Abstract
Background
Podoplanin (PDPN gene) and CLEC-2 are involved in inflammatory hemostasis and have also been related with the pathogenesis of thrombosis. Emerging evidence also suggests that podoplanin can exert protective effects in sepsis and acute lung injury. In the lungs, podoplanin is coexpressed with angiotensin-converting enzyme 2, which is the main entry receptor for SARS-CoV-2.
Objectives
To explore the role of podoplanin and CLEC-2 in COVID-19.
Methods
Circulating levels of podoplanin and CLEC-2 were measured in 30 consecutive patients with COVID-19 admitted due to hypoxia and in 30 age- and sex-matched healthy individuals. Podoplanin expression in lungs from patients who died of COVID-19 was obtained from 2 independent public databases of single-cell RNA sequencing, from which data from control lungs were also available.
Results
Circulating podoplanin levels were lower in patients with COVID-19, whereas no difference was observed in CLEC-2 levels. Podoplanin levels were significantly inversely correlated with markers of coagulation, fibrinolysis, and innate immunity. Single-cell RNA sequencing data confirmed that PDPN is coexpressed with angiotensin-converting enzyme 2 in pneumocytes and showed that PDPN expression is lower in this cell compartment in the lungs of patients with COVID-19.
Conclusion
Circulating levels of podoplanin are lower in patients with COVID-19, and the magnitude of this reduction is correlated with hemostasis activation. We also demonstrate the downregulation of PDPN at the transcription level in pneumocytes. Together, our exploratory study questions whether an acquired podoplanin deficiency could be involved in the pathogenesis of acute lung injury in COVID-19 and warrants additional studies to confirm and refine these findings.
Keywords: acute lung injury, CLEC-2, COVID-19, podoplanin, ScRNAseq
Essentials
-
•
Evidence supports an association between podoplanin, platelet-activating protein, and thrombosis.
-
•
We measured podoplanin in patients with COVID-19 compared with healthy controls.
-
•
Patients with COVID-19 had lower podoplanin levels and lower levels correlated with hemostatic markers.
-
•
Gene expression of podoplanin was lower in pneumocytes of patients with COVID-19.
1. Introduction
COVID-19 is a respiratory disease caused by SARS-CoV-2 [1,2], whose clinical presentation ranges from asymptomatic to paucisymptomatic flu-like symptoms to severe dyspnea and hypoxemia, which can develop into a severe acute respiratory syndrome [3,4].
One of the hallmarks of COVID-19 is the intense activation of hemostasis, evidenced by both laboratory changes, such as increased levels of von Willebrand factor (VWF), platelet activation markers, and D-dimer [3,5], as well as clinical changes, such as higher risk of venous thromboembolism and the presence of pulmonary microvascular thrombosis [6,7]. This activation of hemostasis is considered part of the host response to the presence of SARS-CoV-2 in a process referred to as immunothrombosis, which when localized, can contribute to pathogen eradication but when deregulated and/or systemic, can cause secondary tissue damage [8,9]. Several cells and pathways have been shown to contribute to immunothrombosis, such as platelet and complement activation [10], intravascular expression of tissue factor in monocytes and/or microvesicles [11,12], activation of the intrinsic coagulation pathway, and the generation of neutrophil extracellular traps [13,14].
Podoplanin (PDPN gene) is a membrane glycoprotein with primary expression in the endothelium of lymphatic vessels, type-I pneumocytes, and glomerular podocytes [15,16]. Initially identified as an important mediator of lymphangiogenesis [17], podoplanin has also been shown to be important in platelet activation in the context of inflammatory hemostasis [18,19]. This function involves the platelet receptor CLEC-2, which is the only known ligand of podoplanin [20,21]. More recently, podoplanin has been involved in the pathogenesis of thrombosis, not only in animal models [19,[22], [23], [24]] but also in clinical studies that showed that expression of PDPN in brain tumors is an independent predictor of thrombotic risk [25]. Interestingly, emerging evidence also demonstrated that podoplanin is involved in other compartments of innate immune response, as suggested by its protective effect in animal models of acute lung injury and sepsis [26,27]. Interestingly, both podoplanin and angiotensin-converting enzyme 2 (ACE2), which is the main receptor by which SARS-CoV-2 invades lung epithelial cells [28], are expressed in lungs by alveolar type 1 cells [[29], [30], [31]]. Here, we explored whether levels of podoplanin and CLEC-2 were modulated in COVID-19 as well as their association with other clinical and laboratory characteristics of this condition.
2. Methods
2.1. Overall study design
This was a prospective cohort study carried out with patients admitted to an academic tertiary hospital. Samples were obtained from subjects who were enrolled in a clinical trial at our institution [32], before any study intervention, immediately after enrollment. Inclusion criteria were age over 18 years, a confirmed diagnosis of COVID-19, and need for hospital admission due to hypoxia. COVID-19 was confirmed using real time-polymerase chain reaction, and lung disease was confirmed using computerized tomography scan. Exclusion criteria were symptom onset >12 days, pregnancy, HIV infection or other immunodeficiency, severe kidney and/or liver disease, previous diagnosis of cancer, and previous history of thromboembolism. Patients were enrolled consecutively from April 23 to June 14, 2020, and all patients who fulfilled inclusion criteria were offered to participate, up to a predetermined target of 30 patients. In addition, 30 healthy, asymptomatic, age- and sex-matched individuals were recruited during the same time period in the same geographic region among health care employees of the local blood bank. The study was conducted in accordance with the Declaration of Helsinki and approved by the local institutional ethics committee (CAAE 36528420.3.0000.5404 and 30227920.9.0000.5404). Frozen serum samples from a different cohort of patients with COVID-19 were used to evaluate the effect of sample type on circulating podoplanin measurements. These patients were recruited in the same hospital from April to July 2020. Inclusion criteria were a confirmed diagnosis of COVID-19 using real time-polymerase chain reaction, requiring hospital admission associated with respiratory symptoms attributed to COVID-19. However, abnormalities in lung computerized tomography scan were not required, and patients with comorbidities, such as cancer and liver disease, were not excluded. Samples were obtained within 48 hours of admission. The institutional ethics committee also approved the use of these samples (CAAE 30709620.2.0000.5404), and healthy individuals were the same as the ones used in the first cohort, from whom frozen serum samples were also available.
2.2. Sample collection and processing
Samples were collected in EDTA (BD vacutainer) and 3.2% sodium citrate tubes (BD Vacutainer) immediately after enrollment in the clinical trial. Plasma was obtained after centrifugation at 1800 × g for 15 minutes and frozen at −80 °C until analysis. To obtain platelet free plasma, citrate tubes were centrifuged as described above, plasma was separated in a different polypropylene tube, and submitted to a second centrifugation step.
2.3. Clinical and laboratory results
Clinical and laboratory data were obtained from electronic medical records from the hospital and from case report forms from the clinical trial.
2.4. Laboratory evaluation of hemostasis
Prothrombin time, activated partial thromboplastin time, fibrinogen levels, coagulation factor (F) activities (FVIII:C, FIX:C, FX:C, FXI:C, and FXII:C), VWF antigen, ristocetin A cofactor activity, and antithrombin levels were measured in an automated coagulometer (ACL TOP 550 CTS, Instrumentation Laboratory) using commercial assays available from the same manufacturer (HemosIL reagents). The coefficient of variation of these assays is shown in Supplementary Table 2. The urokinase-type plasminogen activator receptor, P-selectin, and plasminogen activator inhibitor-1 values were obtained by a Luminex assay. D-dimer was measured using an immunoturbidimetric assay (Innovance D-Dimer, Siemens Healthcare).
2.5. Podoplanin and CLEC-2 levels
Podoplanin and CLEC-2 levels were measured in EDTA plasma using commercial enzyme-linked immunosorbent assay (ELISA) Kits (Sigma Aldrich, Cat. RAB 1632-1KT and Cat. RAB 1374-1KT, respectively). PDPN levels were also measured in the same plasma samples using an alternative ELISA Kit (Raybiotech, Cat. ELH-PDPN). Optical density was measured at 450 nm in a plate reader (Thermo Scientific – Multiskan GO). Both assays used for podoplanin measurement are based on the same methodological principle, and the reported interassay and intra-assay coefficients of variance are <12% and <10%, respectively. These values were obtained from the package insert and were not validated in our laboratory.
2.6. Bioinformatic analysis of single-cell RNA sequencing data
Public single-cell RNA sequencing (scRNAseq) data were used for bioinformatic analysis. For the analysis of alveolar cells from healthy donors, a meta-analysis consensus of the healthy human lung was used [33]. As previously described, approximately 130,000 cells that had their transcriptome sequenced were obtained from 3 different studies and integrated using canonical correlation analysis and were classified into cell types through semisupervized analysis by anchor-based label transfer with the Seurat v3 package [34] in the R programming language. Such analysis aims to remove laboratory-specific noise and reinforce common shared biological signals across data sets. Gene expression values (normalized expression) were then visualized through heatmaps and embeddings.
For the analysis of data from patients with COVID-19, 2 different sources from the COVID-19 Cell Atlas were used [35,36]. Both data sets were analyzed with Scanpy [37] in the Python programming language, following the default workflow. First, total gene expression was normalized by count number. Next, expression value was variance-stabilized with a log transformation; highly variable genes with high variance and mean expression were then selected for downstream analysis with principal component analysis for neighborhood graph estimation. Neighborhood graphs were then clustered with the Leiden graph partitioning algorithm and visualized with uniform manifold approximation and projection [38]. Original cell-type labels were retained and used in visualizations. Heatmaps, embeddings, and violin plots of normalized gene expression were generated within Scanpy using default parameters for plotting functions. All source code used for the analysis is available upon request.
2.7. Statistical analysis
Quantitative data were expressed as median, percentiles, and mean and SD. For comparison between groups and experiments, variables were treated as nonparametric and parametric, and comparisons were made using the Mann–Whitney U-test and t-test, respectively. Correlations were evaluated by Spearman’s test. Statistical significance was considered when P < .05. All statistical analyses were performed using Statistical Package for Social Sciences version 26 (IBM) or GraphPad Prism 8.0 software (GraphPad).
3. Results
In total, 30 consecutive patients were enrolled on the basis of the inclusion or exclusion criteria, as shown in the study flowchart (Supplementary Figure 1). The clinical and laboratory characteristics of the study population have been described previously [39] and are summarized in Table 1. Of note, at the time of enrollment, no patient had been exposed to any experimental treatment or to full-dose anticoagulation. Thromboprophylaxis with enoxaparin (40 mg to 1 mg/kg once a day) was used in all patients according to institutional protocols, which was initiated before sample collection in 15 patients (maximum 1 dose) and after sample collection in 15 patients. No demographic or outcome difference was observed between these 2 subgroups (data not shown).
Table 1.
Hematological and clinical characteristics of study participants.
| Characteristics | Patients (n = 30) | Healthy individuals (n = 30) | P value |
|---|---|---|---|
| Agea | 52.7 ± 12.3 | 50.3 ± 9.2 | .40 |
| Sex, male:female | 16:14 | 16:14 | 1.00 |
| Body mass indexa | 30.6 ± 6.6 | 25.9 ± 4.2 | .006 |
| Hemoglobina, g/dL | 13.96 ± 1.91 | 14.30 ± 1.11 | .42 |
| Leukocytesa,b, 109/L | 8.04 ± 3.91 | 5.58 ± 1.58 | .004 |
| Neutrophilsa,b, 109/L | 6.38 ± 3.77 | 3.09 ± 0.93 | <.001 |
| Lymphocytesa,b, 109/L | 1.20 ± 0.55 | 1.79 ± 0.28 | <.001 |
| Monocytesa,b, 109/L | 0.51 ± 0.30 | 0.34 ± 0.11 | .05 |
| Plateletsa,b, 109/L | 216.33 ± 93.02 | 245.59 ± 40.34 | .12 |
| NLRa | 6.19 ± 4.26 | 1.72 ± 0.61 | <.001 |
| D-dimerc, ng/mL | 759 (625-1197) | 243 (150-508) | <.001 |
| C-reactive proteinc, mg/L | 96.3 (53.1-157) | 2.0 (1.0-4.0) | <.001 |
| Troponina, ng/mL | 10.96 ± 11.98 | 4.33 ± 2.74 | .004 |
| Lactatea, mmol/L | 1.44 ± 0.51 | - | - |
| Time from symptom onseta, d | 8.1 ± 2.3 | - | - |
| CT scorea | 17.8 ± 7.3 | - | - |
| O2 saturationa,b | 95.24 ± 2.86 | - | - |
| Need for mechanical ventilation | 9/30 (30%) | - | - |
| Venous thromboembolism | 4/30 (13.3%) | - | - |
| SOFA score | 0.93 ± 0.57 | - | - |
| Mortality | 2/30 (6.7%) | - | - |
| Length of staya, d | 12.9 ± 9.8 | - | - |
| Total disease timea, d | 19.8 ± 8.5 | - | - |
| Need for intensive care, yes (%) | 12/30 (40%) | - | - |
| Length of intensive care stayc, d (n = 12) | 15.5 (4.2-25.2) | - | - |
No patients used extracorporeal membrane oxygenation; all samples were obtained before mechanical ventilation was initiated.
CT, computed tomography; NLR, neutrophil:lymphocyte ratio; O2, oxygen; SOFA, sequential sepsis-related organ failure assessment.
Mean ± SD.
After admission.
Median (IQR).
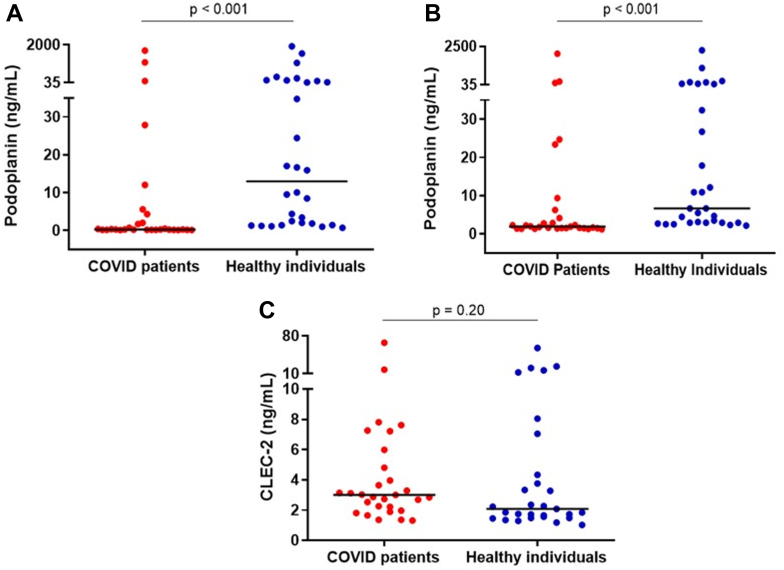
Results of ELISA assays using kits from 2 different manufacturers showed that median podoplanin levels were lower in patients with COVID-19 than in healthy individuals (Figure 1A, B). The Spearman correlation coefficient between results of these 2 assays was 0.954, P < .001. Of note, there was a large range of circulating podoplanin levels in both patients and healthy controls, but fewer of the patients circulated higher podoplanin levels. In contrast, CLEC-2 levels did not differ between patients and healthy controls (Figure 1C).
Figure 1.
(A) Plasma levels of podoplanin in patients and healthy individuals. Medians are depicted as horizontal bars; P values are from Mann-Whitney U-test (n = 29-30 per group). (B) Plasma levels of podoplanin in patients and healthy individuals measured with an enzyme-linked immunosorbent assay from a different manufacturer. Medians are depicted as horizontal bars; P values are from Mann-Whitney U-test (n = 29-30 per group). The size of the difference of plasma podoplanin levels between patients and healthy individuals measured with these 2 assays were 12.69 (95% CI, 1.59-24.14) and 4.77 ng/mL (95% CI, 1.24-9.90), respectively. (C) Plasma levels of CLEC-2 in patients and healthy individuals. Medians are depicted as horizontal bars; P values are from Mann-Whitney U-test (n = 29-30 per group).
Regarding the association of podoplanin levels with clinical outcomes, median podoplanin levels were significantly lower in patients who required intensive care unit (ICU) (0.22; IQR, 0.18-0.30) than in patients who did not require ICU (0.64; IQR, 0.23-7.23). There was no association or correlation of PDPN levels with other relevant clinical outcomes. CLEC-2 levels did not correlate with clinical outcomes.
In contrast, a consistent correlation of podoplanin levels with laboratory markers of disease severity and hemostatic activation was observed, with lower podoplanin levels associated with higher levels of these biomarkers. The magnitude of this correlation was higher for D-dimer, C-reactive protein, and VWF levels and activity. Of note, CLEC-2 levels did not correlate with any of these biomarkers except with platelet counts and PDPN levels (Table 2).
Table 2.
Correlation analyses of clinically and laboratory-relevant endpoints.
| Variable | Correlation variable | Correlation coefficient | P value |
|---|---|---|---|
| Podoplanin | D-dimer CRP Troponin NLR Neutrophil VWF:Ag RistCof FVIII FXI Fibrinogen uPar CLEC-2 |
−0.529 −0.517 −0.348 −0.481 −0.380 −0.513 −0.530 −0.374 −0.361 −0.401 −0.363 0.344 |
<.001 <.001 .007 <.001 .005 <.001 <.001 .003 .006 .002 .004 .008 |
| CLEC-2 | Platelets P-selectin Podoplanin |
0.279 0.149 0.344 |
.04 .27 .008 |
Correlation coefficients (Spearman, according to data distribution) were calculated using data from all participants (patients and healthy individuals). Correlation coefficients that yielded statistically nonsignificant results are reported in Supplementary Table 1.
CRP, C-reactive protein; F, factor; NLR, neutrophil:lymphocyte ratio; PAP, plasmin-antiplasmin complexes; RistCof, ristocetin cofactor activity; uPar, urokinase-type plasminogen activator receptor; VWF:Ag, von Willebrand factor:antigen.
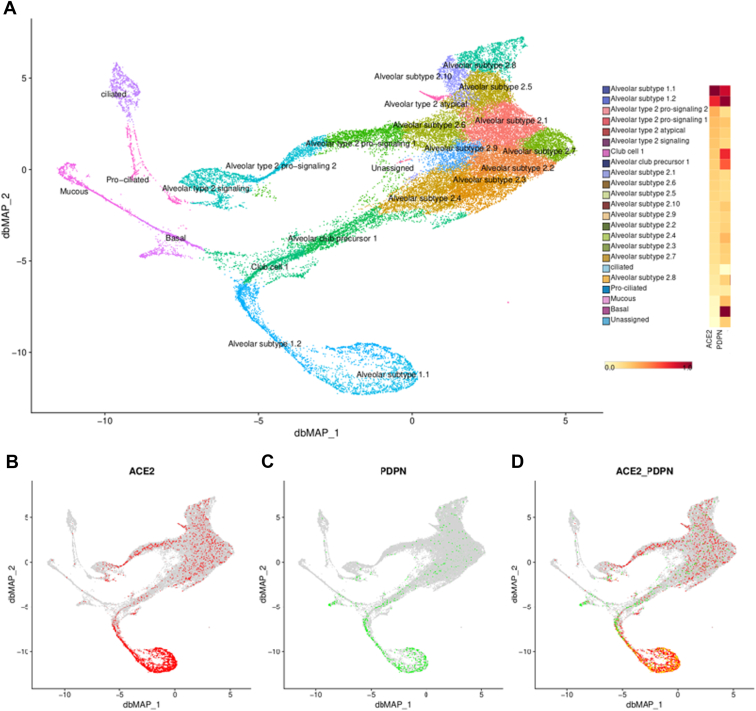
To gain further insights into the association of lower podoplanin levels with COVID-19, we explored the gene expression of PDPN in public databases of scRNAseq of lung cells. We first confirmed that PDPN and ACE2, which codes for the receptor that SARS-CoV-2 uses to invade lung cells, are coexpressed in subtype 1.1 and 1.2 pneumocytes of healthy individuals (Figure 2).
Figure 2.
Gene expression of PDPN and ACE2 in lungs from healthy individuals. (A) In the left panel, the subdivision of cell types are shown, with the relative levels of PDPN and ACE2 expression shown in the right column, confirming the coexpression of these genes, mainly in pneumocytes of subtype 1.1 and 1.2. In the lower panels, the expression of ACE2 (red) and (C) PDPN (green) are highlighted, showing coexpression in the same cell types (D). ACE2, angiotensin-converting enzyme 2; PDPN, podoplanin.
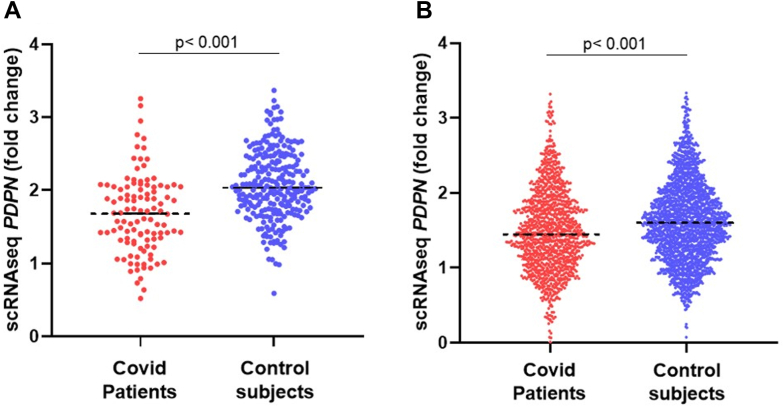
In addition, we also analyzed PDPN expression in lung tissues from patients with COVID-19 using scRNAseq data from 2 independent cohorts of patients. Lower PDPN expression in lung cells from patients who died from COVID-19 was confirmed in both cohorts [35,37], which included 10 and 32 patients, respectively (Figure 3).
Figure 3.
Gene expression of PDPN from scRNAseq databases of 2 independent cohorts. Each point represents an individual cell from lungs of COVID-19 patients or control subjects. Cohort A (A) included 10 patients with COVID-19 and 7 control subjects, represented by 108 and 257 cells, respectively. Cohort B (B) included 32 patients with COVID-19 and 37 control subjects, represented by 1174 and 2039 cells, respectively. Expression levels were compared using t-test. ACE2, angiotensin-converting enzyme 2; PDPN, podoplanin; scRNAseq, single-cell RNA sequencing.
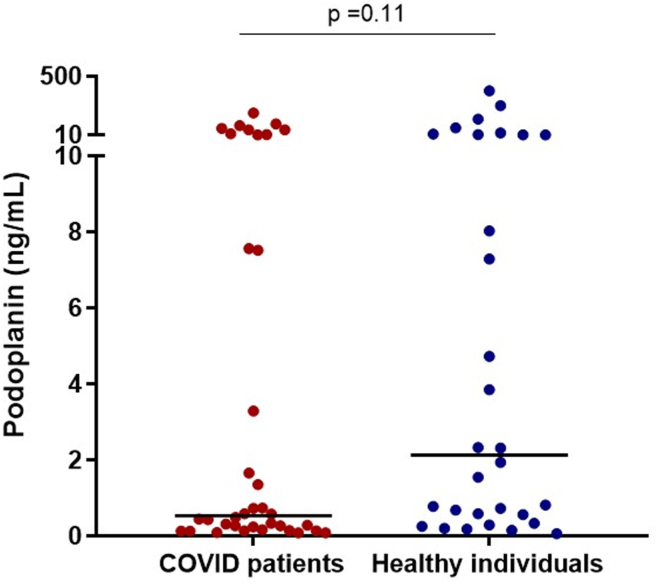
Finally, in order to explore whether the wide distribution of circulating podoplanin levels in both patients and healthy individuals could be attributed to residual platelets in plasma samples and to confirm our results, podoplanin levels were measured using the same ELISA method in frozen serum samples from an independent cohort. Although inclusion and exclusion criteria used for this cohort presented some marked differences, results still presented a skewed distribution, with a lower range of values. Moreover, a trend toward lower podoplanin level was observed (Figure 4), and podoplanin levels were also inversely correlated with D-dimer (Rs = −0.259, P = .03).
Figure 4.
Serum levels of podoplanin in patients and healthy individuals. Medians are depicted as horizontal bars; P values are from Mann-Whitney U-test (n = 30-36 per group).
4. Discussion
Concomitant activation of innate immunity and hemostasis, known as immunothrombosis, is considered an important element in the pathogenesis of COVID-19 and a potential source of novel biomarkers and therapeutic targets. The main contribution of our study was the demonstration that levels of podoplanin, a protein that is involved in platelet activation during inflammation and believed to exert protective effects in models of acute inflammation, are decreased in patients with COVID-19 and that the magnitude of this modulation is at least partially associated with laboratory markers of immunothrombosis in these patients.
Several lines of evidence support that the podoplanin/CLEC-2 pathway is associated not only with platelet activation but also with the pathogenesis of thrombosis. In vivo studies performed in animal models demonstrated that hepatic thrombus formation induced by Salmonella typhimurium infection is dependent on the podoplanin/CLEC-2 pathway [19]. In addition, CLEC-2 deficiency protected, while podoplanin promoted venous thrombosis in different mice models [[22], [23], [24]]. The association of podoplanin with thrombosis and hypercoagulability was also observed in humans. In patients with glioblastoma multiforme, individuals with high expression of PDPN were 5.71 times more likely to develop venous thromboembolism [25]. Interestingly, podoplanin and CLEC-2 have also been reported to present anti-inflammatory effects in animal models of inflammation. A study using 2 different models of sepsis demonstrated that CLEC-2 deficiency was associated with enhanced inflammation and that anti-PDPN antibody mimicked this phenotype [40]. More recently, the anti-inflammatory effects of this pathway in sepsis models were explained by the release of complement inhibitors by monocytes induced by podoplanin [41]. A protective effect of the podoplanin/CLEC-2 pathway was also demonstrated in a model of acute lung injury based on the intratracheal instillation of lipopolysaccharide, in which lung injury was significantly higher in mice with CLEC-2 or podoplanin deficiency [26,27]. Together, these data raise the hypothesis that podoplanin could be involved in both the inflammatory and prothrombotic state observed in patients with COVID-19.
In our study, podoplanin levels were lower in patients than in healthy individuals. Moreover, lower podoplanin levels were associated with ICU needs. These findings are in contrast with studies in patients with cancer, in which higher podoplanin levels were associated with adverse outcomes [25,42]. Importantly, we confirmed our ELISA findings using 2 different commercial reagent kits. Moreover, we also validated these findings using public databases of scRNAseq of lung tissues, in which PDPN gene expression was also lower in patients with COVID-19. If these data are confirmed in independent studies, acquired podoplanin deficiency could be a novel pathogenic element of acute lung injury in COVID-19. The mechanisms underlying the downregulation of PDPN in COVID-19 are yet to be determined. ACE2, which is the receptor used by SARS-CoV-2 to invade lung cells, is coexpressed with PDPN in pneumocytes [29], which could explain the decrease in podoplanin levels in both plasma and tissues. Given the preclinical evidence of the anti-inflammatory effects of PDPN in sepsis and in lipopolysaccharides-induced acute lung injury [26,41], the possibility that this relative deficiency of podoplanin could be playing a role in the pathogenesis of COVID-19 warrants additional studies.
A consistent and relevant finding of our study was the inverse correlation between podoplanin levels and biomarkers of coagulation and fibrinolysis activation. While the role of podoplanin as a mediator of platelet activation is well-established, increasing evidence demonstrates that it could also be involved in the pathogenesis of thrombosis in inflammatory conditions such as infections and cancer [19,25]. In this context, our data showing the modulation of podoplanin in COVID-19, coupled with its association with biomarkers of hemostatic activation, allow us to consider podoplanin as an additional element of the immunothrombotic host response to COVID-19. Again, further studies are warranted to refine our understanding on how modulation of podoplanin could influence the local and systemic hemostatic balance.
Our study has limitations that need to be considered. First, sample size is relatively limited. However, we believe that the fact that samples were obtained from individuals consecutively enrolled in a clinical trial at least partially compensated for this limitation since recruitment followed the consecutive screening of all eligible patients, limiting selection bias, and quality of both data and samples were guaranteed by the more structured routines of a randomized clinical trial. A second limitation is the exploratory nature of our study, which was not designed to identify the cellular and molecular mechanisms of the lower podoplanin levels in COVID-19. As previously mentioned, additional studies are warranted to explore these findings. It should also be mentioned that since recruitment occurred during the first phase of the pandemic, our results cannot necessarily be extrapolated to the current epidemiologic phase. However, our main objective to explore potentially novel elements in the pathogenesis of acute lung injury using a population of patients recruited before vaccination was available, when evolvement to hypoxia and ICU requirement was still relatively common, can also be viewed as an attractive characteristic. A third and important limitation is the relatively high skewness of podoplanin levels among patients and healthy individuals. Few studies have measured circulating podoplanin levels, mainly in patients with cancer, and it is possible that variables, such as sample type (serum vs plasma) and processing, may influence levels of this biomarker, similar to other biomarkers that are either stored or interact with platelets [43]. This limitation was partially addressed by measuring podoplanin levels in serum obtained from a second cohort of patients, whose results demonstrated similar data skewness, with a lower variation range and a trend toward lower podoplanin values. These results suggest that other yet unknown factors contribute to a skewed distribution of podoplanin levels when measured in plasma or serum, which can influence the performance of this biomarker. Accordingly, our results should be viewed as a first step toward understanding the kinetics of this biomarker in conditions associated with immunothrombosis. Finally, 2 minor limitations should be acknowledged. First, although we are not aware that circadian variations influence podoplanin levels, the fact that samples were obtained at different times of the day (following recruitment) may have influenced our results. Second, race/ethnicity was not recorded. Brazil is known for its highly racially admixed populations. Nonetheless, the lack of this information should be considered when interpreting our data.
In conclusion, our results demonstrate that podoplanin levels are modulated in COVID-19, being reduced both at protein level in plasma and the transcriptional level in pneumocytes. The correlation of the magnitude of podoplanin downregulation with markers of coagulation and fibrinolysis activation warrants additional studies to confirm and further explore the hypothesis that an acquired podoplanin deficiency could contribute to the pathogenesis of COVID-19.
Acknowledgments
Funding
This study was funded by the Sao Paulo Research Foundation grants 2016/14172-6, 2020/05985-9, and 2020/05369-6; FAEPEX-Unicamp grant 2404/2020; and Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior–Brasil, finance code 001.
Author contributions
I.T.B.J. performed podoplanin and CLEC-2 assays, contributed to data analysis, and drafted and revised the manuscript. F.L. obtained and processed samples, performed coagulation factor assays, contributed to data analysis, and revised the manuscript. C.R.P.M. obtained and processed samples, performed multiplex assays, and revised the manuscript. D.S.O. performed scRNAseq analysis and revised the manuscript. J.M.A. provided laboratory support and infrastructure for classical coagulation assays and revised the manuscript. E.M. and L.A.V. designed and conducted the clinical trial from which patients were recruited and revised the manuscript. F.T.M.C., F.A.O., and M.L.M. contributed to the study design and data analysis and revised the manuscript. B.B. and A.C.P. were involved in patient recruitment and revised the manuscript. E.V.D.P. designed the study, obtained and processed samples, oversaw and provided resources and infrastructure for enzyme-linked immunosorbent assay analysis, contributed to data analysis, drafted and revised the manuscript. All authors read and approved the final version of the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Kristen Sanfilippo
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100282
Supplementary material
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-jie, Z-yi Ni, Hu Y., Liang W.-hua, Ou C.-quan, He J.-xing, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am J Hematol. 2020;95:1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 9.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verschoor A., Langer H.F. Crosstalk between platelets and the complement system in immune protection and disease. Thromb Haemost. 2013;110:910–919. doi: 10.1160/TH13-02-0102. [DOI] [PubMed] [Google Scholar]
- 11.Osterud B., Flaegstad T. Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb Haemost. 1983;49:5–7. [PubMed] [Google Scholar]
- 12.Grover S.P., Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 13.Noubouossie D.F., Reeves B.N., Strahl B.D., Key N.S. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133:2186–2197. doi: 10.1182/blood-2018-10-862243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooberry M.J., Bradford R., Hobl E.L., Lin F.C., Jilma B., Key N.S. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J Thromb Haemost. 2016;14:1031–1042. doi: 10.1111/jth.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breiteneder-Geleff S., Matsui K., Soleiman A., Meraner P., Poczewski H., Kalt R., et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 16.Rishi A.K., Joyce-Brady M., Fisher J., Dobbs L.G., Floros J., VanderSpek J., et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 17.Wetterwald A., Hoffstetter W., Cecchini M.G., Lanske B., Wagner C., Fleisch H., et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 18.May F., Hagedorn I., Pleines I., Bender M., Vögtle T., Eble J., et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 19.Hitchcock J.R., Cook C.N., Bobat S., Ross E.A., Flores-Langarica A., Lowe K.L., et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Invest. 2015;125:4429–4446. doi: 10.1172/JCI79070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y., Fujita N., Kunita A., Sato S., Kaneko M., Osawa M., et al. Molecular identification of Aggrus/T1α as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki-Inoue K., Kato Y., Inoue O., Kaneko M.K., Mishima K., Yatomi Y., et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 22.Shirai T., Inoue O., Tamura S., Tsukiji N., Sasaki T., Endo H., et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. 2017;15:513–525. doi: 10.1111/jth.13604. [DOI] [PubMed] [Google Scholar]
- 23.Payne H., Ponomaryov T., Watson S.P., Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129:2013–2020. doi: 10.1182/blood-2016-09-742999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasano T., Gonzalez-Delgado R., Muñoz N.M., Carlos-Alcade W., Cho M.S., Sheth R.A., et al. Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer. J Thromb Haemost. 2022;20:104–114. doi: 10.1111/jth.15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedl J., Preusser M., Nazari P.M., Posch F., Panzer S., Marosi C., et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129:1831–1839. doi: 10.1182/blood-2016-06-720714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lax S., Rayes J., Wichaiyo S., Haining E.J., Lowe K., Grygielska B., et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1016–L1029. doi: 10.1152/ajplung.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X., Chen S., Li Y., Liang L., Chen H., Wen T. Dysfunctional O-glycosylation exacerbates LPS-induced ARDS in mice through impairment of podoplanin expression on alveolar macrophages. Mol Immunol. 2022;152:36–44. doi: 10.1016/j.molimm.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inde Z., Croker B.A., Yapp C., Joshi G.N., Spetz J., Fraser C., Deskin B., et al. Age-dependent regulation of SARS-CoV-2 cell entry genes and cell death programs correlates with COVID-19 disease severity. Sci Adv. 2021;7 doi: 10.1126/sciadv.abf8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astarita J.L., Acton S.E., Turley S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immun. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez M.I., Millien G., Hinds A., Cao Y., Seldin D.C., Williams M.C. T1α, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 32.Mansour E., Palma A.C., Ulaf R.G., Ribeiro L.C., Bernardes A.F., Nunes T.A., et al. Safety and outcomes associated with the pharmacological inhibition of the kinin–kallikrein system in severe COVID-19. Viruses. 2021;13:309. doi: 10.3390/v13020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidarta-Oliveira D., Jara C.P., Ferruzzi A.J., Skaf M.S., Velander W.H., Araujo E.P., et al. SARS-CoV-2 receptor is co-expressed with elements of the kinin–kallikrein, renin–angiotensin and coagulation systems in alveolar cells. Sci Rep. 2020;10:19522. doi: 10.1038/s41598-020-76488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballestar E., Farber D.L., Glover S., Horwitz B., Meyer K., Nikolić M., et al. Single cell profiling of COVID-19 patients: an international data resource from multiple tissues. medRxiv. 2020;2020 doi: 10.1101/2020.11.20.20227355. 11.20.20227355. [DOI] [Google Scholar]
- 36.COVID-19 cell atlas. 2020. https://www.covid19cellatlas.org/
- 37.Melms J.C., Biermann J., Huang H., Wang Y., Nair A., Tagore S., et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595:114–119. doi: 10.1038/s41586-021-03569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McInnes L, Healy J, Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiv 2018;arXiv:1802.03426. 10.48550/arXiv.1802.03426 [DOI]
- 39.Henderson M.W., Lima F., Moraes C.R.P., Ilich A., Huber S.C., Barbosa M.S., et al. Contact and intrinsic coagulation pathways are activated and associated with adverse clinical outcomes in COVID-19. Blood Adv. 2022;6:3367–3377. doi: 10.1182/bloodadvances.2021006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayes J., Lax S., Wichaiyo S., Watson S.K., Di Y., Lombard S., et al. The podoplanin-CLEC-2 axis inhibits inflammation in sepsis. Nat Commun. 2017;8:2239. doi: 10.1038/s41467-017-02402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Z., Shao B., Hoover C., McDaniel M., Song J., Jiang M., et al. Monocyte upregulation of podoplanin during early sepsis induces complement inhibitor release to protect liver function. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X., Xu M., Zhao X., Shen F., Ruan C., Zhao Y. The detection of plasma soluble podoplanin of patients with breast cancer and its clinical signification. Cancer Manag Res. 2020;12:13207–13214. doi: 10.2147/CMAR.S281785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb N.J.A., Bottomley M.J., Watson C.J., Brenchley P.E.C. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 1998;94:395–404. doi: 10.1042/cs0940395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.