Abstract
The Coronavirus (COVID-19) Disease Pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has affected millions of people worldwide, prompting a collective effort from the global scientific community to develop a vaccine against it. This study purports to investigate the influence of factors such as sex, age, type of vaccination (Comirnaty, BNT162b2, Pfizer Inc. or Vaxzevria, ChAdOx1-S, Oxford/AstraZeneca), and time since vaccine administration on the process of antibody production. Both of them are based on the introduction of SARS-CoV-2 spike protein (S protein) to the body using different mechanisms (mRNA and recombinant adenovirus, respectively). S protein is responsible for host cell attachment and penetration via its receptor-binding domain (RBD domain). The level of anti-RBD IgG antibodies was tested with an ELISA-based immunodiagnostic assay in serum samples from a total of 1395 patients at 3 time points: before vaccination, after the first dose, and after the second dose. Our novel statistical model, the Generalized Additive Model, revealed variability in antibody production dynamics for both vaccines. Interestingly, no discernible variation in antibody levels between men and women was found.
A nonlinear relationship between age and antibody production was observed, characterized by decreased antibody levels for people up to 30 and over 60 years of age, with a lack of correlation in the middle age range. Collectively, our findings further the understanding of the mechanism driving vaccine-induced immunity. Additionally, we propose the Generalized Additive Model as a standardized way of presenting data in similar research.
1. Introduction
In 2019, a pneumonia outbreak of an obscure cause started spreading worldwide, becoming the highest-priority global threat. A new type of coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the aetiological agent [1], [2].Fig. 1
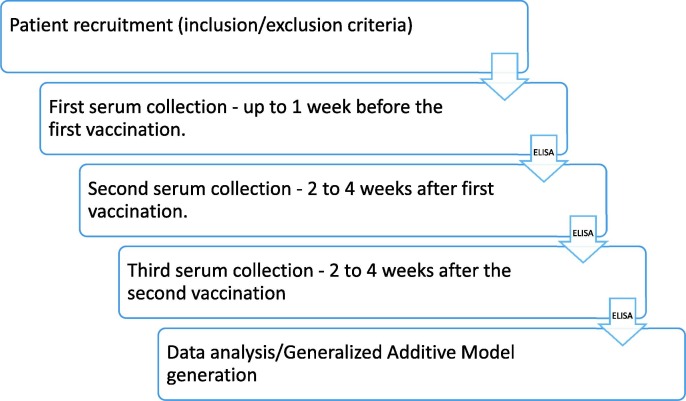
Fig. 1.
A diagram showing the outline of our study.
The symptomatic spectrum of SARS-CoV-2 infection varies from flu-like symptoms, through characteristic temporal ansomy to a multisystem organ failure and death [3], [4]. Widely adopted countermeasures against SARS-CoV-2 transmission such as physical distancing, face masks, and mucosal protection turned out insufficient in preventing the COVID-19 pandemic [5]. Therefore, a vaccination program along with the maintenance of sanitary restrictions has been implemented worldwide [6]. Multiple vaccines against SARS-CoV-2 have been deployed and administered expeditiously to immunize patients and achieve herd immunity.
The use of the polymerase chain reaction and the evaluation of antibody-mediated immunity became the gold standard in molecular diagnostics of the SARS-CoV-2 infection. Although nowadays reverse transcription-polymerase chain reaction is a commonly used molecular diagnostics tool, it is a labor-intensive and intrinsically complex assay that necessitates a relatively high level of expertise. Due to increased testing rates, more laboratory staff was required to be trained at the early stage of the pandemic. Well-trained, but inexperienced laboratory personnel poses a heightened risk of sample cross-contamination leading to false-positive results, which was a crucial issue in COVID-19 testing [7]. Various immunoassay-based systems quantifying specific anti-SARS-CoV-2 antibodies were successfully used to estimate the humoral immune response against the contagion. Numerous studies have assessed the performance of anti-SARS-CoV-2 antibody binding assays based on different target antigens in patients [8], [9], [10], [11]. Testing against various isotypes of anti-SARS-CoV-2 antibodies is a useful prognostic and diagnostic tool for evaluating treatment regimens or diagnosing non-symptomatic patients. The majority of immunoassays are designed for the detection of IgG antibodies. This class of antibodies protects against SARS-CoV-2 infection and is crucial from the standpoint of herd immunity [12].
For the purpose of anti-SARS-CoV-2 antibody detection, the most commonly used antigens are the surface S1-protein (spike protein), and inner nucleocapsid protein (N protein). In plasma, the neutralizing activity of antibodies directed against SARS-CoV-2 is strongly related to the level of IgG and IgA class antibodies recognizing the receptor binding domain (RBD) of the spike protein [12], [13], [14]. RBD is a highly conserved, immunogenic subdomain of S1-protein responsible for virion-cell interaction and subsequent entrance into the host cell. Previous studies have reported that immunoassays targeting RBD, detecting the IgG class of antibodies, showed the highest sensitivity and specificity [9], [15].
Age, male sex, seronegativity, and comorbidities were found to be associated with reduced humoral immune response. These findings indicate the significance of antibody response surveillance following vaccination to ensure fair access to vaccinations, develop more effective vaccination regimens, and underline the importance of administering booster injections in order to lessen the consequences of waning immunity [16], [17], [18]. The research focused on two different types of vaccines: Comirnaty (BNT162b2, Pfizer Inc.) and Vaxzevria (ChAdOx1-S, Oxford/AstraZeneca) with regard to sex, age, and time after vaccination. Comirnaty is a nucleoside-modified RNA vaccine formulated into lipid nanoparticles. The RNA encodes the SARS-CoV-2 full-length S-protein in a prefusion, stabilized state [19], whereas the Vaxzevria consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS-CoV-2 S-protein gene [20]. Based on the obtained data the Generalized Additive Model was used to describe the serological response, providing insights into the dynamics of immune response and immunization efficiency with respect to tested factors, for both abovementioned vaccines. To our knowledge, this is the first large cohort study utilizing the Generalized Additive Model for the investigation of their efficiency.
2. Materials and methods
2.1. Ethics statement
The Study has been approved by the Bioethics Committee of the Poznan University of Medical Sciences on June 17, 2020 (no. 471/20). Patients signed informed consent before recruitment into the study. Approval from the Ethical Committee was granted before starting the study.
2.2. Scope and limitations of the study
The study was designed to monitor the immune response after vaccination with different vaccines against SARS-CoV-2 approved in Poland – Comirnaty, (BNT162b2 Pfizer, Inc.) and Vaxzevria (ChAdOx1-S, Oxford/AstraZeneca), Jcovden (Ad26.COV2-S, J&J), and Spikevax (mRNA-1273, Moderna) in the population of Wielkopolska voivodeship, Poland (population of 3.5 mln people) and provide a statistical model for the immune response regarding gender, age and type of vaccine. Patients were enrolled at the Provincial Specialist Hospital for Lung Diseases and Tuberculosis in Wolica (referred to as ‘Wolica’) and among students, academic staff, and their relatives from Adam Mickiewicz University in Poznań (referred to as ‘AMU’), according to the inclusion and exclusion criteria (Table 1 ).
Table 1.
Table representing inclusion and exclusion criteria for the study.
| Inclusion | Exclusion |
|---|---|
|
|
The samples were taken at three time points defined as pre-vaccination, after the 1st dose, and after the 2nd dose, over a period of eight months (March 2021 to October 2021). A two-week serological window period was taken under consideration while sampling after the 1st and the 2nd dose of vaccination. Patients were divided according to age groups (18–25, 26–45, 46–64, 65+), sex, and type of received vaccine. Blood samples from a total of 1,395 patients were eventually included into the analysis (inclusion/exclusion criteria were met and at least 3 serum samples were collected at the specified time of the study). Amongst them, 1,174 participants were enrolled in Wolica group, and 221 samples originated from AMU group. A detailed information regarding the demography of enrolled patients are presented in Table 2 . Number of samples collected from individuals who received Jcovden (Ad26.COV2-S, J&J) and Spikevax (mRNA-1273, Moderna) vaccination were insufficient to provide reliable statistical analysis, therefore this data had to be excluded from the analysis. Other limitation of our study was age – during the sample acquisition period, in Poland the vaccines were approved only for adults (over 18 years old). We were gathering samples from two facilities which was a limiting factor for the number of participants – only persons who met our criteria, wanted to be vaccinated with two doses and voluntarily donate the blood sample in specified intervals could be included in the study. Cellular and humoral response, are both interacting mechanisms of immunity. Our cohort study aimed to evaluate the humoral immune response after each dose of vaccination, specifically by measurement of generated antibody levels. It would be appropriate to examine the cellular response as well in order to advance the knowledge of the immune response, however, such tests are substantially more expensive and time-consuming than standard serological tests. These requirements are crucial, especially in research investigating numerous study samples like the ones below.
Table 2.
Table representing the number of patients enrolled in the study, with regard to sex, age, and type of vaccination.
| AMU |
WOLICA |
TOGETHER |
|
|---|---|---|---|
|
TOTAL SAMPLES (%) | |||
| 221 (100%) | 1,174 (100%) | 1,395 (100%) | |
| SEX | |||
| MALE | 77 (34.84%) | 474 (40.37%) | 551 (39.50%) |
| FEMALE | 144 (65.16%) | 700 (59.63%) | 844 (60.50%) |
| AGE GROUPS | |||
| 18–25 | 2 (0.9%) | 95 (8.1%) | 97 (6.95%) |
| 26–45 | 119 (53.85%) | 317 (27%) | 436 (31.26%) |
| 46–64 | 84 (38.01%) | 416 (35.43%) | 500 (35.84%) |
| 65+ | 16 (7.24%) | 346 (29.47%) | 362 (25.95%) |
| TYPE OF VACCINE | |||
| COMIRNATY | 37 (16.74%) | 1,042 (88.76%) | 1,079 (77.35%) |
| VAXZEVRIA | 184 (83.26%) | 132 (11.24%) | 316 (22.65%) |
2.3. Sample collection
The whole blood was aspirated into the syringe with a blood clotting activator (S-Monovette® Serum, Sarstedt). After 30 min, the samples were centrifuged at 3500g, 5 min, RT, and the resulting sera were aliquoted before storage. The aliquots were stored at –20 °C and analyzed within one month. One freezing/thawing cycle was applied before testing.
2.4. Antigen production
The antigen used in this study was the receptor binding domain (RBD) of native (WuHan-Hu-1) SARS-CoV-2 S-protein (anti-RBD). The antigen was produced based on the previously described methodology, with modifications [21]. Briefly, the RBD coding sequence, flanked with the signal peptide coding sequence at 5′-end and 6xHis-Tag at 3′-end, was cloned into pCAGGS expression plasmid. Expi293F cell line (ThermoFisher Scientific), a modified HEK293 line, optimized for production of protein exported outside the cell to the cell culture medium, were transfected with expression vector using ExpiFectamine™ reagent (ThermoFisher Scientific) according to the manufacturer’s protocol. After 5 days, the cell suspension was centrifuged at 4000g, 20 min, 4 °C, and the supernatant was collected. The supernatant containing RBD protein was passed through a chromatography column filled with Ni-NTA resin (Therm Fisher Scientific), to immobilize the protein via His-tag. After immobilization, the column was washed with phosphate-buffered saline (PBS), containing 25 mM imidazole and 0.15 M NaCl. The protein was eluted from the resin using PBS containing 230 mM imidazole and 0.2 M NaCl. Subsequently, the protein solution was concentrated and purified with 10 kDa Amicon centrifugal filter columns (Merck, Germany).
2.5. Immunoassay
Anti-RBD IgG levels were evaluated by indirect enzyme-linked immunosorbent assay (ELISA). For the assay, 96-well microtiter Maxisorp plates (Nunc, Thermo, Denmark) were coated overnight at 4 °C with RBD antigen (100 μl per well of 1 μg/ml). The protein solution was then discarded, wells were washed 3 times with phosphate buffered saline with 0.1% Tween 20 (PBS-T), and each well was blocked with 3% skimmed milk in PBS-T, at room temperature (RT) for 60 min. The blocking solution was then discarded and human serum samples or positive and negative controls, or 8 calibrator dilutions (sera and controls diluted 1:100 with PBS-T; calibrator diluted 1:200 with PBS-T), were added to antigen-coated wells in duplicates. The plate was incubated at RT for 60 min., washed 3 times with PBS-T and an HRP-conjugated isotype-specific antibody goat anti-human IgG Fab specific horseradish peroxidase (HRP) conjugate (Sigma-Aldrich) (1:12,000 dilution) was added to each well for 30 min. After washing 3 times with PBS-T, incubation TMB (3,3′,5,5′-tetramethylbenzidine) Substrate Solution (Pierce 1-Step Turbo TMB-ELISA Substrate Solution, Thermo Fisher Scientific) for 30 min, followed by the addition of a stop solution (2 M H2SO4), to yield colorimetric reaction with the HRP enzyme. The OD of each well was measured at 450 nm using a microplate reader (TECAN Infinite M200 Pro).
2.6. ELISA calibration
The first WHO International Reference panel of anti-SARS-CoV-2 immunoglobulin consisted of pooled plasma samples from individuals recovered from COVID-19 and a negative control plasma obtained from healthy blood donors collected before 2019. The panel was evaluated by World Health Organization (WHO) in an international collaborative study [22]. The standard was supplied in a freeze-dried format by the National Institute for Biological Standards and Control (NIBSC)-code 20/136. An arbitrary unit of 1,000 binding antibody units per mL (BAU/mL) is used to compare assays detecting the same class of immunoglobulins with the same specificity. Due to a limited supply of the original reference sample supplied by the NIBSC, an internal reference was prepared from the pooled sera of recovered and vaccinated participants, calibrated against the NIBSC 20/136 standard.
For each microplate, a standard curve for quantitative measurement was calculated. The cut-off value of 35 BAU/mL was calculated based on the mean OD value of negative samples (n = 100) plus two standard deviations (SD). OD values greater than the determined cut-off value were defined as positive. The unit of measurement used is in accordance with the latest notification received from World Health Organization (WHO) NIBSC 20/136 standard. Our immunoassay was validated by an external, independent laboratory. The diagnostic value of the test was defined by determining the following parameters of the method, presented in Table 3 .
Table 3.
The diagnostic value of ELISA assay, determined by the external independent laboratory.
| Parameter | Value |
|---|---|
| Sensitivity | 86% |
| Specificity | 98% |
| Positive predictive value | 98% |
| Negative predictive value | 81% |
Positive reference samples consisted of pooled plasma obtained from 15 strongly seropositive patients with confirmed COVID 19 recovery. Negative reference sample was the plasma collected in 2018, in time the disease has not occurred yet.
2.7. Statistical model
Generalized Additive Models (GAM) [23] were used to investigate what factors influence antibody level (AL). For simplification, the term ‘antibody level’ is used to all the results obtained from the immunoassay, both below and above the set cut-off value. The dependent variables were antibody levels (respectively: pre-vaccination, after the first, and after the second dose), while the independent variables were locations (‘Wolica’ or ‘AMU’), sex, age and antibody level determined in the study (log-transformed), type of the vaccines (Vaxzevria or Comirnaty) and the time since vaccination. Three separate models were fitted (representing AL before, after the first, and after the second dose, respectively). AL was assumed to originate from a compound Poisson Gamma (Tweedie) distribution with the log link function. This approach allows modeling of non-negative real numbers and can effectively account for overdispersion. The patients’ age, AL, and time (both log-transformed) after the previous vaccine dose (if applicable) were fitted as smooth functions. Separate fits were allowed for the time following the vaccination. Additionally, the type of vaccine, sex, and a factor coding sampling locality were used as parametric predictors. Both production and degradation of antibodies are multiplicative processes, therefore ratios of the specific values of parameters were used when calculating effect sizes. To assess contrasts, estimated marginal means were used with the R package mmeans [24]. All analyses were performed with R version 4.2 [25].
3. Results
3.1. Pre-vaccination
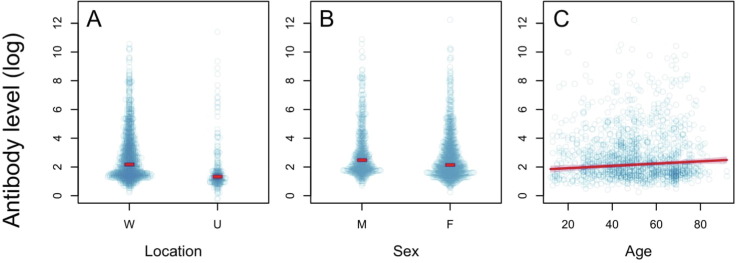
Antibody level (AL) before vaccination (pre-vaccination) was on average 2.31 times higher in the ‘Wolica’ location than in the ‘AMU’ location (95% CI: 1.77–2.86, t = 6.95, p < 0.0001) (Fig. 2 A, S1A). There was a significant difference between sexes - in men, AL is on average 1.40 times higher than in women (95% CI: 1.15–1.64, t = 3.75, p = 0.0002) (Fig. 2 B). Age was another significant factor. The AL increases by an average rate of 7.81% for every 10 years of average age (95% CI: 2.54–13.09, t = 3.75, p = 0.0002) (Fig. 2 C, S1C). Although the relationships found are statistically significant (Table 4 ), the fit of the model is low and the factors considered explain only 3.5% of the total variability of the AL. This means that in the unvaccinated population, AL is highly variable and only slightly affected by factors such as sex, age, and location.Table 5 Table 6
Fig. 2.
The effect of location (W = Wolica, U = AMU), sex (F = women, M = men), and age (in years) on AL before vaccination. The blue dots represent the observed values, the red lines represent fitted values, and shaded regions are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Generalized Additive Model results for pre-vaccination antibody level.
| PARAMETRIC TERMS | DF | F | P |
|---|---|---|---|
| LOCATION | 1 | 48.3 | <0.0001 |
| SEX | 1 | 14.06 | 0.0002 |
| SMOOTH TERMS | EDF | F | P |
| AGE | 1.0 | 8.36 | 0.0038 |
Explanations: DF: degrees of freedom for parametric terms, EDF: estimated degrees of freedom for smooth terms (corresponding to the degree of the polynomial, e.g. 1 is a straight line, 2 is a second-degree polynomial, etc.), F: Snedecor's test statistic, P: p-value (in bold when p < 0.05).
Table 5.
GAM model results for AL after the first dose of vaccination.
| PARAMETRIC TERMS | DF | F | P |
|---|---|---|---|
| VACCINE TYPE | 1 | 0.29 | 0.5900 |
| LOCATION | 1 | 23.1 | <0.0001 |
| SEX | 1 | 0.64 | 0.4220 |
| SMOOTH TERMS | EDF | F | P |
| AL PRE-VACCINATION | 4.3 | 165.8 | <0.0001 |
| AGE | 3.3 | 10.4 | <0.0001 |
| TIME AFTER VACCINE(VAXZEVRIA) | 1.0 | 29.2 | <0.0001 |
| TIME AFTER VACCINE (COMIRNATY) | 1.0 | 3.0 | 0.0817 |
Explanations: see Table 4. The fit of the model is 41.5%.
Table 6.
GAM model results for antibody level after the second dose of vaccination.
| PARAMETRIC TERMS | DF | F | P |
|---|---|---|---|
| VACCINE TYPE | 1 | 377.4 | <0.0001 |
| LOCATION | 1 | 13.7 | 0.0002 |
| SEX | 1 | 0.60 | 0.4372 |
| SMOOTH TERMS | EDF | F | P |
| AL PRE-VACCINATION | 4.1 | 169.7 | <0.0001 |
| AGE | 2.2 | 1.31 | 0.2363 |
| TIME AFTER VACCINATION (VAXZEVRIA) | 1.0 | 0.12 | 0.7317 |
| TIME AFTER VACCINATION (COMIRNATY) | 1.0 | 13.8 | 0.0002 |
Explanations: see Table 1. The fit of the model is 54.0%.
3.2. After the first dose
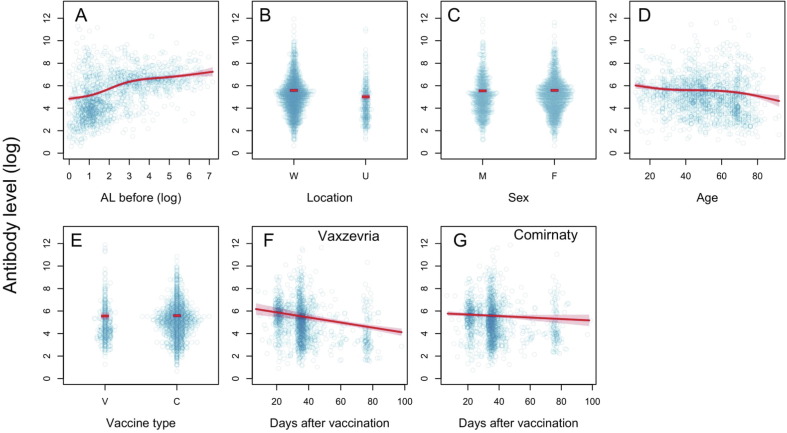
The most important factor determining the antibody level after the first vaccination is the AL pre-vaccination: participants with higher AL before treatment displayed a higher increase of AL after vaccination, which corroborates the dynamics of antibody production after secondary exposure to a specific antigen. As in the pre-vaccinated population, AL values after first dose were higher in the ‘Wolica’ location than in the ‘AMU’ location (mean 1.77 times, 95% CI: 1.36–2.18, t = 4.80, p < 0.0001) (Fig. 3 B, S2B). There were no significant differences in response to the first dose between women and men (mean AL ratio men/women is 0.96, 95% CI: 0.87–1.06, t = −0.80, p = 0.4220) (Fig. 3 C, S2C). Age was demonstrated to be a crucial factor determining the quickness and sustenance of the immune response: in the elderly, antibody production after the first dose of vaccine is slower and declines at an average rate of 9.19% for every 10 years of life (95% CI: 6.08–12.30, t = 3.22, p < 0.0001) (Fig. 3 D, S2D). However, this relationship is non-linear. In participants up to 30 years of age, the production of antibodies decreases with age. In the location of 30–60 yrs. range, the age dependence is insignificant and again, over the age of 60, a negative correlation between age and AL was observed. For example, the mean AL proportion between a 20-year-old and a 70-year-old participant is 1.58 (95% CI: 1.34–1.83, t = 5.79, p < 0.0001) (Fig. 3 D, S2D).
Fig. 3.
The effect of pre-vaccination (logarithm) antibody level on AL after the first dose of vaccination: location (W = Wolica, U = AMU), sex (F = female, M = male) and age (years), and vaccine type (V = Vaxzevria, C = Comirnaty), and time after vaccine was taken (separately for Vaxzevria and Comirnaty). The blue dots represent the observed values, the red lines represent fitted values, and shaded regions are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The mean AL after the first dose (measured in the 3 months post-vaccination period) did not differ with the type of vaccine (mean PP ratio for Vaxzevria/Comirnaty is 0.93, 95% CI: 0.67–1.19, t = −0.54, p = 0.5900) (Fig. 3 E, S2E). There were, however, clear differences in the rate of change in AL after vaccination: for Vaxzevria the initial response was very strong, followed by a decrease in AL at an average rate of 2.26% per day (95% CI: 1.44–3.08, t = −5.40, p < 0.0001) (Fig. 3 F, S2F), while in the case of the Comirnaty, the average rate of decrease in AL was more than 4 times lower, amounting to 0.66% per day. Based on the available data and with the assumed significance level of α = 0.05, there is no evidence backing the assumption that the change (in the case of the Comirnaty) is significantly different from zero (95% CI: −0.08–1.40, t = 1.74, p = 0.0817) (Fig. 3 G, S2G).
Consequently, the average 3-month AL after the first dose did not differ between the two types of vaccines (Fig. 3 E, S2E), while the dynamics of AL in the post-vaccination period differed: the response to Vaxzevria is rapid, followed by the AL decrease (Fig. 3 F, S2F), while for Comirnaty the initial response is lower but the AL remains almost constant (Fig. 3 G, S2G).
3.3. After the second dose
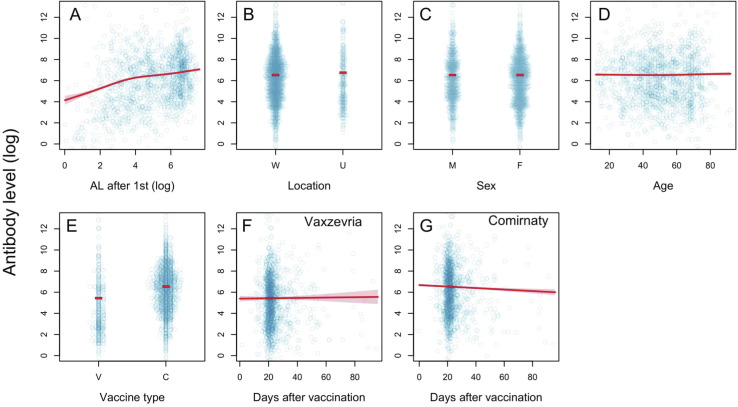
The greater AL following the first dose, the higher AL following the second dose. The location effect again proved to be significant, but this time AL was lower in the ‘Wolica’ location compared to ‘AMU’ (mean Wolica/AMU ratio is 0.81, 95% CI: 0.71–0.90, t = 3.70, p = 0.0002) (Fig. 4 B, S3B). There were no significant differences in response to the second dose between women and men (mean AL ratio men/women is 1.02, 95% CI: 0.96–1.08, t = 0.78, p = 0.4372) (Fig. 4 C, S3C). Age does not appear to be significant for AL obtained after the second dose (t = 1.14, p = 0.2363).
Fig. 4.
Effect of the (logarithmic) antibody level after the first dose of vaccination: location (W = Wolica, U = AMU), sex (F = female, M = male), vaccine type (V = Vaxzevria, C = Comirnaty) and time after the vaccine was taken (separately for Vaxzevria and Comirnaty) on AL after the second dose of vaccine. The blue dots represent the observed values, the red lines represent fitted values, and shaded regions are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
There were significant vaccine-type dependent differences in the mean AL (measured over 3 months) after the second dose of Vaxzevria: the mean AL was 2.9 times lower than after the second dose of Comirnaty (Vaxzevria/Comirnaty AL ratio was 0.34, 95% CI: 0.30–0.38, t = −0.54, p < 0.0001) (Fig. 4 D, S3D). There were also clear differences in the rate of change of AL after the booster: for Vaxzevria, it remains constant (the average rate of change is 0.16% and does not differ significantly from zero: 95% CI: 0.77–1.10, t = 0.34, p = 0.7307) (Fig. 4 E, S3E), while with the Comirnaty, AL decreases at an average rate of 0.71% per day (95% CI: 0.77–1.10, t = −3.74, p = 0.0002) (Fig. 4 F, S3F).
This means that both the average 3-month AL and immune response dynamics vary between the two types of vaccines. The initial response to the Vaxzevria was about 3 times weaker in comparison to the Comirnaty and then remains constant, while for the Comirnaty, the initial response is robust and then declines, leading to a plateau, still higher than the Vaxzevria after 3 months.
4. Discussion
Overall safety and efficacy of Vaxzevria and Comirnaty were assessed in the multinational, placebo-controlled, observer-blinded, pivotal efficacy trial clinical trials [19], [20]. Subsequently, they were approved under emergency use authorization in European Union. As expected aftermath of a worldwide vaccination program, many independently conducted studies have emerged, designed to investigate the serological response upon vaccination of specific subpopulations [26], [27], [28]. In these studies, several variables were considered such as age, sex, co-existing diseases, blood group, and antibody level. In our study, we aimed for the first time, at providing a Generalized Additive Model of immune response for two types of vaccines: Comirnaty and Vaxzevria with the association with age and time after vaccination on a relatively big cohort. Firstly, our model showed, contradictory to many hypotheses, that there is no difference between the serological response between males and females [29], [30]. It is well known that men are more susceptible to infection by e.g. influenza viruses, HIV, and hepatitis [31]. In the case of SARS-CoV-2, the data show that infection rates are very similar globally [32]. However, male COVID-19 patients are twice more likely to require Intensive-Care Unit admission and 30% more likely to die [31]. Studies have shown that innate and adaptive immune responses are stronger in females than males, especially during reproductive age [33], [34]. These discrepancies are linked to divergent sex hormone patterns, which differentially affect certain populations of immune cells [32] Nonetheless, our model reveals a predominant similarity in the immune response, regardless of sex, vaccine type, and the number of administered doses. There were discrepancies in the basal level (pre-vaccination) of antibodies, however, the fit of our model was low in this case. This may be attributed to more people having asymptomatic infections, as well as undiagnosed infections.
Our model reveals that patients with high initial level of antibodies before vaccination exhibit greater potential for boosted serological response afterward. Such patients was not diagnosed with SARS-CoV-2 infection, but may have an asymptomatic one. This confirms the other studies, which showed that a single dose of booster shot from a recovered patient elicits neutralizing titers of antibodies against all variants of SARS-CoV-2 up to 1000-fold, compared to much lower titers of naive donors. [35]. This phenomenon is referred to as “hybrid immunity” and it is attributed to the presence of memory B-cells after an infection, which enhances the serological response after boosting from the vaccine. Moreover, it was discovered that antibodies produced by cells after infection showed longer persistence and higher variability, which enhances the effectiveness of the response against immune-evading variants, compared to the vaccine-only antibodies. This mechanism is utilized in the heterologous vaccination strategy, which was recommended in many countries. It was confirmed that a combination of adenoviral first dose and mRNA booster yielded a better overall serological response. The virus-based preparations reportedly increased the cellular response, while mRNA vaccines yielded a higher humoral response [36], [37], [38].
Interestingly, our model finds age-related AL dependency only after the first dose.
The dynamics of the immune response reveal that the AL decrease with age up to 30 years, then remains constant in the range of 30–60 days of our study, and diminishes again in the 60+ group. This phenomenon was not observed after the second dose. Our study expanded the previous research [39], where statistically significant differences were observed only after the first dose of Comirnaty.
An intriguing behavior of the AL dynamics between the two vaccines was observed. After the first dose, the AL was higher for Vaxzevria than Comirnaty, but it decreased sharply over time, compared to Comirnaty where the decline was moderate. Nonetheless, the average AL was not statistically different after 3 months. After the second dose, the opposite dynamic was observed. The AL for Vaxzevria remained low and constant for 3 months. In comparison, a steep boost of the AL after Comirnaty, with a steady decline afterward, was observed. We found that many more Vaxzevria-vaccinated participants had very low, or even below the threshold AL after the second dose, compared to the Comirnaty-vaccinated ones. Since the RBD antigen was used in the ELISA, one of the two major targets for the neutralizing antibodies of the S protein (along with the N-terminal domain) [40], we attributed the low AL after Vaxzevria due to the lower production of neutralizing antibodies. This assumption can be confirmed with the work by Terpos et al. [41], in which the inhibition (%) of SARS‐CoV‐2 binding to the human host receptor angiotensin-converting enzyme‐2 (ACE-2) was measured. The study demonstrated that the median inhibition score was 91.25% for those receiving the Comirnaty, compared to the median score of 63.09% for those receiving the Vaxzevria, which is a direct measurement of the neutralizing antibodies’ potency. Similar results were presented by Khoury [42], who found the average SARS-CoV-2 AL induced by Vaxzevria to be lower than average post-COVID-19 level, and Karbiener, who showed that the average post-vaccination anti-SARS-CoV-2 neutralizing antibody level were more than twice lower compared to the Comirnaty and Novavax [43].
In our work, the humoral response of RBD against one SARS-CoV-2 variant – the Wuhan-Hu-1, was examined. Since the pandemic outbreak, a few variants of concern were identified, such as Alpha, Beta, Delta, and Omicron, characterized by particular mutations in the capsid proteins, especially in the spike protein [44]. There are studies, which aim at elucidating the cross-immunity between different viral strains [42], [45], [46], [47]. Available studies apply various methodologies to analyze and present the data, which is suboptimal for cross-comparison between different studies. Therefore, we propose the Generalized Additive Model as a standardized way of presenting the data, if such analysis is applicable. Provided model offers a coherent and clear way to describe the complexity of the serological response, simplifying data comparison between various studies. We believe that the presented model could be a good reference for further more applied research on SARS-CoV-2 humoral response, including post-booster reaction, the efficacy of various vaccines against specific viral mutations, and their impact on developing subsequent long COVID immunity. Given that vaccination prevents the severe course of COVID-19, it is conceivable that fully vaccinated patients presented a lower incidence of protracted symptoms. More substantial comparative observational studies and trials are required due to the full influence of vaccination remains undetermined.
5. Conclusions
In this study, 1,395 samples from the two places (Provincial Specialist Hospital for Lung Diseases and Tuberculosis in Wolica and Adam Mickiewicz University in Poznan) from Wielkopolska voivodeship, were collected and grouped based on vaccination status, sex, age, and type of the administered vaccine. Subsequently, “in-house” ELISA was developed, utilizing RBD as the capturing antigen. Lastly, a statistical model was designed to investigate the dynamics of SARS-CoV-2 humoral immune response over time. The models reveal different dynamics of humoral response for Vaxzevria and Comirnaty, as well as the difference in average antibody level after full vaccination regarding age and gender. The presented models complements other studies in the field and may serve as a convenient representation of humoral response after vaccination. Our data provide an important contribution to the understanding of immune response dynamics as a result of a worldwide vaccination effort in the fight against the COVID-19 pandemic.
Funding
The project described was supported by the The National Centre for Research and Development, in the frame of the program ‘SZPITALE JEDNOIMIENNE’, under the Grant agreement number SZPITALE-JEDNOIMIENNE/76/2020.
Uncited references
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jakub Rybka reports financial support was provided by National Centre for Research and Development.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.008.
Data availability
Data will be made available on request.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses-a statement of the Coronavirus Study Group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 3.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/nejmcp2009575. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/nejmcp2009249. [DOI] [PubMed] [Google Scholar]
- 5.Chu D.K., Akl E.A., Duda S., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontanet A., Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20(10):583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albano P.M., Notarte K.I., Macaranas I., et al. Cross-contamination in molecular diagnostic laboratories in low- and middle-income countries: A challenge to COVID-19 testing. PJP. 2020;5(2):7–11. doi: 10.21141/PJP.2020.09. [DOI] [Google Scholar]
- 8.Irsara C., Egger A.E., Prokop W., et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59(8):1453–1462. doi: 10.1515/cclm-2021-0214. [DOI] [PubMed] [Google Scholar]
- 9.Ma H., Zeng W., He H., et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17(7):773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendrone-Junior A., Dinardo C.L., Ferreira S.C., et al. Correlation between SARS-COV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion (Paris) 2021;61(4):1181–1190. doi: 10.1111/trf.16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun S., Ryu J.H., Jang J.H., et al. Comparison of sars-cov-2 antibody responses and seroconversion in covid-19 patients using twelve commercial immunoassays. Ann Lab Med. 2021;41(6):577–587. doi: 10.3343/ALM.2021.41.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2 2021;13. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed]
- 13.Isho B., Abe K.T., Zuo M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterhoff D., Glück V., Vogel M., et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49(1):75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espejo A.P., Akgun Y., al Mana A.F., et al. Review of current advances in serologic testing for COVID-19. Am J Clin Pathol. 2020;154(3):293–304. doi: 10.1093/AJCP/AQAA112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notarte K.I., Catahay J.A., Peligro P.J., et al. Humoral response in hemodialysis patients post-SARS-CoV-2 mRNA vaccination: A systematic review of literature. Vaccines (Basel) 2023;11(4):724. doi: 10.3390/vaccines11040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notarte K.I., Guerrero-Arguero I., Velasco J.V., et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J Med Virol. 2022;94(7):2939–2961. doi: 10.1002/jmv.27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notarte K.I., Ver A.T., Velasco J.V., et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;59(6):373–390. doi: 10.1080/10408363.2022.2038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanat F., Stadlbauer D., Strohmeier S., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristiansen P.A., Page M., Bernasconi V., et al. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood S.N. Generalized additive models: an introduction with R. Chapman and Hall/CRC; 2017. [DOI] [Google Scholar]
- 24.Searle S.R., Speed F.M., Milliken G.A. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34(4):216. doi: 10.2307/2684063. [DOI] [Google Scholar]
- 25.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A language and environment for statistical computing. https://www.R-project.org/ [Google Scholar]
- 26.Ward H., Whitaker M., Flower B., et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-28527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiomar R., Santos A.J., Melo A.M., et al. Monitoring of SARS-CoV-2 specific antibodies after vaccination. Vaccines (Basel) 2022;10(2) doi: 10.3390/vaccines10020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starrfelt J., Danielsen A.S., Buanes E.A., et al. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July–November 2021. BMC Med. 2022;20(1) doi: 10.1186/s12916-022-02480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen A., Stromme M., Moyassari S., et al. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp Clin Trials. 2022;115 doi: 10.1016/j.cct.2022.106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciarambino T., Para O., Giordano M. Immune system and COVID-19 by sex differences and age. Women’s Health. 2021;17 doi: 10.1177/17455065211022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsen H., Klein S.L. Sex differences in immunity to viral infections. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia H.J., Zhang G.H., Wang R.R., Zheng Y.T. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol Immunol. 2009;6(6):433–440. doi: 10.1038/cmi.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villacres M.C., Longmate J., Auge C., Diamond D.J. Predominant type 1 CMV-specific memory T-helper response in humans: Evidence for gender differences in cytokine secretion. Hum Immunol. 2004;65(5):476–485. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Stamatatos L., Czartoski J., Wan Y.H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science (1979) 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozzetto B., Legros V., Djebali S., et al. Immunogenicity and efficacy of heterologous ChAdOx1–BNT162b2 vaccination. Nature. 2021;600(7890):701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 37.Stuart A.S.V., Shaw R.H., Liu X., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borobia A.M., Carcas A.J., Pérez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anastassopoulou C., Antoni D., Manoussopoulos Y., et al. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS One. 2022;17(4):e0266958. doi: 10.1371/journal.pone.0266958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduct Target Ther. 2021;6(1):95. doi: 10.1038/s41392-021-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terpos E., Karalis V., Ntanasis-Stathopoulos I., et al. Comparison of neutralizing antibody responses at 6 months post vaccination with BNT162b2 and AZD1222. Biomedicines. 2022;10(2):338. doi: 10.3390/biomedicines10020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 43.Karbiener M., Farcet M.R., Zollner A., et al. Calibrated comparison of SARS-CoV-2 neutralizing antibody levels in response to protein-, mRNA-, and vector-based COVID-19 vaccines. NPJ Vaccines. 2022;7(1):22. doi: 10.1038/s41541-022-00455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavan M., Bassani D., Sturlese M., Moro S. From the Wuhan-Hu-1 strain to the XD and XE variants: is targeting the SARS-CoV-2 spike protein still a pharmaceutically relevant option against COVID-19? J Enzyme Inhib Med Chem. 2022;37(1):1704–1714. doi: 10.1080/14756366.2022.2081847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suryawanshi R.K., Chen I.P., Ma T., et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. 2022;607(7918):351–355. doi: 10.1038/s41586-022-04865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toh Z.Q., Mazarakis N., Nguyen J., et al. Comparison of antibody responses to SARS-CoV-2 variants in Australian children. Nat Commun. 2022;13(1):7185. doi: 10.1038/s41467-022-34983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernández-Luis P., Aguilar R., Pelegrin-Pérez J., et al. Decreased and heterogeneous neutralizing antibody responses against RBD of SARS-CoV-2 variants after mRNA vaccination. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.816389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notarte K.I., Catahay J.A., Velasco J.V., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byambasuren O., Stehlik P., Clark J., et al. Effect of covid-19 vaccination on long COVID: systematic review. BMJ Med. 2023;2:e000385. doi: 10.1136/bmjmed-2022-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe A., Iwagami M., Yasuhara J., et al. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine. 2023;41(11):1783–1790. doi: 10.1016/j.vaccine.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.