Abstract
Despite exhaustive and fully-financed plans to manage the risks of globally coordinated cessation of oral poliovirus vaccine (OPV) containing type 2 (OPV2) prior to 2016, as of 2022, extensive, continued transmission of circulating vaccine-derived polioviruses (cVDPVs) type 2 (cVDPV2) remains. Notably, cumulative cases caused by cVDPV2 since 2016 now exceed 2,500. Earlier analyses explored the implications of using different vaccine formulations to respond to cVDPV2 outbreaks and demonstrated how different properties of novel OPV2 (nOPV2) might affect its performance compared to Sabin monovalent OPV2 (mOPV2). These prior analyses used fixed assumptions for how outbreak response would occur, but outbreak response implementation can change. We update an existing global poliovirus transmission model to explore different options for responding with different vaccines and assumptions about scope, delays, immunization intensity, target age groups, and number of rounds. Our findings suggest that in order to successfully stop all cVDPV2 transmission globally, countries and the Global Polio Eradication Initiative need to address the deficiencies in emergency outbreak response policy and implementation. The polio program must urgently act to substantially reduce response time, target larger populations — particularly in high transmission areas — and achieve high coverage with improved access to under-vaccinated subpopulations. Given the limited supplies of nOPV2 at the present, using mOPV2 intensively immediately, followed by nOPV2 intensively if needed and when sufficient quantities become available, substantially increases the probability of ending cVDPV2 transmission globally.
Keywords: Polio, Dynamic modeling, Outbreak response, OPV, Eradication
1. Introduction
The polio endgame remains complicated by numerous risks, including some risks associated with using Sabin oral poliovirus vaccine (OPV) strains, to eradicate the transmission of the three types of polioviruses (i.e., types 1, 2, and 3) [1]. Although not noticeable in a background with ongoing transmission of indigenous wild polioviruses (WPVs) [2], following successful efforts to end WPV transmission by achieving high coverage with OPV, the risks of OPV become observable (e.g., as occurred in the US [2]). Specifically, as a live attenuated virus vaccine, OPV induces an infection in vaccine recipients, who can then excrete or shed OPV and OPV-related strains, which may spread secondarily. This secondary spread can effectively serve to induce or boost immunity in the population. However, in very rare instances, OPV may cause vaccine-associated paralytic polio (VAPP) in OPV recipients or their close contacts [1]. In addition, as OPV-related viruses transmit in populations with low immunization coverage, they can lose their attenuating mutations and recombine with other enteroviruses which may lead to outbreaks of circulating vaccine-derived polioviruses (cVDPVs) that cause paralysis and behave like homotypic WPVs [1,3]. Finally, a very small fraction of infected individuals with B-cell-related primary immunodeficiencies can develop prolonged or chronic OPV infections (immunodeficiency-associated VDPVs (iVDPVs), which hypothetically might lead to community transmission) [1,4]. Recognizing that the risks of Sabin OPV become less acceptable after transmission of WPV stops, the Global Polio Eradication Initiative (GPEI) 2013–2018 strategic plan included phased globally-coordinated cessation of each OPV type following the certification of eradication of the homotypic WPV, specifically staring with type 2 [5]. Numerous modeling studies supported the development and evaluation of strategies in support of this plan (see comprehensive review of results from studies published 2000–2019 elsewhere [6]) and helped to support the identification of pre-requisites for type-specific OPV cessation [7].
In 2016, globally coordinated cessation of type 2 OPV (OPV2) [8] meant that OPV-using countries switched from trivalent OPV (tOPV, which contains all three OPV types) to bivalent OPV (bOPV, which contains only types 1 and 3 OPV) for routine immunization (RI) and supplementary immunization activities (SIAs). The creation of a stockpile of monovalent OPV2 (mOPV2) provided a resource to support outbreak response activities in the event of cVDPV2 outbreaks [9,10]. In preparation for the switch from tOPV to bOPV, countries that only used OPV in RI began adding a single dose of inactivated poliovirus vaccine (IPV) to their RI schedules as a strategy to mitigate risks of paralysis in the event of continued type 2 poliovirus transmission after the switch [11]. In 2016, nearly all OPV2-related virus transmission stopped [12], which presumably led to a decline in rare type 2 VAPP cases. However, persistent and increasing transmission of type 2 cVDPVs (cVDPV2s) since 2018 [13,14], including some outbreaks caused by unknown sources [15,16], continue to raise questions about outbreak response strategies, tactics and implementation. Notably, cumulative cases caused by cVDPV2 since 2016 now exceed 2,577 [17]. In addition, insufficient supplies of IPV delayed paralysis risk mitigation efforts for some children born after OPV2 cessation [18]. However, those children who received the limited IPV doses, and consequently did not become paralyzed upon becoming infected, likely reduced the number of cases that otherwise would have occurred, which may have increased the time required to detected outbreaks through a surveillance system that depends on the detection of cases [19]. In the first few years following global OPV2 cessation, numerous cVDPV2 outbreaks and outbreak response SIAs (oSIAs) depleted mOPV2 from the stockpile [13–15,20,21]. In response, GPEI ordered the production of additional mOPV2 in 2018 and tOPV in 2019 with support from modeling studies that recommended using tOPV, if available, to respond to cVDPV2 outbreaks in type 1 WPV (WPV1) endemic countries post OPV2 cessation [22–24]). Use of tOPV for oSIAs in Pakistan and Afghanistan since 2020 substantially reduced transmission and cases caused by both types 1 and 2 polioviruses [25]. Overall, prior modeling studies that focused on oSIA choices consistently suggested the need to start type-specific OPV oSIAs shortly after outbreak detection with sufficient coverage and scope to shut down transmission [6,13,22,26,27].
Concerns about OPV-associated risks also motivated plans to use inactivated poliovirus vaccine (IPV) exclusively for oSIAs after successful OPV cessation (e.g., after 5 years [22,28,29] or 8 years [30], with the shift to later time occurring after delays in succeeding with OPV2 cessation). In parallel, GPEI partners made substantial investments to develop new oral poliovirus vaccines [31–33], including a novel OPV2 (nOPV2) designed with properties that make it less prone to reversion and with a substantially lower risk of neurovirulence as measured in experimental animals [34–37]. The World Health Organization (WHO) granted nOPV2 Emergency Use Listing (EUL) in November 2020, however, due to extensive requirements for countries to meet readiness criteria under the EUL, the first uses of nOPV2 began in March 2021 [38]. Based on the most recent report of the Global Advisory Committee on Vaccine Safety (GACVS), nOPV2 shows an acceptable safety profile and appears more genetically stable than mOPV2 in field use (as designed) [39] with a growing body of clinical evidence related to its characteristics [40]. The Strategic Advisory Group of Experts on Immunization (SAGE) recommended the transition to wider use of nOPV2 in October of 2021, while emphasizing “the importance of a timely response to cVDPV2 outbreaks using any available type 2 oral poliovirus vaccine rather than waiting for requirements for use of the nOPV2 to be met.” [41].
Recent studies considered the potential impacts of using nOPV2 for oSIAs in the polio endgame prior to [16,20,30] and after initial disruptions caused by the COVID-19 pandemic [26,42]. While these studies provided some insights, they assumed that future oSIAs would mimic the performance of oSIAs in the past few years, and did not consider potential changes in oSIA implementation or nOPV2 performance in field use. With nOPV2 playing a prominent role in recent and current GPEI strategic plans and policies [43–47], we recognized the importance of updating our global modeling to reflect actual immunization practices and epidemiological conditions through the end of 2021 and to explore the impact of a wide range of prospective oSIA options.
2. Methods
We updated and extended prior global poliovirus transmission modeling [26] that considered different bounding scenarios for the field properties of nOPV2 recently observed. Briefly, the global model divides the world into 72 blocks of 10 subpopulations of approximately 10.7 million total population each according to World Bank Income Level (low-income, LI; lower middle-income, LMI; upper middle-income, UMI; high-income, HI) and current vaccine use in routine immunization (OPV + IPV, IPV/OPV, IPV-only) [48]. Although the age distributions within the subpopulations vary (consistent with variability in the global population), on average a subpopulation includes approximately 8%, 18%, and 25% of children under 5 years, 10 years, and 15 years of age, respectively (i.e., approximately 1 million children < 5 years, 2 million < 10 years, and 3 million < 15 years of age). Mixing within blocks occurs homogenously in space and heterogeneously by age, while mixing between blocks occurs according to nine varying preferential mixing areas of different size, which in abstract represent larger geographical regions. Unlike the prior analysis [26] that modeled oSIAs only with fixed assumptions consistent with actual GPEI oSIA performance [30], for this analysis we explore a range of different oSIA vaccine choices and performance characteristics (Table 1). We consider a prospective analytical time horizon of T0 of January 1, 2022 to Tend of December 31, 2026, consistent with the duration of the current GPEI Strategic Plan [47]. To update the global model for a new time horizon, we review the epidemiological and immunization data for the time period between the beginning of the prior model time horizon (e.g., 2020 for the time horizon of 2020–2023 [26]) and the beginning of the new time horizon (i.e., 2022). We then convert the years with available data to part of the deterministic run up. This process involves ensuring that the model uses the same number of rounds and types of vaccine for each round in the subpopulations that abstractly represent countries to simulate actual historical performance, and that the model correctly simulates observed retrospective importation events. Thus, for the deterministic run up, we mimic the events that actually occurred, while modeling for the prospective time horizon allows for stochastic importations to occur in different places at different times.
Table 1.
Vaccine options and characteristics inputs (top) and outbreak response supplementary immunization activities (oSIAs) characteristic options (bottom) used in combination for scenarios to characterize the impacts of the changes in (oSIAs) on poliovirus immunization and transmission (see main text for descriptions, each scenario defined by one letter (vaccine option) and one or more number (characteristic option)). Bold highlights the difference from the RC.
| Vaccine option | Time period 1 | Vaccine 1 | Time period 2 | Vaccine 2 | Notes | |
|---|---|---|---|---|---|---|
|
| ||||||
| A (used for the RC) | T0 - Tend | mOPV2 | ||||
| B | T0 - Tend | nOPV2 | No reversion, no VAPP [20] | |||
| C | T0 - Tend | nOPV2 | Some reversion, same VAPP [20] | |||
| D | T0 - Tend | nOPV2 | 90% relative to B | |||
| E | T0 - Tend | nOPV2 | 90% relative to C | |||
| F | T0 – April 30, 2024 | mOPV2 | May 1, 2024 - T end | IPV | SIA intensity reduced by one level [20] | |
| G | T0 – June 30, 2022 | mOPV2 | July 1, 2022 - T end | nOPV2 | No reversion, no VAPP [20] | |
| H | T0 – June 30, 2022 | mOPV2 | July 1, 2022 - T end | nOPV2 | Some reversion, same VAPP [20] | |
| I | T0 – June 30, 2022 | mOPV2 | July 1, 2022 - T end | nOPV2 | 90% relative to G | |
| J | T0 – June 30, 2022 | mOPV2 | July 1, 2022 - T end | nOPV2 | 90% relative to H | |
| Characteristic option | Univariate changes to oSIA characteristics | Scope in R0 >9 blocks* | Days to first round | Number of rounds | Intensity** (TC,PRM) | Target age group (years) |
|
| ||||||
| 0 (used for the RC) | 1 + 4 | 45 | 2 | Varied | <5 | |
| 1 | Subpopulation scope | 1 + 0 | 45 | 2 | Varied | <5 |
| 2 | Block scope | 1 + 9 | 45 | 2 | Varied | <5 |
| 3 | Shorter delay | 1 + 4 | 30 | 2 | Varied | <5 |
| 4 | Longer delay | 1 + 4 | 60 | 2 | Varied | <5 |
| 5 | Three rounds | 1 + 4 | 45 | 3 | Varied | <5 |
| 6 | Four rounds | 1 + 4 | 45 | 4 | Varied | <5 |
| 7 | High intensity | 1 + 4 | 45 | 2 | (0.80, 0.70) | <5 |
| 8 | EAG | 1 + 4 | 45 | 2 | Varied | <10 |
| 9 | Conditional EAG | 1 + 4 | 45 | 2 | Varied | <10 *** |
| 2379 | Combination 1 | 1 + 9 | 30 | 2 | (0.80, 0.70) | <10 *** |
| 23,579 | Combination 2 | 1 + 9 | 30 | 3 | (0.80, 0.70) | <10 *** |
Abbreviations: EAG, expanded age group; IPV, inactivated poliovirus vaccine; mOPV2, type 2 monovalent oral poliovirus vaccine; nOPV2, type 2 novel oral poliovirus vaccine; oSIA, outbreak response supplementary immunization activity; PRM, repeatedly missed probability; R0, basic reproductive number; RC, reference case; TC, true coverage.
1 + X = outbreak subpopulation plus X number of worst performing neighboring subpopulations within the block [13,26].
Immunization intensity varied by subpopulation to match the performance of preventive supplementary immunization activities (pSIAs) using specified SIA levels [13,26].
Expanded target age group to include all birth cohorts born since globally coordinated OPV2 cessation in blocks with no OPV2 use since before OPV2 cessation in 2016.
For the reference case (RC), a modeling benchmark that is well characterized and will facilitate subsequent comparisons, we use the same fixed assumptions from prior analyses for the characteristics of prospective oSIAs [20,26,30], which assume they target children < 5 years of age, start 45 days after detection, and include 2 rounds of mOPV2 separated by 30 days with 2 additional rounds after any breakthrough transmission. Although the GPEI uses the term outbreak on the scale of geopolitical units, we defined an outbreak in the model on the scale of a subpopulation, which we note may represent more than one country (for small countries) or a small part of a large country. In most modeled subpopulations, for which WPV1 R0 < 10, if an outbreak occurs, the oSIA scope would include only the outbreak subpopulation. However, for subpopulations modeled with WPV1 R0 ≥ 10, which represent the highest-risk outbreak areas, the oSIA scope includes the outbreak subpopulation and its four worst-performing neighbor subpopulations within the same block [30]. The oSIA intensity varies for different subpopulations, ranging from 15% true coverage and 95% repeatedly missed probability to 80% true coverage and 50% repeatedly missed probability. We use mOPV2 for the RC since it represented the primary vaccine available and recommended for use for type 2 oSIAs in 2021, with the understanding that the actual vaccine use data will likely differ given the evolving landscape of vaccines and outbreak response activities. Specifically, although GPEI plans a complete shift to nOPV2 for oSIAs at some point in the near future [47] and tOPV may be used in some outbreak responses, at the beginning of the time horizon for the model (January 1, 2022), mOPV2 served as the primary vaccine available for rapid use for type 2 oSIAs and it represents an important baseline for comparison given historical experience with mOPV2 and its well-known properties.
Since receiving the EUL, nOPV2 use occurred broadly in Africa, although uncertainty remains about its field use characteristics and the ultimate timing of its licensure. Pakistan, Afghanistan, and Yemen used tOPV in some oSIAs in 2020–2022. Consistent with actual prior use, the model includes tOPV use for oSIAs by Pakistan and Afghanistan in 2021 [49]. Prospective use of tOPV (instead of mOPV2) does not impact the RC results for cVDPV2 due to the same assumptions about type 2 take rate, although it may improve the control and eradication outcomes for WPV1 and type 1 cVDPVs (cVDPV1). Prior modeling recognized the challenges of managing co-circulation of both types 1 and 2 polioviruses, issues with competition between oSIAs and preventive SIAs (pSIAs), and the benefits of using tOPV to increase population immunity [22–24]. Building on prior modeling specific to Pakistan and Afghanistan that explored prospective scenarios [49], we assumed that improved vaccination intensity for the RC in the remaining endemic block could lead to elimination of WPV1 in early 2022, although uncertainty remained about what intensity would actually occur in 2022. Given this assumption, despite continued WPV1 transmission in Pakistan and Afghanistan as of the end of 2021 [49] and reported cases in 2022 [50], we also focus the results of this analysis on type 1 cVDPVs (cVDPV1s). The visibility and importance of cVDPVs increases as transmission of WPVs declines. Notably, cVDPV1 outbreaks led to more cases in 2021 than WPV1 (i.e., 14 cVDPV1 cases vs 5 WPV1 cases in 2021 [17,50]).
2.1. Vaccine options and characteristics
The top rows of Table 1 describe the different vaccine options and characteristics that we considered, each scenario designated by a letter; for example, “A” indicates the use of mOPV2 for the entire time horizon, as assumed for the RC. Recognizing the limited available information about how nOPV2 will actually perform in the field, we consider different assumptions to bound the performance of nOPV2 in oSIAs based on a prior analysis [20,26]. We do not consider the possibility of any countries restarting the use of Sabin type 2 OPV in RI [16,51]. In the prior modeling, we assumed the same field effectiveness of nOPV2 as mOPV2 for recipients based on non-inferiority in seroconversion studies compared to mOPV2 [20]. We previously considered four alternative scenarios assuming the use of the low-dose candidate 1 nOPV2 vaccine granted the EUL [36,37,52] (referred to as nOPV2) and we compared them to using mOPV2 for all oSIAs for cVDPV2s starting in January 1, 2021 [20]. For this analysis we again consider two of these nOPV2 characteristics bounding scenarios. Scenario B (previously called “no reversion, no VAPP” [20,26]) assumes the same effectiveness as mOPV2, no reversion despite transmissibility, and no VAPP2, and Scenario C (previously called “some reversion, same VAPP”) assumes the same effectiveness as mOPV2, prior assumptions for reduced reversion [26], and VAPP2 occurring at the mOPV2 rate for vaccine recipients [26]. For this analysis, we also add two lower bound scenarios that allow for slightly lower effectiveness of nOPV2 compared to mOPV2. Scenario D assumes 90% effectiveness of mOPV2, no reversion despite transmissibility, and paralysis occurring at the 90% of mOPV2 VAPP2 rate for vaccine recipients, and Scenario E assumes 90% of the effectiveness of mOPV2, prior assumptions for reduced reversion [26] further reduced by 10%, and VAPP2 occurring at 90% of that for mOPV2 rate in vaccine recipients.
The very small contribution of VAPP cases to the total cases shown elsewhere [20], led us to ignore VAPP in Scenarios B and D. We note that by comparison to Scenario B, a “no reversion, same VAPP” scenario would give the same behavior and results as scenario B except that the total incidence would increase by<10 VAPP cases.
Prior modeling assumed that all type-specific OPV use in oSIAs would cease within 5 years [22,28,29] or 8 years [30] of its globally coordinated cessation (e.g., in 2021 or 2024 for OPV2). This assumption changed over time, and is no longer consistent with the current GPEI strategic plan, but shifting to IPV for oSIAs remains a possible option [47]. Recognizing the possibility, we also consider scenario F that assumes mOPV2 use prior to May 1, 2024 (i.e., within 8 years of OPV2 cessation [30]) followed by IPV exclusively for oSIAs for the remainder of the time horizon, albeit with lower oSIA intensity [30].
For all scenarios, we assume no possibility of coordinated or uncoordinated OPV2 restart (of any formulation) in routine childhood immunization, and we assume no constraints on vaccine availability (i.e., unlimited stockpiles), which allows us to characterize potential vaccine supply needs, which we report without adjustment for wastage [30]. Management of vaccine supplies is essential to ensure timely responses [9,10,22,53], and GPEI encountered multiple challenges that led to insufficient type 2-containing vaccine supplies in the stockpile to meet demand since 2019 [21]. Recognizing that as of early 2022, mOPV2 supplies exceeded 300 million doses and nOPV2 supplies remain constrained, we also included scenarios G-J that assume mOPV2 used during the first six months of 2022 (instead of throughout the time horizon in scenario A), followed by a shift to nOPV2 with the properties of scenarios B-E, respectively, from July 1, 2022, throughout the remainder of the time horizon.
2.2. oSIA characteristics options
We also consider several options that vary individual oSIA characteristics as shown in the bottom of Table 1, all of which use scenario A vaccine (i.e., mOPV2) for direct comparison with the RC. We use “0′′ to indicate the characteristics of oSIAs for the RC as described in earlier studies [20,30]. We explore the impact of changing the scope of the oSIA in population blocks with high transmission potential (i.e., WPV1 R0 ≥ 10) by using option 1 that restricts the oSIA scope in high R0 blocks to the outbreak subpopulation only, and option 2 that expands the oSIA scope in high R0 blocks to the whole block containing the outbreak subpopulation. We also consider the impact of the time delay between outbreak detection to the first oSIA round, for which we explore options 3 and 4 that respectively decrease or increase the delay by 15 days compared to the RC to either 30 or 60 days, respectively. We recognize that the assumption of 45 days of delay to the first oSIA round better reflects pre-COVID-19 activities than experienced during the COVID-19 pandemic and response. However, GPEI standard operating procedures recommend implementing oSIAs as promptly as possible after outbreak confirmation [54], and GPEI partners anticipate a return to pre-COVID oSIA performance during 2022. A recent study also shows potential promise of the use of direct detection methods to speed up detection and confirmation of polio cases [55]; therefore, we did not change the assumption for the RC for this analysis.
Since prior modeling studies argued for aggressive oSIAs immediately after OPV2 cessation using at least four rounds [22,28], we considered options 5 and 6 that assume the use of three or four oSIA rounds, respectively, instead of the two rounds in the RC as has been the standard operating procedure (SOP). Similarly, since prior studies argued for aggressive oSIAs with high intensity, we consider option 7 that assumes 80% for true coverage and 70% of repeatedly missing the same children for all oSIAs. Finally, taking into account the time elapsed since globally coordinated OPV2 cessation in 2016 (i.e., more than 5 years), and the expectation by prior modeling that oSIAs would include all age cohorts born since OPV cessation, we consider options 8 and 9 that involved an expanded age group (EAG) for the oSIA target population of all children aged < 10 years. For this analysis, we consider option 8 that uses the target age group of children < 10 years without consideration of any mOPV2 use since OPV2 cessation, and option 9 that expands the oSIA target age group to children < 10 years only in blocks for which more than 5 years have passed since the last OPV2 use in outbreak response.
Recognizing the potential for oSIA changes that combine the individual effects explored by the preceding scenarios, we consider the joint effect of two vaccine options: A (i.e., mOPV2) or B (i.e., nOPV2, no reversion, no VAPP2) and group the best performing univariate changes (i.e., SIA options “2379” or “23579” that show the combination of the characteristics with these options in the bottom of Table 1). We also consider the combinations with vaccine characteristics in scenarios G-J given the reality of constrained vaccine supplies. Recognizing the importance of improving quality, we also repeated the runs with the best performing characteristics except for improving quality using scenarios G239 and G2369. Similarly, we simulated the effect of reduced potential seeding effects when using nOPV2 instead of mOPV2 for larger (i.e., blockwide) oSIAs using scenarios B2 and G2.
We performed all simulations using JAVA™ programming language in the integrated development environment Eclipse™, and we simulated 100 stochastic iterations starting with the same random number seeds and initial conditions for each scenario. We estimated the probability of transmission die-out of cVDPV2 by counting the number of iterations with no ongoing transmission of type 2 at the end of the time horizon (i.e., December 31, 2026) and the expected number of affected modeled subpopulations out of 720 modeled subpopulations as a surrogate for geographical spread.
3. Results
For this analysis, we focus primarily on cVDPV2 cases, which dominate the global polio incidence as of the end of 2021. For the time horizon of 2022–2026, the model estimates an average of over 9,523 expected cVDPV2 cases and 9,775 total cases for the RC (Table 2), leads to an estimated probability of die out of 1% for cVDPV (Table 3), and comes with expected use of an average of 437 million mOPV2 doses (Table 4). The estimates in Table 4 do not adjust for any wastage and they are intended for direct comparison of the modeled scenarios, not for use for estimating GPEI prospective vaccine needs. Modeling insights are presented in the context of results shown in Figs. 1 through 4, which compare alternative scenarios to the RC from a global perspective. Now 6 years after OPV2 cessation, seeding of type 2 live poliovirus vaccines introduced from any source can restart transmission in many subpopulations, and the model integrates over all possible risks of reintroduction.
Table 2.
Estimated expected value ((median) and [range]) of poliovirus cases in 100 stochastic iterations for 2022–2026 for the scenarios modeled (see main text for descriptions).
| Scenario | Estimated expected global cVDPV1 cases (median) [range] | Estimated expected global total* type 1 cases (median) [range] | Estimated expected global cVDPV2 cases (median) [range] | Estimated expected global total* type 2 cases (median) [range] |
|---|---|---|---|---|
| RC (A0) | 231 (229) [166–364] | 315 (314) [251–450] | 9,523 (4,178) [156–42,959] | 9,775 (4,395) [172–43,690] |
| B0 | 230 (229) [229–233] | 314 (313) [313–318] | 2,295 (1,160) [359–16,852] | 2,345 (1,195) [371–16,889] |
| C0 | 234 (229) [207–477] | 318 (313) [292–564] | 9,062 (4,057) [173–59,370] | 9,106 (4,072) [181–59,430] |
| D0 | 232 (229) [229–393] | 317 (314) [313–479] | 5,496 (13,175) [428–38,594] | 5,544 (3,192) [441–38,630] |
| E0 | 233 (229) [156–348] | 318 (314) [240–433] | 16,592 (8,353) [563–63,319] | 16,637 (14,266) [573–63,365] |
| F0 | 210 (229) [97–232] | 293 (312) [179–314] | 18,474 (8,353) [263–94,704] | 18,629 (8,368) [275–94,864] |
| A1 | 230 (229) [228–275] | 315 (314) [313–363] | 20,882 (12,147) [2,888–97,275] | 20,967 (12,167) [2,897–97,374] |
| A2 | 228 (229) [189–237] | 312 (313) [273–321] | 1,238 (284) [52–12,231] | 1,359 (353) [100–12,445] |
| A3 | 164 (153) [143–564] | 248 (238) [227–650] | 7,550 (2,971) [61–54,124] | 7,795 (3,107) [73–54,930] |
| A4 | 232 (224) [154–572] | 316 (308) [238–656] | 11,594 (5,832) [163–49,955] | 11,840 (5,874) [185–50,575] |
| A5 | 228 (213) [183–517] | 313 (297) [267–602] | 10,297 (6,248) [35–45,940] | 10,584 (6,375) [49–46,852] |
| A6 | 95 (64) [64–924] | 180 (149) [147–1,012] | 11,412 (7,598) [35–42,229] | 11,690 (7,702) [45–42,763] |
| A7 | 61 (54) [53–651] | 145 (137) [137–737] | 8,494 (4,304) [17–41,109] | 8,727 (4,443) [28–41,824] |
| A8 | 222 (229) [138–314] | 307 (313) [224–398] | 8,314 (4,730) [31–31,333] | 8,618 (4,963) [43–31,924] |
| A9 | 230 (229) [160–397] | 314 (314) [247–482] | 5,718 (2,922) [164–32,784] | 5,930 (3,056) [183–33,552] |
| A2379 | 50 (47) [47–75] | 133 (130) [130–159] | 350 (36) [11–5,898] | 437 (75) [40–6,022] |
| B2379 | 61 (48) [47–76] | 144 (131) [130–159] | 115 (48) [11–2,493] | 134 (54) [15–2,516] |
| A23579 | 49 (45) [45–150] | 131 (128) [127–233] | 305 (35) [11–5,604] | 390 (74) [39–5,727] |
| B23579 | 73 (45) [45–150] | 155 (128) [127–233] | 70 (20) [11–2,072] | 100 (33) [15–2,084] |
| G2379 | 51 (47) [47–76] | 134 (130) [130–159] | 90 (20) [11–2,423] | 115 (37) [23–2,455] |
| H2379 | 50 (47) [47–75] | 133 (130) [130–159] | 178 (24) [11–4,051] | 223 (57) [36–4,094] |
| I2379 | 51 (47) [47–78] | 134 (130) [130–159] | 70 (21) [11–1,245] | 94 (37) [23–1,263] |
| J2379 | 50 (47) [47–75] | 133 (130) [130–159] | 104 (21) [11–4,236] | 154 (51) [34–4,281] |
| B2 | 230 (229) [229–233] | 314 (313) [312–317] | 904 (605) [208–5,961] | 950 (616) [222–5,995] |
| G2 | 229 (229) [201–233] | 313 (313) [285–317] | 1,016 (517) [238–14,966] | 1,076 (539) [264–14,996] |
| G239 | 156 (145) [143–177] | 240 (229) [227–261] | 519 (408) [190–2,732] | 547 (423) [203–2,751] |
| G2369 | 57 (56) [56–61] | 140 (140) [139–146] | 158 (114) [14–1,993] | 183 (131) [25–2,016] |
Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC, reference case, for details about scenarios see Table 1.
Notes:
Includes all cases, i.e., totals from all infections with live polioviruses, including wild polioviruses, vaccine-derived polioviruses, vaccine-associated paralytic polio, and cases associated with rare, but non-zero stochastic risks such as containment breaches.
Table 3.
Estimated probability of type 2 transmission die-out number of iterations with no ongoing transmission as of December 31, 2026) and the expected number of affected modeled subpopulations out of 720 in 100 stochastic iterations for the scenarios modeled (see main text for descriptions).
| Scenario | Probability of die out | Estimated expected affected modeled subpopulations out of 720 (median) [range] |
|---|---|---|
| RC (A0) | 1% | 40 (23) [5 – 158] |
| B0 | 0% | 17 (11) [5 – 82] |
| C0 | 1% | 36 (21) [5 – 198] |
| D0 | 0% | 27 (19) [6 – 136] |
| E0 | 0% | 53 (49) [6 – 197] |
| F0 | 0% | 63 (49) [5 – 261] |
| A1 | 0% | 69 (49) [15 – 279] |
| A2 | 2% | 13 (7) [5 – 63] |
| A3 | 1% | 33 (18) [5 – 187] |
| A4 | 0% | 46 (28) [5 – 183] |
| A5 | 13% | 42 (28) [5 – 174] |
| A6 | 15% | 44 (32) [5 – 160] |
| A7 | 25% | 34 (19) [4 – 155] |
| A8 | 20% | 37 (26) [5 – 132] |
| A9 | 1% | 27 (18) [5 – 116] |
| A2379 | 81% | 6 (4) [3 – 49] |
| B2379 | 95% | 4 (4) [3 –15] |
| A23579 | 85% | 6 (4) [3 –48] |
| B23579 | 96% | 4 (3) [3 –14] |
| G2379 | 95% | 4 (4) [3 –15] |
| H2379 | 88% | 5 (4) [3 –41] |
| I2379 | 97% | 4 (4) [3 –13] |
| J2379 | 93% | 4 (4) [3 –19] |
| B2 | 1% | 9 (7) [5 –41] |
| G2 | 0% | 9 (7) [5 –47] |
| G239 | 0% | 7 (6) [5 –17] |
| G2369 | 58% | 5 (5)[5 –16] |
Abbreviations: RC, reference case, for details about scenarios see Table 1.
Table 4.
Estimated expected value ((median) and [range]) of vaccine use for outbreak response in 100 stochastic iterations for 2022–2026 for the scenarios modeled (see main text for descriptions).
| Scenario | Estimated expected millions of outbreak response supplementary immunization activities (oSIAs) doses used (median) [range] |
|||
|---|---|---|---|---|
| bOPV | mOPV2 | nOPV2 | IPV | |
| RC (A0) | 59 (59) [49–107] | 430 (365) [129–1,042] | NA | 0.2 (0) [0–9] |
| B0 | 59 (59) [59 –59] | NA | 202 (179) [111–501] | 0.0 (0) [0–1] |
| C0 | 60 (59) [49–116] | NA | 390 (325) [122–1,221] | 0.0 (0) [0–1] |
| D0 | 59 (59) [59 –59] | NA | 323 (254) [162–884] | 0.2 (0) [0–14] |
| E0 | 59 (59) [49–64] | NA | 500 (495) [213–1,194] | 0.2 (0) [0–15] |
| F0 | 56 (59) [40 –59] | 197 (200) [102–434] | NA | 190 (120) [5–821] |
| A1 | 14 (14) [13 –15] | 206 (149) [51–681] | NA | 0.1 (0) [0–3] |
| A2 | 150 (150) [124–163] | 749 (736) [512–1,190] | NA | 0.1 (0) [0–3] |
| A3 | 52 (49) [49–93] | 419 (359) [94–1,258] | NA | 0.1 (0) [0–2] |
| A4 | 60 (59) [49–116] | 439 (395) [115–1,197] | NA | 0.2 (0) [0–8] |
| A5 | 65 (60) [59–145] | 583 (553) [139–1,281] | NA | 0.3 (0) [0–17] |
| A6 | 47 (41) [39–103] | 652 (632) [158–1,588] | NA | 0.4 (0) [0–16] |
| A7 | 34 (34) [33 –53] | 453 (421) [99–1,253] | NA | 0.1 (0) [0–2] |
| A8 | 62 (60) [50–91] | 830 (768) [191–2,120] | NA | 0.4 (0) [0–13] |
| A9 | 60 (59) [49–102] | 408 (342) [138–1,083] | NA | 0.4 (0) [0–13] |
| A2379 | 63 (63) [62–65] | 769 (752) [509–1,434] | NA | 0.3 (0) [0–12] |
| B2379 | 64 (63) [63–65] | NA | 223 (221) [144–365] | 0.1 (0) [0–7] |
| A23579 | 95 (94) [94–98] | 1,010 (982) [667–1,765] | NA | 0.3 (0) [0–12] |
| B23579 | 95 (94) [94–98] | NA 129 (128) | 284 (279) [209–581] | 0.2 (0) [0–11] |
| G2379 | 63 (63) [63–65] | [60–229] | 178 (181) [102–216] | 0.1 (0) [0–7] |
| H2379 | 63 (63) [62–65] | 129 (128) [60–229] | 599 (586) [406–1,046] | 0.2 (0) [0–7] |
| I2379 | 63 (63) [63–65] | 129 (128) [60–229] | 180 (177) [102–216] | 0.1 (0) [0–7] |
| J2379 | 63 (63) [63–65] | 129 (128) [60–229] | 599 (541) [406–810] | 0.1 (0) [0–7] |
| B2 | 150 (150) [150–150] | NA 74 (80) | 294 (288) [197–543] | 0.0 (0) [0–1] |
| G2 | 150 (150) [125–150] | [42–127] | 286 [275] (184–535] | 0.0 (0) [0–1] |
| G239 | 124 (124) [124–125] | 119 (117) [55–215] | 271 (264) [214–324] | 0.2 (0) [0–8] |
| G2369 | 100 (100) [98–100] | 136 (138 ) [62–264] | 359 (349) [270–483] | 0.3 (0) [0–17] |
Abbreviations: bOPV, bivalent oral poliovirus vaccine; IPV, inactivated poliovirus vaccine; mOPV2, type 2 monovalent oral poliovirus vaccine; nOPV2, type 2 novel oral poliovirus vaccine; RC, reference case, for details about scenarios see Table 1.
Fig. 1.
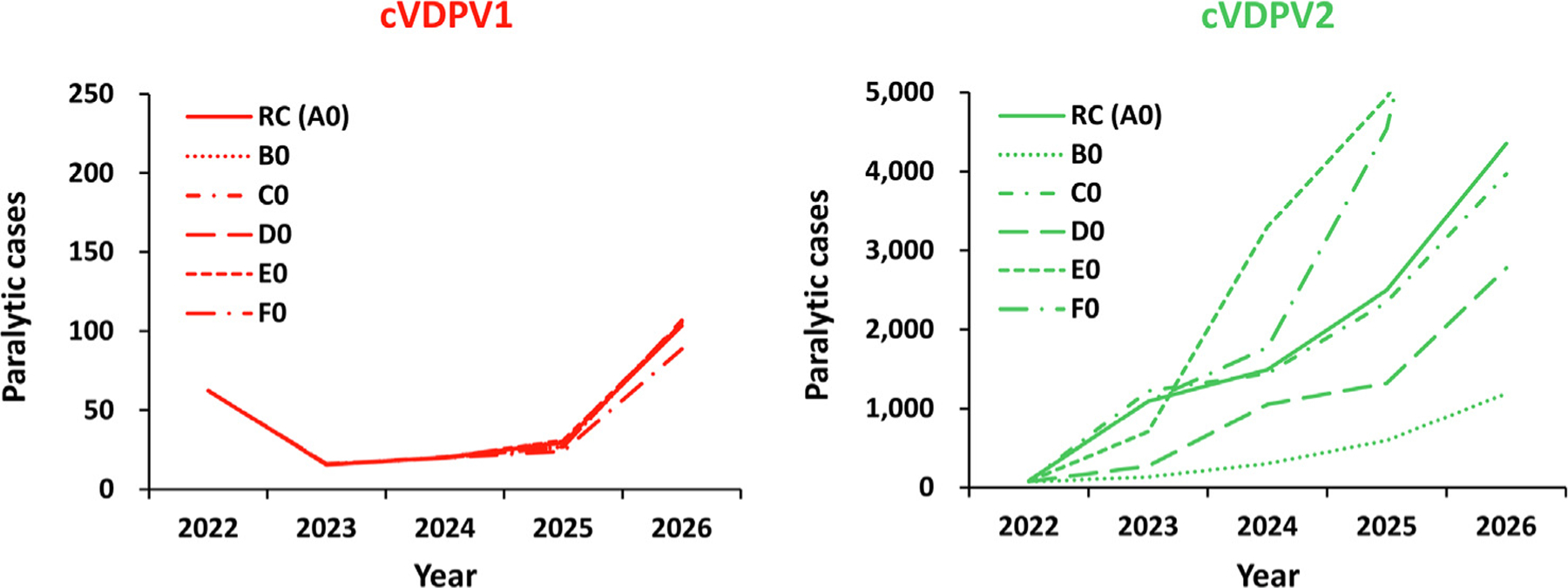
Expected global number of polio cases by year for 100 stochastic iterations of the different vaccine characteristics scenarios for 2022–2026 compared to the reference case (see main text for descriptions). Notes: All scenarios use the characteristics of the reference case (Table 1 bottom, row 0). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case, which uses monovalent Sabin OPV2 (A); B0 indicates the use of novel OPV2 (nOPV2) assuming the same effectiveness, no reversion, and no vaccine-associated paralytic polio (VAPP) [16]; C0 indicates the use of nOPV2 assuming the same effectiveness and same VAPP as mOPV2, but reduced reversion relative to mOPV2; D0 assumes nOPV2 with reduced effectiveness, no reversion, and no VAPP; E0 assumes nOPV2 with reduced effectiveness and VAPP and less reversion relative to mOPV2; and F0 uses mOPV2 until April 30, 2024 and then inactivated poliovirus vaccine (IPV) for the rest of the time horizon.
Fig. 4.
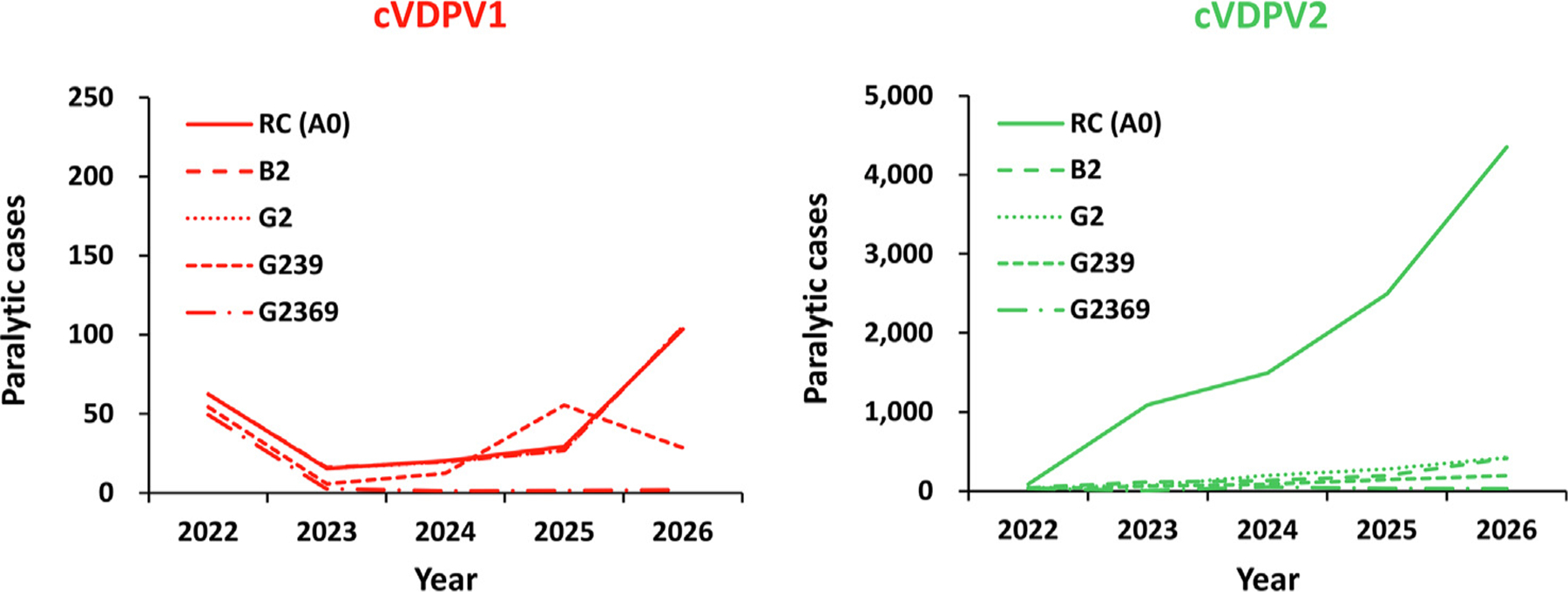
Expected global value of polio cases by year for 100 stochastic iterations of some nOPV2 oSIA options with larger (blockwide) response and some other improvements characteristics that exclude improvement of quality for 2022–2026 compared to the reference case (see main text for descriptions). Notes: All scenarios use the best combination of univariate changes (see Table 1, bottom) and novel OPV2 (nOPV2) with the most favorable properties (Table 1, row B), or the use of mOPV2 for six months followed by nOPV2 with the most favorable properties (Table 1, row G). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case.
Fig. 1 shows the expected value of the estimated annual incidence of global polio cases for the modeled primary vaccine choice options (Table 1, top B-F) compared to the RC (Table 1, vaccine option A) for cVDPV1 and cVDPV2. (All simulations shown in Fig. 1 use oSIA option characteristic 0 (Table 1, bottom), hence the legends A0, B0, and so on for the different curves in Fig. 1.) Scenario B0, the best nOPV2 scenario, shows lower expected cVDPV2 cases than expected with mOPV2 use in the RC, with an immediate drop from 2022 and no new cVDPV2s seeded afterwards. However, simply using nOPV2 instead of mOPV2 (independent of the properties assumed (B0-E0)) while maintaining the other oSIA characteristics the same as the RC, fails to stop the cVDPV2s already seeded and leads to a similar probability of die out. Scenario C0 performs marginally better compared to the RC due to the counteracting effects of lower reversion and lower secondary spread associated with less transmissibility of the vaccine itself, leading to the expected annual cVDPV2 cases closely following the RC results, including the same probability of die out. Scenario D0 shows lower expected cVDPV2 cases than with mOPV2 use in RC, but higher than Scenario B0 due to lower vaccine effectiveness. Scenario E0 performs the worst due to combination of lower vaccine effectiveness and diminished secondary spread, leading to substantially higher expected annual cVDPV2 cases compared to the RC. Scenario F0 shows that switching to IPV from 2024 starts to diverge from and perform worse than the RC as soon as IPV oSIAs begin. This reflects the properties of IPV, which offers protection from paralysis to recipients with no prior immunity induced by prior live poliovirus exposure, but does not lead to indirect community vaccination due to secondary transmission (as occurs for OPV) [56]. In addition, the model assumes lower intensity SIAs for IPV.
Fig. 2 shows the expected value of the total number of global polio cases for the modeled oSIA characteristic options (Table 1, bottom 1–9) compared to the RC (Table 1, bottom option 0) for cVDPV1 and cVDPV2. (All simulations shown in Fig. 2 use vaccine option A (Table 1), hence the legends A1, A2, and so on for the different curves in Fig. 2). Scenario A1 shows that a subpopulation response in high R0 blocks overall performs worse than the RC. While limiting the geographical scope of outbreak response to avoid seeding of new cVDPV2s may save some expected cases during early years, in the long run, diminishing population immunity to transmission in surrounding areas allows for the wider virus spread (i.e., more than a 60% increase in the number of affected modeled subpopulations compared to the RC). In contrast, the results for scenario A2 show that using larger (i.e., blockwide oSIAs) in high R0 blocks performs better compared to the RC. This occurs because wider geographical scope increases population immunity in surrounding subpopulations within the block, which prevents potential outbreak virus spread and new outbreaks in other subpopulations within the block and beyond (i.e., more than a 60% decrease in the number of affected modeled subpopulations compared to RC). The results from scenario A3 show that shorter delay to the first round (by 15 days compared to the RC) leads to around a 20% decrease in cVDPV2 cases and around 15% reduction in affected modeled subpopulations compared to the RC. Scenario A4 shows that a longer delay to the first round (by 15 days compared to the RC) leads to around a 20% increase in cVDPV2 cases and around 15% more affected modeled subpopulations compared to the RC. As noted, recent oSIA experience included substantially longer delays for some oSIAs for various reasons, and thus future modeling may need to consider average delays of over 60 days.
Fig. 2.
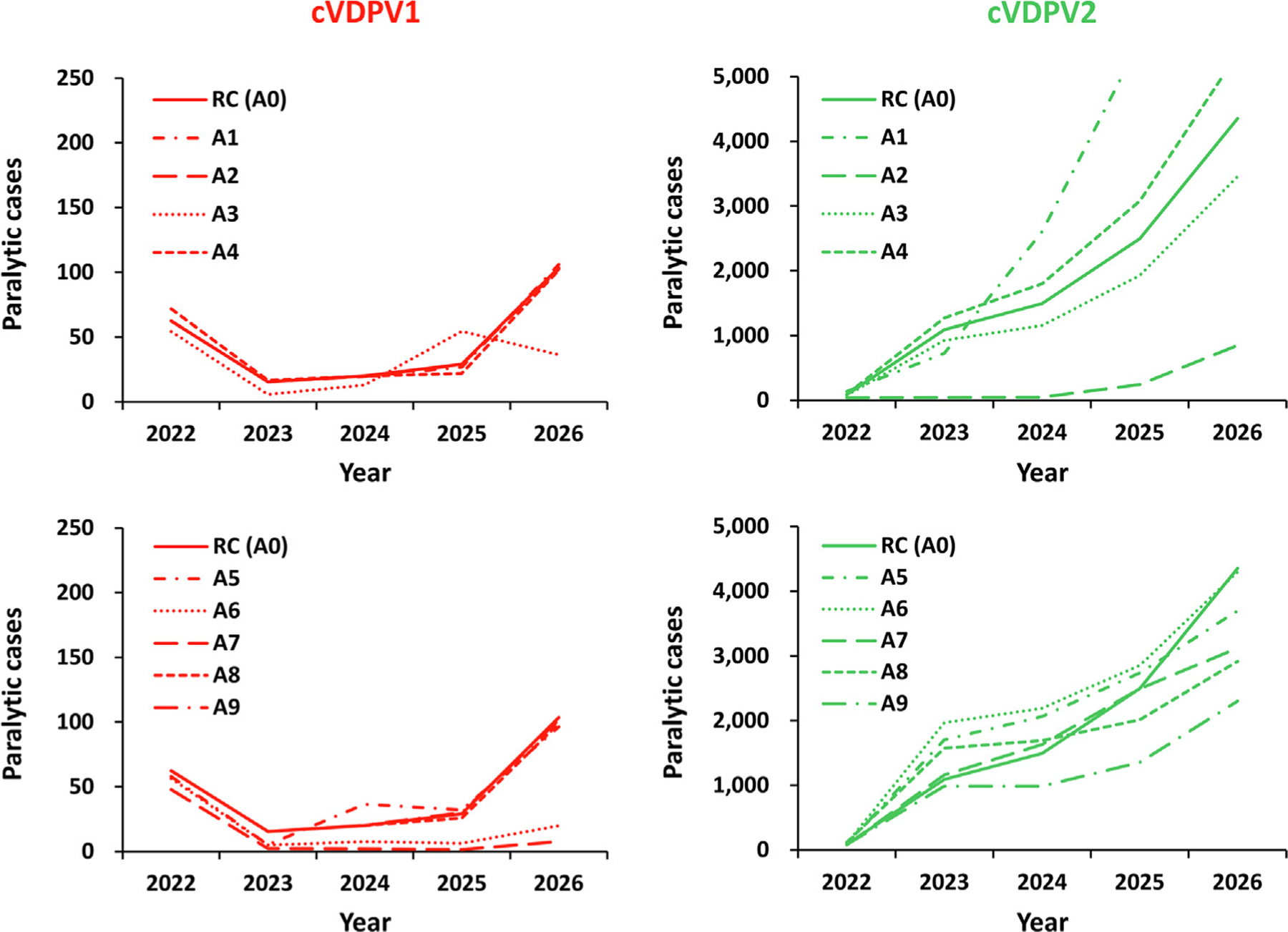
Expected global number of polio cases by year for 100 stochastic iterations of univariate outbreak response SIA change scenarios using mOPV2 for direct comparison to the reference case for 2022–2026 (see main text for descriptions). Notes: All scenarios use monovalent Sabin OPV2 (Table 1, top, row A) and all or all but one of the reference case characteristics (Table 1, bottom, row 0). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case, which assumes response in the outbreak subpopulation and 4 subpopulations in the same block at highest-risk (1 + 4) in blocks with high transmissibility that starts 45 days after case detection with 2 rounds with immunization intensity consistent with recent experience in preventive campaigns, and target children < 5 years of age; A1 reduces the scope of the response to just the outbreak subpopulation (1 + 0) in blocks with high transmissibility; A2 increase the scope to all 10 subpopulations in the block (1 + 9) in blocks with high transmissibility; A3 assumes a shorter delay of 30 days to the first round; A4 assumes a longer delay of 60 days to the first round; A5 assumes three rounds; A6 assumes four rounds; A7 assumes high intensity rounds (i.e., 80% immunization coverage, 70% repeated miss probability) for all response rounds; A8 assumes an expanded target age group of children < 10 years of age for all responses; and A9 assumes an expanded target age group of children < 10 years of age for outbreaks in subpopulations with no OPV2 use since 2016.
Scenario A5 shows that using three rounds for oSIAs initially performs worse compared to the RC, since more rounds lead to more cVDPV2s seeded starting from last quarter of 2022. This occurs due to the increased vulnerability of the population to mOPV2 transmission now 6 years after OPV2 cessation [57,58], which makes OPV2 exportations from outbreak subpopulations to other areas more likely to seed new cVDPV2 emergences and motivated prior analyses that explored restarting Sabin OPV2 in routine immunization [6,10,28,51]. However, the expected number of cases for scenario A5 starts to decrease after 2 years due to the overall increase in population immunity, leading to net improvement relative to the RC from 2026 onwards. Similarly, scenario A6 shows that using four oSIA rounds initially performs worse compared to the RC starting from the last quarter of 2022, with improvement relative to the RC from 2026 onwards, albeit more slowly. Scenario A7 shows that changing oSIA intensity closely follows the RC, with an initial (2022) decrease in cVDPV2 cases followed by a slight increase (2023–24) due to the trade-off between an increase in the amount of vaccine that can lead to potential seeding early on versus a threshold amount of population immunity that can shut down the transmission. The change in oSIA intensity eventually leads to a substantial decrease in cases by the end of the time horizon.
The results from scenario A8 show that always targeting an expanded age group of children < 10 initially performs worse compared to the RC, since the oSIAs seed new cVDPV2s outbreaks (particularly in already poorly-performing populations) starting from last quarter of 2022. However, the expected number of cases starts to decrease after 2 years due to the overall increase in population immunity, and scenario A8 outperforms the RC from 2025 onwards. The results of scenario A9 show that only targeting expanded age groups for populations that have not implemented oSIAs since OPV cessation performs better compared to always targeting the expanded age group. Better performance is accomplished by avoiding unnecessary seeding of new cVDPV2s in poorly-performing populations that experienced prior outbreaks, effectively producing fewer expected cVDPV2 cases compared to the RC.
Table 2 shows the estimated expected values and ranges of cases for 2022–2026 for the oSIA options modeled. While none of the simulated scenarios that simply change the vaccine used or make a univariate change in an oSIA characteristic succeeds at completely containing the cVDPV2 outbreaks alone (low probabilities of die out, see Table 3), some of the scenarios show a substantial decrease in the estimated expected number of cases (up to 87% reduction while responding by block in high R0 blocks).
Table 4 shows estimated expected values and ranges of vaccine use for outbreak response over the 5-year time horizon for the scenarios modeled. Since all scenarios use the same set of random number seeds for long range exportations, all non-IPV scenarios include iterations with a few exportations of cVDPV2 viruses into high-income countries that perform oSIAs with IPV. This leads to low numbers of IPV doses used for oSIAs shown for these scenarios. Comparing the performance on expected incidence (Table 2) to the respective vaccine needs for each scenario (Table 4) reveals some notable trade-offs. While some scenarios require a substantial increase of available vaccine doses, they offer high returns with respect to reducing expected cases. For example, although blockwide response in high R0 blocks (scenario A2) requires around 1.75 times the amount of vaccine dose over 5-year time horizon compared to the RC, scenario A2 leads to an expected 87% reduction in cases. At the same time, if nOPV2 behaves as assumed in scenario B0, its use may substantially reduce the amount of vaccine doses needed (around 50%) while simultaneously substantially reducing the number of cVDPV2 cases (decreased around 75%) compared to the RC. The use of nOPV2 with blockwide response (B2 or G2) reduce the number of expected cases and doses compared to scenario A2, which provides an indication of the benefits offered by reduced seeding, albeit in the context of inadequate oSIAs to shut down the outbreaks.
Fig. 3 shows the results of the different oSIA option combinations modeled. Combining the best univariate oSIA characteristic options (2, 3, 7, and 9, with or without 5) and using either vaccine choice A or B shows that with block response in high R0 blocks, shorter delay, three oSIA rounds, high intensity, and conditional EAG leads to the best performance in terms of epidemiology of poliovirus transmission (i.e., highest probability of die out – up to 96%, reduction in the number of affected modeled subpopulations – 88%, and cases avoided – 99%). As shown for the combinations that use vaccine characteristics in scenarios G-J (see Table 1, top), first using the available mOPV2 and then shifting to nOPV2 with the best performing oSIA characteristics could strike a balance between scenario performance in terms of epidemiological targets and vaccine dosses needed to achieve them. Specifically, given current mOPV2 stockpile levels as well as projected production of nOPV2 and its delivery times, considering two high-intensity oSIAs with shorter delays to the first round, wider response geographies in high R0 settings that expand the target age group if appropriate, and using immediately available mOPV2 followed by the use of nOPV2 (once available in sufficient quantities), emerges as an important option. Notably this option yields a 97% probability of die out, an 89% reduction in the number of affected modeled subpopulations, and a 99% reduction in expected cases, while avoiding waste of doses already purchased and ultimately saving almost 30% of overall doses (relative to the RC).
Fig. 3.
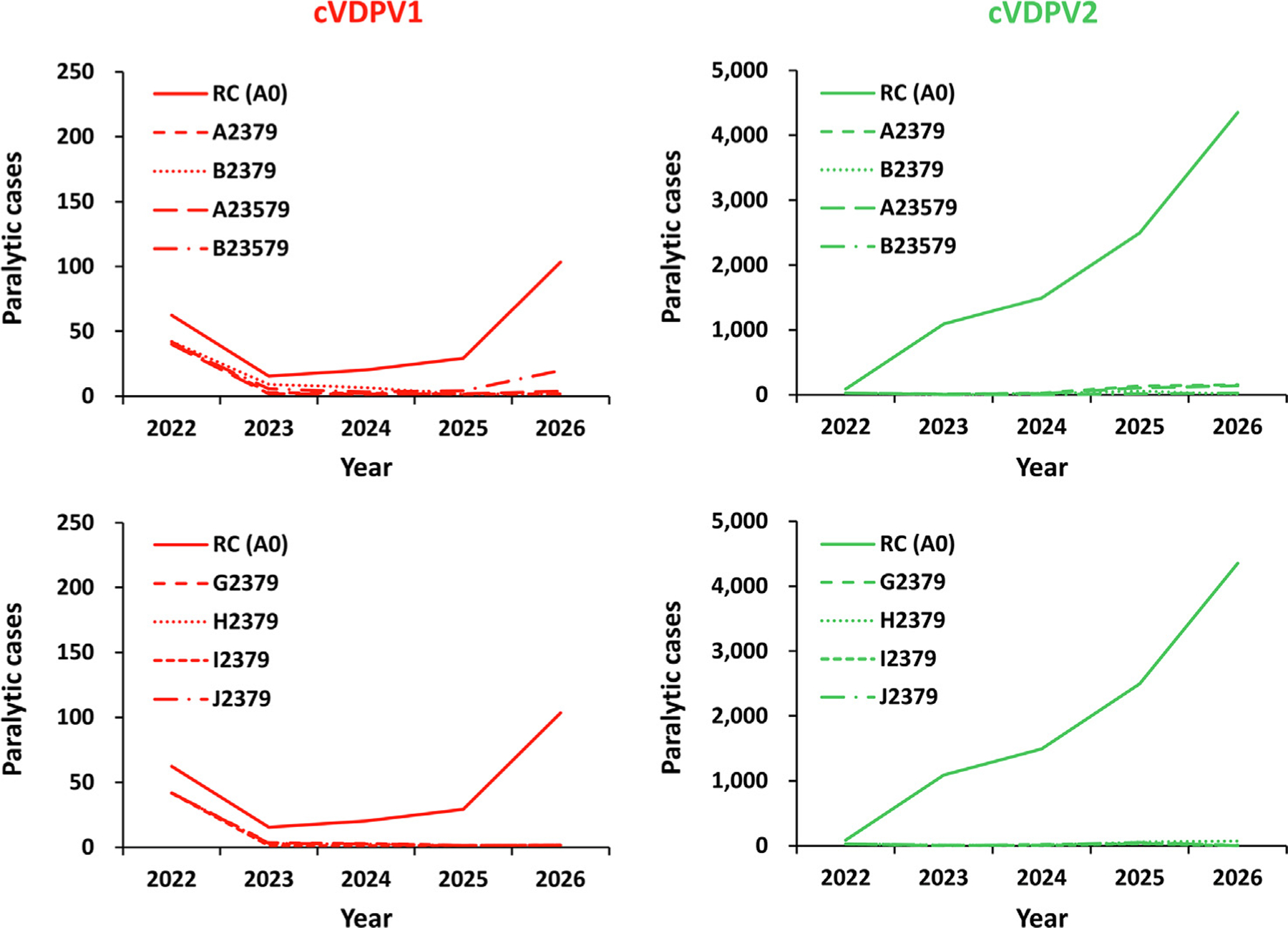
Expected global value of polio cases by year for 100 stochastic iterations of the combination of SIA options for 2022–2026 compared to the reference case (see main text for descriptions). Notes: All scenarios use the best combination of univariate changes (see Table 1, bottom) and either monovalent Sabin OPV2 (Table 1, top, row A), novel OPV2 (nOPV2) with the most favorable properties (Table 1, top, row B), or the use of mOPV2 for six months followed by nOPV2 with different properties (G, H, I, and J). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case.
Fig. 4 shows the results of the additional scenarios that use nOPV2, but do not improve quality. B2 and G2 show the substantial reductions that occur with blockwide response, and G239 and G2369. These scenarios show lower expected cases due to the larger oSIAs, but transmission continues in most of the stochastic iterations, which leads to lower PODs than those estimated when quality improves (see Table 4).
Although we focused on cVDPV2s, even with the optimistic assumption of WPV1 dying out in 2022, the risks of type 1 poliovirus transmission (i.e., WPV1 and/or cVDPV1) may potentially require simultaneous management of two types. The cVDPV1 results in Figs. 1–4 hint at this by showing some stochastic differences between the results that reflect the displacement of bOPV pSIAs by cVDPV2 outbreak response in some subpopulations for some oSIA characteristics.
4. Discussion
Despite suggestions from prior modeling about the need to treat any type 2 poliovirus transmission as global emergencies after OPV2 cessation and to aggressively shut these outbreaks down [7,22,26], global cases caused by type 2 increased over time [14] and success of OPV2 cessation for the current trajectory looks unlikely based on the RC.
The results of our analyses suggest that none of the simulated oSIA options with univariate changes succeed at completely containing the cVDPV2 outbreaks alone; however, some of them provide a substantial decrease in estimated expected number of cases. As anticipated by the prior modeling, increased time since OPV2 cessation makes larger outbreak response activities more important due to declines in population immunity [22]. To reach a high probability of ending global cVDPV2 transmission, countries need to employ a combination of changes to the implementation of oSIAs that substantially improve field performance. Countries need to use available OPV2s (mOPV2 or nOPV2) with shorter delays, wider response geographies in high R0 settings (e.g., sub-Saharan Africa), and focus on high intensity rounds with higher oSIA intensity (i.e., high coverage and low repeatedly missed children) that expand the target age group, if necessary. This approach requires substantial increases in vaccine doses needed over the currently planned stockpiles, but offers high probability of die out and a low number of cases.
Using increased amounts of mOPV2 for outbreak response comes with a risk of seeding new outbreaks if exported into populations with low and decreasing population immunity to transmission. However, the failure to contain existing outbreaks results in potential exportation of the outbreak virus and represents a greater risk [26], consistent with the most recent observations and recommendations of the SAGE [41]. Using tOPV, if available, instead of mOPV2 or nOPV2 would come with substantial benefits of also boosting immunity for types 1 and 3, with no or minimal impact on the effectiveness of type 2 responses [49]. With the co-circulation of types 1 and 2 reported in Mozambique in 2022 [17,50], the simultaneously management of two types will likely emerge as a priority and motivate discussions about the use of tOPV for oSIAs outside of Pakistan, Afghanistan, and Yemen.
The results of our analyses depend on a number of assumptions, with known limitations of modeling poliovirus transmission and OPV evolution, and available information (see details in appendix of [30]). The limitations include the conceptual characterization of global variability using block/subpopulation structure and the simplified modeling structures used to simulate effective poliovirus introductions during exportation to new block/subpopulation, transmission die-out, waning of immunity, OPV evolution in general and the field characteristics of nOPV2 specifically. In particular, assuming the best potential properties of nOPV2 (including the same individual protection as for mOPV2) while clinical trials in infants are still ongoing may prove too optimistic and therefore underestimate the actual vaccine needs. Moreover, trying to estimate the probability of die out of the scenarios by counting the number of iterations with no ongoing transmission of type 2 at the end of the time horizon may lead to both over- or underestimation due to artificial truncation of the time horizon.
Active reporting and field investigation of experiences with using nOPV2 should resolve some remaining questions. For example, ongoing clinical and field studies will provide insights about the safety and immunogenicity of nOVP2 in naïve infants, field effectiveness in outbreak populations, the impact of coadministration with bOPV, and further characterization of nOPV2 reversion properties with longer potential times of observation for transmission in populations.
With respect to delays to the first round of oSIAs, several issues warrant mention. First, disruptions in national surveillance activities and transportation networks continues to extend the time between sample collection and sequencing results in some countries (e.g., the confirmation of recent outbreaks in Guinea Bissau and Yemen followed long delays due to challenges in transporting specimens). Second, gaps in surveillance in detecting cases of acute flaccid paralysis in children, delays in investigation and sample collection, and delays in transporting and processing samples singly or in combination contributed to substantial delays in some national responses to cVDPVs. Third, limited supplies of mOPV2 from 2018 to 2020 and nOPV2 in 2021 further contributed to delays in many oSIAs. Fourth, despite the SAGE recommendation urging rapid response with any available OPV2-containing vaccine [41], many countries have delayed outbreak response and chosen not to use available supplies of mOPV2 due to an expressed preference to request nOPV2 even though limited supplies are available. As recovery from the disruption of COVID-19 continues, questions remain about how quickly oSIA activities will occur and with what effectiveness in reaching the target population.
Although not a limitation of the analysis as performed, we emphasize that our assumption about unlimited vaccine supplies influences the outcomes of the modeled scenarios. The reality of insufficient supplies makes actual implementation of some strategies impractical in the short term, even with efficient use (i.e., low wastage). Vaccine supply management for polio vaccines remains one of the most significant polio eradication areas for risk management.
Our implicit assumptions about the prospective trajectories of WPV1 and cVDPV1 also come with limitations. While the trajectory of interrupting WPV1 transmission in Pakistan and Afghanistan remains uncertain, particularly given the ongoing transmission observed through September 2022 [50], the RC assumes WPV1 dies out globally in 2022. The recent detection of a confirmed WPV1 case in Malawi, for which the closest genetic relative in Pakistan indicated transmission somewhere for almost two years [59], and cases in Mozambique [50] raises concerns about the quality of surveillance and the possibility of undetected transmission of WPV1 outside of Pakistan and Afghanistan. If transmission of WPV1 continues in Pakistan, Afghanistan, and/or other countries, then this could lead to changes in the model results due to operational interference that could occur when national responses to type 1 or type 2 outbreaks delay oSIA rounds that use OPV vaccine containing the other serotypes, because historically oSIA rounds have disrupted the timing and occurrence of preventive SIAs, as mentioned in the results. Since these results do not consider the impact of bOPV cessation, which could further increase the risks of cVDPV1, further modeling should consider the consequences of co-circulation of types 1 and 2 under conditions with even less (or no) bOPV use. The overall reduction in preventive SIAs that has occurred since 2016 increases the vulnerability to cVDPV1 and WPV1 risks in populations with poor RI as the population immunity to transmission for types 1 and 3 declines in these areas [51,60–62], while increasing use of IPV both partially off-sets some of this risk and may make any transmission occurring more difficult to detect. Cocirculation of multiple poliovirus types could lead to increasing demand for tOPV for oSIAs and reconsideration of restarting OPV2 in routine immunization.
In order to successfully stop all cVDPV2 transmission globally, countries and GPEI need to address the deficiencies in emergency outbreak response policy and implementation. This will require rapidly achieving and then maintaining much better program performance in areas experiencing outbreaks, and ensuring the maintenance of high polio immunization coverage in polio-free areas. These results should help decision makers at all levels recognize that using a different vaccine and partial or incremental improvements will not suffice to shut down global cVDPV2 transmission. If national and global leaders act urgently to substantially reduce response times, target larger populations — particularly in high transmission areas, achieve high coverage with improved access to under-vaccinated subpopulations, and use available type-2 containing vaccines, then this could substantially increase the probability of die-out of cVDPV2 transmission globally.
Acknowledgments
The first (D.A.K.) and last two authors (K.B. and K.M.T.) acknowledge support for this publication under Cooperative Agreement Number 5NU2RGH001915 funded by the US Centers for Disease Control and Prevention. The views expressed are solely those of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention or Department of Health and Human Services. We thank Frank Mahoney, John Vertefeuille, and members of the GPEI Strategy Committee for helpful comments and discussions.
Footnotes
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
All of the data that the authors can share is available in the public domain and appropriate citations are provided.
References
- [1].Tebbens RJD, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal 2006;26(6):1471–505. [DOI] [PubMed] [Google Scholar]
- [2].Thompson KM, Tebbens RJD. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal 2006;26(6):1423–40. [DOI] [PubMed] [Google Scholar]
- [3].Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, et al. Review: Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal 2013;33(4):680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kalkowska DA, Pallansch MA, Thompson KM. Updated modelling of the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. Epidemiol Infect 2019;147:e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame Strategic Plan (2013–2018) http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf; 2013. [accessed Jun 4, 2019].
- [6].Thompson KM, Kalkowska DA. Review of poliovirus modeling performed from 2000–2019 to support global polio eradication. Expert Rev Vaccines 2020;19:661–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thompson KM, Tebbens RJD. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines 2012;11(4):449–59. [DOI] [PubMed] [Google Scholar]
- [8].Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide, 2016. MMWR 2016;65(35):934–8. [DOI] [PubMed] [Google Scholar]
- [9].Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: Stockpile needs and coordinated OPV cessation. Med J Med 2008;10:190. [PMC free article] [PubMed] [Google Scholar]
- [10].Duintjer Tebbens RJ, Thompson KM. Poliovirus vaccination during the endgame: Insights from integrated modeling. Expert Rev Vaccines 2017;16 (6):577–86. [DOI] [PubMed] [Google Scholar]
- [11].World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2015 - conclusions and recommendations. Wkly Epidemiol Rec 2015;90:681–700. [PubMed] [Google Scholar]
- [12].Diop OM, Asghar H, Gavrilin E, Moeletsi NG, Benito GR, Paladin F, et al. Virologic Monitoring of Poliovirus Type 2 after Oral Poliovirus Vaccine Type 2 Withdrawal in April 2016 - Worldwide, 2016–2017. Morb Mortal Wkly Rep 2017;66(20):538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper LV, Bandyopadhyay A, Gumede-Moeletsi N, Mach O, Mkanda P, Ndoutabé M, et al. Risk factors for spread of vaccine-derived type 2 polioviruses in Africa following global withdrawal of trivalent oral poliovirus vaccine and impact of outbreak response with monovalent vaccine: a retrospective analysis of surveillance data. Lancet Infect Dis 2022;22:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thompson KM. Polio eradication: what kind of world do we want?. Lancet Infect Dis 2022;22(2):161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Macklin GR, O’Reilly KM, Grassly NC, Edmunds WJ, Mach O, Santhana Gopala Krishnan R, et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020;368 (6489):401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kalkowska DA, Pallansch MA, Cochi SL, Kovacs SD, Wassilak SGF, Thompson KM. Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal 2021;41(2):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].World Health Organization Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus https://polioeradication.org/wp-content/uploads/2022/09/weekly-polio-analyses-cVDPV-20220906.pdf; 2022. [accessed 9 Sep, 2022].
- [18].World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2017 - conclusions and recommendations. Wkly Epidemiol Rec 2017;92:301–20. [PubMed] [Google Scholar]
- [19].Duintjer Tebbens RJ, Thompson KM. Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J Infect Dis 2014;210:S485–97. [DOI] [PubMed] [Google Scholar]
- [20].Kalkowska DA, Pallansch MA, Wilkinson A, Bandyopadhyay AS, Konopka-Anstadt JL, Burns CC, et al. Updated characterization of poliovirus outbreak response strategies for 2019–2029: Impacts of the use of novel OPV2 strains. Risk Anal 2021;41:329–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harutyunyan V QA, Pallansch M, Zipursky S, Woods D, Ottosen A, Vertefeuille J, Lewis I. Global oral poliovirus vaccine stockpile management as an essential preparedness and response mechanism for type 2 poliovirus outbreaks following global oral poliovirus vaccine type 2 withdrawal. Vaccine 2022; S0264-410X(22)00204-3. [DOI] [PMC free article] [PubMed]
- [22].Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, Cochi SL, Thompson KM. Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infecti Dis 2016;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Ehrhardt DT, Farag NH, Hadler SC, et al. Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Anal 2018;38 (8):1701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thompson KM, Duintjer Tebbens RJ. The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission. BMC Infect Dis 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kalkowska DA, Pallansch MA, Cochi SL, Thompson KM. Updated characterization of poliovirus transmission in Pakistan and Afghanistan and the impacts of different outbreak response options. J Infect Dis 2021;224:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kalkowska DA, Pallansch MA, Wassilak SGF, Cochi SL, Thompson KM. Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine 2021. 10.1016/j.vaccine.2021.04.061. online May 14, 2021. In this issue. [DOI] [PMC free article] [PubMed]
- [27].Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal 2006;26(6):1541–56. [DOI] [PubMed] [Google Scholar]
- [28].Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Thompson KM. An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect Dis 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thompson KM, Duintjer Tebbens RJ. Lessons from globally-coordinated cessation of serotype 2 oral poliovirus vaccine for the remaining serotypes. J Infect Dis 2017;216:S168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kalkowska DA, Wassilak SGF, Cochi SL, Pallansch MA, Thompson KM. Global transmission of live polioviruses: Updated integrated dynamic modeling of the polio endgame. Risk Anal 2021;41:248–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Macadam AJ, Ferguson G, Stone DM, Meredith J, Knowlson S, Auda G, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J Virol 2006;80 (17):8653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol 2015;10(5):791–808. [DOI] [PubMed] [Google Scholar]
- [33].Duintjer Tebbens RJ, Thompson KM. The potential benefits of a new poliovirus vaccine for long-term poliovirus risk management. Future Microbiol 2016;11 (12):1549–61. [DOI] [PubMed] [Google Scholar]
- [34].Sáez-Llorens X, Bandyopadhyay AS, Gast C, Leon TD, DeAntonio R, Jimeno J, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials. The Lancet 2021;397(10268):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Coster I, Leroux-Roels I, Bandyopadhyay AS, Gast C, Withanage K, Steenackers K, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials. The Lancet 2021;397 (10268):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Konopka-Anstadt JL, Campagnoli R, Vincent A, Shaw J, Wei L, Wynn NT, et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. npj Vaccines 2020;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gast C, Bandyopadhyay AS, Sáez-Llorens X, De Leon T, DeAntonio R, Jimeno J, et al. Fecal shedding of two novel live attenuated oral poliovirus type 2 vaccines candidates by healthy bOPV/IPV-vaccinated infants: two randomized clinical trials. J Infect Dis 2022;226(5):852–61. 10.1093/infdis/jiab507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].World Health Organization. cVDPV2 outbreaks and the type 2 novel oral poliovirus vaccine (nOPV2) https://polioeradication.org/wp-content/uploads/2022/01/GPEI_cVDPV2-nOPV2_Factsheet_13-Jan-2022-EN.pdf; 2022. [accessed Feb 24, 2022].
- [39].World Health Organization. Global Advisory Committee on Vaccine Safety, 17 September 2021. Wkly Epidemiol Rec 2022;97:17–24. [Google Scholar]
- [40].Global Polio Eradication Initiative. nOPV2: Clinical development summary updated June 2022 https://polioeradication.org/wp-content/uploads/2022/06/nOPV2-Clinical-Development-Summary_June-2022-Update_Final-EN.pdf; 2022. [accessed 11 Aug 2022].
- [41].World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2021: conclusions and recommendations. Wkly Epidemiol Rec 2021;96:613–32. [Google Scholar]
- [42].Kalkowska DA, Voorman A, Pallansch MA, Wassilak SGF, Cochi SL, Badizadegan K, et al. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine 2021:S0264-410X(21)00473-4. [DOI] [PMC free article] [PubMed]
- [43].World Health Organization Global Polio Eradication Initiative. Standard operating procedures: Responding to a poliovirus event or outbreak: Part 2: Protocol for poliovirus type 2 http://polioeradication.org/wp-content/uploads/2018/01/pol-sop-responding-polio-event-outbreak-part2-20180117.pdf; 2018. [accessed August 20, 2020].
- [44].World Health Organization Global Polio Eradication Initiative. Standard operating procedures: Responding to a poliovirus event or outbreak: Version 3.1, March 2020 http://polioeradication.org/wp-content/uploads/2020/04/POL-SOP-V3.1-20200424.pdf; 2020. [accessed August 20, 2020].
- [45].World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2019–2023) https://polioeradication.org/wp-content/uploads/2019/06/english-polio-endgame-strategy.pdf; 2019. [accessed Jun 4, 2019].
- [46].World Health Organization Global Polio Eradication Initiative. Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: Addendum to the Polio eradication and endgame strategic plan (2019–2023) http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf; 2020. [accessed Mar 10, 2020].
- [47].World Health Organization Global Polio Eradication Initiative. Polio eradication strategy 2022–2026: Delivering on a promise https://polioeradication.org/wp-content/uploads/2021/06/polio-eradication-strategy-2022-2026-pre-publication-version-20210609.pdf; 2021. [accessed Jun 11, 2021].
- [48].World Health Organization. WHO/UNICEF estimated coverage time series http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls; 2020. [accessed Jul 24, 2020].
- [49].Kalkowska DA, Badizadegan K, Thompson KM. Modeling scenarios for ending poliovirus transmission in Pakistan and Afghanistan. Risk Anal 2022. 10.1111/risa.13983. online June 23, 2022. [DOI] [PMC free article] [PubMed]
- [50].World Health Organization. Wild poliovirus list: List of wild poliovirus by country and year https://polioeradication.org/wp-content/uploads/2022/09/weekly-polio-analyses-WPV-20220906.pdf; 2022. [accessed 9 Sep, 2022].
- [51].Duintjer Tebbens RJ, Thompson KM. Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines 2018;17:739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Van Damme P, De Coster I, Bandyopadhyay AS, Revets H, Withanage K, De Smedt P, et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 2019;394:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Duintjer Tebbens RJ, Pallansch MA, Alexander JP, Thompson KM. Optimal vaccine stockpile design for an eradicated disease: Application to polio. Vaccine 2010;28:4312–27. [DOI] [PubMed] [Google Scholar]
- [54].World Health Organization Global Polio Eradication Initiative. Standard operating procedures - Responding to a poliovirus event or outbreak – version 4 https://polioeradication.org/wp-content/uploads/2022/07/Standard-Operating-Procedures-For-Responding-to-a-Poliovirus-Event-Or-Outbreak-20220807-EN-Final.pdf; 2022. [accessed 20 Aug, 2022].
- [55].Shaw AG, Cooper LV, Gumede N, Bandyopadhyay AS, Grassly NC, Blake IM. Time taken to detect and respond to polio outbreaks in Africa and the potential impact of direct molecular detection and nanopore sequencing. J Infect Dis 2022;226:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Estivariz CF, Pallansch MA, Anand A, Wassilak SG, Sutter RW, Wenger JD, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Current Opinion in Virology 2013;3:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of potential non-synchronous cessation. BMC Infect Dis 2016;16:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infect Dis 2016;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].World Health Organization Global Polio Eradication Initiative. GPEI statement on WPV1 in Malawi https://polioeradication.org/news-post/gpei-statement-on-wpv1-in-malawi/; 2022. [accessed Feb 22, 2022].
- [60].Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, Pallansch MA, Cochi SL, Thompson KM. Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. J Vaccines Vaccination 2016;7:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kalkowska DA, Thompson KM. Expected implications of globally-coordinated cessation of serotype 3 oral poliovirus vaccine (OPV) before serotype 1 OPV. Risk Anal 2021;41:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thompson KM, Kalkowska DA, Duintjer Tebbens RJ. Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses. Vaccine 2015;33:1568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data that the authors can share is available in the public domain and appropriate citations are provided.


