 than non-SOT children. Our findings support the need for tailored strategies for these high-risk children.
Keywords: solid organ transplant, children, severe COVID-19, relative risk, Korea
Background
Since the report of first cases in December 2019, the coronavirus disease 2019 (COVID-19) pandemic has become endemic, and many countries are shifting public health strategies from universal prevention and control to policies targeting people at an increased risk of developing severe COVID-19 [1, 2].
Meanwhile, most children with COVID-19 have mild clinical symptoms [3-5]. A study analyzing pediatric COVID-19 deaths until February 2021 in seven high-income countries reported that COVID-19 mortality in 0–19-year-olds was 0.17 per 100,000, accounting for 0.5% of all pediatric deaths [6]. This is less than 1% of the reported COVID-19 mortality rate in the adult population of Western Europe and the United States [7, 8]. This mild severity, coupled with the relatively low vaccine effectiveness against the Delta and Omicron variants upon introduction of immunization in children, and parents’ hesitation regarding the “new platform” vaccines, has resulted in a lower coverage of COVID-19 vaccination in children [9-11]. This poor vaccine uptake is also applicable to pediatric solid organ transplant recipients (SOTRs), another high-risk group for severe COVID-19 owing to being immunocompromised [12]. Paradoxically, these pediatric SOTRs may be less protected than adult SOTRs as many antiviral therapies or targeted monoclonal antibodies are often unavailable due to age and/or weight restrictions [13, 14].
In Korea, approximately 4,500 solid organ transplants (SOTs) have been performed annually for the past 7 years, of which approximately 120 (3%) are in children [15]. These SOTRs are registered as patients with rare and incurable diseases and are supported and managed through the National Health Insurance Service (NHIS). Additionally, COVID-19 was classified as a category I infectious disease until May 2022, and all COVID-19 confirmed records were managed using the Korea Disease Control and Prevention Agency (KDCA) registry [15]. This scenario provides a niche for evaluating the risk of COVID-19 in these high-risk pediatric populations at the national level. We established a convergence big data cohort and investigated the additional risk of severe COVID-19 in pediatric SOTRs compared with non-SOT children. Risk factors for severe COVID-19 were also explored.
Materials and methods
Data source
The K-COV-N cohort (KDCA-COVID19-NHIS) was constructed by merging the COVID-19 registry data of the KDCA with the claims data of the NHIS. The COVID-19 registry data comprised confirmed case and vaccination data in Korea. As of March 30, 2022, this registry contained data on 12,965,556 individuals with confirmed COVID-19, and 119,433,355 doses of COVID-19 vaccination in 44,534,225 individuals. By converging with the claims data of the NHIS, this cohort provides detailed information on treatment behaviors and underlying diseases in individuals with confirmed COVID-19.
Study design
This retrospective, matched-control study used propensity score matching. We first extracted pediatric SOTRs (<18 years old) with confirmed COVID-19. Next, non-SOT individuals (<18 years old) with COVID-19, corresponding to 100 × the number of pediatric SOTRs, were extracted through random sampling. Among them, those who matched for age, sex, Pediatric Comorbidity Index (PCI), and time of COVID-19 confirmation (pre-Omicron vs. Omicron period) were selected using the 1:4 greedy nearest neighbor matching and selected as a control group. The primary outcome was the occurrence of severe COVID-19 within 30 days of COVID-19 confirmation. The total primary outcome was considered up to April 30, 2022, 1 month after the last date of the study (March 30, 2022). This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Severance Children's Hospital, Yonsei University College of Medicine, Seoul, South Korea (No. 4-2020-0240).
Definitions
The confirmed date of COVID-19 was defined as the collection date of the SARS-CoV-2 specimen. Given the low likelihood of severe COVID-19 in children, severe COVID-19 was defined in three categories based on conditions requiring respiratory support beyond supplemental O2, death, or prolonged hospitalization for ≥6 days. Severe I: requiring respiratory support above a high-flow nasal cannula or hospitalization for ≥6 days; Severe II: requiring respiratory support with any oxygen supplement including a nasal prong (Same as WHO's severe criteria); and Severe III: requiring respiratory support with any oxygen supplement or hospitalization for ≥6 days. SOTRs were defined as those who had SOT procedure codes between January 1, 2008, and January 31, 2020 (Supplementary Table S1). SOTs were classified into kidney, liver, heart, lung, pancreas, and intestinal transplantation. Heart-lung transplantation was considered as a lung transplant. Patients who had undergone sclera or corneal transplantations were excluded. We excluded (1) individuals confirmed to have COVID-19 after death (due to the possibility of SARS-CoV-2 being incidentally detected in deceased children with other causes of death), (2) those with no claims data within the last 5 years, and (3) SOTRs who also underwent hematopoietic cell transplantation. Comorbidity was defined as a disease claimed to be a primary or secondary disease at least once within 5 years prior to COVID-19. We used the previously developed and validated PCI categories and scores as comorbidity indices [16]. The use of immunosuppressive drugs was defined as having a prescription within 3 months prior to COVID-19 onset. The generic name codes for immunosuppressive drugs are presented in Supplementary Table S2. The Omicron period was defined as the period from January 15, 2022, to March 30, 2022 (the last date of the study), considering the time point when the Omicron variant was announced as the dominant species in Korea [17].
Statistical analyses
Categorical variables are presented as frequencies and percentages, whereas continuous variables are presented as means with standard deviations and/or medians with interquartile ranges. The number of COVID-19 cases was divided by the annual mid-year population obtained from the Korean Statistical Information Service (http://kosis.kr) to calculate the monthly incidence per 1,000,000 individuals. The 1:4 propensity score matching with a greedy nearest neighbor matching method was used to assess the severity risk in the SOT group but not the non-SOT group. We adjusted for factors including age, sex, PCI score, Omicron period, and COVID-19 vaccination status. Logistic regression analysis was performed to explore the risk factors for severe infection in SOTRs with COVID-19. Multivariate analysis included factors such as age, sex, time between transplantation and COVID-19 onset, PCI score, Omicron period, type of SOT, and COVID-19 vaccination status. All tests were two-tailed, and P-values <0.05 were considered statistically significant. Statistical analyses were performed using SAS Enterprise Guide 8.2 (SAS Institute Inc., NC, USA).
Results
Overall, 2,998,782 children with COVID-19 were registered in the K-COV-N cohort, of which 41,459 met the exclusion criteria and the remaining 2,957,323 were included in the study. Among these, 206 pediatric SOTRs with COVID-19 were identified. Moreover, among the remaining study participants, 20,600 participants were extracted through a 100 × random sampling process. The 803 participants that matched with 205 SOTRs were selected as non-SOT controls, excluding one SOTR with difficulty in propensity matching (Fig. 1 ).
Fig. 1.
Selection flow of study participants
The mean age of the 205 SOTRs was 10.7 years (SD±3.7). In addition, 47.8% (n=98) were males, and 92.2% (n=189) underwent liver and kidney transplantations. The mean time from SOT to COVID-19 confirmation was 84 months (SD±42.3). Approximately 23.4% of SOTRs (n=48) received at least one COVID-19 vaccine dose, whereas most received two doses (21.0%, n=43); only 1.0% (n=2) received three doses. Other details and characteristics of the SOT and non-SOT groups before and after matching are described in Table 1 . The detailed distribution of comorbidities is presented in Supplementary Table S3.
Table 1.
Baseline characteristics of pediatric SOT and non-SOT groups before and after propensity score matching
| Variable |
Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Pediatric SOT group | Non-SOT group | SMD | Pediatric SOT group | Non-SOT group | SMD | |
| N = 206 | N = 20,600 | N = 205 | N = 803 | |||
| Sex | -0.121 | −0.005 | ||||
| Male | 98 (47.6) | 11039 (53.6) | 98 (47.8) | 386 (48.1) | ||
| Female | 108 (52.4) | 9561 (46.4) | 107 (52.2) | 417 (51.9) | ||
| Age (years), mean (SD) | 10.65 (3.7) | 9.54 (4.4) | 0.273 | 10.66 (3.7) | 10.69 (3.7) | −0.008 |
| Age (years), median (IQR) | 11 (8–14) | 10 (6–13) | 11 (8–14) | 11 (8–14) | ||
| 0–4 y | 12 (5.8) | 3124 (15.2) | 12 (5.9) | 48 (6.0) | ||
| 5–11 y | 105 (51.0) | 10236 (49.7) | 104 (50.7) | 402 (50.1) | ||
| 12–17 y | 89 (43.2) | 7240 (35.2) | 89 (43.4) | 353 (44.0) | ||
| Type of SOT | 0.716 | 0.707 | ||||
| Liver Transplantation | 149 (72.3) | 148 (72.2) | ||||
| Kidney Transplantation | 41 (19.9) | 41 (20.0) | ||||
| Heart Transplantation | 15 (7.3) | 15 (7.3) | ||||
| Lung Transplantation | 1 (0.5) | 1 (0.5) | ||||
| COVID-19 Vaccination (dose) | 0.46 (0.9) | 0.45 (0.8) | 0.011 | 0.46 (0.9) | 0.56 (0.9) | −0.110 |
| 0 | 158 (76.7) | 15906 (77.2) | 0.025 | 157 (76.6) | 576 (71.7) | 0.119 |
| 1 | 3 (1.5) | 250 (1.2) | 3 (1.5) | 11 (1.4) | ||
| 2 | 43 (20.9) | 4269 (20.7) | 43 (21.0) | 209 (26.0) | ||
| 3 | 2 (1.0) | 175 (0.9) | 2 (1.0) | 7 (0.9) | ||
| Comorbidities | ||||||
| PCI, mean score (SD) | 4.45 (3.3) | 1.40 (1.52) | 1.188 | 4.39 (3.2) | 3.92 (2.7) | 0.157 |
| PCI, median score (IQR) | 4 (2–6) | 1 (0–2) | 4 (2–6) | 4 (2–5) | ||
| PCI ≥ 5 | 91 (44.2) | 765 (3.7) | 1.077 | 90 (43.9) | 320 (39.9) | 0.082 |
| Timing of SARS-CoV-2 Infection | −0.008 | −0.010 | ||||
| Before Omicron-predominant | 7 (3.4) | 669 (3.3) | 7 (3.4) | 26 (3.2) | ||
| Omicron-predominant | 199 (96.6) | 19931 (96.8) | 198 (96.6) | 777 (96.8) | ||
Numbers indicate the number of cases and parentheses indicate (%). The notation for continuous variables is described for each variable item.
SOT, solid organ transplantation; SMD, standardized mean difference; SD, standard deviation; IQR, interquartile range; PCI, pediatric comorbidity index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
COVID-19 outbreak and risk of severe COVID-19
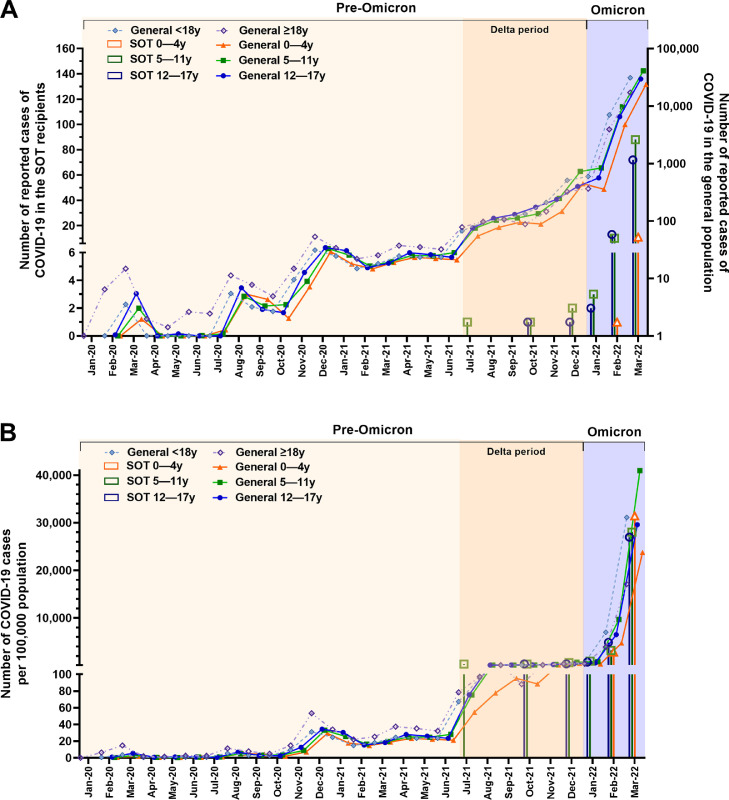
The first COVID-19 case in pediatric SOTRs occurred in July 2021 during the Delta period. Thereafter, the trend in monthly incidence rate of COVID-19 in pediatric SOTRs was similar to that in the general pediatric population in Korea (Fig. 2 A and 2B). Most COVID-19 cases, reaching 96.6%, occurred during the Omicron period, whereas the remaining 3.4% occurred during the Delta period (Fig. 2A and Table 1).
Fig. 2.
Monthly incidence of COVID-19 in Korea by age group
(A) Monthly number of COVID-19 cases in Korea. The left Y-axis represents the number of COVID-19 cases in solid organ transplant recipients, while the right Y-axis represents the number of COVID-19 cases in the general population. The right Y-axis is plotted on a logarithmic scale as the number of patients rapidly increases in the Omicron period. (B) Monthly incidence of COVID-19 in Korea as cases per 100,000 people in the age group.
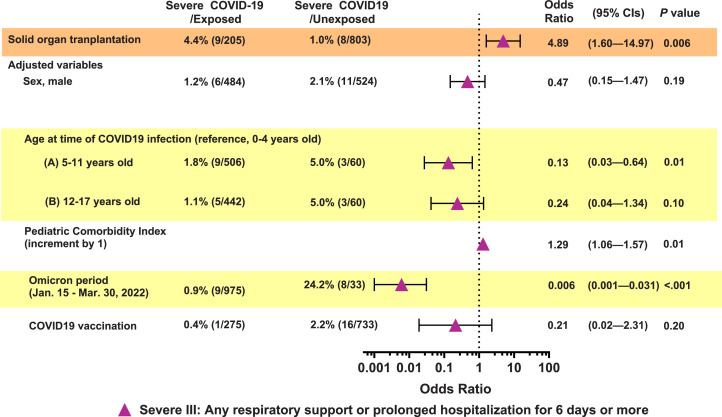
Among the 205 pediatric SOTRs included in propensity score matching, seven (3.4%), six (2.9%), and nine (4.4%) cases were identified and classified as Severe I, Severe II, and Severe III, respectively. No cases of mortality were observed. Of the six Severe II cases requiring support beyond oxygen supplementation, two required mechanical ventilator support or intensive care unit hospitalization, two required high-flow nasal cannulas, and two required oxygen supplementation using nasal prong. Based on the Severe I definition, severe COVID-19 risk was significantly higher (3.6-fold) in the pediatric SOT group than in the non-SOT group (95% CI, 1.1–11.7, P=0.03). (Supplementary Fig. S1) In the case of Severe III, the risk of severe COVID-19 was 4.9-fold higher in the pediatric SOT group than in the non-SOT group (95% CI, 1.6–15.0, P=0.006). (Fig. 3 ) Notably, regarding Severe II, there were no severe cases occurred in the non-SOT group; therefore, risk analysis could not be performed. (Supplementary Fig. S1)
Fig. 3.
Adjusted relative risk of severe COVID-19 in pediatric solid organ transplant recipients (Severe III)
Risk factors for severe COVID-19
A total of 206 children with COVID-19 who had undergone SOT were included in the risk factor analysis for severe COVID-19 (Fig. 1). In the multivariate analysis, COVID-19 during the Omicron period significantly lowered the risk of severe COVID-19 by 0.03–0.05 times, regardless of the definition of severe COVID-19 (Table 2 ). Moreover, among SOT patients, there were no severe cases of COVID-19 in the group that received at least one dose of COVID-19 vaccine (all Severe I to III), and all severe cases occurred exclusively in non-vaccinated patients; however, the difference did not reach statistical significance.
Table 2.
Risk factors for severe COVID-19 infection in pediatric SOTRs (n = 206)
| Variable |
Number of cases | Univariable analysis | Multivariable analysis a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-severe | Severe | P | OR | 95% CIs | P | OR | 95% CIs | P | |
| Severe I | 199 | 7 | |||||||
| Sex, male | 96 (48.2) | 2 (28.6) | 0.31 | 0.43 | 0.08–2.26 | 0.32 | 0.46 | 0.08–2.88 | 0.41 |
| Transplantation to COVID-19, months, mean (SD) | 84.7 (42.1) | 72.9 (49.1) | 0.47 | 0.99 | 0.97–1.01 | 0.47 | 1.01 | 0.98–1.03 | 0.65 |
| Age. years, mean (SD) | 10.7 (3.7) | 8.3 (4.3) | 0.08 | ||||||
| 0–4 y | 10 (5.0) | 2 (28.6) | 0.03 | Ref | Ref | ||||
| 5–11 y | 102 (51.3) | 3 (42.9) | 0.15 | 0.2–0.99 | 0.048 | 0.14 | 0.01–1.51 | 0.10 | |
| 12–17 y | 87 (43.7) | 2 (28.6) | 0.12 | 0.02–0.91 | 0.04 | 0.12 | 0.01–2.72 | 0.18 | |
| PCI score (≥5) | 86 (43.2) | 5 (71.4) | 0.14 | 3.29 | 0.62–17.34 | 0.16 | 4.34 | 0.61–31.07 | 0.14 |
| COVID vaccine (≥1 dose) | 48 (24.1) | 0 (0.0) | 0.14 | ||||||
| SOT | 0.04 | ||||||||
| LT + KT | 185 (93.0) | 5 (71.4) | Ref | Ref | |||||
| LuT + HT | 14 (7.0) | 2 (28.6) | 5.29 | 0.94–29.74 | 0.06 | 4.93 | 0.61–39.57 | 0.13 | |
| Omicron period | 193 (97.0) | 6 (85.7) | 0.11 | 0.19 | 0.02–1.80 | 0.15 | 0.05 | 0.003–0.74 | 0.03 |
| Severe II | 200 | 6 | |||||||
| Sex, male | 97 (48.5) | 1 (16.7) | 0.12 | 0.21 | 0.02–1.85 | 0.16 | 0.13 | 0.01–1.80 | 0.13 |
| Transplantation to COVID-19, months, mean (SD) | 85.3 (42.2) | 51 (31.2) | 0.05 | 0.97 | 0.94–1.003 | 0.08 | 0.97 | 0.94–1.01 | 0.20 |
| Age. years, mean (SD) | 10.7 (3.7) | 7.7 (3.5) | 0.04 | ||||||
| 0–4 y | 10 (5.0) | 2 (33.3) | 0.02 | Ref | Ref | ||||
| 5–11 y | 102 (51.0) | 3 (50.0) | 0.15 | 0.02–0.99 | 0.048 | 0.35 | 0.03–4.06 | 0.40 | |
| 12–17 y | 88 (44.0) | 1 (16.7) | 0.06 | 0.005–0.68 | 0.02 | 0.30 | 0.02–5.90 | 0.43 | |
| PCI score (≥5) | 87 (43.5) | 4 (66.7) | 0.26 | 2.6 | 0.47–14.5 | 0.28 | 2.53 | 0.33–19.60 | 0.37 |
| COVID vaccine (≥1 dose) | 48 (24.0) | 0 (0.0) | 0.17 | ||||||
| SOT | 0.02 | ||||||||
| LT + KT | 186 (93.0) | 4 (66.7) | Ref | Ref | |||||
| LuT + HT | 14 (7.0) | 2 (33.3) | 6.64 | 1.12–39–48 | 0.04 | 4.67 | 0.56–39.10 | 0.15 | |
| Omicron period | 194 (97.0) | 5 (83.3) |
0.07 | 0.16 | 0.02–1.54 | 0.11 | 0.03 | 0.002–0.56 | 0.02 |
| Severe III | 197 | 9 | |||||||
| Sex, male | 96(48.7) | 2 (22.2) | 0.12 | 0.3 | 0.06–1.48 | 0.14 | 0.29 | 0.05–1.72 | 0.17 |
| Transplantation to COVID-19, months, mean (SD) | 85.3 (42.0) | 63.8 (46.2) | 0.14 | 0.99 | 0.97–1.01 | 0.15 | 1.00 | 0.98–1.03 | ≥ 0.99 |
| Age. years, mean (SD) | 10.8 (3.6) | 7.9 (4.0) | 0.02 | ||||||
| 0–4 y | 9 (4.6) | 3 (33.3) | 0.01 | Ref | Ref | ||||
| 5–11 y | 101 (51.3) | 4 (44.4) | 0.12 | 0.02–0.62 | 0.01 | 0.13 | 0.01–1.13 | 0.06 | |
| 12–17 y | 87 (44.2) | 2 (22.2) | 0.07 | 0.01–0.47 | 0.006 | 0.10 | 0.006–1.69 | 0.11 | |
| PCI score (≥5) | 84 (42.6) | 7 (77.8) | 0.04 | 4.71 | 0.95–23.2 | 0.06 | 6.14 | 0.93–40.74 | 0.06 |
| COVID vaccine (≥ 1 dose) | 48 (24.3) | 0 (0.0) | 0.09 | ||||||
| SOT | 0.003 | ||||||||
| LT + KT | 184 (93.4) | 6 (66.7) | Ref | Ref | |||||
| LuT + HT | 13 (6.6) | 3 (33.3) | 7.08 | 1.59–31.6 | 0.01 | 6.37 | 0.96–42.06 | 0.05 | |
| Omicron period | 191 (97.0) | 8 (88.9) | 0.19 | 0.25 | 0.03–2.34 | 0.23 | 0.05 | 0.003–0.68 | 0.03 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; PCI, pediatric comorbidity index; SOT, solid organ transplantation; LT, liver transplantation; KT, kidney transplantation; LuT, lung transplantation; HT, heart transplantation; Ref, reference, COVID, coronavirus disease.
In the multivariable analysis, the COVID-19 vaccination status was not included as a variable since none of the patients in the severe group received any COVID-19 vaccine doses.
In the case of lung or heart transplantation, the risk of severe infection was significantly higher by 6.6–7.1 times (Severe II, P=0.04; Severe III, P=0.01) compared to that with liver or kidney transplantation in the univariable analysis; however, there was no significant difference in the multivariable analysis. In addition, age <5 years and a PCI score of ≥5 were identified as risk factors for severe COVID-19; however, significance was not achieved in the multivariate analysis (Table 2).
Discussion
Our findings show that pediatric SOTRs have a higher burden of severe COVID-19 than non-SOT children. To our knowledge, this is the largest comparative study evaluating the risk of severe COVID-19 in pediatric SOTRs.
We have shown that pediatric SOTRs are at a high-risk of severe COVID-19. Except for a few case reports, most previous studies of pediatric SOTRs with COVID-19 reported no or minimal risk of severe COVID-19 despite their immunocompromised status [18-21]. Matthew et al. conducted a retrospective observational study in five centers in 2021 and reported no cases requiring oxygen supplementation among 26 pediatric SOTRs with COVID-19 [22]. Canpolat et al. also reported only three cases of oxygen supplementation in 29 pediatric SOTRs with COVID-19 in Turkey [23]. This may be due to the absolute risk of severe COVID-19 in children being much lower than in adults, and an insufficient number of pediatric SOTRs in previous studies. Additionally, to assess the vulnerability of pediatric SOTRs to severe COVID-19, they must be compared with children with similar underlying comorbidities and not with adults. Our study is meaningful as it is the first to report that the burden of severe COVID-19 is 3.6–4.9 times higher in pediatric SOTRs than in non-SOT children, using the national cohort registry. This result also supports the current health authorities' guidelines recommending prioritized vaccination and targeted antivirals against COVID-19 in pediatric SOTRs as a high-risk population.
Despite our inability to demonstrate a statistically significant protective effect of the COVID-19 vaccine against severe infections in pediatric SOTR, it is notable that no severe cases were observed among SOT children who received at least one dose of vaccination against COVID-19. The limited number of vaccinated children with SOT and insufficient immunization doses for these immunocompromised children may have reduced the statistical power of our study. In Korea, the introduction of the COVID-19 vaccine for 5–11-year-olds was implemented from March 31, 2022; therefore, the vaccinated children included in our study were limited to 12–17-year-olds. Most had received two vaccine doses, which was a suboptimal primary series for these immunocompromised children. However, no severe COVID-19 cases (0.0%, 0/48) occurred in pediatric SOTRs who received the COVID-19 vaccine, whereas all severe cases occurred in the unvaccinated SOTRs. In addition, although there was no statistical significance when both the SOT and non-SOT groups were included, the rate of severe COVID-19 among vaccinated SOT children was lower than in non-vaccinated children by 21–22% (Fig. 3). This result is similar to that of a previous study that reported that hospitalization was reduced by 82.7% when two or more doses of BNT162b2 were administered to children aged 5–11 years in Singapore during the Omicron period [24]. This protective effect against severe COVID-19, despite the insufficient primary series of vaccinations for SOT children, is likely due to the maintenance of memory T cell and B cell functions, although the neutralizing antibody titers against the Omicron strain are reduced [25, 26]. Our data do not support the sufficiency of two doses of COVID-19 vaccine to avoid severe infection in children with SOT [27]. Furthermore, in pediatric SOTRs these humoral and cell-mediated responses are enhanced and maintained after the third dose of SARS-CoV-2 mRNA vaccination [28]. Therefore, it is necessary to investigate the protective effect of vaccination against severe COVID-19 in pediatric SOTRs who have been vaccinated with the third or additional dose of COVID-19 vaccine through follow-up studies.
A similar or lower COVID-19 vaccine coverage rate compared to that in non-SOT children is a worrisome finding in our study. Low adherence to COVID-19 vaccinations among children, including SOTRs, has been reported in several countries [10, 12]. Mild COVID-19 symptoms in children compared to adults may be one reason for low vaccine uptake; however, caregivers' concerns regarding COVID-19 vaccine safety and lack of confidence in the vaccine's effectiveness could also contribute to vaccine hesitancy for children [3, 10]. Zheng et al. reported that the proportion of pediatric SOTRs in China who had received at least one dose of a COVID-19 vaccine was as low as 9.4%, whereas that of their caregivers was as high as 94.8%. The major reason for vaccine avoidance in their children included concerns about vaccine safety [12]. Therefore, a proper COVID-19 vaccination campaign accompanied by credible information and education targeting these high-risk children and their caregivers is required [11, 29, 30].
This study has some limitations. Despite being a national-level cohort, it was difficult to perform subgroup analysis or logistic analysis for certain variables due to the few cumulative pediatric SOT cases, as well as cases of severe COVID-19 in children. Second, our study did not include SOTRs with COVID-19 in the early period after transplantation; therefore, the risk of severe COVID-19 may be underestimated. This study was conducted on children who underwent SOT until January 2020; however, most COVID-19 cases occurred after January 2022 in the Omicron-predominant period in Korea. Therefore, pediatric SOTRs who were stable for more than 2 years after transplantation were included. Nevertheless, our study is meaningful in that it shows that the risk of severe COVID-19 remains high in children with SOT who were stable over time after transplantation. Third, even if COVID-19 is a mandatory notifiable disease, there may be asymptomatic and mild, unreported cases. We presume that these trends were the same in both groups or occurred more often in the non-SOT control group, which could have affected the underestimation of the risk of severe infection in children with SOT. Fourth, patients re-infected with COVID-19 were not included in this study. Fifth, the low risk of severity in the pediatric population limited the evaluation of the relative risk between SOT and non-SOT children by defining severe disease as the use of supplemental oxygen or higher respiratory support according to the WHO criteria. In fact, there were no cases of severe COVID-19 in the non-SOT children based on this definition [31]. To overcome this limitation, we established multiple criteria for defining severe cases, including prolonged hospitalization. During the Omicron-predominant period in Korea, mild confirmed COVID-19 cases were managed through self-quarantine, and hospitalized patients were discharged within 5 days when no further hospital stay was necessary. Any need for extended hospitalization was evaluated by the health authorities. Therefore, we determined that prolonged hospitalization could serve as an indicator of greater disease severity as it necessitates additional medical attention [32].
Nonetheless, this is the first national big data convergence study to evaluate the risk of severe COVID-19 in pediatric SOTRs. Results show that children with SOT are at a greater risk of developing severe COVID-19 than non-SOT children. Thus, strategies should be established to increase COVID-19 vaccine uptake in these high-risk children. Additionally, follow-up studies are required in these high-risk children on the effects of additional protective measures, such as vaccination against COVID-19 and the use of targeted anti-COVID-19 treatments.
Funding
This research was supported by the Core Research Institute Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2019R1A6A1A03032869).
The funder was not involved in the study design, analysis and interpretation of data, writing of the report, or the decision to submit the study results for publication.
Data Statement
Deidentified data licensed for this analysis will be made available upon reasonable request to the corresponding author.
Author Contributions
Drs. JM Kang, J Jung, K Hub, and M Kang had full access to the study data and take responsibility for its integrity and accuracy of analysis. Drs. Kang JM and M Kang contributed equally to this study. Concept and design: JM Kang, J Jung, and K Hub, Acquisition: YE Kim, Y Choi, SJ An, J Seong, and MJ Go, Analysis: K Hub and M Kang
Statistical analysis: M Kang, Interpretation of data: all authors, Drafting of the manuscript: JM Kang and M Kang
References
[1] Huang, C., Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020. 395(10223): p. 497-506.
[2] El-Sadr, W.M., A. Vasan, and A. El-Mohandes, Facing the New Covid-19 Reality. N Engl J Med, 2023. 388(5): p. 385-7.
[3] Hani, E., M. Bertran, A. Powell, H. Williams, P. Birrell, D. DeAngelis, et al., Significantly lower infection fatality rates associated with SARS-CoV-2 Omicron (B.1.1.529) infection in children and young people: Active, prospective national surveillance, January-March 2022, England. J Infect, 2023.
[4] Bahl, A., N. Mielke, S. Johnson, A. Desai, and L. Qu, Severe COVID-19 outcomes in pediatrics: An observational cohort analysis comparing Alpha, Delta, and Omicron variants. Lancet Reg Health Am, 2023. 18: p. 100405.
[5] Funk, A.L., T.A. Florin, N. Kuppermann, D.J. Tancredi, J. Xie, K. Kim, et al., Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study. JAMA Netw Open, 2022. 5(1): p. e2142322.
[6] Bhopal, S.S., J. Bagaria, B. Olabi, and R. Bhopal, Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health, 2021. 5(5): p. e12-e3.
[7] Collaborators, C.-E.M., Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet, 2022. 399(10334): p. 1513-36.
[8] UNICEF, Child mortality and COVID-19. https://data.unicef.org/topic/child-survival/covid-19/, 2023: p. Last Access Date: Feb 28, 2023.
[9] Lin, D.Y., Y. Gu, Y. Xu, D. Zeng, B. Wheeler, H. Young, et al., Effects of Vaccination and Previous Infection on Omicron Infections in Children. N Engl J Med, 2022. 387(12): p. 1141-3.
[10] Lee, M., S. Seo, S. Choi, J.H. Park, S. Kim, Y.J. Choe, et al., Parental Acceptance of COVID-19 Vaccination for Children and Its Association With Information Sufficiency and Credibility in South Korea. JAMA Netw Open, 2022. 5(12): p. e2246624.
[11] Hoshen, M., V. Shkalim Zemer, S. Ashkenazi, Z. Grossman, M. Gerstein, N. Yosef, et al., How to increase COVID-19 vaccine uptake among children? determinants associated with vaccine compliance. Front Pediatr, 2022. 10: p. 1038308.
[12] Zheng, Z., Y. Lu, M. Wang, Y. Luo, P. Wan, T. Zhou, et al., Low COVID-19 vaccine coverage and guardian acceptance among pediatric transplant recipients. J Med Virol, 2023. 95(1): p. e28377.
[13] Chiotos, K., M. Hayes, D.W. Kimberlin, S.B. Jones, S.H. James, S.G. Pinninti, et al., Multicenter Interim Guidance on Use of Antivirals for Children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc, 2021. 10(1): p. 34-48.
[14] Vora, S.B., J.A. Englund, I. Trehan, A. Waghmare, A. Kong, A. Adler, et al., Monoclonal Antibody and Antiviral Therapy for Mild-to-Moderate COVID-19 in Pediatric Patients. Pediatr Infect Dis J, 2023. 42(1): p. 32-4.
[15] KONOS, The National Institute of Organ, Tissue and Blood Management https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_1&boardId=30, Last Access Date: March 1, 2023 (in Korean). 2020.
[16] Sun, J.W., F.T. Bourgeois, S. Haneuse, S. Hernandez-Diaz, J.E. Landon, B.T. Bateman, et al., Development and Validation of a Pediatric Comorbidity Index. Am J Epidemiol, 2021. 190(5): p. 918-27.
[17] Boyeong Ryu, E.S., Na-Young Kim, Dong Hwi Kim, HyunJu Lee, Ahra Kim, Shin Young Park, Seonhee Ahn, Jinhwa Jang, Seong-Sun Kim, Donghyok Kwon, Severity of COVID-19 Associated with SARS-CoV-2 Variants Circulating in the Republic of Korea. Public Health Weekly Report, 2022. 15(47): p. 2873-95.
[18] Freitas, A.F., R.P.S. Pugliese, F. Feier, I.K. Miura, V.L.B. Danesi, E.N. Oliveira, et al., Impact of COVID-19 Infection on Children and Adolescents after Liver Transplantation in a Latin American Reference Center. Microorganisms, 2022. 10(5).
[19] Levenson, E., T.N. Shepherd, D. Aviles, R. Craver, A. Ehlayel, G.L. Love, et al., De novo collapsing glomerulopathy in a pediatric kidney transplant recipient with COVID-19 infection. Pediatr Transplant, 2021. 25(4): p. e14013.
[20] Petters, L.M., T.P. Vogel, F.M. Munoz, J.A. Hernandez, S. Koohmaraie, M.J. Nowicki, et al., Multisystem inflammatory syndrome in children associated with SARS-CoV-2 in a solid organ transplant recipient. Am J Transplant, 2021. 21(7): p. 2596-9.
[21] Varnell, C., Jr., L.A. Harshman, C. Liu, L. Smith, S. Al-Akash, G.M. Barletta, et al., COVID-19 in pediatric kidney transplantation: a follow-up report of the Improving Renal Outcomes Collaborative. Pediatr Nephrol, 2023. 38(2): p. 537-47.
[22] Goss, M.B., N.T.N. Galvan, W. Ruan, F.M. Munoz, E.D. Brewer, C.A. O'Mahony, et al., The pediatric solid organ transplant experience with COVID-19: An initial multi-center, multi-organ case series. Pediatr Transplant, 2021. 25(3): p. e13868.
[23] Canpolat, N., Z.Y. Yildirim, N. Yildiz, M. Tasdemir, N. Goknar, H. Evrengul, et al., COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur J Pediatr, 2022. 181(1): p. 117-23.
[24] Tan, S.H.X., A.R. Cook, D. Heng, B. Ong, D.C. Lye, and K.B. Tan, Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N Engl J Med, 2022. 387(6): p. 525-32.
[25] Tarke, A., C.H. Coelho, Z. Zhang, J.M. Dan, E.D. Yu, N. Methot, et al., SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell, 2022. 185(5): p. 847-59 e11.
[26] Muik, A., B.G. Lui, A.K. Wallisch, M. Bacher, J. Muhl, J. Reinholz, et al., Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science, 2022. 375(6581): p. 678-80.
[27] Kang, J.M., J. Lee, K.H. Huh, D.J. Joo, J.G. Lee, H.R. Kim, et al., Matched Versus Mixed COVID-19 Vaccinations in Korean Solid Organ Transplant Recipients: An Observational Study. Transplantation, 2022. 106(9): p. e392-e403.
[28] Bratic, J.S., H.A. Gans, S.F. Chen, N. Banaei, E.M. Johnston, K. Sear, et al., Pediatric solid organ transplant recipients demonstrate robust cell-mediated and humoral responses to three doses of mRNA SARS-CoV-2 vaccine. Am J Transplant, 2022. 22(12): p. 3047-52.
[29] Shkalim Zemer, V., Z. Grossman, H.A. Cohen, M. Hoshen, M. Gerstein, Y. Richenberg, et al., Variables Associated With COVID-19 Vaccination Among Israeli Adolescents and the Need for Targeted Interventions. Pediatr Infect Dis J, 2022. 41(11): p. 927-32.
[30] Woodruff, R.C., A.P. Campbell, C.A. Taylor, S.J. Chai, B. Kawasaki, J. Meek, et al., Risk Factors for Severe COVID-19 in Children. Pediatrics, 2022. 149(1).
[31] Buonsenso, D., N. Parri, C. De Rose, P. Valentini, and C.-t. Gemelli-pediatric, Toward a clinically based classification of disease severity for paediatric COVID-19. Lancet Infect Dis, 2021. 21(1): p. 22.
[32] Cheng, D.R., S. Schrader, A. McMinn, N.W. Crawford, S. Tosif, S. McNab, et al., Paediatric admissions with SARS-CoV-2 during the Delta and Omicron waves: an Australian single-centre retrospective study. BMJ Paediatr Open, 2023. 7(1).
Declaration Competing of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was conducted as part of the public-private joint research on COVID-19, co-hosted by the KDCA and the NHIS. This study used the database of the KDCA and the NHIS for policy and academic research. The research number of this study is KDCA-NHIS-2022-1-532.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.06.016.
Appendix. Supplementary materials
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.